Abstract
Objectives:
Assess the potential role of differential diuretic use in preventing incident acute decompensated heart failure (ADHF) in SPRINT [Systolic Blood Pressure (SBP) Intervention Trial].
Background
SPRINT showed that intensive BP reduction in older patients (50–97 years) results in 36% fewer incident ADHF cases. However, some have questioned whether this was due merely to intergroup differences in diuretic medications.
Methods
Detailed medication use data prospectively collected throughout the trial were examined.
Results
ADHF events occurred in 173 of 9361 participants. Diuretic medication increased in both arms from screening to baseline visit (from 45% to 50% standard arm; 43% to 63% intensive arm) and then remained steady. The lowest use of diuretics was among participants in the standard arm who never had an ADHF event. Withdrawal of diuretics at baseline visit occurred in 6.1% (n=284) of standard arm and 2.3% (n=107) of intensive arm participants. Of these, only 11 developed ADHF during the trial (10 standard arm; 1 intensive arm), and only 1 occurred ≤1 month after diuretic withdrawal. The benefit of ADHF reduction remained significant even after excluding those 11 participants (HR: 0.69; CI: 0.5–0.94, p=0.02). Most ADHF events occurred in participants who were on prescribed diuretic at the last visit prior to the ADHF event. There was limited use of loop (<6%) and potassium-sparing diuretics (2%). Diuretic use was not a predictor of ADHF (HR: 0.96 [0.66–1.40], p-value 0.83).
Conclusion
We found no evidence that the reduction in new ADHF events in SPRINT was due to differential diuretic use.
Keywords: Systolic Hypertension, Heart Failure, Aging, Diuretics, SPRINT Clinical Trial
Introduction
Among older persons, systolic hypertension is one of the strongest risk factors for development of heart failure (HF). The large multicenter Systolic Blood Pressure (SBP) Intervention Trial (SPRINT) examined older patients (mean age 68, 28%≥ 75 years) with systolic hypertension and at high risk for cardiovascular disease (CVD) events. The trial, which was stopped early due to benefit, showed that intensive BP treatment targeting a SBP<120 mmHg, compared with standard BP treatment goal <140 mm Hg, reduced the primary outcome (a composite of a myocardial infarction [MI], acute coronary syndrome, stroke, acute decompensated HF [ADHF], or death from CV causes) by 25% and reduced total mortality by 27%.1 A major contributor to the strongly positive primary outcome was a statistically significant (p=0.002) 36% reduction in hospitalized ADHF events.2 Participants who developed ADHF had a 26-fold increased risk of subsequent CVD events and death.2 The intensive intervention was generally well tolerated, even in frail, older participants.3 These results represent an important new strategy for prevention of HF and are reflected in the 2017 ACC/AHA/HFSA guidelines.4
However, the validity of the SPRINT results has been questioned based on speculation that the ADHF events may have been due merely to withdrawal of diuretics from the standard treatment group5 and other differences in diuretic prescriptions.6 Participants in the standard arm were postulated to have had diuretics discontinued shortly after randomization, thereby ‘unmasking’ underlying HF, and the intensive group had increased diuretic use after randomization, thereby ‘masking’ or hiding the detection of HF.5 In order to test this hypothesis, we analyzed the large volume of detailed data regarding antihypertensive medication use that was prospectively collected during SPRINT.
METHODS
Study population
SPRINT design, methods, and primary results were previously reported in detail.1;7 Briefly, 9361 participants ≥50 years of age with SBP ≥130mm Hg, without a history of diabetes mellitus or stroke, and an increased risk of CV events were enrolled. Increased CV risk was defined by one or more of the following: clinical or subclinical CVD; chronic kidney disease (CKD); 10-year risk of CVD ≥15% by Framingham risk score; or age ≥75 years. Clinical CVD was defined as: a) previous MI, percutaneous intervention, coronary artery bypass grafting, carotid endarterectomy, or carotid stenting; peripheral arterial disease with revascularization; b) acute coronary syndrome with or without resting ECG change, ECG changes on an exercise test, or positive cardiac imaging study; c) at least a 50% diameter stenosis of a coronary, carotid, or lower extremity artery; or abdominal aortic aneurysm ≥5cm with or without repair. Subclinical CVD was defined as: a) coronary artery calcium score ≥400 Agatston units; b) ankle brachial index ≤0.90; c) left ventricular (LV) hypertrophy by ECG, echocardiogram report, or other cardiac imaging procedure. CKD was defined as estimated glomerular filtration rate (eGFR) <60ml/min/1.73m2 using the four-variable Modification of Diet in Renal Disease (MDRD) equation.1 Patients with symptomatic HF within 6 months or known LV ejection fraction (EF)<35% were excluded.
Intervention
Extensive details regarding the randomization and intervention have been previously reported.1;3;7 Participants were randomly assigned to have SBP treatment targeted to <140mmHg (n=4683) or <120mmHg (n=4678). Doses were increased and/or additional antihypertensive medications were added at monthly visits until SBP<120 mm Hg was reached or the investigator decided no further antihypertensive medications should be added. For participants in the standard-treatment group, medications were adjusted to target a SBP of 135–139 mm Hg, and the dose was reduced if SBP <130 mmHg on a single visit or <135 mmHg on two consecutive visits.7
Study Measurements
BP measurements were conducted using methods that were recommended by professional societies and BP guideline committees.8 SPRINT BP was the average of 3 BP measurements obtained using an automated measurement device (Omron 907XL) after a 5-minute quiet rest period and appropriate cuff size.7;8 Clinical information, laboratory data, and antihypertensive medications were recorded at screening and monthly for the first three months after randomization, then every three months for the duration of the trial.
Study Outcomes
Potential outcomes were assessed every 3 months in both treatment arms using a standard protocol that included a structured interview to minimize ascertainment bias7 with centralized monitoring by the coordinating center.
All clinical events, including ADHF, were formally adjudicated by a Morbidity and Mortality committee using a detailed manual of operations for adjudication based on that used and validated in the ARIC study.9 Adjudicators were blinded to treatment assignment. ADHF was defined as combination of: hospitalization or emergency department (ED) visit; a clinical syndrome that presented with multiple signs and symptoms (see below) consistent with cardiac decompensation and inadequate cardiac pump function; evidence in the treating physician’s notes that the primary reason for the hospitalization or ED visit was ADHF; new or increased treatment specifically for ADHF (which had to be intravenously administered diuretic or inotrope if ADHF was an ED visit); and documented response to therapy. Symptoms supporting ADHF included: evidence of increasing or new onset shortness of breath, peripheral edema, orthopnea, or paroxysmal nocturnal dyspnea. Signs supporting ADHF included: hypoxia; pulmonary rales on clinical examination; pulmonary vascular congestion on chest x-ray; elevation of biomarker B-type natriuretic peptide (BNP) or pro-N-terminal BNP (NTpro-BNP) above diagnostic threshold; reduced LVEF or diastolic dysfunction. Chronic stable HF was not considered an endpoint in SPRINT. Reduced LVEF in the absence of symptoms, right sided HF, and volume overload due to inadequate dialysis in patients with end-stage renal disease were not considered ADHF endpoints.
Statistical analyses
If a participant had multiple ADHF events, only the first event was used. Use of medication to manage BP was collected during screening (1–45 days prior to randomization); at treatment initiation (baseline), at first month, at second month, at third month and every three months during the trial. Medication use during the trial (ever vs. never) was compared between participants in each treatment arm, and between participants who experienced ADHF during the trial vs. those who did not. Continuous variables are presented as mean with standard deviation (SD) if normally distributed or as median with 25th-75th percentile range if not normally distributed; categorical variables are presented as number with percent. Medication use was compared with Student’s t-test or Wilcoxon rank sum test for continuous variables, and chi-squared test for discrete or categorical variables.
Diuretic use was ascertained from detailed medication logs which included the type of diuretics and patterns of use, including both addition and withdrawal of diuretics at baseline and during the trial. Medications taken during screening but stopped at the baseline visit as a consequence of randomization are referred to as withdrawn at baseline. Among the participants withdrawn from diuretics at baseline who subsequently developed ADHF during the trial, the number of months from baseline until ADHF event was determined. Time until first occurrence of ADHF was analyzed with the intention-to-treat approach with Cox proportional-hazards regression with two-sided tests at the 5% level of significance stratified by clinical site. Follow-up time was censored on the date of the last event ascertainment or end of trial. A covariate indicating use of diuretics for BP management during the trial was added to the model to evaluate the effect on ADHF. Diuretic use after an ADHF event was excluded. Statistical analyses were conducted at the coordinating center with the use of SAS software, v.9.4.
Results
Baseline characteristics were previously reported; age ranged 50–97 (mean 68±9) years.1;2 Overall, 173 participants developed ADHF during the trial: 105 in the standard arm; 68 in the intensive arm [HR: 0.63, {0.47–0.86}, p=value=0.004)] (Table 1; Figure 1). Baseline characteristics and medications use between the participants with and without ADHF during the trial were given in Supplemental table 1. Separation of the curves of ADHF events between the two arms became apparent at 6 months and increased steadily with no inflection point, including in the first 12 months when anti-hypertensive medications were the most actively titrated. ADHF event characteristics were similar in both arms (Table 1); ADHF was confirmed by elevated BNP or NT pro-BNP in 89% of participants.
Table 1.
Heart failure characteristics at the time of acute decompensation
| Symptoms | Overall (n=167)♦ | Treatment Arm | p-value | |
|---|---|---|---|---|
| Standard (n=101) | Intensive (n=66) | |||
| Increasing or new onset shortness of breath | 147 (88.0%) | 94 (93.1%) | 53 (80.3%) | 0.01 |
| at rest | 60 (43.2%) | 38 (45.2%) | 22 (40.0%) | 0.54 |
| with exertion | 82 (59.0%) | 52 (61.9%) | 30 (54.5%) | 0.39 |
| not specified | 41 (29.5%) | 25 (29.8%) | 16 (29.1%) | 0.93 |
| Increasing or new onset peripheral edema | 91 (54.5%) | 54 (53.5%) | 37 (56.1%) | 0.74 |
| Increasing or new onset paroxysmal nocturnal dyspnea | 29 (17.4%) | 15 (14.9%) | 14 (21.2%) | 0.29 |
| Increasing or new onset orthopnea | 60 (35.9%) | 35 (34.7%) | 25 (37.9%) | 0.67 |
| Signs | ||||
| Increasing or new onset hypoxia: 02 sat < 90% room air | 44 (26.3%) | 24 (23.8%) | 20 (30.3%) | 0.35 |
| Physician reported rales or crackles | 85 (61.2%) | 51 (60.7%) | 34 (61.8%) | 0.9 |
| Chest x-ray: pulmonary edema or venous congestion or vascular engorgement or congestion or HF | 89 (65.4%) | 55 (67.9%) | 34 (61.8%) | 0.46 |
| BNP* | 749 (407–1326) | 758 (459–1440) | 655 (392–984) | 0.66 |
| NT ProBNP≠ | 4734 (1590–12700) | 4357 (1928–11781) | 5109 (1060–12700) | 0.71 |
| Treatment | ||||
| Treated with additional medication | 159 (95.2%) | 96 (95.0%) | 63 (95.5%) | 0.91 |
| Intravenous or oral diuretics | 127 (96.9%) | 79 (98.8%) | 48 (94.1%) | 0.13 |
| Intravenous vasodilators pressors or inotropes | 8 (6.1%) | 4 (5.0%) | 4 (7.8%) | 0.51 |
| Beta blockers | 45 (28.5%) | 34 (35.4%) | 11 (17.7%) | 0.02 |
| Digitalis | 4 (2.5%) | 4 (4.2%) | 0 (0.0%) | 0.10 |
| ACE inhibitors or ARBs | 20 (15.3%) | 13 (16.3%) | 7 (13.7%) | 0.7 |
| Oral vasodilators | 12 (9.2%) | 6 (7.5%) | 6 (11.8%) | 0.41 |
HF=heart failure; ACE=angiotensin converting enzymes; ARB=angiotensin receptor blockers; BNP =Brain natriuretic peptide; NT pro BNP= N-terminal pro hormone BNP
BNP available in 54% (n=90) participants.
NT pro BNP available in 35% (n=59) participants [18 (11%) participants did not have biomarkers]
Please note: HF characteristics at the time of ADHF were available for non-fatal ADHF events and for fatal events where death did not occur within 7 days of the event.
Figure 1. Kaplan–Meier curves for development of ADHF by treatment group.
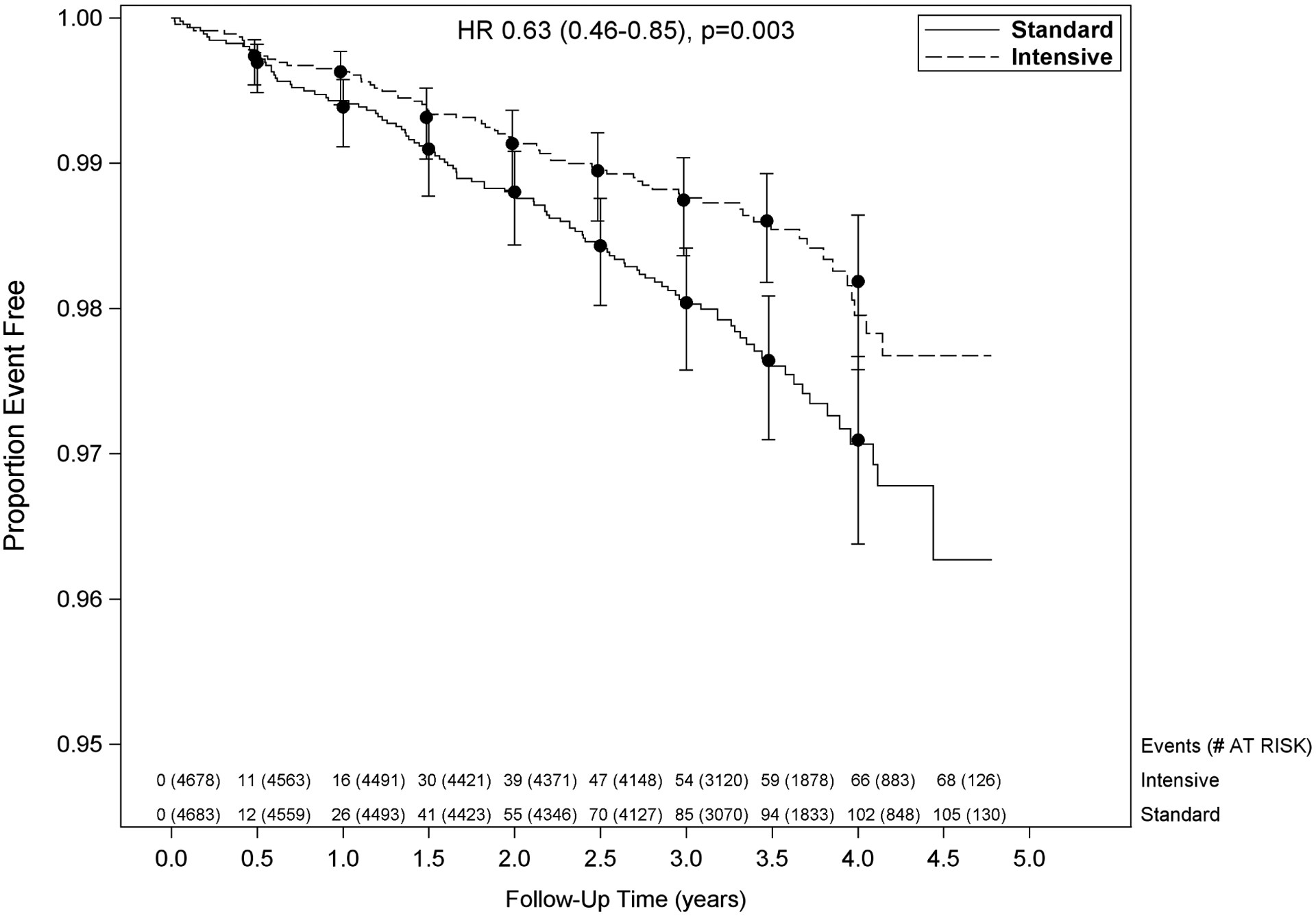
Vertical bars indicate 95% confidence intervals. P-value is from Cox proportional hazards model stratified by clinical site. Number at risk and number of events shown every 6 months.
Diuretic use for BP management over time
Classes of medication used during the trial are shown in Table 2 and Supplemental Table 2. The percentage of participants who were prescribed any diuretic, by visit and treatment arm, is shown in Figure 2. The percentage of participants prescribed diuretics in the standard arm increased from 45% during the pre-randomization screening period to 50% upon initiation of the intervention at baseline visit, before decreasing to an average of about 45% diuretic use throughout the rest of the trial. Prescription of diuretics in the intensive arm increased from 43% during screening to 63% at the baseline visit and then stabilized at about 70% during the remainder of the trial. As shown in Figure 2, both arms remained at a steady percentage of diuretic use throughout the course of the trial.
Table 2.
Blood Pressure Management Medication Use During the Trial by Randomized Group
| Medications | Overall (n=9361) | Treatment Arm | p-value | |
|---|---|---|---|---|
| Standard (n=4683) | Intensive (n=4678) | |||
| Any diuretic | 7335 (78.4%) | 3227 (68.9%) | 4108 (87.8%) | <.0001 |
| Any ACE-I or ARB | 7830 (83.6%) | 3628 (77.5%) | 4202 (89.8%) | <.0001 |
| Any beta blockers | 4413 (47.1%) | 2006 (42.8%) | 2407 (51.5%) | <.0001 |
| Any Calcium channel blockers | 6146 (65.7%) | 2670 (57.0%) | 3476 (74.3%) | <.0001 |
ACE-I =Angiotensin converting enzymes Inhibitors; ARB=Angiotensin II antagonists.
This table shows the medication and percentage that ever used from randomization until August 20, 2015
Figure 2: Percentage of participants prescribed diuretics at each time point by treatment arm.
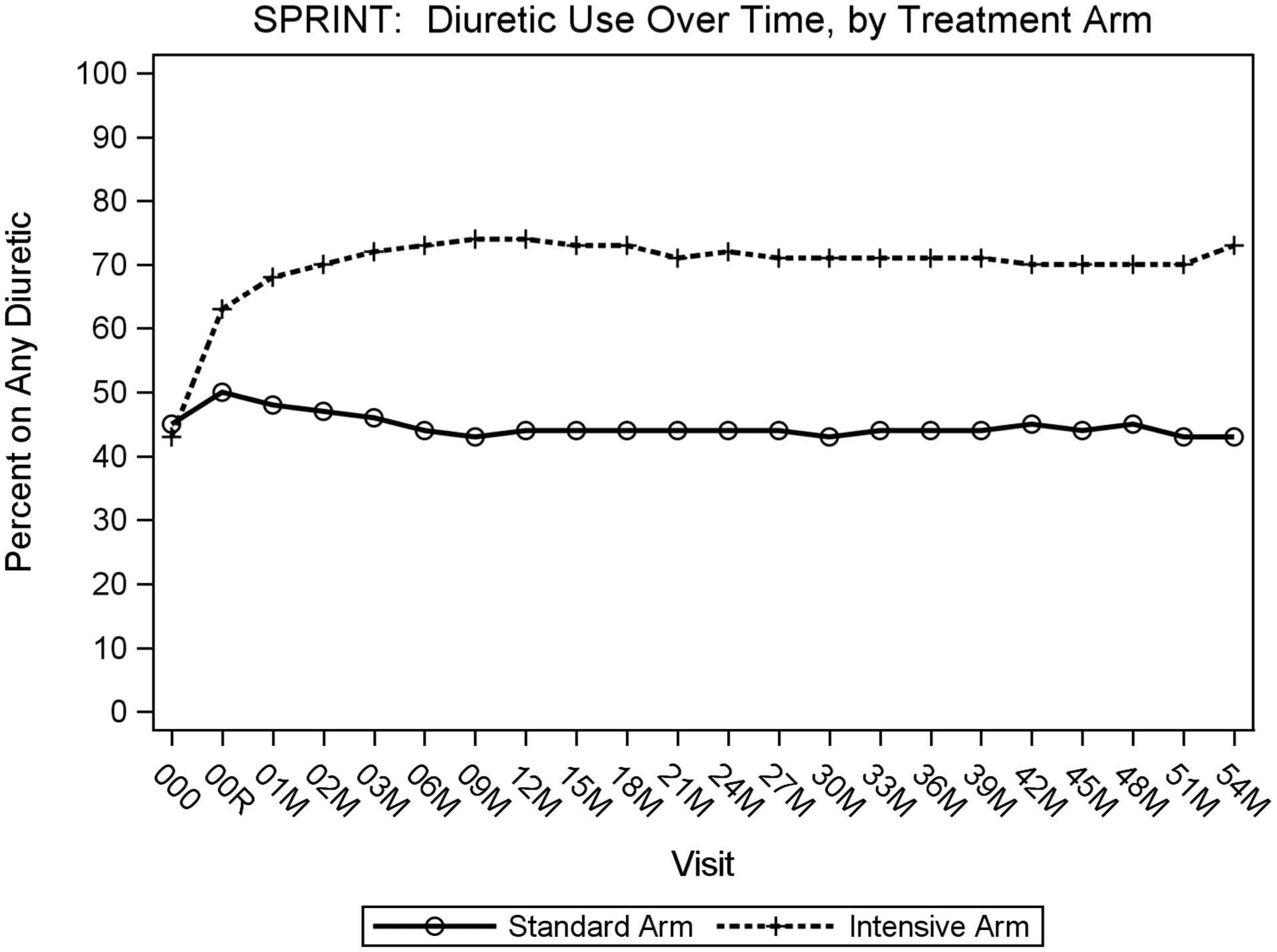
X-axis shows visit number in months from screening visit(000) and baseline visit(00R) to 60-months after baseline visit. Y-axis shows percentage of participants prescribed any diuretic.
In exploratory analyses, when adding diuretic use during the trial to the primary analysis, it was not a predictor of ADHF (HR: 0.96 [0.66–1.40], p-value 0.83). In addition, adding a variable for “withdrawal of diuretics at baseline” to the primary analysis did not change the relationship between treatment arm and ADHF (HR: 0.65, {0.48–0.88}, p-value=0.006), nor was the “withdrawal of diuretics at baseline” variable statistically significant (p-value=0.18). (Supplemental Table 3). Further examination of diuretic use during the trial by pre-specified subgroups indicated a similar prescription of diuretics among participants with or without CKD, CVD and age ≥ 75 years (Supplemental Table 4).
Withdrawal from Diuretics for BP Management
Among the 4,683 standard arm participants, 2098(45%) had a diuretic prescription at the time of screening and 284(6.1%) had diuretics withdrawn at baseline visit. Of these 284, 10(3.5%) developed ADHF at some point during the trial. Among the 4,678 Intensive arm participants, 1997(43%) had a diuretic prescription at the time of screening and 107 out of 4678(2.3%) had diuretics withdrawn at baseline visit. Of these 107, 1(0.9%) developed ADHF at some point during the trial. Among the 11 participants with ADHF who had diuretics withdrawn at baseline visit, the time to first on-study ADHF event after diuretics withdrawal is shown in Figure 3. Only two events occurred <6 months (0.8 and 3 months respectively) after the baseline visit and both were in the standard arm.
Figure 3. Scatter plot showing number of months until ADHF event for participants who developed ADHF after withdrawal of diuretics at baseline visit.
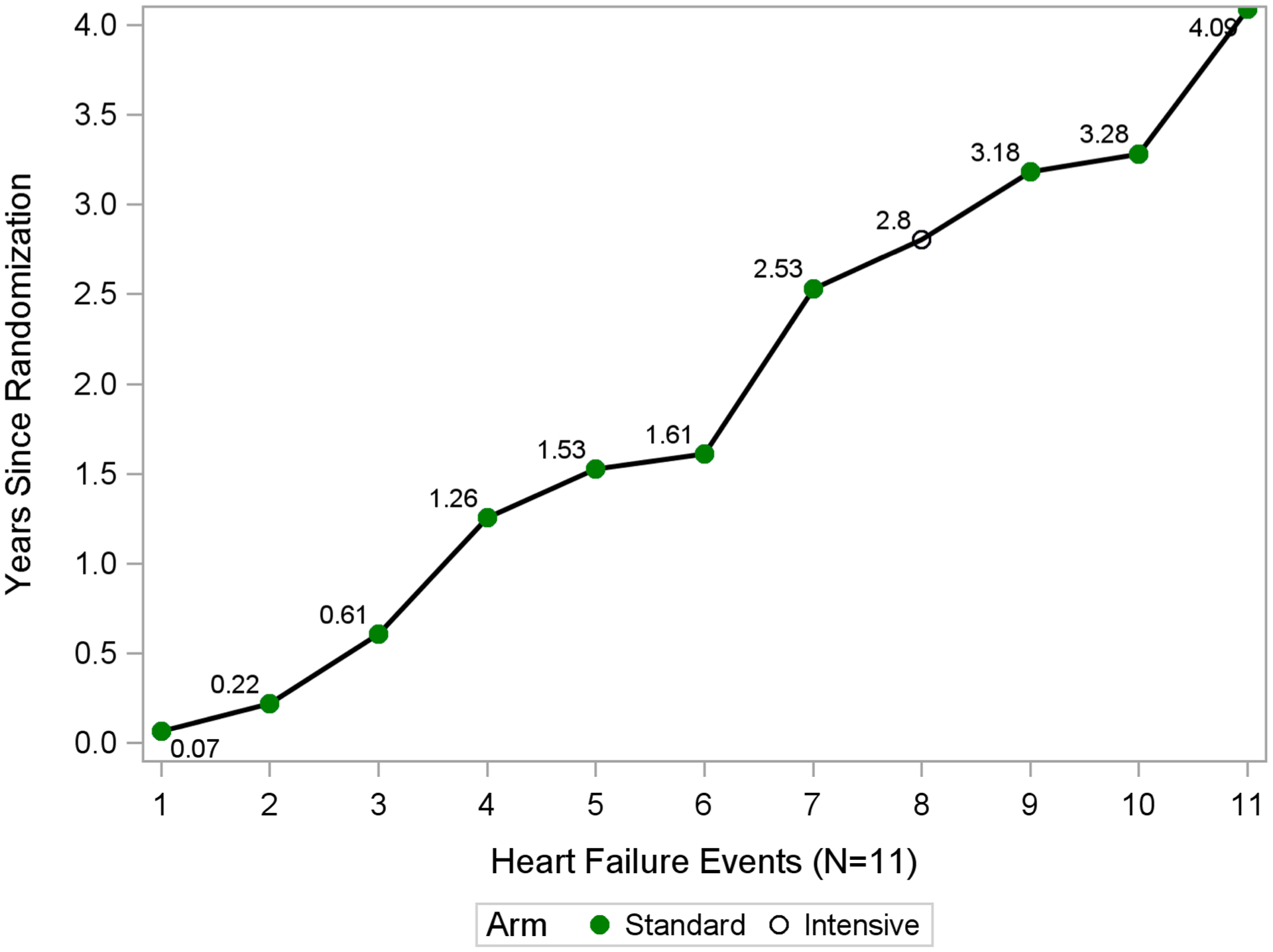
X-axis shows visit number in months; Y-axis shows percentage of participants prescribed any diuretic. Plot depicts four combinations of development of heart failure and treatment arm
Among the 173 participants who developed ADHF, diuretic use information within the year prior to the ADHF event was available for 165(95%). The 11 participants who had diuretic withdrawal after the baseline visit and developed ADHF during the trial represented only 6.3% of 173 participants who developed ADHF. In an analysis that excluded these 11 participants, there was minimal change in the magnitude of benefit of intensive BP reduction for reducing new ADHF events (exclusion of 11 people; HR: 0.69; CI: 0.5–0.94, p=0.02 and whole cohort; HR: 0.63; CI: 0.47–0.86, p=0.004). Similarly there was minimal change in the magnitude of benefit of intensive BP reduction for reducing new ADHF events even after excluding 391 (284+107) participants who had diuretics withdrawn from the primary analysis (exclusion of 391 people; HR: 0.66; CI: 0.48–0.90, p=0.01 and whole cohort; HR: 0.63; CI: 0.47–0.86, p=0.004).
Diuretic Use for BP management by ADHF and Treatment Arms
Diuretic use was more prevalent in participants who developed ADHF regardless of treatment arm compared to participants without an ADHF event (Supplemental tables 5, 6; Figures 4; supplemental Figure 1). Irrespective of treatment arm, participants with ADHF were more likely to have been prescribed diuretics for BP management during the trial than those without an ADHF event. The lowest use of diuretics was among participants in the standard arm who never had an ADHF event, while the group that most commonly used diuretics for BP management was those assigned to the intensive group who developed ADHF (Figure 4).
Figure 4. Percentage of participants prescribed diuretics by treatment arm at each time point.
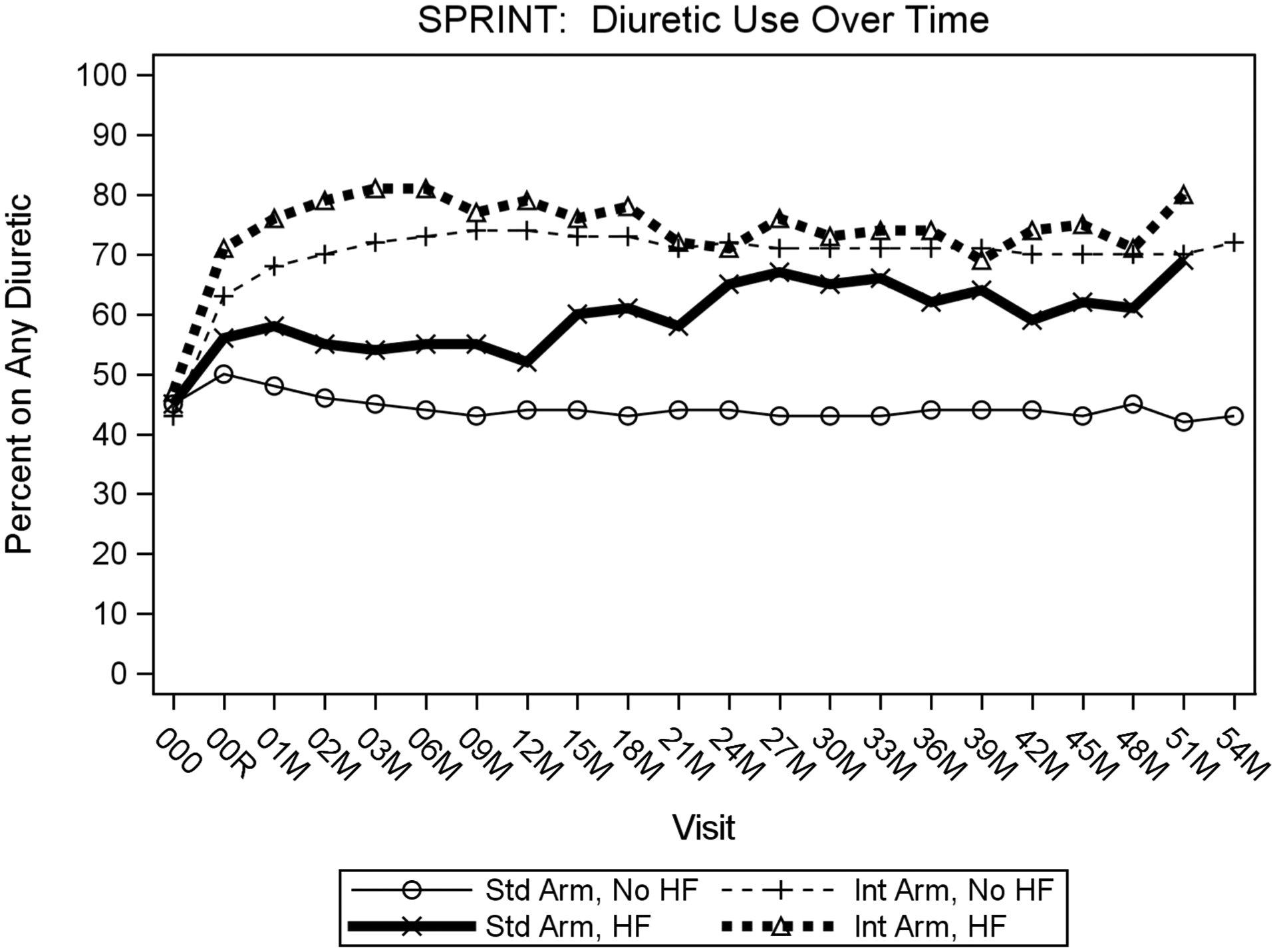
X-axis shows visit number in months; Y-axis shows percentage of participants prescribed any diuretic. Plot depicts four combinations of development of heart failure and treatment arm
Classes of Diuretic use for BP management
Thiazide (hydrochlorothiazide) and thiazide-like diuretics (chlorthalidone) were the most commonly used diuretics (71% of all diuretic use) for BP control in both treatment arms. As expected, intensive arm participants were prescribed thiazide diuretics at a higher rate than in the standard arm. The prescription of thiazide diuretics initially increased in both arms at baseline visit, with a higher rate of rise in the intensive arm. After this initial increase, both arms remained at a steady percentage use throughout the course of the trial (Supplemental Figure 2). Average daily dose of chlorthalidone was 20±9 mg with no significant intergroup dose difference. As shown in Supplemental figures 3 and 4, ~2% of participants in both arms were prescribed potassium sparing diuretics, with no difference by treatment arm. Similarly, <6% of participants in both arms were prescribed loop diuretics throughout the trial. There was a very gradual increase over time with no significant intergroup difference.
Other BP Medication Use by ADHF and Treatment arms
We also examined use of angiotensin converting enzyme inhibitors (ACE –I) and angiotensin receptor blockers (ARBs). Use of ACE-I and ARB increased from screening to the baseline visit in both treatment arms and was maintained at this level throughout the trial (Table 3).
Table 3:
ACE-I or ARBs use between two arms at screening, baseline and post baseline-12 months
| ACE-I | ARB | |
|---|---|---|
| At screening | ||
| Standard arm | 1693 (36%) | 992 (21%) |
| Intensive arm | 1763 (38%) | 993 (21%) |
| At baseline visit | ||
| Standard | 1797 (38%) | 1130 (24%) |
| Intensive | 2083 (45%) | 1245 (27%) |
| Post baseline -12 months | ||
| Standard | 2405 (51%) | 1710 (37%) |
| Intensive | 2766 (59%) | 2224 (48%) |
ACE-I =angiotensin converting enzyme inhibitors; ARB=angiotensin receptor blockers
In SPRINT, the preferred regimens included a thiazide type diuretic (hydrochlorothiazide or chlorthalidone), calcium channel blocker, ACE inhibitor or ARB based on CVD and hypertension practice guidelines.10 page in the intensive treatment arm used more classes of antihypertensive agents compared to those in the standard arm; the number of BP medications averaged 2.8(intensive arm) and 1.8(standard arm). As a result, all classes of antihypertensives agents were used more frequently in the intensive arm than the standard arm. But the distribution of antihypertensive medication classes used was similar in the two treatment groups (Table 2). Thus, the higher diuretic use in the intensive arm was not out of proportion to use of any other antihypertensive medication class. Similarly, participants who developed ADHF were more likely to be on prescribed diuretics, beta blockers, aldosterone antagonists or vasodilators (supplemental tables 5, 6) compared to those without ADHF.
Discussion
On average, diuretics were not withdrawn from standard arm participants at baseline or during the trial. Both treatment arms remained at a steady percentage of diuretic use, although higher in those assigned to intensive control throughout the course of the trial. Similarly, the relative distribution of antihypertensive medication classes, including diuretics, was similar between treatment groups. Thus, higher diuretic use in the intensive arm was not out of proportion to that of any other antihypertensive medication class. The lowest use of diuretics was among participants in the standard arm who never had an ADHF event. Among the 391 participants in whom diuretics were withdrawn at baseline visit, only 11 developed ADHF (and only 1 occurred ≤1 month after diuretic withdrawal). This represents only 6% of the 173 participants who developed ADHF; an analysis excluding these participants had minimal effect on the benefit of intensive BP reduction in reducing ADHF. In addition, in a separate analysis, diuretic use during the trial was not a predictor of ADHF. Further, the majority of ADHF events occurred in participants who were already on a prescribed diuretic at the time ADHF developed. These data indicate that the marked reduction in ADHF events among older patients randomized to intensive BP treatment in SPRINT was not due to differential diuretic use.
Multiple other lines of evidence further support the conclusion that the reduction in ADHF events in SPRINT was not an artifact of the study design or due to differential use of diuretics. The type (hydrochlorothiazide and chlorthalidone) and dose of diuretics used in SPRINT provide only modest diuresis and are generally ineffective treatments for ADHF. When these are used in ADHF it is in combination with loop diuretics in cases of diuretic resistance and refractory edema.4;11 Hydrochlochlorothiazide and chlorthalidone were the only thiazide/thiazide-like diuretics on the SPRINT formulary; metolazone was not included. Loop diuretics were not first-line drugs and were used very infrequently. Any ADHF event caused primarily by volume shifts from differential diuretic prescription or diuretic withdrawal would have been expected to occur relatively early after randomization, however most of the ADHF events in SPRINT occurred much later in the study. There was no sudden increase in ADHF events at any point in the trial, particularly in the first 6 months when anti-hypertensive medications were being actively titrated. Importantly, no ADHF events developed shortly (<2 weeks) after diuretic withdrawal.
Data from prior large landmark systolic hypertension trials, all of which used diuretics and found large reductions in ADHF events, also support the present conclusions.12–15 The Systolic Hypertension in the Elderly Program (SHEP) was a 2-arm study with thiazide diuretic as first step and a comparator arm of no treatment which resulted in a 100% difference in diuretic use between arms.12 Similar to SPRINT, several prior landmark HTN trials also adjusted or changed anti-hypertensives drugs upon entry.12–15 In ALLHAT, although chlorthalidone had somewhat greater effect on HF events compared to the doses used for amlodipine and lisinopril, this was explained by the fact that it had a greater effect on reducing SBP.15 Supporting the primacy of the degree of BP reduction for reducing HF events is a recent meta-analysis of randomized antihypertensive trials which showed that BP lowering by any of the five major classes of drugs significantly reduced the risk of new-onset HF.16 The meta-analysis concluded that the preventive effect of antihypertensive therapy on HF was primarily due to BP lowering effects and not to differences in drug classes.16 The fact that all classes of antihypertensive drug therapy reduce ADHF events provides additional compelling evidence that BP-lowering rather than drug-induced volume changes underpins the beneficial effect of antihypertensive drug therapy for HF prevention.16;17 Thus, our SPRINT findings are consistent with other systolic hypertension trials.
The magnitude of reduction in ADHF events in SPRINT is similar to that observed in the other large, landmark trials in older participants with systolic hypertension: 64% in HYVET; 50% in SHEP; and 36% in Syst-Eur.12–14 Overall, HF has been an important outcome in >35 prior hypertension treatment trials and in some has been a component of the primary outcome.16;17 In a recent meta-analysis, every 10mmHg reduction in SBP reduced the risk of HF by 28% (HR:0.72, 95%CI: 0.67–0.78,p<0.001).17 The proportional reductions per 10 mmHg decrease in SBP were greater for HF and stroke than for coronary disease, and there was a trend toward decreased HF events even with baseline SBP<130 mm Hg.17 Similarly, a meta-analysis of 35 hypertension trials that reported HF events showed a significant correlation between the degree of BP reduction and the reduction in HF events.16 In addition, Shapiro et al, showed that in SPRINT, SBP lowering appears to benefit across the spectrum of baseline SBP, even among those in the lowest tertile of SBP at baseline and even with low levels of DBP.18
ADHF events require a combination of progressive impairments in function of the heart, kidney, and other organs that result from chronic elevation of SBP and are retarded by reducing BP.2;16 While volume overload participates in the pathogenesis of ADHF, increased volume alone is generally not adequate to elicit an ADHF event. For instance, rapid infusion of very large volumes of normal saline directly into the central circulation in healthy persons results in only modest, transient increases in pulmonary capillary pressure and no symptoms.19;20 A healthy CV system accommodates excess volume by increasing pulmonary and systemic vascular compliance and by enhancing LV relaxation.21 Thus, development of ADHF events depends on the presence of multiple CV abnormalities, not merely or solely increased volume.2;16;17 Other abnormalities contributing to HF development include increased afterload, LV hypertrophy and fibrosis, ischemia, and increased arterial stiffness, as well as inflammation, oxidative stress and endothelial dysfunction.22
Some commentators have also questioned the validity of the ADHF diagnosis in SPRINT.23 As previously reported,24 ADHF events in SPRINT were based on well-established, objective, reliable criteria: high BNP or NTpro-BNP, chest x-ray showing lung congestion, pulmonary rales, and symptom relief following treatment for ADHF.2;9 These adjudication methods have been validated and used in many large landmark clinical trials and population studies.9;12;13;15 Although diagnosis of chronic HF in outpatients can be challenging, hospitalization for ADHF, the event used in SPRINT, is a less difficult and more objective outcome. Hospitalized ADHF has been used as an important outcome in more than 35 prior hypertension trials.12;13;15–17 In SPRINT, prognosis was markedly worsened after a positively adjudicated ADHF event, with a 27-fold increase in CV death and 10-fold higher risk of all-cause death.2 This further supports the validity of ADHF diagnosis in SPRINT, and as noted in the updated HF management guideline that cited SPRINT,4 highlights the importance of preventing new-onset ADHF in high-risk patients with appropriate BP reduction.
Limitations
SPRINT tested a treatment strategy for different SBP goals and did not test specific medications. The BP treatment protocol had an approved formulary but allowed flexibility in choice and doses of antihypertensive medications,10 which could confound analyses of relationships between medication use and study outcomes. SPRINT investigators could also prescribe other antihypertensive medications (not provided by the study). The treatment protocol encouraged, but did not mandate, the use of drug of different classes including diuretics. Participants with a history of ADHF or LVEF<35% were excluded from SPRINT, potentially limiting generalizability of our results, but enabling the study to focus on the important goal of ADHF prevention. Even though ADHF events that are caused primarily by volume shifts from diuretic withdrawal would usually be expected to have occurred relatively early after randomization, it is possible for ADHF events to also occur later, particularly with dietary sodium indiscretion which can be episodic. We did not assess the reasons why specific drugs were originally prescribed to participants before enrolment.
Conclusion
In SPRINT, intensive BP reduction markedly reduced new onset acute HF and improved mortality. The marked reduction in ADHF events was not merely due to withdrawal of diuretics from the standard treatment group or relative differential use of diuretics between groups. These results are concordant with hypertension trials that have shown that effective treatment of hypertension is a highly effective strategy for preventing HF, particularly among older adults in whom systolic hypertension and HF are highly prevalent and associated with poor outcomes.
Supplementary Material
Central Illustration. Intensive BP Reduction for Preventing Heart Failure: Potential Role of Diuretics.
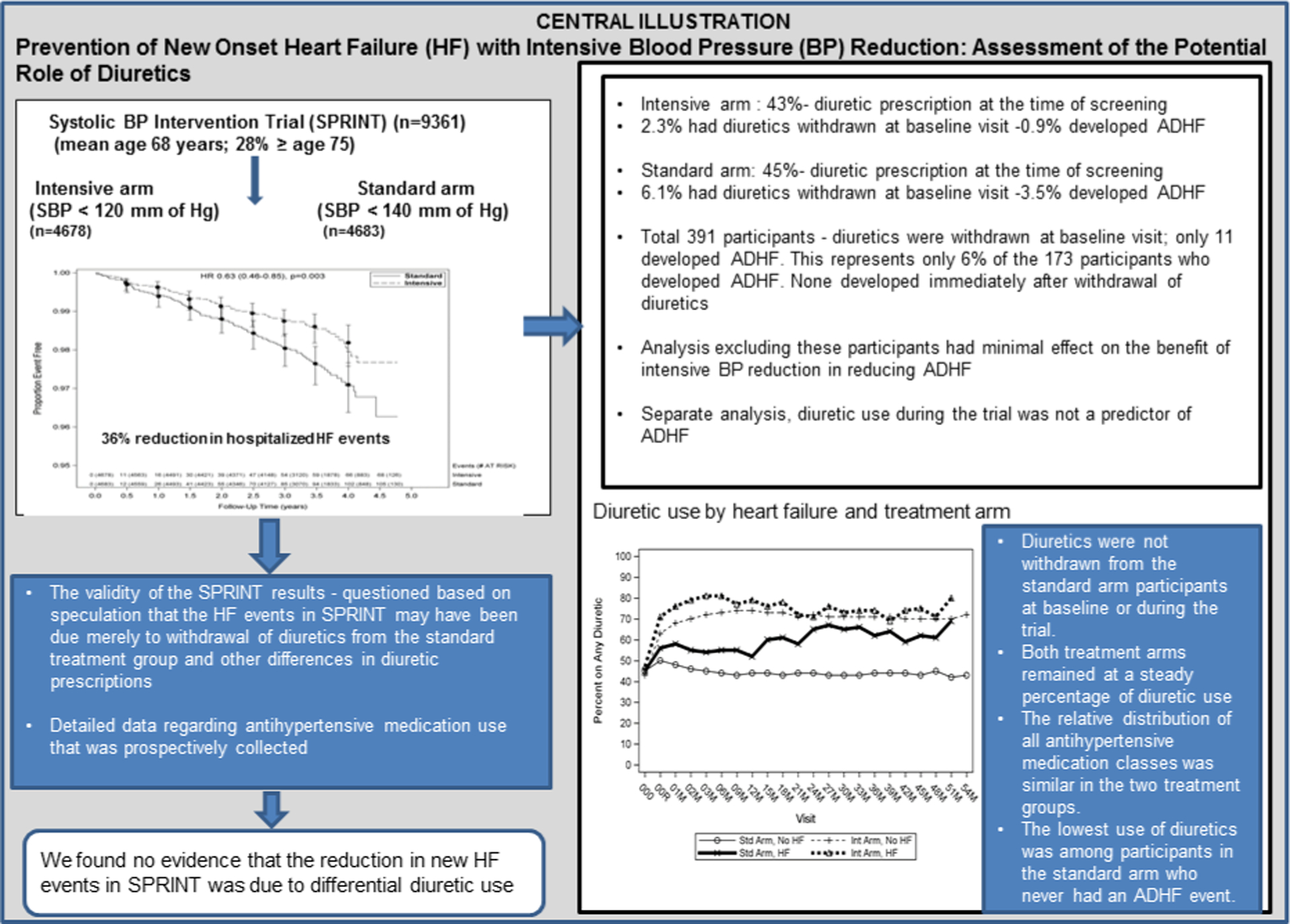
SPRINT showed that intensive blood pressure reduction in older patients results in 36% fewer incident acute decompensated heart failure events (ADHF). However, some have questioned whether this was due merely to intergroup differences in diuretic medications. Detailed medication use data prospectively collected throughout the trial were examined and we found no evidence that the reduction in new ADHF events in SPRINT was due to differential diuretic use.
Perspectives.
Competency in Medical Knowledge
The reduction in ADHF events in SPRINT was not due to differential use of diuretics. Our results are concordant with a large body of evidence from prior hypertension trials where effective hypertension treatment was highly effective for prevention HF, particularly among older adults.
Translational Outlook
Identifying the optimal BP target for treatment of older adults with systolic hypertension is a priority for healthcare providers, because of the high prevalence of hypertension in this population and the more adverse outcomes compared with younger persons.
Sources of Funding:
National Institutes of Health (NHLBI, NIDDK, NIA, NINDS) Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN2682009000 49C, and Inter-Agency Agreement A-HL-13-002-001. Also resources and facilities through Department of Veterans Affairs. Also supported by CTSA awards from NCATS: UL1TR000439; UL1RR025755; UL1RR024134; UL1TR000003; UL1RR025771; UL1TR000093; UL1RR025752; UL1TR000073; UL1TR001064; UL1TR000050; UL1TR000005; 9U54TR000017-06; UL1TR000105-05; UL1 TR000445; UL1TR000075; UL1 TR000002; UL1 TR000064; UL1TR000433; P30GM103337 COBRE Award NIGMS. Also in part by NIH grants R01AG18915, R01HL107257, P30AG021332, U24AG05964, R01AG045551 and Kermit G. Phillips II Chair, Wake Forest School of Medicine.
Disclosures:
Dr. Kitzman: Consultant:Abbvie, Bayer, Merck, Medtronic, Relypsa, Merck, Corvia Medical, Boehringer-Ingelheim, GSK, AstraZeneca, CinRx, Novartis, Actavis. Grant funding: Novartis, Bayer, and AstraZeneca. Stock ownership: Gilead Sciences.
Dr. Upadhya: Novartis; Corvia.
Dr.Kostis: Consultant: Abbott, Abbvie and Sanofi. Grant funding: Sanofi, AstraZeneca, Hamilton Health Sciences, Bayer.
Other authors: none
Abbreviations list:
- ACE-I
angiotensin converting enzyme inhibitors
- ADHF
acute decompensated heart failure
- ARBs
angiotensin receptor blockers
- BNP
B-type natriuretic peptide
- BP
blood pressure
- CCB
calcium channel blockers
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- HF
heart failure
- LV
left ventricle
- MI
myocardial infarction
- NIH
National Institues of Health
- NT pro-BNP
N-terminal pro BNP
- SBP
systolic blood pressure
- SPRINT
systolic blood pressure intervention trial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trials Information: ClinicalTrials.gov number, NCT01206062
References
- (1).Wright JT Jr, Williamson JD, Whelton PK et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Upadhya B, Rocco M, Lewis CE et al. Effect of intensive blood pressure treatment on heart failure events in the systolic blood pressure reduction intervention trial. Circ Heart Fail 2017;10:e003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Williamson JD, Supiano MA, Applegate WB et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged >/=75 Years: A Randomized Clinical Trial. JAMA 2016;315:2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Yancy CW, Jessup M, Bozkurt B et al. 2017. ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. J Am Coll Cardiol 2017;[In print]. [Google Scholar]
- (5).Kjeldsen SE, Narkiewicz K, Hedner T, Mancia G. The SPRINT study: Outcome may be driven by difference in diuretic treatment demasking heart failure and study design may support systolic blood pressure target below 140 mmHg rather than below 120 mmHg. Blood Press 2016;25:63–66. [DOI] [PubMed] [Google Scholar]
- (6).Jones DW, Weatherly L, Hall JE. SPRINT: What Remains Unanswered and Where Do We Go From Here? Hypertension 2016;67:261–262. [DOI] [PubMed] [Google Scholar]
- (7).Ambrosius WT, Sink KM, Foy CG et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014;11:532–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Johnson KC, Whelton PK, Cushman WC et al. Blood Pressure Measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension 2018;71:848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Rosamond WD, Chang PP, Baggett C et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail 2012;5:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).James P, Oparil S, Carter B. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (jnc 8). JAMA 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- (11).Felker GM, Lee KL, Bull DA et al. Diuretic Strategies in Patients with Acute Decompensated Heart Failure. N Engl J Med 2011;364:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Kostis J, Davis BR, Cutler JA, et al. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA 1997;278:212–216. [PubMed] [Google Scholar]
- (13).Beckett S, Peters R, Fletcher AE et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887–1898. [DOI] [PubMed] [Google Scholar]
- (14).Staessen JA, Fagard R, Thijs L et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997;350:757–764. [DOI] [PubMed] [Google Scholar]
- (15).Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- (16).Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure-lowering treatment. 6. Prevention of heart failure and new-onset heart failure - meta-analyses of randomized trials. J Hypertens 2016;34:373–384. [DOI] [PubMed] [Google Scholar]
- (17).Ettehad D, Emdin CA, Kiran A et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957–967. [DOI] [PubMed] [Google Scholar]
- (18).Shapiro BP, Ambrosius WT, Blackshear JL et al. Impact of Intensive Versus Standard Blood Pressure Management by Tertiles of Blood Pressure in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension 2018;71:1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Fujimoto N, Borlaug BA, Lewis GD et al. Hemodynamic Responses to Rapid Saline Loading: The Impact of Age, Sex, and Heart Failure. Circulation 2013;127:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Andersen MJ, Olson TP, Melenovsky V, Kane GC, Borlaug BA. Differential hemodynamic effects of exercise and volume expansion in people with and without heart failure. Circ Heart Fail 2015;8:41–48. [DOI] [PubMed] [Google Scholar]
- (21).Cheng CP, Igarashi Y, Little WC. Mechanism of augmented rate of left ventricle filling during exercise. Circ Res 1992;70:9–19. [DOI] [PubMed] [Google Scholar]
- (22).Cohen RA, Tong X. Vascular oxidative stress: the common link in hypertensive and diabetic vascular disease. J Cardiovasc Pharmacol 2010;55:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Kjeldsen SE, Mariampillai JE, Nilsson PM. Optimal Blood Pressure Target in Diabetic and Nondiabetic Hypertensive Patients. Circ Res 2018;123:528–530. [DOI] [PubMed] [Google Scholar]
- (24).Oparil S, Cushman WC, Johnson KC, Kitzman DW, Whelton PK, Wright JT Jr. SPRINTING toward the optimal blood pressure target for hypertensive patients. Circulation 2018;123:531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


