Abstract
The treatment of lung infection in the context of cystic fibrosis (CF) is limited by a biofilm mode of growth of pathogenic organisms. When compared to planktonically grown bacteria, bacterial biofilms can survive extremely high levels of antimicrobials. Within the lung, bacterial biofilms are aggregates of microorganisms suspended in a matrix of self-secreted proteins within the sputum. These structures offer both physical protection from antibiotics as well as a heterogeneous population of metabolically and phenotypically distinct bacteria. The bacteria themselves and the components of the extracellular matrix, in addition to the signaling pathways that direct their behaviour, are all potential targets for therapeutic intervention discussed in this review. This review touches on the successes and failures of current anti-biofilm strategies, before looking at emerging therapies and the mechanisms by which it is hoped they will overcome current limitations.
Keywords: Pseudomonas aeruginosa, biofilm matrix, anti-biofilm, exopolysaccharides, adjunctive therapies, antimicrobial resistance
1. Introduction
Cystic fibrosis (CF) is a life limiting disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. CF lung disease is characterized by inflammation as well as chronic bacterial infection, where bacteria become host-adapted pathogens. In CF, the lives of affected individuals are punctuated by pulmonary exacerbations in which bacterial driven inflammatory changes can lead to permanent scarring and decline in lung function [1,2]. Pseudomonas aeruginosa and Staphylococcus aureus are two most common pathogens in CF airways, although other organisms such as Burkholderia spp., non-tuberculous mycobacteria (NTM) spp., Stenotrophomonas maltophilia, Achromobacter spp., and fungi such as Aspergillus spp., have also been implicated in CF lung infection [3].
Understanding how bacteria survive in the lungs of patients despite years of antimicrobial therapy is important and may help designing new approaches to target chronic infections. Within the context of CF lung disease, bacterial antimicrobial resistance (AMR) increases over the lives of patients and limits the efficacy of antibiotics [4,5,6]. Higher rates of AMR, driven in large part by the selective pressures of antimicrobial treatments, are associated with decline in pulmonary function (as measured by forced expiratory volume in 1s, FEV1), as well as decreased quality of life outcomes and earlier mortality as disease progresses [7,8,9]. Resistance in the context of antimicrobial susceptibility testing (AST) involves measuring both innate properties of the bacteria like membrane permeability [10], as well as the expression of efflux pumps and the production of enzymes like β-lactamases that degrade antibiotics [11]. Conventional planktonic-based AST fails to take into account the biofilm lifestyle and resistance that result from social adaptation to the local environment. Although conventional AST is the laboratory cornerstone for antimicrobial selection in the treatment of acute infections, this method falls short of predicting clinical outcomes in CF [12,13].
Bacteria exhibit social behaviours and live in aggregates or communities of organisms suspended within a matrix of self-secreted proteins—termed a biofilm. The main constituents of this matrix are extracellular DNA, exopolysaccharides, lipids, proteins and metabolites [14]. These constituents surround the bacterial community, offering physical and electrostatic protection from antimicrobials, host defenses and other environmental stressors [15]. Channels for nutrients and metabolites running through the structure of protein scaffolding allow for differential access to nutrients, which creates a heterogeneity of bacterial populations with differing rates of metabolic activity and cell division [16]. The high level of population heterogeneity within the biofilm gives rise to ‘persister cells’, a metabolically dormant phenotype. Persister cells represent a small fraction of the bacterial population but are present in higher numbers within slow-growing biofilms compared to planktonically grown bacteria, perhaps due to differences in access to nutrients or as a population level survival mechanism. These sub populations of cells can survive high doses of bactericidal agents [17,18,19].
We now understand that the predominant mode of growth of bacteria in chronic infections is within biofilms rather than planktonically, which may be a transitional phase in the growth and spread of bacteria. Biofilms are a recognized hurdle to the successful treatment of chronic wound, ear and eye infections, as well as infections involving prosthetic medical devices [20]. Bacterial biofilms require a substratum to bind to, like that of a wound, or the surface of a foreign medical device, like a urinary catheter. However, in the lungs of individuals with CF, bacteria often aggregate to themselves, forming clusters of communities suspended within the airway mucus [21,22,23].
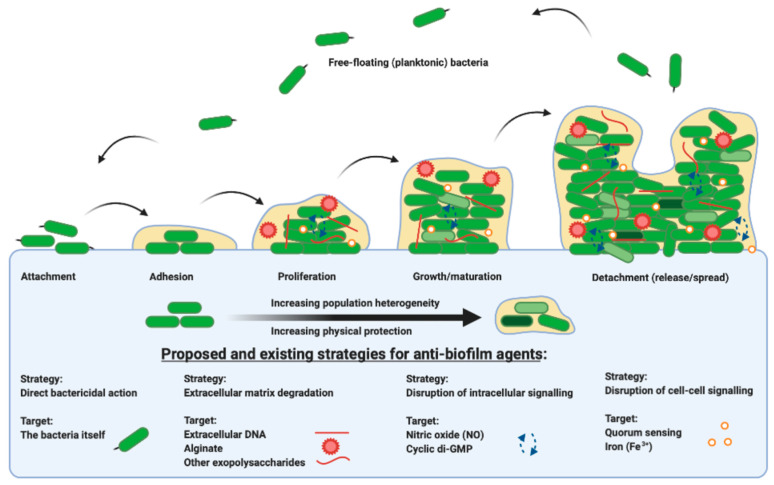
Each stage in the life cycle of a biofilm is summarized in Figure 1 from attachment, maturation, dispersal and colonization. The bacteria themselves and the components of the extracellular matrix, in addition to the signaling pathways that direct their behaviour, are all potential targets for therapeutic intervention. This review outlines the evidence for anti-biofilm agents currently in use and explores upcoming therapies and their targets. Recognizing the diversity of agents and targets, there is an emerging emphasis on the development of specific anti-biofilm therapies for adjuvant use with current antibiotics, for the exploitation of dual mechanism bacterial killing.
Figure 1.
A representation of the bacterial biofilm life cycle, from the point at which free-floating planktonic cells attach to a substrate or surface, to adhesion, to proliferation and microcolony formation, through to growth and maturation and subsequent dispersal, propagating and beginning the life cycle once more. There are many existing and proposed strategies for targeting biofilm grown organisms, from direct action on the bacteria themselves, to the extracellular matrix, to intracellular signaling pathways, to the disruption of cell-to-cell signaling. Figure created in BioRender.com.
2. Strategy: Direct Antibacterial Action
It has been shown that minimum biofilm eradication concentrations can be 100–1000 fold higher than the minimum inhibitory concentration (MIC) of antibiotics against planktonic grown bacteria [24]. Achieving these concentrations of antibiotics either systemically, through oral or intravenous administration, or via nebulization and inhalation, is difficult [25,26,27]. Thus, it becomes necessary to evaluate ways in which antibiotics can be effective as antibiofilm agents and where they fail, in order to optimize the efficacy of our treatments. Many antimicrobial agents are limited in action by the characteristics of bacterial biofilms. For instance, antibiotics including β-lactams, fluoroquinolones and aminoglycosides target actively dividing or metabolically active cells. Persister cells, cells with low metabolic activity embedded within the matrix, are refractory to treatment. Furthermore, components of the matrix itself provide a physical barrier to the dispersal and penetration of antimicrobials. In vitro P. aeruginosa work has demonstrated that the use of the aminoglycoside tobramycin—a mainstay in the treatment of Gram-negative infections—kills only the metabolically active layer of cells at the extremity of the biofilm, without the ability to penetrate the interior of the aggregate [28]. The polymyxin antibiotic colistimethate sodium (colistin), on the other hand, acts as a cationic detergent and binds to the negatively charged lipopolysaccharide present in the outer membrane of P. aeruginosa and has no such requirement for metabolic activity to take effect [28].
2.1. Dual Antibiotic Administration
The current practice of using antibiotic combinations that target different mechanisms of action to treat CF pulmonary infections may be more effective at killing bacterial biofilms than single antibiotics use. Colistin-tobramycin combinations demonstrated superior efficacy in comparison to monotherapy in the eradication of P. aeruginosa biofilms, both in vitro and in a rat lung infection model [28]. In addition, colistin-ceftolozane/tazobactam and colistin/meropenem combinations demonstrated both reduced bacterial counts of biofilm-embedded P. aeruginosa, and less colistin resistance when compared to colistin administration alone [29]. Biofilm models of both Acinetobacter baumannii and P. aeruginosa also showed that colistin-aminoglycoside combinations were efficacious in the eradication of persister cells otherwise unaffected by monotherapy [30,31].
2.2. Enhancing Antibiotic Efficacy and Delivery
One strategy to optimize antibiotic delivery to cells distributed within the biofilm is with liposomal preparations. In this approach, antibiotics are encapsulated in phospholipid vesicles that can fuse to cell membranes, allowing for improved drug delivery. Early studies showed that both charge [32] and size [33] were important factors in reaching bacterial cells within the biofilm. Imaging studies have confirmed penetration of liposomal amikacin particles deep into the layers of both non-tuberculous mycobacterial and P. aeruginosa biofilms [34,35]. Randomized trials of inhaled liposomal amikacin demonstrated non inferiority to tobramycin inhalation solution in P. aeruginosa lung infection in patients with CF [36,37]. More recently, a randomized trial using the same preparation for CF patients with NTM infection, resulted in culture conversion and improved performance on a 6 min-walk-test, although it did not meet the primary endpoint of reduction of sputum bacterial density [38]. The approach of developing liposomal formulations of antibiotics is the focus of other extensive reviews on the subject [37,39,40].
Antimicrobial peptides (AMPs) are a class of natural and synthetic proteins that vary greatly in size and function. They have been the focus of research looking into many diverse therapeutic applications and have shown promise both as antimicrobials in their own right, but also as adjuncts to standard antibiotics in combatting biofilm infections [41,42]. One such novel cationic AMP, 6K-F17, was seen to effectively kill biofilms and reduce biofilm biomass when used with tobramycin against multi-drug resistant CF P. aeruginosa isolates [43]. Still other AMPs have been shown to reduce LPS-induced pro-inflammatory responses [44,45]. The multitude of proposed functions—from direct antimicrobial action, to potentiation of antibiotics to the modulation of inflammatory responses known to be detrimental in infection—make AMPs an exciting adjunctive therapy in CF biofilm infections.
2.3. Bacteriophage
Bacteriophages are viruses that use bacteria as their hosts to complete their life cycle. There are different types of such viruses, including those that are lysogenic or filamentous. Lysogenic phages incorporate themselves into the host genome and can confer resistance genes [46] while filamentous phages are themselves a component of the biofilm matrix [47,48]. Recently, there has been a resurgence in interest of lytic phages used as an anti-biofilm agent. Lytic phages bind to, replicate within and lyse specific bacterial hosts and are present in the environment wherever there are bacteria. There are many proposed benefits to bacteriophage therapy—among them, the ability to replicate at sites with the highest burden of infection and the ability to target specific bacterial species, leaving the remaining host microbial community intact. In contrast to most conventional antibiotics that require actively dividing bacterial cells, bacteriophages have the ability to lyse metabolically dormant cells. A study investigating certain S. aureus phages demonstrated infection and lysis of metabolically dormant persister cells [49]. Some lytic phages specific to P. aeruginosa employ the use of exopolysaccharide degrading enzymes that can degrade biofilms, which may prove beneficial in chronic, mucoid infections. Early reports of P. aeruginosa lytic phages demonstrated expression of alginate lyase, an enzyme that degrades alginate (exopolysaccharide produced by many types of bacteria, but particularly P. aeruginosa), allowing the phages to penetrate into deeper layers of the bacterial aggregates seen within the CF lung [50].
Within the context of P. aeruginosa infection, a randomized, double-blinded, placebo-controlled trial of a topical phage preparation in patients with otitis media refractory to antibiotic treatment demonstrated both safety and efficacy [51]. In vitro work has similarly demonstrated the efficacy of bacteriophages in reducing the colony count of P. aeruginosa in sputum samples from CF patients [52]. In animal models of P. aeruginosa lung infection (both planktonic and biofilm), the administration of lytic phage preparations lowered both bacterial load and inflammatory markers in the airway [53,54]. Although there have been reports of the use of lytic phages in antibiotic refractory infections [55,56], there are no published clinical trials in humans demonstrating the efficacy of this therapy within the context of CF lung infection. There are, however, trials underway, including a phase 1a/2b trial exploring the safety and efficacy of a nebulized preparation of anti-pseudomonal bacteriophages in CF patients with chronic infection (NCT04596319) [57].
3. Strategy: Targeting the Biofilm Matrix
The components of the extracellular matrix are potential therapeutic targets to free bacteria and expose them to both host immune cells, as well as antibiotics. Although there are many components of the extracellular matrix of biofilms, this paragraph will focus on those targeted by drugs with promising results thus far.
3.1. Alginate
One example of a biofilm matrix target is the P. aeruginosa exopolysaccharide, alginate. P. aeruginosa can express a mucoid phenotype through copious production of alginate, which encases bacterial communities, providing a barrier to antimicrobials and host defences [58,59]. Alginate is one exopolysaccharide comprising the extracellular matrix of the biofilm, and is overexpressed in many—but not all—chronic isolates of P. aeruginosa from airways of patients with CF. Such a phenotypic change is an example of a survival mechanism that can be driven by factors inherent in the CF lung [58,60], but also by external factors, including antibiotic administration [61].
In the context of CF, there are a number of compounds designed to break through this protective layer. Many have examined the therapeutic potential of degrading enzyme alginate lyase to break through the sticky matrix of polysaccharides. There are several in vitro studies demonstrating the efficacy of this compound at disrupting the architecture of the biofilm and increasing susceptibility to antibiotic therapy [62]. More recently, alginate lyase has been combined with antibiotics to degrade biofilms in order to improve penetration of antimicrobials deep into the biofilm. The fluoroquinolone ciprofloxacin, for example, has been formulated for co-administration with alginate lyase in several novel preparations [63,64]. In one, chitosan nanoparticles co-immobilized with ciprofloxacin and alginate lyase effectively reduced the biomass and cell density of biofilm grown P. aeruginosa [64].
Alginate itself can be hydrolyzed into low molecular weight (mW]) oligomers that have been used in different biological applications. OligoG CF-5/20 (OligoG) is one such small (mW 2600 Da) alginate oligosaccharide derived from seaweed that has been shown to alter the structure of mucin and increase pore size in the mucus layer, increasing particle diffusion and modifying the viscoelasticity of sputum in CF patients [65]. Early in vitro work showed that OligoG can potentiate the action of antibiotics against multi-drug resistant (MDR) Gram-negative bacteria (including Pseudomonas, Acinetobacter, and Burkholderia spp.) as well as in fungal species (including Aspergillus and Candida spp.) [66,67]. Further work confirmed that OligoG was able to inhibit biofilm formation of Gram-negative organisms [68] and disrupt established biofilm matrix in vitro [69]. OligoG demonstrated an ability to inhibit biofilm formation in a mouse model of P. aeruginosa infection in a dose-dependent manner [70]. A randomized, double-blind, placebo-controlled, crossover study investigating a dry powder formulation of OligoG in 65 CF patients with chronic P. aeruginosa infection demonstrated safety, and a trend towards an increase in lung function, although results did not reach significance [71]. This approach is still actively being studied, with a dose finding study of OligoG underway (NCT03698448) [72].
3.2. Other Exopolysaccharides
In addition to alginate, P. aeruginosa produces two other exopolysaccharides: Psl and Pel. Psl and Pel are key components in the formation and maintenance of the biofilm structural framework that provide physical protection to bacteria [73,74]. There is promising work investigating the biofilm dispersion potential of glycoside hydrolases in targeting and degrading Psl and Pel, thereby liberating bacteria trapped within the biofilm and exposing cells to antimicrobial therapy [73]. In a porcine wound model of chronic P. aeruginosa infection, PslG hydrolase co-administered with antibiotics demonstrated increased antibiotic penetration into the biofilm as well as improved immune-mediated clearance, when compared to monotherapy [74].
Poly-b-1,6-N-acetyl-D-glucosamine (PNAG/PIA) is another exopolysaccharide important in the structural integrity of biofilms in both Gram-positive and Gram-negative organisms. A hydrolase targeting PNAG called DispersinB showed therapeutic potential in the biofilm dispersal of several different bacterial species (S. aureus, S. epidermidis, Enterococci, A. baumannii, Klebsiella pneumoniae, and P. aeruginosa) in laboratory models of biofilm infection [75].
3.3. Extracellular DNA (eDNA)
Extracellular DNA (eDNA) is a key component of the biofilm matrix. Recombinant human deoxyribonuclease I (dornase alfa or DNase) cleaves DNA, released from neutrophils as well as bacteria, that provide a structure to the biofilm. DNase therapy has been shown in meta-analyses to be effective both at reducing the rate of lung function decline, as well as reducing the number of pulmonary exacerbations [76]. DNase also disrupts pre-formed bacterial biofilms, which may account for some of its effect [77]. Although the predominant mechanism has been attributed to the reduction in mucous viscosity and improved airway clearance, DNase also disperses bacteria and increases antimicrobial susceptibility in staphylococcal species [77] as well as in mixed bacterial species models of biofilm infection [78]. Furthermore, the discovery of bacterial expressed eDNA binding proteins that crosslink with eDNA strands within the matrix provides potential new therapeutic targets for the eradication of biofilms [79]. As a proof of concept, it has been shown that Burkholderia cenocepacia biofilms were dispersed by targeting the DNA binding integration host factor (IHF) but could not be dispersed by DNase treatment [80].
4. Strategy: Targeting Intracellular Signaling Pathways
Bis-(3′-5′)-cyclic dimeric GMP (c-di-GMP) is the principle second messenger involved in the regulation of genes that are expressed in a biofilm lifestyle [81]. Altering intracellular signalling mechanisms has the potential to catalyze the transition of bacteria from a biofilm lifestyle into a planktonic one, thus rendering the dispersed bacteria more susceptible to antimicrobials. There are many signalling pathways with intracellular mediators that have been implicated in biofilm formation and bacterial virulence, but here we focus on the pathways that have yielded the most promise in terms of pharmaceutical intervention.
4.1. Compounds Reducing Intracellular Cyclic-di-GMP
Increased levels of c-di-GMP promote transition from planktonic to a biofilm mode of growth, whereas reduction in levels results in biofilm dispersal [82]. Cells are disseminated from P. aeruginosa biofilms through the modulation of specific phosphodiesterases, which are activated in response to environmental cues. Blocking or downregulating this signalling pathway has therefore been proposed as a promising drug target to aid in the eradication of biofilms.
Previous studies demonstrated that a decrease in c-di-GMP level, led to the dispersal of established P. aeruginosa biofilms on infected silicone implants in the peritoneal cavity of mice [83]. In the treated mice, bacteria accumulated in the spleen, and although no mice died or experienced sepsis, this finding would suggest adjunctive use of antimicrobials to minimize the risk of widespread bacterial dispersal. Different groups have since used high throughput methods to detect compounds that reduce the levels of c-di-GMP in a number of different bacterial species [84]. Azathioprine, an immunosuppressive drug already in clinical use, is a compound shown to decrease di-GMP, correlating with a lack of expression of extracellular matrix proteins in E. coli [85].
4.2. Other Compounds Reducing Cyclic-di-GMP: Nitric Oxide (NO)
Nitric oxide (NO), while long recognised as a vital signalling compound in eukaryote biology [86], has now been implicated as one of the key intracellular signalling molecules involved in bacterial growth—namely, in the transition between planktonic and biofilm lifestyles. Highly water soluble and diffusible in biological systems, NO is ideal for intracellular communication. Although high levels of NO can be toxic to bacteria, some bacterial species encounter high levels of this compound in denitrification, a process whereby certain prokaryotes can use nitrate or nitrate for respiration in oxygen-limited conditions [87]. Exogenous NO has been shown to improve antibiotic susceptibility in resistant bacteria and has therefore been suggested as an adjuvant therapy [88]. It is believed that NO works by increasing concentrations of specific PDEs which, in turn, reduce the levels of c-di-GMP and disperse cells from the biofilm [89,90,91]. Treatment of P. aeruginosa biofilms with NO has been shown to result in biofilm dispersal [89,90], but not kill planktonically grown cells [92]. There are currently three approaches to using NO therapeutically in bacterial infection: Delivering NO donor prodrugs, direct inhalation of NO, and increasing endogenous NO formation.
NO donor prodrugs are designed to release NO locally, thereby triggering biofilm dispersion. Screening of chemical libraries first led to the identification of sulfathiazole [93], an antimetabolite drug, as a potential compound targeting this pathway. More recently, cephalosporin-3′-diazeniumdiolates (C3Ds) have been shown to release NO following cleavage of their β-lactam moiety with bacterial β-lactamases. In vitro work demonstrated that this compound caused significant P. aeruginosa biofilm dispersal on its own, improved dispersal when co-administered with tobramycin, and nearly completely eradicated biofilms when combined with colistin [94]. The donor NO compound spermine NONOate (S150), has also shown efficacy in reducing biofilm biomass of P. aeruginosa clinical isolates from CF patients [92].
Inhaled gaseous NO has also been studied; results from a small pilot study in CF patients with P. aeruginosa infection yielded lower numbers of bacterial aggregates in the sputum when compared with controls when used as adjunctive therapy to antibiotics [95]. A clinical trial of high dose (160 ppb) inhaled NO in CF patients is currently underway (NCT02498535) in the USA [96].
Another approach is to increase endogenous NO production. One way of doing this is through inhibition of arginase, an enzyme that competes with nitric oxide synthase (NOS) for L-arginine as substrate. A phase 1B randomized, double-blind, placebo-controlled trial investigating a compound called CB-280, a potent arginase inhibitor, is currently being conducted (NCT04279769) [97].
5. Strategy: Targeting Cell-Cell Signalling
Quorum sensing (QS) is a method by which bacteria can communicate with one another through the production, secretion, and sensing of small molecules called autoinducers, which allows for regulation of population level phenotypic change [98]. QS allows for a bacterium to modulate gene expression in response to gradients of bacterial density, directing lifestyle choices appropriate to a given environment. Many cellular processes, including but not limited to, the transition to a biofilm mode of growth and virulence factor expression, are known to be controlled by the QS system [99]. As the primary method of communication between bacteria, QS has also been implicated as a key factor in the intracellular pathways mentioned previously. It has been shown to regulate levels of c-di-GMP, the principle second messenger involved in the regulation of genes that are expressed in a biofilm lifestyle [81]. Bacteria transitioning from a biofilm lifestyle into a dispersion phase, change phenotypically [100], enabling it to move to new areas and colonize different regions of the lung.
In many Gram-negative bacteria, QS is controlled by two N-acyl-homoserine lactone (AHL) signalling systems—the las and rhl systems [101,102]. Whereas the las system is activated in cells undergoing early biofilm formation [103], RhlR/RhlI is activated in the maturation stages of biofilm formation [100]. These systems are hierarchically arranged, whereby the las system has the power to up-regulate or downregulate rhl [104]. In Gram-positive bacteria, autoinducing peptides (AIPs) fulfill the same role, whereas both Gram-positive and negative organisms produce autoinducer-2 (AI-2) [105]. These QS pathways are ubiquitous in bacterial cells and have specific roles in biofilm development and maintenance.
5.1. Compounds That Disrupt Quorum Sensing
The seaweed Delisea pulchra was observed to avoid bacterial colonization by the production of halogenated furanone compounds similar in structure to the homoserine lactones used in QS in Gram-negative organisms [106]. These findings led to the concept of using such compounds therapeutically. Kim et al. [107] synthesized furanone derivatives that were noted to inhibit QS and impair biofilm formation, suggesting that compounds inhibiting QS may aid in bacterial eradication. Subsequently, several more compounds were identified through high throughput screening methods, detected through suppression of genes normally expressed in QS [108]. Compounds have also been identified by computerized structural analysis models [109]. One promising compound that inhibited QS was garlic extract—in particular the compound ajoene [110,111]. In a mouse model of pulmonary P. aeruginosa biofilm infection, ajoene was effective in blocking the production of rhamnolipids needed for biofilm maintenance [108]. However, a small, randomized pilot study exploring the effects of oral garlic in CF patients with chronic P. aeruginosa infection showed no significant effect on pulmonary function [112].
Several antibiotics already in use have effects on QS. For example, the macrolide azithromycin, whilst not bactericidal against P. aeruginosa, can disrupt quorum sensing and block alginate production in a mouse model of infection with this organism [113]. In a meta-analysis including four trials of CF patients, those treated with azithromycin had improved lung function, especially in the subgroup of patients colonized with P. aeruginosa [114]. It is worth noting that there are other hypotheses regarding the clinical efficacity of azithromycin, including effects on airway inflammation [115,116]. Furthermore, the antibiotics ceftazidime and ciprofloxacin have also been shown to disrupt QS [117]. On this basis, identification of compounds using this mechanism for targeting biofilm bacteria remains one that holds promise.
5.2. Compounds Disrupting Iron Metabolism
Successful pathogenic bacteria often employ the use of iron scavenging systems to obtain iron, an essential nutrient for bacterial growth, from host tissues [118]. Iron is also an important signalling molecule involved in bacterial adherence and biofilm maturation in certain bacterial pathogens. Previous studies demonstrated that iron depletion in models of P. aeruginosa and B. cenocepacia infection led to widespread transition from biofilm to a motile planktonic mode of growth, while increasing the availability of iron led to bacterial aggregation [119].
Gallium (Ga3+) is a metal that interferes with iron signalling. Ga3+ and Fe3+ have many similarities in size and other electrochemical properties that allow gallium to be mistaken for iron and taken up by bacterial cells, subsequently disrupting cellular processes promoting biofilm formation [120,121]. This approach has been shown to be efficacious in murine models of P. aeruginosa infection—in two models of lung infection [122] and in a thermally induced wound infection [123]—while simultaneously associated with lower rates of bacterial resistance compared to other small molecule antibiotic therapies [120]. In an experimental model using lung epithelial cells, a liposomal formulation of gentamicin co-encapsulated with gallium eradicated P. aeruginosa biofilms more efficaciously than liposomal aminoglycoside alone [124]. In human clinical trials, results from a recent Phase 2 study in 23 centres in the U.S. demonstrated trends towards improved lung function and lower P. aeruginosa density in sputum in CF patients with chronic P. aeruginosa infection treated with intravenous (IV) gallium [125]. There are also clinical trials in development to evaluate IV gallium in the treatment of NTM pulmonary infection (NCT04294043) [126].
6. Conclusions
Effectively treating bacterial infections in the context of CF entails a thorough examination of the environments in which these pathogens survive despite repeated, broad spectrum courses of antibiotics. The biofilm is an adaptive structure that provides bacterial populations with both physical protection and reservoirs of phenotypically distinct subpopulations that have the ability to withstand antimicrobials and immune response. Though existing therapies have yielded improved patient outcomes, including lung function, survival and quality of life, the biofilm lifestyle represents a barrier that limits benefit. There are a number of therapies currently in use that have anti-biofilm properties beyond their initially understood mechanism of action—from the eDNA lysing dornase alfa to the anti-quorum sensing properties of azithromycin. Such therapies, when used in conjunction with standard antimicrobials, serve as examples of using multiple modes of action against biofilms to improve clinical outcomes.
It is also important to consider what impact improved CFTR function will have on microbial communities, in an era where an increasing number of people with CF have access to effective CFTR modulator therapy. Though we do not yet have the answer, the multifactorial nature of the question has been the focus of several reviews [127,128]. However effective these new therapies prove to be, the need for anti-biofilm agents will continue to be relevant in the context of CF. The biofilm lifestyle is also but one of many factors that need to be taken into account when designing laboratory methods to more accurately predict response to treatment, as studies investigating antimicrobial selection based on biofilm versus standard AST in the treatment of CF patients have not yielded improved clinical outcomes [129,130]. It may be that the next breakthrough in targeting microbial biofilms lies in a personalized approach to bacterial eradication, accounting for the influence of polymicrobial infection or other patient-specific characteristics in models of disease.
Author Contributions
Conceptualization, I.M.; investigation, I.M.; writing—original draft preparation, I.M.; writing—review and editing, H.G. and V.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aaron S.D., Vandemheen K.L., Ramotar K., Giesbrecht-Lewis T., Tullis E., Freitag A., Paterson N., Jackson M., Lougheed M.D., Dowson C., et al. Infection with transmissible strains of Pseudomonas aeruginosa and clinical outcomes in adults with cystic fibrosis. JAMA. 2010;304:2145–2153. doi: 10.1001/jama.2010.1665. [DOI] [PubMed] [Google Scholar]
- 2.Cantin A.M., Hartl D., Konstan M.W., Chmiel J.F. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J. Cyst. Fibros. 2015;14:419–430. doi: 10.1016/j.jcf.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Doring G., Flume P., Heijerman H., Elborn J.S., Consensus Study G. Treatment of lung infection in patients with cystic fibrosis: Current and future strategies. J. Cyst. Fibros. 2012;11:461–479. doi: 10.1016/j.jcf.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Parkins M.D., Floto R.A. Emerging bacterial pathogens and changing concepts of bacterial pathogenesis in cystic fibrosis. J. Cyst. Fibros. 2015;14:293–304. doi: 10.1016/j.jcf.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Lucca F., Guarnieri M., Ros M., Muffato G., Rigoli R., Da Dalt L. Antibiotic resistance evolution of Pseudomonas aeruginosa in cystic fibrosis patients (2010-2013) Clin. Respir J. 2018;12:2189–2196. doi: 10.1111/crj.12787. [DOI] [PubMed] [Google Scholar]
- 6.Lechtzin N., John M., Irizarry R., Merlo C., Diette G.B., Boyle M.P. Outcomes of Adults with Cystic Fibrosis Infected with Antibiotic-Resistant Pseudomonas aeruginosa. Respiration. 2006;73:27–33. doi: 10.1159/000087686. [DOI] [PubMed] [Google Scholar]
- 7.Conway S.P., Brownlee K.G., Denton M., Peckham D.G. Antibiotic treatment of multidrug-resistant organisms in cystic fibrosis. Am. J. Respir. Med. 2003;2:321–332. doi: 10.1007/BF03256660. [DOI] [PubMed] [Google Scholar]
- 8.Stefani S., Campana S., Cariani L., Carnovale V., Colombo C., Lleo M.M., Iula V.D., Minicucci L., Morelli P., Pizzamiglio G., et al. Relevance of multidrug-resistant Pseudomonas aeruginosa infections in cystic fibrosis. Int. J. Med. Microbiol. 2017;307:353–362. doi: 10.1016/j.ijmm.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch E.B., Tam V.H. Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes. Expert Rev. Pharmacoecon. Outcomes Res. 2010;10:441–451. doi: 10.1586/erp.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breidenstein E.B., de la Fuente-Nunez C., Hancock R.E. Pseudomonas aeruginosa: All roads lead to resistance. Trends Microbiol. 2011;19:419–426. doi: 10.1016/j.tim.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Tenover F.C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Infect. Control. 2006;34:S3–S10. doi: 10.1016/j.ajic.2006.05.219. [DOI] [PubMed] [Google Scholar]
- 12.Smith A.L., Fiel S.B., Mayer-Hamblett N., Ramsey B., Burns J.L. Susceptibility testing of Pseudomonas aeruginosa isolates and clinical response to parenteral antibiotic administration: Lack of association in cystic fibrosis. Chest. 2003;123:1495–1502. doi: 10.1378/chest.123.5.1495. [DOI] [PubMed] [Google Scholar]
- 13.Hurley M.N., Amin A.H., Bertenshaw A.C., Bhatt J., Smyth A.R. Results of antibiotic susceptibility testing do not influence clinical outcome in children with cystic fibrosis. J. Cyst. Fibros. 2012;11:288–292. doi: 10.1016/j.jcf.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Branda S.S., Vik S., Friedman L., Kolter R. Biofilms: The matrix revisited. Trends Microbiol. 2005;13:20–26. doi: 10.1016/j.tim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Sun F., Qu F., Ling Y., Mao P., Xia P., Chen H., Zhou D. Biofilm-associated infections: Antibiotic resistance and novel therapeutic strategies. Future Microbiol. 2013;8:877–886. doi: 10.2217/fmb.13.58. [DOI] [PubMed] [Google Scholar]
- 16.Wimpenny J., Manz W., Szewzyk U. Heterogeneity in biofilms. FEMS Microbiol. Rev. 2000;24:661–671. doi: 10.1111/j.1574-6976.2000.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 17.Mulcahy L.R., Burns J.L., Lory S., Lewis K. Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J. Bacteriol. 2010;192:6191–6199. doi: 10.1128/JB.01651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keren I., Kaldalu N., Spoering A., Wang Y., Lewis K. Persister cells and tolerance to antimicrobials. FEMS Microbiol. Lett. 2004;230:13–18. doi: 10.1016/S0378-1097(03)00856-5. [DOI] [PubMed] [Google Scholar]
- 19.Somayaji R., Parkins M.D., Shah A., Martiniano S.L., Tunney M.M., Kahle J.S., Waters V.J., Elborn J.S., Bell S.C., Flume P.A., et al. Antimicrobial Resistance in Cystic Fibrosis InternationalWorking, G. Antimicrobial susceptibility testing (AST) and associated clinical outcomes in individuals with cystic fibrosis: A systematic review. J. Cyst. Fibros. 2019;18:236–243. doi: 10.1016/j.jcf.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Boudarel H., Mathias J.D., Blaysat B., Grediac M. Towards standardized mechanical characterization of microbial biofilms: Analysis and critical review. NPJ Biofilms Microbiomes. 2018;4:17. doi: 10.1038/s41522-018-0062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alhede M., Kragh K.N., Qvortrup K., Allesen-Holm M., van Gennip M., Christensen L.D., Jensen P.O., Nielsen A.K., Parsek M., Wozniak D., et al. Phenotypes of non-attached Pseudomonas aeruginosa aggregates resemble surface attached biofilm. PLoS ONE. 2011;6:e27943. doi: 10.1371/journal.pone.0027943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Secor P.R., Michaels L.A., Ratjen A., Jennings L.K., Singh P.K. Entropically driven aggregation of bacteria by host polymers promotes antibiotic tolerance in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 2018;115:10780–10785. doi: 10.1073/pnas.1806005115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reichhardt C., Jacobs H.M., Matwichuk M., Wong C., Wozniak D.J., Parsek M.R. The Versatile Pseudomonas aeruginosa Biofilm Matrix Protein CdrA Promotes Aggregation through Different Extracellular Exopolysaccharide Interactions. J. Bacteriol. 2020;202 doi: 10.1128/JB.00216-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olson M.E., Ceri H., Morck D.W., Buret A.G., Read R.R. Biofilm bacteria: Formation and comparative susceptibility to antibiotics. Can. J. Vet. Res. 2002;66:86–92. [PMC free article] [PubMed] [Google Scholar]
- 25.Moriarty T.F., McElnay J.C., Elborn J.S., Tunney M.M. Sputum antibiotic concentrations: Implications for treatment of cystic fibrosis lung infection. Pediatr. Pulmonol. 2007;42:1008–1017. doi: 10.1002/ppul.20671. [DOI] [PubMed] [Google Scholar]
- 26.Conway S.P. Nebulized antibiotic therapy: the evidence. Chron. Respir. Dis. 2005;2:35–41. doi: 10.1191/1479972305cd045rs. [DOI] [PubMed] [Google Scholar]
- 27.Chmiel J.F., Aksamit T.R., Chotirmall S.H., Dasenbrook E.C., Elborn J.S., LiPuma J.J., Ranganathan S.C., Waters V.J., Ratjen F.A. Antibiotic management of lung infections in cystic fibrosis. I. The microbiome, methicillin-resistant Staphylococcus aureus, gram-negative bacteria, and multiple infections. Ann. Am. Thorac. Soc. 2014;11:1120–1129. doi: 10.1513/AnnalsATS.201402-050AS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrmann G., Yang L., Wu H., Song Z., Wang H., Hoiby N., Ulrich M., Molin S., Riethmuller J., Doring G. Colistin-tobramycin combinations are superior to monotherapy concerning the killing of biofilm Pseudomonas aeruginosa. J. Infect. Dis. 2010;202:1585–1592. doi: 10.1086/656788. [DOI] [PubMed] [Google Scholar]
- 29.Gomez-Junyent J., Benavent E., Sierra Y., El Haj C., Soldevila L., Torrejon B., Rigo-Bonnin R., Tubau F., Ariza J., Murillo O. Efficacy of ceftolozane/tazobactam, alone and in combination with colistin, against multidrug-resistant Pseudomonas aeruginosa in an in vitro biofilm pharmacodynamic model. Int J. Antimicrob. Agents. 2019;53:612–619. doi: 10.1016/j.ijantimicag.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Baek M.S., Chung E.S., Jung D.S., Ko K.S. Effect of colistin-based antibiotic combinations on the eradication of persister cells in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2020;75:917–924. doi: 10.1093/jac/dkz552. [DOI] [PubMed] [Google Scholar]
- 31.Chung E.S., Ko K.S. Eradication of persister cells of Acinetobacter baumannii through combination of colistin and amikacin antibiotics. J. Antimicrob. Chemother. 2019;74:1277–1283. doi: 10.1093/jac/dkz034. [DOI] [PubMed] [Google Scholar]
- 32.Robinson A.M., Bannister M., Creeth J.E., Jones M.N. The interaction of phospholipid liposomes with mixed bacterial biofilms and their use in the delivery of bactericide. Colloids Surf. A Physicochem. Eng. Asp. 2001;186:43–53. doi: 10.1016/S0927-7757(01)00481-2. [DOI] [Google Scholar]
- 33.Martin C., Low W.L., Gupta A., Amin M.C., Radecka I., Britland S.T., Raj P., Kenward K.M. Strategies for antimicrobial drug delivery to biofilm. Curr. Pharm. Des. 2015;21:43–66. doi: 10.2174/1381612820666140905123529. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J., Leifer F., Rose S., Chun D.Y., Thaisz J., Herr T., Nashed M., Joseph J., Perkins W.R., DiPetrillo K. Amikacin Liposome Inhalation Suspension (ALIS) Penetrates Non-tuberculous Mycobacterial Biofilms and Enhances Amikacin Uptake Into Macrophages. Front. Microbiol. 2018;9:915. doi: 10.3389/fmicb.2018.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meers P., Neville M., Malinin V., Scotto A.W., Sardaryan G., Kurumunda R., Mackinson C., James G., Fisher S., Perkins W.R. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J. Antimicrob. Chemother. 2008;61:859–868. doi: 10.1093/jac/dkn059. [DOI] [PubMed] [Google Scholar]
- 36.Ehsan Z., Wetzel J.D., Clancy J.P. Nebulized liposomal amikacin for the treatment of Pseudomonas aeruginosa infection in cystic fibrosis patients. Expert Opin. Investig. Drugs. 2014;23:743–749. doi: 10.1517/13543784.2014.895322. [DOI] [PubMed] [Google Scholar]
- 37.Bilton D., Pressler T., Fajac I., Clancy J.P., Sands D., Minic P., Cipolli M., Galeva I., Sole A., Quittner A.L., et al. Amikacin liposome inhalation suspension for chronic Pseudomonas aeruginosa infection in cystic fibrosis. J. Cyst. Fibros. 2020;19:284–291. doi: 10.1016/j.jcf.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olivier K.N., Griffith D.E., Eagle G., McGinnis J.P., 2nd, Micioni L., Liu K., Daley C.L., Winthrop K.L., Ruoss S., Addrizzo-Harris D.J., et al. Randomized Trial of Liposomal Amikacin for Inhalation in Nontuberculous Mycobacterial Lung Disease. Am. J. Respir. Crit. Care Med. 2017;195:814–823. doi: 10.1164/rccm.201604-0700OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rukavina Z., Vanic Z. Current Trends in Development of Liposomes for Targeting Bacterial Biofilms. Pharmaceutics. 2016;8:18. doi: 10.3390/pharmaceutics8020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bassetti M., Vena A., Russo A., Peghin M. Inhaled Liposomal Antimicrobial Delivery in Lung Infections. Drugs. 2020;80:1309–1318. doi: 10.1007/s40265-020-01359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parisien A., Allain B., Zhang J., Mandeville R., Lan C.Q. Novel alternatives to antibiotics: Bacteriophages, bacterial cell wall hydrolases, and antimicrobial peptides. J. Appl. Microbiol. 2008;104:1–13. doi: 10.1111/j.1365-2672.2007.03498.x. [DOI] [PubMed] [Google Scholar]
- 42.Batoni G., Maisetta G., Esin S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta. 2016;1858:1044–1060. doi: 10.1016/j.bbamem.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Beaudoin T., Stone T.A., Glibowicka M., Adams C., Yau Y., Ahmadi S., Bear C.E., Grasemann H., Waters V., Deber C.M. Activity of a novel antimicrobial peptide against Pseudomonas aeruginosa biofilms. Sci. Rep. 2018;8:14728. doi: 10.1038/s41598-018-33016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mookherjee N., Brown K.L., Bowdish D.M., Doria S., Falsafi R., Hokamp K., Roche F.M., Mu R., Doho G.H., Pistolic J., et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J. Immunol. 2006;176:2455–2464. doi: 10.4049/jimmunol.176.4.2455. [DOI] [PubMed] [Google Scholar]
- 45.Bezzerri V., Avitabile C., Dechecchi M.C., Lampronti I., Borgatti M., Montagner G., Cabrini G., Gambari R., Romanelli A. Antibacterial and anti-inflammatory activity of a temporin B peptide analogue on an in vitro model of cystic fibrosis. J. Pept. Sci. 2014;20:822–830. doi: 10.1002/psc.2674. [DOI] [PubMed] [Google Scholar]
- 46.Davies E.V., James C.E., Kukavica-Ibrulj I., Levesque R.C., Brockhurst M.A., Winstanley C. Temperate phages enhance pathogen fitness in chronic lung infection. ISME J. 2016;10:2553–2555. doi: 10.1038/ismej.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rice S.A., Tan C.H., Mikkelsen P.J., Kung V., Woo J., Tay M., Hauser A., McDougald D., Webb J.S., Kjelleberg S. The biofilm life cycle and virulence of Pseudomonas aeruginosa are dependent on a filamentous prophage. ISME J. 2009;3:271–282. doi: 10.1038/ismej.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Secor P.R., Sweere J.M., Michaels L.A., Malkovskiy A.V., Lazzareschi D., Katznelson E., Rajadas J., Birnbaum M.E., Arrigoni A., Braun K.R., et al. Filamentous Bacteriophage Promote Biofilm Assembly and Function. Cell Host Microbe. 2015;18:549–559. doi: 10.1016/j.chom.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tkhilaishvili T., Lombardi L., Klatt A.B., Trampuz A., Di Luca M. Bacteriophage Sb-1 enhances antibiotic activity against biofilm, degrades exopolysaccharide matrix and targets persisters of Staphylococcus aureus. Int. J. Antimicrob. Agents. 2018;52:842–853. doi: 10.1016/j.ijantimicag.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Glonti T., Chanishvili N., Taylor P.W. Bacteriophage-derived enzyme that depolymerizes the alginic acid capsule associated with cystic fibrosis isolates of Pseudomonas aeruginosa. J. Appl. Microbiol. 2010;108:695–702. doi: 10.1111/j.1365-2672.2009.04469.x. [DOI] [PubMed] [Google Scholar]
- 51.Wright A., Hawkins C.H., Anggard E.E., Harper D.R. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiot ic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin. Otolaryngol. 2009;34:349–357. doi: 10.1111/j.1749-4486.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- 52.Saussereau E., Vachier I., Chiron R., Godbert B., Sermet I., Dufour N., Pirnay J.P., De Vos D., Carrie F., Molinari N., et al. Effectiveness of bacteriophages in the sputum of cystic fibrosis patients. Clin. Microbiol. Infect. 2014;20:O983–O990. doi: 10.1111/1469-0691.12712. [DOI] [PubMed] [Google Scholar]
- 53.Pabary R., Singh C., Morales S., Bush A., Alshafi K., Bilton D., Alton E.W., Smithyman A., Davies J.C. Antipseudomonal Bacteriophage Reduces Infective Burden and Inflammatory Response in Murine Lung. Antimicrob. Agents Chemother. 2016;60:744–751. doi: 10.1128/AAC.01426-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waters E.M., Neill D.R., Kaman B., Sahota J.S., Clokie M.R.J., Winstanley C., Kadioglu A. Phage therapy is highly effective against chronic lung infections with Pseudomonas aeruginosa. Thorax. 2017;72:666–667. doi: 10.1136/thoraxjnl-2016-209265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Law N., Logan C., Yung G., Furr C.L., Lehman S.M., Morales S., Rosas F., Gaidamaka A., Bilinsky I., Grint P., et al. Successful adjunctive use of bacteriophage therapy for treatment of multidrug-resistant Pseudomonas aeruginosa infection in a cystic fibrosis patient. Infection. 2019;47:665–668. doi: 10.1007/s15010-019-01319-0. [DOI] [PubMed] [Google Scholar]
- 56.Dedrick R.M., Guerrero-Bustamante C.A., Garlena R.A., Russell D.A., Ford K., Harris K., Gilmour K.C., Soothill J., Jacobs-Sera D., Schooley R.T., et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019;25:730–733. doi: 10.1038/s41591-019-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.A Phase 1b/2a, Multi-Center, Double-Blind, Randomized, Placebo-Controlled, Single and Multiple Ascending Dose Study to Evaluate the Safety and Tolerability of AP-PA02 Multi-Phage Therapeutic Candidate for Inhalation in Subjects With Cystic Fibrosis and Chronic Pulmonary Pseudomonas aeruginosa (Pa) Infection. [(accessed on 5 December 2020)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04596319?term=bacteriophage&cond=Cystic+Fibrosis&draw=2&rank=2.
- 58.Folkesson A., Jelsbak L., Yang L., Johansen H.K., Ciofu O., Hoiby N., Molin S. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: An evolutionary perspective. Nat. Rev. Microbiol. 2012;10:841–851. doi: 10.1038/nrmicro2907. [DOI] [PubMed] [Google Scholar]
- 59.Hentzer M., Teitzel G.M., Balzer G.J., Heydorn A., Molin S., Givskov M., Parsek M.R. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 2001;183:5395–5401. doi: 10.1128/JB.183.18.5395-5401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mowat E., Paterson S., Fothergill J.L., Wright E.A., Ledson M.J., Walshaw M.J., Brockhurst M.A., Winstanley C. Pseudomonas aeruginosa population diversity and turnover in cystic fibrosis chronic infections. Am. J. Respir. Crit. Care Med. 2011;183:1674–1679. doi: 10.1164/rccm.201009-1430OC. [DOI] [PubMed] [Google Scholar]
- 61.Fernandez-Barat L., Ciofu O., Kragh K.N., Pressler T., Johansen U., Motos A., Torres A., Hoiby N. Phenotypic shift in Pseudomonas aeruginosa populations from cystic fibrosis lungs after 2-week antipseudomonal treatment. J. Cyst. Fibros. 2017;16:222–229. doi: 10.1016/j.jcf.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 62.Alkawash M.A., Soothill J.S., Schiller N.L. Alginate lyase enhances antibiotic killing of mucoid Pseudomonas aeruginosa in biofilms. APMIS. 2006;114:131–138. doi: 10.1111/j.1600-0463.2006.apm_356.x. [DOI] [PubMed] [Google Scholar]
- 63.Islan G.A., Bosio V.E., Castro G.R. Alginate lyase and ciprofloxacin co-immobilization on biopolymeric microspheres for cystic fibrosis treatment. Macromol. Biosci. 2013;13:1238–1248. doi: 10.1002/mabi.201300134. [DOI] [PubMed] [Google Scholar]
- 64.Patel K.K., Tripathi M., Pandey N., Agrawal A.K., Gade S., Anjum M.M., Tilak R., Singh S. Alginate lyase immobilized chitosan nanoparticles of ciprofloxacin for the improved antimicrobial activity against the biofilm associated mucoid P. aeruginosa infection in cystic fibrosis. Int J. Pharm. 2019;563:30–42. doi: 10.1016/j.ijpharm.2019.03.051. [DOI] [PubMed] [Google Scholar]
- 65.Pritchard M.F., Powell L.C., Menzies G.E., Lewis P.D., Hawkins K., Wright C., Doull I., Walsh T.R., Onsoyen E., Dessen A., et al. A New Class of Safe Oligosaccharide Polymer Therapy To Modify the Mucus Barrier of Chronic Respiratory Disease. Mol. Pharm. 2016;13:863–872. doi: 10.1021/acs.molpharmaceut.5b00794. [DOI] [PubMed] [Google Scholar]
- 66.Khan S., Tondervik A., Sletta H., Klinkenberg G., Emanuel C., Onsoyen E., Myrvold R., Howe R.A., Walsh T.R., Hill K.E., et al. Overcoming drug resistance with alginate oligosaccharides able to potentiate the action of selected antibiotics. Antimicrob. Agents Chemother. 2012;56:5134–5141. doi: 10.1128/AAC.00525-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tondervik A., Sletta H., Klinkenberg G., Emanuel C., Powell L.C., Pritchard M.F., Khan S., Craine K.M., Onsoyen E., Rye P.D., et al. Alginate oligosaccharides inhibit fungal cell growth and potentiate the activity of antifungals against Candida and Aspergillus spp. PLoS ONE. 2014;9:e112518. doi: 10.1371/journal.pone.0112518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Powell L.C., Sowedan A., Khan S., Wright C.J., Hawkins K., Onsoyen E., Myrvold R., Hill K.E., Thomas D.W. The effect of alginate oligosaccharides on the mechanical properties of Gram-negative biofilms. Biofouling. 2013;29:413–421. doi: 10.1080/08927014.2013.777954. [DOI] [PubMed] [Google Scholar]
- 69.Powell L.C., Pritchard M.F., Ferguson E.L., Powell K.A., Patel S.U., Rye P.D., Sakellakou S.M., Buurma N.J., Brilliant C.D., Copping J.M., et al. Targeted disruption of the extracellular polymeric network of Pseudomonas aeruginosa biofilms by alginate oligosaccharides. NPJ Biofilms Microbiomes. 2018;4:13. doi: 10.1038/s41522-018-0056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hengzhuang W., Song Z., Ciofu O., Onsoyen E., Rye P.D., Hoiby N. OligoG CF-5/20 Disruption of Mucoid Pseudomonas aeruginosa Biofilm in a Murine Lung Infection Model. Antimicrob. Agents Chemother. 2016;60:2620–2626. doi: 10.1128/AAC.01721-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Koningsbruggen-Rietschel S., Davies J.C., Pressler T., Fischer R., MacGregor G., Donaldson S.H., Smerud K., Meland N., Mortensen J., Fosbol M.O., et al. Inhaled dry powder alginate oligosaccharide in cystic fibrosis: A randomised, double-blind, placebo-controlled, crossover phase 2b study. ERJ Open Res. 2020;6 doi: 10.1183/23120541.00132-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.A Dose-finding Study of Inhaled OligoG vs. Placebo in Patients With Cystic Fibrosis (SMR3372) [(accessed on 5 December 2020)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT03698448?term=oligog&cond=Cystic+Fibrosis&draw=2&rank=4.
- 73.Baker P., Hill P.J., Snarr B.D., Alnabelseya N., Pestrak M.J., Lee M.J., Jennings L.K., Tam J., Melnyk R.A., Parsek M.R., et al. Exopolysaccharide biosynthetic glycoside hydrolases can be utilized to disrupt and prevent Pseudomonas aeruginosa biofilms. Sci. Adv. 2016;2:e1501632. doi: 10.1126/sciadv.1501632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pestrak M.J., Baker P., Dellos-Nolan S., Hill P.J., Passos da Silva D., Silver H., Lacdao I., Raju D., Parsek M.R., Wozniak D.J., et al. Treatment with the Pseudomonas aeruginosa Glycoside Hydrolase PslG Combats Wound Infection by Improving Antibiotic Efficacy and Host Innate Immune Activity. Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.00234-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gawande P.V., Leung K.P., Madhyastha S. Antibiofilm and antimicrobial efficacy of DispersinB(R)-KSL-W peptide-based wound gel against chronic wound infection associated bacteria. Curr. Microbiol. 2014;68:635–641. doi: 10.1007/s00284-014-0519-6. [DOI] [PubMed] [Google Scholar]
- 76.Yang C., Chilvers M., Montgomery M., Nolan S.J. Dornase alfa for cystic fibrosis. Cochrane Database Syst. Rev. 2016;4:CD001127. doi: 10.1002/14651858.CD001127.pub3. [DOI] [PubMed] [Google Scholar]
- 77.Kaplan J.B., LoVetri K., Cardona S.T., Madhyastha S., Sadovskaya I., Jabbouri S., Izano E.A. Recombinant human DNase I decreases biofilm and increases antimicrobial susceptibility in staphylococci. J. Antibiot. 2012;65:73–77. doi: 10.1038/ja.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sharma K., Pagedar Singh A. Antibiofilm Effect of DNase against Single and Mixed Species Biofilm. Foods. 2018;7:42. doi: 10.3390/foods7030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okshevsky M., Regina V.R., Meyer R.L. Extracellular DNA as a target for biofilm control. Curr. Opin. Biotechnol. 2015;33:73–80. doi: 10.1016/j.copbio.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 80.Novotny L.A., Amer A.O., Brockson M.E., Goodman S.D., Bakaletz L.O. Structural stability of Burkholderia cenocepacia biofilms is reliant on eDNA structure and presence of a bacterial nucleic acid binding protein. PLoS ONE. 2013;8:e67629. doi: 10.1371/journal.pone.0067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ueda A., Wood T.K. Connecting Quorum Sensing, c-di-GMP, Pel Polysaccharide, and Biofilm Formation in Pseudomonas aeruginosa through Tyrosine Phosphatase TpbA (PA3885) PLoS Pathog. 2009;5:e1000483. doi: 10.1371/journal.ppat.1000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chua S.L., Hultqvist L.D., Yuan M., Rybtke M., Nielsen T.E., Givskov M., Tolker-Nielsen T., Yang L. In vitro and in vivo generation and characterization of Pseudomonas aeruginosa biofilm-dispersed cells via c-di-GMP manipulation. Nat. Protoc. 2015;10:1165–1180. doi: 10.1038/nprot.2015.067. [DOI] [PubMed] [Google Scholar]
- 83.Christensen L.D., van Gennip M., Rybtke M.T., Wu H., Chiang W.-H., Alhede M., Høiby N., Nielsen T.E., Givskov M., Tolker-Nielsena T. Clearance of Pseudomonas aeruginosa Foreign-Body Biofilm Infections through Reduction of the Cyclic Di-GMP Level in the Bacteria. Infect. Immun. 2013;81:2705–2713. doi: 10.1128/IAI.00332-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sambanthamoorthy K., Gokhale A.A., Lao W., Parashar V., Semmelhack M.F., Lee I., Waters C.M. Identification of a novel benzimidazole that inhibits bacterial biofilm formation in a broad-spectrum manner. Antimicrob. Agents Chemother. 2011;55:4369–4378. doi: 10.1128/AAC.00583-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Antoniani D., Rossi E., Rinaldo S., Bocci P., Lolicato M., Paiardini A., Raffaelli N., Cutruzzola F., Landini P. The immunosuppressive drug azathioprine inhibits biosynthesis of the bacterial signal molecule cyclic-di-GMP by interfering with intracellular nucleotide pool availability. Appl. Microbiol. Biotechnol. 2013;97:7325–7336. doi: 10.1007/s00253-013-4875-0. [DOI] [PubMed] [Google Scholar]
- 86.Derbyshire E.R., Marletta M.A. Structure and regulation of soluble guanylate cyclase. Annu. Rev. Biochem. 2012;81:533–559. doi: 10.1146/annurev-biochem-050410-100030. [DOI] [PubMed] [Google Scholar]
- 87.Arora D.P., Hossain S., Xu Y., Boon E.M. Nitric Oxide Regulation of Bacterial Biofilms. Biochemistry. 2015;54:3717–3728. doi: 10.1021/bi501476n. [DOI] [PubMed] [Google Scholar]
- 88.Rouillard K.R., Novak O.P., Pistiolis A.M., Yang L., Ahonen M.J.R., McDonald R.A., Schoenfisch M.H. Exogenous Nitric Oxide Improves Antibiotic Susceptibility in Resistant Bacteria. ACS Infect. Dis. 2021;7:23–33. doi: 10.1021/acsinfecdis.0c00337. [DOI] [PubMed] [Google Scholar]
- 89.Barraud N., Schleheck D., Klebensberger J., Webb J.S., Hassett D.J., Rice S.A., Kjelleberg S. Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and enhanced dispersal. J. Bacteriol. 2009;191:7333–7342. doi: 10.1128/JB.00975-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barraud N., Hassett D.J., Hwang S.H., Rice S.A., Kjelleberg S., Webb J.S. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J. Bacteriol. 2006;188:7344–7353. doi: 10.1128/JB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barraud N., Kelso M.J., Rice S.A., Kjelleberg S. Nitric oxide: a key mediator of biofilm dispersal with applications in infectious diseases. Curr. Pharm. Des. 2015;21:31–42. doi: 10.2174/1381612820666140905112822. [DOI] [PubMed] [Google Scholar]
- 92.Cai Y.M., Webb J.S. Optimization of nitric oxide donors for investigating biofilm dispersal response in Pseudomonas aeruginosa clinical isolates. Appl. Microbiol. Biotechnol. 2020;104:8859–8869. doi: 10.1007/s00253-020-10859-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Antoniani D., Bocci P., Maciag A., Raffaelli N., Landini P. Monitoring of diguanylate cyclase activity and of cyclic-di-GMP biosynthesis by whole-cell assays suitable for high-throughput screening of biofilm inhibitors. Appl. Microbiol. Biotechnol. 2010;85:1095–1104. doi: 10.1007/s00253-009-2199-x. [DOI] [PubMed] [Google Scholar]
- 94.Soren O., Rineh A., Silva D.G., Cai Y., Howlin R.P., Allan R.N., Feelisch M., Davies J.C., Connett G.J., Faust S.N., et al. Cephalosporin nitric oxide-donor prodrug DEA-C3D disperses biofilms formed by clinical cystic fibrosis isolates of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2020;75:117–125. doi: 10.1093/jac/dkz378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Howlin R.P., Cathie K., Hall-Stoodley L., Cornelius V., Duignan C., Allan R.N., Fernandez B.O., Barraud N., Bruce K.D., Jefferies J., et al. Low-Dose Nitric Oxide as Targeted Anti-biofilm Adjunctive Therapy to Treat Chronic Pseudomonas aeruginosa Infection in Cystic Fibrosis. Mol. Ther. 2017;25:2104–2116. doi: 10.1016/j.ymthe.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Prospective, Randomized, Placebo Controlled Trial of the Efficacy and Safety of Inhaled Nitric Oxide (NO) in Cystic Fibrosis (CF) Patients. [(accessed on 5 December 2020)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT02498535?term=nitric+oxide&cond=Cystic+Fibrosis&draw=5.
- 97.Study to Evaluate the Safety of CB-280 in Patients With Cystic Fibrosis. [(accessed on 14 January 2021)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04279769?cond=NCT04279769&draw=2&rank=1.
- 98.Camilli A., Bassler B.L. Bacterial small-molecule signaling pathways. Science. 2006;311:1113–1116. doi: 10.1126/science.1121357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Waters C.M., Bassler B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 100.Sauer K., Camper A.K., Ehrlich G.D., Costerton J.W., Davies D.G. Pseudomonas aeruginosa Displays Multiple Phenotypes during Development as a Biofilm. J. Bacteriol. 2002;184:1140–1154. doi: 10.1128/jb.184.4.1140-1154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schuster M., Greenberg E.P. A network of networks: Quorum-sensing gene regulation in Pseudomonas aeruginosa. Int J. Med. Microbiol. 2006;296:73–81. doi: 10.1016/j.ijmm.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 102.Venturi V. Regulation of quorum sensing in Pseudomonas. FEMS Microbiol Rev. 2006;30:274–291. doi: 10.1111/j.1574-6976.2005.00012.x. [DOI] [PubMed] [Google Scholar]
- 103.De Kievit T.R., Gillis R., Marx S., Brown C., Iglewski B.H. Quorum-Sensing Genes in Pseudomonas aeruginosa Biofilms: Their Role and Expression Patterns. Appl. Environ. Microbiol. 2001;67:1865–1873. doi: 10.1128/AEM.67.4.1865-1873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Latifi A., Foglino M., Tanaka K., Williams P., Lazdunski A. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol. Microbiol. 1996;21:1137–1146. doi: 10.1046/j.1365-2958.1996.00063.x. [DOI] [PubMed] [Google Scholar]
- 105.Jiang Y., Chen J., Yang L., Yin Y., Yao K. Quorum Sensing: A Prospective Therapeutic Target for Bacterial Diseases. Biomed. Res. Int. 2019 doi: 10.1155/2019/2015978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Nys R., Steinberg P.D., Willemsen P., Dworjanyn S.A., Gabelish C.L., King R.J. Broad spectrum effects of secondary metabolites from the red alga Delisea pulchra in antifouling assays. Biofouling. 1995;8:259–271. doi: 10.1080/08927019509378279. [DOI] [Google Scholar]
- 107.Kim C., Kim J., Park H.Y., Park H.J., Lee J.H., Kim C.K. Furanone derivatives as quorum-sensing antagonists of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2008;80:37–47. doi: 10.1007/s00253-008-1474-6. [DOI] [PubMed] [Google Scholar]
- 108.Muh U., Schuster M., Heim R., Singh A., Olson E.R., Greenberg E.P. Novel Pseudomonas aeruginosa quorum-sensing inhibitors identified in an ultra-high-throughput screen. Antimicrob. Agents Chemother. 2006;50:3674–3679. doi: 10.1128/AAC.00665-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bottomley M.J., Muraglia E., Bazzo R., Carfi A. Molecular insights into quorum sensing in the human pathogen Pseudomonas aeruginosa from the structure of the virulence regulator LasR bound to its autoinducer. J. Biol. Chem. 2007;282:13592–13600. doi: 10.1074/jbc.M700556200. [DOI] [PubMed] [Google Scholar]
- 110.Bjarnsholt T., Jensen P.O., Rasmussen T.B., Christophersen L., Calum H., Hentzer M., Hougen H.P., Rygaard J., Moser C., Eberl L., et al. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology (Reading) 2005;151:3873–3880. doi: 10.1099/mic.0.27955-0. [DOI] [PubMed] [Google Scholar]
- 111.Jakobsen T.H., van Gennip M., Phipps R.K., Shanmugham M.S., Christensen L.D., Alhede M., Skindersoe M.E., Rasmussen T.B., Friedrich K., Uthe F., et al. Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob. Agents Chemother. 2012;56:2314–2325. doi: 10.1128/AAC.05919-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smyth A.R., Cifelli P.M., Ortori C.A., Righetti K., Lewis S., Erskine P., Holland E.D., Givskov M., Williams P., Camara M., et al. Garlic as an inhibitor of Pseudomonas aeruginosa quorum sensing in cystic fibrosis--a pilot randomized controlled trial. Pediatr. Pulmonol. 2010;45:356–362. doi: 10.1002/ppul.21193. [DOI] [PubMed] [Google Scholar]
- 113.Hoffmann N., Lee B., Hentzer M., Rasmussen T.B., Song Z., Johansen H.K., Givskov M., Hoiby N. Azithromycin Blocks Quorum Sensing and Alginate Polymer Formation and Increases the Sensitivity to Serum and Stationary-Growth-Phase Killing of Pseudomonas aeruginosa and Attenuates Chronic P. aeruginosa Lung Infection in Cftr−/− Mice. Antimicrob Agents Chemother. 2007;51:3677–3687. doi: 10.1128/AAC.01011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Florescu D.F., Murphy P.J., Kalil A.C. Effects of prolonged use of azithromycin in patientswith cystic fibrosis: A meta-analysis. Pulmon. Pharmaco. Ther. 2009;22:467–472. doi: 10.1016/j.pupt.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 115.Bystrzycka W., Manda-Handzlik A., Sieczkowska S., Moskalik A., Demkow U., Ciepiela O. Azithromycin and Chloramphenicol Diminish Neutrophil Extracellular Traps (NETs) Release. Int. J. Mol. Sci. 2017;18:2666. doi: 10.3390/ijms18122666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bosnar M., Cuzic S., Bosnjak B., Nujic K., Ergovic G., Marjanovic N., Pasalic I., Hrvacic B., Polancec D., Glojnaric I., et al. Azithromycin inhibits macrophage interleukin-1beta production through inhibition of activator protein-1 in lipopolysaccharide-induced murine pulmonary neutrophilia. Int. Immunopharmacol. 2011;11:424–434. doi: 10.1016/j.intimp.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 117.Skindersoe M.E., Alhede M., Phipps R., Yang L., Jensen P.O., Rasmussen T.B. Effects of antibiotics on quorum sensing in Pseudomo- nas aeruginosa. Antimicrob. Agents Chemother. 2008;52:3648–3663. doi: 10.1128/AAC.01230-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schaible U.E., Kaufmann S.H. Iron and microbial infection. Nat. Rev. Microbiol. 2004;2:946–953. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- 119.Berlutti F., Morea C., Battistoni A., Sarli S., Cipriani P., Superti F., Ammendolia M.G., Valenti P. Iron availability influences aggregation, biofilm, adhesion and invasion of Pseudomonas aeruginosa and Burkholderia cenocepacia. Int. J. Immunopathol. Pharmacol. 2005;18:661–670. doi: 10.1177/039463200501800407. [DOI] [PubMed] [Google Scholar]
- 120.Bonchi C., Imperi F., Minandri F., Visca P., Frangipani E. Repurposing of gallium-based drugs for antibacterial therapy. Biofactors. 2014;40:303–312. doi: 10.1002/biof.1159. [DOI] [PubMed] [Google Scholar]
- 121.Minandri F., Bonchi C., Frangipani E. Promises and failures of gallium as an antibacterial agent. Future Microbiol. 2014;9:379–397. doi: 10.2217/fmb.14.3. [DOI] [PubMed] [Google Scholar]
- 122.Kaneko Y., Thoendel M., Olakanmi O., Britigan B.E., Singh P.K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 2007;117:877–888. doi: 10.1172/JCI30783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.DeLeon K., Balldin F., Watters C., Hamood A., Griswold J., Sreedharan S., Rumbaugh K.P. Gallium maltolate treatment eradicates Pseudomonas aeruginosa infection in thermally injured mice. Antimicrob. Agents Chemother. 2009;53:1331–1337. doi: 10.1128/AAC.01330-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Halwani M., Yebio B., Suntres Z.E., Alipour M., Azghani A.O., Omri A. Co-encapsulation of gallium with gentamicin in liposomes enhances antimicrobial activity of gentamicin against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2008;62:1291–1297. doi: 10.1093/jac/dkn422. [DOI] [PubMed] [Google Scholar]
- 125.Goss C., Heltshe S., Aitken M.L., Hornick D., Lechtzin N., McCoy K., Skalland M., Mayer-Hamblett N., Teresi M., Singh P. IV gallium nitrate demonstrates biological activity for chronic Pseudomonas aeruginosa infection in cystic fibrosis. J. Cyst. Fibros. 2019;18:S1–S38. doi: 10.1016/S1569-1993(19)30119-5. [DOI] [Google Scholar]
- 126.A Phase 1b, Multi-center Study of Intravenous (IV) Gallium Nitrate in Patients With Cystic Fibrosis (CF) Who Are Colonized With Nontuberculous Mycobacteria (NTM) (The ABATE Study) [(accessed on 5 December 2020)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04294043?term=gallium&cond=Cystic+Fibrosis&draw=2&rank=4.
- 127.Davies J.C., Martin I. New anti-pseudomonal agents for cystic fibrosis- still needed in the era of small molecule CFTR modulators? Expert Opin. Pharmacother. 2018;19:1327–1336. doi: 10.1080/14656566.2018.1505864. [DOI] [PubMed] [Google Scholar]
- 128.Saiman L. Improving outcomes of infections in cystic fibrosis in the era of CFTR modulator therapy. Pediatr Pulmonol. 2019;54:S18–S26. doi: 10.1002/ppul.24522. [DOI] [PubMed] [Google Scholar]
- 129.Yau Y.C., Ratjen F., Tullis E., Wilcox P., Freitag A., Chilvers M., Grasemann H., Zlosnik J., Speert D., Corey M., et al. Randomized controlled trial of biofilm antimicrobial susceptibility testing in cystic fibrosis patients. J. Cyst. Fibros. 2015;14:262–266. doi: 10.1016/j.jcf.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 130.Waters V., Ratjen F. Standard versus biofilm antimicrobial susceptibility testing to guide antibiotic therapy in cystic fibrosis. Cochrane Database Syst. Rev. 2017;10:CD009528. doi: 10.1002/14651858.CD009528.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]