The human body has long been considered a superorganism composed of host cells and symbiotic microorganisms. The number of microbes in the human body exceeds 10 times that of human cells, both of which collectively determine health and disease. The microflora dysbiosis can cause the disease or modulate the progression of human disease.1 Recent human microbiome studies have shed some important insights into the contributions of the microbiome on lung diseases. Previous beliefs regarding the sterility of the lungs were debunked with the improvement of microorganism culture conditions and the development of next-generation sequencing technologies.2 The continuous migration of oronasal microbes and the elimination of microorganisms through ciliary activity result in a dynamic microflora in the lungs that influence the lung’s health. Moreover, the gut microbe can affect the respiratory immunity and health through the gut–lung axis, and although the microbial density is lower in the lung compared with the gut, the change of microflora in the lung can affect the gut microbiome. However, it is difficult to demonstrate the causative relationship between individual bacteria in the lung microflora and the biological phenotype because of the diversity of the lung microbiota and complexity of the human body. Many studies are using mouse models where the specific human microbiome are transplanted into mice to evaluate respiratory health.3 They are valuable tools to understand the possible cause and effect of the lung microbiota in the disease because single bacteria monocolonization in mice is achievable, and particular genotype or phenotype related to certain phenotypes can be made using mice models.
The mouse is the most commonly used animal model and has become an invaluable tool in studying human diseases, including the relation between the microbiome and lung disease, because of their 97% homologous genes, convenient operation, fast procreation, and ability to genetic manipulation. Even with these advantages, the mouse model has certain limitations, especially with it comes to studying the effects of lung microbiota, and warrants special attention in extrapolating these results to human health. Two widely used mouse models to study the host–microbiome interactions are germ-free (GM) and antibiotic-treated (AT) mouse (Fig 1). Both of these models provide unique advantages, but each suffers from their inherent limitations.
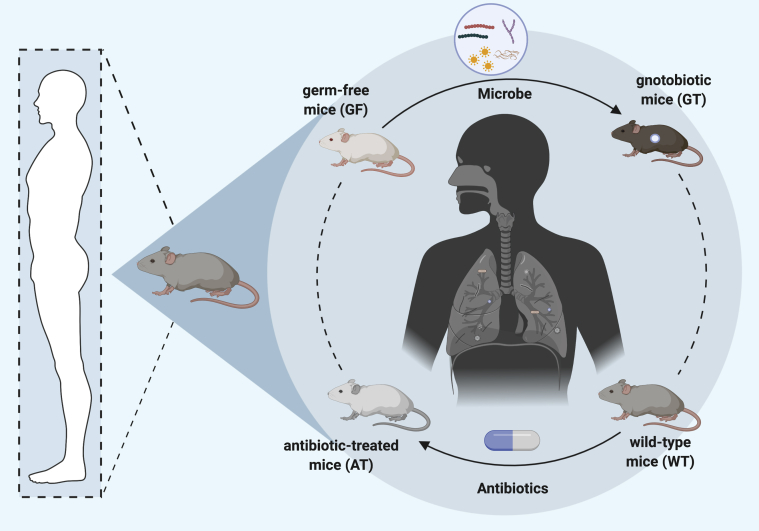
Figure 1.
Murine microbiome models commonly used to study the host-microbiome interactions. Antibiotic-treated mice (AT) can be obtained by depleting gut microbiota of wild-type mice (AT) with broad-spectrum antibiotics. Gnotobiotic mice (GT) are derived by colonization of germ-free mice (GF) with a defined microbe. GT mice can be produced using bacterial species associated with human microbiome.
GM mice do not have any microorganisms in their bodies or on their skin because they are born by hysterectomy and raised under strict husbandry protocols to ensure germ-free conditions. The birth of the first GM mouse in 1959 accelerated the progress in the field of microbiota and illness, which was considered a milestone in studying the human microbiome.4 These mice can be modified with specific genes or implanted with specific microbiota to understand their effects on various pathophysiological outcomes. When GM mice are inoculated with microbes, they are referred to as gnotobiotic mice, which are continuously maintained under sterile conditions to avoid contaminating the desired microbiome in these mice. Because of the transplantation of single bacteria, GM mice are an excellent tool for studying species or genus-specific effects of microbiota on respiratory health. Additionally, the interaction between specific genes and the microbiome can be analyzed by making germ-free transgenic mice. However, the requirements of specialized facilities, as well as the costs and skills required to regularly monitor these GM mice for contamination, limit their widespread applicability. Furthermore, GM mice are born and grown in sterile conditions that impair their development and immune system. For example, GM mice need to consume more food because they lack gut microorganisms providing extra energy from dietary fibers.
To avoid these limitations, another model widely used in microbiome research uses pseudo-sterile mice by using antibiotics that deplete the microorganisms in the mice.5 For example, to investigate the potential role of lung microbiota in COPD, the pulmonary microbiota isolated from diseased mice were transferred to antibiotic-treated mice via intranasal administration; this study demonstrated a causal link between the lung microbiome and the inflammation present in COPD.3 Additional benefits of this model include its lower cost, no requirement for special facilities before antibiotic treatment, and the application in any genotype. However, the generation of AT mice depends on initial bacterial composition, antibiotic mixture, duration of treatment, and phenotypic interpretation. Furthermore, the antibiotic treatment can affect other organs, especially liver and kidney, that play roles in metabolism and excretion and lead to the evolution of drug-resistant microbes. These limitations can make experimental results variable besides generating potential threatening pathogens. Despite the low cost and availability, the results from this model come with warnings of potential off-target effects of antibiotics and incomplete or inconsistent microbial depletion. Therefore, AT mice are more challenging and require strict standardized antibiotic treatment.
Despite the different limitations in murine microbiome models, they are currently the best in vivo model systems available for studying the effects of the microbiome on lung diseases. The main issues in this field include how the lung microbiota establish and maintain themselves, how to determine the microbiota composition and distribution, and how they affect the lung’s health and pathogenesis of lung diseases. It is essential to address the causal relationship between lung microbiome and disease to pave the way for developing new microbial regulation strategies. When interpreting the results from using mouse microbiota models, scientists should take into account that the findings cannot be directly applied to humans because of the distinct physiology, anatomy, and behaviors that influence microbial communities. In future studies on microbiome and lung health, to harness the murine microbiome models, the following aspects should be considered: monitoring the microbial loads to ensure no contamination in GM and AT mice models; evaluating the replicability of finding in GM and AT mice models to exclude off-target effects or developmental differences; comparing the structure and composition of the overall microbial community and assessing the function of the transplanted microbiome at the molecular levels; and developing new models that truly reflect host–microbiota interaction in humans closely. Overall, the murine lung microbiome models have great potential in providing insights into the cause-and-effect relationship between the microbiome and human lung diseases.
Acknowledgments
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FINANCIAL/NONFINANCIAL DISCLOSURES: The authors have reported to CHEST the following: They received financial support from China Scholarship Council (D. C.; No. 201809112037), Beijing Nova Program (D. C.; No. Z171100001117012), Beijing Nova Program Interdisciplinary Cooperation Project (D. C.; No. Z191100001119021), and National Institutes of Health National Heart, Lung, and Blood Institute (C. D. C.; No. R01HL126094), American Lung Association (L. S.; No. 513385) and American Thoracic Society (L. S.; No. 2018-18).
References
- 1.Thomas S., Izard J., Walsh E. The host microbiome regulates and maintains human health: a primer and perspective for non-microbiologists. Cancer Res. 2017;77:1783–1812. doi: 10.1158/0008-5472.CAN-16-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wypych T.P., Wickramasinghe L.C., Marsland B.J. The influence of the microbiome on respiratory health. Nat Immunol. 2019;20:1279–1290. doi: 10.1038/s41590-019-0451-9. [DOI] [PubMed] [Google Scholar]
- 3.Yadava K., Pattaroni C., Sichelstiel A.K. Microbiota promotes chronic pulmonary inflammation by enhancing IL-17A and autoantibodies. Am J Respir Crit Care Med. 2016;193:975–987. doi: 10.1164/rccm.201504-0779OC. [DOI] [PubMed] [Google Scholar]
- 4.Macpherson A., McCoy K. Standardised animal models of host microbial mutualism. Mucosal Immunol. 2015;8:476. doi: 10.1038/mi.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayer F., Ascher S., Pontarollo G., Reinhardt C. Antibiotic treatment protocols and germ-free mouse models in vascular research. Front Immunol. 2019;10:2174. doi: 10.3389/fimmu.2019.02174. [DOI] [PMC free article] [PubMed] [Google Scholar]