Abstract
Sleep consolidates episodic content of emotional memories. Whether it likewise preserves or, to the contrary, depotentiates the emotional response associated with memory content is unclear, as there is conflicting evidence. In the current study, we investigated the influence of an afternoon nap (2-hr nap opportunity) on emotional responses of memories using multiple simultaneous measures. Young adults viewed 45 negative and 45 neutral pictures before taking a nap (measured with polysomnography) or remaining awake. Following the nap or wake period, participants viewed the same pictures intermixed with novel ones and indicated whether they remembered each picture. Emotional response to each picture was measured at both time points both subjectively, with valence and arousal ratings, and objectively, with recordings of electrodermal activity, electrocardiography, and corrugator supercilii electromyography. Compared to waking, a nap led to preserved subjective valence for negative pictures and preserved/increased skin conductance response in general. On the other hand, heart rate deceleration response decreased over the nap compared to wake interval, and this result was not influenced by picture type. These data suggest that sleep consolidates aspects of both subjective and physiological emotional response associated with episodic memory content. While sympathetic response appears to be preserved over sleep, parasympathetic response may be diminished.
1. Introduction
Emotional memory consists of episodic content (details of what, when, and where) as well as the emotional tone that accompanies this content. Emotional tone has components of subjective feelings as well as physiological responses (Bradley & Lang, 2006). Abundant evidence indicates that sleep strengthens or preserves emotional memory content (Baran et al., 2012, Nishida et al., 2009, Payne et al., 2008, Tempesta et al., 2018, Wagner et al., 2006). However, its influence on emotional response associated with memory content is less clear, as there is conflicting evidence.
The sleep to remember, sleep to forget hypothesis posits that sleep consolidates memory content while attenuating emotional tone (Walker & van Der Helm, 2009). In particular, processing during rapid-eye-movement (REM) sleep is proposed to depotentiate the emotional tone of memories. In support of this hypothesis, amygdala activity in response to viewing emotional pictures declines over a night of sleep but not over a day of wakefulness, and this decline is correlated with gamma activity during REM sleep (van der Helm et al., 2011). A decline in reactivity over a night of sleep compared to a day of wakefulness has also been observed in skin conductance and heart rate deceleration responses, which are measures of sympathetic and parasympathetic activity, respectively (Cunningham et al., 2014). Finally, a decline in skin conductance and corrugator electromyography responses has been observed over an afternoon nap compared to an equivalent time spent awake (Pace-Schott et al., 2011). These findings suggest that sleep may depotentiate the emotional tone of memories. However, there is also evidence that sleep, and REM sleep in particular, may preserve or perhaps strengthen the emotional tone of memories. Compared to a day spent awake, a night of sleep preserved valence ratings of negative pictures, and this preservation was correlated with time in REM sleep (Baran et al., 2012). Selective REM sleep deprivation led to reduced reactivity compared to normal overnight sleep (Lara-Carrasco, Nielsen, Solomonova, Levrier, & Popova, 2009). Reactivity to a scary movie was preserved over a night of sleep compared to a day of wake, with preservation linked to REM sleep (Werner, Schabus, Blechert, Kolodyazhniy, & Wilhelm, 2015). Finally, ratings of emotional texts increased after REM-rich (late night) sleep (Wagner, Fischer, & Born, 2002).
Thus, it remains unclear whether sleep depotentiates or preserves the emotional tone of memories. One factor that could have caused discrepancies in past findings is circadian effects, as emotion responses can vary by time of day (Hot, Leconte, & Sequeira, 2005), and many studies compared changes over a night to changes over a day. Another potential source of discrepancy is differences in emotional measures, as some studies assessed only subjective ratings and some only physiology. Here, we sought to address these issues by controlling for time of day with a nap design and incorporating measures of both subjective and physiological emotional response. We hypothesized that non-REM sleep would be implicated in consolidating (strengthening/preserving) memory content, while REM sleep would be implicated in preserving emotional tone (Baran et al., 2012, Genzel et al., 2015, Jones et al., 2016).
2. Materials and methods
2.1. Participants
Participants were 77 young adults between 18 and 28 years of age (nap group: n = 50, M = 20.94, SD = 2.29, 35 females; wake group: n = 27, M = 20.33, SD = 2.08, 20 females). More participants were recruited in the nap group because we anticipated variability in nap/REM sleep duration and exclusions based on insufficient nap duration. Participants had normal or corrected-to-normal vision and no history of neurological disease, sleep disorders, head injury, or use of medications known to affect sleep or cognitive function. Participants were instructed to refrain from alcohol, sleep at least 6 h the night before the experiment, wake up no later than 8:00 AM the morning of the experiment, and limit caffeine intake the day of the experiment. All participants were compensated with payment or course credit. Experimental procedures were approved by the University of Massachusetts, Amherst Institutional Review Board and written informed consent was obtained before the experiment.
Fourteen participants were excluded from all analyses for missing data files (n = 2), falling asleep during the behavior task (n = 2), misunderstanding the task instructions (n = 2), sleeping less than 45 min (half of a typical sleep cycle) during the 2-hr nap opportunity (n = 7), and multiple awakenings due to construction noise near the sleep lab (n = 1). The resulting sample consisted of 38 participants in the nap group (M ± SD = 20.79 ± 2.11 years of age, 25 females) and 25 in the wake group (M ± SD = 19.96 ± 1.62 years of age, 19 females). Due to data loss, sleep stage scoring was not possible for 11 participants. Thus, sleep stage analyses are based on 27 participants. Three additional participants were excluded from sigma and delta activity analyses due to poor recording quality at electrode site F3 and/or F4 (where these measures were calculated), leaving 24 participants for these analyses. REM theta activity was calculated for the 20 participants who obtained REM sleep. Finally, due to data loss, some participants were excluded from psychophysiology analyses (see final sample sizes for these analyses in Table 1).
Table 1.
Baseline emotional response (M(SE))
| Nap | Wake | |||||
|---|---|---|---|---|---|---|
| N | Negative | Neutral | N | Negative | Neutral | |
| Valence | 36 | 1.86 (0.09) | 5.01 (0.02) | 25 | 1.73 (0.10) | 4.97 (0.02) |
| Arousal | 36 | 6.07 (0.26) | 2.02 (0.16) | 25 | 6.53 (0.26) | 2.03 (0.17) |
| SCR | 27 | 0.06 (0.02) | 0.05 (0.03) | 21 | 0.09 (0.03) | 0.03 (0.02) |
| HRD | 25 | 0.16 (0.01) | 0.15 (0.01) | 21 | 0.17 (0.01) | 0.17 (0.01) |
| cEMG | 25 | 0.15 (0.02) | 0.12 (0.02) | 17 | 0.13 (0.02) | 0.10 (0.02) |
2.2. Materials
Stimuli were 90 emotionally negative and 90 emotionally neutral pictures. The majority of stimuli were obtained from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2005). The rest were from an in-house set and were chosen to match the IAPS pictures in content and emotionality (Baran et al., 2012). Based on normative data and previous work in our lab (Jones et al., 2016), negative pictures were moderate to high in arousal, and neutral pictures were low in arousal.
2.3. Procedure
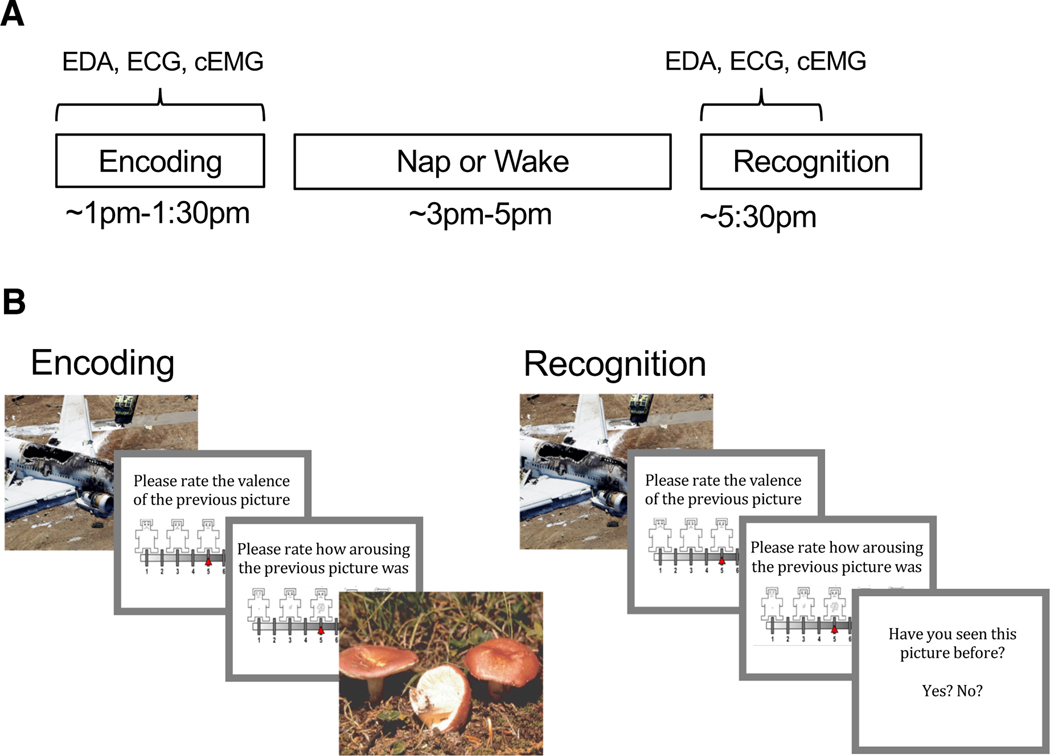
Participants arrived for the Encoding session between 12:30 and 1:00 PM. Following Encoding, participants in the nap group underwent application of polysomnography (PSG) and were given a 2-hour nap opportunity followed by 30 min of waking to allow for dissipation of sleep inertia. Participants in the wake group watched nature documentaries and were allowed to rest quietly for approximately 3 h, such that the delay interval was equivalent for nap and wake groups. Following the nap or wake interval, participants completed the Recognition session. Electrodermal activity (EDA), electrocardiography (ECG), and corrugator supercilii electromyography (cEMG) were measured during Encoding and the first half of Recognition (Fig. 1A).
Fig. 1.
Experimental procedure and task. (A) Encoding took place in the early afternoon followed by a 2-hr nap opportunity or wake period and then Recognition. Electrodermal activity (EDA), electrocardiography (ECG), and corrugator supercilii electromyography (cEMG) were measured during the entirety of Encoding and the first half of Recognition. (B) During Encoding participants viewed 90 pictures (targets) and rated the valence and arousal of each on 9-point self-assessment manikin scales. During Recognition, participants viewed 180 pictures, a mixture of target and novel foil pictures, and rated each one on valence and arousal. Participants indicated whether or not they recognized the picture by responding yes/no.
During Encoding, participants viewed 90 target stimuli (45 negative, 45 neutral) in random order (Fig. 1B). Each picture appeared on the computer screen for 2 s, followed by a black screen for 6 s. After this black screen, participants were first prompted to rate the valence of the picture on a nine-item self-assessment manikin (SAM) valence scale (1 = negative, 5 = neutral, 9 = positive), and then prompted to rate its arousability on a nine-item SAM arousal scale (1 = no arousal, 9 = highly arousing; Bradley & Lang, 1994). Ratings were entered using numbers on a keyboard without any time limit. Following the rating scales, another black screen appeared for 10–14 s before the next picture. A long inter-stimulus interval was needed to collect emotion physiology data. Participants were not informed that their memory for the pictures would be tested later.
During Recognition, participants were shown 180 pictures: the same 90 targets seen during Encoding intermixed with 90 novel pictures (foils; 45 neutral and 45 negative). The stimulus presentation procedure was identical to Encoding with the following exceptions: (1) following valence and arousal ratings, participants were prompted to indicate whether they had seen each picture before by pressing “y” for yes and “n” for no, and (2) a 1-s inter-stimulus interval was used during the second half of the session in order to prevent fatigue. Target pictures were evenly distributed across the first and second halves of the session (45 in each half). Stimuli were presented using E-Prime 2.0 (Psychology Software Tools, Inc.).
2.4. Psychophysiology
EDA, ECG, and cEMG were recorded with a sampling rate of 2000 Hz using the MP150 data acquisition system and AcqKnowledge 4.1 software (BIOPAC systems, Inc., Goleta, CA). Stimulus onset markers were transmitted via the BIOPAC STP100C digital interface. EDA was measured using two disposable electrodes placed on the hypothenar surface of the non-dominate hand. ECG was measured using two disposable electrodes placed over the left first intercostal space and below the right lowest rib the participant could feel. cEMG was measured using two Ag-AgCl shielded electrodes placed over the left corrugator supercilii muscle.
2.5. Polysomnography
PSG was recorded in the sleep laboratory using the Comet Plus PSG system (Grass Technologies) combined with a 32-electrode cap (EasyCap; Brain Products GmbH, Germany) that included two electrooculography (EOG; right and left ocular canthi), two chin electromyography (EMG), and 25 electroencephalography (EEG) leads (Fz, F3, F4, F7, F8, FCz, FC1, FC2, FC5, FC6, Cz, C3, C4, CP1, CP2, CP5, CP6, Pz, P3, P4, P7, P8, POz, O1, O2). PSG data were collected at a sampling rate of 200 Hz with a bandpass of 0.1–100 Hz. EOG and EEG channels were referenced to Cz during recording and re-referenced to the contralateral mastoid for scoring. Recordings were obtained and scored according to the specifications provided by the American Academy of Sleep Medicine (Iber, Ancoli-Israel, Chesson, & Quan, 2007).
2.6. Data analysis
Participants’ individual valence ratings of the pictures were used to categorize stimuli for analyses (as in St. Jacques et al., 2009, Baran et al., 2012). Due to individual differences in emotional response, individualized categorization may provide the most accurate measures. Targets were categorized based on ratings during the Encoding session, and foils were categorized based on ratings during the Recognition session. Negative and neutral pictures were defined as those rated 1–3 and 4–6 on valence, respectively. Hence, the analyzed picture sets were unique for each participant. On average, participants rated 36.50 ± 8.71 target pictures as negative, 43.53 ± 10.99 target pictures as neutral, 37.95 ± 8.85 foil pictures as negative, and 46.68 ± 11.0 foil pictures as neutral. Number of pictures in each category did not differ between nap and wake groups (all p’s > 0.62). In order to account for performance on both targets and foils, we chose corrected recognition as the memory outcome. Corrected recognition was calculated as the hit rate (percentage of target pictures correctly identified as previously seen) minus the false alarm rate (percentage of foil pictures incorrectly identified as previously seen).
Skin conductance response (SCR) was calculated for each picture by subtracting the mean skin conductance level during the 2 s prior to picture onset from the maximum level during 1–6 s after picture onset. The value was square-root transformed, and if the untransformed value was negative, then the negative sign was added to the transformed value. Heart rate deceleration (HRD) was calculated for each picture by subtracting the mean heart rate during the 2 s prior to picture onset from the minimum heart rate during the 2 s of picture presentation. cEMG activity was integrated over a 250 ms time constant, and the response to each picture was calculated by subtracting the mean amplitude during the 2 s prior to picture onset from the maximum amplitude during the 2 s of picture presentation. To account for individual differences in reactivity, the psychophysiological responses of each participant during encoding and recognition were range corrected by dividing each response by the participant’s maximum respective response at encoding (Lykken & Venables, 1971). Notably, this transformation resulted in decelerations of heart rate having a positive value.
EEG amplitude density was measured in the delta (0.5–4 Hz), theta (4–8 Hz), and sigma (12–16 Hz) bands over frontal scalp regions (F3, F4) by extracting the amplitude envelope of bandpass-filtered EEG, summing it within identified sleep stages, and normalizing by time. The use of the Hilbert-transformation-derived amplitude envelope to quantify signal dynamics is a common method in engineering that has been previously applied to EEG analysis in multiple contexts, including sleep (Clochon et al., 1996, Díaz et al., 2018, Freeman, 2004). We opt to use this approach over the short-time Fourier transform or wavelet decomposition methods of signal dynamics quantification because it is more computationally efficient, and because recent evidence suggests that Hilbert-transformation-derived envelopes may more accurately capture arrhythmic elements of the EEG (Díaz et al., 2018).
EEG was first re-referenced offline to the averaged mastoid recording, then filtered separately into delta activity using a Butterworth infinite-impulse response filter (order = 2) that did not remove mean recording bias and theta activity and sigma activity using a forward impulse response filter (order = 164) that did remove mean recording bias. Regions of continuous filtered EEG exceeding frequency-band specific thresholds (delta: ±250 μV, theta: ±100 μV, sigma: ±75 μV) within a moving 500 ms window were marked as artifact. Delta, theta, and sigma amplitude envelopes were then calculated for each electrode as the magnitude (absolute value) of the analytic signal (z) of the filtered EEG, where the analytic signal is the sum of the filtered EEG and its discrete Hilbert transformation multiplied by the imaginary unit: z(EEG) = EEG + i * Hilbert(EEG). Amplitude envelopes were then averaged across electrodes. For delta and sigma, samples not previously marked as artifact at either electrode were summed across stage 2 non-rapid eye movement (NREM2) sleep and SWS epochs, and divided by the combined number of artifact-free seconds spent in NREM2 sleep and SWS. For theta, samples not previously marked as artifact were summed across REM sleep epochs and divided by the number of artifact-free seconds spent in REM sleep. Less than 0.04%, 0.02%, and 0.02% of samples were marked as artifact for any participant for delta, theta, and sigma, respectively. EEG analyses were conducted in MATLAB using a combination of EEGLAB (Delorme & Makeig, 2004), ERPLAB (Lopez-Calderon & Luck, 2014), and custom in-house functions (available upon request).
Within-subject comparisons of means were conducted using repeated-measures analyses of variance (ANOVAs), and post-hoc pairwise comparisons were made using Student’s independent-samples t-tests. Pearson’s r was used to assess bivariate linear relationships. For each variable of interest, data points falling outside 3 SD of the mean were identified as outliers and removed from analyses of that variable (n = 0–2 for each variable, see Results and Table 1 for final sample sizes). Significance levels were set to p < 0.05. Statistical analyses were conducted in SPSS.
3. Results
3.1. Sleep
Nap characteristics are reported in Table 2. Of the 27 participants for whom sleep stage scoring was possible, 25 obtained SWS, and 20 obtained REM sleep. Eighteen participants were in NREM2 when they woke from the nap, 6 were in NREM3, and 3 were in REM sleep.
Table 2.
Nap characteristics
| Mean | SE | |
|---|---|---|
| TST (min) | 93.59 | 4.23 |
| SL (min) | 11.02 | 1.68 |
| SE (%) | 81.12 | 2.93 |
| RL (min) | 64.67 | 3.62 |
| NREM1 (%) | 11.46 | 1.66 |
| NREM2 (%) | 49.77 | 3.11 |
| SWS (%) | 26.99 | 3.98 |
| REM (%) | 11.79 | 2.49 |
| Delta | 20.71 | 1.62 |
| Sigma | 3.19 | 0.11 |
| Theta | 5.68 | 0.31 |
TST=total sleep time, SL=sleep latency, SE=sleep efficiency, RL=REM latency
3.2. Memory
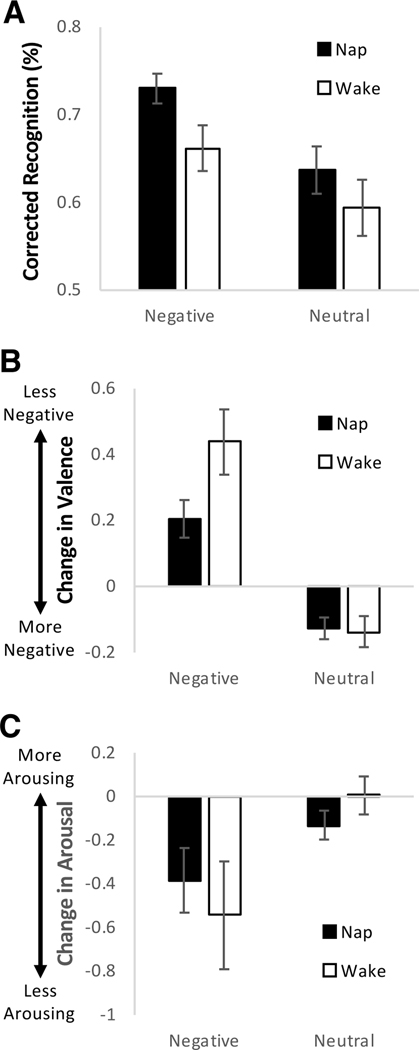
A 2 × 2 repeated-measures ANOVA with Group (nap, wake) as a between-subjects factor and Valence (negative, neutral) as a within-subjects factor was conducted on corrected recognition. Negative pictures were better remembered than neutral pictures (main effect of Valence: F(1,60) = 19.476, p < 0.001), and this effect was not modulated by nap or wake (Valence × Group interaction: p = 0.481; Fig. 2A). As expected, the nap group performed better than the wake group, but this effect did not reach statistical significance (main effect of Group: F(1,60) = 3.084, p = 0.084). Based on our hypothesis that episodic content of emotional memory is consolidated during NREM sleep, correlation analyses were conducted on memory for negative pictures (corrected recognition) and NREM variables (percent NREM2, percent SWS, sigma activity, delta activity). There was a positive relationship between sigma activity and negative memory (r = 0.458, p = 0.024; not corrected for multiple testing). The other correlations were not significant (p’s > 0.2). Sigma activity was not related to neutral memory (r = 0.122, p = 0.569), perhaps suggesting a specific association with negative memory.
Fig. 2.
Memory performance and change in subjective emotional response. (A) Average percent corrected recognition (hit rate-false alarm rate) for negative and neutral pictures. (B) Average inter-session change in valence ratings for negative and neutral pictures. Valence was rated on a 9-point Likert-type scale: 1 = most negative, 5 = neutral, 9 = most positive. A positive change in valence indicates ratings became less negative. (C) Average inter-session change in arousal ratings for negative and neutral pictures. Arousal was rated on a 9-point Likert-type scale: 1 = no arousal, 9 = highest arousal. Error bars represent standard errors of means.
3.3. Subjective emotional response
There was a non-significant difference between the nap and wake groups on valence ratings of neutral pictures at Encoding (t = 1.680, p = 0.098). The difference was very slight (see Table 1), and neither group’s rating was different than absolute neutral on the SAM valence scale (5; p’s > 0.13, one-sample t-tests). Additionally, neutral valence rating was not significantly correlated with any of the outcome measures (p’s > 0.1). Other ratings did not differ between groups (p’s > 0.23, independent-samples t-tests; Table 1).
2 × 2 repeated-measures ANOVAs with Group (nap, wake) as a between-subjects factor and Valence (negative, neutral) as a within-subjects factor were conducted on change in valence ratings and change in arousal ratings. With regard to valence ratings, there was a Valence × Group interaction (F(1,59) = 4.472, p = 0.039) such that ratings of negative pictures (t = −2.183, p = 0.033) but not neutral pictures (t = 0.171, p = 0.865) were preserved in the nap relative to wake group (Fig. 2B). Change in valence ratings differed between negative and neutral pictures, with ratings becoming less negative for negative pictures and more negative (less neutral) for neutral pictures (main effect of Valence: F(1,59) = 62.145, p < 0.001). The main effect of Group did not reach statistical significance (F(1,59) = 3.158, p = 0.081). With regard to arousal ratings, there was a greater decline in ratings (signifying decreased arousal) of negative pictures than neutral pictures (main effect of Valence: F(1,59) = 9.129, p = 0.004; Fig. 2C). This effect was not modulated by nap or wake (Valence × Group interaction), nor was there an overall difference between nap and wake groups (main effect of Group; p’s > 0.26).
These results suggest that sleep preserves aspects of subjective emotional valence associated with episodic memory. Based on our hypothesis that emotional tone of memories is processed during REM sleep, correlation analyses were conducted on change in valence ratings for negative pictures and REM sleep variables (percent REM, REM theta activity). We did not observe any significant correlations (p’s > 0.59).
3.4. Physiological emotional response
There were no significant differences between groups in SCR, HRD, or cEMG response at Encoding (all p’s > 0.3, independent-samples t-tests; Table 1). 2 × 2 repeated-measures ANOVAs with Group (nap, wake) as a between-subjects factor and Valence (negative, neutral) as a within-subjects factor were conducted on change in SCR, change in HRD, and change in cEMG response.
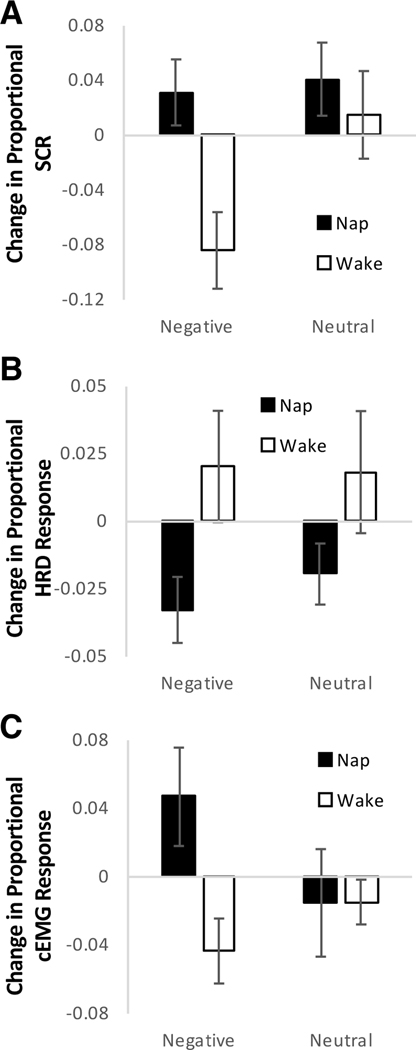
SCR increased more/decreased less over the nap relative to wake interval (main effect of Group: F(1,46) = 6.232, p = 0.016), and this effect was not significantly modulated by picture valence (Valence × Group interaction: p = 0.104; Fig. 3A). There was some evidence that SCR declined more/increased less for negative than neutral pictures overall, though this effect did not reach statistical significance (main effect of Valence: F(1,46) = 4.061, p = 0.050). A positive change in SCR reflects an increase in response between Encoding and Recognition, whereas a negative change reflects a reduction in response between Encoding and Recognition. Thus, response to negative pictures declined over the wake interval but not the nap interval, and response to neutral pictures increased to some extent over both intervals.
Fig. 3.
Change in physiological emotional response. (A) Average inter-session change in range-corrected skin conductance responses (SCR) for negative and neutral pictures. (B) Average inter-session change in range-corrected heart rate deceleration (HRD) responses for negative and neutral pictures. A positive change reflects an increase in response (greater deceleration) between Encoding and Recognition, whereas a negative change reflects a decrease in response (less deceleration) between Encoding and Recognition. (C) Average inter-session change in range-corrected corrugator supercilii EMG (cEMG) responses for negative and neutral pictures. Range correction was performed within individuals by dividing the response to each picture by the maximum response during Encoding. Error bars represent standard errors of means.
HRD declined more over the nap than wake interval (main effect of Group: F(1,44) = 6.147, p = 0.017; Fig. 3B). This effect was not modulated by picture valence (Valence × Group interaction: p = 0.531), and change in HRD did not differ between negative and neutral pictures overall (main effect of Valence: p = 0.623). A positive change in HRD reflects an increase in response (greater deceleration) between Encoding and Recognition, whereas a negative change reflects a decrease in response (less deceleration) between Encoding and Recognition. Thus, there was an increase in deceleration response to both picture types in the wake group and a decrease in deceleration response to both picture types in the nap group. With regard to cEMG response, there was some evidence that differences between nap and wake groups depended on picture valence, though this effect did not reach statistical significance (Valence × Group interaction: F(1,40) = 3.185, p = 0.082). The response to negative pictures increased more/declined less in the nap group relative to the wake group (t = 2.353, p = 0.024), while the change in response to neutral pictures did not differ between groups (t = −0.012, p = 0.990; Fig. 3C). The main effects of Group and Valence were not significant (p’s > 0.13). A positive change in cEMG response reflects an increase in response between Encoding and Recognition, whereas a negative change reflects a decrease in response between Encoding and Recognition. Thus, response to negative pictures declined over the wake interval and increased over the nap interval, and response to neutral pictures decreased to some extent over both intervals.
These results suggest that sleep preserves aspects of sympathetic and somatic emotional response associated with episodic memory, whereas aspects of parasympathetic response may be diminished by sleep. Based on our hypothesis that emotional tone of memories is processed during REM sleep, correlation analyses were conducted on change in SCR, change in HRD, and change in cEMG response for negative pictures and REM sleep variables (percent REM, REM theta activity). The relationship between REM theta activity and change in SCR over the nap was in the expected (positive) direction, though the correlation did not reach statistical significance (r = 0.554, p = 0.077; not corrected for multiple testing). Other correlations were not significant (p’s > 0.24). REM theta activity was not related to change in SCR for neutral pictures (r = −0.095, p = 0.780), perhaps suggesting a specific association with negative memories. It should be noted that correlations of psychophysiological variables with REM theta activity are based on only 10–11 data points (participants having both REM sleep and psychophysiological data).
4. Discussion
Many of our results support that sleep preserves not only emotional memory content but also emotional tone. Compared to an equivalent interval of waking, an afternoon nap led to preserved subjective valence, increased SCR, and increased cEMG. This pattern was specific to negative pictures for subjective valence and cEMG, though it should be kept in mind that the valence by group interaction did not reach statistical significance for cEMG. However, contrary to this pattern, HRD decreased over the nap compared to wake interval, and this result was not influenced by picture type.
With regard to memory, we observed a trend for better performance in the nap group than wake group. This finding is in line with a large body of evidence indicating that sleep consolidates memory (Rasch & Born, 2013). We did not see a preferential benefit for negative memory, which is sometimes observed (Nishida et al., 2009, Payne et al., 2008, Wagner et al., 2006). However, a non-selective benefit is consistent with many past findings (Baran et al., 2012, Cellini et al., 2016, Lehmann et al., 2016, Lewis et al., 2011), and a mixed presentation of emotional and neutral stimuli could be conducive to a carry-over effect between emotional and neutral items (Jones et al., 2016, Tambini et al., 2017). Sigma activity, the frequency band of sleep spindles, during NREM sleep was positively related to negative memory performance. Consistent with previous studies (Alger et al., 2018, Cairney et al., 2014 Groch et al., 2011; Hauner et al., 2013, Kaestner et al., 2013, Payne et al., 2015, Rolls et al., 2013), this result suggests that mechanisms during NREM sleep may contribute to consolidation of episodic content of emotional memory.
Regarding subjective emotional response, we observed that valence ratings of negative pictures were preserved over the nap compared to wake interval. This finding replicates those of overnight studies in young adults (Baran et al., 2012, Mantua et al., 2017). Here, we did not observe sleep-related preservation of arousal ratings, though this has been observed in some past overnight studies (Jones et al., 2016, Lara-Carrasco et al., 2009; though see van der Helm et al., 2011). Preservation or strengthening of valence and arousal ratings has been linked to REM sleep (Baran et al., 2012, Lara-Carrasco et al., 2009, Wagner et al., 2002). However, we did not observe a relationship between preservation of subjective emotional response and REM sleep in this study. It is possible that the limited amount of REM sleep achieved in the nap was not sufficient for a relationship to emerge. Future studies could compare the influence of naps with and without REM sleep to better determine the effects of this sleep stage on subjective emotional response.
We observed that SCR declined over the wake interval but was preserved over the nap. Most nap participants obtained REM sleep, and there was a trend for a positive relationship between REM theta activity and change in SCR. This finding is consistent with that of a previous study in which participants who exhibited REM sleep in a nap had preserved SCR to negative pictures compared to those who did not exhibit REM sleep (Pace-Schott et al., 2011). Our finding is also consistent with previous results showing that SCR to emotional film clips increases over a night of sleep, with the increase positively correlated with the amount of REM sleep obtained at the end of the night (Werner et al., 2015). However, our results are at odds with those of a previous study showing a reduced number of SCRs in response to negative and neutral objects following a night of sleep compared to a day of wakefulness (Cunningham et al., 2014). Since SCR has been shown to be higher in the evening than morning (Hot et al., 2005), an overnight reduction in number may reflect fewer responses reaching the magnitude cutoff used in this analysis in the morning compared to the evening. Overall, the majority of evidence suggests that sleep, and REM sleep in particular, is involved in preservation or possibly strengthening of sympathetic reactivity to previously encountered emotional stimuli.
Contrary to our hypothesis, we observed that HRD decreased over the nap relative to wake. This finding is in line with that of a previous study in which HRD decreased over a night of sleep but not over a day of wakefulness (Cunningham et al., 2014). However, our results contradict findings of preserved HRD over a nap compared to an equivalent wake period in the context of a habituation task (Pace-Schott et al., 2011). HRD is under parasympathetic control and reflects perceptual processing associated with the orienting response (Graham & Clifton, 1966). Both emotional valence and novelty influence HRD (Bradley, 2009). In episodic memory tasks (stimuli presented once prior to sleep/wake as in the current study and Cunningham et al., 2014), consolidation of memory during sleep would increase the familiarity of the stimuli and perhaps reduce subsequent perceptual processing. In a habituation task (stimuli presented several times prior to sleep/wake as in Pace-Schott et al., 2011), novelty would be diminished in both sleep and wake groups, and change in HRD may reflect differential influence of sleep and wake on emotional response. Future research could investigate this possibility.
We observed that cEMG response to negative pictures increased over the nap and decreased over wake. While this pattern was not observed for neutral pictures, it should be kept in mind that the valence by group interaction did not reach statistical significance. cEMG activity is an objective index of valence, with the highest reactivity in response to negative stimuli (Bradley & Lang, 2006). Thus, this result is consistent with our finding that the nap preserved ratings of subjective valence compared to the wake interval. REM sleep has been linked to preservation of cEMG response to negative stimuli (Werner et al., 2015), whereas SWS has been linked to decline in cEMG (Pace-Schott et al., 2011), perhaps suggesting different emotion-relevant processing in different sleep stages.
Recent evidence suggests that sleep may attenuate cognitive emotional response (measured by subjective valence and the late positive potential) and preserve automatic emotional response (measured by HRD) in children (Bolinger, Born, & Zinke, 2018). While it is difficult to directly compare this finding in children to the current results due to methodological differences including analysis approach, it nonetheless raises the possibility that the influence of sleep on emotion may change across the lifespan. Along this line, sleep did not preserve emotional response of negative memories in middle-aged or older adults (Jones et al., 2016, Jones et al., 2018). Future studies could investigate whether developmental/age-related changes in sleep and emotion regulation may influence the effect of sleep on emotional tone of memories.
Overall, results of this study suggest that sleep preserves or strengthens many aspects of emotional response associated with episodic memory content. Preservation/strengthening occurs along dimensions of both valence (subjective ratings, cEMG) and arousal (SCR) and is specific to emotional (negative) stimuli, at least in the case of subjective valence. These results do not support the sleep to remember, sleep to forget hypothesis (Walker & van Der Helm, 2009), but rather are consistent with an account that sleep consolidates the emotional tone of episodic memories (Baran et al., 2012, Groch, Wilhelm, Diekelmann, & Born, 2013, Lara-Carrasco et al., 2009, Wagner et al., 2002, Werner et al., 2015). However, sleep may generally depotentiate parasympathetic response (HRD), although this result may be confounded by the influence of sleep on stimulus novelty. Limitations of this study include a small amount of REM sleep obtained in a nap relative to overnight sleep and only assessing memory after one short sleep episode. Whether emotional response changes further over additional sleep bouts is unknown and warrants investigation.
Acknowledgements
This work was supported by NIH R01 AG040133. We thank Ed Pace-Schott for guidance on psychophysiology recording and analysis and Ahren Fitzroy for providing custom functions used in EEG analyses.
Footnotes
Disclosure statement
The authors declare no conflicts of interest. All procedures were approved by the Institutional Review Board of the University of Massachusetts, Amherst.
References
- Alger SE, Kensinger EA, Payne JD. Preferential consolidation of emotionally salient information during a nap is preserved in middle age Neurobiology of Aging, 68 (2018), pp. 34–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran B, Pace-Schott EF, Ericson C, Spencer RMC. Processing of emotional reactivity and emotional memory over sleep Journal of Neuroscience, 32 (2012), pp. 1035–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolinger E, Born J, Zinke K. Sleep divergently affects cognitive and automatic emotional response in children Neuropsychologia, 117 (2018), pp. 84–91 [DOI] [PubMed] [Google Scholar]
- Bradley MM. Natural selective attention: Orienting and emotion Psychophysiology, 46 (2009), pp. 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: The Self-Assessment Manikin and the semantic differential Journal of Behavior Therapy and Experimental Psychiatry, 25 (1994), pp. 49–59 [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Motivation and emotion J.T. Cacioppo, L.G. Tassinary, G. Berntson (Eds.), Handbook of psychophysiology (2nd ed.), Cambridge University Press, New York (2006) [Google Scholar]
- Cairney SA, Durrant SJ, Power R, Lewis PA. Complementary roles of slow-wave sleep and rapid eye movement sleep in emotional memory consolidation Cerebral Cortex (2014), 10.1093/cercor/bht349 [DOI] [PubMed] [Google Scholar]
- Cellini N, Torre J, Stegagno L, Sarlo M. Sleep before and after learning promotes the consolidation of both neutral and emotional information regardless of REM presence Neurobiology of Learning and Memory, 133 (2016), pp. 136–144 [DOI] [PubMed] [Google Scholar]
- Clochon P, Fontbonne J-M, Lebrun N, Etévenon P. A new method for quantifying EEG event-related desynchronization: Amplitude envelope analysis Electroencephalography and Clinical Neurophysiology, 98 (1996), pp. 126–129 [DOI] [PubMed] [Google Scholar]
- Cunningham TJ, Crowell CR, Alger SE, Kensinger EA, Villano MA, Mattingly SM, P ayne JD. Psychophysiological arousal at encoding leads to reduced reactivity but enhanced emotional memory following sleep Neurobiology of Learning and Memory, 114 (2014), pp. 155–164 [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis Journal of Neuroscience Methods, 134 (2004), pp. 9–21 [DOI] [PubMed] [Google Scholar]
- Díaz J, Bassi A, Coolen A, Vivaldi EA, Letelier J-C. Envelope analysis links oscillatory and arrhythmic EEG activities to two types of neuronal synchronization NeuroImage, 172 (2018), pp. 575–585 [DOI] [PubMed] [Google Scholar]
- Freeman WJ. Origin, structure, and role of background EEG activity. Part 1. Analytic amplitude Clinical Neurophysiology, 115 (2004), pp. 2077–2088 [DOI] [PubMed] [Google Scholar]
- Genzel L, Spoormaker VI, Konrad BN, Dresler M. The role of rapid eye movement sleep for amygdala-related memory processing Neurobiology of Learning and Memory, 122 (2015), pp. 110–121 [DOI] [PubMed] [Google Scholar]
- Graham FK, Clifton RK. Heart-rate change as a component of the orienting response Psychological Bulletin, 65 (1966), pp. 305–320 [DOI] [PubMed] [Google Scholar]
- Groch S, Wilhelm I, Diekelmann S, Born J. The role of REM sleep in the processing of emotional memories: Evidence from behavior and event-related potentials Neurobiology of Learning and Memory, 99 (2013), pp. 1–9 [DOI] [PubMed] [Google Scholar]
- Groch S, Wilhelm I, Diekelmann S, Sayk F, Gais S, Born J. Contribution of norepinephrine to emotional memory consolidation during sleep Psychoneuroendocrinology, 36 (9) (2011), pp. 1342–1350 [DOI] [PubMed] [Google Scholar]
- Hauner KK, Howard JD, Zelano C, Gottfried JA. Stimulus-specific enhancement of fear extinction during slow-wave sleep Nature Neuroscience, 16 (2013), pp. 1553–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hot P, Leconte P, Sequeira H. Diurnal autonomic variations and emotional reactivity Biological Psychology, 69 (2005), pp. 261–270 [DOI] [PubMed] [Google Scholar]
- Kaestner EJ, Wixted JT, Mednick SC. Pharmacologically increasing sleep spindles enhances recognition for negative and high-arousal memories Journal of Cognitive Neuroscience, 25 (2013), pp. 1597–1610 [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, & Quan SF (2007). The AASM manual for the scoring of sleep and associated events: Rules, terminology, and technical specification, 1st ed. American Academy of Sleep Medicine, Westchester, IL. [Google Scholar]
- Jones BJ, Mackay A, Mantua J, Schultz KS, Spencer RMC The role of sleep in emotional memory processing in middle age Neurobiology of Learning and Memory, 155 (2018), pp. 208–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BJ, Schultz KS, Adams S, Baran B, Spencer RMC. Emotional bias of sleep-dependent processing shifts from negative to positive with aging Neurobiology of Aging, 45 (2016), pp. 178–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (2005). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Tech. Rep. A-6, Univ. Florida, Gainesville, FL. [Google Scholar]
- Lara-Carrasco J, Nielsen TA, Solomonova E, Levrier K, Popova A. Overnight emotional adaptation to negative stimuli is altered by REM sleep deprivation and is correlated with intervening dream emotions Journal of Sleep Research, 18 (2009), pp. 178–187 [DOI] [PubMed] [Google Scholar]
- Lehmann M, Seifritz E, Rasch B. Sleep benefits emotional and neutral associative memories equally Somnologie, 20 (2016), pp. 47–53 [Google Scholar]
- Lewis PA, Cairney S, Manning L, Critchley HD. The impact of overnight consolidation upon memory for emotional and neutral encoding contexts Neuropsychologia, 49 (2011), pp. 2619–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Calderon J, Luck SJ. ERPLAB: An open-source toolbox for the analysis of event-related potentials Frontiers in Human Neuroscience, 8 (2014), 10.3389/fnhum.2014.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykken DT, Venables PH. Direct measurement of skin conductance: A proposal for standardization Psychophysiology, 8 (1971), pp. 656–672 [DOI] [PubMed] [Google Scholar]
- Mantua J, Henry OS, Garskovas NF, Spencer RMC. Mild traumatic brain injury chronically impairs sleep- and wake-dependent emotional processing Sleep, 40 (2017), 10.1093/sleep/zsx062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory Cerebral Cortex, 19 (2009), pp. 1158–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PaceSchott EF, Shepherd E, Spencer RM, Marcello M, Tucker M, Propper RE, Stickgol R. dNapping promotes inter-session habituation to emotional stimuli Neurobiology of Learning and Memory, 95 (2011), pp. 24–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Kensinger EA, Wamsley EJ, Spreng RN, Alger SE, Gibler K, ..., Stickgold R. Napping and the selective consolidation of negative aspects of scenes Emotion, 15 (2015), pp. 176–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Stickgold R, Swanberg K, Kensinger EA. Sleep preferentially enhances memory for emotional components of scenes Psychological Science, 19 (2008), pp. 781–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, Born J. About sleep’s role in memory Physiological Reviews, 93 (2013), pp. 681–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A, Makam M, Kroeger D, Colas D, de Lecea L, Heller HC. Sleep to forget: Interference of fear memories during sleep Molecular Psychiatry, 18 (2013), pp. 1166–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: A network analysis of functional magnetic resonance imaging data Psychological Science, 20 (2009), pp. 74–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Rimmele U, Phelps EA, Davachi L. Emotional brain states carry over and enhance future memory formation Nature Neuroscience, 20 (2017), pp. 271–278 [DOI] [PubMed] [Google Scholar]
- Tempesta D, Socci V, De Gennaro L, Ferrara M. Sleep and emotional processing Sleep Medicine Reviews, 40 (2018), pp. 183–195 [DOI] [PubMed] [Google Scholar]
- van der Helm E, Yao J, Dutt S, Rao V, Saletin JM, Walker MP. REM sleep depotentiates amygdala activity to previous emotional experiences Current Biology, 21 (2011), pp. 2029–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Fischer S, Born J. Changes in emotional responses to aversive pictures across periods rich in slow-wave sleep versus rapid eye movement sleep Psychosomatic Medicine, 64 (2002), pp. 627–634 [DOI] [PubMed] [Google Scholar]
- Wagner U, Hallschmid M, Rasch B, Born J. Brief sleep after learning keeps emotional memories alive for years Biological Psychiatry, 60 (2006), pp. 788–790 [DOI] [PubMed] [Google Scholar]
- Walker MP, van Der Helm E. Overnight therapy? The role of sleep in emotional brain processing Psychological Bulletin, 135 (2009), pp. 731–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner GG, Schabus M, Blechert J, Kolodyazhniy V, Wilhelm FH. Pre- to postsleep change in psychophysiological reactivity to emotional films: Late-night REM sleep is associated with attenuated emotional processing Psychophysiology, 52 (2015), pp. 813–825 [DOI] [PubMed] [Google Scholar]