Abstract
Introduction
Discovering non‐invasive and easily acquired biomarkers that are conducive to the accurate diagnosis of dementia is an urgent area of ongoing clinical research. One promising approach is retinal imaging, as there is homology between retinal and cerebral vasculature. Recently, optical coherence tomography angiography (OCT‐A) has emerged as a promising new technology for imaging the microvasculature of the retina.
Methods
A systematic review and meta‐analysis was conducted to examine the application of OCT‐A in dementia.
Results
Fourteen studies assessing OCT‐A in preclinical Alzheimer's disease (AD), mild cognitive impairment, or AD were included. Exploratory meta‐analyses revealed a significant increase in the foveal avascular zone area and a significant decrease in superficial parafoveal and whole vessel density in AD, although there was significant heterogeneity between studies.
Discussion
Although certain OCT‐A metrics may have the potential to serve as biomarkers for AD, the field requires further standardization to allow conclusions to be reached regarding their clinical utility.
Keywords: Alzheimer's disease, dementia, diagnostic tool, foveal avascular zone, mild cognitive impairment, optical coherence tomography angiography, perfusion density, preclinical, retinal imaging, retinal vasculature, vessel density
1. INTRODUCTION
Alzheimer's disease (AD) and other forms of dementia are rising rapidly in prevalence and subsequently pose growing challenges to individuals and families as well as to societal and healthcare systems globally. 1 As such, discovering non‐invasive biomarkers that can be measured objectively and that are conducive to effective screening and diagnosis of dementia is an urgent area of clinical research. 2 Within the disease continuum, the pathological processes of dementia are believed to begin years before signs of cognitive symptoms appear. 3 In particular, AD pathology is characterized by the formation of extracellular amyloid beta (Aβ) plaques and intracellular neurofibrillary tangles of hyperphosphorylated tau, as well as changes to the cerebral vasculature such as cerebral amyloid angiopathy, atherosclerosis and arteriosclerosis, reduced capillary density, and altered capillary morphology. 4 , 5 , 6 , 7 It has been shown that Aβ plaque deposition is almost at its peak by the time cognitive symptoms manifest, whereas the acceleration of tau tangle accumulation may mark the transition period from the preclinical stage to clinically detectable symptoms, after which those affected often experience a period of mild cognitive impairment (MCI) before progressing to AD. 8 , 9 However, the effects of Aβ and tau deposition on synaptic dysfunction and neuronal survival do not peak until moderate to severe stages are reached. 10 Thus early intervention is of utmost importance for developing and administering preventive therapies for AD that preserve cognitive abilities or delay decline.
Due to the shared diencephalic origin of the retina and brain, there is homology between retinal and cerebral vasculature, and the retina is thus regarded as an extension of the central nervous system (CNS). 11 Some CNS disorders, such as cerebral small vessel disease and AD, are accompanied by ocular manifestations that reflect changes occurring in the brain. 12 , 13 , 14 , 15 For example, retinal microvascular changes such as venular dilation are observed with cerebral small vessel disease, 16 and narrow venular caliber and increased venular tortuosity have been observed in AD. 17 Changes to metrics that quantify aspects of the retinal microvasculature, such as reduced venous blood flow rate, may even be seen in earlier stages of disease. 18
Although cerebrovascular imaging is often expensive and requires the use of specialized techniques such as positron emission tomography (PET), retinal imaging provides an opportunity for a non‐invasive and quick modality to diagnose dementia or identify a need for early intervention. 19 Previous systematic reviews and meta‐analyses have been conducted on the use of retinal imaging techniques such as optical coherence tomography (OCT) and fundus photography as sources of biomarkers for dementia. 20 , 21 Recently, OCT angiography (OCT‐A) has emerged as a promising new technology for imaging the retina, which builds on established OCT technology and provides high‐resolution images of the retinal microvasculature and choroid. 22 Previous reviews have reported on the uses of OCT‐A in neurological research. 23 , 24 , 25 However, to the best of our knowledge, our review constitutes the first meta‐analytic approach to explore the use of OCT‐A metrics in dementia. We gave consideration to the different metrics that various studies featuring OCT‐A and persons with dementia have reported, and we aimed, where possible, to compare quantitatively across studies and highlight agreements or differences in their outputs. Furthermore, we propose several methods of standardization that may improve comparability among future studies.
2. METHODS
2.1. Search strategy
Studies were identified through systematic searches of the Medical Literature Analysis and Retrieval System Online (MEDLINE, from 1966), PubMed (from 1946), and the Excerpta Medica Database (EMBASE, from 1980) for all studies published through August 2020, in all languages. The search terms were “optical coherence tomography angiography” with “dementia” or “Alzheimer” or “Lewy body disease” or “vascular dementia” or “frontotemporal dementia” or “small vessel disease” or “mild cognitive impairment” or “cognitive” or “cognition” or “memory.” A forward citation search was also conducted using Google Scholar, although no further studies were identified.
HIGHLIGHTS
Most studies using optical coherence tomography angiography (OCT‐A) metrics in dementia have focused on Alzheimer's disease (AD)
Changes in the foveal avascular zone (FAZ) area and superficial vessel density may reflect AD progression
Standardization is needed before meaningful conclusions can be drawn
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using MEDLINE, PubMed, EMBASE, and Google Scholar. We identified 14 studies using the optical coherence tomography angiography imaging modality to assess changes in the retinal microvasculature along the Alzheimer's disease continuum.
Interpretation: Although our findings suggest that measurements of the foveal avascular zone area and vessel density of the superficial retinal layer may hold promise as potential biomarkers for Alzheimer's disease, methodological standardization is required before studies can be meaningfully compared and conclusions drawn as to the clinical utility of these novel metrics.
Future directions: We propose several steps to standardize future studies conducted in this area. Examples include: (a) reaching a consensus on terminology and anatomical boundaries used to describe and define macular and optic disc regions of study; (b) stratification of stages along the Alzheimer's disease continuum; (c) standardization of ophthalmological imaging protocols; and (d) standardization of vessel density calculation algorithms.
2.2. Inclusion and exclusion criteria
This review aimed to include all published studies utilizing OCT‐A to examine the association between the retinal microvasculature and any stage of dementia, including the preclinical stage. In this review, we defined preclinical dementia as the stage at which biomarkers, for example, Aβ+ or tau+ for preclinical AD, are present, but cognition or the ability to perform activities of daily living are not yet impaired. 26 , 27 Inclusion criteria were (1) original study that had undergone peer review; (2) written in English; (3) inclusion of retinal microvascular metrics using OCT‐A; (4a) diagnosis of any form of dementia based on established criteria such as that of the National Institute on Aging and Alzheimer's Association (NIA‐AA) or the National Institute of Neurological, Communicative Disorders and Stroke‐Alzheimer Disease and Related Disorders Association (NINCDS‐ADRDA) 28 , 29 or (4b) diagnosis of preclinical dementia by biomarker status, for example, Aβ+ or tau+; and (5) inclusion of a control group. Exclusion criteria were (1) reviews; (2) case reports; (3) non‐human research; (4) non–English‐language studies; (5) conference presentations or summaries; (6) studies without details of diagnostic criteria; (7) studies without OCT‐A; and (8) studies without a control group.
2.3. Data extraction
The identified studies were screened by title and abstract for duplication and relevance. The remaining studies were then subjected to full‐text review, and inclusion and exclusion criteria were applied (Supplementary Figure S1 ). Data extracted were: (1) title; (2) first author; (3) year of publication; (4) study aim; (5) study design; (6) number of participants; (7) mean age; (8) diagnostic criteria; (9) participant selection criteria; (10) method of imaging and analysis used; (11) results; and (12) conclusions.
2.4. Statistical analysis
Study‐specific OCT‐A measurement results are reported as mean difference (MD) with a P‐value for significance. Meta‐analyses of continuous outcomes were conducted with Review Manager Software Version 5.3 (Cochrane, Oxford) 30 using an inverse variance (IV) random‐effects model to calculate summary estimates of mean difference from extracted means, standard deviations (SDs), and sample sizes (Total) with 95% confidence intervals (CIs). Heterogeneity was tested using a χ 2 test with a significance threshold of P < 0.05. Analyses stratified by disease stage subgroups (ie, preclinical AD, MCI, AD) were conducted where possible.
3. RESULTS
The literature search yielded 177 results, of which 53 were unique studies. Of these, 34 were removed after they were determined to not meet the inclusion/exclusion criteria after a title and abstract screen. The full‐text versions of the remaining 19 studies were retrieved. One study was omitted as it mentioned OCT‐A in the abstract but not elsewhere in the article. One study was omitted because it lacked a control group. Two studies were omitted because they did not meet the appropriate diagnostic criteria, for example, if only Mini Mental State Examination (MMSE) scores were used. A final study was omitted because it was the only study featuring cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) and would thus not make for a suitable comparison with the other studies (all of which featured preclinical AD, MCI, or AD). See Supplementary Figure S1 for a flow diagram of the paper selection process. The populations sampled were from the United States (5), The Netherlands (2), Italy (2), Germany (1), Poland (1), Turkey (1), South Korea (1), and China (1). Table 1 describes the final set of 14 studies.
TABLE 1.
Included studies using optical coherence tomography angiography (OCT‐A) to assess retinal microvasculature in preclinical Alzheimer's disease (AD), mild cognitive impairment (MCI), and Alzheimer's disease (AD)
| Study | Study design (Total sample size) | Dementia outcome (Diagnostic criteria) (# of cases) | Additional Scoring | Mean (SD) age | Female (%) | Type of OCT‐A machine |
|---|---|---|---|---|---|---|
| Bulut et al., 2018 | Observational, case‐control (n = 52) |
AD (DSM‐IV and NIA‐AA) (n = 26) C (n = 26) |
MMSE a |
C: 72.58(6.28) AD: 74.23(7.55) Age‐matched |
C: 50% AD: 58% Sex‐matched |
RTVue XR Avanti |
| Criscuolo et al., 2020 | Observational, case‐control (n = 56) |
aMCI (NIA‐AA, ApoE4 and PET considered) (n = 27) C (n = 29) |
MMSE a |
C: 73.1(7) aMCI: 73(6) Adjusted for age in analyses |
C: 52% aMCI: 56% Adjusted for sex in analyses |
RTVue XR Avanti |
| den Haan et al., 2019 | Observational, case‐control (n = 86) |
AD (NIA‐AA and Aβ/tau/phospho‐tau+ CSF a or Aβ+ PET) (n = 48) C (n = 38) |
MMSE a WMH a phospho‐tau CSF a |
C: 60.6(5.0) AD: 65.4(8.1) P < 0.01 Adjusted for age in analyses |
C: 37% AD: 48% ns Adjusted for sex in analyses |
Zeiss Cirrus 5000 |
| Jiang et al., 2018 | Observational, case‐control (n = 52) |
AD (NIA‐AA) (n = 12) MCI (NIA‐AA) (n = 19) C (n = 21) |
MMSE a |
C: 67.6(8.3) MCI: 69.6(9.8) AD: 73.3(9.6) ns |
C: 67% MCI: 63% AD: 42% ns |
Zeiss Cirrus 5000 |
| Lahme et al., 2018 | Observational, case‐control (n = 74) |
AD (NIA‐AA) (n = 36) C (n = 38) |
MMSE a WMH a Aβ and tau CSF a |
C: 66.08(10.11) AD: 67.97(9.30) Age‐matched |
C: 62% AD: 58% |
RTVue XR Avanti |
| Lee et al., 2020 | Observational, case‐control (n = 42) |
ADCI (NIA‐AA and Aβ+ PET) (n = 28) C (n = 14) |
WMH a |
C: 67.2(6.1) ADCI: 67.5(9.5) Adjusted for age in analyses |
C: 71% ADCI: 61% |
DRI OCT Triton Plus |
| O'Bryhim et al., 2018 | Observational, case‐control (n = 32) |
preAD (Aβ+ CSF and/or PET) (n = 14) C (n = 18) |
‐ |
C: 75.2(6.6) preAD: 73.5(4.7) Adjusted for age in analyses |
C: 62% preAD: 43% |
RTVue XR Avanti |
| Querques et al., 2019 | Observational, case‐control (n = 56) |
AD (NIA‐AA) (n = 12) MCI (NIA‐AA) (n = 12) C (n = 32) |
MMSE Aβ, tau and phospho‐tau CSF a |
C: 71.6(5.9) MCI: 76.3(6.9) AD: 72.9(7.2) Age‐matched |
C: 47% MCI: 58% AD: 67% Sex‐matched |
Zeiss Cirrus 5000 |
| Sadda et al., 2019 | Observational, case‐control (n = 15) |
preAD (either cognitively normal with Aβ/tau+ CSF or cognitively impaired MMSE/MoCA but Aβ/tau‐ CSF and no MCI): 7 C (n = 8) |
MMSE, MoCA |
C: 76.3(11.9) preAD: 82.4(6.8) Age‐matched |
C: 63% preAD: 43% |
Zeiss Cirrus 5000 |
| van de Kreeke et al., 2020 | Observational, case‐control, population‐based (n = 124) |
preAD (Aβ+ PET)‡ (n = 13) C (n = 111) |
MMSE |
all participants: 68.6(6.3) Adjusted for age in analyses |
all participants: 66% Adjusted for sex in analyses |
Zeiss Cirrus 5000 |
| Wu et al., 2020 | Observational, case‐control (n = 60) |
AD (NINCDS‐ADRDA) (n = 21) MCI (Petersen) (n = 21) C (n = 18) |
MMSE |
C: 68.67(5.85) MCI: 67.81(5.96) AD: 69.94(6.39) Age‐matched |
C: 48% MCI: 43% AD: 47% Sex‐matched |
RTVue XR Avanti |
| Yoon et al., 2019 |
Observational, case‐control (n = 209) |
AD (NIA‐AA) (n = 39) MCI (NIA‐AA) (n = 37) C (n = 133) |
MMSE a |
C: 69.2(7.8) MCI: 71.1(7.6) AD: 72.8(7.7) P < 0.05 Adjusted for age in analyses |
C: 73% MCI: 54% AD: 67% ns |
Zeiss Cirrus 5000 |
| Zabel et al., 2019 | Observational, case‐control (n = 54) |
AD (NIA‐AA and Aβ+ PET) (n = 27) C (n = 27) |
MMSE a |
C: 74.26(7.66) AD: 74.11(5.87) ns |
C: 70% AD: 78% ns |
RTVue XR Avanti |
| Zhang et al., 2019 | Observational, case‐control (n = 32) |
aMCI/eAD (NIA‐AA) (n = 16) C (n = 16) |
MoCA a |
C: 73.60(7.69) aMCI/eAD: 73.03(8.24) Age‐matched |
C: 81% aMCI/eAD: 81% Sex‐matched |
RTVue XR Avanti |
indicates that associations or correlations were calculated with OCT‐A metrics. Ns indicates that differences in age or sex between groups were determined to be non‐significant. Abbreviations: AD, Alzheimer's disease; ADCI, Alzheimer's disease‐related cognitive impairment; aMCI, amnestic MCI; ApoE4, apolipoprotein E4; Aβ+, amyloid beta‐positive; C, control; CSF, cerebrospinal fluid; DSM‐IV, Diagnostic and Statistical Manual of Mental Disorders 4th edition; eAD, early‐stage AD; MCI, mild cognitive impairment; MMSE, Mini Mental State Examination; MoCA, Montreal Cognitive Assessment; NIA‐AA, National Institute on Aging and Alzheimer's Association; NINCDS‐ADRDA, National Institute of Neurological, Communicative Disorders and Stroke‐Alzheimer Disease and Related Disorders Association; PET, positron emission tomography; preAD, preclinical Alzheimer's disease; WMH, white matter hyperintensity.
3.1. Study design and population
All included studies were observational, 13 of which were case‐control studies 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 and one of which was a case‐control, population‐based study 44 (Table 1). Some studies also described a prospective design, 34 , 36 , 37 , 38 , 41 , 42 although no longitudinal results have been published at the time of this review. The number of unique participants across all studies was 942. The number of participants with AD ranged from 12 to 48, the number of participants with MCI ranged from 12 to 37, and the number of participants with preclinical AD ranged from 7 to 14 among studies. The average age of participants with AD ranged from 65.4 to 74.2 years, the average age of participants with MCI ranged from 67.8 to 76.3 years, the average age of participants with preclinical AD ranged from 68.6 to 82.4 years, and the average age of control participants ranged from 60.6 to 76.3 years. Studies defined AD as meeting NIA‐AA criteria, 31 , 33 , 34 , 36 , 39 meeting NINCDS‐ADRDA criteria, 38 or meeting NIA‐AA criteria and Aβ/tau+ confirmed through cerebrospinal fluid (CSF) analysis or amyloid positron emission tomography (amyloid‐PET). 29 , 32 , 40 Studies defined MCI as MCI meeting NIA‐AA criteria, 33 , 36 , 39 amnestic MCI (aMCI) meeting NIA‐AA criteria, 43 aMCI or early stage AD (eAD) meeting NIA‐AA criteria, 41 or MCI meeting Petersen criteria. 29 , 38 , 45 One study described an Alzheimer's disease–related cognitive impairment (ADCI) group, which combined AD and aMCI meeting NIA‐AA criteria, confirmed through Aβ+ PET. 29 , 42 Studies defined preclinical AD as cognitively normal with Aβ+ status confirmed through CSF or PET, 35 , 44 except for one study that also included participants who were cognitively impaired according to MMSE and Montreal Cognitive Assessment (MoCA) but not with MCI and with Aβ/tau‐ status confirmed through CSF. 37 Studies imposed an inconsistent range of exclusion criteria regarding conditions affecting the body and eye, a list of which can be found in Supplementary Table S1 .
3.2. Metrics and terminology
Six of the included studies mentioned pupillary dilation as being included as part of their ophthalmological examination. 32 , 37 , 40 , 41 , 43 , 44 Seven studies performed OCT‐A measurements with the Optovue RTVue XR Avanti (Optovue, Fremont, CA) with AngioVue software, which operates using a split‐spectrum amplitude decorrelation angiography algorithm (SSADA). Six studies used the Zeiss Cirrus 5000 (Carl Zeiss Meditech, Dublin, CA) with AngioPlex software, which uses an optical microangiography (OMAG) approach. One study used the Topcon DRI OCT Triton Plus (Topcon Medical Systems, Tokyo, Japan), which employs OCT‐A ratio analysis (OCTARA). It must also be noted that although 10 studies reported measurements automatically calculated by OCT‐A device software, three employed a semi‐automated method that involved non‐standardized thresholding protocols in ImageJ (https://imagej.nih.gov/ij/), 36 , 37 , 41 and one used a custom software. 33
Due to the differences in OCT‐A technology and boundaries for the segmentation of retinal layers among the three types of devices, results were not compared between studies that used different machines, with the exception of the measurement of foveal avascular zone (FAZ) area, which has been shown previously to be comparable across platforms. 46 , 47 Discrepancies considered, this review refers to measurements taken from the layer referred to as superficial (retinal) vascular plexus or superficial (retinal) capillary plexus as measurements in the “superficial retinal layer” and measurements taken from the layer referred to as deep (retinal) vascular plexus or deep (retinal) capillary plexus as measurements in the “deep retinal layer” for simplicity, although the boundaries for these layers may vary between studies (Figure 1, Supplementary Table S2 ). 47 , 48
FIGURE 1.
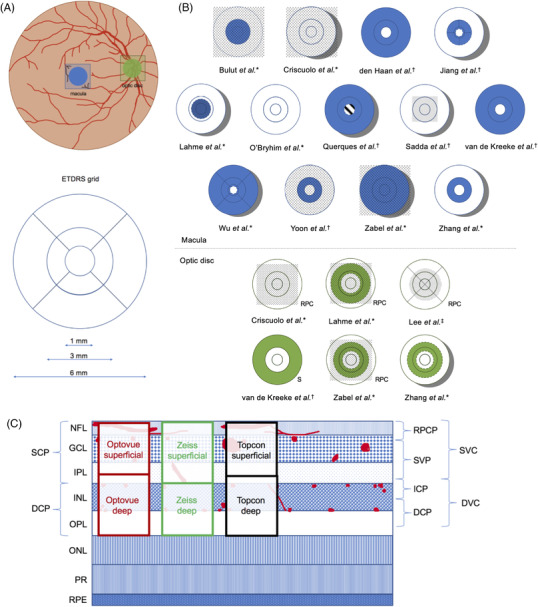
Regions measured for perfusion or vessel density by included studies compared to the Early Treatment Diabetic Retinopathy Study (ETDRS) grid. A) Illustration of the fundus showing the location of the macula (blue) and optic disc (green) where OCT‐A scans are commonly acquired and an ETDRS grid to compare with B. B) Macular (blue) regions and optic disc (green) regions measured in each study. * indicates studies that used an Optovue machine, † indicates studies that used a Zeiss machine, and ‡ indicates studies that used a Topcon machine. A dotted overlay indicates whole measurements. Dashed lines indicate the use of a regional boundary inconsistent with those of the ETDRS grid. A dotted line indicates that boundary measurements were not described (Bulut et al.). A striped center indicates that FAZ area was subtracted from the outer regions to calculate the boundary. C) A diagram of the anatomical layers of the retina with drawn vascular plexuses in red, currently used and proposed 4‐layer OCT‐A segmentation based on microvasculature (adapted from Campbell et al. with information from Munk et al. 47 , 48 ). Automatic segmentation of superficial and deep layers by Optovue, Zeiss, and Topcon OCT‐A machines are shown in red, green, and black respectively. DCP, deep capillary plexus; DVC, deep vascular complex; GCL, ganglion cell layer; ICP, intermediate capillary plexus; INL, inner nuclear layer; IPL, inner plexiform layer; NFL, nerve fiber layer; ONL, outer nuclear layer; OPL, outer plexiform layer; PR, photoreceptor layers; RPC, radial peripapillary capillaries; RPCP, radial peripapillary capillary plexus; RPE, retinal pigment epithelium; S, superficial; SCP, superficial capillary plexus; SVC, superficial vascular complex; SVP, superficial vascular plexus.
Studies reported either area‐based measurements (ie, total area of vasculature per unit area), length‐based measurements (ie, total length of vasculature per unit area), or both. It must be noted that for area‐based measurements, larger vessels have a greater influence on the measurement, whereas for length‐based measurements, all vessels influence the measurement equally. Thus length‐based measurements are more sensitive to changes in smaller capillaries. 49 Supplementary Table S2 lists the terminology used by each study and the corresponding definitions. Metrics that studies derived from the Optovue machine were: FAZ area; a density measurement (area‐based) referred to as vessel density, vascular density, or microvascular density that will herein be referred to as “vessel density”; vessel length density (length‐based, bespoke post‐processing calculation); and flow index. Metrics that studies derived from the Zeiss machine were: FAZ area; perfusion density (area‐based); and a density measurement (length‐based) referred to as vessel density or vascular density, which will herein be referred to as “vessel density.” Metrics that studies derived from the Topcon machine were: a density measurement (area‐based) referred to as capillary density that will herein be referred to as “vessel density.” It is important to note that vessel density calculated with an Optovue or Topcon machine is area‐based, whereas for Zeiss machines, vessel density is length‐based and perfusion density is the area‐based metric.
OCT‐A measurements were taken from regions (eg, foveal, parafoveal, perifoveal, peripapillary, whole en face) within the superficial and deep layers of the macula and the superficial and radial peripapillary capillary layers of the optic disc (also known as the optic nerve head), although fields of view and boundaries used to measure these regions were not standardized, thereby limiting opportunities to compare results among studies (Figure 1; Supplementary Tables S2‐3 ). Generally, the parafovea is defined as the annular area immediately surrounding the foveal avascular zone, whereas the perifovea is defined as the annular area surrounding the parafovea. The peripapillary region is generally defined as the annular area surrounding the optic nerve head. Although some studies do not explicitly name these areas as such, for example, 3‐mm ring instead of parafovea, 39 this review refers to these measurements using the aforementioned terminology for simplicity. Whole density measurements include both the FAZ and its surrounding area and are either taken from a standardized circular region of interest or from the whole field of view encompassed by the square en face image, depending on the study (Figure 1).
3.3. FAZ area
FAZ area measured in square millimeters (mm 2 ) was the most commonly included OCT‐A measurement across the studies; of the 14 studies included, 10 published results featuring FAZ area differences between case and control groups. Meta‐analysis revealed an increase in FAZ area in AD (mean difference [MD], 0.07 mm2; 95% CI, −0.00 to 0.13; Z, P = 0.06) with significant heterogeneity among studies (χ 2 , P < 0.001) (Figure 2). Heterogeneity was still present when only studies using an Optovue machine were analyzed together (χ 2 , P < 0.001), although in this analysis, there was a significant increase in FAZ area in the AD group (MD, 0.11 mm2; 95% CI, 0.02 to 0.19; Z, P < 0.01) (Supplementary Figure S2 ). Significant increases were found individually by Bulut et al. (MD, 0.14 mm2; P < 0.05), Wu et al. (MD, 0.18 mm2; P < 0.05), and Zabel et al. (MD, 0.11 mm2; P < 0.001), studies which had an older average AD participant age (74.2 ± 7.6 years, 69.9 ± 6.4 years, and 74.1 ± 5.9 years, respectively), and used Optovue machines (Tables 1, 2). By contrast, no evidence of significant differences in FAZ area between participants with AD and controls was found by den Haan et al. (MD, −0.02 mm2; P > 0.05), Lahme et al., (MD, 0.00 mm2; P > 0.05), and Yoon et al. (MD, 0.00 mm2; P > 0.05). These are studies that had a younger average age for participants with AD (65.4 ± 8.1 years, 68.0 ± 9.3 years, and 72.8 ± 7.7 years, respectively), and of which two used the Zeiss machine (Tables 1, 2). Studies where participants were age‐matched, 31 , 34 , 38 where age was adjusted for, 32 , 39 or where neither was performed 40 do not appear to segregate clearly with either significant or non‐significant results, in the present and following sections.
FIGURE 2.
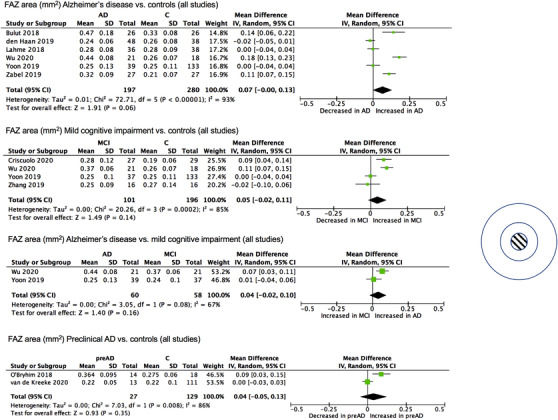
Meta‐analysis of foveal avascular zone (FAZ) measurements (mm 2 ) for Alzheimer's disease (AD), mild cognitive impairment (MCI), and preclinical AD (preAD) participants versus controls (C) and AD versus MCI. Mean and standard deviation (SD) are included, with 95% confidence intervals (CIs), heterogeneity scores, and overall effect in an inverse variance (IV) random effects model. The green square size represents the weight attributed to each study based on relative sample size. N.B. Results from van de Kreeke et al. are unadjusted and were obtained through personal correspondence with authors.
TABLE 2.
Direction of effects reported in the included studies using optical coherence tomography angiography (OCT‐A) to examine retinal changes in preclinical Alzheimer's disease (AD), mild cognitive impariment (MCI), and Alzheimer's disease (AD)
| Study (Optovue) | FAZ area | Superficial parafoveal VD | Superficial perifoveal VD | Superficial whole VD | Deep parafoveal VD | Deep whole VD | RPC peripapillary VD | RPC whole VD |
|---|---|---|---|---|---|---|---|---|
| Bulut et al. | ↑ AD | ↓ AD | ‐ | ↓ AD | ‐ | ‐ | ‐ | ‐ |
| Criscuolo et al. | ↑ aMCI | ‐ | ‐ | ↓ aMCI | ‐ | ↓ aMCI | ‐ | ↓ aMCI |
| Lahme et al. | ∼AD | ↓ AD | ‐ | ↓ AD | ∼AD | ∼AD | ∼AD | ↓ AD |
| O'Bryhim et al. | ↑ preAD | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Wu et al. | ↑ AD ↑ MCI | ∼AD ∼MCI | ∼AD ∼MCI | ‐ | ↓ AD ↓ MCI | ‐ | ‐ | ‐ |
| Zabel et al. | ↑ AD | n.d. | n.d. | ∼AD | ‐ | ↓ AD | ∼AD | ∼AD |
| Zhang et al. | ∼aMCI/eAD | ↓ aMCI/eAD | ‐ | ‐ | ∼aMCI/eAD | ‐ | ∼aMCI/eAD | ‐ |
| Study (Zeiss) | FAZ area | Superficial parafoveal VD†/PD‡ | Superficial perifoveal VD†/PD‡ | Superficial whole VD†/PD‡ | Deep parafoveal VD†/PD‡ | Deep whole VD†/PD‡ | RPC peripapillary VD†/PD‡ | RPC whole VD†/PD‡ |
|---|---|---|---|---|---|---|---|---|
| den Haan et al. | ∼AD | ∼AD† | ∼AD† | ‐ | ‐ | ‐ | ‐ | ‐ |
| Jiang et al. | ‐ | ↓ AD ∼MCI† | ‐ | ‐ | ↓ AD ∼MCI† | ‐ | ‐ | ‐ |
| Querques et al. | ‐ | ∼AD ∼MCI‡ | ∼AD ∼MCI‡ | ‐ | ∼AD ∼MCI‡ | ‐ | ‐ | ‐ |
| Sadda et al. | ‐ | ‐ | ‐ | ∼preAD† | ‐ | ∼preAD† | ‐ | ‐ |
| van de Kreeke et al. | ∼preAD | ↑ preAD† | ↑ preAD† | ‐ | ‐ | ‐ | ‐ | ‐ |
| Yoon et al. | ∼AD ∼MCI | ↓ AD ∼MCI†/‡ | ‐ | ↓ AD ∼MCI†/‡ | ‐ | ‐ | ‐ | ‐ |
| Study (Topcon) | FAZ area | Superficial parafoveal VD | Superficial perifoveal VD | Superficial whole VD | Deep parafoveal VD | Deep whole VD | RPC peripapillary VD | RPC whole VD |
|---|---|---|---|---|---|---|---|---|
| Lee et al. | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ∼ADC |
Measurements that were included in at least 3 studies are shown, divided between studies using the Optovue and the Zeiss machines. N.B. Due to differences in machines and software, measurements are not comparable between machines. ↑ indicates evidence of a significant increase in the case group compared to the control group, ↓ indicates a significant decrease, and ∼ indicates no evidence of a significant difference. † indicates that vessel density was measured and ‡ indicates that perfusion density was measured in studies using the Zeiss machine. N.d. indicates that a measurement of this type was mentioned, but the effect was not described. Abbreviations: AD, Alzheimer's disease; ADCI, Alzheimer's disease‐related cognitive impairment; aMCI, amnestic mild cognitive impairment; eAD, early Alzheimer's disease; FAZ, foveal avascular zone; MCI, mild cognitive impairment; PD, perfusion density; preAD, preclinical Alzheimer's disease; RPC, radial peripapillary capillaries; VD, vessel density.
For MCI, four studies provided measurements, of which Criscuolo et al. (MD, 0.09 mm2; P < 0.001) and Wu et al. (MD, 0.11 mm2; P < 0.05) found a significant increase in MCI, whereas Yoon et al. (MD, −0.01 mm2; P > 0.05) and Zhang et al. (MD, −0.02 mm2; P > 0.05) found no evidence of a significant difference compared to controls (Table 2). Meta‐analysis (MD, 0.05 mm2; 95% CI, −0.02 to 0.11, Z, P = 0.14) found significant heterogeneity among these studies (χ 2 , P = 0.0002) (Figure 2). Of these, Wu et al. (MD, 0.07 mm2; significance not described) and Yoon et al. (MD, 0.01 mm2; P > 0.05) included FAZ measurements for both AD and MCI (MD, 0.04 mm2; 95% CI, −0.02 to 0.10; Z, P = 0.16) with no significant heterogeneity between them (χ 2 , P = 0.08). For preclinical AD, two studies provided measurements for preclinical AD (MD, 0.04 mm2; 95% CI, −0.05 to 0.13; Z, P = 0.35) with significant heterogeneity between them (χ 2 , P = 0.008), of which O'Bryhim et al. found a significant increase in FAZ area in preclinical AD (MD, 0.08 mm2; P < 0.01) and van de Kreeke et al. found no evidence of a difference between preclinical AD and control groups (MD, 0.00 mm2; P > 0.05) (Figure 2; Table 2).
3.4. Density measurements in the superficial retinal layer
Density (%) measurements were taken from a diverse range of retinal areas and layers across studies resulting in limited opportunities for direct comparison (Figure 1, Supplementary Table S2 ). Studies were therefore compared only in groups that used the same machine and software, as it has been shown that vessel density measurements from different machines are not equivalent. 46 , 50 The most commonly characterized layer of the retina across the included studies was the superficial layer, for which the most popular measurement was in the parafoveal region. This included three Optovue studies of which Bulut et al. (MD, −3.16%; P < 0.05) and Lahme et al. (MD, −2.62%; P < 0.01) independently found a significant decrease in superficial parafoveal vessel density in AD compared to controls (Table 2). Wu et al. (MD, −0.91%; P > 0.05) found no evidence of a significant difference in vessel density in the whole parafovea but when divided into sectors, did find a significant decrease in the superior sector (MD, −1.69%; P < 0.05). Meta‐analysis revealed no significant heterogeneity between studies (χ 2 , P = 0.24) and a significant overall effect of AD on superficial parafoveal vessel density (MD, −2.10%; 95% CI, −3.42 to −0.77; Z, P = 0.002) (Figure 3). Five Zeiss studies could not be compared quantitatively due to differences in metrics and calculation methods. Of these, Jiang et al. (fractal analysis: MD, not described; P < 0.05) and Yoon et al. (vessel density: MD, −1.2%; P < 0.01; perfusion density: −0.02%; P < 0.01) independently found a significant decrease in superficial parafoveal vessel density in AD, whereas den Haan et al. (vessel density: MD, −0.1%; P > 0.05) and Querques et al. (perfusion density: MD, −0.26%; P > 0.05) found no evidence of a significant difference (Table 2).
FIGURE 3.
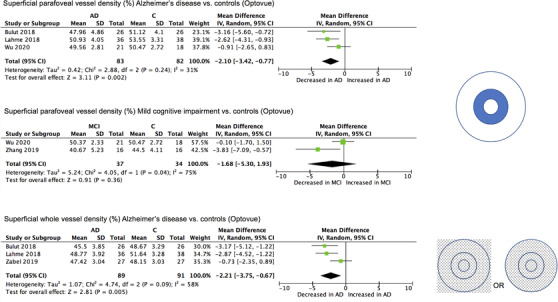
Meta‐analysis of superficial parafoveal and whole vessel density (%) for participants with Alzheimer's disease (AD) and participants with mild cognitive impairment (MCI) versus controls (C). Mean and standard deviation (SD) are included, with 95% confidence intervals (CIs), heterogeneity scores, and overall effect in an inverse variance (IV) random effects model. The green square size represents the weight attributed to each study based on relative sample size.
Two studies using Optovue provided a measurement for MCI participants; Zhang et al. found a significant decrease in vessel density in MCI compared to controls (MD, −3.83%; P < 0.05), whereas Wu et al. found no evidence of a significant difference (MD, −0.10%; P > 0.05). Meta‐analysis revealed significant heterogeneity (χ 2 , P = 0.04) between these two studies (MD, −1.68%; 95% CI, −5.30 to 1.93; Z, P = 0.36); this comparison should be considered with caution, as Zhang et al. used a calculation protocol with external software (ImageJ, an open‐source image‐processing program) in addition to AngioVue software, which may affect the equivalence of their measurements to those reported by other studies. Three studies using Zeiss also provided a measurement for MCI participants, of which Jiang et al. (fractal analysis: MD, not described; P > 0.05) and Querques et al. (perfusion density: MD, 1.21%; P > 0.05) found no evidence of a significant difference between MCI and control participants. Of interest, Yoon et al. found a significant decrease in AD compared to MCI (vessel density: MD, −1.3%; P < 0.01; perfusion density: MD, −0.02%; P < 0.01). Similarly, one study using Zeiss found that superficial parafoveal vessel density was significantly increased in preclinical AD compared to control participants (vessel density: MD, 0.81%; P < 0.001). 44
Another common superficial vessel density measurement was whole (en face) vessel density, which includes the FAZ (Figure 3). Two studies using Optovue, Bulut et al. (MD, −3.17%; P < 0.01) and Lahme et al. (MD, −2.87%; P < 0.001), independently found this measure to be significantly decreased in AD compared to controls, whereas Zabel et al. found no evidence of a significant difference (MD, −0.73%; P > 0.05) (Table 2). Meta‐analysis of three Optovue studies revealed no significant heterogeneity (χ 2 , P = 0.09) and a significant overall effect of AD for this metric (MD, −2.21%; 95% CI, −3.75 to −0.67; Z, P = 0.005). This comparison should be regarded with a degree of caution, however, as there were differences in the field of view from which these measurements were obtained. As for MCI, Criscuolo et al. found a significant decrease in whole vessel density in MCI compared to controls (MD, −3.14%; P < 0.01). The study of Yoon et al. using Zeiss found, similar to their parafoveal measurements, a significant decrease in AD compared to MCI (vessel density: MD, −1.3%; P < 0.01; perfusion density: MD, −0.019%; P < 0.01) and a significant decrease in AD compared to controls (vessel density: MD, −1.2%; P < 0.05; perfusion density: MD, −0.018%; P < 0.01). A smaller study using a Zeiss machine looked at a preclinical AD group that included both cognitively normal biomarker‐positive (Aβ or tau), and cognitively impaired (MMSE/MoCA but not MCI) biomarker‐negative, and found no evidence of a significant difference in superficial whole vessel density between their groups (vessel density: MD, 1%; P > 0.05). 37
Superficial foveal vessel density measurements were reported by studies that used Optovue machines, of which Bulut et al. did (MD, −5.76%; P < 0.01) and Lahme et al. did not (MD, −1.66%; P > 0.05) find evidence of a significant difference in AD compared to controls. Superficial perifoveal density measurements were reported in four studies (Table 2). One study using Optovue (AD vs C: MD, −0.84%; P > 0.05; MCI vs C: MD, 0.36%; P > 0.05) and one study using Zeiss (perfusion density: AD vs C: MD, 0.12%; P > 0.05; MCI vs C: MD, 0.39%; P > 0.05) found no evidence of a significant difference between AD or MCI and controls. 36 , 38 Similarly, den Haan et al. found no evidence of a significant difference between AD and controls (MD, −0.4%; P > 0.05). Of interest, van de Kreeke et al. found a significant increase in superficial perifoveal vessel density in their preclinical AD group compared to their control group (MD, 0.50%; P < 0.05).
3.5. Density measurements in the deep retinal layer, choriocapillaris, and choroid
The most commonly reported density measurement for the deep retinal layer was in the parafoveal region (Table 2). Both Wu et al. (MD, −9.18%; P < 0.001) and Jiang et al. (vessel density: MD, not described; P < 0.05) reported a significant decrease in vessel density in this region in AD compared to controls, whereas neither Lahme et al. (MD, −0.41%; P > 0.05) nor Querques et al. (MD, −1.99%; P > 0.05) found evidence of a significant difference in vessel density and perfusion density, respectively. Regarding MCI, Wu et al. reported a significant decrease in vessel density compared to controls (MD, −4.19%; P < 0.001), whereas Jiang et al. (vessel density: MD, not described; P > 0.05), Querques et al. (perfusion density: MD, −0.72%; P > 0.05), and Zhang et al. (vessel density: MD, −0.54%; P > 0.05) did not find evidence of a significant difference. Wu et al. also found a significant decrease in AD compared to MCI (MD, −4.99%; P < 0.001). Other regions measured in this layer included the fovea, perifovea, and whole measurements. Lahme et al. provided a foveal vessel density measurement, which was not found to be significantly different between AD and controls (MD, 1.89%; P > 0.05). Wu et al. reported a perifoveal vessel density measurement and found deep perifoveal vessel density to be significantly decreased in both AD (MD, −7.64%; P < 0.001) and MCI (MD, −2.25%; P < 0.001) compared to controls, as well as in AD compared to MCI (MD, −5.39%; P < 0.001). Querques et al. included a perifoveal perfusion density measurement, which was not found to be significantly different between AD (MD, −0.46%; P > 0.05) or MCI (MD, −0.33%; P > 0.05) and controls. Deep whole measurements of vessel density from Zabel et al. found a significant decrease in AD compared to controls (MD, −5.51%; P < 0.001), whereas Lahme et al. found no evidence of a significant difference (MD, −1.37%; P > 0.05). Criscuolo et al. found a significant decrease in deep whole vessel density in MCI compared to controls (MD, −5.48%; P < 0.001), whereas Sadda et al. found no evidence of a significant difference between preclinical AD and controls (MD, −1%, P > 0.05).
Querques et al. and Sadda et al. (two studies using Zeiss) included measurements in other layers such as the choriocapillaris and choroid. Querques et al. found no evidence of a significant difference in perfusion density of the choriocapillaris (perfusion density (3 x 3 mm): AD vs C: MD, 0.05%; P > 0.05; MCI vs C: MD, −0.22%; P > 0.05; AD vs MCI: MD, 0.27%; P > 0.05) or choroid (perfusion density (3 x 3 mm): AD vs C: MD, ‐0.16%; P > 0.05; MCI vs C: 0.11%; P > 0.05; AD vs MCI: MD, −0.27%; P > 0.05) between AD, MCI, and controls. Sadda et al. found no evidence of a significant difference in the vessel density of the choriocapillaris in the macular region between preclinical AD and controls (MD, −1%; P > 0.05).
3.6. Density measurements in the optic disc region
Three studies using Optovue provided measurements of vessel density for the peripapillary region in the radial peripapillary capillary layer of the optic disc, two of which compared participants with AD and controls, 34 , 40 and one of which compared participants with MCI to controls. 41 None of these found evidence of significant differences between case and control groups (Lahme: MD, ‐1.94%; P > 0.05; Zabel: MD, 1.05%; P > 0.05; Zhang: vessel density: MD, ‐0.27%; P > 0.05; vessel length density: MD, ‐0.84%; P > 0.05). Two studies provided a measurement of the peripapillary region in the superficial layer. One study found no evidence of a significant difference between MCI and controls (vessel density: MD, ‐1.84%; P > 0.05; vessel length density: MD, ‐0.58%; P > 0.05). 41 The other study provided this measurement for preclinical AD in the superficial layer and did find evidence of a significant difference between preclinical AD and control participants (MD, 0.83%; P < 0.05). 44
Four studies provided a whole vessel density measurement in the radial peripapillary capillary layer. Lahme et al. found a significant decrease in whole vessel density in AD compared to controls (MD, ‐2.32%; P < 0.05) in this region, whereas Zabel et al. found no evidence of a difference (MD, 1.64%; P > 0.05). Criscuolo et al. found a significant decrease in whole vessel density in MCI compared to controls (MD, ‐2.04%; P < 0.05). One study, which compared an ADCI group including both AD and MCI participants to controls, found no evidence of a significant difference in whole vessel density between their ADCI group and control participants in any of the measured quadrants (superior: MD, 0.99%; P > 0.05; inferior: MD, 3.76%; P > 0.05; temporal: MD, ‐2.69%; P > 0.05; nasal: ‐0.82%; P > 0.05). 42
3.7. Blood flow velocity measurements
Two studies using Optovue included unitless metrics that indicate blood flow velocity. Bulut et al. reported an outer retinal (MD, ‐0.01; P > 0.05) and a choroidal (MD, ‐0.01; P > 0.05) flow (index) rate, which are described to be lower, although not significantly, in AD versus controls. Zhang et al. reported a significantly lower adjusted flow index in the parafoveal superficial capillary plexus in aMCI/eAD compared to controls (MD, ‐0.031; P < 0.05). Studies involving preclinical AD participants did not report a flow index measurement.
4. DISCUSSION
Although many studies support the correlation of blood and CSF biomarkers with amyloid pathology and dementia diagnosis, retinal imaging is less invasive and could be more widely applicable as a screening tool if validated. 51 , 52 Thus the possibility that OCT‐A could be used for preclinical or clinical dementia diagnosis is exciting, as it would be an efficient and economical method for determining where there is need for early intervention. Informal comparisons and meta‐analyses in the current review reveal that FAZ area, superficial parafoveal, and whole vessel density may have the potential to serve as indicators of AD; effect sizes of significant findings in individual studies are very large for FAZ area (Cohen's d = 1.01 to 2.39) and medium to large for superficial parafoveal (Cohen's d = 0.67 to 0.81) and whole vessel densities (Cohen's d = 0.61 to 0.89), which suggests that the differences are non‐trivial. 53 However, the significant heterogeneity among studies, which may be attributed to several factors discussed later, prevents an inference from being made at present regarding their clinical utility (Figure 4). In addition, it will be important for this technique to be sufficiently sensitive to pick up changes that occur in preclinical AD or MCI to provide opportunities for early intervention. Meta‐analyses in this study reveal no evidence of a significant difference between preclinical AD or MCI and controls for these measures, although such analyses are limited, and retinal microvascular changes that occur in preclinical AD or MCI may become clearer through increased standardization.
FIGURE 4.
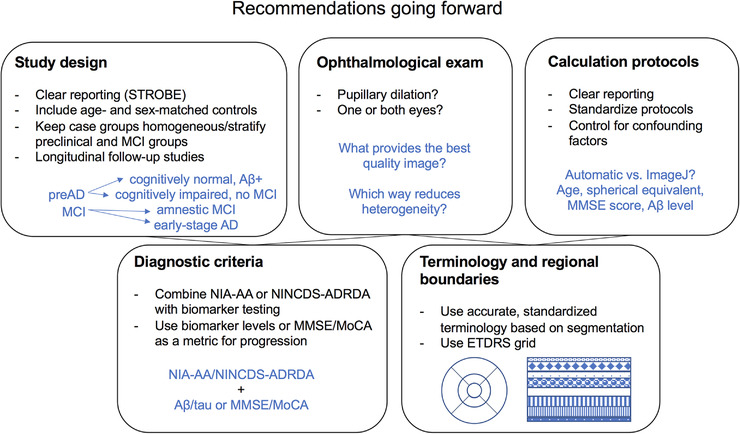
Recommendations for standardization of studies using optical coherence tomography angiography (OCT‐A) to detect changes in the retinal microvasculature in preclinical Alzheimer's disease (AD), mild cognitive impairment (MCI), and Alzheimer's disease (AD). Abbreviations: Aβ+, amyloid beta‐positive; ETDRS, Early Treatment Diabetic Retinopathy Study; MMSE, Mini Mental State Examination; MoCA, Montreal Cognitive Assessment; NIA‐AA, National Institute on Aging and Alzheimer's Association; NINCDS‐ADRDA, National Institute of Neurological, Communicative Disorders and Stroke‐Alzheimer Disease and Related Disorders Association; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology.
A major obstacle preventing the comparison of studies was the different types of OCT‐A machines used to perform measurements; different algorithms are used to reconstruct the images, and different terminology is employed between Optovue, Zeiss, and Topcon. This is further compounded by differences in retinal layer segmentation, leading to uncomparable vessel density calculations 46 , 47 , 50 , 54 (Figure 1, Supplementary Table S2 ). Furthermore, one study has shown that different AngioVue software updates generate significantly different results due to a change in segmentation boundaries calculated between software versions. 55 In order to be able to confidently compare and meta‐analyze measurements obtained from OCT‐A for an overall effect of any disease on these metrics, it is crucially important to standardize the boundaries of the retinal layers being measured, the language that is used to describe them, and the metrics that are subsequently derived. 48 Ideally, those who are using different machines should describe in clear detail the retinal layers that are being investigated, the boundaries that define them, how these compare to those calculated by other machines, and their current software version. It appears that FAZ area differences tend to be more significant between case and control groups in studies that featured Optovue, and a significant overall effect of AD on superficial parafoveal and whole vessel density was found in the meta‐analysis of studies using Optovue. However, this tendency must be considered with caution as there was heterogeneity in parafoveal boundaries and participant populations, and the numbers of participants in the studies were small. Furthermore, although some studies reported automated calculations, others included semi‐automated procedures that involved image analysis with additional software; this is another source of heterogeneity, but one that could be reduced by establishing a standardized protocol for calculating certain density metrics, a set of inter‐machine correction factors, or perhaps the development of a platform‐independent software, such as that which has been developed for spectral‐domain OCT (SD‐OCT). 56 Using a machine‐learning approach such as deep learning may be an option for circumventing the limitations of current OCT‐A algorithms, as classifiers of disease stage can be learned based on images only and without the need for post‐processing and quantification. 57 , 58 However, in the field of neurodegenerative research, it may be challenging to collect the sizeable volume of clinical data needed to achieve an acceptable level of machine‐learning performance, as well as to discern which discriminating features such a system is utilizing in the classifying process.
Reaching a consensus regarding the boundaries that define areas such as the fovea, parafovea, and perifovea in the macula and the peripapillary region of the optic disc, as well as fields of view (3 × 3 mm vs 6 × 6 mm macula; 4.5 × 4.5 mm optic disc) used to image these regions and whether to include the fovea or optic nerve head in the center, would achieve further standardization. This could perhaps be achieved in a manner similar to what was achieved with SD‐OCT. 59 At present, studies contain different sets of measurements and discrepancies in defined boundaries, which prevents conclusions from being reached regarding the reproducibility of findings and the potential for certain metrics to be used as biomarkers for different stages of disease (Supplementary Table S3 ). Going forward, it will be important to establish a set of metrics with comprehensive descriptions recommended for inclusion in studies featuring OCT‐A so that the overall effects of disease progression on vessel density in these regions can be more readily discerned. One standard that could be used (and that has been used by some of the included studies) is the Early Treatment Diabetic Retinopathy Study (ETDRS) grid, which delineates nine sectors in the macula: a centered circle with a 1 mm radius, an inner ring 1‐3 mm from the center divided into four quadrants, and an outer ring 3‐6 mm from the center divided into four quadrants. These could serve as standard boundaries for the fovea, parafovea, and perifovea, respectively. Two studies included measurements for sectors within areas such as the parafovea and perifovea and found changes specific to certain sectors, although the way that they were defined varied between the studies, that is, superior, inferior, temporal, and nasal versus superior temporal, superior nasal, inferior temporal, and inferior nasal (Figure 1). 33 , 38 , 42 It is possible that changes occurring through the dementia disease course are sector‐specific and are consequently overlooked when calculations are made over larger areas. Thus it would be worthwhile to include measurements for individual sectors so that any potential changes of this type are not disregarded. Along with this, we recommend that OCT‐A images are included in the studies, so that differences between case and control groups may be visualized. Comparison images between case and control groups were included in only seven of the studies reviewed. 33 , 34 , 35 , 39 , 40 , 42 , 43
Another likely source of heterogeneity among the included studies is the diversity in participant populations. Although a number of studies matched cases and controls based on age and sex or include an age or sex adjustment, it is possible that differences in these factors contributed to some of the inconsistent results between studies, especially as FAZ area and vessel density have been found to change with normal aging. 60 For example, studies that found a significant enlargement of FAZ area in AD 31 , 38 , 40 tended to have an older average AD participant age than studies that did not, so it is possible that these participants had more severe AD than those at a younger age. With regard to sex, lower superficial vessel density was significantly associated with male sex in a recent population‐based study. 61 It would be of value to include sex‐stratified data in future studies in order to determine whether certain findings are sex‐specific, as well as to reduce variation within groups so that calculations can be more sensitive to smaller effect sizes. Furthermore, although the included studies selected participants based on established NIA‐AA or NINCDS‐ADRDA diagnostic criteria, it is possible that an AD group could be heterogeneous; for example, there may be differences in Aβ or tau levels within the group, or other forms of dementia present instead of, or alongside, AD that may act as confounding variables. Although some studies excluded participants with other types of dementia, neurodegeneration, or neurological disease, 31 , 36 , 38 , 39 , 40 , 42 , 43 others did not make mention of this. In addition, confirmation of AD through biomarker testing was a diagnostic requirement for participants in only three studies. 32 , 40 , 42 It will be important to utilize biomarker levels as a covariate and a requirement for comprehensive AD selection criteria, since the NIA‐AA research framework was recently revised to include CSF and imaging biomarkers. 27 This may be challenging, however, without funding given their expense. It may also be helpful to include MMSE and MoCA scores as another metric for AD staging after diagnosis using biomarkers.
How preclinical AD and MCI groups were defined varied considerably among studies. Although two studies defined their preclinical group as cognitively normal Aβ+, 35 , 44 one study involved a preclinical AD group that was referred to as the “AD group” and contained both cognitively normal Aβ/tau+ participants and cognitively impaired Aβ/tau‐ participants without MCI. 37 Many studies included MCI participants meeting NIA‐AA or Petersen criteria, although one study of the former type refers to both amnestic MCI and early stage AD and combines them into one group. 41 One study combined MCI participants and AD participants into one group. 42 These groupings appeared to be heterogeneous, and future studies would benefit from further standardization and stratification of groupings to investigate the retinal changes occurring in the different stages preceding AD. The need for this is accentuated by the possibility of a biphasic effect occurring, whereby vessel density increases in preclinical AD or MCI and decreases in AD, leading to mixed results across studies. Such a model is supported by findings from van de Kreeke et al., where an increase in vessel density was reported in cognitively normal Aβ+ participants compared to controls. This may also occur in the progression from MCI to AD, as Yoon et al. found a decrease in vessel density in AD compared to MCI. Thus standardization of participant characterization as outlined by McKhann et al. is of the utmost importance to determine whether OCT‐A is sufficiently sensitive to detect changes between different stages along the disease continuum.
Regarding the ophthalmological examination, pupillary dilation was mentioned in only some of the included studies. 31 , 32 , 35 , 36 , 37 , 40 , 41 , 43 , 44 Although pupillary dilation is not required to perform OCT‐A, it is important to consider whether this has an impact on image quality and the ensuing measurements. We therefore recommend that whether or not pupillary dilation was performed prior to OCT‐A imaging should be explicitly mentioned when reporting a study. Further investigation is now needed to reach a consensus on whether pupillary dilation should be performed in future studies to yield consistent image quality and potentially more reliable measurements, or whether undilated examination is sufficient, considering that scan quality has been shown to improve with dilation. 62 Furthermore, there were differences in the decisions made across studies about whether to include one or both eyes in analyses. Some studies chose one eye consistently, some chose one eye randomly or based on best image quality, some averaged values for both eyes, and some included values for both eyes where possible but used a generalized estimating equation (GEE) correction to account for sample size inflation. We recommend that details about eye selection are clearly reported and that this is as consistent as possible among participants, as right and left eyes for a person may not be interchangeable due to interocular asymmetry. 63 Finally, ophthalmological confounders should be considered where possible; for example, axial length has been shown to impact both foveal avascular zone area and superficial vessel density measurements, yet only two studies described a range of axial length measurements in their inclusion criteria 31 , 38 , 64 (Supplementary Table S1 ). Other studies chose to statistically adjust for potential confounders such as age and spherical equivalent. 32 , 35 , 39 , 42 , 43 , 44
External sources of heterogeneity notwithstanding, it must also be noted that the metrics discussed in this review have inherent limitations that may contribute to the inconsistent results observed across the literature. For example, FAZ area measurements are limited to a few deep layers of capillaries of the foveal pit, and thus the detection of disease‐associated changes may only be possible within a certain window during which these particular layers are affected. 65 It is important to note that the FAZ area does not take into account the ganglion cell layer, which is known to be impacted by neurodegeneration. 66 Measuring vessel density of the parafovea and perifovea can provide some of this information to which the FAZ area metric may be less sensitive, although vessel density measurements may also be influenced by noise in the image, or variable anatomic features, and are thus dependent upon the calculation method used. 67 It may also be advantageous to explore the use of more peripheral retinal metrics, which may be less affected by the above limitations as well as any central media opacity. However, we acknowledge that this could require machines with larger fields of view or different gaze positions for the participant, and the images may be more challenging to segment accurately, correct for distortion, and analyze. 65 In any case, we recommend using multiple metrics to create a more holistic picture of the retinal microvasculature, and we stress that obtaining good quality images with clear ocular media is criticial for all metrics. Furthermore, there can be natural variability of these parameters in healthy adults, and thus we emphasize the need for longitudinal monitoring of the same participants to determine how individuals are changing over time. 68 , 69 Finally, further consideration must be made regarding the appropriateness of measuring computed blood flow velocity using OCT‐A rather than methods that show live blood flow metrics, such as adaptive optics scanning laser ophthalmoscope (AOSLO) imaging, adaptive optics (AO) OCT‐A, the retinal functional imager (RFI) system, or laser speckle flowgraphy (LSFG), as OCT‐A measurements may be picking up projection artifacts. 70 , 71
Finally, although study design could often be inferred from the study descriptions presented in each of the articles we reviewed, the language used to describe study designs varied. It is therefore recommended that reporting guidelines, such as those of Strengthening the Reporting of Observational Studies in Epidemiology (STROBE), are followed to ensure clear and comprehensive descriptions of study design. 72 Sample sizes in most of the studies were relatively small (though we recognize the challenges involved in conducting studies with these particular participant groups), so it will be important to conduct similar investigations with larger populations to increase the statistical power when investigating associations between OCT‐A metrics and AD. In addition, a major advantage of standardization would be inter‐study comparability, which can be used to overcome the limitations of small sample sizes in individual studies. Furthermore, a consistent approach would also benefit research into associations of OCT‐A metrics with biomarkers derived from other neuroimaging techniques, such as MRI and PET, as better understanding of how these techniques can be used in conjunction with each other may allow for improved risk assessment. Finally, no longitudinal studies matching the inclusion and exclusion criteria were found in our literature search. Because OCT‐A is still a relatively new technology, this may be unsurprising, but it is vital for future work to include follow‐up measurements on the same participant populations while remaining wary of the aforementioned sources of variance. This way, changes in these measurements can be effectively tracked over time in order to gain insight into the dynamics of the retinal microvasculature with disease progression.
CONFLICTS OF INTEREST
The authors do not have any conflicts of interest.
INFORMED CONSENT OF HUMAN SUBJECTS
This study reviews only findings from already published studies, hence no additional consent from participants (human subjects) was necessary to carry out this work.
Supporting information
Supplementary information
Supplementary information
Supplementary information
Supplementary information
Supplementary information
Supplementary information
ACKNOWLEDGMENTS
This work was supported by the Wellcome Translational Neuroscience PhD Programme (108890/Z/15/Z), the Karen L. Wrenn Alzheimer's Disease Travel Award (Duke University), and by the Alzheimer's Drug Discovery Foundation (ADDF) GDAPB‐201808‐2016196 “Delivering novel neuro‐retinal biomarkers for the early diagnosis of Alzheimer's disease.” The funding sources had no involvement in designing, conducting, or submitting this work. Support from NHS Lothian R&D, and Edinburgh Imaging and Edinburgh Clinical Research Facility at the University of Edinburgh is gratefully acknowledged.
Rifai OM, McGrory S, Robbins CB, et al. The application of optical coherence tomography angiography in Alzheimer's disease: A systematic review. Alzheimer's Dement. 2021;13:e12149. 10.1002/dad2.12149
REFERENCES
- 1. Wu Y‐T, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time — current evidence. Nat Rev Neurol. 2017;13(6):327‐339. [DOI] [PubMed] [Google Scholar]
- 2. Wright CF, Hall A, Matthews FE, Brayne C. Biomarkers, dementia, and public health. Ann N Y Acad Sci. 2009;1180:11‐19. [DOI] [PubMed] [Google Scholar]
- 3. Holtzman DM, Morris JC, Goate AM. Alzheimer's disease: the challenge of the second century. Sci Transl Med. 2011;3(77):77sr71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of cerebral vessel disease to Alzheimer's disease dementia and cognitive function in elderly people: a cross‐sectional study. Lancet Neurol. 2016;15(9):934‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37(1):56‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith EE, Greenberg SM. Beta‐amyloid, blood vessels, and brain function. Stroke. 2009;40(7):2601‐2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thal DR, Attems J, Ewers M. Spreading of amyloid, tau, and microvascular pathology in Alzheimer's disease: findings from neuropathological and neuroimaging studies. J Alzheimers Dis. 2014;42 Suppl 4:S421‐429. [DOI] [PubMed] [Google Scholar]
- 8. Fischer P, Jungwirth S, Zehetmayer S, et al. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology. 2007;68(4):288‐291. [DOI] [PubMed] [Google Scholar]
- 9. Morris JC, Price JL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early‐stage Alzheimer's disease. J Mol Neurosci. 2001;17(2):101‐118. [DOI] [PubMed] [Google Scholar]
- 10. Jack CR, Jr. , Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9(1):119‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. London A, Benhar I, Schwartz M. The retina as a window to the brain‐from eye research to CNS disorders. Nat Rev Neurol. 2013;9(1):44‐53. [DOI] [PubMed] [Google Scholar]
- 12. Heringa SM, Bouvy WH, van den Berg E, Moll AC, Kappelle LJ, Biessels GJ. Associations between retinal microvascular changes and dementia, cognitive functioning, and brain imaging abnormalities: a systematic review. J Cereb Blood Flow Metab. 2013;33(7):983‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koronyo Y, Biggs D, Barron E, et al. Retinal amyloid pathology and proof‐of‐concept imaging trial in Alzheimer's disease. JCI Insight. 2017;2(16):e93621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoon SP, Thompson AC, Polascik BW, et al. Correlation of OCTA and Volumetric MRI in Mild Cognitive Impairment and Alzheimer's Disease. Ophthalmic Surg Lasers Imaging Retina. 2019;50(11):709‐718. [DOI] [PubMed] [Google Scholar]
- 15. den Haan J, Janssen SF, van de Kreeke JA, Scheltens P, Verbraak FD, Bouwman FH. Retinal thickness correlates with parietal cortical atrophy in early‐onset Alzheimer's disease and controls. Alzheimers Dement (Amst). 2018;10:49‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ikram MK, De Jong FJ, Van Dijk EJ, et al. Retinal vessel diameters and cerebral small vessel disease: the Rotterdam Scan Study. Brain. 2006;129(Pt 1):182‐188. [DOI] [PubMed] [Google Scholar]
- 17. Cheung CY, Ong YT, Ikram MK, et al. Microvascular network alterations in the retina of patients with Alzheimer's disease. Alzheimers Dement. 2014;10(2):135‐142. [DOI] [PubMed] [Google Scholar]
- 18. Feke GT, Hyman BT, Stern RA, Pasquale LR. Retinal blood flow in mild cognitive impairment and Alzheimer's disease. Alzheimers Dement (Amst). 2015;1(2):144‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005;206(4):319‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thomson KL, Yeo JM, Waddell B, Cameron JR, Pal S. A systematic review and meta‐analysis of retinal nerve fiber layer change in dementia, using optical coherence tomography. Alzheimers Dement (Amst). 2015;1(2):136‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGrory S, Cameron JR, Pellegrini E, et al. The application of retinal fundus camera imaging in dementia: A systematic review. Alzheimers Dement (Amst). 2017;6:91‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wylęgała A. Principles of OCTA and Applications in Clinical Neurology. Curr Neurol Neurosci Rep. 2018;18(12):96‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsokolas G, Tsaousis KT, Diakonis VF, Matsou A, Tyradellis S. Optical coherence tomography angiography in neurodegenerative diseases: a review. Eye Brain. 2020;12:73‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alber J, Goldfarb D, Thompson LI, et al. Developing retinal biomarkers for the earliest stages of Alzheimer's disease: What we know, what we don't, and how to move forward. Alzheimers Dement. 2020;16(1):229‐243. [DOI] [PubMed] [Google Scholar]
- 26. Dubois B, Hampel H, Feldman HH, et al. Preclinical Alzheimer's disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12(3):292‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jack CR, Jr. , Bennett DA, Blennow K, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939‐944. [DOI] [PubMed] [Google Scholar]
- 29. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- 31. Bulut M, Kurtuluş F, Gözkaya O, et al. Evaluation of optical coherence tomography angiographic findings in Alzheimer's type dementia. Br J Ophthalmol. 2018;102(2):233‐237. [DOI] [PubMed] [Google Scholar]
- 32. den Haan J, van de Kreeke JA, van Berckel BN, et al. Is retinal vasculature a biomarker in amyloid proven Alzheimer's disease? Alzheimers Dement (Amst). 2019;11:383‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiang H, Wei Y, Shi Y, et al. Altered Macular Microvasculature in Mild Cognitive Impairment and Alzheimer Disease. J Neuroophthalmol. 2018;38(3):292‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lahme L, Esser EL, Mihailovic N, et al. Evaluation of Ocular Perfusion in Alzheimer's Disease Using Optical Coherence Tomography Angiography. J Alzheimers Dis. 2018;66(4):1745‐1752. [DOI] [PubMed] [Google Scholar]
- 35. O'Bryhim BE, Apte RS, Kung N, Coble D, Van Stavern GP. Association of Preclinical Alzheimer Disease With Optical Coherence Tomographic Angiography Findings. JAMA Ophthalmol. 2018;136(11):1242‐1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Querques G, Borrelli E, Sacconi R, et al. Functional and morphological changes of the retinal vessels in Alzheimer's disease and mild cognitive impairment. Sci Rep. 2019;9(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sadda SR, Borrelli E, Fan W, et al. A pilot study of fluorescence lifetime imaging ophthalmoscopy in preclinical Alzheimer's disease. Eye. 2019;33(8):1271‐1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu J, Zhang X, Azhati G, Li T, Xu G, Liu F. Retinal microvascular attenuation in mental cognitive impairment and Alzheimer's disease by optical coherence tomography angiography. Acta Ophthalmologica. 2020;98(6):e793‐e794. [DOI] [PubMed] [Google Scholar]
- 39. Yoon SP, Grewal DS, Thompson AC, et al. Retinal microvascular and neurodegenerative changes in Alzheimer's disease and mild cognitive impairment compared with control participants. Ophthalmol Retina. 2019;3(6):489‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zabel P, Kaluzny JJ, Wilkosc‐Debczynska M, et al. Comparison of retinal microvasculature in patients with Alzheimer's disease and primary open‐angle glaucoma by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2019;60(10):3447‐3455. [DOI] [PubMed] [Google Scholar]
- 41. Zhang YS, Zhou N, Knoll BM, et al. Parafoveal vessel loss and correlation between peripapillary vessel density and cognitive performance in amnestic mild cognitive impairment and early Alzheimer's Disease on optical coherence tomography angiography. PLoS One. 2019;14(4):e0214685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee J‐Y, Kim JP, Jang H, et al. Optical coherence tomography angiography as a potential screening tool for cerebral small vessel diseases. Alzheimers Res Ther. 2020;12(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Criscuolo C, Cennamo G, Montorio D, et al. Assessment of retinal vascular network in amnestic mild cognitive impairment by optical coherence tomography angiography. PLOS ONE. 2020;15(6):e0233975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van de Kreeke JA, Nguyen H‐T, Konijnenberg E, et al. Optical coherence tomography angiography in preclinical Alzheimer's disease. Br J Ophthalmol. 2020;104(2):157‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Kokmen E, Tangelos EG. Aging, memory, and mild cognitive impairment. Int Psychogeriatr. 1997;9 Suppl 1:65‐69. [DOI] [PubMed] [Google Scholar]
- 46. Lu Y, Wang JC, Zeng R, et al. Quantitative Comparison Of Microvascular Metrics On Three Optical Coherence Tomography Angiography Devices In Chorioretinal Disease. Clin Ophthalmol. 2019;13:2063‐2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Munk MR, Giannakaki‐Zimmermann H, Berger L, et al. OCT‐angiography: A qualitative and quantitative comparison of 4 OCT‐A devices. PLOS ONE. 2017;12(5):e0177059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Campbell JP, Zhang M, Hwang TS, et al. Detailed vascular anatomy of the human retina by projection‐resolved optical coherence tomography angiography. Sci Rep. 2017;7(1):42201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Durbin MK, An L, Shemonski ND, et al. Quantification of retinal microvascular density in optical coherence tomographic angiography images in diabetic retinopathy. JAMA Ophthalmol. 2017;135(4):370‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lei J, Pei C, Wen C, Abdelfattah NS. Repeatability and reproducibility of quantification of superficial peri‐papillary capillaries by four different optical coherence tomography angiography devices. Sci Rep. 2018;8(1):17866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sweeney MD, Sagare AP, Zlokovic BV. Cerebrospinal fluid biomarkers of neurovascular dysfunction in mild dementia and Alzheimer's disease. J Cereb Blood Flow Metab. 2015;35(7):1055‐1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Toombs J, Zetterberg H. In the blood: biomarkers for amyloid pathology and neurodegeneration in Alzheimer's disease. Brain Commun. 2020;2(1):fcaa054. 10.1093/braincomms/fcaa054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sullivan GM, Feinn R. Using effect size‐or why the P value is not enough. J Grad Med Educ. 2012;4(3):279‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Spaide RF, Curcio CA. Evaluation of segmentation of the superficial and deep vascular layers of the retina by optical coherence tomography angiography instruments in normal eyes. JAMA Ophthalmol. 2017;135(3):259‐262. [DOI] [PubMed] [Google Scholar]
- 55. Sampson DM, Ali N, Au Yong A, et al. RTVue XR angiovue optical coherence tomography angiography software upgrade impacts on retinal thickness and vessel density measurements. Transl Vis Sci Technol. 2020;9(3):10‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Willoughby AS, Chiu SJ, Silverman RK, et al. Platform‐independent cirrus and spectralis thickness measurements in eyes with diabetic macular edema using fully automated software. Transl Vis Sci Technol. 2017;6(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee CS, Tyring AJ, Wu Y, et al. Generating retinal flow maps from structural optical coherence tomography with artificial intelligence. Sci Rep. 2019;9(1):5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jiang Z, Huang Z, Qiu B, et al. Comparative study of deep learning models for optical coherence tomography angiography. Biomed Opt Express. 2020;11(3):1580‐1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Staurenghi G, Sadda S, Chakravarthy U, Spaide RF. Proposed lexicon for anatomic landmarks in normal posterior segment spectral‐domain optical coherence tomography: the IN•OCT consensus. Ophthalmology. 2014;121(8):1572‐1578. [DOI] [PubMed] [Google Scholar]
- 60. Iafe NA, Phasukkijwatana N, Chen X, Sarraf D. Retinal capillary density and foveal avascular zone area are age‐dependent: quantitative analysis using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(13):5780‐5787. [DOI] [PubMed] [Google Scholar]
- 61. You QS, Chan JCH, Ng ALK, et al. Macular vessel density measured with optical coherence tomography angiography and its associations in a large population‐based study. Invest Ophthalmol Vis Sci. 2019;60(14):4830‐4837. [DOI] [PubMed] [Google Scholar]
- 62. Holló G. Influence of posterior subcapsular cataract on structural OCT and OCT angiography vessel density measurements in the peripapillary retina. J Glaucoma. 2019;28(4):e61‐e63. [DOI] [PubMed] [Google Scholar]
- 63. Cameron JR, Megaw RD, Tatham AJ, et al. Lateral thinking ‐ Interocular symmetry and asymmetry in neurovascular patterning, in health and disease. Prog Retin Eye Res. 2017;59:131‐157. [DOI] [PubMed] [Google Scholar]
- 64. Sampson DM, Gong P, An D, et al. Axial length variation impacts on superficial retinal vessel density and foveal avascular zone area measurements using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017;58(7):3065‐3072. [DOI] [PubMed] [Google Scholar]
- 65. Arthur E, Elsner AE, Sapoznik KA, Papay JA, Muller MS, Burns SA. Distances from capillaries to arterioles or venules measured using OCTA and AOSLO. Invest Ophthalmol Vis Sci. 2019;60(6):1833‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. La Morgia C, Di Vito L, Carelli V, Carbonelli M. Patterns of retinal ganglion cell damage in neurodegenerative disorders: parvocellular vs magnocellular degeneration in optical coherence tomography studies. Front Neurol. 2017;8:710‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Terheyden JH, Wintergerst MWM, Falahat P, Berger M, Holz FG, Finger RP. Automated thresholding algorithms outperform manual thresholding in macular optical coherence tomography angiography image analysis. PLOS ONE. 2020;15(3):e0230260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Samara WA, Say EAT, Khoo CTL, et al. Correlation of foveal avascular zone size with foveal morphology in normal eyes using optical coherence tomography angiography. Retina. 2015;35(11):2188‐2195. [DOI] [PubMed] [Google Scholar]
- 69. Coscas F, Sellam A, Glacet‐Bernard A, et al. Normative data for vascular density in superficial and deep capillary plexuses of healthy adults assessed by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT211‐OCT223. [DOI] [PubMed] [Google Scholar]
- 70. Braaf B, Gräfe MGO, Uribe‐Patarroyo N, et al. OCT‐based velocimetry for blood flow quantification. In: Bille JF, ed. High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics. Cham: Springer International Publishing; 2019:161‐179. [PubMed] [Google Scholar]
- 71. Wang J, Zhang M, Hwang TS, et al. Reflectance‐based projection‐resolved optical coherence tomography angiography [Invited]. Biomed Opt Express. 2017;8(3):1536‐1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495‐1499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Supplementary information
Supplementary information
Supplementary information
Supplementary information
Supplementary information


