Abstract
OSA occurs in approximately 1% to 5% of children in the United States. Long-term cardiovascular risks associated with OSA in the adult population are well documented. Although changes in BP regulation occur in children with OSA, the pathways leading to chronic cardiovascular risks of OSA in children are less clear. Risk factors associated with cardiovascular disease in adult populations could carry the same future risk for children. It is imperative to determine whether known mechanisms of cardiovascular diseases in adults are like those that lead to pediatric disease. Early pathophysiologic changes may lead to a lifetime burden of cardiovascular disease and early mortality. With this perspective in mind, our review discusses pathways leading to cardiovascular pathology in children with OSA and provides a comprehensive overview of recent research findings related to cardiovascular sequelae in the pediatric population.
Key Words: cardiovascular, OSA, pediatrics, risk factors
Abbreviations: AMBP, ambulatory BP; CRP, C-reactive protein; HRV, heart rate variability; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; OAHI, obstructive apnea-hypopnea index; PSG, polysomnography; PTT, pulse transit time
The prevalence of OSA in children varies with the severity of the disorder.1 In a population of 5- to 12-year-old children, the prevalence of mild OSA (an obstructive apnea-hypopnea index [OAHI] between one and five events per hour) reaches 25%. The prevalence of moderate OSA (an OAHI > 5 and < 10 events per hour2) is approximately 1.2%. Pediatric OSA was first recognized as a serious clinical diagnosis following publication of several case reports describing children with severe pulmonary hypertension and irregular breathing during sleep.3, 4 The improvement in nocturnal breathing patterns and pulmonary hypertension, as well as their cardiopulmonary health, following tracheostomy represented the first link of upper airway obstruction during sleep to cardiopulmonary disease in the pediatric population.3 Due to the relatively early diagnosis of OSA in children, the clinical presentation, including pulmonary hypertension and cardiac dysfunction, is not routinely encountered in today’s practice.
The change in the clinical phenotype of children presenting with OSA to one that lacks an overt manifestation of cardiovascular disease leads to the important question of whether OSA in children still poses a cardiovascular risk. To address this question, the prevalence of known risk factors leading to cardiovascular disease in adults is being evaluated in children with OSA. This approach assumes that risk factors associated with cardiovascular disease in adult populations carry the same future risk for children. However, it is important to recognize that numerous childhood cohorts studied over the last several decades were created to determine the association between cardiovascular risk factors identified during childhood and adult cardiovascular diseases5, 6; this goal has not yet been completely achieved. One important reason for this delay is the dynamic change in these risk factors over the span of several decades. Specifically, the incidence of cardiovascular disease during adulthood may depend on whether a certain risk factor is sustained from childhood to adulthood or whether it waxes and wanes over time.
Notwithstanding our inability to describe the continuum of cardiovascular health throughout life, there is strong evidence that some cardiovascular risk factors may continue from childhood into adulthood. For example, in a longitudinal study of 493 boys and girls aged 5 to 18 years, elevated systolic BP during childhood was associated with hypertension and metabolic syndrome as adults.5 It is therefore imperative to determine whether known mechanisms of adult cardiovascular diseases are also important for the development of pediatric disease. With these issues in mind, the present review discusses pathways leading to cardiovascular pathology in children with OSA. Tables 1 through 4 summarize some of the major studies described in this review.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24
BP Dysregulation
As a result of defining BP ≥ 95th percentile,25 the diagnosis of childhood hypertension has been made more frequently over the last several decades.26, 27 Hypertension and BP dysregulation, well-known risk factors for cardiovascular diseases, have been investigated in both cross-sectional and longitudinal studies of children with OSA. Many of these studies examined BP in healthy normotensive children without comorbid conditions. Fewer studies examined the prevalence of OSA in children with hypertension. However, it is important to recognize that the American Academy of Pediatrics revised its definition of pediatric prehypertension in 2017,25 and the prevalence of prehypertension and/or hypertension in children with OSA may change accordingly. In addition, it is important to note that variability in cardiovascular outcomes in children can be influenced by the age of study participants. Phenotypic changes in preschool-aged children are not as marked as changes in older children, and this factor may be related to the length of time children are exposed to untreated disease.
Prevalence of OSA in Children With Hypertension
The prevalence of OSA in adults with resistant hypertension is as high as 80%, with approximately 50% having moderate to severe OSA.28 However, there are few data on the prevalence of OSA in children with hypertension. A recent retrospective study29 that examined 446 children aged 10 to 17 years referred to a hypertension clinic reported a snoring prevalence of 23%. In a subset of children with snoring who underwent polysomnography (PSG), 55% had OSA. Fifty-two percent of children with severe OSA (an apnea-hypopnea index [AHI] ≥ 10 events per hour2) had stage 2 hypertension based on in-office BP measurements.
BP in Normotensive Children
There are several commonly used approaches to study BP control in children with OSA. These include measurements of in-office BP, 24-h ambulatory BP (AMBP) measurements, and pulse transit time (PTT) as a surrogate marker of BP.7 However, some previous studies also used continuous BP recordings from overnight PSG. In addition to measuring BP during wakefulness and sleep, studies examined BP variability and nocturnal dipping. Marcus et al,9 for example, first published the results of BP recording during overnight PSG and reported an increase in diastolic BP during both wakefulness and sleep. In children aged 2 to 12 years, systolic and diastolic BPs were associated with the severity of OSA. Additional studies of 24-h AMBP in school-aged and adolescent children showed an increase in diastolic and/or systolic BPs.10, 11, 12, 13 In one study of 24-h AMBP,10 BP load (defined as the number of measurements exceeding the 95th percentile) was positively correlated with the severity of OSA. Similarly, children with OSA had a higher morning BP surge also associated with the severity of OSA, as measured by using OAHI. In almost all case-control studies that examined BP control, children with OSA had a significantly higher BMI compared with control subjects. As a result, the relative contribution of obesity vs OSA to 24-h AMBP findings was examined. The results revealed that OSA and BMI have similar effects on diurnal systolic BP, diastolic BP, and sleep systolic BP. However, OSA had a significantly greater effect on nocturnal diastolic BP than BMI. Evidence supports the association between OSA and changes in BP in the pediatric population (Table 1).7, 8, 9, 10, 11, 12, 13
Table 1.
Summary of Studies Discussing General Effects of OSA on BP
| Study | Year | Study Type | No. of Subjects | Age | What Is Evaluated | Findings |
|---|---|---|---|---|---|---|
| Nisbet et al7 | 2013 | Prospective | 81 children | 3-5 y | PTT | Obstructive events elicit acute cardiovascular changes in preschool-aged children |
| Horne et al8 | 2018 | Prospective | 98 children | 8-18 y | PTT | BMI has combined and independent effects on BP and heart rate in children with OSA |
| Marcus et al9 | 1998 | Prospective | 67 children | 2-12 y | BP using arm cuff | Childhood OSA is associated with systemic diastolic hypertension |
| Amin et al10 | 2008 | Prospective | 140 children | 7-13 y | 24-h AMBP | SDB in children associated with increase in morning BP surge, BP load, and 24-h AMBP |
| Li et al11 | 2008 | Prospective | 306 children | 6-13 y | 24-h AMBP | OSA associated with elevated daytime and nocturnal HTN |
| Leung et al12 | 2006 | Prospective | 96 children | 6-15 y | 24-h AMBP | Increased desaturation index is associated with elevation of diastolic BP elevation |
| Kang et al13 | 2016 | Prospective | 163 children | 4-16 y | 24-h AMBP | Prevalence of nocturnal systolic HTN higher in children with OSA |
AMBP = ambulatory BP; HTN = hypertension; PTT = pulse transit time; SDB = sleep-disordered breathing.
Because children with OSA have higher BMIs compared with control subjects,10 and obesity significantly affects the cardiovascular system, some studies were designed to distinguish the effects of OSA vs obesity. In a study of children aged 8 to 18 years, BP and PTT were evaluated in normal weight and obese control subjects and those with OSA. In this study, BMI exhibited combined and independent effects on BP in those with OSA.8 Other studies have co-varied for BMI,30, 31, 32, 33, 34 all showing that OSA and obesity are independently associated with adverse cardiovascular outcomes. In a 35-year longitudinal study of preadolescent children without OSA, participants who were overweight at the initial visit were significantly more likely to develop OSA in middle age,6 highlighting the significance of childhood obesity on the development of OSA in adulthood. Although additive effects of obesity and OSA on the cardiovascular system are difficult to distinguish, improvement of cardiovascular sequelae in children with OSA also requires control of obesity.
Nocturnal dipping of BP is an important factor that has a protective effect against cardiovascular disease. In both children and adults, nondipping is defined as a fall in less than 10% of the nocturnal (asleep) BP compared with the daytime (awake) BP. Dipping status, however, can vary depending on the measured BP index.35 It is well established that a nondipping pattern of diurnal BP variation in adults is an independent predictor of adverse cardiovascular outcomes.36 In fact, in a meta-analysis of four prospective studies of adults with hypertension, nocturnal BP was a better predictor of cardiovascular outcomes than daytime BP.37 Several mechanisms have been proposed to explain nondipping of nocturnal BP, including attenuated nocturnal decreases in systemic vascular resistance, increases in sympathetic tone, and decreases in baroreflex sensitivity during sleep.38, 39 In adults with OSA, nondipping BP is well documented and correlates with the severity of the disease.40, 41, 42 Similar observations were made in children with OSA.14, 15 Nondipping nocturnal BP in children 5 to 17 years of age with OSA has been reported in previous studies.15, 16, 43 However, in a group of 7- to 13-year-old Australian children with OSA, BP dipping was preserved.17 Similarly, preserved nocturnal BP dipping is seen in children aged 3 to 5 years with OSA even in the presence of sleep fragmentation.18 Evidence supports a link between OSA and nondipping BP in children (Table 2).14, 15, 16, 17, 18
Table 2.
Summary of Studies Discussing Nondipping BP in OSA
| Study | Year | Study Type | No. of Subjects | Age | What Is Evaluated | Findings |
|---|---|---|---|---|---|---|
| Horne et al14 | 2011 | Prospective | 105 children | 7-13 y | Continuous overnight BP | SDB associated with nondipping BP |
| Amin et al15 | 2004 | Prospective | 60 children | 7-14 y | Continuous overnight BP | Nocturnal BP dipping was predicted by desaturation index |
| Xu et al16 | 2013 | Prospective | 145 children | 5-14 y | 24-h AMBP | Children with OSA had decreased nocturnal dipping |
| Horne et al17 | 2013 | Prospective | 141 children | 7-12 y | Continuous overnight BP | SDB does not alter nocturnal BP dipping |
| Nisbet et al18 | 2014 | Prospective | 192 children | 3-5 y | PTT | Nocturnal dipping is preserved in young children with OSA |
See Table 1 legend for expansion of abbreviations.
Another parameter of BP control is the variability of diurnal and nocturnal BP. It is evident that the variability of BP between clinic visits and in 24-h AMBP in the adult population carries an increased risk of cardiovascular disease and all-cause mortality.44, 45 Increases in BP variability have also been reported in 5- to 17-year-old children with OSA, and this finding correlated with the severity of OSA.15 The role of autonomic dysfunction in BP dysregulation in children with OSA has been explored in several studies. There is now evidence that a broad range of children (from toddlers to teenagers) with OSA have heightened sympathetic tone and decreased baroreceptor sensitivity.19, 20, 22, 23
Autonomic balance can also be evaluated by using other measures such as heart rate variability (HRV).46 Many studies have used HRV to assess sympathetic tone in children with OSA. In a study of children with OSA,21 autonomic balance, as determined by HRV from overnight PSG, correlated with respiratory disturbance index. In addition, HRV, as a measure of sympathetic activity of the autonomic nervous system, decreased in 2- to 7-year-old children following treatment of OSA with adenotonsillectomy.47 Morning urinary catecholamine levels also seem to be associated with severity of OSA, indicating that OSA leads to increased sympathetic tone.19, 48 OSA leads to changes in both sympathetic tone and baroreflex sensitivity in the pediatric population (Table 3).19, 20, 21, 22, 23
Table 3.
Summary of Studies Discussing Changes in Sympathetic Tone and Baroreflex Sensitivity in OSA
| Category | Study | Year | Study Type | No. of Subjects | Age | What is Evaluated | Findings |
|---|---|---|---|---|---|---|---|
| Sympathetic tone | O’Driscoll et al19 | 2011 | Prospective | 96 children | 3-12 y | Overnight urinary catecholamine levels | Overnight urinary noradrenaline (sympathetic tone) related to severity of OSA |
| Montesano et al20 | 2010 | Prospective | 50 children | 7-12 y | Ewing test battery | Increase in basal sympathetic activity during wakefulness, dependent on severity of OSA | |
| Baharav et al21 | 1999 | Prospective | 20 children | 3-14 y | Heart rate fluctuation in PSG | Children with OSA exhibit enhanced sympathetic activity | |
| Baroreflex sensitivity | Chaicharn et al22 | 2009 | Prospective | 20 children | 7-14 y | Baroreflex gain | Vagal modulation remains normal in children with OSA, but baseline sympathetic activity is elevated |
| McConnell et al23 | 2009 | Prospective | 169 children | 7-12 y | Baroreflex gain | OSA associated with decrease in nocturnal baroreflex gain and increase in BP variability |
PSG = polysomnography.
Most studies of children with OSA reported increased systolic and diastolic BPs, increased BP variability, and decreased BP dipping. It is important to recognize that not all studies report all three abnormalities in BP. This lack of agreement between studies highlights the fundamental question about the genetic predisposition and gene-environment interactions that lead to a phenotype with increased cardiovascular risk.
The causal relationship between OSA and elevated BP level has been explored through longitudinal follow-up studies of untreated children with OSA and by examining the change in BP following treatment. In a 4-year prospective follow-up study of untreated childhood OSA,49 baseline OAHI in 9- and 10-year-old children was positively associated with follow-up awake and sleep systolic and diastolic BPs. The change in OAHI was also positively associated with sleep systolic and diastolic BPs. The effect of treatment on BP control has also been reported previously. In multiple cross-sectional studies, treatment with adenotonsillectomy or CPAP was associated with a decrease in BP.50, 51, 52, 53, 54, 55, 56 These studies suggest that a causal relationship between elevated BP and OSA in children might indeed exist. However, these previous observations have not been confirmed through more rigorous randomized controlled trials.
The Rationale for Monitoring BP in Children With OSA
Studies have shown that the BP trajectory from childhood to young adulthood is closely related to end-organ structure and function. In a 23-year longitudinal study of BP in children without OSA recruited between the ages of 5 and 16 years,24 BP could be successfully tracked into adulthood. The study also showed that the trajectory of systolic BP was a significant predictor of both carotid intimal thickness and left ventricular mass index (Table 4).5, 6, 24 Furthermore, studies that examined left ventricular geometry in prehypertensive and hypertensive children between the ages of 5 and 19 years found that both groups had increased left ventricular remodeling and mass compared with normotensive children and that the change in left ventricular geometry increased with increasing BP.57, 58 Left ventricular remodeling and hypertrophy have been described in 5- to 12-year-old children with OSA59 and were also associated with increasing BP.10 Although most children are normotensive based on the American Academy of Pediatrics guidelines that precede the 2017 report,25 observing the BP trajectory of children with OSA is essential to identifying those at risk for developing clinically significant elevated BP later in life.
Table 4.
Summary of Several Longitudinal Studies
| Study | Year | Study Type | No. of Subjects | Age | What Is Evaluated | Findings |
|---|---|---|---|---|---|---|
| Sun et al5 | 2007 | Longitudinal cohort | 493 children into adulthood | 5-18 y | Standard BP measurements | Children with elevated BP at increased risk of HTN as adults |
| Bazzano et al6 | 2016 | Longitudinal cohort | 844 children into adulthood | 7-12 y | Anthropometric measurements | Overweight in childhood increases risk for OSA in middle age |
| Hao et al24 | 2017 | Longitudinal cohort | 683 children into adulthood | 5-16 y | Standard BP measurements, echo | Childhood systolic BP trajectories are associated with subclinical cardiovascular risk |
See Table 1 legend for expansion of abbreviation.
OSA and Endothelial Dysfunction
Vascular tone, platelet activity, leukocyte adhesion, and angiogenesis are regulated by the vascular endothelium. Nitric oxide, along with other regulatory factors, is essential to preserving endothelial function. A decrease in nitric oxide bioavailability induces endothelial inflammation that promotes atherosclerosis.60, 61 Therefore, endothelial dysfunction correlates with cardiovascular disease progression and predicts cardiovascular events.61, 62, 63, 64 In children, several studies described an association between endothelial dysfunction and OSA.65, 66, 67, 68 Treatment of nonobese 6- to 11-year-old children with OSA by using adenotonsillectomy seems to have a beneficial effect on endothelial function in a subset of children with no family history of hypertension.65
Inflammation and OSA in Children
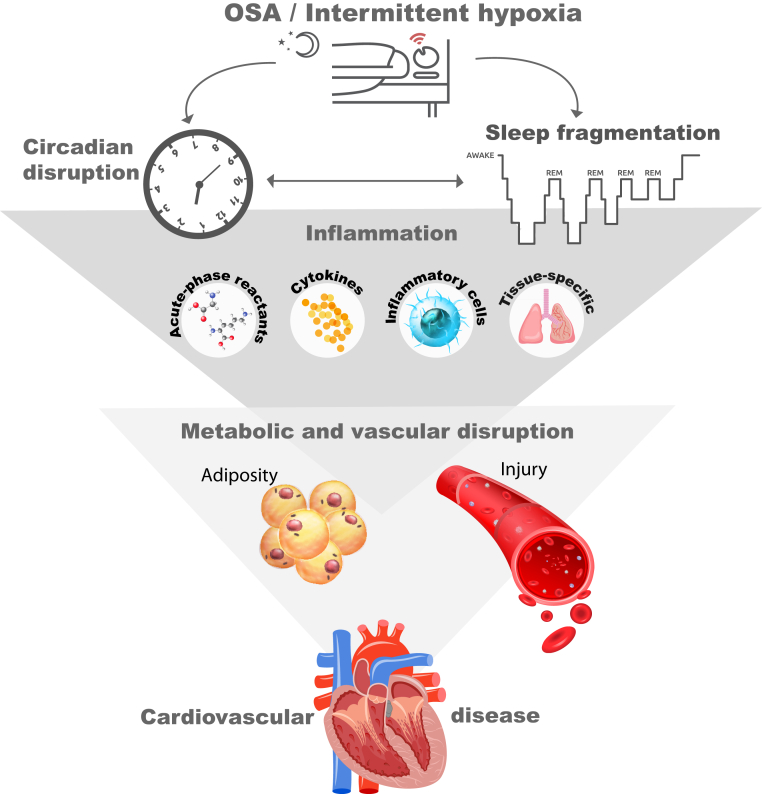
The relationship between inflammatory pathways and OSA is complex and likely to be bidirectional. There are multiple facets to the inflammatory response in the context of OSA. Evidence exists of systemic inflammation that is quantified by circulating cytokines, acute-phase reactants, and inflammatory cells. Evidence also exists of tissue- or organ-specific inflammation that mediates some of the phenotypic characteristics observed in children with OSA. Lastly, there is a potential role of inflammation in the development of OSA (Fig 1).
Figure 1.
Relationship between inflammation and OSA. Systemic and tissue-specific inflammation mediates cardiovascular and metabolic disturbances that also contribute to the relationship between central adiposity and OSA. REM = rapid eye movement.
Systemic Inflammation
Multiple studies have reported increased levels of plasma cytokines in children with OSA across many age groups.69, 70, 71, 72, 73, 74, 75 However, fewer studies have examined the relationship between circulating cytokines and cardiovascular end points. In children with OSA, circulating cytokines and/or inflammatory cells were found to correlate with the degree of endothelial dysfunction and PTT.66, 74, 76 Parallel to the increase in proinflammatory cytokines is an increase in acute-phase reactants,77, 78, 79 such as C-reactive protein (CRP), and adipokines.74, 80 Although the role of acute-phase reactants in mediating and or protecting from cardiovascular injury needs further investigation, some evidence suggests that acute-phase reactants such as CRP may play dual roles: one as proinflammatory in the process of endothelial injury, and the second to protect from the proinflammatory effects of circulating cytokines.74
Tissue Inflammation in OSA
Preclinical studies show that intermittent hypoxia induces endothelial inflammation and dysfunction through upregulation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), a transcription factor that upregulates > 200 genes.81, 82, 83, 84, 85 The pathways mediating endothelial inflammation and injury observed in preclinical studies have been tested in children with OSA. These studies show that the same inflammatory pathways were closely associated with endothelial dysfunction.67, 86
Adenoid and tonsillar tissue from children with OSA have characteristic distributions of lymphocyte subsets.87 There is also high expression of leukotrienes and their receptors in the adenotonsillar tissues,87, 88, 89 which elicit a cellular proliferation of tonsillar tissue. Demain and Goetz90 showed that long-term use of nasal corticosteroids in 5- to 11-year-old children could reduce the size of adenoid tissue and treat isolated nasal airway obstruction. A similar treatment regimen showed a significant decrease in obstructive respiratory events in children aged 1 to 10 years with OSA.91 Several prospective clinical studies have also reported a therapeutic role for leukotriene modifiers in pediatric OSA.92, 93 These data suggest that the inflammatory response observed in children with OSA may also contribute to further airway obstruction by inducing hypertrophy of tonsillar and adenoid tissue.
Contribution of Inflammation to OSA
The strong association between obesity and OSA in children and adults has been repeatedly reported in multiple studies that have examined the risk factors for OSA, with central obesity playing an important role in mediating this relationship. Several explanations for the potential causal association between obesity and OSA have also been proposed. Data suggest that the association between central adiposity and OSA is mediated through an inflammatory process.80 In this longitudinal study examining the change in obesity, OSA, and inflammatory biomarkers from early childhood to adolescence, changes in CRP were closely associated with changes in waist circumference and follow-up OAHI. These results suggest that inflammation may explain the association between increasing central obesity and OSA severity. Additional studies reported that genetic polymorphisms of IL-6, tumor necrosis factor α, and CRP contribute to OSA.74, 94, 95, 96, 97 Based on these observations, it is plausible that obesity-related inflammation represents one mechanism of OSA in overweight and obese children.
Insulin Resistance and Glucose Homeostasis in Pediatric OSA
It is plausible that even in the absence of obesity, OSA may increase the risk for cardiovascular disease by inducing insulin resistance. Insulin resistance is directly associated with an increased risk for atherosclerosis.98 Artificially induced sleep fragmentation is associated with decreased morning insulin sensitivity,99 and the degree of sleep disruption is correlated with the level of insulin resistance.100 Intermittent hypoxia-induced inflammation led to insulin resistance in a murine model of OSA.101 Intermittent hypoxia also induces arousal in mice.102 Furthermore, intermittent hypoxia in healthy adults decreases insulin sensitivity.103 In a large pediatric cohort of normal and overweight children aged 5 to 12 years, sleep fragmentation was independently and positively associated with insulin resistance measures.104 Although obesity is also associated with insulin resistance, complicating causal relationships between OSA, obesity, and cardiovascular disease, it is apparent that sleep fragmentation associated with OSA may play a role in disrupted homeostasis commonly seen in children with OSA.
Circadian Misalignment in Pediatric OSA
Advances in research on control of the circadian clock have expanded our understanding of the impact of circadian misalignment on human disease.105 Variations of the endogenous circadian rhythm or misalignment between the clock and environmental time cues negatively affect the sleep-wake cycle and can lead to cardiovascular disease.106, 107 Humans have developed an endogenous clock that follows the light-dark cycle.108 This system is hierarchically organized109, 110 and is composed of the central clock, located in the hypothalamus,111 and peripheral clocks throughout the body that contain autonomous oscillators.112, 113, 114 This feedback loop comprises activators and repressors that orchestrate transcription for thousands of genes in all cells over the 24-h rhythm.109, 115
Virtually all cardiovascular and metabolic functions have a daily rhythm that is regulated by clock genes. Diurnal changes in BP and heart rate, cardiac remodeling, and contractility follow a diurnal rhythm.116 Furthermore, glucose homeostasis is dependent on alignment of the circadian rhythm of multiple organs.117, 118, 119 Sleep fragmentation in adolescents with sleep disorders led to clock gene dysregulation and decreased glucose tolerance, showing the role of circadian rhythm disturbances in carbohydrate metabolic dysfunction.120 Cytokines that mediate cardiovascular end-organ damage also follow a diurnal rhythm.121, 122, 123 Given the number of cardiac, metabolic, and immunologic functions that exhibit circadian rhythmicity, it is possible that circadian misalignment is closely affiliated with other mechanisms which are associated with cardiovascular disease in children with OSA.
Preclinical studies reported increased left ventricular end-systolic and end-diastolic dimensions and reduced cardiac contractility in circadian rhythm-disturbed animals.124, 125 In humans, the impact of circadian misalignment on the cardiovascular system has been largely investigated in adult shift workers.126 Sleep restriction and circadian misalignment in adults are associated with increased CRP levels and insulin resistance,127 decreases in cortisol levels and changes in proinflammatory and antiinflammatory cytokines,128 and increases in 24-h systolic and diastolic BPs.129 There is limited evidence of circadian misalignment in adults and children with OSA. One study in adults showed that OSA has a significant effect on peak serum melatonin levels and that 3-month CPAP use restores the physiologic rhythm of melatonin secretion.130 In a large prospective study of > 13,000 adults, shortened sleep duration and OSA were independently associated with major coronary events.131 Interestingly, OSA had an additive effect to short sleep and shift work hours on the risk of cardiovascular disease.
Evidence regarding the risks of circadian misalignment that accompany OSA has been identified from the change in the diurnal rhythm of mediators of inflammation and immunity. It has been suggested that phases of rest vs activity influence the balance between proinflammatory and antiinflammatory mediators.132 The active phase favors an antiinflammatory state, whereas rest favors a proinflammatory state. Although there are no data from adult OSA populations regarding changes in the diurnal rhythm of inflammatory mediators, a preliminary study in children showed changes in cytokine rhythmicity because of OSA.74 In healthy control subjects, tumor necrosis factor α, IL-6, and IL-8 levels were higher in the evening (Fig 2). However, children 6 to 13 years old with OSA exhibited higher levels in the morning. This reversal of diurnal rhythmicity occurred in addition to overall higher cytokine levels in children with OSA compared with control subjects. Although these data are derived from one single cross-sectional study, the results may suggest that the loss of a normal diurnal rhythm of inflammation in OSA could be a mechanism of sustained inflammatory response throughout a 24-h period.
Figure 2.
Cytokine levels (evening [blue lines] vs morning [red lines]) for healthy participants and children with mild vs severe OSA; data are reported as mean ± SD. Healthy participants and children with OSA aged 5 to 13 years were recruited to the study. All participants underwent overnight polysomnography with evening and morning blood draws for cytokine and acute-phase reactant level measurements. Cytokine levels were measured at 6:00 pm and 6:00 am. Serum IL-6, IL-8, and TNF-α exhibited a difference in diurnal variation in children with OSA compared with healthy control subjects. In control patients, cytokine levels decreased from evening to morning. In children with mild and severe OSA, cytokine levels increased from evening to morning. TNF-α = tumor necrosis factor α. The figure was created by using data presented previously by Smith et al.74
The effects of OSA on the circadian rhythm of hormone levels could be associated with pathophysiologic consequences seen with untreated disease. Nocturnal awakenings seen in OSA lead to alterations in the hypothalamic-pituitary-adrenal axis and increased pulsatile cortisol release.133 As the major product of the hypothalamic-pituitary-adrenal axis, cortisol plays a significant role in metabolic and BP regulation.134 Although previous large-scale studies have not identified a difference in cortisol levels in patients with OSA, demonstration of changes in rhythmicity would require careful and repeatedly timed measurements.135 Recent research in mice has shown that flattening of daily glucocorticoid oscillations (as seen in chronic conditions that alter glucocorticoid secretion) results in increases in fat mass and weight gain.136 It is possible that changes in hormone rhythmicity in the presence of OSA could be linked to weight gain.
Future Directions
As the prevalence of OSA increases in the pediatric population, the long-term socioeconomic impact and burden for patients, families, and the medical community will only worsen. Much research is now focused on identification of the novel mechanistic pathways that lead to pathophysiologic progression of untreated disease. For example, evaluation of microRNA profiles and transcriptome profiling in patients with OSA have uncovered potential target genes for future medical intervention.137, 138, 139, 140, 141 Identification of other upstream pathways, such as those that regulate the circadian clock, may lead to the development of new diagnostic techniques and medical therapies.142
Conclusions
Published literature supports the hypothesis that children with OSA have cardiovascular and inflammatory processes such as those associated with cardiovascular disease in adults. Further research is needed to determine the causal relationship between OSA and the presence of cardiovascular risk factors as well as the reversibility of these processes with adequate treatment. Furthermore, there is a gap in knowledge pertaining to factors that determine whether these processes that begin during childhood translate into cardiovascular diseases during adulthood.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
References
- 1.Bixler E.O., Vgontzas A.N., Lin H.M. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32(6):731–736. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redline S., Tishler P.V., Schluchter M., Aylor J., Clark K., Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159(5 pt 1):1527–1532. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 3.Lugaresi E., Coccagna G., Mantovani M., Brignani F. Effects of tracheostomy in two cases of hypersomnia with periodic breathing. J Neurol Neurosurg Psychiatry. 1973;36(1):15–26. doi: 10.1136/jnnp.36.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coccagna G., Mantovani M., Brignani F., Parchi C., Lugaresi E. Tracheostomy in hypersomnia with periodic breathing. Bull Physiopathol Respir. 1972;8(5):1217–1227. [PubMed] [Google Scholar]
- 5.Sun S.S., Grave G.D., Siervogel R.M., Pickoff A.A., Arslanian S.S., Daniels S.R. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119(2):237–246. doi: 10.1542/peds.2006-2543. [DOI] [PubMed] [Google Scholar]
- 6.Bazzano L.A., Hu T., Bertisch S.M. Childhood obesity patterns and relation to middle-age sleep apnoea risk: the Bogalusa Heart Study. Pediatr Obes. 2016;11(6):535–542. doi: 10.1111/ijpo.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nisbet L.C., Yiallourou S.R., Nixon G.M. Characterization of the acute pulse transit time response to obstructive apneas and hypopneas in preschool children with sleep-disordered breathing. Sleep Med. 2013;14(11):1123–1131. doi: 10.1016/j.sleep.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Horne R.S.C., Shandler G., Tamanyan K. The impact of sleep disordered breathing on cardiovascular health in overweight children. Sleep Med. 2018;41:58–68. doi: 10.1016/j.sleep.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Marcus C.L., Greene M.G., Carroll J.L. Blood pressure in children with obstructive sleep apnea. Am J Respir Crit Care Med. 1998;157(4 pt 1):1098–1103. doi: 10.1164/ajrccm.157.4.9704080. [DOI] [PubMed] [Google Scholar]
- 10.Amin R., Somers V.K., McConnell K. Activity-adjusted 24-hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathing. Hypertension. 2008;51(1):84–91. doi: 10.1161/HYPERTENSIONAHA.107.099762. [DOI] [PubMed] [Google Scholar]
- 11.Li A.M., Au C.T., Sung R.Y.T. Ambulatory blood pressure in children with obstructive sleep apnoea: a community based study. Thorax. 2008;63(9):803–809. doi: 10.1136/thx.2007.091132. [DOI] [PubMed] [Google Scholar]
- 12.Leung L.C.K., Ng D.K., Lau M.W. Twenty-four-hour ambulatory BP in snoring children with obstructive sleep apnea syndrome. Chest. 2006;130(4):1009–1017. doi: 10.1378/chest.130.4.1009. [DOI] [PubMed] [Google Scholar]
- 13.Kang K.T., Chiu S.N., Weng W.C., Lee P.L., Hsu W.C. Comparisons of office and 24-hour ambulatory blood pressure monitoring in children with obstructive sleep apnea. J Pediatr. 2017;182 doi: 10.1016/j.jpeds.2016.11.032. 177-183.e2. [DOI] [PubMed] [Google Scholar]
- 14.Horne R.S.C., Yang J.S.C., Walter L.M. Elevated blood pressure during sleep and wake in children with sleep-disordered breathing. Pediatrics. 2011;128(1):e85–e92. doi: 10.1542/peds.2010-3431. [DOI] [PubMed] [Google Scholar]
- 15.Amin R.S., Carroll J.L., Jeffries J.L. Twenty-four–hour ambulatory blood pressure in children with sleep-disordered breathing. Am J Respir Crit Care Med. 2004;169(8):950–956. doi: 10.1164/rccm.200309-1305OC. [DOI] [PubMed] [Google Scholar]
- 16.Xu Z., Li B., Shen K. Ambulatory blood pressure monitoring in Chinese children with obstructive sleep apnea/hypopnea syndrome. Pediatr Pulmonol. 2013;48(3):274–279. doi: 10.1002/ppul.22595. [DOI] [PubMed] [Google Scholar]
- 17.Horne R.S.C., Yang J.S.C., Walter L.M. Nocturnal dipping is preserved in children with sleep disordered breathing regardless of its severity. Pediatr Pulmonol. 2013;48(11):1127–1134. doi: 10.1002/ppul.22727. [DOI] [PubMed] [Google Scholar]
- 18.Nisbet L.C., Nixon G.M., Yiallourou S.R. Sleep-disordered breathing does not affect nocturnal dipping, as assessed by pulse transit time, in preschool children: evidence for early intervention to prevent adverse cardiovascular effects? Sleep Med. 2014;15(4):464–471. doi: 10.1016/j.sleep.2013.11.787. [DOI] [PubMed] [Google Scholar]
- 19.O’Driscoll D.M., Horne R.S.C., Davey M.J. Increased sympathetic activity in children with obstructive sleep apnea: cardiovascular implications. Sleep Med. 2011;12(5):483–488. doi: 10.1016/j.sleep.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Montesano M., Miano S., Paolino M.C. Autonomic cardiovascular tests in children with obstructive sleep apnea syndrome. Sleep. 2010;33(10):1349–1355. doi: 10.1093/sleep/33.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baharav A., Kotagal S., Rubin B.K., Pratt J., Akselrod S. Autonomic cardiovascular control in children with obstructive sleep apnea. Clin Auton Res. 1999;9(6):345–351. doi: 10.1007/BF02318382. [DOI] [PubMed] [Google Scholar]
- 22.Chaicharn J., Lin Z., Chen M.L., Ward S.L.D., Keens T., Khoo M.C.K. Model-based assessment of cardiovascular autonomic control in children with obstructive sleep apnea. Sleep. 2009;32(7):927–938. doi: 10.1093/sleep/32.7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McConnell K., Somers V.K., Kimball T. Baroreflex gain in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2009;180(1):42–48. doi: 10.1164/rccm.200808-1324OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hao G., Wang X., Treiber F.A., Harshfield G., Kapuku G., Su S. Blood pressure trajectories from childhood to young adulthood associated with cardiovascular risk: results from the 23-year longitudinal Georgia Stress and Heart Study. Hypertension. 2017;69(3):435–442. doi: 10.1161/HYPERTENSIONAHA.116.08312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flynn J.T., Kaelber D.C., Baker-Smith C.M. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3) doi: 10.1542/peds.2017-1904. pii: e20171904. [DOI] [PubMed] [Google Scholar]
- 26.Rosner B., Cook N.R., Daniels S., Falkner B. Childhood blood pressure trends and risk factors for high blood pressure: the NHANES experience 1988-2008. Hypertension. 2013;62(2):247–254. doi: 10.1161/HYPERTENSIONAHA.111.00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Din-Dzietham R., Liu Y., Bielo M.V., Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116(13):1488–1496. doi: 10.1161/CIRCULATIONAHA.106.683243. [DOI] [PubMed] [Google Scholar]
- 28.Muxfeldt E.S., Margallo V.S., Guimarães G.M., Salles G.F. Prevalence and associated factors of obstructive sleep apnea in patients with resistant hypertension. Am J Hypertens. 2014;27(8):1069–1078. doi: 10.1093/ajh/hpu023. [DOI] [PubMed] [Google Scholar]
- 29.Hinkle J., Connolly H.V., Adams H.R., Lande M.B. Severe obstructive sleep apnea in children with elevated blood pressure. J Am Soc Hypertens. 2018;12(3):204–210. doi: 10.1016/j.jash.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enright P.L., Goodwin J.L., Sherrill D.L., Quan J.R., Quan S.F. Tucson Children’s Assessment of Sleep Apnea Study. Blood pressure elevation associated with sleep-related breathing disorder in a community sample of white and Hispanic children: the Tucson Children’s Assessment of Sleep Apnea study. Arch Pediatr Adolesc Med. 2003;157(9):901–904. doi: 10.1001/archpedi.157.9.901. [DOI] [PubMed] [Google Scholar]
- 31.Kohyama J., Ohinata J.S., Hasegawa T. Blood pressure in sleep disordered breathing. Arch Dis Child. 2003;88(2):139–142. doi: 10.1136/adc.88.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwok K.L., Ng D.K.K., Cheung Y.F. BP and arterial distensibility in children with primary snoring. Chest. 2003;123(5):1561–1566. doi: 10.1378/chest.123.5.1561. [DOI] [PubMed] [Google Scholar]
- 33.Isacco L., Roche J., Quinart S. Cardiometabolic risk is associated with the severity of sleep-disordered breathing in children with obesity. Physiol Behav. 2017;170:62–67. doi: 10.1016/j.physbeh.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 34.Van Eyck A., Van Hoorenbeeck K., De Winter B.Y., Van Gaal L., De Backer W., Verhulst S.L. Sleep disordered breathing and autonomic function in overweight and obese children and adolescents. ERJ Open Res. 2016;2(4) doi: 10.1183/23120541.00038-2016. pii: 00038-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavelaars M, Tulen JHM, Bemmel JH van, Meiracker AH van den Physical activity, dipping and haemodynamics. J Hypertens. 2004;22(12):2303–2309. doi: 10.1097/00004872-200412000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Salles G.F., Reboldi G., Fagard R.H. Prognostic effect of the nocturnal blood pressure fall in hypertensive patients: the Ambulatory Blood Pressure Collaboration in Patients With Hypertension (ABC-H) Meta-Analysis. Hypertension. 2016;67(4):693–700. doi: 10.1161/HYPERTENSIONAHA.115.06981. [DOI] [PubMed] [Google Scholar]
- 37.Fagard R.H., Celis H., Thijs L. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51(1):55–61. doi: 10.1161/HYPERTENSIONAHA.107.100727. [DOI] [PubMed] [Google Scholar]
- 38.Sherwood A., Hill L.K., Blumenthal J.A., Hinderliter A.L. Circadian hemodynamics in men and women with high blood pressure: dipper vs. nondipper and racial differences. J Hypertens. 2018;36(2):250–258. doi: 10.1097/HJH.0000000000001533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen C.W., Kuo T.B.J., Chen C.Y., Yang C.C.H. Reduced capacity of autonomic and baroreflex control associated with sleep pattern in spontaneously hypertensive rats with a nondipping profile. J Hypertens. 2017;35(3):558–570. doi: 10.1097/HJH.0000000000001205. [DOI] [PubMed] [Google Scholar]
- 40.Seif F., Patel S.R., Walia H.K. Obstructive sleep apnea and diurnal nondipping hemodynamic indices in patients at increased cardiovascular risk. J Hypertens. 2014;32(2):267–275. doi: 10.1097/HJH.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sasaki N., Ozono R., Edahiro Y. Impact of non-dipping on cardiovascular outcomes in patients with obstructive sleep apnea syndrome. Clin Exp Hypertens. 2015;37(6):449–453. doi: 10.3109/10641963.2015.1057833. [DOI] [PubMed] [Google Scholar]
- 42.Marcus J.A., Pothineni A., Marcus C.Z., Bisognano J.D. The role of obesity and obstructive sleep apnea in the pathogenesis and treatment of resistant hypertension. Curr Hypertens Rep. 2014;16(1):411. doi: 10.1007/s11906-013-0411-y. [DOI] [PubMed] [Google Scholar]
- 43.Weber SAT, Santos VJB dos, Semenzati G de O, Martin LC Ambulatory blood pressure monitoring in children with obstructive sleep apnea and primary snoring. Int J Pediatr Otorhinolaryngol. 2012;76(6):787–790. doi: 10.1016/j.ijporl.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 44.Stevens S.L., Wood S., Koshiaris C. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ. 2016;354:i4098. doi: 10.1136/bmj.i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ke X., Sun Y., Yang R. Association of 24 h-systolic blood pressure variability and cardiovascular disease in patients with obstructive sleep apnea. BMC Cardiovasc Disord. 2017;17(1):287. doi: 10.1186/s12872-017-0723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 47.Muzumdar H.V., Sin S., Nikova M., Gates G., Kim D., Arens R. Changes in heart rate variability after adenotonsillectomy in children with obstructive sleep apnea. Chest. 2011;139(5):1050–1059. doi: 10.1378/chest.10-1555. [DOI] [PubMed] [Google Scholar]
- 48.Snow A.B., Khalyfa A., Serpero L.D. Catecholamine alterations in pediatric obstructive sleep apnea: effect of obesity. Pediatr Pulmonol. 2009;44(6):559–567. doi: 10.1002/ppul.21015. [DOI] [PubMed] [Google Scholar]
- 49.Li A.M., Au C.T., Ng C., Lam H.S., Ho C.K.W., Wing Y.K. A 4-year prospective follow-up study of childhood OSA and its association with BP. Chest. 2014;145(6):1255–1263. doi: 10.1378/chest.13-1333. [DOI] [PubMed] [Google Scholar]
- 50.Amin R., Anthony L., Somers V. Growth velocity predicts recurrence of sleep-disordered breathing 1 year after adenotonsillectomy. Am J Respir Crit Care Med. 2008;177(6):654–659. doi: 10.1164/rccm.200710-1610OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vlahandonis A., Yiallourou S.R., Sands S.A. Long-term changes in blood pressure control in elementary school-aged children with sleep-disordered breathing. Sleep Med. 2014;15(1):83–90. doi: 10.1016/j.sleep.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 52.Vlahandonis A., Nixon G.M., Davey M.J., Walter L.M., Horne R.S.C. Improvement of sleep-disordered breathing in children is associated with a reduction in overnight blood pressure. Sleep Med. 2013;14(12):1295–1303. doi: 10.1016/j.sleep.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 53.Ng D.K., Wong J.C., Chan C.H., Leung L.C.K., Leung S.Y. Ambulatory blood pressure before and after adenotonsillectomy in children with obstructive sleep apnea. Sleep Med. 2010;11(7):721–725. doi: 10.1016/j.sleep.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 54.Lee C.H., Kang K.T., Chiu S.N. Association of adenotonsillectomy with blood pressure among hypertensive and nonhypertensive children with obstructive sleep apnea. JAMA Otolaryngol Head Neck Surg. 2018;144(4):300–307. doi: 10.1001/jamaoto.2017.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsu W.C., Kang K.T., Chiu S.N., Weng W.C., Lee P.L., Lin C.Y. 24-Hour ambulatory blood pressure after adenotonsillectomy in childhood sleep apnea. J Pediatr. 2018;199:112–117.e6. doi: 10.1016/j.jpeds.2018.03.072. [DOI] [PubMed] [Google Scholar]
- 56.DelRosso L.M., King J., Ferri R. Systolic blood pressure elevation in children with obstructive sleep apnea is improved with positive airway pressure use. J Pediatr. 2018;195:102–107.e1. doi: 10.1016/j.jpeds.2017.11.043. [DOI] [PubMed] [Google Scholar]
- 57.Stabouli S., Kotsis V., Rizos Z. Left ventricular mass in normotensive, prehypertensive and hypertensive children and adolescents. Pediatr Nephrol. 2009;24(8):1545–1551. doi: 10.1007/s00467-009-1165-2. [DOI] [PubMed] [Google Scholar]
- 58.Dušan P., Tamara I., Goran V., Gordana M.L., Amira P.A. Left ventricular mass and diastolic function in obese children and adolescents. Pediatr Nephrol. 2015;30(4):645–652. doi: 10.1007/s00467-014-2992-3. [DOI] [PubMed] [Google Scholar]
- 59.Amin R.S., Kimball T.R., Bean J.A. Left ventricular hypertrophy and abnormal ventricular geometry in children and adolescents with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165(10):1395–1399. doi: 10.1164/rccm.2105118. [DOI] [PubMed] [Google Scholar]
- 60.Vita J.A., Keaney J.F., Jr. Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106(6):640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 61.Halcox J.P.J., Donald A.E., Ellins E. Endothelial function predicts progression of carotid intima-media thickness. Circulation. 2009;119(7):1005–1012. doi: 10.1161/CIRCULATIONAHA.108.765701. [DOI] [PubMed] [Google Scholar]
- 62.Yeboah J., Crouse J.R., Hsu F.C., Burke G.L., Herrington D.M. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115(18):2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 63.Cooper L.L., Palmisano J.N., Benjamin E.J. Microvascular Function contributes to the relation between aortic stiffness and cardiovascular events: the Framingham Heart Study. Circ Cardiovasc Imaging. 2016;9(12) doi: 10.1161/CIRCIMAGING.116.004979. pii: e004979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeboah J., Folsom A.R., Burke G.L. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2009;120(6):502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gozal D., Kheirandish-Gozal L., Serpero L.D., Sans Capdevila O., Dayyat E. Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation. 2007;116(20):2307–2314. doi: 10.1161/CIRCULATIONAHA.107.696823. [DOI] [PubMed] [Google Scholar]
- 66.Kheirandish-Gozal L., Bhattacharjee R., Kim J., Clair H.B., Gozal D. Endothelial progenitor cells and vascular dysfunction in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2010;182(1):92–97. doi: 10.1164/rccm.200912-1845OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khalyfa A., Kheirandish-Gozal L., Khalyfa A.A. Circulating plasma extracellular microvesicle microRNA cargo and endothelial dysfunction in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2016;194(9):1116–1126. doi: 10.1164/rccm.201602-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gozal D., Kheirandish-Gozal L., Bhattacharjee R., Spruyt K. Neurocognitive and endothelial dysfunction in children with obstructive sleep apnea. Pediatrics. 2010;126(5):e1161–e1167. doi: 10.1542/peds.2010-0688. [DOI] [PubMed] [Google Scholar]
- 69.Tam C.S., Wong M., McBain R., Bailey S., Waters K.A. Inflammatory measures in children with obstructive sleep apnoea. J Paediatr Child Health. 2006;42(5):277–282. doi: 10.1111/j.1440-1754.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- 70.Gozal D., Lipton A.J., Jones K.L. Circulating vascular endothelial growth factor levels in patients with obstructive sleep apnea. Sleep. 2002;25(1):59–65. doi: 10.1093/sleep/25.1.59. [DOI] [PubMed] [Google Scholar]
- 71.Gozal D., Serpero L.D., Kheirandish-Gozal L., Capdevila O.S., Khalyfa A., Tauman R. Sleep measures and morning plasma TNF-alpha levels in children with sleep-disordered breathing. Sleep. 2010;33(3):319–325. doi: 10.1093/sleep/33.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Israel L.P., Benharoch D., Gopas J., Goldbart A.D. A pro-inflammatory role for nuclear factor kappa B in childhood obstructive sleep apnea syndrome. Sleep. 2013;36(12):1947–1955. doi: 10.5665/sleep.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramesh V., Nair D., Zhang S.X.L. Disrupted sleep without sleep curtailment induces sleepiness and cognitive dysfunction via the tumor necrosis factor-α pathway. J Neuroinflammation. 2012;9:91. doi: 10.1186/1742-2094-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith D.F., Hossain M.M., Hura A. Inflammatory milieu and cardiovascular homeostasis in children with obstructive sleep apnea. Sleep. 2017;40(4) doi: 10.1093/sleep/zsx022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ye J., Liu H., Li P. CD4+T-lymphocyte subsets in nonobese children with obstructive sleep apnea syndrome. Pediatr Res. 2015;78:165. doi: 10.1038/pr.2015.76. [DOI] [PubMed] [Google Scholar]
- 76.Kim J., Bhattacharjee R., Snow A.B., Capdevila O.S., Kheirandish-Gozal L., Gozal D. Myeloid-related protein 8/14 levels in children with obstructive sleep apnoea. Eur Respir J. 2010;35(4):843–850. doi: 10.1183/09031936.00075409. [DOI] [PubMed] [Google Scholar]
- 77.Tam C.S., Wong M., Tam K., Aouad L., Waters K.A. The effect of acute intermittent hypercapnic hypoxia treatment on IL-6, TNF-alpha, and CRP levels in piglets. Sleep. 2007;30(6):723–727. doi: 10.1093/sleep/30.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tauman R., O’Brien L.M., Gozal D. Hypoxemia and obesity modulate plasma C-reactive protein and interleukin-6 levels in sleep-disordered breathing. Sleep Breath. 2007;11(2):77–84. doi: 10.1007/s11325-006-0085-7. [DOI] [PubMed] [Google Scholar]
- 79.Gozal D., Kheirandish-Gozal L., Bhattacharjee R., Kim J. C-reactive protein and obstructive sleep apnea syndrome in children. Front Biosci. 2012;4:2410–2422. doi: 10.2741/e553. [DOI] [PubMed] [Google Scholar]
- 80.Gaines J., Vgontzas A.N., Fernandez-Mendoza J. Inflammation mediates the association between visceral adiposity and obstructive sleep apnea in adolescents. Am J Physiol Endocrinol Metab. 2016;311(5):E851–E858. doi: 10.1152/ajpendo.00249.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Recoquillon S., Gómez-Guzmán M., Rodier M. Non-muscular myosin light chain kinase triggers intermittent hypoxia-induced interleukin-6 release, endothelial dysfunction and permeability. Sci Rep. 2017;7(1):13664. doi: 10.1038/s41598-017-13268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Polotsky V.Y., Savransky V., Bevans-Fonti S. Intermittent and sustained hypoxia induce a similar gene expression profile in human aortic endothelial cells. Physiol Genomics. 2010;41(3):306–314. doi: 10.1152/physiolgenomics.00091.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee M.Y.K., Wang Y., Mak J.C.W., Ip M.S.M. Intermittent hypoxia induces NF-κB-dependent endothelial activation via adipocyte-derived mediators. Am J Physiol Cell Physiol. 2016;310(6):C446–C455. doi: 10.1152/ajpcell.00240.2015. [DOI] [PubMed] [Google Scholar]
- 84.Han Q., Yeung S.C., Ip M.S.M., Mak J.C.W. Intermittent hypoxia-induced NF-κB and HO-1 regulation in human endothelial EA.hy926 cells. Cell Biochem Biophys. 2013;66(3):431–441. doi: 10.1007/s12013-012-9491-6. [DOI] [PubMed] [Google Scholar]
- 85.Badran M., Golbidi S., Devlin A., Ayas N., Laher I. Chronic intermittent hypoxia causes endothelial dysfunction in a mouse model of diet-induced obesity. Sleep Med. 2014;15(5):596–602. doi: 10.1016/j.sleep.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 86.Kim J., Gozal D., Bhattacharjee R., Kheirandish-Gozal L. TREM-1 and pentraxin-3 plasma levels and their association with obstructive sleep apnea, obesity, and endothelial function in children. Sleep. 2013;36(6):923–931. doi: 10.5665/sleep.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sade K., Fishman G., Kivity S., DeRowe A., Langier S. Expression of Th17 and Treg lymphocyte subsets in hypertrophied adenoids of children and its clinical significance. Immunol Invest. 2011;40(6):657–666. doi: 10.3109/08820139.2011.575426. [DOI] [PubMed] [Google Scholar]
- 88.Dayyat E., Serpero L.D., Kheirandish-Gozal L. Leukotriene pathways and in vitro adenotonsillar cell proliferation in children with obstructive sleep apnea. Chest. 2009;135(5):1142–1149. doi: 10.1378/chest.08-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim J., Bhattacharjee R., Dayyat E. Increased cellular proliferation and inflammatory cytokines in tonsils derived from children with obstructive sleep apnea. Pediatr Res. 2009;66(4):423–428. doi: 10.1203/PDR.0b013e3181b453e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Demain J.G., Goetz D.W. Pediatric adenoidal hypertrophy and nasal airway obstruction: reduction with aqueous nasal beclomethasone. Pediatrics. 1995;95(3):355–364. [PubMed] [Google Scholar]
- 91.Brouillette R.T., Manoukian J.J., Ducharme F.M. Efficacy of fluticasone nasal spray for pediatric obstructive sleep apnea. J Pediatr. 2001;138(6):838–844. doi: 10.1067/mpd.2001.114474. [DOI] [PubMed] [Google Scholar]
- 92.Goldbart A.D., Greenberg-Dotan S., Tal A. Montelukast for children with obstructive sleep apnea: a double-blind, placebo-controlled study. Pediatrics. 2012;130(3):e575–e580. doi: 10.1542/peds.2012-0310. [DOI] [PubMed] [Google Scholar]
- 93.Kheirandish-Gozal L., Bandla H.P.R., Gozal D. Montelukast for children with obstructive sleep apnea: results of a double-blind, randomized, placebo-controlled trial. Ann Am Thorac Soc. 2016;13(10):1736–1741. doi: 10.1513/AnnalsATS.201606-432OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khalyfa A., Serpero L.D., Kheirandish-Gozal L., Capdevila O.S., Gozal D. TNF-α gene polymorphisms and excessive daytime sleepiness in pediatric obstructive sleep apnea. J Pediatr. 2011;158(1):77–82. doi: 10.1016/j.jpeds.2010.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang Y.S., Guilleminault C., Hwang F.M. Inflammatory cytokines in pediatric obstructive sleep apnea. Medicine. 2016;95(41):e4944. doi: 10.1097/MD.0000000000004944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaditis A.G., Gozal D., Khalyfa A. Variants in C-reactive protein and IL-6 genes and susceptibility to obstructive sleep apnea in children: a candidate-gene association study in European American and Southeast European populations. Sleep Med. 2014;15(2):228–235. doi: 10.1016/j.sleep.2013.08.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu Y., Cao C., Wu Y. TNF-α-308G/A polymorphism contributes to obstructive sleep apnea syndrome risk: evidence based on 10 case-control studies. PLoS One. 2014;9(9):e106183. doi: 10.1371/journal.pone.0106183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Howard G., O’Leary D.H., Zaccaro D. Insulin sensitivity and atherosclerosis. Circulation. 1996;93:1809–1817. doi: 10.1161/01.cir.93.10.1809. [DOI] [PubMed] [Google Scholar]
- 99.Stamatakis K.A., Punjabi N.M. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137(1):95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tasali E., Leproult R., Ehrmann D.A., Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105(3):1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ryan S., Taylor C.T., McNicholas W.T. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112(17):2660–2667. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 102.Polotsky V.Y., Rubin A.E., Balbir A. Intermittent hypoxia causes REM sleep deficits and decreases EEG delta power in NREM sleep in the C57BL/6J mouse. Sleep Med. 2006;7(1):7–16. doi: 10.1016/j.sleep.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 103.Louis M., Punjabi N.M. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol. 2009;106(5):1538–1544. doi: 10.1152/japplphysiol.91523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koren D., Gozal D., Philby M.F., Bhattacharjee R., Kheirandish-Gozal L. Impact of obstructive sleep apnoea on insulin resistance in nonobese and obese children. Eur Respir J. 2016;47(4):1152–1161. doi: 10.1183/13993003.01430-2015. [DOI] [PubMed] [Google Scholar]
- 105.Zhu L., Zee P.C. Circadian rhythm sleep disorders. Neurol Clin. 2012;30(4):1167–1191. doi: 10.1016/j.ncl.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Young M.E., Bray M.S. Potential role for peripheral circadian clock dyssynchrony in the pathogenesis of cardiovascular dysfunction. Sleep Med. 2007;8(6):656–667. doi: 10.1016/j.sleep.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bray M.S., Young M.E. Circadian rhythms in the development of obesity: potential role for the circadian clock within the adipocyte. Obes Rev. 2007;8(2):169–181. doi: 10.1111/j.1467-789X.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 108.Pittendrigh C.S. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:16–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- 109.Dunlap J.C., Loros J.J., Liu Y., Crosthwaite S.K. Eukaryotic circadian systems: cycles in common. Genes Cells. 1999;4(1):1–10. doi: 10.1046/j.1365-2443.1999.00239.x. [DOI] [PubMed] [Google Scholar]
- 110.Lowrey P.L., Takahashi J.S. Genetics of the mammalian circadian system: photic entrainment, circadian pacemaker mechanisms, and posttranslational regulation. Annu Rev Genet. 2000;34:533–562. doi: 10.1146/annurev.genet.34.1.533. [DOI] [PubMed] [Google Scholar]
- 111.Ripperger J.A., Jud C., Albrecht U. The daily rhythm of mice. FEBS Lett. 2011;585(10):1384–1392. doi: 10.1016/j.febslet.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 112.Yagita K., Tamanini F., Der Horst GT van, Okamura H. Molecular mechanisms of the biological clock in cultured fibroblasts. Science. 2001;292(5515):278–281. doi: 10.1126/science.1059542. [DOI] [PubMed] [Google Scholar]
- 113.Yamazaki S., Numano R., Abe M. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288(5466):682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 114.Balsalobre A., Brown S.A., Marcacci L. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289(5488):2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 115.Takahashi J.S. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18(3):164–179. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Durgan D.J., Hotze M.A., Tomlin T.M. The intrinsic circadian clock within the cardiomyocyte. Am J Physiol Heart Circ Physiol. 2005;289(4):H1530–H1541. doi: 10.1152/ajpheart.00406.2005. [DOI] [PubMed] [Google Scholar]
- 117.Bescos R., Boden M.J., Jackson M.L. Four days of simulated shift work reduces insulin sensitivity in humans. Acta Physiol. 2018;223(2):e13039. doi: 10.1111/apha.13039. [DOI] [PubMed] [Google Scholar]
- 118.Reinke H., Asher G. Circadian clock control of liver metabolic functions. Gastroenterology. 2016;150(3):574–580. doi: 10.1053/j.gastro.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 119.Rakshit K., Qian J., Ernst J., Matveyenko A.V. Circadian variation of the pancreatic islet transcriptome. Physiol Genomics. 2016;48(9):677–687. doi: 10.1152/physiolgenomics.00019.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tomoda A., Kawatani J., Joudoi T., Hamada A., Miike T. Metabolic dysfunction and circadian rhythm abnormalities in adolescents with sleep disturbance. Neuroimage. 2009;47(suppl 2):T21–T26. doi: 10.1016/j.neuroimage.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 121.Geiger S.S., Fagundes C.T., Siegel R.M. Chrono-immunology: progress and challenges in understanding links between the circadian and immune systems. Immunology. 2015;146(3):349–358. doi: 10.1111/imm.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Krueger J.M., Fang J., Taishi P., Chen Z., Kushikata T., Gardi J. Sleep: a physiologic role for IL-1beta and TNF-alpha. Annals NY Acad Sci. 1998;856:148–159. doi: 10.1111/j.1749-6632.1998.tb08323.x. [DOI] [PubMed] [Google Scholar]
- 123.Vgontzas A.N., Bixler E.O., Lin H.M., Prolo P., Trakada G., Chrousos G.P. IL-6 and its circadian secretion in humans. Neuroimmunomodulation. 2005;12(3):131–140. doi: 10.1159/000084844. [DOI] [PubMed] [Google Scholar]
- 124.Martino T.A., Tata N., Belsham D.D. Disturbed diurnal rhythm alters gene expression and exacerbates cardiovascular disease with rescue by resynchronization. Hypertension. 2007;49(5):1104–1113. doi: 10.1161/HYPERTENSIONAHA.106.083568. [DOI] [PubMed] [Google Scholar]
- 125.Bray M.S., Shaw C.A., Moore M.W.S. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol. 2008;294(2):H1036–H1047. doi: 10.1152/ajpheart.01291.2007. [DOI] [PubMed] [Google Scholar]
- 126.Wang D., Ruan W., Chen Z., Peng Y., Li W. Shift work and risk of cardiovascular disease morbidity and mortality: a dose-response meta-analysis of cohort studies. Eur J Prev Cardiol. 2018;25(12):1293–1302. doi: 10.1177/2047487318783892. [DOI] [PubMed] [Google Scholar]
- 127.Leproult R., Holmbäck U., Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63(6):1860–1869. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wright K.P., Jr., Drake A.L., Frey D.J. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav Immun. 2015;47:24–34. doi: 10.1016/j.bbi.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morris C.J., Purvis T.E., Hu K., Scheer F.A.J.L. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. 2016;113(10):E1402–E1411. doi: 10.1073/pnas.1516953113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barnaś M., Maskey-Warzęchowska M., Bielicki P., Kumor M., Chazan R. Diurnal and nocturnal serum melatonin concentrations after treatment with continuous positive airway pressure in patients with obstructive sleep apnea. Pol Arch Intern Med. 2017;127(9):589–596. doi: 10.20452/pamw.4062. [DOI] [PubMed] [Google Scholar]
- 131.Barger L.K., Rajaratnam S.M.W., Cannon C.P. Short sleep duration, obstructive sleep apnea, shiftwork, and the risk of adverse cardiovascular events in patients after an acute coronary syndrome. J Am Heart Assoc. 2017;6(10) doi: 10.1161/JAHA.117.006959. pii: e006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Aschoff J., Fatranská M., Giedke H., Doerr P., Stamm D., Wisser H. Human circadian rhythms in continuous darkness: entrainment by social cues. Science. 1971;171(3967):213–215. doi: 10.1126/science.171.3967.213. [DOI] [PubMed] [Google Scholar]
- 133.Späth-Schwalbe E., Gofferje M., Kern W., Born J., Fehm H.L. Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biol Psychiatry. 1991;29(6):575–584. doi: 10.1016/0006-3223(91)90093-2. [DOI] [PubMed] [Google Scholar]
- 134.Tomfohr L.M., Edwards K.M., Dimsdale J.E. Is obstructive sleep apnea associated with cortisol levels? A systematic review of the research evidence. Sleep Med Rev. 2012;16(3):243–249. doi: 10.1016/j.smrv.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lattova Z., Keckeis M., Maurovich-Horvat E. The stress hormone system in various sleep disorders. J Psychiatr Res. 2011;45(9):1223–1228. doi: 10.1016/j.jpsychires.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 136.Bahrami-Nejad Z., Zhao M.L., Tholen S. A transcriptional circuit filters oscillating circadian hormonal inputs to regulate fat cell differentiation. Cell Metab. 2018;27(4):854–868.e8. doi: 10.1016/j.cmet.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li K., Wei P., Qin Y., Wei Y. MicroRNA expression profiling and bioinformatics analysis of dysregulated microRNAs in obstructive sleep apnea patients. Medicine. 2017;96(34):e7917. doi: 10.1097/MD.0000000000007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ortega F.J., Mercader J.M., Catalán V. Targeting the circulating microRNA signature of obesity. Clin Chem. 2013;59(5):781–792. doi: 10.1373/clinchem.2012.195776. [DOI] [PubMed] [Google Scholar]
- 139.Gharib S.A., Khalyfa A., Abdelkarim A., Bhushan B., Gozal D. Integrative miRNA-mRNA profiling of adipose tissue unravels transcriptional circuits induced by sleep fragmentation. PLoS One. 2012;7(5):e37669. doi: 10.1371/journal.pone.0037669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gharib S.A., Seiger A.N., Hayes A.L., Mehra R., Patel S.R. Treatment of obstructive sleep apnea alters cancer-associated transcriptional signatures in circulating leukocytes. Sleep. 2014;37(4):709–714. doi: 10.5665/sleep.3574. 714A-714T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen Y.C., Chen T.W., Su M.C. Whole genome DNA methylation analysis of obstructive sleep apnea: IL1R2, NPR2, AR, SP140 methylation and clinical phenotype. Sleep. 2016;39(4):743–755. doi: 10.5665/sleep.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Allmen DC von, Francey L.J., Rogers G.M. Circadian dysregulation: the next frontier in obstructive sleep apnea research. Otolaryngol Head Neck Surg. 2018 doi: 10.1177/0194599818797311. 194599818797311. [DOI] [PubMed] [Google Scholar]