Abstract
Previous studies indicate that musical instrument training may improve the cognitive function of older adults. However, little is known about the neural origins of training‐related improvement in cognitive function. Here, we assessed the effects of instrumental training program on cognitive functions and neural efficiency in musically naïve older adults (61–85 years old). Participants were assigned to either the intervention group, which received a 4‐month instrumental training program using keyboard harmonica, or a control group without any alternative training. Cognitive measurements and functional magnetic resonance imaging during visual working memory (VWM) task were administered before and after the intervention in both groups. Behavioral data revealed that the intervention group significantly improved memory performance on the test that measures verbal recall compared to the control group. Neuroimaging data revealed that brain activation in the right supplementary motor area, left precuneus, and bilateral posterior cingulate gyrus (PCgG) during the VWM task decreased after instrumental training only in the intervention group. Task‐related functional connectivity (FC) analysis revealed that the intervention group showed decreased FC between the right PCgG and left middle temporal gyrus, and between the left putamen and right superior temporal gyrus (lPu‐rSTG) during a VWM task after the intervention. Furthermore, a greater improvement in memory performance in the intervention group was associated with a larger reduction in lPu‐rSTG FC, which might be interpreted as improved neural efficiency. Our results indicate that the musical instrument training program may contribute to improvements in verbal memory and neural efficiency in novice older adults.
Keywords: aging, instrumental intervention, neural efficiency, randomized controlled trial, verbal memory
The study demonstrated increased neural efficiency (decreased task‐related brain activation and functional connectivity) in older adults after the instrumental training program. Moreover, this was accompanied by improved behavioral performances in non‐musical verbal memory. The present findings provide new causal evidence for instrumental training‐related plasticity in older adults.
1. INTRODUCTION
Aging is associated with declines in certain cognitive functions, such as processing speed, working memory (WM), and episodic memory (Nyberg, Lövdén, Riklund, Lindenberger, & Bäckman, 2012; Park et al., 2002; Park & Reuter‐Lorenz, 2009). Such age‐related cognitive reduction has emerged as one of the significant threats to elderly health (Hebert, Scherr, Bienias, Bennett, & Evans, 2003; Hebert, Weuve, Scherr, & Evans, 2013). However, some studies support the idea that protection against age‐related perceptual and cognitive decline may occur through engagement in specific activities or training (Anderson, White‐Schwoch, Parbery‐Clark, & Kraus, 2013; Fratiglioni, Paillard‐Borg, & Winblad, 2004; Hall et al., 2009; Scarmeas & Stern, 2003; Small, Dixon, McArdle, & Grimm, 2012), which can maintain or even enhance “cognitive reserve” (Akbaraly et al., 2009; Scarmeas & Stern, 2003; Stern, 2012; Stern et al., 1994), a key factor that can explain individual differences in preserved cognitive functions among elderly people.
Training to play a musical instrument is one of the effective activities that can help preserve cognitive functions in elderly people. Playing a musical instrument is a multimodal cognitive activity that requires the integration of complex sensory information with processes associated with attention, fine motor control, memory storage and retrieval, and emotion (Herholz & Zatorre, 2012; Janata & Grafton, 2003; Koelsch, 2014; Zatorre, Chen, & Penhune, 2007). In fact, accumulated evidence indicated the effects of performing multimodal cognitive activities, such as playing musical instruments, on various cognitive functions. For example, musicians had greater working memory capacity than age‐matched nonmusician controls (Parbery‐Clark, Skoe, Lam, & Kraus, 2009). Interestingly, adults who received music training before the age of 12 years had a better verbal memory than those who did not (Chan, Ho, & Cheung, 1998). Furthermore, studies have raised the possibility that complex multimodal interventions, such as dual task‐based multimodal exercise (Nishiguchi et al., 2015) or music‐based multitask training (Hars, Herrmann, Gold, Rizzoli, & Trombetti, 2014), induce more extensive cognitive transfer effects than does a strategy‐focused approach that is relevant to single cognitive domain (Basak, Boot, Voss, & Kramer, 2008; Noice, Noice, & Staines, 2004; for a review, see Lustig, Shah, Seidler, & Reuter‐Lorenz, 2009). Thus, musical training has emerged as a valuable model for the investigation of training‐related brain plasticity (Herholz & Zatorre, 2012; Kraus & Chandrasekaran, 2010; Münte, Altenmüller, & Jäncke, 2002; Wan & Schlaug, 2010; Zatorre, 2005).
While most studies have focused on investigating the effects of musical training on children's cognitive function (Fujioka, Ross, Kakigi, Pantev, & Trainor, 2006; Guo, Ohsawa, Suzuki, & Sekiyama, 2018; Moreno et al., 2009; Moreno et al., 2011; Schellenberg, 2004), there is evidence that music training may help keeping the adult brain in a younger state (Rogenmoser, Kernbach, Schlaug, & Gaser, 2018), and may reduce age‐related declines in cognitive and neural functions (for reviews, see Kraus & White‐Schwoch, 2014; Wan & Schlaug, 2010). Consistent with this idea, an epidemiological study reported that elderly people who frequently played a musical instrument were less likely to develop dementia than those who participated in other types of leisure activities (Verghese et al., 2003). In a cross‐sectional study, the association between long‐term musical instrumental participation and cognitive aging was investigated; it was found that older musicians are superior to age‐matched nonmusicians on nonverbal memory, naming, and executive function (Hanna‐Pladdy & MacKay, 2011). Parbery‐Clark and colleagues (Parbery‐Clark, Strait, Anderson, Hittner, & Kraus, 2011) also demonstrated that musicians show less age‐related decline in cognitive ability, including better speech‐in‐noise perception and auditory WM capacity. Furthermore, the musical experience is reported to offset the age‐related decline in neural responses to speech (Parbery‐Clark, Anderson, Hittner, & Kraus, 2012; White‐Schwoch, Carr, Anderson, Strait, & Kraus, 2013).
Further evidence comes from several randomized controlled trials (RCTs) and longitudinal intervention studies. However, the previous findings on whether musical instrument training may improve cognitive functions in healthy older adults are mixed. For example, an RCT study by Bugos, Perlstein, McCrae, Brophy, and Bedenbaugh (2007) demonstrated that musically naïve healthy older adults who received 6 months of individualized piano instruction improved executive function when compared to a control group that did not receive such lessons. In a non‐RCT intervention study, Seinfeld, Figueroa, Ortiz‐Gil, and Sanchez‐Vives (2013) found that 4 months of a piano lesson program improved executive function in healthy older adults. Our research team also conducted an RCT study to investigate the effects of musical instrument training on verbal memory and other cognitive functions in older adults (Wada et al., 2017), using keyboard harmonica as an intervention instrument. After 3 months of intervention, the intervention group significantly improved verbal memory but did not improve executive function compared to the untrained control group. Thus, while the previous studies have suggested cognitive improvements in older adults following a musical instrument training program, the exact cognitive domains (i.e., executive function or verbal memory) showing an intervention effect are likely to vary depending on the contents of the training program. An additional RCT is needed to confirm a link between keyboard harmonica training and improvement in verbal memory.
A more important point to be pursued is the effect of musical instrument training on brain activity in elderly people. At the task‐based functional level, several cross‐sectional studies have reported an “increase” in neural activity in musicians compared to nonmusicians. For example, pianists exhibit higher functional coupling between auditory (superior temporal gyrus) and motor areas (premotor cortex) compared to nonmusicians, when listening to piano music or when playing piano music without auditory feedback (Bangert et al., 2006; Baumann et al., 2007; Jäncke, 2012). Musicians also showed better WM performance, and larger BOLD responses in neural networks associated with sustained attention and cognitive control than nonmusicians during the WM task of musical sounds. Moreover, compared to nonmusicians, musicians had a more positive relationship between WM task performance and BOLD signals in several regions, including the right putamen, right supplementary motor cortex, right insula, and right middle cingulate gyrus (Pallesen et al., 2010). Another neuroimaging study reported that musicians activated specific subcomponents only during verbal (insula) or only during tonal WM (basal ganglia and cerebellum), and a positive correlation was found between the musicians' performance during the tonal WM and activation in the basal ganglia and cerebellum (Schulze, Zysset, Mueller, Friederici, & Koelsch, 2011).
The “increase” of neural activity in the aforementioned studies has been observed in a cross‐sectional comparison where the increased neural activity was typically associated with a better task performance. In contrast, another study has reported a “decrease” in neural activity caused by music training (Chen, Rae, & Watkins, 2012), where the decreased neural activity along with comparable or better task performance can be interpreted as improved neural efficiency. For example, in a longitudinal study by Chen et al. (2012), brain activation in the right superior temporal gyrus was reduced post‐ compared to pretraining in musically naïve younger adults listening to trained melody; similarly, brain activation in the left dorsal and ventral premotor cortex was reduced post‐ compared to pretraining when playing melodies. Importantly, decreased brain activation in the premotor cortex was associated with increased melody learning performance scores, which suggests increased efficiency in the neural processing of a learned stimulus (Chen et al., 2012). Other non‐musical intervention studies have also shown decreased neural activity after longitudinal comparison (Heinzel et al., 2014; Nishiguchi et al., 2015; Schneiders, Opitz, Krick, & Mecklinger, 2011). Taken together, these findings indicated that the training‐related effects are likely to be better reflected by decreased rather than by increased neural activity, as depicted in studies with a longitudinal design.
While the training‐related decrease in neural activity has been reported for young participants' musical instrumental training, RCT studies targeting older adults have not yet assessed the effect of musical instrument training on brain activity, especially brain activation during a general cognitive task other than musical‐based auditory or motor task. WM is among the first to be affected by dementia (Huntley & Howard, 2010), and the n‐back task is the most commonly used experimental paradigm for functional neuroimaging of WM (e.g., Grady, Yu, & Alain, 2008; Heinzel, Lorenz, Duong, Rapp, & Deserno, 2017; Suzuki et al., 2018; for a review, see Rottschy et al., 2012). Therefore, in this study, we used an n‐back task of non‐music stimuli to investigate the potential improvement of neural efficiency ‐ decreased neural activity with comparable behavioral performance. In fact, using n‐back tasks combined with functional magnetic resonance imaging (fMRI), our previous study (Nishiguchi et al., 2015) could successfully detect improved neural efficiency caused by multimodal exercise intervention, pointing to the efficacy of a WM task to measure neural efficiency caused by the intervention. Consistent with this finding, a longitudinal study using the n‐back intervention revealed specific training‐related decreases in the activity in the right middle frontal gyrus after training (Schneiders et al., 2011).
In the present study, we conducted an RCT to investigate whether a 16‐week keyboard harmonica instrument training (Key‐HIT) program could improve cognitive performance and neural efficiency measured by fMRI in musically naïve healthy older adults. To reduce the difficulty of playing a musical instrument such as bimanual coordination for older adults with no musical experience, based on our previous behavioral studies (Guo et al., 2018; Wada et al., 2017), we used Key‐HIT program in which an instrument usually played with one hand is used (Supplementary Figure 1). Based on the previous findings described above, we hypothesized that after the Key‐HIT program, participants in the intervention group would exhibit improvement in cognitive functions, especially in verbal memory (Wada et al., 2017), and less brain activation in the regions associated with WM (Nishiguchi et al., 2015). In addition to investigating regional brain activity, we also focused on functional connectivity (FC) during the WM task. Playing a musical instrument requires information integration between different brain regions, and FC provides information on how anatomically distinct brain regions work cohesively (Friston, 2011). We therefore predicted that the intervention group show FC changes during the WM task in the regions associated with both WM and the playing of a musical instrument and the correlations between FC changes and behavioral changes.
2. MATERIALS AND METHODS
2.1. Participants and procedure
Sixty‐six Japanese older adults (53 females), 61–85 years of age, participated in this study (Figure 1). They were recruited at the Kyoto City Sakyo Elderly Welfare Center, Japan. The center had approximately 1,000 users (eligibility was 60 years or older) who gathered regularly for various leisure activities such as table tennis, hula dance, light exercise, magic tricks, hand craft, painting, and so on. Three participants were excluded after the pretest because they scored more than two standard deviations (SD) below their age‐appropriate means in the Wechsler Memory Scale‐Revised (WMS‐R) Logical Memory II. Because the Japanese version of the WMS‐R does not provide standardized means for participants above the age of 75, age means were acquired from Kawano (2012). The remaining 63 participants were divided into either the intervention group (n = 30) or control (n = 33) group in a pseudo‐randomized manner to balance socio‐demographics (sex, age, education years), Mini‐Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975), exercise frequency (measured with a social lifestyle questionnaire), and memory (delayed verbal recall). The intervention group participants received a 1‐h keyboard harmonica lesson once per week for 16 weeks. Control group participants did not receive any music‐related lesson during the intervention phase, and received only health education at the beginning of the intervention in order to maintain the social relationship between the researchers and the participants and maintain the motivation of study participation. They were instructed to spend their time as usual during the intervention phase. The participants of each group were informed what the other group was doing during the intervention period. Ten participants were excluded from the analysis because they either did not attend the posttest, dropped out due to health problems, turned out to have experience in musical training, had a history of depression, showed brain damage on structural MRI, or had a participation rate in the keyboard harmonica lesson under two‐thirds. Therefore, 53 participants were included in the final statistical analysis. Twenty‐seven participants in the intervention group (21 females, mean age 73.3 ± 5.66 years old) and 26 in the control group (21 females, mean age 72.85 ± 5.15 years old) were analyzed. They were all right‐handed on the Edinburgh handedness inventory (Oldfield, 1971), had normal or corrected‐to‐normal vision, had no history of neurological or psychiatric disorders, and no or less than three‐year experience in musical performance training. Applicants with instrumental music training in the recent 5 years were declined. According to recruitment policy, the participants in the control group were provided with the same keyboard harmonica lesson after completion of the study. The ethics committee of Kyoto University approved this study. All participants provided written informed consent.
FIGURE 1.
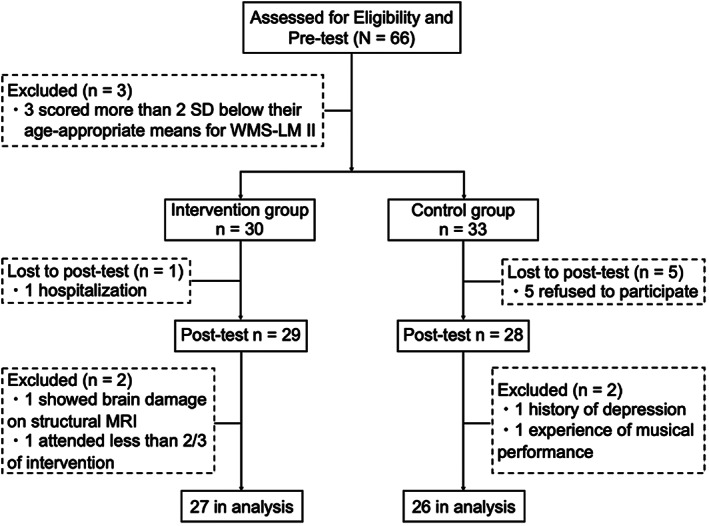
Study flow diagram. MRI, magnetic resonance imaging; SD, standard deviation; WMS‐LM II, Wechsler Memory Scale‐Revised Logical Memory II test
Before and after the intervention, questionnaires on mental health and social lifestyle and a series of behavioral measurements were administered to all participants in a session separate from the fMRI scan session. The same tests were performed in the pretest and posttest. Behavioral measurements consisted of the MMSE (Folstein et al., 1975), the WMS‐R Logical Memory (Sugishita, 2000; Wechsler, 1987), the Digit Span (DS; Wechsler, 1997), the Verbal Fluency Test (VFT; Lezak, Howieson, Bigler, & Tranel, 2012), the Trail Making Test (TMT; Reitan, 1992), the Pegboard Test (PEG; Sakai Medical CO. Ltd., Tokyo, Japan), the World Health Organization Five Well‐Being Index (WHO‐5; http://www.who-5.org/), and the Kessler Psychological Distress Scale (K6; Kessler et al., 2002). In the fMRI scan session, we performed the n‐back task to measure VWM. In addition, a questionnaire to assess the outing frequency of two groups during the intervention phase was administered after the intervention.
2.2. Behavioral measurements
2.2.1. Screening test
The MMSE (Folstein et al., 1975) was a 30‐point paper‐based test that is used to measure global cognitive function (cut‐off point, 23/24).
2.2.2. Cognitive and motor function
The Logical Memory (LM), from the Japanese version of WMS‐R (Sugishita, 2000; Wechsler, 1987), was used to assess verbal memory. In this test, two short stories were read aloud to the participants. They were then required to recall each story immediately (WMS‐LM I, immediate recall) and 30 min after the first recall (WMS‐LM II, delayed recall). Phrases correctly recalled were scored.
The DS was a subtest from the Wechsler Adult Intelligence Scale‐III (WAIS III; Wechsler, 1997) to assess WM. This test asked participants to repeat a sequence of digits in the same order (digit span forward) or in the reverse order (digit span backward) immediately after the examiner orally gave a sequence.
The VFT was used to assess verbal functioning (e.g., Lezak et al., 2012). For the VFT, participants were asked to produce as many words as possible from a category or letter in 60 seconds. This test consisted of two tasks: category fluency asked participants to produce names from a specified category (i.e., animals), and the letter fluency asked participants to produce words beginning with a specified letter (i.e., “か”).
The TMT (Reitan, 1992) was used to measure executive function. This test consisted of two parts: in part A, participants were instructed to connect 25 numbered circles in ascending order; part B was more difficult than part A and required to connect numbers and letters in an alternating fashion (“1” → “あ (a)” → “2” → “い (i)”, and so on). Time to complete each task was measured.
The PEG (Sakai Medical CO. Ltd., Tokyo, Japan) was used to measure manual dexterity. This test asked participants to turn all 20 pegs (diameter 0.5 cm × height 3.5 cm) up‐side‐down as fast as possible with each of the left and right hand, and the performance time was recorded.
2.2.3. Mental health
The WHO‐5 was a 5‐item questionnaire that is used to measure current mental well‐being. The K6 (Kessler et al., 2002) was a 6‐item scale that is used to measure psychological distress.
2.2.4. Social lifestyle questionnaire
The social lifestyle questionnaire included five question items: (i) frequency of cognitive activities (painting, reciting poems, etc.), (ii) frequency of exercise, (iii) frequency of volunteer or paid work, (iv) frequency of family care (parents, grandchildren, etc.), (v) lifestyle (“no cohabitation” or “cohabitation”).
2.2.5. Outing frequency questionnaire
The outing frequency (such as leisure activities, volunteer or paid work, shopping, visiting relatives, and so on) of the two groups during the intervention phase was examined by a questionnaire on actual calendar records only in the posttest (less than 0.5 times/week = 1, 0.5 to less than 1.5 times/week = 2; 1.5 to less than 2.5 times/week = 3; 2.5 to less than 4.5 times/week = 4; more than 4.5 times/week = 5).
2.3. fMRI scan session
2.3.1. Image acquisition
Whole‐brain imaging was performed on a 3 T Siemens Magnetom Verio MRI scanner (Siemens, Erlangen, Germany). Functional images were acquired with a T2*‐weighted echo‐planar imaging (EPI) sequence (39 axial slices; thickness = 3.5 mm; in‐plane resolution = 3.5 × 3.5 mm; repetition time [TR] = 2000 ms; echo time [TE] = 25 ms; flip angle = 75°; field of view [FOV] = 224 × 224 mm; matrix = 64 × 64). The first five volumes were discarded to allow for T1 equilibration effects. After the functional scan combined with VWM task, a resting‐state fMRI scan was also recorded (results not reported here). The high‐resolution brain structural images were collected using a T1‐weighted, 3D magnetization prepared rapid‐acquisition gradient echo (MP‐RAGE) pulse sequence (voxel size = 1 × 1 × 1 mm3; 208 axial slices; FOV = 256 × 256 mm; matrix = 256 × 256).
2.3.2. Task‐related fMRI
A block‐design fMRI was conducted using an n‐back task for face stimuli (Figure 2; Kawagoe et al., 2015; Nishiguchi et al., 2015). We used only 1‐back and 0‐back tasks due to following two reasons. First, our previous study showed that 2‐back task using the stimuli of faces, numbers, or dots (location memory) are too demanding for older adults (accuracy below 70%; Kawagoe & Sekiyama, 2014). Second, we wanted to maintain relatively high accuracy of behavioral performance during fMRI scanning, in order to derive meaningful and valid interpretations on neural efficiency (i.e., decreased neural activity with comparable behavioral performance) from the block design fMRI experiment. In our previous study (Nishiguchi et al., 2015), we were able to successfully detect improved neural efficiency caused by a multimodal intervention, using the 1‐back task with the faces and dots stimuli. Therefore, based on our previous studies (Kawagoe & Sekiyama, 2014; Nishiguchi et al., 2015), we decided to use 1‐back and 0‐back tasks with facial stimuli in the present study.
FIGURE 2.
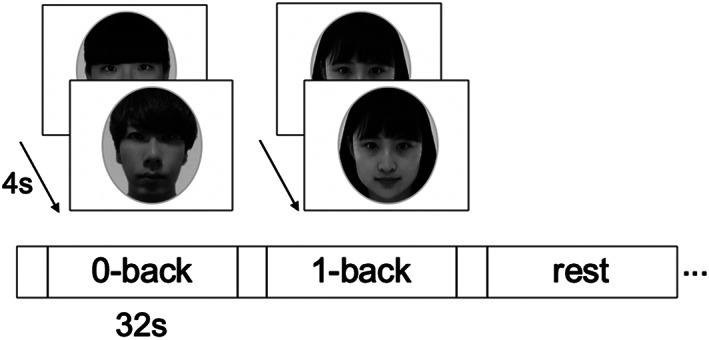
Experimental design of the n‐back tasks. This task was composed of 4 blocks for the 0‐back condition, 4 blocks for the 1‐back condition, and 4 blocks for the rest condition (a total of 12 blocks). Each block consisted of eight trials, lasting a total of 32 s, preceded by instruction lasting 4 s. In the 0‐back task, participants were instructed to respond when the displayed face stimuli disappeared. In the 1‐back task, participants were asked to monitor a series of stimuli and to indicate whether the stimulus was the same as the one presented 1‐trial prior. In the rest condition, participants were instructed to maintain their attention on a fixation cross in the middle of screen. s, second
The face stimuli were composed of neutral faces of Japanese university students (26 females and 26 males). They were presented in a sequence, where each stimulus appeared on the screen for 2000 ms, with an SOA (stimulus onset asynchrony) of 4,000 ms. A black central fixation cross (+) was presented between the stimuli. If participants could not respond until the next stimulus presentation, the response was recorded as a miss. Before the fMRI scanning, participants were given practice trials outside the scanner with stimuli different from those used during fMRI. In the 0‐back task, participants were instructed to respond when the displayed face stimuli disappeared by pressing the left button with an index finger of the right hand using an MRI‐compatible keypad. In the 1‐back task, participants were asked to monitor a series of stimuli and to indicate whether the stimulus was the same as the one presented 1‐trial before. The responses were to be made with the index (“same”) and middle (“different”) finger of the right hand. Both hit and correct rejection responses (for the same and different trials, respectively) were considered as correct. Participants were instructed to respond as quickly as possible without sacrificing accuracy. While we note that the repetition of the same facial stimulus in the 1‐back task is likely to cause sensory adaption in brain regions responsible for face processing (e.g., fusiform gyrus), we emphasize that our interest is to examine intervention‐related changes in brain activity in the regions associated with VWM. In the rest condition, participants were instructed to relax and keep their attention on a fixation cross in the middle of the screen. Three conditions (1‐back, 0‐back, and rest) were blocked and the blocks were alternated in one fMRI run. The run was composed of 12 blocks (4 blocks for each condition) in total. Each block consisted of eight trials lasting a total of 32 s, preceded by instruction lasting a total of 4 s.
2.4. Keyboard harmonica instrument training (Key‐HIT)
The keyboard harmonica is a keyboard instrument that produces sound by exhalation. In the present study, we used a 32‐key keyboard harmonica instrument (YAMAHA P‐32EP, alto‐model; Supplementary Figure 2). The Key‐HIT program was implemented by a professional piano teacher and 4–5 teaching assistants. The intervention group participants were divided into two classes with 15 participants per class. Both classes were taught by the same teacher and teaching assistants successively on the same day (a 1‐h group lesson per week for 16 weeks). In addition, the participants were asked to practice every day as much as possible and to record the practice time per day on a distributed diary.
The Key‐HIT program provided instructions regarding reading basic music scores, finger dexterity/independence exercises, and progressive difficulty in musical performance. Reading music scores included note reading and a corresponding fingering, positional relationship between notes and key position on the keyboard, duration of note and rest, music symbols, and so on. Finger exercises were practiced by playing ascendant and descendent progressions of the musical scale and melodic intervals. Regarding the musical performance, first, participants were taught how to use their breath to produce a sound with a keyboard harmonica. Next, participants were taught to play simple melodies. For example, how to reproduce familiar melodies that were mostly composed of only five musical notes (C‐D‐E‐F‐G; all using white keys) that do not require a hand position change. Then, the complexity and range of the melody were increased gradually. Participants were taught how to play melodies that contained dotted notes and hand position changes. Gradually, they learned to extend their fingers up or down from the Middle C‐Position and to change their hand position to play the melodies; they also learned to play the melodies with accidentals (sharp ♯ or flat ♭). Finally, participants practiced ensemble and added playing with intended expressive dynamics. In addition, we distributed a few novel and more challenging music scores each time, starting from the tenth lesson, for participants who wanted more stimulating practice at home.
In each lesson, teachers first checked the previous week's practice records before the lesson began. Then, the lesson began with finger exercises. Next, the contents learned in the previous lesson were reviewed. After that, finger gymnastics were administered as a break of performance practice. In the latter half of the lesson, participants learned new music theory knowledge and songs. Specifically, to learn how to play a new song, participants first listened to the song played by the teacher, then participants attempted to sing the lyrics. Next, they were taught to sing with the melody (by using note names). Then, participants were taught the music theory knowledge of the learned song. After that, in order to play the melody with precise rhythms, participants were asked to sing with the melody, while playing the rhythm with their hand clapping. Finally, participants were taught to play the melody on the keyboard harmonica.
In total, 11 songs familiar to participants were learned during 16 lessons and participants practiced on average 266.58 min (SD = 178.27) per week across these 16 weeks. All participants in the intervention group were able to play” Goin' Home” (excerpt from Symphony No. 9; Antonín Dvořák, 1893),” Ode to Joy” (excerpt from Symphony No. 9; Ludwig van Beethoven, 1824),” Furusato (Home)” (Teiichi Okano, 1914) and” Aura Lee” (George Rodway Poulton, 1861) at the final musical performance.
2.5. Statistical analysis
2.5.1. Behavioral data analysis
Demographic data were compared using a two‐sample t‐test between the intervention and control groups to evaluate whether the pseudorandom grouping resulted in homogeneous groups. The intervention effects on the behavioral measures were determined using a two‐way mixed ANOVA with group (intervention and control groups) as a between‐subject factor and time (before and after intervention) as a within‐subject factor. When interactions were significant, the simple effects were investigated for each group. For all analyses, p < .05 was considered statistically significant.
Multiple tests may induce a type I error for overestimating significant effects under no‐correction or a type II error for underestimating significant effects under correction. Therefore, we conducted a permutation test (Nichols & Holmes, 2002) for the validation of original interaction effects detected under the uncorrected α‐level threshold. For each behavioral measure, all of the 106 observed samples (53 participants × 2 times) were randomized together and were resampled to obtain a dummy group‐by‐time data. These data were introduced into the same two‐way mixed ANOVA as in the original ANOVA to obtain a dummy F‐value for the group‐by‐time interaction. This procedure was repeated 10,000 times for each of the 13 behavioral measures. We pooled a total of 130,000 F‐values (10,000 resampling×13 measures) and created a unique permutation F‐distribution to obtain the single adjusted α‐level threshold (the top five percentile rank in the distribution) of the F‐value.
Finally, a Pearson's correlation analysis was performed to investigate the relationship between cognitive changes (only for those in which the interaction was significant) and the practice time of keyboard harmonica instrument in the intervention group.
2.5.2. Task‐related fMRI data preprocessing and statistical analysis
Functional data preprocessing and statistical analyses were conducted using Statistical Parametric Mapping 12 (SPM12, Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB R2016b (MathWorks), except for the FC analysis (see below). The preprocessing steps included (i) slice‐timing correction for acquisition time differences, (ii) time series image realignment of all images to the first functional image to correct for head motion, (iii) coregistration of the functional images to the T1 structural image, (iv) spatial normalization of functional images to a Montreal Neurological Institute (MNI) space, and (v) smoothing with an 8‐mm full width at half maximum Gaussian filter.
For the task‐related fMRI data collected in the blocked design, activated voxels in each experimental condition (0‐back task, 1‐back task, and rest) were modeled using a statistical model containing a boxcar function convolved with a canonical hemodynamic response function. A high‐pass filter (1/128 Hz) was used to remove low‐frequency noise, and an autoregressive (AR [1]) model was employed to correct for temporal autocorrelations. First, in order to identify the brain regions related to the VWM in pretest, we carried out an SPM12‐based whole brain analysis using a one‐sample t‐test for 1‐back task vs. 0‐back task with the pre data of all participants (N = 53), where the participant was treated as a random effect. Significance levels were set at p < .05 family‐wise error (FWE) corrected at the voxel level. Next, to examine the intervention effects on brain activation, using the MarsBaR toolbox, parameter estimates were extracted from spherical regions of interest (ROIs) centering on the local maxima peak of significant clusters (for overly large cluster, we also used its sub‐clusters) with an 8‐mm radius from the regions identified in the aforementioned analysis. We then used a two‐way mixed ANOVA in IBM SPSS Statistics software (version 23, SPSS, Inc) to determine whether the group‐by‐time interactions were significant. Validation tests for original interaction effects were also performed in a permutation method. For each ROI, all of 106 observed samples for the pre‐ and postintervention periods in both participant groups were randomized together and were resampled to obtain a dummy group‐by‐time data (53 participants × 2 times). These data were introduced into the same two‐way repeated‐measure ANOVA as in the original ANOVA to obtain, in particular, the F‐value for the group‐by‐time interaction. This procedure was repeated 10,000 times for each of the 14 ROIs. We pooled a total of 140,000 F‐values and created a permutation F‐distribution to obtain the single adjusted α‐level threshold (the top five percentile rank in the distribution) of the F‐value.
2.5.3. Functional connectivity MRI preprocessing and statistical analysis
Task‐related FC data analysis was performed using the seed‐to‐voxel analysis (i.e., seed‐based approach with all other brain voxels) in CONN toolbox 18.b (https:// www.nitrc.org/projects/conn). The analyses compute the correlation between the mean time series from a given seed and the average time series at other voxels (seed‐to‐voxel analysis). Seeds were defined based on the previous studies and results of the brain activation (see below). Task‐related FC data were preprocessed within the toolbox. The preprocessing steps included: (i) subject motion estimation and correction; (ii) functional center to (0,0,0) coordinates; (iii) functional slice‐timing correction; (iv) ART (the Artifact Rejection Toolbox)‐based identification of outlier scans for scrubbing; (v) segmentation of functional image into gray matter, white matter, and cerebrospinal fluid (CSF), and MNI normalization; (vi) structural center to (0,0,0) coordinates; (vii) segmentation of structural image into gray matter, white matter, and CSF, and MNI normalization; (viii) spatial smoothing with an 8‐mm Gaussian kernel. Data were bandpass filtered (0.008–0.09 Hz). First‐level analyses were performed using the general linear model within CONN. Second‐level analyses were performed using a 2x2 mixed ANOVA interaction in CONN to assess the intervention effects (group × time interaction) for each of the 1‐back and 0‐back conditions. The statistical thresholds were set at p < .001 uncorrected for multiple comparisons at the voxel level, and p < .05, FWE corrected for multiple comparisons at the cluster level.
2.5.4. Seed selection
Based on the results of the 1‐back >0‐back contrast of the task‐related fMRI analysis, four regions that showed an intervention effect (i.e., group x time interaction) were selected as seeds (spherical regions with 8 mm radius). The four regions and their center MNI coordinates were: the right supplementary motor area (SMA) (6, 24, 48); the left precuneus (−4, −64, 48); the left posterior cingulate gyrus (PCgG) (−4, −28, 30); and the right PCgG (10, −30, 34).
In addition to these four regions, the putamen was selected as a seed region. The putamen is associated with both musical instrument performance (Vaquero et al., 2016; Wollman, Penhune, Segado, Carpentier, & Zatorre, 2018) and WM (Pallesen et al., 2010; Rottschy et al., 2012; Salmi, Nyberg, & Laine, 2018; Voytek & Knight, 2010). Activity in the putamen is also known to be associated with repetitive and well‐learned movements that require little cognitive effort (Haber, 2016). Therefore, the putamen is the candidate region that can reflect changes in FC caused by the musical instrument training during the VWM task. The left and right putamen were defined based on the FSL Harvard‐Oxford atlas maximum likelihood subcortical atlas provided by the CONN toolbox.
2.5.5. Correlation analysis
To evaluate whether changes in task‐related FC were related to improvements in behavioral outcomes, we used a Pearson's correlation analysis to investigate the relationship between task‐related FC changes and cognitive changes. For cognitive changes, we focused on the cognitive measurements that measured WM (DS, WMS‐LM I) or showed an intervention effect (WMS‐LM II) with ANOVA. For FC changes, extraction of individual‐level FC change values (beta values; post‐pre) was performed within CONN. The extracted values and cognitive change scores (post‐pre) were then processed with the IBM SPSS Statistics software for correlation analyses. Analyses were performed separately for the two groups. Validation tests for significant correlations were also performed in a permutation method. Because degrees of freedom differed between the two participant groups, the validation test was separately conducted for each group. Initially, we combined two significant FC data sets (rPCgG‐lMTG FC and lPu‐rSTG FC) randomly, resampling them to produce dummy data (26 and 27 FC changes for the control and the intervention groups). The two resampled data sets were repeatedly correlated with behavioral data. We pooled a total of 60,000 coefficients (10,000 resampling × 2 FC × 3 behavioral data) and created a permutation |r|‐distribution to obtain the single adjusted α‐level threshold (the top five percentile rank in the distribution) of the |r|‐value.
3. RESULTS
3.1. Demographics
Demographic data for the intervention and control groups are shown in Table 1. There were no significant differences in the age, sex, years of education, MMSE, and exercise frequency between the two groups in the pretest period, indicating the two groups were equivalent. Furthermore, concerning the frequency of outings during the intervention phase, no significant difference was found between the two groups (Table 2). Results of the other behavioral data for the intervention and control groups are shown in Table 3. No significant differences were found between the two groups at the time of pretest (except for TMT‐A), suggesting that cognitive functions were virtually comparable between the two groups before the intervention period (results of t‐tests are summarized in Supplementary Table 1).
TABLE 1.
Demographic data for intervention and control groups at the time of the pretest. Values are mean (SD), unless otherwise indicated
| Intervention (n = 27) | Control (n = 26) | p‐value | |
|---|---|---|---|
| Number of males/females | 6/21 | 5/21 | |
| Age | 73.30 (5.66) | 72.85 (5.15) | .768 |
| Years of education | 13.00 (2.05) | 13.08 (2.11) | .896 |
| MMSE | 28.85 (1.38) | 28.46 (1.08) | .267 |
| Exercise a | 3.48 (1.29) | 3.62 (1.21) | .704 |
Frequency of exercise: less than 0.5 times per week = 1; 0.5 to less than 1.5 times per week = 2; 1.5 to less than 2.5 times per week = 3; 2.5 to less than 4.5 times per week = 4; more than 4.5 times per week = 5. MMSE, Mini‐Mental State Examination; SD, standard deviation.
TABLE 2.
Outing frequency (mean [SD]) of studied groups during the intervention phase
| Intervention (n = 27) | Control (n = 26) | p‐value | |
|---|---|---|---|
| Outing frequency a | 4.48 (0.63) | 4.27 (0.71) | .264 |
Frequency of outing: less than 0.5 times per week = 1; 0.5 to less than 1.5 times per week = 2; 1.5 to less than 2.5 times per week = 3; 2.5 to less than 4.5 times per week = 4; more than 4.5 times per week = 5. SD, standard deviation.
TABLE 3.
Behavioral outcomes of the intervention and control groups. Values are mean (SD)
| Measures | Intervention (n = 27) | Control (n = 26) | Group × time | |||
|---|---|---|---|---|---|---|
| Pretest | Posttest | Pretest | Posttest | F‐value | p‐value | |
| WMS‐LM I | 20.07 (5.33) | 23.63 (5.89) | 19.69 (5.28) | 21.39 (6.69) | 2.281 | .137 |
| WMS‐LM II | 14.85 (5.22) | 20.33 (6.50) | 14.96 (6.60) | 17.04 (6.94) | 5.582 | .022* |
| VFT (letter) | 12.30 (3.60) | 11.63 (2.75) | 12.12 (3.07) | 12.39 (3.57) | 1.015 | .319 |
| VFT (animal) | 17.00 (3.90) | 17.78 (4.77) | 17.73 (3.84) | 18.27 (4.43) | 0.036 | .851 |
| DS | 12.56 (2.15) | 13.48 (2.39) | 13.35 (2.70) | 13.50 (2.83) | 1.616 | .210 |
| TMT‐A (seconds) | 43.90 (16.58) | 38.02 (10.41) | 35.76 (10.28) | 34.52 (10.39) | 1.494 | .227 |
| TMT‐B (seconds) | 107.76 (38.86) | 100.21 (35.70) | 92.00 (39.77) | 90.71 (34.26) | 0.447 | .507 |
| Δ TMT (seconds) | 63.86 (29.23) | 62.19 (31.60) | 56.25 (34.91) | 56.19 (29.56) | 0.038 | .846 |
| PEG (left hand; seconds) | 42.46 (7.52) | 42.66 (7.92) | 42.14 (7.79) | 41.23 (8.68) | 0.520 | .474 |
| PEG (right hand; seconds) | 38.99 (6.87) | 38.16 (6.00) | 38.35 (5.66) | 37.76 (6.95) | 0.044 | .836 |
| WHO‐5 | 18.22 (4.82) | 17.37 (5.14) | 17.39 (3.48) | 16.77 (4.17) | 0.044 | .835 |
| K6 | 4.44 (4.52) | 5.93 (4.94) | 4.42 (3.38) | 5.00 (3.77) | 0.472 | .495 |
| 1‐back correct (%) | 93.8 (7.5) | 93.8 (7.31) | 92.6 (12.6) | 91.3 (12.8) | 0.253 | .617 |
Abbreviations: DS, digit span; K6, Kessler Psychological Distress Scale; PEG, Pegboard Test; SD, standard deviation; TMT, Trail Making Test; VFT, Verbal Fluency Test; WMS‐LM, Wechsler Memory Scale‐Revised Logical Memory; WHO‐5, World Health Organization Five Well‐Being Index.
3.2. Effects of intervention on verbal memory
Among the 13 behavioral measures, only the WMS‐LM II (Figure 3), as a measure of delayed verbal recall, showed an improvement in the intervention group (mean and SD in the intervention group: pretest = 14.85 ± 5.22, posttest = 20.33 ± 6.50; mean and SD in the control group: pretest = 14.96 ± 6.60, posttest = 17.04 ± 6.94). The initial ANOVA yielded a significant group‐by‐time interaction [F (1, 51) = 5.582, p = 0.022, generalized η 2 = 0.018], and this F‐value was higher than the adjusted α‐level (.05) threshold F (1, 51) = 4.003 obtained in the permutation test (Table 3). The simple main effects analysis on time revealed that both groups had significantly improved WMS‐LM II performances at the posttest period [intervention: F (1, 26) = 24.969, p < 0.001, generalized η 2 = 0.178; control: F (1, 25) = 5.026, p = 0.034, generalized η 2 = 0.023], suggesting that delayed LM performance could be affected by repeated testing. However, we emphasize that the significant group‐by‐time interaction indicates that the improvement of WMS‐LM II scores in the intervention group cannot be explained by only the repetition effect. Instead, the improvement in WMS‐LM II is likely to be caused by the combination of the intervention effect and the repetition effect. Furthermore, although the simple main effects analysis on group revealed no significant between‐group difference at the pretest period [F (1, 51) = 0.004, p = 0.948, generalized η 2 < 0.001], the between‐group comparison of the posttest performances suggested that the intervention group, compared to the control group, showed a marginally higher WMS‐LM II scores [F (1, 51) = 3.065, p = 0.086, generalized η 2 = 0.057]. Our interpretation of the combination of the intervention effect and repetition effect was also supported by the planned comparison of difference scores (post minus pre) between the groups [intervention: 5.48 ± 5.59; control: 2.08 ± 4.63; t (51) = 2.363, p = 0.022, d = 0.663]. As regards the association between improvement in WMS‐LM II and practice time with the keyboard harmonica at home, there was no significant correlation (r = −0.246, p = 0.217). A significant main effect of time was found on the WMS‐LM I [F (1, 51) = 18.093, p < 0.001, generalized η 2 = 0.048] without an interaction effect [F (1, 51) = 2.281, p = 0.137, generalized η 2 = 0.006], raising the possibility that immediate LM performance was affected by repeated testing. The remaining 11 measures yielded no significant group‐by‐time interaction or time effects (Table 3).
FIGURE 3.
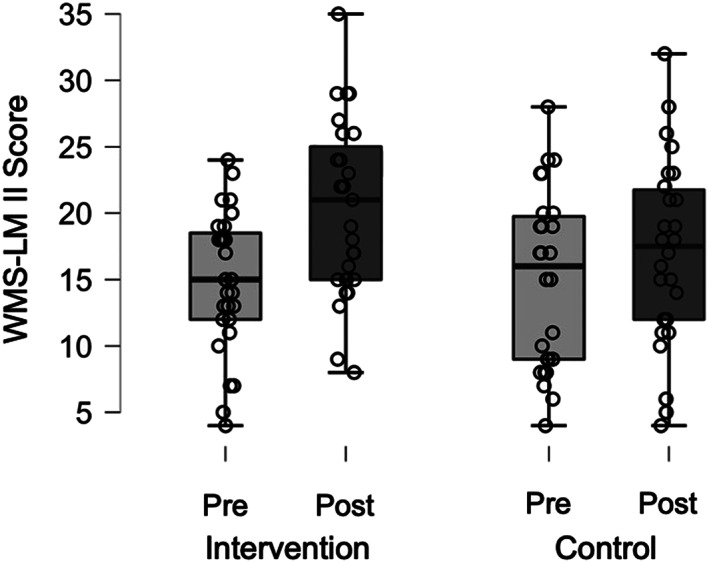
WMS‐LM II results at pre‐ and posttest. The box plots display the range of individual scores presented by points. Within each box, the horizontal black lines denote the median scores. WMS‐LM II, Wechsler Memory Scale‐Revised Logical Memory II
3.3. Effects of intervention on brain activation during the visual working memory
For the behavioral performance of the n‐back task, there was no significant group‐by‐time interaction in 1‐back task performance after the intervention (Table 3), possibly because of a ceiling effect (% correct, mean and SD in the intervention group: pretest = 93.8 ± 7.5, posttest = 93.8 ± 7.31; mean and SD in the control group: pretest = 92.6 ± 12.6, posttest = 91.3 ± 12.8).
For brain activation patterns associated with the 1‐back versus 0‐back task in the pretest, analyses indicated involvement of the bilateral SMA, bilateral PCgG, left anterior insula, left superior parietal lobule, left angular gyrus, left precuneus, left middle temporal gyrus, left inferior temporal gyrus, right middle temporal gyrus, and right cerebellum (Supplementary Table 2, Supplementary Figure 3), which are brain regions characteristically associated with WM.
Regarding the intervention effect on brain activation during WM, a significant group‐by‐time interaction was observed in the right SMA [F (1, 51) = 5.717, p = 0.021, generalized η 2 = 0.032], left precuneus [F (1, 51) = 5.266, p = 0.026, generalized η 2 = 0.024], left PCgG [F (1, 51) = 4.166, p = 0.046, generalized η 2 = 0.020], and right PCgG [F (1, 51) = 4.913, p = 0.031, generalized η 2 = 0.032] (Table 4). Here, we state that our statistical inferences were based on the permutation tests, rather than on p‐values. The obtained F‐values for the interaction effect were higher than the adjusted α‐level threshold F (1, 51) = 4.017 obtained in the permutation test. After the intervention, the intervention group showed a reduction in brain activation in these regions (Figure 4), indicating less effort for the same performance of the VWM task. The group‐by‐time interaction effects were also observed in the left SMA [F (1, 51) = 4.070, p = 0.049, generalized η 2 = 0.028] and left anterior insula [F (1, 51) = 4.098, p = 0.048, generalized η 2 = 0.027]. However, the interaction effects observed in these regions were mainly driven by the activity difference at the time of pretest between two groups. Therefore, the results in these two regions are not further discussed.
TABLE 4.
Brain regions in which activations during VWM showed a significant group‐by‐time interaction indicative of an intervention effect
| Group × time | |||||
|---|---|---|---|---|---|
| Region | BA | MNI coordinates | F‐value | p‐value | Generalized η 2 |
| Right supplementary motor area | 8 | 6, 24, 48 | 5.717 | .021 | 0.032 |
| Left precuneus | 7 | −4, −64, 48 | 5.266 | .026 | 0.024 |
| Left posterior cingulate gyrus | 23 | −4, −28, 30 | 4.166 | .046 | 0.020 |
| Right posterior cingulate gyrus | 23 | 10, −30, 34 | 4.913 | .031 | 0.032 |
Abbreviations: BA, Brodmann area; MNI, Montreal Neurological Institute; VWM, visual working memory.
FIGURE 4.
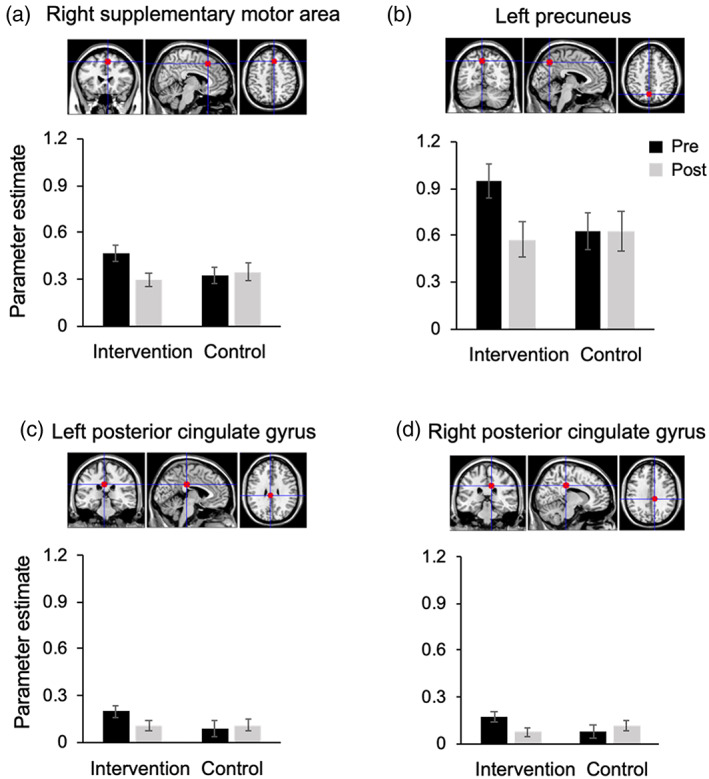
The parameter estimates obtained from fMRI analyses for the VWM task in the (a) right supplementary motor area, (b) left precuneus, (c) left posterior cingulate gyrus and (d) right posterior cingulate gyrus. These four regions showed significant group‐by‐time interactions. VWM, visual working memory
3.4. Effects of intervention on task‐related functional connectivity during the visual working memory
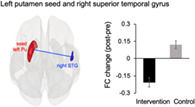
The results of the task‐related FC analysis are shown in Figure 5 and Table 5. The right SMA, left precuneus, bilateral PCgG and putamen were selected as seeds (see Materials and Methods) in the FC analyses for the 0‐ and 1‐back tasks. Among these seeds, significant group‐by‐time interactions were found only for two seeds. First, a significant interaction effect has been found in FC between the right PCgG seed and left middle temporal gyrus (rPCgG‐lMTG) during the 1‐back task, but not the 0‐back task. We also found a significant interaction effect in FC between the left putamen seed and right superior temporal gyrus (lPu‐rSTG) during the 1‐back task, but not the 0‐back task. Consistent with the results of task‐related activation, the intervention group, compared to the control group, showed decreased rPCgG‐lMTG (Figure 5(a)) and lPu‐rSTG (Figure 5(b)) FCs during the 1‐back task after the intervention.
FIGURE 5.
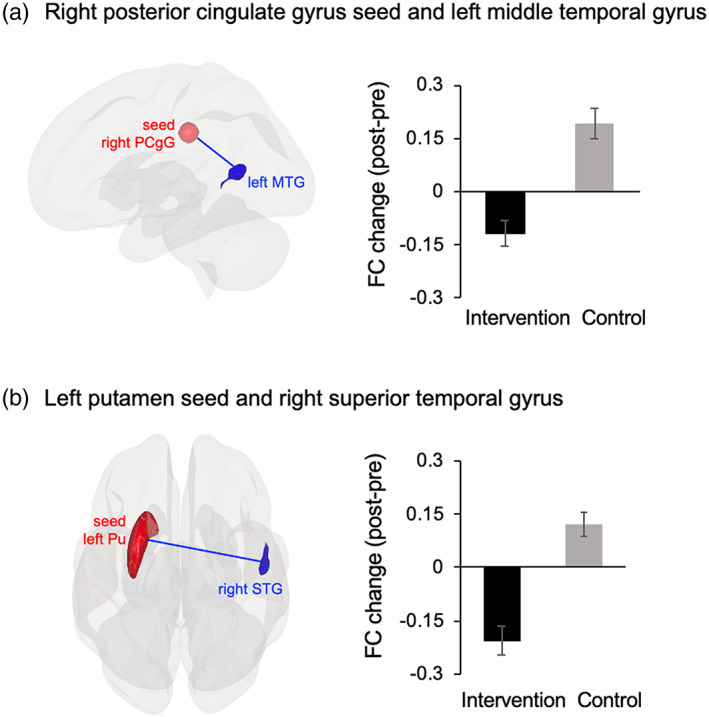
Results of the FC analysis. (a) Decreased rPCgG‐lMTG FC in the intervention group vs. the control group during the 1‐back task after the intervention. (b) Decreased lPu‐rSTG FC in the intervention group vs. the control group during the 1‐back task after the intervention. FC, functional connectivity; rPCgG, right posterior cingulate gyrus; lMTG, left middle temporal gyrus; lPu, left putamen seed; rSTG, right superior temporal gyrus
TABLE 5.
The regions which showed decreased task‐related functional connectivity postintervention in the intervention group compared to the control group (cluster‐level p < .05, FWE corrected)
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Region | BA | x | y | z | p‐value (FWE correction) | Cluster size |
| Seed: Right posterior cingulate gyrus | ||||||
| Left middle temporal gyrus | 37 | −56 | −60 | 8 | .009 | 140 |
| Seed: Left putamen | ||||||
| Right superior temporal gyrus | 22 | 52 | −16 | −8 | .007 | 145 |
Abbreviations: BA, Brodmann area; FWE, family‐wise error; MNI, Montreal Neurological Institute.
3.5. Relationship between FC changes and behavioral changes
For the intervention group, lPu‐rSTG FC change (post ‐ pre) during the 1‐back task was negatively correlated with behavioral changes. That is, larger improvement in memory performance measured by DS and WMS‐LM II was associated with reduced lPu‐rSTG FC during the 1‐back task (DS: r = −0.430, p = 0.025, Figure 6(a); WMS‐LM II: r = −0.418, p = 0.030, Figure 6(b)). These coefficients were higher than the adjusted α‐level threshold, |r| = 0.384, which was obtained in the permutation test. We confirmed that the lPu‐rSTG FC change was not correlated with age in either the intervention group (r = −0.251, p = 0.206) or the control group (r = −0.197, p = 0.335). In addition, no significant correlations were found between lPu‐rSTG FC and WMS‐LM II performance at both pretest (Intervention group: r = 0.215, p = 0.282; Control group: r = 0.078, p = 0.704) and posttest (Intervention group: r = 0.074, p = 0.712; Control group: r = 0.036, p = 0.861), indicating that the lPu‐rSTG FC specifically contributes to the change induced by intervention, not by the memory performance per se. In the control group, no significant correlation between lPu‐rSTG FC change and behavioral changes was found (DS: r = 0.235, p = 0.247, Figure 6(c); WMS‐LM II: r = −0.374, p = 0.060, Figure 6(d)). Moreover, WMS‐LM I change did not correlate with lPu‐rSTG FC change in the either group (intervention: r = −0.282, p = 0.154; control: r = −0.115, p = 0.451).
FIGURE 6.
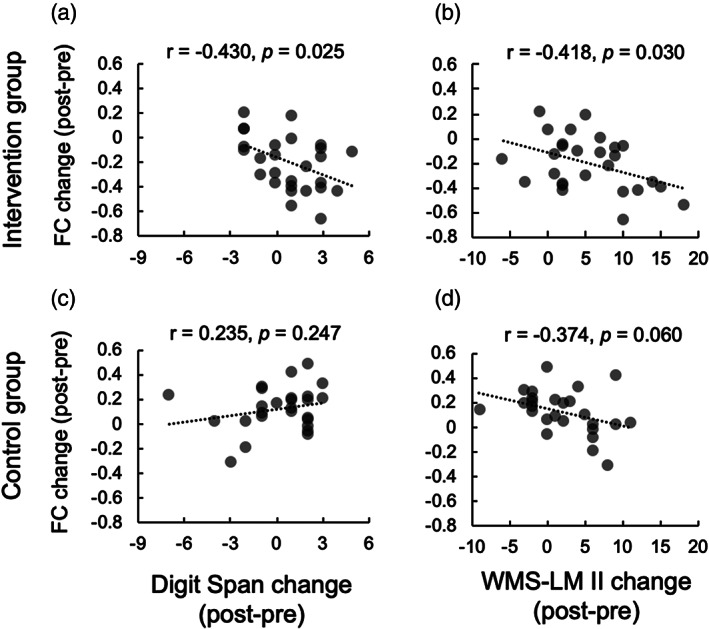
Correlation analyses between task‐related functional connectivity change and behavioral change. (a) lPu‐rSTG FC change during the 1‐back task was negatively correlated with Digit Span performance change in the intervention group. (b) lPu‐rSTG FC change during the 1‐back task was negatively correlated with WMS‐LM II performance change in the intervention group. (c) No significant correlation was found between lPu‐rSTG FC change and Digit Span performance change in the control group. (d) No significant correlation was found between lPu‐rSTG FC change and WMS‐LM II performance change in the control group. Although this effect was marginal, the coefficient did not surpass the adjusted α‐level threshold (|r| = 0.384) obtained in the permutation test. FC, functional connectivity; lPu, left putamen seed; rSTG, right superior temporal gyrus; WMS‐LM II, Wechsler Memory Scale‐Revised Logical Memory II
For the correlation between rPCgG‐lMTG FC change and behavioral changes, there was no significant correlation for either the intervention (DS: r = 0.080, p = 0.690; WMS‐LM I: r = −0.241, p = 0.225; WMS‐LM II: r = −0.059, p = 0.770) or the control group (DS: r = 0.177, p = 0.388; WMS‐LM I: r = −0.012, p = 0.953; WMS‐LM II: r = 0.060, p = 0.772).
4. DISCUSSION
The present study investigated the effects of the Key‐HIT program on cognitive functions and neural efficiency in novice healthy older adults. Our results revealed that after the Key‐HIT intervention, the intervention group showed (1) a significant improvement in the WMS‐LM II test; (2) decreased brain activation in the right SMA, left precuneus and bilateral PCgG during the WM task; and (3) decreased rPCgG‐lMTG and lPu‐rSTG FCs during the WM task. Moreover, improvement in memory performance was associated with a reduction in lPu‐rSTG FC in the intervention group. Our results indicate that decreased WM‐related activity and FC in older adults after musical instrument training, which might be interpreted as improved neural efficiency, was accompanied by improved behavioral performances in non‐musical verbal memory. The present findings provide new causal evidence for training‐related plasticity in older adults, and further support the notion that playing a musical instrument provides robust neuroplasticity across one's lifespan.
Consistent with our previous study (Wada et al., 2017), this study revealed that the Key‐HIT program significantly improved older adults' WMS‐LM II scores in the intervention group. The improvement in verbal memory performance might be explained by the shared components between the music making and the language production, both of which are based on some kind of syntax (Koelsch, Rohrmeier, Torrecuso, & Jentschke, 2013; Rohrmeier, 2011). In addition, our Key‐HIT program included singing the melody, a sort of practice of verbal memory, which might be, to some extent, related to the verbal memory improvement. Alternatively, the multimodal nature of our Key‐HIT program may contribute to improving the verbal memory of older adults. That is, our Key‐HIT program included not only the cognitively demanding processes such as understanding tonal scale or reading musical notation, but also the learning of the association between melody and sequential finger movement patterns for key presses. Such aspects of multimodal learning in the program may have transferred to the improvement in verbal memory. In line with this idea, our previous study on multimodal exercise intervention showed that a 12‐week physical and cognitive exercise program improved verbal memory (WMS‐LM II) among older adults (Nishiguchi et al., 2015).
In contrast to the results of previous studies using piano intervention showing significant improvement in the TMT or Stroop task (Bugos et al., 2007; Seinfeld et al., 2013), we did not find similar improvements in executive functions. One possible reason for these differences is the level of instrument difficulty. Playing the piano requires higher cognitive functions, such as coordinating the movements of both hands, while simultaneously reading the treble clef (G‐clef) and bass clef (F‐clef) scores. In contrast, only the right hand was used in our Key‐HIT program, which may have a lower cognitive load compared to piano practice.
Despite the fact that we did not observe an intervention effect on behavioral performance of 1‐back task during fMRI scanning, the intervention group showed decreased task‐related brain activation in the right SMA, left precuneus and bilateral PCgG after the intervention, indicating less effort for the same performance. These results were consistent with previous longitudinal studies revealing the decrease in brain activation after intervention (Chen et al., 2012; Heinzel et al., 2014; Nishiguchi et al., 2015; Schneiders et al., 2011). A few previous studies (Persson et al., 2006; Pudas, Josefsson, Rieckmann, & Nyberg, 2018) have demonstrated that increases in frontal activation observed among older individuals are related to age‐related decline in cognitive function. Moreover, Morcom, Li, and Rugg (2007) suggested that when the level of behavioral accuracy is matched between age groups, over‐recruitment reflects less neural efficiency in the older group. Taken together, our findings indicate that the Key‐HIT program resulted in increased neural efficiency, given that less brain activation was needed for the same performance of VWM task after the intervention.
Concerning FC, the intervention group showed decreased rPCgG‐lMTG FC and lPu‐rSTG FC during the VWM task. These findings can be interpreted as the additional evidence for improved neural efficiency caused by the intervention. Musical training results in a greater gray matter volume (Groussard et al., 2014) or increased cortical surface area (Elmer, Hänggi, Meyer, & Jäncke, 2013) in the temporal or cingulate cortex for musicians. The putamen and STG have also been repeatedly shown to have a larger gray matter volume in musicians than nonmusicians (Bermudez, Lerch, Evans, & Zatorre, 2009; Vaquero et al., 2016). Therefore, we speculate that the Key‐HIT program improves the function of the putamen, temporal and cingulate regions related to instrumental training, leading to the decreased rPCgG‐lMTG FC and lPu‐rSTG FC during WM task.
Further analyses revealed that, in the intervention group, an improvement in verbal memory performance was associated with a reduction of the lPu‐rSTG FC during the WM task. This correlation may be explained by the shared resources in musical training and verbal memory. Musical instrument training is associated with multiple brain regions, including the putamen and the temporal cortex (Vaquero et al., 2016; Wollman et al., 2018). The basal ganglia region is one of the critical regions for motor sequence learning (Lehéricy et al., 2005), and the striatal system including the putamen is associated with learning of predictive stimulus–response associations (Penhune & Steele, 2012). The temporal area, such as the STG, is involved in music processing (Koelsch et al., 2002), acquiring musical instrumental skills (Chen et al., 2012), and language processing (Hickok & Poeppel, 2004). These regions have also been associated with WM (Langer, Von Bastian, Wirz, Oberauer, & Jäncke, 2013; Proskovec, Wiesman, Heinrichs‐Graham, & Wilson, 2019; Salmi et al., 2018; Voytek & Knight, 2010) and verbal memory (Garrido et al., 2002; Ystad, Eichele, Lundervold, & Lundervold, 2010). In light of these observations, we propose that our Key‐HIT program, which may broadly engage the neural networks including lPu‐rSTG, induces neurocognitive improvement not specific to musical skills, such as improvements in verbal memory and neural efficiency associated with VWM.
The present study has several limitations. First, while the effects of the Key‐HIT program have been demonstrated, it is still unclear which aspect of the program is effective. Second, control group participants did not receive any group intervention, which limits the interpretation of findings. Thus, social engagement, other than the musical instrument training, may have partially influenced the intervention group. However, it is unlikely that these intervention effects are merely due to an increase in outings in the intervention group because the two groups did not differ in outing frequency. Third, as the same tests were used in the pretest and posttest, there may have been a repetition effect on some of our results (i.e., WMS‐LM). However, our main finding on the training‐related effects on WMS‐LM II was substantiated by a group x time interaction. Furthermore, decreased VWM‐related activity and FC, and the association between behavioral changes and FC changes, were only observed in the intervention group, suggesting that the changes in memory performance and neural efficiency are caused by our intervention program, rather than by repeated testing. Fourth, the participants in this study had an active lifestyle before the start of this investigation; therefore, the results may not be generalizable to older adults with inactive lifestyle. Finally, there were few male participants in our sample. Therefore, the present results may not be generalizable to both genders. We leave these questions as topics for future research.
5. CONCLUSION
The present findings provide important new insight for training‐related plasticity by demonstrating that the Key‐HIT program may improve verbal memory and neural efficiency in older adults. Moreover, these results demonstrate that such instrumental training‐related neuroplasticity is not restricted to children or younger adults, but rather extends across an individual's lifespan.
CONFLICT OF INTERESTS
The authors declare that there are no conflicts of interest regarding the publication of this article.
ETHICS APPROVAL STATEMENT
The ethics committee of Kyoto University approved this study. All participants provided written informed consent.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGMENTS
This study was supported by Grant‐in‐Aid (No. 16H06325 to Kaoru Sekiyama) for Scientific Research from the Japan Society for the Promotion of Science (JSPS). Xia Guo was supported by Research Fellowship for Young Scientists from JSPS. We thank Dr. Mamoru Hashimoto for visual inspection of abnormalities in anatomical brain MRI. We also thank Dr. Makiko Sadakata for her contribution in programming; Mariko Higuchi and Takashi Hiroi for their assistance in data collection and music intervention; Daiki Yamasaki, Shiori Tanaka, Kyoko Toyohara, Natsuho Hirakawa, Sachie Motoki, and Hikaru Hirasawa for their assistance in data collection and Chieko Sadakata, Chiaki Tan, and Masae Ikuma for their support in the music intervention. This study was conducted using the MRI scanner and related facilities of the Kokoro Research Center, Kyoto University.
Guo X, Yamashita M, Suzuki M, et al. Musical instrument training program improves verbal memory and neural efficiency in novice older adults. Hum Brain Mapp. 2021;42:1359–1375. 10.1002/hbm.25298
Funding information Kyoto University; Japan Society for the Promotion of Science, Grant/Award Number: 16H06325
DATA AVAILABILITY STATEMENT
The functional data used in the present study can be accessed upon request to the corresponding authors.
REFERENCES
- Akbaraly, T. N. , Portet, F. , Fustinoni, S. , Dartigues, J. F. , Artero, S. , Rouaud, O. , … Berr, C. (2009). Leisure activities and the risk of dementia in the elderly: Results from the Three‐City study. Neurology, 73, 854–861. 10.1212/WNL.0b013e3181b7849b [DOI] [PubMed] [Google Scholar]
- Anderson, S. , White‐Schwoch, T. , Parbery‐Clark, A. , & Kraus, N. (2013). Reversal of age‐related neural timing delays with training. Proceedings of the National Academy of Sciences, 110, 4357–4362. 10.1073/pnas.1213555110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangert, M. , Peschel, T. , Schlaug, G. , Rotte, M. , Drescher, D. , Hinrichs, H. , … Altenmüller, E. (2006). Shared networks for auditory and motor processing in professional pianists: Evidence from fMRI conjunction. NeuroImage, 30, 917–926. 10.1016/j.neuroimage.2005.10.044 [DOI] [PubMed] [Google Scholar]
- Basak, C. , Boot, W. R. , Voss, M. W. , & Kramer, A. F. (2008). Can training in a real‐time strategy video game attenuate cognitive decline in older adults? Psychology and Aging, 23, 765–777. 10.1037/a0013494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann, S. , Koeneke, S. , Schmidt, C. F. , Meyer, M. , Lutz, K. , & Jancke, L. (2007). A network for audio–motor coordination in skilled pianists and non‐musicians. Brain Research, 1161, 65–78. 10.1016/j.brainres.2007.05.045 [DOI] [PubMed] [Google Scholar]
- Bermudez, P. , Lerch, J. P. , Evans, A. C. , & Zatorre, R. J. (2009). Neuroanatomical correlates of musicianship as revealed by cortical thickness and voxel‐based morphometry. Cerebral Cortex, 19, 1583–1596. 10.1093/cercor/bhn196 [DOI] [PubMed] [Google Scholar]
- Bugos, J. A. , Perlstein, W. M. , McCrae, C. S. , Brophy, T. S. , & Bedenbaugh, P. H. (2007). Individualized piano instruction enhances executive functioning and working memory in older adults. Aging and Mental Health, 11, 464–471. 10.1080/13607860601086504 [DOI] [PubMed] [Google Scholar]
- Chan, A. S. , Ho, Y. C. , & Cheung, M. C. (1998). Music training improves verbal memory. Nature, 396, 128. 10.1038/24075 [DOI] [PubMed] [Google Scholar]
- Chen, J. L. , Rae, C. , & Watkins, K. E. (2012). Learning to play a melody: An fMRI study examining the formation of auditory‐motor associations. NeuroImage, 59, 1200–1208. 10.1016/j.neuroimage.2011.08.012 [DOI] [PubMed] [Google Scholar]
- Elmer, S. , Hänggi, J. , Meyer, M. , & Jäncke, L. (2013). Increased cortical surface area of the left planum temporale in musicians facilitates the categorization of phonetic and temporal speech sounds. Cortex, 49, 2812–2821. 10.1016/j.cortex.2013.03.007 [DOI] [PubMed] [Google Scholar]
- Folstein, M. F. , Folstein, S. E. , & McHugh, P. R. (1975). “Mini‐mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Fratiglioni, L. , Paillard‐Borg, S. , & Winblad, B. (2004). An active and socially integrated lifestyle in late life might protect against dementia. The Lancet Neurology, 3, 343–353. 10.1016/S1474-4422(04)00767-7 [DOI] [PubMed] [Google Scholar]
- Friston, K. J. (2011). Functional and effective connectivity: A review. Brain Connectivity, 1, 13–36. 10.1089/brain.2011.0008 [DOI] [PubMed] [Google Scholar]
- Fujioka, T. , Ross, B. , Kakigi, R. , Pantev, C. , & Trainor, L. J. (2006). One year of musical training affects development of auditory cortical‐evoked fields in young children. Brain, 129, 2593–2608. 10.1093/brain/awl247 [DOI] [PubMed] [Google Scholar]
- Garrido, G. E. J. , Furuie, S. S. , Buchpiguel, C. A. , Bottino, C. M. C. , Almeida, O. P. , Cid, C. G. , … Busatto, G. F. (2002). Relation between medial temporal atrophy and functional brain activity during memory processing in Alzheimer's disease: A combined MRI and SPECT study. Journal of Neurology, Neurosurgery & Psychiatry, 73, 508–516. 10.1136/jnnp.73.5.508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady, C. L. , Yu, H. , & Alain, C. (2008). Age‐related differences in brain activity underlying working memory for spatial and nonspatial auditory information. Cerebral Cortex, 18, 189–199. 10.1093/cercor/bhm045 [DOI] [PubMed] [Google Scholar]
- Groussard, M. , Viader, F. , Landeau, B. , Desgranges, B. , Eustache, F. , & Platel, H. (2014). The effects of musical practice on structural plasticity: The dynamics of grey matter changes. Brain and Cognition, 90, 174–180. 10.1016/j.bandc.2014.06.013 [DOI] [PubMed] [Google Scholar]
- Guo, X. , Ohsawa, C. , Suzuki, A. , & Sekiyama, K. (2018). Improved digit span in children after a 6‐week intervention of playing a musical instrument: An exploratory randomized controlled trial. Frontiers in Psychology, 8, 2303. 10.3389/fpsyg.2017.02303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber, S. N. (2016). Corticostriatal circuitry. Dialogues in Clinical Neuroscience, 18, 7–21. 10.1007/978-1-4939-3474-4_135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, C. B. , Lipton, R. B. , Sliwinski, M. , Katz, M. J. , Derby, C. A. , & Verghese, J. (2009). Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology, 73, 356–361. 10.1212/WNL.0b013e3181b04ae3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna‐Pladdy, B. , & MacKay, A. (2011). The relation between instrumental musical activity and cognitive aging. Neuropsychology, 25, 378–386. 10.1037/a0021895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hars, M. , Herrmann, F. R. , Gold, G. , Rizzoli, R. , & Trombetti, A. (2014). Effect of music‐based multitask training on cognition and mood in older adults. Age and Ageing, 43, 196–200. 10.1093/ageing/aft163 [DOI] [PubMed] [Google Scholar]
- Hebert, L. E. , Scherr, P. A. , Bienias, J. L. , Bennett, D. A. , & Evans, D. A. (2003). Alzheimer disease in the US population: Prevalence estimates using the 2000 census. Archives of Neurology, 60, 1119–1122. 10.1001/archneur.60.8.1119 [DOI] [PubMed] [Google Scholar]
- Hebert, L. E. , Weuve, J. , Scherr, P. A. , & Evans, D. A. (2013). Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology, 80, 1778–1783. 10.1212/WNL.0b013e31828726f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel, S. , Lorenz, R. C. , Brockhaus, W. R. , Wüstenberg, T. , Kathmann, N. , Heinz, A. , & Rapp, M. A. (2014). Working memory load‐dependent brain response predicts behavioral training gains in older adults. Journal of Neuroscience, 34, 1224–1233. 10.1523/JNEUROSCI.2463-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel, S. , Lorenz, R. C. , Duong, Q. L. , Rapp, M. A. , & Deserno, L. (2017). Prefrontal‐parietal effective connectivity during working memory in older adults. Neurobiology of Aging, 57, 18–27. 10.1016/j.neurobiolaging.2017.05.005 [DOI] [PubMed] [Google Scholar]
- Herholz, S. C. , & Zatorre, R. J. (2012). Musical training as a framework for brain plasticity: Behavior, function, and structure. Neuron, 76, 486–502. 10.1016/j.neuron.2012.10.011 [DOI] [PubMed] [Google Scholar]
- Hickok, G. , & Poeppel, D. (2004). Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition, 92, 67–99. 10.1016/j.cognition.2003.10.011 [DOI] [PubMed] [Google Scholar]
- Huntley, J. D. , & Howard, R. J. (2010). Working memory in early Alzheimer's disease: A neuropsychological review. International Journal of Geriatric Psychiatry, 25, 121–132. 10.1002/gps.2314 [DOI] [PubMed] [Google Scholar]
- Janata, P. , & Grafton, S. T. (2003). Swinging in the brain: Shared neural substrates for behaviors related to sequencing and music. Nature Neuroscience, 6, 682–687. 10.1038/nn1081 [DOI] [PubMed] [Google Scholar]
- Jäncke, L. (2012). The dynamic audio–motor system in pianists. Annals of the new York Academy of Sciences, 1252, 246–252. 10.1111/j.1749-6632.2011.06416.x [DOI] [PubMed] [Google Scholar]
- Kawagoe, T. , & Sekiyama, K. (2014). Visually encoded working memory is closely associated with mobility in older adults. Experimental Brain Research, 232, 2035–2043. 10.1007/s00221-014-3893-1 [DOI] [PubMed] [Google Scholar]
- Kawagoe, T. , Suzuki, M. , Nishiguchi, S. , Abe, N. , Otsuka, Y. , Nakai, R. , … Sekiyama, K. (2015). Brain activation during visual working memory correlates with behavioral mobility performance in older adults. Frontiers in Aging Neuroscience, 7, 186. 10.3389/fnagi.2015.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano, N. (2012). A pilot study of standardization of WMS‐R logical memory for Japanese old‐old people: Differences between the story a and story B. Otsuma Journal of Social Information Studies, 21, 223–231. [Google Scholar]
- Kessler, R. C. , Andrews, G. , Colpe, L. J. , Hiripi, E. , Mroczek, D. K. , Normand, S. L. , … Zaslavsky, A. M. (2002). Short screening scales to monitor population prevalences and trends in non‐specific psychological distress. Psychological Medicine, 32, 959–976. 10.1017/S0033291702006074 [DOI] [PubMed] [Google Scholar]
- Koelsch, S. (2014). Brain correlates of music‐evoked emotions. Nature Reviews Neuroscience, 15, 170–180. 10.1038/nrn3666 [DOI] [PubMed] [Google Scholar]
- Koelsch, S. , Gunter, T. C. , von Cramon, D. Y. , Zysset, S. , Lohmann, G. , & Friederici, A. D. (2002). Bach speaks: A cortical" language‐network" serves the processing of music. NeuroImage, 17, 956–966. 10.1006/nimg.2002.1154 [DOI] [PubMed] [Google Scholar]
- Koelsch, S. , Rohrmeier, M. , Torrecuso, R. , & Jentschke, S. (2013). Processing of hierarchical syntactic structure in music. Proceedings of the National Academy of Sciences, 110, 15443–15448. 10.1073/pnas.1300272110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus, N. , & Chandrasekaran, B. (2010). Music training for the development of auditory skills. Nature Reviews Neuroscience, 11, 599–605. 10.1038/nrn2882 [DOI] [PubMed] [Google Scholar]
- Kraus, N. , & White‐Schwoch, T. (2014). Music training: Lifelong investment to protect the brain from aging and hearing loss. Acoustics Australia, 42, 117–123. [Google Scholar]
- Langer, N. , Von Bastian, C. C. , Wirz, H. , Oberauer, K. , & Jäncke, L. (2013). The effects of working memory training on functional brain network efficiency. Cortex, 49, 2424–2438. 10.1016/j.cortex.2013.01.008 [DOI] [PubMed] [Google Scholar]
- Lehéricy, S. , Benali, H. , Van de Moortele, P. F. , Pélégrini‐Issac, M. , Waechter, T. , Ugurbil, K. , & Doyon, J. (2005). Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proceedings of the National Academy of Sciences, 102, 12566–12571. 10.1073/pnas.0502762102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak, M. D. , Howieson, D. B. , Bigler, E. D. , & Tranel, D. (2012). Neuropsychological assessment (5th). New York, NY: Oxford University Press. [Google Scholar]
- Lustig, C. , Shah, P. , Seidler, R. , & Reuter‐Lorenz, P. A. (2009). Aging, training, and the brain: A review and future directions. Neuropsychology Review, 19, 504–522. 10.1007/s11065-009-9119-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcom, A. M. , Li, J. , & Rugg, M. D. (2007). Age effects on the neural correlates of episodic retrieval: Increased cortical recruitment with matched performance. Cerebral Cortex, 17, 2491–2506. 10.1093/cercor/bhl155 [DOI] [PubMed] [Google Scholar]
- Moreno, S. , Bialystok, E. , Barac, R. , Schellenberg, E. G. , Cepeda, N. J. , & Chau, T. (2011). Short‐term music training enhances verbal intelligence and executive function. Psychological Science, 22, 1425–1433. 10.1177/0956797611416999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, S. , Marques, C. , Santos, A. , Santos, M. , Castro, S. L. , & Besson, M. (2009). Musical training influences linguistic abilities in 8‐year‐old children: More evidence for brain plasticity. Cerebral Cortex, 19, 712–723. 10.1093/cercor/bhn120 [DOI] [PubMed] [Google Scholar]
- Münte, T. F. , Altenmüller, E. , & Jäncke, L. (2002). The musician's brain as a model of neuroplasticity. Nature Reviews Neuroscience, 3, 473–478. 10.1038/nrn843 [DOI] [PubMed] [Google Scholar]
- Nichols, T. E. , & Holmes, A. P. (2002). Nonparametric permutation tests for functional neuroimaging: A primer with examples. Human Brain Mapping., 15, 1–25. 10.1002/hbm.1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi, S. , Yamada, M. , Tanigawa, T. , Sekiyama, K. , Kawagoe, T. , Suzuki, M. , … Aoyama, T. (2015). A 12‐week physical and cognitive exercise program can improve cognitive function and neural efficiency in community‐dwelling older adults: A randomized controlled trial. Journal of the American Geriatrics Society, 63, 1355–1363. 10.1111/jgs.13481 [DOI] [PubMed] [Google Scholar]
- Noice, H. , Noice, T. , & Staines, G. (2004). A short‐term intervention to enhance cognitive and affective functioning in older adults. Journal of Aging and Health, 16, 562–585. 10.1177/0898264304265819 [DOI] [PubMed] [Google Scholar]
- Nyberg, L. , Lövdén, M. , Riklund, K. , Lindenberger, U. , & Bäckman, L. (2012). Memory aging and brain maintenance. Trends in Cognitive Sciences, 16, 292–305. 10.1016/j.tics.2012.04.005 [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Pallesen, K. J. , Brattico, E. , Bailey, C. J. , Korvenoja, A. , Koivisto, J. , Gjedde, A. , & Carlson, S. (2010). Cognitive control in auditory working memory is enhanced in musicians. PLoS One, 5, e11120. 10.1371/journal.pone.0011120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parbery‐Clark, A. , Anderson, S. , Hittner, E. , & Kraus, N. (2012). Musical experience offsets age‐related delays in neural timing. Neurobiology of Aging, 33, 1483–1483.e4. 10.1016/j.neurobiolaging.2011.12.015 [DOI] [PubMed] [Google Scholar]
- Parbery‐Clark, A. , Skoe, E. , Lam, C. , & Kraus, N. (2009). Musician enhancement for speech‐in‐noise. Ear and Hearing, 30, 653–661. 10.1097/AUD.0b013e3181b412e9 [DOI] [PubMed] [Google Scholar]
- Parbery‐Clark, A. , Strait, D. L. , Anderson, S. , Hittner, E. , & Kraus, N. (2011). Musical experience and the aging auditory system: Implications for cognitive abilities and hearing speech in noise. PLoS One, 6, e18082. 10.1371/journal.pone.0018082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, D. C. , Lautenschlager, G. , Hedden, T. , Davidson, N. S. , Smith, A. D. , & Smith, P. K. (2002). Models of visuospatial and verbal memory across the adult life span. Psychology and Aging, 17, 299–320. 10.1037/0882-7974.17.2.299 [DOI] [PubMed] [Google Scholar]
- Park, D. C. , & Reuter‐Lorenz, P. (2009). The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology, 60, 173–196. 10.1146/annurev.psych.59.103006.093656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhune, V. B. , & Steele, C. J. (2012). Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behavioural Brain Research, 226, 579–591. 10.1016/j.bbr.2011.09.044 [DOI] [PubMed] [Google Scholar]
- Persson, J. , Nyberg, L. , Lind, J. , Larsson, A. , Nilsson, L. G. , Ingvar, M. , & Buckner, R. L. (2006). Structure–function correlates of cognitive decline in aging. Cerebral Cortex, 16, 907–915. 10.1093/cercor/bhj036 [DOI] [PubMed] [Google Scholar]
- Proskovec, A. L. , Wiesman, A. I. , Heinrichs‐Graham, E. , & Wilson, T. W. (2019). Load effects on spatial working memory performance are linked to distributed alpha and beta oscillations. Human Brain Mapping, 40, 3682–3689. 10.1002/hbm.24625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudas, S. , Josefsson, M. , Rieckmann, A. , & Nyberg, L. (2018). Longitudinal evidence for increased functional response in frontal cortex for older adults with hippocampal atrophy and memory decline. Cerebral Cortex, 28, 936–948. 10.1093/cercor/bhw418 [DOI] [PubMed] [Google Scholar]
- Reitan, R. M. (1992). Trail making test: Manual for administration and scoring. Tucson, AZ: Reitan Neuropsychology Laboratory. [Google Scholar]
- Rogenmoser, L. , Kernbach, J. , Schlaug, G. , & Gaser, C. (2018). Keeping brains young with making music. Brain Structure and Function, 223, 297–305. 10.1007/s00429-017-1491-2 [DOI] [PubMed] [Google Scholar]
- Rohrmeier, M. (2011). Towards a generative syntax of tonal harmony. Journal of Mathematics and Music, 5, 35–53. 10.1080/17459737.2011.573676 [DOI] [Google Scholar]
- Rottschy, C. , Langner, R. , Dogan, I. , Reetz, K. , Laird, A. R. , Schulz, J. B. , … Eickhoff, S. B. (2012). Modelling neural correlates of working memory: A coordinate‐based meta‐analysis. NeuroImage, 60, 830–846. 10.1016/j.neuroimage.2011.11.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi, J. , Nyberg, L. , & Laine, M. (2018). Working memory training mostly engages general‐purpose large‐scale networks for learning. Neuroscience & Biobehavioral Reviews, 93, 108–122. 10.1016/j.neubiorev.2018.03.019 [DOI] [PubMed] [Google Scholar]
- Scarmeas, N. , & Stern, Y. (2003). Cognitive reserve and lifestyle. Journal of Clinical and Experimental Neuropsychology, 25, 625–633. 10.1076/jcen.25.5.625.14576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg, E. G. (2004). Music lessons enhance IQ. Psychological Science, 15, 511–514. 10.1111/j.0956-7976.2004.00711.x [DOI] [PubMed] [Google Scholar]
- Schneiders, J. A. , Opitz, B. , Krick, C. M. , & Mecklinger, A. (2011). Separating intra‐modal and across‐modal training effects in visual working memory: An fMRI investigation. Cerebral Cortex, 21, 2555–2564. 10.1093/cercor/bhr037 [DOI] [PubMed] [Google Scholar]
- Schulze, K. , Zysset, S. , Mueller, K. , Friederici, A. D. , & Koelsch, S. (2011). Neuroarchitecture of verbal and tonal working memory in nonmusicians and musicians. Human Brain Mapping, 32, 771–783. 10.1002/hbm.21060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seinfeld, S. , Figueroa, H. , Ortiz‐Gil, J. , & Sanchez‐Vives, M. V. (2013). Effects of music learning and piano practice on cognitive function, mood and quality of life in older adults. Frontiers in Psychology, 4, 810. 10.3389/fpsyg.2013.00810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small, B. J. , Dixon, R. A. , McArdle, J. J. , & Grimm, K. J. (2012). Do changes in lifestyle engagement moderate cognitive decline in normal aging? Evidence from the Victoria longitudinal study. Neuropsychology, 26, 144–155. 10.1037/a0026579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, Y. (2012). Cognitive reserve in ageing and Alzheimer's disease. The Lancet Neurology, 11, 1006–1012. 10.1016/S1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, Y. , Gurland, B. , Tatemichi, T. K. , Tang, M. X. , Wilder, D. , & Mayeux, R. (1994). Influence of education and occupation on the incidence of Alzheimer's disease. Jama, 271, 1004–1010. 10.1001/jama.1994.03510370056032 [DOI] [PubMed] [Google Scholar]
- Sugishita, M. (2000). Wechsler memory scale ‐ revised. Tokyo, Japan: Nihon Bunka Kagakusha. [Google Scholar]
- Suzuki, M. , Kawagoe, T. , Nishiguchi, S. , Abe, N. , Otsuka, Y. , Nakai, R. , … Sekiyama, K. (2018). Neural correlates of working memory maintenance in advanced aging: Evidence from fMRI. Frontiers in Aging Neuroscience, 10, 358. 10.3389/fnagi.2018.00358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero, L. , Hartmann, K. , Ripollés, P. , Rojo, N. , Sierpowska, J. , François, C. , … Münte, T. F. (2016). Structural neuroplasticity in expert pianists depends on the age of musical training onset. NeuroImage, 126, 106–119. 10.1016/j.neuroimage.2015.11.008 [DOI] [PubMed] [Google Scholar]
- Verghese, J. , Lipton, R. B. , Katz, M. J. , Hall, C. B. , Derby, C. A. , Kuslansky, G. , … Buschke, H. (2003). Leisure activities and the risk of dementia in the elderly. New England Journal of Medicine, 348, 2508–2516. 10.1056/NEJMoa022252 [DOI] [PubMed] [Google Scholar]
- Voytek, B. , & Knight, R. T. (2010). Prefrontal cortex and basal ganglia contributions to visual working memory. Proceedings of the National Academy of Sciences, 107, 18167–18172. 10.1073/pnas.1007277107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada, R., Hisanaga, S., Kaku, K., Kimura, H., Suzuki, M., Kawagoe, T., & Sekiyama, K. (2017). The effects of playing the keyboard harmonica on older adults verbal memory. In 15th World Congress of Music Therapy, Tsukuba.
- Wan, C. Y. , & Schlaug, G. (2010). Music making as a tool for promoting brain plasticity across the life span. The Neuroscientist, 16, 566–577. 10.1177/1073858410377805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, D. (1987). WMS‐R: Wechsler memory scale–revised: Manual. San Antonio, TX: The Psychological Corporation/Harcourt Brace Jovanovich. [Google Scholar]
- Wechsler, D. (1997). Wechsler Adult Intelligence Scale—3rd Edition (WAIS‐3). San Antonio, TX: Harcourt Assessment. [Google Scholar]
- White‐Schwoch, T. , Carr, K. W. , Anderson, S. , Strait, D. L. , & Kraus, N. (2013). Older adults benefit from music training early in life: Biological evidence for long‐term training‐driven plasticity. Journal of Neuroscience, 33, 17667–17674. 10.1523/JNEUROSCI.2560-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman, I. , Penhune, V. , Segado, M. , Carpentier, T. , & Zatorre, R. J. (2018). Neural network retuning and neural predictors of learning success associated with cello training. Proceedings of the National Academy of Sciences, 115, E6056–E6064. 10.1073/pnas.1721414115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystad, M. , Eichele, T. , Lundervold, A. J. , & Lundervold, A. (2010). Subcortical functional connectivity and verbal episodic memory in healthy elderly—A resting state fMRI study. NeuroImage, 52, 379–388. 10.1016/j.neuroimage.2010.03.062 [DOI] [PubMed] [Google Scholar]
- Zatorre, R. (2005). Music, the food of neuroscience? Nature, 434, 312–315. 10.1038/434312a [DOI] [PubMed] [Google Scholar]
- Zatorre, R. J. , Chen, J. L. , & Penhune, V. B. (2007). When the brain plays music: Auditory–motor interactions in music perception and production. Nature Reviews Neuroscience, 8, 547–558. 10.1038/nrn2152 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Data Availability Statement
The functional data used in the present study can be accessed upon request to the corresponding authors.


