Abstract
Study Objectives:
OSA is a common sleep disorder. There is a strong link between sleep-related breathing disorders and cardiovascular and cerebrovascular diseases. Matrix metalloproteinase-9 (MMP-9) is a biological marker for extracellular matrix degradation, which plays a significant role in systemic hypertension, myocardial infarction and postmyocardial infarction heart failure, and ischemic stroke. This article reviews MMP-9 as an inflammatory mediator and a potential messenger between OSA and OSA-induced comorbidities.
Methods:
We reviewed the MEDLINE database (PubMed) for publications on MMP-9, OSA, and cardiovascular disease, identifying 1,592 studies and including and reviewing 50 articles for this work.
Results:
There is strong evidence that MMP-9 and tissue inhibitor of metalloproteinase-1 levels are elevated in patients with OSA (mainly MMP-9), systemic hypertension, myocardial infarction, and postmyocardial infarction heart failure. Our study showed variable results that could be related to the sample size or to laboratory methodology.
Conclusions:
MMP-9 and its endogenous inhibitor, tissue inhibitor of metalloproteinase-1, are a common denominator in OSA, systemic hypertension, myocardial infarction, and heart failure. This characterization makes MMP-9 a target for developing novel selective inhibitors that can serve as adjuvant therapy in patients with OSA, which may ameliorate the cardiovascular and cerebrovascular mortality associated with OSA.
Citation:
Mashaqi S, Mansour HM, Alameddin H, et al. Matrix metalloproteinase-9 as a messenger in the cross talk between obstructive sleep apnea and comorbid systemic hypertension, cardiac remodeling, and ischemic stroke: a literature review. J Clin Sleep Med. 2021;17(3):567–591.
Keywords: OSA, matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1, hypertension, remodeling, myocardial infarction, ischemic stroke
INTRODUCTION
OSA is a common sleep disorder that is characterized by repetitive episodes of collapse in the pharyngeal airway during sleep. This collapse is usually related to a crowded upper airway secondary to multiple factors (eg, obesity,1 enlarged tonsils,2 retrognathia,3 high arched palate,4 and other craniofacial features5). The prevalence of OSA has increased over the last 2 decades. In 2014, the prevalence of OSA was 26% with more than 26 million adults in the United States diagnosed with OSA between ages 30 and 70 years.6 Men are twice as likely to be diagnosed with OSA compared to women.7 Sleep disruption associated with OSA results in excessive daytime sleepiness, fatigue, and other neurocognitive, mood, and behavioral symptoms leading to impairment in numerous facets of life including work, academic achievement, and social interaction.8–10
Over the last few decades, the effects of OSA on other comorbid conditions have become clearer. Untreated OSA can worsen systemic hypertension (HTN), coronary artery disease, obesity, cerebrovascular diseases, and metabolic syndrome.11–13 OSA-induced cardiovascular diseases in particular have been evaluated extensively in numerous studies. OSA increases the risk of incident and prevalent HTN,14,15 coronary artery disease,16,17 prevalent congestive heart failure (HF),17 atrial fibrillation,18 pulmonary HTN,19 and incident stroke.20
The exact pathophysiology correlating OSA and cardiovascular diseases is not fully understood. Intermittent hypoxia, which is a major feature of OSA, seems to play a critical role through different pathways. Stimulation of the sympathetic nervous system and the surge of noradrenaline associated with chronic intermittent hypoxia can contribute to the risk of developing systemic HTN and coronary artery disease.21 Intermittent hypoxia has been shown to cause endothelial dysfunction, which can be related to the production of inflammatory mediators, reactive oxygen species, vascular adhesive molecules, and alteration in endothelial vasoactive mediators (such as an increase in endothelin-1 and a decrease in nitrous oxide).22,23 The hypoxia/reoxygenation process per se can facilitate endothelial dysfunction.
Systemic inflammation is a common denominator between OSA and these comorbid diseases. Dysregulation of the extracellular matrix (ECM) proteins is a significant element in the inflammatory process. Inflammatory stress induces the production of proteolytic enzymes (ie, proteinases) intracellularly. Proteinases primarily degrade collagen, laminin, and elastin. Any imbalance between the synthesis and degradation of collagen can lead to abnormal extra deposition of collagen in different tissues.24 When this process takes place in the wall of major blood vessel walls and the myocardial interstitium, it is known as “remodeling.” Remodeling is an ongoing, insidious process that can yield an increased stiffness in major blood vessel walls and the myocardium, which ultimately increases the risk of systemic HTN and HF, respectively.25
One family of these proteolytic enzymes is the matrix metalloproteinases (MMPs). MMP-9, also known as gelatinase B, is a significant protease that plays a critical role in systemic inflammation in patients with OSA and cardiovascular and cerebrovascular diseases. In this article, we review the role of MMP-9 in OSA, systemic HTN, myocardial infarction (MI), post-MI systolic and diastolic HF, and ischemic stroke. Therapeutic interventions that selectively inhibit MMP-9 are a potential novel therapy to mitigate OSA-induced cardiovascular and cerebrovascular effects.
MMP-9 (structural and functional review)
MMPs are a group of zinc-containing proteinases that degrade the ECM components.26 They are produced intracellularly in the form of pro-MMP (inactive zymogen) and become activated extracellularly (except for MMP-11).27 MMPs are classified into 6 groups (collagenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs, and others). MMPs are under tight control at different levels (such as transcription, pro-MMP activation, posttranslational modification, and inhibition by endogenous inhibitors).28 There are 26 different types of vertebrate MMPs, and 23 have been identified in humans.29
MMP-9 is a 92-kDa peptide30 that is produced and released by different inflammatory cells such as neutrophils, monocytes, and macrophages. It has different domains (the pro-MMP-9 domain, the catalytic domain, the linker domain, and a hemopexin-like domain; Figure 1).30 Activation takes place extracellularly via proteolysis by different enzymes (eg, plasmin) or by chemical substances (eg, thiol-modifying agents, oxidized glutathione, reactive oxygen).31 All MMPs contain zinc ions in the catalytic domain that are essential for proteolytic activity. The activation process starts with an injury that stimulates the release of cytokines (eg, tumor necrosis factor-α and interleukin-1β) from attracted neutrophils and monocytes.32,33 Subsequently, transcription factors are activated (eg, activator protein-1, Nuclear Factor kappa-light-chain-enhancer of activated B cells, Serum amyloid A-activating factor-1),34 leading to the production of MMP-9 in the inactive form (pro-MMP-9). Pro-MMP-9 binds specific endogenous inhibitory factors (tissue inhibitors of metalloproteinases [TIMPs]) in the Golgi apparatus, which keeps it inactivated.35 The pro-MMP-9/TIMP complex is released extracellularly and becomes activated by different proteinases such as plasmin and tissue-type plasminogen activator.36 There are 4 identified TIMPs (TIMP-1, TIMP-2, TIMP-3, and TIMP-4).37 TIMPs are 21 to 29 kDa.38 Each has a wedge-shaped domain that binds the catalytic site like any other substrate, which results in the deactivation of MMPs.39
Figure 1. MMP-9 structure.
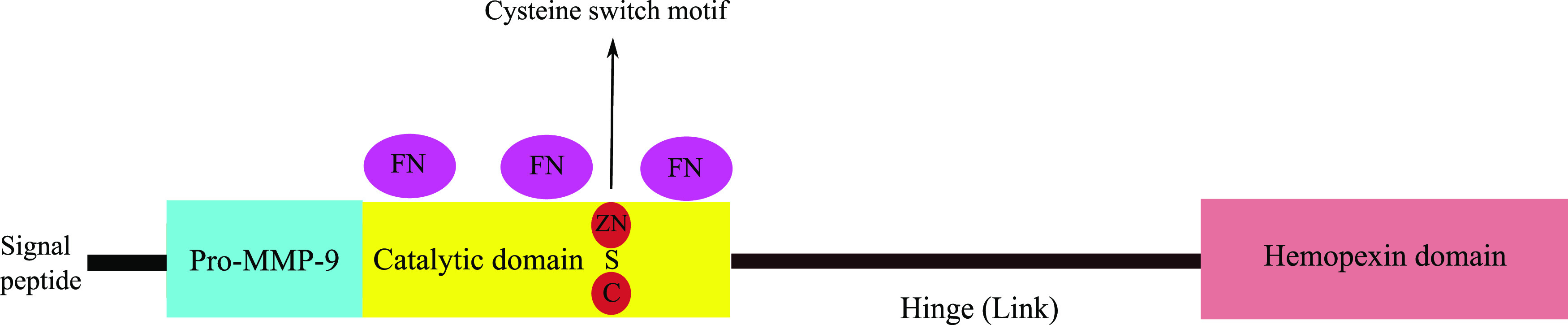
C = cysteine, FN = fibronectin, MMP-9 = matrix metalloproteinase-9, S = sulphur, ZN = zinc.
The degradation of ECM by MMP-9 triggers angiogenesis via several proangiogenic factors such as vascular endothelial growth factor and fibroblast growth factors.40 For example, in cardiac fibroblasts and myofibroblasts, MMP-9 degrades collagen IV and V, which ultimately lead to scar formation post-MI.41 It has been shown that MMP-9 plays a critical role in atheroma formation. It attracts neutrophils and monocytes while enhancing the invasion of monocytes via endothelial cells into the subendothelial layer and the formation of foam cells, which is considered the cornerstone step in the process of atherosclerosis.42 Furthermore, these foam cells start producing more MMP-9, leading to the progression of atheroma and the thinning of the fibrous cap that covers the atheroma.43
METHODS
We searched the MEDLINE database (PubMed) using the following phrases: “matrix metalloproteinase-9 and obstructive sleep apnea,” “matrix metalloproteinase-9 and intermittent hypoxia,” “matrix metalloproteinase-9 and hypertension,” “matrix metalloproteinase-9 and myocardial infarction,” “matrix metalloproteinase-9 and cardiac remodeling,” “matrix metalloproteinase-9 and heart failure,” and “matrix metalloproteinase-9 and ischemic stroke.” Exclusion criteria included (1) articles written in a non-English language, (2) studies using animal models, (3) all MMP-9 studies in patients with noncardiovascular or cerebrovascular disorders, (4) all trials that included other MMPs but not MMP-9, and (5) studies in a pediatric population aged < 18 years (because cardiovascular diseases in children are typically congenital rather than ischemic or inflammatory). The search yielded a total of 50 studies. Table 1 summarizes the literature search.
Table 1.
Total number of studies searched, excluded, and included in the review (PubMed).
| Total Studies | Excluded | Included | |
|---|---|---|---|
| MMP-9 and OSA | 21 | 8 | 13 |
| MMP-9 and HTN | 537 | 521 | 16 |
| MMP-9 and heart failure | 326 | 315 | 11 |
| MMP-9 and ischemic stroke | 708 | 698 | 10 |
HTN= hypertension, MMP-9= matrix metalloproteinase-9.
REVIEW
OSA and MMP-9
Few clinical trials have investigated the link between OSA and MMP-9 in adult humans. Most of these trials have shown a correlation between OSA and increased MMP-9 levels. Fang et al44 conducted a meta-analysis that included 18 trials and concluded that MMP-9 levels are higher in participants with severe OSA compared with those with mild and moderate OSA. They found a positive correlation between MMP-9 and the severity of OSA. The meta-analysis included 3 trials that examined the association between MMP-9 (−1562C/T) gene polymorphism and OSA but were unable to show an association between MMP-9 gene polymorphism and the susceptibility for OSA. Most of these studies measured MMP-9 levels in the peripheral venous blood at night before sleep study and in the morning after polysomnography.
Wang, Li, et al45 showed that elevated MMP-9 levels in patients with OSA correlate with cardiovascular disease (HTN and left ventricular hypertrophy). They followed participants for 12 months, and 34% of them developed systemic HTN. The risk was positively correlated with the severity of OSA. Because MMP-9 is under tight regulation by its endogenous inhibitor (TIMP-1), evaluating MMP-9 levels and activity is more comprehensive with TIMP-1 levels and the MMP-9/TIMP-1 ratio. All studies examined (except Hopps et al46) showed no significant change in TIMP-1 levels in OSA (Table 2). Hopps et al46 showed that TIMP-1 and MMP 9 levels are elevated in OSA and correlated with OSA severity.
Table 2.
MMP-9 in patients with OSA.
| Study | Sample Size (men) | OSA Severity (n unless otherwise indicated) | MMP-9 ng/mL | Other Inflammatory Markers | Laboratory Method | Intervention | Results Summary |
|---|---|---|---|---|---|---|---|
| Wang et al,45 China | 47 (36) | Mild = 16; moderate = 12; severe = 19 | ↑ | N/A | Gelatin zymography | No | • Higher MMP-9 levels in patients with OSA correlated with HTN and LVH occurrence |
| • MMP-9 levels positively correlated with AHI, ODI, and MAP | |||||||
| • Risk factors for developing HTN in patients with OSA and without any cardiovascular dysfunction: OSA severity, nadir of oxygen saturation, and MMP-9 | |||||||
| Volna et al,106 Czech Republic | 51 (51) | Mean values: AHI = 31; ODI = 32; SpO2 = 93%; T90% = 15% | ↑ | MMP-2, copper, zinc, hsCRP, sRAGE | ELISA | No | • Strongest correlation between MMP-9, copper, and hsCRP and OSA parameters ODI and mean SpO2, which were higher in patients with severe OSA |
| • Strongest biological markers of oxidative stress in patients with OSA were copper and hsCRP, independent of BMI | |||||||
| • MMP-9 associated with OSA severity, based on ODI, mean SpO2, and T90% | |||||||
| • AHI positively correlated with hsCRP only | |||||||
| • sRAGE protective against oxidative stress in patients with OSA (associated with AHI and ODI) and negatively correlated with BMI | |||||||
| Maeder et al,47 Switzerland | 98 (71) | None/mild = 65; moderate/severe = 33 | No Δ | BNP; NT-proBNP; VEGF; IL-6; insulin | Erenna immunoassay** | Yes; CPAP 1 night | • Increased insulin levels before sleep |
| • Increased IL-6 levels after sleep | |||||||
| • No Δ in BNP, NT-proBNP, or VEGF in none/mild OSA compared with moderate/severe OSA | |||||||
| • No Δ in levels of all these markers after 1 night of CPAP use | |||||||
| Tazaki et al,49 Japan | 66 (66) no women | Control patients/patients who were obese = 18; mild = 24; moderate/severe = 24 | ↑ | TIMP-1; IL-6; TNF-α (2 sets of samples taken before and after sleep) | Gelatin zymography | Yes; CPAP 1 mo | • MMP-9 levels and activity higher in patients with OSA compared with control patients |
| • MMP-9 levels correlated with IL-6 and TNF-α levels in patients with OSA | |||||||
| • TIMP-1 levels did not change between patients with OSA and control patients | |||||||
| • OSA severity related to MMP-9 levels and activity | |||||||
| • Treatment with CPAP in patients with moderate/severe OSA improved MMP-9, IL-6, and TNF-α levels | |||||||
| • Treatment with CPAP did not affect TIMP-1 levels | |||||||
| • Positive correlation between MMP-9 levels and activity and duration of hypoxia in patients with OSA (ie, T90%) | |||||||
| Tamaki et al,50 Nara, Japan | 46 (42) | Control patients = 13; mild/moderate = 13; severe = 20 | ↑ | MCP-1; TNF-α (2 sets of samples taken before and after sleep) | ELISA | Yes; CPAP 3 mo | • MMP-9, MCP-1, and TNF-α levels increased in patients with severe OSA compared with control patients |
| • MMP-9, MCP-1, and TNF-α levels did not change significantly in patients with mild or moderate OSA compared with control patients | |||||||
| • TNF-α levels correlated with OSA severity | |||||||
| • Treatment with CPAP (3 months) decreased MMP-9, MCP-1, and TNF-α levels | |||||||
| Bonanno et al,52 Italy | 50 (50) no women | RDI < 30 = 25; RDI > 30 = 25; normotensive = 13; HTN = 13 | No Δ | Relaxin; TIMP-1; MMP-9/TIMP-1; MMP-2; TIMP-2; MMP-2/TIMP-2; VEGF | ELISA | No | • Relaxin did not differ between OSA patients who were normotensive and hypertensive suggesting that relaxin does not lay a role in OSA-induced hypertension |
| • MMP-9 and TIMP-1 did not differ between different degrees of OSA (however, there was a trend for increased MMP-9 in severe OSA, not statistically significant) | |||||||
| • MMP-2 was lower in patients with severe OSA | |||||||
| • Detectable relaxin levels not associated with higher VEGF levels | |||||||
| • VEGF positively correlated with MMP-9 | |||||||
| • VEGF levels higher in patients with severe OSA | |||||||
| Hopps et al,105 Italy | 79 (53) | Control patients = 31; mild = 21; severe = 27 | ↑ | TIMP-1; MMP-9/TIMP-1; MMP-2; TIMP-2; MMP-2/TIMP-2 | ELISA | No | • MMP-9, TIMP-1, MMP-2, and TIMP-2 elevated in patients with OSA compared with control patients |
| • MMP-9, TIMP-1, MMP-2, and TIMP-2 elevated more in severe OSA than in mild OSA | |||||||
| • MMP-9/TIMP-1 ratio decreased in patients with OSA compared with control patients | |||||||
| • MMP-9/TIMP-1 ratio decreased more in severe OSA than in mild OSA | |||||||
| • No significant variation in MMP-2/TIMP-2 ratio between OSA group and control group or between subgroups of OSA | |||||||
| • Positive correlation between MMP-9 and AHI | |||||||
| • Positive correlation between MMP-9 and ODI | |||||||
| • Negative correlation between MMP-9 and mean SpO2 | |||||||
| • Positive correlation between MMP-9 and neck circumference | |||||||
| Hopps et al46 | 48 (37) | Low grade group (mild and moderate) = 21; High grade group (severe) = 27 | ↑ | Gelatinases and their inhibitors: MMP-2, MMP-9, TIMP-1, TIMP-2; oxidative status; lipid peroxidation (TBARS); protein peroxidation (PC); TAS; NOx metabolites | ELISA | No | • Significant increase in lipid and protein peroxidation and decrease in NOx metabolites in H group compared with L group |
| • Significant increase in MMP-9 and TIMP-1 levels in H group compared with L group | |||||||
| • No significant difference in MMP-2 and TIMP-2 between H and L groups | |||||||
| • Correlations between MMPs, oxidative stress, and OSA parameters | |||||||
| • Positive correlation between MMP-9 and TBARS | |||||||
| • Positive correlation between MMP-2 and TAS | |||||||
| • Negative correlation between TIMP-1 and TBARS | |||||||
| • Positive correlation between MMP-9 and AHI and ODI but negative correlation with mean SpO2 | |||||||
| Chuang et al,107 Taiwan | 28 (28) no women | AI 0 = 11; AI 1–5 = 9; AI > 5 = 8 | ↑ | MMP-1; MMP-2; MMP-3; TIMP-1 (2 sets of samples taken before and after sleep) | ELISA; zymography (for MMP-9 activity); real-time PCR (for MMP-9 gene expression in monocytes) | No | • MMP-9 levels and activity increased in all groups, especially in patients with severe OSA |
| • MMP-9 levels correlated with OSA severity | |||||||
| • MMP-9 gene expression in monocytes correlated with OSA severity and MMP-9 levels | |||||||
| • MMP-1, MMP-2, MMP-3, and TIMP-1 did not change between OSA groups | |||||||
| Feng et al,108 China | 100 | Control patients= 50; OSA = 50 | ↑ | FFA | ELISA | No | • Both MMP-9 and FFA levels higher in patients with OSA compared with control patients |
| • Both MMP-9 and FFA levels in OSA together associated with higher cardiovascular risk compared with either one or the other alone | |||||||
| Vuralkan et al,51 Turkey | 25 (14) | Mean AHI = 17 (sleep studies and blood samples obtained before surgery [UPF] and 6 months after surgery) | ↑ | MDA | ELISA | Yes; UPF | • MMP-9 and MDA higher in patients with OSA before UPF surgery compared with postoperative levels |
| Nizam et al,48 Turkey | 50 (30) | Control patients = 13; mild/moderate = 17; severe = 20 | No Δ | MMP-8; TIMP-1; MMP-8/TIMP-1; pro-MMP-9; pro-MMP-2; NE; (serum and salivary levels) | ELISA; gelatin zymography; IFMA | No | • In serum samples, only pro-MMP-9 lower in patients with severe OSA compared with control patients; other MMPs did not show significant difference in the 3 groups |
| • In salivary samples, NE significantly lower in patients with mild-moderate and severe OSA compared with control patients | |||||||
| • Serum and salivary NE and pro-MMP-2 lower in patients with OSA compared with control patients | |||||||
| Ye al,109 China | 76 | Patients who were obese/control patients= 25; mild = 23; moderate-severe = 28 | ↑ | CRP | ELISA | No | • MMP-9 significantly higher in patients with moderate to severe OSA than in patients with mild OSA and mild OSA than patients who were obese and control patients |
| • CRP and MMP-9 positively moderately correlated with AHI even after adjusting for age and BMI | |||||||
| • CRP levels moderately correlated with levels of MMP-9 in patients with OSA |
BMI = body mass index, BNP = brain natriuretic peptide, ELISA = enzyme-linked immunosorbent assay, FFA = free fatty acids, hsCRP = high sensitivity C-Reactive Protein, HTN = hypertension, IFMA = immunofluorometric assays, LVH = left ventricular hypertrophy, MAP = mean arterial pressure, MCP-1 = monocyte chemoattractant protein-1, MDA = malondialdehyde, MMP = matrix metalloproteinase, NE = neutrophil elastase, NOx = nitric oxide metabolites, ODI = oxygen desaturation index, PC = protein carbonyl, PCR = polymerase chain reaction, RDI = Respiratory Disturbance Index, SPO2= oxygen saturation, sRAGE = soluble Receptor of Advanced Glycation end Products, TAS = total antioxidant status, TBARS = thiobarbituric acid-reactive substances, TIMP = tissue inhibitor of matrix metalloproteinase, TNF-α = tumor necrosis factor-alpha, T-90% = Time spent with SO2 < 90%, UPF = uvulopalatal flap, VEGF = vascular endothelial growth factor. **Erenna instruments - MilliporeSigma, Billerica, MA.
On the contrary, Maeder et al47 did not show a correlation between MMP-9 levels and OSA or OSA severity (moderate/severe OSA compared to no/mild OSA). They also did not show any impact of PAP therapy on MMP-9 levels. However, PAP therapy was used only for 1 night in this trial. Nizam et al48 did not show any difference in MMP-9 levels or MMP-9 activity in both serum and saliva among all OSA groups in their study.
Two other studies evaluated the impact of PAP therapy on MMP-9 levels and activity for a short period (Table 1). Tazaki et al49 and Tamaki et al50 tested CPAP for 1 and 2 months, respectively, and both showed that PAP therapy reduced MMP-9 levels in patients with severe OSA compared with patients with mild OSA and control patients. Vuralkan et al51 showed that uvulopalatal flap (a surgical intervention for OSA) reduced MMP-9 levels.
Because there is a cross-reactivity between MMP-9 and other MMPs, some studies evaluated the levels of other MMPs in addition to MMP-9. Bonanno et al52 showed that MMP-2 levels were lower in patients with severe OSA. However, all other MMP levels did not show any significant correlation with OSA severity. Other inflammatory mediators and cytokines measured in these trials were increased in participants with OSA compared with control participants (eg, interleukin-1, interleukin-6, tumor necrosis factor-α, monocyte chemoattractant protein-1, and C-reactive protein). Table 2 lists these studies with more detailed results and methodology.
The variation in MMP-9 levels in some of these studies could be related to several reasons. The sample size in all these studies was small (< 100 participants). Furthermore, the laboratory assays used to assess MMP-9 levels were not standard. Some laboratories used enzyme-linked immunosorbent assay (ELISA) kits, and others used gelatin zymography. In addition, all studies measured venous blood (serum or plasma) levels but not tissue MMP-9 levels. MMP-9 blood levels do not necessarily reflect the tissue level: For example, an elevated MMP-9 blood level does not mean elevated levels in tissue.
Systemic HTM and MMP-9
Many trials have evaluated the role of MMP-9 and TIMP-1 as significant players in the remodeling process that takes place in the walls of major blood vessels and myocardial tissues leading to HTN heart diseases. In the very early stages of HTN, MMP-9 degrades fibrillar collagen in the wall of blood vessels, leading to distensibility, which allows the blood vessels to accommodate more blood volume with a lower increase in blood pressure. This phase is followed by a compensated phase in which collagen deposition increases, leading ultimately to arterial wall stiffness.53 MMP-9 levels are increased in a linear relationship with arterial wall stiffness.54,55 Concomitantly, more collagen deposition takes place in the myocardial tissues (remodeling).56 MMP-9 levels further increase during the transition from ventricular hypertrophy to congestive HF.57
The results of studies conducted to assess MMP-9 levels in patients with HTN are conflicting. Yasmin et al58 evaluated aorta media stiffness in patients with isolated systolic HTN and concluded that MMP-9 was the most important predictor of aortic wall stiffness using pulse wave velocity regardless of patient age. In addition to MMP-9, serum elastase activity increased, contributing to the degradation of elastin, the major component of the elastic laminae of the aorta, and subsequently increasing arterial wall stiffness. Furthermore, elevated levels of C-reactive protein suggest that other inflammatory pathways could be potential sources of collagen deposition in the aortic wall.
It also seems that the size of the blood vessel plays a role. Large-sized arteries show higher MMP-9 and TIMP-1 levels compared with smaller muscular arteries. Tan et al54 showed that a more prominent elevation in MMP-9 and TIMP-1 correlated positively with carotid-femoral pulse wave velocity but not the MMP-9/TIMP-1 ratio. The role of TIMP-1 in HTN and cardiac remodeling is not completely understood. Because TIMP-1 exerts an inhibitory action on all MMPs (not only MMP-9), TIMP-1 may inhibit MMP-1 and MMP-8, decreasing the turnover of ECM and enhancing collagen deposition in the wall of large arteries and subsequently increasing stiffness.
A meta-analysis conducted by Marchesi et al59 concluded that MMP-9, in addition to TIMP-1 and MMP-2, plays a major role in the remodeling process in patients with HTN. Although both MMP-9 and TIMP-1 are elevated in HTN without HF, the authors noticed much higher elevation in TIMP-1 levels compared with MMP-9 levels (a lower MMP-9/TIMP-1 ratio) in patients with HTN heart diseases, suggesting a significant role of TIMP-1 in cardiac remodeling in patients with HTN. Ahmed et al57 used MMP-9 and TIMP-1 levels to predict the risk of developing HF in patients with HTN. They found that whereas patients with HTN but normal left ventricular function had normal MMP-9 and normal TIMP-1 levels, those with TIMP-1 levels higher than MMP-9 levels were more likely to develop congestive HF. Accordingly, they concluded that the MMP-9/TIMP-1 ratio can be used to predict the risk of developing HF in patients with HTN. Tayebjee, Nadar, MacFadyen, et al60 showed elevated MMP-9 and TIMP-1 levels in patients with HTN but showed only elevated TIMP-1 in patients with HTN and left ventricular hypertrophy, raising the possibility that MMP-9 may play a more significant role in vascular remodeling and tone compared to cardiac remodeling. However, Saglam et al61 showed contradictory results where both MMP-9 and MMP-3 were elevated in patients with HTN and MMP-9 levels were correlated with left ventricular hypertrophy.
Some trials have also evaluated the impact of treatment with antihypertensive agents on MMP-9 levels. Onal et al62 showed that MMP-9 and TIMP-1 levels were elevated in patients with HTN and that treatment with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers decreased MMP-9 levels. Tayebjee, Nadar, Blann, et al63 had a similar conclusion where they noticed a reduction in MMP-9 levels after treatment with antihypertensive agents. However, they also noticed an elevation in TIMP-1 with treatment, which was not statistically significant yet still surprising. This finding may be related to the activation of other MMPs (eg, MMP-1, MMP-2, MMP-3, or MMP-10) by antihypertensive agents. For example, MMP-1 is one of the collagenases that directly degrade collagens type I and III that form most of the elastic laminae of blood vessel walls.
On the contrary, some trials have shown decreased levels of MMP-9 in patients with HTN. Li-Saw-Hee et al64 noticed that both MMP-9 and TIMP-1 are reduced in patients with HTN, and treatment with ACE inhibitors or ARB did not affect their levels. Zervoudaki, Economou, Stefanadis, et al65 and Zervoudaki, Economou, Pitsavos, et al66 showed similar results; however, they noticed that MMP-9 levels increased with the use of amlodipine compared with felodipine or diltiazem. Table 3 lists these studies with more detailed results and methodology.
Table 3.
MMPs, MMP inducers, and MMP inhibitor roles in ECM metabolism in patients with HTN.
| Study | Study Design | Sample Size (men) | Treatment (duration) | MMP-9 ng/mL (before Rx) | TIMP-1 ng/mL (before Rx) | Other MMPs and Inflammatory Markers | Laboratory Method | Results Summary |
|---|---|---|---|---|---|---|---|---|
| Laviades et al110 | Case-controlled | 60 (41): control patients = 23; HTN = 37 | Lisinopril (1 y) | N/A | ↑ | MMP-1 ↓ | ELISA | • Patients with HTN and LVH had more depressed extracellular degradation of type I collagen |
| • Treatment resulted in increased MMP-1 and decreased TIMP-1 | ||||||||
| Li-Saw-Hee et al64 | Case-controlled | 56 (16): control patients = 24; HTN = 32 | Enalapril or losartan (8 wks) | ↓ | ↓ | None | ELISA | • Treatment did not impact MMP-9 or TIMP-1 levels |
| • No relationships between MMP-9 or TIMP-1 and LV mass or diastolic dysfunction | ||||||||
| Zervoudaki et al,65 | Case-controlled | 67 (29): control patients = 25; HTN = 42 | Amlodipine (6 mo) | ↓ | Not tested | MMP-2 ↓ | ELISA | • Patients with HTN and higher SVR (> 1,440 dynes/s/cm-5) showed further lower MMP-9 and MMP-2 levels |
| • Treatment with amlodipine increased MMP-9 levels only | ||||||||
| Zervoudaki et al,66 | Randomized controlled trial (randomized to diltiazem and felodipine) | 117 (68): control patients = 45; HTN = 72 | Diltiazem and felodipine (6 mo) | ↓ | Not tested | MMP-2 ↓ | ELISA | • Treatment with felodipine increased MMP-2 levels only |
| • Treatment with diltiazem did not affect MMP-2 or MMP-9 | ||||||||
| • Intensity of alteration of MMP-2 levels independent of felodipine, suggesting a pressure-independent mechanism | ||||||||
| Tayebjee et al,63 recruited participants from the ASCOT trial | Case-controlled | 141 (108): control patients = 45; HTN = 96 (untreated = 12, treated = 84) | N/A (3 y) | ↑ | ↑ | None | ELISA | • MMP-9 levels decreased and TIMP-1 levels increased in treated group vs untreated group (change not statistically significant) |
| Yasmin et al58 | Case-controlled | 240 (117): Isolated systolic hypertension (ISH) = 116, Control = 114 | N/A | ↑ | N/A | MMP-2: ↑; TIMP-2: no Δ; SEA: ↑ | ELISA | • Trial used ISH to study arterial wall stiffness |
| • MMP-9, MMP-2, and SEA increased in patients with ISH | ||||||||
| • MMP-9, MMP-2, and SEA correlated with aortic PWV (MMP-9 was strongest) | ||||||||
| • CRP independently correlated with PWV | ||||||||
| • Positive association between MMP-9 and CRP in healthy group | ||||||||
| Saglam et al61 | Case-controlled | 50 (34): all HTN (LVH = 27, no LVH = 23) | N/A | ↑ with LVH | N/A | MMP-3 ↑ with LVH | EIA | • MMP-9 and MMP-3 levels increased in patients with HTN and LVH |
| • MMP-9 and MMP-3 levels correlated with LV diastolic dysfunction | ||||||||
| Tayebjee et al60 | Case-controlled | 108 (81): control patients = 34; HTN = 74 (untreated = 27, treated = 47) | N/A (only noted patients who were antihypertensive) | ↑ | ↑ | None | ELISA | • MMP-9 and TIMP-1 levels increased in patients with HTN (treated or untreated) |
| • TIMP-1 only correlated with LV mass and diastolic dysfunction but not MMP-9 | ||||||||
| Ahmed et al57 | Case-controlled | 102 (64): control patients = 53 (HTN = 14, no HTN = 39); LVH = 49 (CHF = 26, no CHF = 23) | Different agents by PCP: diuretics, ACE inhibitors, ARB, aldosterone blockers, vasodilators, α blockers, β blockers | ELISA | • Patients with HTN and normal LV structure and function had normal MMP/TIMP profile | |||
| • Patients with HTN and LVH without CHF had decreased MMP-2, MMP-13, and MMP-9 | ||||||||
| • Elevated TIMP-1 strongly associated and a strong predictor for LVH and CHF (especially levels ≥ 1,200 ng/mL) | ||||||||
| Derosa et al,111 | Case-controlled | 192 (97): control patients = 96; HTN = 96 | N/A | ↑ | ↑ | MMP-2 ↑ | ELISA | • Levels and activities of MMP-2, MMP-9, and TIMP-1 increased in patients with HTN |
| Onal et al62 | Case-controlled | 49 (18): control patients = 16; HTN = 33 | Lisinopril or candesartan (3 mo); patients on previous antihypertensive medications stopped for 1 wk before enrollment | ↑ not statistically significant | ↓not statistically significant | N/A | ELISA | • MMP-9 decreased, TIMP-1 increased after treatment |
| • 24 h urinary albumin excretion did not significantly change | ||||||||
| • No Δ between lisinopril and candesartan groups | ||||||||
| • Changes in MMP-9 and TIMP-1 not correlated with changes in BP measurements or 24 h urinary albumin excretion | ||||||||
| Collier et al,112 | Cross-sectional; observational | HTN with HFpEF = 181; HTN asymptomatic = 94 (LAVI ≥ 34 mL/m2 = 30, LAVI < 34 mL/m2 = 64) | N/A | ↑ in HFpEF | ↓ in HFpEF | MMP-2; CITP; PIIINP; PINP; PICP; MCP-1; IL-6; IL-8; TNF-α (for levels, see Results Summary) | ELISA, EIA, ECIA | • Patients who were AH and with LAVI ≥ 34 mL/m2 had higher levels of MMP-2 and lower levels of TIMP-1 than those with LAVI < 34 mL/m2 |
| • MMP-9, MMP-2, BNP, PIIINP, and CITP levels significantly correlated with LAVI | ||||||||
| • Patients with HTN and HFpEF had higher MMP-9, MMP-2, BNP, PIIINP, CITP, IL-6, IL-8, and MCP-1 | ||||||||
| • No significant Δ in TNF-α between HFpEF and AH | ||||||||
| • MMP-9/TIMP-1 significantly identified patients with AH with risk of LVDD compared with BNP | ||||||||
| Tan et al54 | Cross-sectional | 256 (197): control patients = 54; HTN = 202 (never treated = 67, treated = 135) | Antihypertensive medications not specified (median 5 y) | ↑ | ↑ | IL-6: no Δ; sCD40L: no Δ | ELISA | • MMP-9 and TIMP-1 levels increased in HTN |
| • MMP-9 and TIMP-1 levels positively correlated with large arterial stiffness measures | ||||||||
| • MMP-9/TIMP-1 ratio not different between patients with HTN and control patients and not correlated with arterial stiffness | ||||||||
| • IL-6 and sCD40L not correlated with large arterial stiffness | ||||||||
| Ergul et al67 2004 | Case-controlled | 32 (24): control patients = 13; HTN = 19 | CCB, ACEI, β blockers, diuretics, ARB | ↓ | N/A | MMP-2 ↓; EMMRIN ↓; MT-1-MMP ↓; FGF-2 ↑ | Zymography | • In addition to reduction in MMPs, their inducers and activators were also downregulated |
| • Increased deposition of ECM proteins (FGF-2) | ||||||||
| Ritter et al113 | Cross-sectional | 122 (40): all TRH (patients who were obese = 67, patients who were not obese = 55) | Three anti-hypertensive agents at optimal doses, the exact medications used are not available | ↑ | No Δ | MMP-2: no Δ; TIMP-1: no Δ; TIMP-2: no Δ | ELISA | • MMP-9 and MMP-9/TIMP-1 increased in patients who were obese with TRH compared with patients who were not obese with TRH |
| • MMP-9 and MMP-9/TIMP-1 associated with LVH in patients who were obese with TRH | ||||||||
| • CRP increased in patients who were obese with TRH | ||||||||
| • Positive correlation between MMP-9 and fat mass | ||||||||
| Friese et al,114 | Cross-sectional | 68 (52): control patients = 18; HTN = 20; HTN and ESRD = 30 | ACEI, α antagonists, ARB, diuretics, β blockers, CCB | ↑ | N/A | MMP-1; MMP-2; MMP-3; MMP-10 (for levels, see Results Summary) | ELISA, ECIA | • MMP-9 increased in patients with HTN compared with control patients |
| • No Δ in MMP-9 between ESRD and HTN | ||||||||
| • MMP-2 and MMP-10 higher in patients with HTN-ESRD compared with patients with HTN and control patients | ||||||||
| • No Δ in MMP-2 and MMP-10 between patients with HTN and control patients | ||||||||
| • Positive correlation between MMP-2 and MMP-10 | ||||||||
| • Positive correlation between MMP-9 and systolic BP | ||||||||
| • Negative correlation between MMP-2 and BMI |
ACEI = angiotensin-converting enzyme inhibitors, AH = asymptomatic with HTN, ARB = angiotensin receptor blockers, BMI = body mass index, BNP = brain natriuretic peptide, BP = blood pressure, CCB = calcium channel blockers, CHF = congestive heart failure, CITP = carboxy-terminal telopeptide of collagen-I, CRP = C-reactive protein, ECIA = electrochemiluminescence immunoassay, ECM = extracellular matrix, EIA = enzyme immunoassay, ELISA = enzyme-linked immunosorbent assay, ESRD = end-stage renal disease, FGF-2 = fibroblast growth factor-2, HFpEF = heart failure with preserved ejection fraction, HTN = hypertension, IL = interleukin, ISH = isolated systolic hypertension, LAVI = left atrium volume index, LV = left ventricular, LVDD = left ventricular diastolic dysfunction, LVH = left ventricular hypertrophy, MCP-1 = monocyte chemoattractant protein-1, MMP = matrix metalloproteinase, MT-1-MMP = Membrane type 1-matrix metalloproteinase, PICP = carboxy-terminal propeptide of collagen type I, PIIINP = amino-terminal propeptide of procollagen type III, PINP = procollagen type I N propeptide, PWV = pulse wave velocity, Rx = prescription, sCD40L = soluble CD40L, SEA = serum elastase activity, SVR = systemic vascular resistance, TIMP = tissue inhibitor of matrix metalloproteinase, TNF = tumor necrosis factor, TRH = treatment-resistant hypertension.
The change in MMP-9 and TIMP-1 levels in different trials can be related to multiple factors. For example, different methodologies are used in different laboratories (eg, ELISA, gelatin zymography, or reverse zymography). Many patients were on medications at the time of enrollment that could potentially alter these levels. Most of the studies used a small sample size, which can affect the power of the test. Finally, the levels of MMP-9 and TIMP-1 may vary at different stages of HTN. In early stages of the disease, MMP-9 levels are high to facilitate smooth muscle cell migration; however, at a late stage of the disease, endogenous inhibitors predominate, which suppresses MMP-9, allows for much lower ECM turnover, and enhances collagen deposition in the arterial walls.67
In summary, MMP-9 seems to play a major role in blood vessel wall remodeling and blood vessel tone and TIMP-1 seems to play a major role in cardiac remodeling and developing HF.
MI, cardiac remodeling, and MMP-9
As mentioned earlier herein, MMP-9 plays a critical role in atheroma formation through endothelial dysfunction, neutrophil and monocyte attraction, monocyte invasion, smooth muscle cell migration, and foam cell formation. Furthermore, foam cells synthesize and release more MMP-9, which enhances ECM degradation (collagen I, III, IV, V, VI, and XVI),68 leading to rupture of the fibrous cap.69,70 This process exposes the injured part of the fibrous cap to the bloodstream, which is an extremely thrombogenic process leading to thrombus formation and subsequent coronary artery occlusion. Depending on the setting, MMP-9 is protective (the healing process in the fibrous cap) and harmful at the same time (degrading the surface of the fibrous cap). The same principle applies clinically to MI. In the early stages of MI (the first 24 hours), elevated MMP-9 helps in the healing process;71,72 however, further activation of MMP-9 will destabilize and further expand MI.73
Opstad et al74 conducted the NORDISTEMI study, a randomized controlled trial examining MMP-9, TIMP-1, and the MMP inducer Extracellular matrix metalloproteinase inducer in patients with acute ST-elevation MI at 3 days and 3 months post-MI. They concluded that Extracellular matrix metalloproteinase inducer, MMP-9, and TIMP-1 levels and the MMP-9/TIMP-1 ratio declined from day 3 to 3 months. Furthermore, TIMP-1 levels at day 3 correlated significantly with infarct size, troponin T, and amino-terminal pro-B-type natriuretic peptide. These findings further support the role of TIMP-1 in the remodeling process.
One of the most deleterious complications post-MI is HF, which is classified into 2 categories: HF with reduced ejection fraction, which is usually seen as a complication of myocardial ischemia, and HF with preserved ejection fraction, which is usually seen as a complication of long-standing HTN. All trials showed evidence that MMP-9 is upregulated in both reduced and preserved HF; however, TIMP-1 was upregulated in some trials and downregulated in others (Table 4). Li, Feldman, et al75 concluded that the reduction in TIMP-1 levels correlated with the elevation in MMP-9 levels to favor ECM degradation in both HF with reduced ejection fraction and HF with preserved ejection fraction. Martos et al76 showed a correlation of MMP-9 levels at different stages of HF with preserved ejection fraction (impaired relaxation, pseudo-normal, and restrictive-like filling). Morishita et al77 concluded that MMP-9 levels correlated with disease activity in both types of HF despite low levels of B-type natriuretic peptide. Lakhani et al78 emphasized the elevation in MMP-9 levels in patients with post-MI HF. This elevation was more prominent with reduced ejection fraction compared to preserved ejection fraction. Franz et al79 investigated MMP-9 levels in 2 subtypes of HF with preserved ejection fraction and noticed that MMP-9 levels were higher in concentric HF than in eccentric HF. MMP-9 levels were univariate predictors of all-cause mortality in patients with systolic HF. In a trial conducted by Dini et al,80 MMP-9 was the only biomarker that was independently associated with prognosis in patients with HF with reduced ejection fraction. Yan et al81 concurred with these results and showed that MMP-9 levels were correlated with lower left ventricular ejection fraction and subsequent deterioration of left ventricular function. However, no causation was established in this trial. Table 4 summarizes the trials addressing the role of MMP-9 in cardiac remodeling.
Table 4.
MMP-9 levels and association with ischemic and nonischemic congestive heart failure.
| Study | Sample Size (men) | Cardiovascular Disease | MMP-9 | Other MMPs | Laboratory Method | Results Summary |
|---|---|---|---|---|---|---|
| Thomas et al,115 | Control patients = 8 donors; DCM = 7 explanted hearts | Nonischemic DCM | ↑ | MMP-1; MMP-2; MMP-3; TIMP-1; TIMP-2 | Zymography (activity); hs chemiluminescence detection (MMP); immunoblotting (TIMP) | • ↓ zymographic activity of MMP-1 and ↓ relative abundance by 80% |
| • ↑ zymographic activity of MMP-9 and MMP-3 and relative abundance by 4-fold | ||||||
| • No Δ in MMP-2 activity | ||||||
| • ↑ abundance of TIMP-1 and TIMP-2 | ||||||
| • ↑ TIMP-1/MMP-1 | ||||||
| • ↑ TIMP-2/MMP-2 | ||||||
| Reinhardt et al,116 | Control patients = 6 donors; DCM = 29 explanted hearts (ischemic = 13, idiopathic = 16) | DCM (ischemic and nonischemic) | ↑ | MMP-2 | Bio-Rad dye binding assay *Bio-Rad Laboratories, Inc. 2000 Alfred Nobel Dr., Hercules, CA; zymography | • MMP-9 upregulated in DCM regardless of etiology |
| • MMP-2 upregulated only in idiopathic DCM | ||||||
| Li, Feldman,et al75 | 46 (32): control patients = 15; DCM = 15; ICM = 16 (EF not available) | DCM, ICM | ↑ | TIMP-1; TIMP-3; TIMP-4 | ELISA; gelatin zymography | • Upregulation in MMP-9 in both DCM and ICM |
| • Downregulation in TIMP-1 and TIMP-3 in both ICM and DCM | ||||||
| • Downregulation in TIMP-4 only in ICM | ||||||
| • Correlation of decreased TIMP-1 and increased MMP-9 favoring ECM degradation in DCM and ICM | ||||||
| George et al,117 | 118 (63): control patients = 30; CHF = 88 | CHF | ↑ | MMP-2; TIMP-1; BNP | ELISA | • MMP-9, MMP-2, and TIMP-1 |
| • MMP-2 levels correlated with symptoms (NYHA) | ||||||
| • MMP-2 > 352 ng/mL predicted death or hospitalization secondary to HF | ||||||
| • MMP-2 and NT-proBNP independent predictors of mortality in patients with CHF | ||||||
| Martos et al76 | 86: control patients = 20; DD = 64 (impaired relaxation = 38, pseudonormal = 10, restrictive-like filling = 16) | DHF (3 phases of DD): impaired relaxation, pseudonormal, restrictive-like filling | ↑ | PICP; PIIINP; CITP; MMP-1; MMP-2; TIMP-1; PINP; BNP | ELISA; RIA | • PICP, PIIIPN, CITP, MMP-2, and MMP-9 with advance of DD phase to DHF |
| • Positive correlation between MMP-2 and PICP levels and LAVI | ||||||
| Morishita et al77 | 173 (114): chronic HF = 173; + HF events = 35; – HF events = 138 | Ischemic and nonischemic DCM | ↑ | TIMP-1; IL-6; TNF-α; ANP; BNP; NA | Sandwich enzyme immunoassay | • MMP-9, TIMP-1, and MMP-9/TIMP-1 ratios correlated with disease severity |
| • MMP-9 levels correlated with IL-6, NA, and TNF-α | ||||||
| • MMP-9 levels strong predictors of HF events in the long term, even with low BNP | ||||||
| • BNP and MMP-9/TIMP-1 ratios lower in patients with HFpEF compared with HFrEF | ||||||
| • MMP-9 and TIMP-1 levels similar in patients with HFpEF and HFrEF | ||||||
| • No correlation between MMP-9 and BNP | ||||||
| Lakhani et al78 | 43 (26): control patients = 14; MI + pEF = 13; MI + rEF = 16 (only WV population) | Post-MI HF | ↑ | TGF-β; IL-6; TNF-α; IL-10 | ELISA | • MMP-9 levels higher in patients post-MI with rEF compared with patients post-MI with pEF and control patients |
| • TGF-β levels higher in patients post-MI with rEF compared with patients post-MI with pEF and control patients | ||||||
| • TNF-α levels higher in patients post-MI with rEF compared with patients post-MI with pEF and control patients | ||||||
| • IL-6 levels significantly higher in patients post-MI with both rEF and pEF compared with control patients and in patients post-MI with rEF compared with pEF | ||||||
| • IL-10 levels directly correlated with EF in the first 24 h post-MI in patients who progress to HF | ||||||
| Franz et al79 | 107 (73): control patients = 12; HHD = 95 | HHD (concentric LVH and eccentric LVH) | ↑ | Tenascin-C (B domain); tenascin-C (C domain); TIMP-1; TIMP-2; TIMP-4 | ELISA | • MMP-9 levels higher in both CH and EH compared with control patients and higher in CH compared to EH |
| • No Δ in TIMP-1, TIMP-2, and TIMP-4 between all groups | ||||||
| • TIMP-2 levels higher in EH and CH groups than in control patients | ||||||
| • Tenascin-C (B domain) levels increased in both CH and EH compared with control patients and higher in EH than in CH | ||||||
| Buralli et al,118 | 134 (102): control patients (nl MMP-3, MMP-9) = 87; DCM (abn MMP-3, MMP-9) = 47 | DCM (ischemic and nonischemic) | ↑ | MMP-3 | ELISA | • MMP-3 and MMP-9 levels univariate predictors of all-cause mortality in patients with systolic HF |
| • Only MMP-9 was independent risk factor for adverse prognosis | ||||||
| • Addition of MMP-9 to echo parameters (Doppler analysis and mitral annular velocity) may improve prognostic stratification in both ischemic and nonischemic DCM | ||||||
| Dini et al80 | 127 (99): control patients = 58; cardiac events = 69 (all participants had systolic HF with previous hospital admission) | Cardiac events defined as all-cause mortality and hospitalization for worsening HF | ↑ | MMP-3; BNP | ELISA | • MMP-3, MMP-9, and BNP predicted outcome in patients with HF |
| • MMP-9 only biomarker independently associated with prognosis; higher levels associated with worse prognosis | ||||||
| Yan et al81 | 768 (640): study = 184; participants from RESOLVD trial = 584 | Ischemic and nonischemic DCM | ↑ | MMP-2; TIMP-2 | ELISA | • MMP-9 levels correlated with lower LVEF and ESV and subsequent deterioration of LV function (no causal association) |
| • MMP-2 and TIMP-1 levels not associated with LV function |
abn = abnormal, ANP = Atrial Natriuretic Peptide, BNP = brain natriuretic peptide, CH = concentric hypertrophy, CHF = congestive heart failure, CITP = carboxy-terminal telopeptide of collagen-I, DCM = dilated cardiomyopathy, DD = Diastolic Dysfunction, DHF = diastolic heart failure, EF = ejection fraction, EH = eccentric hypertrophy, ELISA = enzyme-linked immunosorbent assay, ESV = end-systolic volume, HF = heart failure, HFpEF = heart failure with preserved ejection fraction, HFrEF = heart failure with reduced ejection fraction, HHD = hypertensive heart disease, ICM = ischemic cardiomyopathy, IL = interleukin, LAVI = left atrium volume index, LVEF = left ventricular ejection fraction, LVH = left ventricular hypertrophy, MI = myocardial infarction, MMP = matrix metalloproteinase, NA = noradrenaline, nl = normal, NT-proBNP = N-terminal pro-brain natriuretic peptide, NYHA = New York Heart Association, PICP = carboxy-terminal propeptide of collagen type I, PIIINP = amino-terminal propeptide of procollagen type III, PINP = procollagen type I N propeptide, pEF = preserved ejection fraction, rEF = reduced ejection fraction, TGF = tumor growth factor, TIMP = tissue inhibitor of matrix metalloproteinase, TNF = tumor necrosis factor, WV = West Virginia.
Cerebrovascular diseases (ischemic stroke) and MMP-9
MMP-9 has been studied heavily in cerebrovascular diseases, especially in acute ischemic stroke, and several trials confirmed its role as a biological marker in ischemic stroke. Montaner et al,82 Zhong et al,83 and Horstmann et al84 concluded that MMP-9 levels are positively correlated with stroke severity (defined by the National Institutes of Health Stroke Scale) at 12, 24, and 48 hours from the onset of stroke symptoms. They also noticed a positive correlation between MMP-9 levels and the size of the infarcted area in addition to a correlation with neurological impairment post-stroke. Lucivero et al85 compared MMP-2 and MMP-9 levels in different stroke types and concluded that whereas elevated MMP-2 is usually associated with lacunar infarcts, elevated MMP-9 levels are associated with more severe stroke. They also concluded that MMP-9 is associated with worse prognosis compared to MMP-2.
Hypertensive cerebrovascular disease is a well-known pathophysiology in the development of cerebral small-vessel disease and lacunar infarction. Research has shown that not all lacunar infarctions are associated with embolic phenomenon. In some cases, systemic HTN can induce pathological changes in the wall of small perforating arterioles that can lead to severe and sometimes complete occlusion, leading to the loss of autoregulatory mechanisms in cerebral blood vessels and subsequently lacunar infarction. These changes include vessel enlargement, hemorrhage, and fibrinoid deposition.86 They were first described by Fisher in the 1960s87 and called lipohyalinosis. It is unknown whether lipohyalinosis severity is correlated with MMP levels.
Moldes et al88 examined the role of MMP-9 in patients who develop cerebral edema after acute ischemic stroke and concluded that MMP-9 levels are higher in patients with cerebral edema (although this finding was not statistically significant). Reynolds et al89 analyzed different biological markers, trying to achieve an algorithmic approach to diagnose stroke within the first 12 hours of symptoms. They concluded that an algorithm of 3/5 positive markers (MMP-9, von Willebrand factor, S100 calcium-binding protein B, B-type neutrotrophic growth factor, and monocyte chemoattractant protein-1) had a 92% sensitivity and 93% specificity. This algorithm was favorably comparable to a computed tomography scan. Another important role of MMP-9 is as a predictive tool of the risk of hemorrhagic transformation after thrombolytic therapy.82,90,91A summary of these studies with detailed results and methodology is listed in Table 5.
Table 5.
MMP-9 levels in patients with ischemic stroke.
| Study | Study Design | Sample Size (men) | MMP-9 ng/mL | Other MMPs | Laboratory Method (assay) | Results Summary |
|---|---|---|---|---|---|---|
| Zhong et al83 | Observational randomized trial | 3,186 (2,008) | ↑ | None | ELISA | • Dose-dependent association between higher MMP-9 levels and increased risk of major disability and mortality |
| Montaner et al82 | Prospective observational trial | 39 (20) | ↑ | MMP-2: no Δ | ELISA | • Stroke severity (defined as NIHSS score) correlated with MMP-9 levels at different timepoints (12, 24, and 48 h) |
| • Neurological impairment from embolic stroke correlated with MMP-9 | ||||||
| • Size of infarcted area correlated with MMP-9 | ||||||
| • No correlation between these measures and MMP-2 levels | ||||||
| Horstmann et al84 | Prospective observational | 100 (66): stroke = 50 (33); control patients = 50 (33) | ↑ | MMP-2 ↓; MMP-3 ↑; MMP-13: no Δ; laminin ↓; TIMP-1: no Δ; TIMP-2 ↓ | Zymography | • MMP-9 levels elevated over the course of acute stroke |
| Zhu et al,119 | Prospective observational | 638 (448) | ↑ | RF ↑; total homocysteine ↑ | ELISA | • MMP-9 combined with RF and total homocysteine improved risk-predictive ability for poststroke cognitive impairment in patients with HTN |
| Rodriguez-yanez et al,120 | Retrospective | 844 (568): normotensive; chronic HTN; new-onset HTN | ↑ | IL-6 ↑; TNF-α ↑; ICAM-1 ↑; VCAM-1 ↑ | ELISA | • Patients with new-onset HTN within 24 h of onset of stroke had poorer neurological outcome |
| • Patients had higher MMP-9 levels in addition to higher IL-6, TNF-α, ICAM-1, VCAM-1 | ||||||
| Reynolds et al89 | Prospective observational | 437: control patients = 213; stroke = 224 (AIS = 85) | ↑ | BNGF; vWF; MCP-1; S-100b | ELISA | • With algorithm panel using these 5 biomarkers, if 3/5 markers are elevated in the first 6 h of stroke symptoms then the sensitivity of approach is 92% at 93% specificity |
| • Approach provides a useful adjunct to computed tomography scanning in emergency setting | ||||||
| Lynch et al,121 | Prospective observational | 222: control patients = 157; AIS = 65 | ↑ | 26 markers of glial activation, inflammatory mediators, thrombosis, cell injury and myelin breakdown, apoptosis, and growth factors | ELISA | • Four markers highly correlated with stroke (MMP-9, VCAM, S100β, and vWF) |
| Rosell et al,122 | Prospective observational | 65 received t-PA; 24 underwent study protocol before and after t-PA; total number of patients = 24 | ↑ | MMP-1; MMP-2; MMP-3; MMP-8; MMP-10; MMP-13; TIMP-1; TIMP-2 | ELISA | • Baseline levels of both MMP-9 and MMP-13 independent predictors of final increase in brain infarct volume at 24 h |
| • MMP-9 and MMP-13 involved in tissue injury and cell death and subsequently counteract benefit from thrombolytics | ||||||
| • MMP-9 levels predict neurological outcome later after symptom onset of AIS and not initially | ||||||
| Lucivero et al85 | Prospective observational | 66: control patients = 37; AIS within 12 h of symptoms = 29 | ↑ | MMP-2 | ELISA | • MMP-2 levels increased only in lacunar strokes |
| • MMP-9 increased only in patients with more severe stroke | ||||||
| • Basal MMP-2 levels higher in patients with stable or recovering symptoms | ||||||
| • MMP-9 values at day 7 correlated with worse clinical outcome | ||||||
| • No differences related to presence of HT found | ||||||
| • MMP-9 values at day 7 correlated with worse mRS at 3-mo follow-up | ||||||
| Moldes et al88 | Prospective observational | 134 with AIS treated with t-PA | ↑ | ET-1 cellular fibronectin | ELISA | • MMP-9 levels higher in patients with AIS and cerebral edema (not statistically significant) |
| • Elevated ET-1 serum levels associated with severe brain edema in patients with AIS treated with t-PA |
AIS = acute ischemic stroke, BNGF = B-type neurotrophic growth factor, ELISA = enzyme-linked immunosorbent assay, ET-1 = endothelin-1, HT = hemorrhagic transformation, HTN = hypertension, ICAM = intercellular adhesion molecule, IL = interleukin, MCP-1 = monocyte chemoattractant protein-1, MMP = matrix metalloproteinase, mRS = modified Rankin Scale, NIHSS = National Institutes of Health Stroke Scale, RF = rheumatoid factor, S100B = S100 calcium-binding protein B, TIMP = tissue inhibitor of matrix metalloproteinase, TNF = tumor necrosis factor, t-PA = Tissue plasminogen activator, VCAM = vascular cell adhesion molecule, vWF = von Willebrand factor.
DISCUSSION
This review provides strong evidence that MMP-9 is elevated in OSA, systemic HTN, MI, and ischemic stroke. However, it also shows that TIMP-1 plays a more critical role in cardiac remodeling and infarct size and subsequently the risk of developing post-MI HF.
Studies to date have shown strong evidence that positively correlates OSA with the risk of incident and prevalent HTN, incident and prevalent congestive HF, and ischemic stroke. Because the development of clinical HTN, atherosclerosis, cardiac remodeling, and HF is insidious, it is plausible that in patients with OSA, MMP-9 may be activated in different tissues at variable time frames. If so, then targeting MMP-9 and TIMP-1 to develop specific and selective MMP-9 inhibitors will be novel and may ameliorate the progression of cardiovascular and cerebrovascular diseases induced by OSA. However, several points applied to studies discussed in this review are worth scrutiny.
The laboratory methodology and assays used to determine MMP-9 and TIMP-1 levels are not standardized in all studies. Although most studies utilized ELISA, some studies used traditional substrate degradation assays (such as gelatin zymography). ELISA is more sensitive in quantitating MMP-9 (ie, a much smaller sample volume of plasma is needed for direct analysis) than gelatin zymography, which requires a higher sample volume.92 ELISA is also specific, with < 1% MMP-9 immunoassay cross-reactivity with the MMP-1, MMP-2, or MMP-3 antigens.93 Measurements for MMP-9 using ELISA are usually reproducible, but achieving good reproducibility with gelatin chromatography is challenging.93,94 On the other hand, gelatin zymography is superior to ELISA in detecting the active and latent forms (ie, pro-MMP-9) of MMP-9. It is also much cheaper than ELISA but is labor-intensive.92 Another benefit of ELISA over zymography is that ELISA is not affected by the binding of TIMP-1 to MMP-9 and still can detect active or latent MMP-9 despite binding to TIMP-1, a feature that gelatin zymography lacks.95 These differences between ELISA and gelatin zymography make MMP-9 levels that are detected by both methods not accurately comparable.
Although the meta-analysis conducted by Fang et al44 showed a correlation between MMP-9 and OSA severity, the impact of PAP therapy on MMP-9 levels is still not clear. Three trials in their meta-analysis used PAP therapy. Two trials used PAP therapy for 1 and 3 months, respectively, and showed a reduction in MMP-9 levels compared with results from the control arm, and 1 trial used PAP for 1 night and did not show a reduction in MMP-9 levels. It is possible that 1 night of PAP therapy was not enough to downregulate MMP-9 but was enough to upregulate MMP-9, shown by the significant elevation in MMP-9 between the samples obtained during the night of the sleep study and the samples obtained the next morning. More clinical trials focusing on the role of PAP therapy in MMP-9 levels may confirm causality between OSA and MMP-9.
All studies used peripheral venous blood to measure MMP-9 levels. MMP-9 tissue levels are generally more accurate than MMP-9 blood levels, but it is not feasible to obtain tissue samples for this purpose. This condition raises the argument as to whether MMP-9 blood levels are the same as MMP-9 tissue levels, higher, or lower and how the distribution of MMP-9 in tissue can be predicted based on MMP-9 blood levels.
MMP-9 gene polymorphism is a contributing factor to elevated MMP-9 levels. It is interesting to note that MMP-9 (−1562C/T) gene polymorphism has been studied in OSA, ischemic stroke, MI, HF, and systemic HTN. Fang et al44 included 3 trials that used 3 genetic models (total 238 patients and 313 control patients) and showed no significant association between OSA and MMP-9 (−1562C/T) gene polymorphism. However, the number of studies included was very limited. Another meta-analysis included 29 case-control studies—17 evaluated the correlation between MMP-9 (−1562C/T) gene polymorphism and the risk of ischemic stroke (total of 3,741 patients and 3,648 control patients)96 and found a significant association. Five out of the 17 studies included White participants, and 12 studies included Asian participants. Moreover, a meta-analysis that included 6 studies (4 from China, 1 from Turkey, and 1 from Brazil) showed a significant correlation between systemic HTN and MMP-9 (−1562C/T) gene polymorphism in the dominant and codominant analyses but not in the recessive analysis.97 Furthermore, another meta-analysis showed a significant association between the risk of aortic dissection in patients with HTN.98 Finally, Wang, Xu, et al99 conducted a meta-analysis of 7 studies evaluating the association between matrix metalloproteinase polymorphism and MI (total of 1,126 participants with MI and 734 control participants) and found a significant association between MMP-9 (−1562C/T) gene polymorphism and MI. These findings raise the need to further confirm the role of MMP-9 (−1562C/T) gene polymorphism in OSA. Accordingly, more experimental studies are needed in this regard.
Although MMP-9 has been discussed so far as a potential marker for many diseases, note that MMP-9 has potential benefits in tissue healing after injury. This capacity is determined by the time of release from tissues after an inflammatory insult. For example, MMP-9 levels in MI have been shown to have a biphasic peak.100 The early peak happens very shortly after acute MI—ie, within 12 hours.101 This peak is beneficial because it helps with the normal healing process of infarcted tissues.73 The late peak, occurring after a few days, seems to plateau. Higher MMP-9 levels that occur late after MI can leave the normal collagen in the healthy myocardium that is remote from the infarcted area exposed to MMP-9. This process leads to scarring formation locally in the infarcted and peri-infarcted zone, leading to left ventricular dilatation, rupture, and reduced ejection fraction.102,103 The development of new pharmacological agents that target MMP-9 should consider these circumstances to avoid blocking the beneficial effects of MMP-9.
MMPs have cross-reactivity. This fact is critical for the development of potential MMP inhibitors. Although a broad-spectrum MMP inhibitor would seem to have a better outcome, it has a high potential for toxicity and adversely affecting the healthy musculoskeletal system.104 A selective MMP-9 inhibitor would be ideal.
Future directions
The role of TIMP-1 in OSA is still not clear. Only 1 trial has shown elevated TIMP-1 levels in patients with OSA and a positive correlation with OSA severity.105 Revealing more about this endogenous inhibitor for MMP-9 will help researchers and physicians to further understand the correlation between these comorbid diseases and OSA. More clinical trials examining the impact of PAP therapy on MMP-9 and TIMP-1 may help establish a causality between OSA and MMP-9.
Furthermore, the lack of association between OSA and MMP-9 gene polymorphism in the context of strong evidence correlating the risk of systemic HTN, MI, cardiac remodeling, and ischemic stroke to MMP-9 gene polymorphism mandates more experimental studies to further clarify the role of MMP-9 gene polymorphism in OSA.
CONCLUSIONS
This review provides clear, strong evidence of the role of MMP-9 in the pathophysiology of OSA and comorbid systemic HTN, MI, cardiac remodeling, and ischemic stroke. Fang et al44 showed a correlation between MMP-9 levels and the severity of OSA but not between MMP-9 gene polymorphism and OSA. We suggest that we may take the conclusions of Fang et al to another level based on the known clinical association between OSA and comorbid systemic HTN, MI, cardiac remodeling, and ischemic stroke. It is plausible that MMP-9 is activated in different tissues at different times during the course of OSA-HTN-ischemic stroke-MI and HF and may serve as a messenger in the cross talk between OSA and OSA-induced diseases. This process can open the door for developing novel selective MMP-9 inhibitors to serve as adjuvant therapy for OSA, which may mitigate the risk of cardiovascular and cerebrovascular diseases in patients with OSA.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at the University of Arizona College of Medicine, Tucson, AZ. Dr. Mansour reports research grants from the National Institutes of Health (grants NIA R44AG059279, NIAID R21AI135935, NHLBI R01HL137282, NIDA UG3DA047717, NHLBI P01HL103453, NCI P01CA229112, and NIA U01AG066623). Dr. Combs reports current research funding from the National Institutes of Health, the American Heart Association, the Lumind Foundation, and University of Arizona Health Sciences and prior research funding from the American Academy of Sleep Medicine Foundation. Dr. Patel reports research supported by grants from the American Sleep Medicine Foundation (203-JF-18), the National Institutes of Health (HL126140), a University of Arizona Health Sciences Career Development Award, and a Faculty Seed Grant Award. Dr. Parthasarathy reports research grants funded by the National Institutes of Health (HL138377, U01HL128954, IPA-014264-00001, and UG3HL140144), Patient-Centered Outcomes Research Institute (PPRND-1507-31666), the American Sleep Medicine Foundation (ASMF-169-SR-17), and Philips (HRC-1504-RETROPAP-UAZ) and Whoop. Inc. He is a co-investigator on research funded by the National Institutes of Health (MD011600), PCORI (PCS-1504-30430), and the U.S. Department of Defense (W81XWH-14-1-0570). The remaining authors report no conflicts of interest.
ABBREVIATIONS
- ECM
extracellular matrix
- ELISA
enzyme-linked immunosorbent assay
- HF
heart failure
- HTN
hypertension
- MI
myocardial infarction
- MMP-9
matrix metalloproteinase-9
- TIMP
tissue inhibitor of matrix metalloproteinase
REFERENCES
- 1.Katz I, Stradling J, Slutsky AS, Zamel N, Hoffstein V. Do patients with obstructive sleep apnea have thick necks? Am Rev Respir Dis. 1990;141(5 Pt 1):1228–1231. 10.1164/ajrccm/141.5_Pt_1.1228 [DOI] [PubMed] [Google Scholar]
- 2.Moser RJ III, Rajagopal KR. Obstructive sleep apnea in adults with tonsillar hypertrophy. Arch Intern Med. 1987;147(7):1265–1267. 10.1001/archinte.1987.00370070079012 [DOI] [PubMed] [Google Scholar]
- 3.Lyberg T, Krogstad O, Djupesland G. Cephalometric analysis in patients with obstructive sleep apnoea syndrome. I. Skeletal morphology. J Laryngol Otol. 1989;103(3):287–292. 10.1017/S0022215100108734 [DOI] [PubMed] [Google Scholar]
- 4.Liu SY, Guilleminault C, Huon LK, Yoon A. Distraction osteogenesis maxillary expansion (DOME) for adult obstructive sleep apnea patients with high arched palate. Otolaryngol Head Neck Surg. 2017;157(2):345–348. 10.1177/0194599817707168 [DOI] [PubMed] [Google Scholar]
- 5.Cakirer B, Hans MG, Graham G, Aylor J, Tishler PV, Redline S. The relationship between craniofacial morphology and obstructive sleep apnea in whites and in African-Americans. Am J Respir Crit Care Med. 2001;163(4):947–950. 10.1164/ajrccm.163.4.2005136 [DOI] [PubMed] [Google Scholar]
- 6.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. 10.1056/NEJM199304293281704 [DOI] [PubMed] [Google Scholar]
- 8.Jehan S, Auguste E, Pandi-Perumal SR, et al. Depression, obstructive sleep apnea and psychosocial health. Sleep Med Disord. 2017;1(3):00012. [PMC free article] [PubMed] [Google Scholar]
- 9.Galland B, Spruyt K, Dawes P, McDowall PS, Elder D, Schaughency E. Sleep disordered breathing and academic performance: a meta-analysis. Pediatrics. 2015;136(4):e934–e946. 10.1542/peds.2015-1677 [DOI] [PubMed] [Google Scholar]
- 10.Omachi TA, Claman DM, Blanc PD, Eisner MD. Obstructive sleep apnea: a risk factor for work disability. Sleep. 2009;32(6):791–798. 10.1093/sleep/32.6.791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall NS, Wong KK, Phillips CL, Liu PY, Knuiman MW, Grunstein RR. Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? J Clin Sleep Med. 2009;5(1):15–20. 10.5664/jcsm.27387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137(3):711–719. 10.1378/chest.09-0360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durgan DJ, Bryan RM Jr. Cerebrovascular consequences of obstructive sleep apnea. J Am Heart Assoc. 2012;1(4):e000091. 10.1161/JAHA.111.000091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836. 10.1001/jama.283.14.1829 [DOI] [PubMed] [Google Scholar]
- 15.Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307(20):2169–2176. 10.1001/jama.2012.3418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. 10.1016/S0140-6736(05)71141-7 [DOI] [PubMed] [Google Scholar]
- 17.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(1):19–25. 10.1164/ajrccm.163.1.2001008 [DOI] [PubMed] [Google Scholar]
- 18.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110(4):364–367. 10.1161/01.CIR.0000136587.68725.8E [DOI] [PubMed] [Google Scholar]
- 19.Arias MA, García-Río F, Alonso-Fernández A, Martínez I, Villamor J. Pulmonary hypertension in obstructive sleep apnoea: effects of continuous positive airway pressure: a randomized, controlled cross-over study. Eur Heart J. 2006;27(9):1106–1113. 10.1093/eurheartj/ehi807 [DOI] [PubMed] [Google Scholar]
- 20.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182(2):269–277. 10.1164/rccm.200911-1746OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi K, Ueda S, Kobayashi T, et al. Chronic intermittent hypoxia-mediated renal sympathetic nerve activation in hypertension and cardiovascular disease. Sci Rep. 2018;8(1):17926. 10.1038/s41598-018-36159-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budhiraja R, Parthasarathy S, Quan SF. Endothelial dysfunction in obstructive sleep apnea. J Clin Sleep Med. 2007;3(4):409–415. 10.5664/jcsm.26864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23 Suppl 1):III27–III32. [DOI] [PubMed] [Google Scholar]
- 24.Korpos E, Wu C, Sorokin L. Multiple roles of the extracellular matrix in inflammation. Curr Pharm Des. 2009;15(12):1349–1357. 10.2174/138161209787846685 [DOI] [PubMed] [Google Scholar]
- 25.Feihl F, Liaudet L, Levy BI, Waeber B. Hypertension and microvascular remodelling. Cardiovasc Res. 2008;78(2):274–285. 10.1093/cvr/cvn022 [DOI] [PubMed] [Google Scholar]
- 26.Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem. 1999;274(31):21491–21494. 10.1074/jbc.274.31.21491 [DOI] [PubMed] [Google Scholar]
- 27.Pei D, Weiss SJ. Furin-dependent intracellular activation of the human stromelysin-3 zymogen. Nature. 1995;375(6528):244–247. 10.1038/375244a0 [DOI] [PubMed] [Google Scholar]
- 28.Yadav L, Puri N, Rastogi V, Satpute P, Ahmad R, Kaur G. Matrix metalloproteinases and cancer—roles in threat and therapy. Asian Pac J Cancer Prev. 2014;15(3):1085–1091. 10.7314/APJCP.2014.15.3.1085 [DOI] [PubMed] [Google Scholar]
- 29.Verma RP, Hansch C. Matrix metalloproteinases (MMPs): chemical-biological functions and (Q)SARs. Bioorg Med Chem. 2007;15(6):2223–2268. 10.1016/j.bmc.2007.01.011 [DOI] [PubMed] [Google Scholar]
- 30.Papazafiropoulou A, Tentolouris N. Matrix metalloproteinases and cardiovascular diseases. Hippokratia. 2009;13(2):76–82. [PMC free article] [PubMed] [Google Scholar]
- 31.Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997;378(3–4):151–160. [PubMed] [Google Scholar]
- 32.Alexander JP, Acott TS. Involvement of protein kinase C in TNFalpha regulation of trabecular matrix metalloproteinases and TIMPs. Invest Ophthalmol Vis Sci. 2001;42(12):2831–2838. [PubMed] [Google Scholar]
- 33.Long CS. The role of interleukin-1 in the failing heart. Heart Fail Rev. 2001;6(2):81–94. 10.1023/A:1011428824771 [DOI] [PubMed] [Google Scholar]
- 34.Crawford HC, Matrisian LM. Mechanisms controlling the transcription of matrix metalloproteinase genes in normal and neoplastic cells. Enzyme Protein. 1996;49(1–3):20–37. 10.1159/000468614 [DOI] [PubMed] [Google Scholar]
- 35.Roderfeld M, Graf J, Giese B, et al. Latent MMP-9 is bound to TIMP-1 before secretion. Biol Chem. 2007;388(11):1227–1234. 10.1515/BC.2007.123 [DOI] [PubMed] [Google Scholar]
- 36.Lijnen HR. Plasmin and matrix metalloproteinases in vascular remodeling. Thromb Haemost. 2001;86(1):324–333. [PubMed] [Google Scholar]
- 37.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477(1–2):267–283. 10.1016/S0167-4838(99)00279-4 [DOI] [PubMed] [Google Scholar]
- 38.Murphy G, Houbrechts A, Cockett MI, Williamson RA, O’Shea M, Docherty AJ. The N-terminal domain of tissue inhibitor of metalloproteinases retains metalloproteinase inhibitory activity. Biochemistry. 1991;30(33):8097–8102. Published correction appears in Biochemistry. 1991;30(42): 10362. 10.1021/bi00247a001 [DOI] [PubMed] [Google Scholar]
- 39.Fernandez-Catalan C, Bode W, Huber R, et al. Crystal structure of the complex formed by the membrane type 1-matrix metalloproteinase with the tissue inhibitor of metalloproteinases-2, the soluble progelatinase A receptor. EMBO J. 1998;17(17):5238–5248. 10.1093/emboj/17.17.5238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bekes EM, Schweighofer B, Kupriyanova TA, et al. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol. 2011;179(3):1455–1470. 10.1016/j.ajpath.2011.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7(1):30–37. 10.1038/nrcardio.2009.199 [DOI] [PubMed] [Google Scholar]
- 42.Newby AC, George SJ, Ismail Y, Johnson JL, Sala-Newby GB, Thomas AC. Vulnerable atherosclerotic plaque metalloproteinases and foam cell phenotypes. Thromb Haemost. 2009;101(6):1006–1011. 10.1160/TH08-07-0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sluijter JP, Pulskens WP, Schoneveld AH, et al. Matrix metalloproteinase 2 is associated with stable and matrix metalloproteinases 8 and 9 with vulnerable carotid atherosclerotic lesions: a study in human endarterectomy specimen pointing to a role for different extracellular matrix metalloproteinase inducer glycosylation forms. Stroke. 2006;37(1):235–239. 10.1161/01.STR.0000196986.50059.e0 [DOI] [PubMed] [Google Scholar]
- 44.Fang X, Chen J, Wang W, et al. Matrix metalloproteinase 9 (MMP9) level and MMP9 -1562C>T in patients with obstructive sleep apnea: a systematic review and meta-analysis of case-control studies. Sleep Med. 2020;67:110–119. 10.1016/j.sleep.2019.11.1247 [DOI] [PubMed] [Google Scholar]
- 45.Wang S, Li S, Wang B, Liu J, Tang Q. Matrix metalloproteinase-9 is a predictive factor for systematic hypertension and heart dysfunction in patients with obstructive sleep apnea syndrome. BioMed Res Int. 2018;2018:1569701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hopps E, Lo Presti R, Montana M, Canino B, Calandrino V, Caimi G. Analysis of the correlations between oxidative stress, gelatinases and their tissue inhibitors in the human subjects with obstructive sleep apnea syndrome. J Physiol Pharmacol. 2015;66(6):803–810. [PubMed] [Google Scholar]
- 47.Maeder MT, Strobel W, Christ M, et al. Comprehensive biomarker profiling in patients with obstructive sleep apnea. Clin Biochem. 2015;48(4–5):340–346. 10.1016/j.clinbiochem.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 48.Nizam N, Basoglu OK, Tasbakan MS, et al. Do salivary and serum collagenases have a role in an association between obstructive sleep apnea syndrome and periodontal disease? A preliminary case-control study. Arch Oral Biol. 2015;60(1):134–143. 10.1016/j.archoralbio.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 49.Tazaki T, Minoguchi K, Yokoe T, et al. Increased levels and activity of matrix metalloproteinase-9 in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;170(12):1354–1359. 10.1164/rccm.200402-193OC [DOI] [PubMed] [Google Scholar]
- 50.Tamaki S, Yamauchi M, Fukuoka A, et al. Production of inflammatory mediators by monocytes in patients with obstructive sleep apnea syndrome. Intern Med. 2009;48(15):1255–1262. 10.2169/internalmedicine.48.2366 [DOI] [PubMed] [Google Scholar]
- 51.Vuralkan E, Mutlu M, Firat IH, et al. Changes in serum levels of MDA and MMP-9 after UPF in patients with OSAS. Eur Arch Otorhinolaryngol. 2014;271(5):1329–1334. 10.1007/s00405-013-2821-5 [DOI] [PubMed] [Google Scholar]
- 52.Bonanno A, Riccobono L, Bonsignore MR, et al. Relaxin in obstructive sleep apnea: relationship with blood pressure and inflammatory mediators. Respiration. 2016;91(1):56–62. 10.1159/000443182 [DOI] [PubMed] [Google Scholar]
- 53.Weber KT, Pick R, Jalil JE, Janicki JS, Carroll EP. Patterns of myocardial fibrosis. J Mol Cell Cardiol. 1989;21(Suppl 5):121–131. 10.1016/0022-2828(89)90778-5 [DOI] [PubMed] [Google Scholar]
- 54.Tan J, Hua Q, Xing X, Wen J, Liu R, Yang Z. Impact of the metalloproteinase-9/tissue inhibitor of metalloproteinase-1 system on large arterial stiffness in patients with essential hypertension. Hypertens Res. 2007;30(10):959–963. 10.1291/hypres.30.959 [DOI] [PubMed] [Google Scholar]
- 55.Lehoux S, Lemarié CA, Esposito B, Lijnen HR, Tedgui A. Pressure-induced matrix metalloproteinase-9 contributes to early hypertensive remodeling. Circulation. 2004;109(8):1041–1047. 10.1161/01.CIR.0000115521.95662.7A [DOI] [PubMed] [Google Scholar]
- 56.Li H, Simon H, Bocan TM, Peterson JT. MMP/TIMP expression in spontaneously hypertensive heart failure rats: the effect of ACE- and MMP-inhibition. Cardiovasc Res. 2000;46(2):298–306. 10.1016/S0008-6363(00)00028-6 [DOI] [PubMed] [Google Scholar]
- 57.Ahmed SH, Clark LL, Pennington WR, et al. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113(17):2089–2096. 10.1161/CIRCULATIONAHA.105.573865 [DOI] [PubMed] [Google Scholar]
- 58.Yasmin, McEniery CM, Wallace S, et al. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(2):372. Published correction appears in Arterioscler Thromb Vasc Biol. 2005;25(4):875. 10.1161/01.ATV.0000151373.33830.41 [DOI] [PubMed] [Google Scholar]
- 59.Marchesi C, Dentali F, Nicolini E, et al. Plasma levels of matrix metalloproteinases and their inhibitors in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30(1):3–16. 10.1097/HJH.0b013e32834d249a [DOI] [PubMed] [Google Scholar]
- 60.Tayebjee MH, Nadar SK, MacFadyen RJ, Lip GY. Tissue inhibitor of metalloproteinase-1 and matrix metalloproteinase-9 levels in patients with hypertension: relationship to tissue Doppler indices of diastolic relaxation. Am J Hypertens. 2004;17(9):770–774. 10.1016/S0895-7061(04)00902-1 [DOI] [PubMed] [Google Scholar]
- 61.Saglam M, Karakaya O, Esen AM, et al. Contribution of plasma matrix metalloproteinases to development of left ventricular hypertrophy and diastolic dysfunction in hypertensive subjects. Tohoku J Exp Med. 2006;208(2):117–122. 10.1620/tjem.208.117 [DOI] [PubMed] [Google Scholar]
- 62.Onal IK, Altun B, Onal ED, Kirkpantur A, Gul Oz S, Turgan C. Serum levels of MMP-9 and TIMP-1 in primary hypertension and effect of antihypertensive treatment. Eur J Intern Med. 2009;20(4):369–372. 10.1016/j.ejim.2008.10.003 [DOI] [PubMed] [Google Scholar]
- 63.Tayebjee MH, Nadar S, Blann AD, Gareth Beevers D, MacFadyen RJ, Lip GY. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in hypertension and their relationship to cardiovascular risk and treatment: a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT). Am J Hypertens. 2004;17(9):764–769. 10.1016/S0895-7061(04)00855-6 [DOI] [PubMed] [Google Scholar]
- 64.Li-Saw-Hee FL, Edmunds E, Blann AD, Beevers DG, Lip GY. Matrix metalloproteinase-9 and tissue inhibitor metalloproteinase-1 levels in essential hypertension. Relationship to left ventricular mass and anti-hypertensive therapy. Int J Cardiol. 2000;75(1):43–47. 10.1016/S0167-5273(00)00274-6 [DOI] [PubMed] [Google Scholar]
- 65.Zervoudaki A, Economou E, Stefanadis C, et al. Plasma levels of active extracellular matrix metalloproteinases 2 and 9 in patients with essential hypertension before and after antihypertensive treatment. J Hum Hypertens. 2003;17(2):119–124. 10.1038/sj.jhh.1001518 [DOI] [PubMed] [Google Scholar]
- 66.Zervoudaki A, Economou E, Pitsavos C, et al. The effect of Ca2+ channel antagonists on plasma concentrations of matrix metalloproteinase-2 and -9 in essential hypertension. Am J Hypertens. 2004;17(3):273–276. 10.1016/j.amjhyper.2003.11.007 [DOI] [PubMed] [Google Scholar]
- 67.Ergul A, Portik-Dobos V, Hutchinson J, Franco J, Anstadt MP. Downregulation of vascular matrix metalloproteinase inducer and activator proteins in hypertensive patients. Am J Hypertens. 2004;17(9):775–782. 10.1016/j.amjhyper.2004.06.025 [DOI] [PubMed] [Google Scholar]
- 68.Rekhter MD. Collagen synthesis in atherosclerosis: too much and not enough. Cardiovasc Res. 1999;41(2):376–384. 10.1016/S0008-6363(98)00321-6 [DOI] [PubMed] [Google Scholar]
- 69.Kramsch DM, Franzblau C, Hollander W. The protein and lipid composition of arterial elastin and its relationship to lipid accumulation in the atherosclerotic plaque. J Clin Invest. 1971;50(8):1666–1677. 10.1172/JCI106656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown DL, Hibbs MS, Kearney M, Loushin C, Isner JM. Identification of 92-kD gelatinase in human coronary atherosclerotic lesions. Association of active enzyme synthesis with unstable angina. Circulation. 1995;91(8):2125–2131. 10.1161/01.CIR.91.8.2125 [DOI] [PubMed] [Google Scholar]
- 71.Heymans S, Luttun A, Nuyens D, et al. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med. 1999;5(10):1135–1142. 10.1038/13459 [DOI] [PubMed] [Google Scholar]
- 72.Frantz S, Ducharme A, Sawyer D, et al. Targeted deletion of caspase-1 reduces early mortality and left ventricular dilatation following myocardial infarction. J Mol Cell Cardiol. 2003;35(6):685–694. 10.1016/S0022-2828(03)00113-5 [DOI] [PubMed] [Google Scholar]
- 73.Creemers EE, Cleutjens JP, Smits JF, Daemen MJ. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure? Circ Res. 2001;89(3):201–210. 10.1161/hh1501.094396 [DOI] [PubMed] [Google Scholar]
- 74.Opstad TB, Seljeflot I, Bøhmer E, Arnesen H, Halvorsen S. MMP-9 and its regulators TIMP-1 and EMMPRIN in patients with acute ST-elevation myocardial infarction: a NORDISTEMI substudy. Cardiology. 2018;139(1):17–24. 10.1159/000481684 [DOI] [PubMed] [Google Scholar]
- 75.Li YY, Feldman AM, Sun Y, McTiernan CF. Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation. 1998;98(17):1728–1734. 10.1161/01.CIR.98.17.1728 [DOI] [PubMed] [Google Scholar]
- 76.Martos R, Baugh J, Ledwidge M, et al. Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007;115(7):888–895. 10.1161/CIRCULATIONAHA.106.638569 [DOI] [PubMed] [Google Scholar]
- 77.Morishita T, Uzui H, Mitsuke Y, et al. Association between matrix metalloproteinase-9 and worsening heart failure events in patients with chronic heart failure. ESC Heart Fail. 2017;4(3):321–330. 10.1002/ehf2.12137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lakhani HV, Khanal T, Gabi A, et al. Developing a panel of biomarkers and miRNA in patients with myocardial infarction for early intervention strategies of heart failure in West Virginian population. PLoS One. 2018;13(10):e0205329. 10.1371/journal.pone.0205329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Franz M, Berndt A, Altendorf-Hofmann A, et al. Serum levels of large tenascin-C variants, matrix metalloproteinase-9, and tissue inhibitors of matrix metalloproteinases in concentric versus eccentric left ventricular hypertrophy. Eur J Heart Fail. 2009;11(11):1057–1062. 10.1093/eurjhf/hfp128 [DOI] [PubMed] [Google Scholar]
- 80.Dini FL, Buralli S, Bajraktari G, et al. Plasma matrix metalloproteinase-9 better predicts outcome than N-terminal protype-B natriuretic peptide in patients with systolic heart failure and a high prevalence of coronary artery disease. Biomed Pharmacother. 2010;64(5):339–342. 10.1016/j.biopha.2009.09.008 [DOI] [PubMed] [Google Scholar]
- 81.Yan AT, Yan RT, Spinale FG, et al. Plasma matrix metalloproteinase-9 level is correlated with left ventricular volumes and ejection fraction in patients with heart failure. J Card Fail. 2006;12(7):514–519. 10.1016/j.cardfail.2006.05.012 [DOI] [PubMed] [Google Scholar]
- 82.Montaner J, Alvarez-Sabín J, Molina C, et al. Matrix metalloproteinase expression after human cardioembolic stroke: temporal profile and relation to neurological impairment. Stroke. 2001;32(8):1759–1766. 10.1161/01.STR.32.8.1759 [DOI] [PubMed] [Google Scholar]
- 83.Zhong C, Yang J, Xu T, et al. Serum matrix metalloproteinase-9 levels and prognosis of acute ischemic stroke. Neurology. 2017;89(8):805–812. 10.1212/WNL.0000000000004257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Horstmann S, Kalb P, Koziol J, Gardner H, Wagner S. Profiles of matrix metalloproteinases, their inhibitors, and laminin in stroke patients: influence of different therapies. Stroke. 2003;34(9):2165–2170. 10.1161/01.STR.0000088062.86084.F2 [DOI] [PubMed] [Google Scholar]
- 85.Lucivero V, Prontera M, Mezzapesa DM, et al. Different roles of matrix metalloproteinases-2 and -9 after human ischaemic stroke. Neurol Sci. 2007;28(4):165–170. [DOI] [PubMed] [Google Scholar]
- 86.Regenhardt RW, Das AS, Lo EH, Caplan LR. Advances in understanding the pathophysiology of lacunar stroke: a review. JAMA Neurol. 2018;75(10):1273–1281. 10.1001/jamaneurol.2018.1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fisher CM. The arterial lesions underlying lacunes. Acta Neuropathol. 1968;12(1):1–15. 10.1007/BF00685305 [DOI] [PubMed] [Google Scholar]
- 88.Moldes O, Sobrino T, Millán M, et al. High serum levels of endothelin-1 predict severe cerebral edema in patients with acute ischemic stroke treated with t-PA. Stroke. 2008;39(7):2006–2010. 10.1161/STROKEAHA.107.495044 [DOI] [PubMed] [Google Scholar]
- 89.Reynolds MA, Kirchick HJ, Dahlen JR, et al. Early biomarkers of stroke. Clin Chem. 2003;49(10):1733–1739. 10.1373/49.10.1733 [DOI] [PubMed] [Google Scholar]
- 90.Heo JH, Kim SH, Lee KY, Kim EH, Chu CK, Nam JM. Increase in plasma matrix metalloproteinase-9 in acute stroke patients with thrombolysis failure. Stroke. 2003;34(6):e48–e50. 10.1161/01.STR.0000073788.81170.1C [DOI] [PubMed] [Google Scholar]
- 91.Castellanos M, Leira R, Serena J, et al. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003;34(1):40–46. 10.1161/01.STR.0000046764.57344.31 [DOI] [PubMed] [Google Scholar]
- 92.Zucker S, Mancuso P, DiMassimo B, Lysik RM, Conner C, Wu CL. Comparison of techniques for measurement of gelatinases/type IV collagenases: enzyme-linked immunoassays versus substrate degradation assays. Clin Exp Metastasis. 1994;12(1):13–23. 10.1007/BF01784329 [DOI] [PubMed] [Google Scholar]
- 93.Zucker S, Lysik RM, Zarrabi MH, et al. Type IV collagenase/gelatinase (MMP-2) is not increased in plasma of patients with cancer. Cancer Epidemiol Biomarkers Prev. 1992;1(6):475–479. [PubMed] [Google Scholar]
- 94.Bergmann U, Michaelis J, Oberhoff R, Knäuper V, Beckmann R, Tschesche H. Enzyme linked immunosorbent assays (ELISA) for the quantitative determination of human leukocyte collagenase and gelatinase. J Clin Chem Clin Biochem. 1989;27(6):351–359. [DOI] [PubMed] [Google Scholar]
- 95.Rajabi M, Dean DD, Woessner JF Jr. High levels of serum collagenase in premature labor—a potential biochemical marker. Obstet Gynecol. 1987;69(2):179–186. [PubMed] [Google Scholar]
- 96.Misra S, Talwar P, Kumar A, et al. Association between matrix metalloproteinase family gene polymorphisms and risk of ischemic stroke: a systematic review and meta-analysis of 29 studies. Gene. 2018;672:180–194. 10.1016/j.gene.2018.06.027 [DOI] [PubMed] [Google Scholar]
- 97.Yang W, Lu J, Yang L, Zhang J. Association of matrix metalloproteinase-9 gene -1562C/T polymorphism with essential hypertension: a systematic review and meta-analysis article. Iran J Public Health. 2015;44(11):1445–1452. [PMC free article] [PubMed] [Google Scholar]
- 98.Song WH, Dang AM, Zhu JM, Lü NQ, Liu GZ, Hui RT. MMP-9 gene -1562C/T polymorphism in aortic dissection in Chinese hypertensive patients. Article in Chinese. Zhonghua Nei Ke Za Zhi. 2006;45(5):376–378. [PubMed] [Google Scholar]
- 99.Wang J, Xu D, Wu X, et al. Polymorphisms of matrix metalloproteinases in myocardial infarction: a meta-analysis. Heart. 2011;97(19):1542–1546. 10.1136/heartjnl-2011-300342 [DOI] [PubMed] [Google Scholar]
- 100.Mukherjee R, Mingoia JT, Bruce JA, et al. Selective spatiotemporal induction of matrix metalloproteinase-2 and matrix metalloproteinase-9 transcription after myocardial infarction. Am J Physiol. Heart Circ. Physiol. 2006;291(5):H2216–H2228. 10.1152/ajpheart.01343.2005 [DOI] [PubMed] [Google Scholar]
- 101.Herzog E, Gu A, Kohmoto T, Burkhoff D, Hochman JS. Early activation of metalloproteinases after experimental myocardial infarction occurs in infarct and non-infarct zones. Cardiovasc Pathol. 1998;7(6):307–312. 10.1016/S1054-8807(98)00008-8 [DOI] [PubMed] [Google Scholar]
- 102.Squire IB, Evans J, Ng LL, Loftus IM, Thompson MM. Plasma MMP-9 and MMP-2 following acute myocardial infarction in man: correlation with echocardiographic and neurohumoral parameters of left ventricular dysfunction. J Card Fail. 2004;10(4):328–333. 10.1016/j.cardfail.2003.11.003 [DOI] [PubMed] [Google Scholar]
- 103.Kelly D, Khan SQ, Thompson M, et al. Plasma tissue inhibitor of metalloproteinase-1 and matrix metalloproteinase-9: novel indicators of left ventricular remodelling and prognosis after acute myocardial infarction. Eur Heart J. 2008;29(17):2116–2124. 10.1093/eurheartj/ehn315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Drummond AH, Beckett P, Brown PD, et al. Preclinical and clinical studies of MMP inhibitors in cancer. Ann N Y Acad Sci. 1999;878:228–235. 10.1111/j.1749-6632.1999.tb07688.x [DOI] [PubMed] [Google Scholar]
- 105.Hopps E, Canino B, Montana M, et al. Gelatinases and their tissue inhibitors in a group of subjects with obstructive sleep apnea syndrome. Clin Hemorheol Microcirc. 2016;62(1):27–34. 10.3233/ch-151928 [DOI] [PubMed] [Google Scholar]
- 106.Volná J, Kemlink D, Kalousová M, et al. Biochemical oxidative stress-related markers in patients with obstructive sleep apnea. Med Sci Monit. 2011;17(9):CR491-CR497. 10.12659/msm.881935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chuang LP, Chen NH, Lin SW, et al. Increased C-C chemokine receptor 2 gene expression in monocytes of severe obstructive sleep apnea patients and under intermittent hypoxia. PLoS One. 2014;9(11):e113304. 10.1371/journal.pone.0113304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Feng X, Liu B, Wang J, Xiao X. [Research on the serum levels of matrix metalloproteinase-9 and free fatty acids in OSAHS cases]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011;25(3):109–113. [PubMed] [Google Scholar]
- 109.Ye J, Liu H, Li Y, et al. Increased serum levels of C-reactive protein and matrix metalloproteinase-9 in obstructive sleep apnea syndrome. Chin Med J (Engl). 2007 Sep 5;120(17):1482–6. [PubMed] [Google Scholar]
- 110.Laviades C, Varo N, Fernández J, et al. Abnormalities of the extracellular degradation of collagen type I in essential hypertension. Circulation. 1998;98(6):535–540. 10.1161/01.cir.98.6.535 [DOI] [PubMed] [Google Scholar]
- 111.Derosa G, D'Angelo A, Ciccarelli L, et al. Matrix metalloproteinase-2, -9, and tissue inhibitor of metalloproteinase-1 in patients with hypertension. Endothelium. 2006;13(3):227–231. 10.1080/10623320600780942 [DOI] [PubMed] [Google Scholar]
- 112.Collier P, Watson CJ, Voon V, et al. Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? Eur J Heart Fail. 2011;13(10):1087–1095. 10.1093/eurjhf/hfr079 [DOI] [PubMed] [Google Scholar]
- 113.Ritter AM, de Faria AP, Barbaro N, et al. Crosstalk between obesity and MMP-9 in cardiac remodelling -a cross-sectional study in apparent treatment-resistant hypertension. Blood Press. 2017;26(2):122–129. 10.1080/08037051.2016.1249336 [DOI] [PubMed] [Google Scholar]
- 114.Friese RS, Rao F, Khandrika S, et al. Matrix metalloproteinases: discrete elevations in essential hypertension and hypertensive end-stage renal disease. Clin Exp Hypertens. 2009;31(7):521–533. 10.3109/10641960802668730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thomas CV, Coker ML, Zellner JL, Handy JR, Crumbley AJ 3rd, Spinale FG. Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation. 1998;97(17):1708–1715. 10.1161/01.cir.97.17.1708 [DOI] [PubMed] [Google Scholar]
- 116.Reinhardt D, Sigusch HH, Hensse J, Tyagi SC, Körfer R, Figulla HR. Cardiac remodelling in end stage heart failure: upregulation of matrix metalloproteinase (MMP) irrespective of the underlying disease, and evidence for a direct inhibitory effect of ACE inhibitors on MMP. Heart. 2002;88(5):525–530. 10.1136/heart.88.5.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.George J, Patal S, Wexler D, Roth A, Sheps D, Keren G. Circulating matrix metalloproteinase-2 but not matrix metalloproteinase-3, matrix metalloproteinase-9, or tissue inhibitor of metalloproteinase-1 predicts outcome in patients with congestive heart failure. Am Heart J. 2005;150(3):484–487. 10.1016/j.ahj.2004.11.016 [DOI] [PubMed] [Google Scholar]
- 118.Buralli S, Dini FL, Ballo P, et al. Circulating matrix metalloproteinase-3 and metalloproteinase-9 and tissue Doppler measures of diastolic dysfunction to risk stratify patients with systolic heart failure. Am J Cardiol. 2010;105(6):853–856. 10.1016/j.amjcard.2009.11.038 [DOI] [PubMed] [Google Scholar]
- 119.Zhu Z, Zhong C, Guo D, et al. Multiple biomarkers covering several pathways improve predictive ability for cognitive impairment among ischemic stroke patients with elevated blood pressure. Atherosclerosis. 2019;287:30–37. 10.1016/j.atherosclerosis.2019.05.028 [DOI] [PubMed] [Google Scholar]
- 120.Rodríguez-Yáñez M, Castellanos M, Blanco M, et al. New-onset hypertension and inflammatory response/poor outcome in acute ischemic stroke. Neurology. 2006;67(11):1973–1978. 10.1212/01.wnl.0000247064.53130.91 [DOI] [PubMed] [Google Scholar]
- 121.Lynch JR, Blessing R, White WD, Grocott HP, Newman MF, Laskowitz DT. Novel diagnostic test for acute stroke. Stroke. 2004;35(1):57–63. 10.1161/01.str.0000105927.62344.4c [DOI] [PubMed] [Google Scholar]
- 122.Rosell A, Alvarez-Sabín J, Arenillas JF, et al. A matrix metalloproteinase protein array reveals a strong relation between MMP-9 and MMP-13 with diffusion-weighted image lesion increase in human stroke. Stroke. 2005;36(7):1415–1420. 10.1161/01.str.0000170641.01047.cc [DOI] [PubMed] [Google Scholar]


