Abstract
Study Objectives:
The effects of serotonergic agents on respiration neuromodulation may vary according to differences in the serotonin system, such as those linked to depression. This study investigated how sleep-related respiratory disturbances relate to depression and the use of medications commonly prescribed for depression.
Methods:
Retrospective polysomnography was collated for all 363 individuals who met selection criteria out of 2,528 consecutive individuals referred to a specialized sleep clinic (Ottawa, Canada) between 2006 and 2016. The apnea-hypopnea index (AHI), oxygen saturation nadir, and oxygen desaturation index during REM and NREM sleep were analyzed using mixed analyses of covariance comparing 3 main groups: (1) medicated individuals with depressive disorders (antidepressant group; subdivided into the selective serotonin reuptake inhibitor and norepinephrine-dopamine reuptake inhibitor subgroups), (2) non-medicated individuals with depressive disorders (non-medicated group), and (3) mentally healthy control patients (control group).
Results:
Individuals with depressive disorders (on antidepressants or not) had significantly higher AHIs compared to control patients (both P ≤ .007). The antidepressant group had a lower NREM sleep oxygen saturation nadir and a higher NREM sleep oxygen desaturation index than the control and non-medicated groups (all P ≤ .009). Within individuals with depressive disorders, independent of depression severity, the selective serotonin reuptake inhibitor group had a lower oxygen saturation nadir and a higher oxygen desaturation index during NREM sleep than the norepinephrine-dopamine reuptake inhibitor (both P ≤ .045) and non-medicated groups (both P < .001) and a higher NREM sleep AHI than the non-medicated group (P = .014).
Conclusions:
These findings suggest that the use of selective serotonin reuptake inhibitors may be associated with impaired breathing and worse nocturnal oxygen saturation in individuals with depressive disorders and sleep complaints, but this needs to be confirmed by prospective studies.
Citation:
Robillard R, Saad M, Ray RB, et al. Selective serotonin reuptake inhibitor use is associated with worse sleep-related breathing disturbances in individuals with depressive disorders and sleep complaints: a retrospective study. J Clin Sleep Med. 2021;17(3):505–513.
Keywords: selective serotonin reuptake inhibitor, antidepressants, depression, sleep-related breathing disturbances, OSA
BRIEF SUMMARY
Current Knowledge/Study Rationale: Considering alterations in the serotonin system that are linked to depression, and the high comorbidity overlap between depression and sleep-related breathing disorders, there is a need to better understand how selective serotonin reuptake inhibitors may interact with nocturnal respiratory functions in the context of depression.
Study Impact: This retrospective study provides the first empirical preliminary evidence suggesting a link between antidepressant use and sleep-related breathing disturbances in individuals with depression and sleep complaints. If confirmed by future prospective studies, the findings point to an unsuspected adverse effect of the most-prescribed antidepressants, which may have important implications for the clinical management of depression.
INTRODUCTION
Medications commonly prescribed for mood disorders, such as selective serotonin reuptake inhibitors (SSRIs), markedly impact sleep.1,2 In addition to altering sleep architecture, SSRIs have been shown to modulate breathing patterns during sleep in animal models and in humans with sleep-related breathing disorders (SRBDs).3–5 Considering the high comorbidity between depression and SRBDs,6 there is a need to better understand how SSRIs may interact with respiratory functions in the context of depression.
Depression is accompanied by dysfunctions in the serotonin (5-HT) system, both centrally and peripherally. For instance, individuals with depression have low levels of plasma tryptophan (a 5-HT precursor)7,8 and 5-HT metabolites in the cerebrospinal fluid, low 5-HT1A receptor binding,9–11 and low availability of 5-HT reuptake sites in the brainstem.12 These 5-HT abnormalities could interfere with respiration, especially during sleep. Most 5-HT neurons are located in the raphe nuclei, a structure heavily involved in the regulation of respiration and sleep.13,14 The serotonergic input to motor neurons regulating upper-airway muscles decreases during sleep,15,16 which is thought to play a role in upper airway obstruction, the basis of OSA.17
Pharmacological manipulations of the 5-HT system may also impact respiratory functions.18 Although conflicting effects have also been reported,5,19 exogenous 5-HT can trigger apneas20 and increase breathing disruptions during sleep in a dose-dependent manner,21,22 whereas 5-HT antagonists actively reduce sleep apneas.23 In humans, small (from 8 to 17 participants) trials attempting to use SSRIs to improve breathing in people with OSA have yielded mixed results; some have reported reduced apnea-hypopnea index (AHI) in a subset of their sample (ie, 33%–65%),3,24 and some have reported an overall slight (nonsignificant) increase in AHI.25 These inconsistencies could result from individual differences in the vulnerability to 5-HT agents. The direction of serotonergic effects on respiration may also vary with the expression of 5-HT receptors.26,27 Agonism at 5-HT3 receptors can have inhibitory effects on upper-airway dilators, thereby reducing patency and airflow,23,28 and agonism at 5-HT1A receptors can have excitatory effects on respiratory centers.29,30 From this perspective, the postulated dysregulation of 5-HT receptors in depression may shape the respiratory response to 5-HT agents. For instance, there are some indications of increased 5-HT3 receptor activity in depression,31 a factor that may potentiate the inhibition of respiratory muscles. In addition, depression-related impairments in 5-HT1A binding may diminish the excitatory effects of 5-HT agents on the respiratory system.
Based on a retrospective cross-sectional chart review in individuals who were referred for a sleep disorder assessment and underwent diagnostic polysomnography (PSG), the present study investigated how sleep-related respiratory disturbances relate to depression and the use of medications commonly prescribed for depression. It was anticipated that people with depression who take antidepressants, more specifically SSRIs, would have a higher AHI and lower blood oxygen saturation compared with those not taking any psychotropic medication and those taking nonserotonergic medication. Supplemental analyses were conducted to explore whether the relationship between antidepressant use and sleep-related respiratory functions is less pronounced in people with a history of depression who are currently asymptomatic for depression compared with those who are currently symptomatic (see supplemental material (36.9KB, pdf) ).
METHODS
Study design and patients
Data were retrospectively collated from a pool of 2,528 constitutive individuals who underwent diagnostic overnight level 1 PSG at the Sleep Disorders Clinic of the Royal Ottawa Mental Health Centre (Ottawa, Ontario, Canada) between 2006 and 2016 and who met the following selection criteria: (1) individuals with depressive disorders (on antidepressant or not) and (2) mentally healthy control patients (see Figure 1). Descriptive information for each group and medication subtypes are reported in Table 1. This retrospective study was approved by the Human Research Ethics Board of the Royal Ottawa Health Care Group (REB# 2015028).
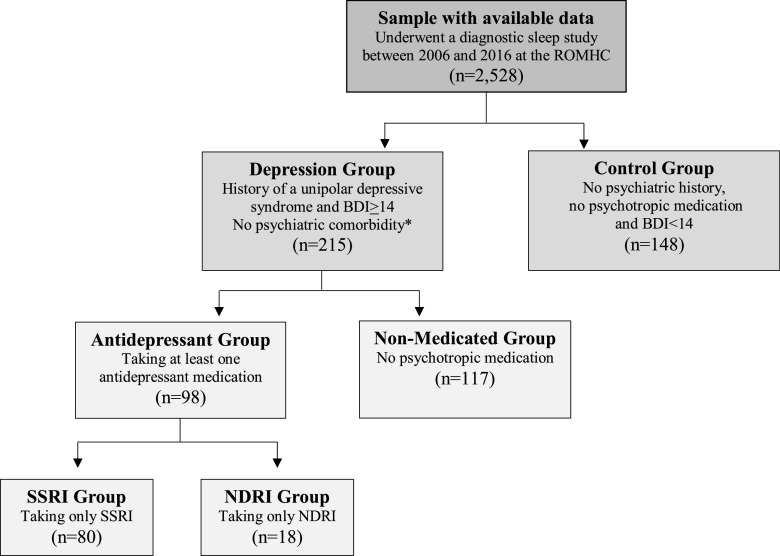
Figure 1. Study groups for the primary study aim.
Flowchart of cases meeting inclusion and exclusion criteria for each group. *Psychiatric comorbidities included bipolar disorder, psychotic disorder, posttraumatic stress disorder, or neurocognitive disorder. A group of 74 people with a history of a unipolar depressive syndrome who were taking an SSRI but were asymptomatic on the BDI-II (score < 14) was also created (see supplemental material (36.9KB, pdf) ). BDI-II = Beck Depression Inventory-II, ROMHC = Royal Ottawa Mental Health Centre.
Table 1.
Sleep architecture and descriptive information across main groups.
| Variable | Antidepressant | Non-medicated | Control | F/χ2 | P Value |
|---|---|---|---|---|---|
| n | 98 | 117 | 148 | — | — |
| Sex distribution (% female) | 64.3 | 67.5 | 45.9 | 14.8 | .001 |
| Age (y) | 43.4 ± 15.0 | 43.7 ± 13.7 | 52.8 ± 15.2 | 17.2 | < .001 |
| BMI (kg/m2) | 29.6 ± 6.6 | 28.9 ± 6.5 | 28.2 ± 5.5 | 1.2 | .310 |
| Sleep architecture (x̅ ± SD) | |||||
| Sleep efficiency (%) | 84.0 ± 11.0 | 84.0 ± 11.9 | 79.8 ± 12.2 | 5.5 | .004 |
| TST (min) | 357.2 ± 60.8 | 357.8 ± 75.0 | 342.9 ± 63.0 | 2.1 | .120 |
| Sleep onset latency (min) | 25.2 ± 24.8 | 17.7 ± 19.1 | 14.7 ± 15.4 | 8.1 | < .001 |
| REM sleep latency (min)a | 208.9 ± 103.9 | 130.2 ± 74.6 | 136.0 ± 81.5 | 26.2 | <.001 |
| NREM sleep (%) | 86.8 ± 7.6 | 83.2 ± 5.8 | 85.5 ± 6.0 | 9.1 | <.001 |
| REM sleep (%) | 13.2 ± 7.6 | 16.8 ± 5.8 | 14.5 ± 6.0 | 9.1 | <.001 |
aThere was no REM sleep noted in 1 individual from the non-medicated group and in 8 in from the antidepressant group. F/χ2 and P values reflect global omnibus statistics (statistics for paired comparisons are reported in the text; please see “Main groups analyses” in the Results section). SD = standard deviation, TST = total sleep time.
Depression group
Individuals included in the depression group (n = 215) had to have documented in their medical chart a history of a unipolar depressive syndrome (eg, major depression, dysthymic disorder, depressive disorder not otherwise specified), and current depressive symptoms of at least mild severity (Beck Depression Inventory-II [BDI-II] ≥ 14] around the time of the sleep assessment. Patients were systematically excluded if they had comorbid bipolar disorder, psychotic disorder, posttraumatic stress disorder, or neurocognitive disorder. A psychologist reviewed and classified all diagnostic information.
The depression group was first split into 2 groups based on the use of psychotropic medications at the time of PSG: the antidepressant group (individuals who reported taking at least 1 antidepressant medication and no other psychotropic medication on the night of the sleep study; n = 98), and the nonmedicated group (individuals who were free of psychotropic medication on the night of the sleep study; n = 117). The antidepressant group was subsequently split into 2 subgroups based on the class of antidepressant used: the SSRI group (n = 80) and the norepinephrine-dopamine reuptake inhibitor (NDRI) group (n = 18). Individuals taking any other psychotropic drug were systematically excluded. In addition, participants were free of other serotonergic drugs including triptans, 5-HT-3 receptor antagonists such as ondansetron, and muscle relaxants such as cyclobenzaprine.
Control group
Individuals included in the control group (n = 148) had no history of mood disorders, anxiety disorders, psychotic disorders, posttraumatic stress disorder, or neurocognitive disorders documented in their medical records. To be included in the control group, participants additionally had to have a score in the asymptomatic range on the BDI-II (< 14) and be free of psychotropic medication.
Procedures
All patients underwent height and weight measurement to determine body mass index (BMI), rated the severity of their depressive symptoms on the BDI-II, and underwent level 1 PSG. PSG was recorded with the Sandman SD32 acquisition software (Sandman 10.1; Natus Medical Incorporated, San Carlos, California) using 3 electroencephalogram channels (F3, C3, and O1), left and right electrooculogram, chin and leg electromyogram (EMG), and 2 electrocardiogram channels. Respiration was monitored with an airflow cannula (pressure transducer) and nasal-oral thermistor plus chest and abdomen plethysmography. Pulse oximetry data were used to calculate the minimum oxygen saturation (minSpO2) percentage reached during sleep.
Sleep stages, apneas/hypopneas, and arousals were visually scored by registered sleep technologists according to guidelines established by the American Academy of Sleep Medicine.32 Two types of breathing events were defined: apneas (≥ 10-second pause in breathing with at least a 90% drop in baseline breathing amplitude for 90% of the event), and hypopneas (at least 10 seconds of a > 30% reduction in airflow, with a ≥ 3% drop in oxygen saturation from pre-event baseline, with 90% of the event’s duration meeting the amplitude reduction criteria). The following indices of respiratory function were computed: AHI (number of apnea and hypopnea events per hour), oxygen desaturation index (ODI; number of blood oxygen desaturations ≥ 3% per hour), and minSpO2 across total sleep time and during REM and NREM sleep.
Statistical analysis
For descriptive purposes, χ2 and one-way analyses of variance were used to identify significant differences in sex distribution, age, BMI, and sleep architecture across groups. In addition, BDI-II scores were compared across the 3 depression groups.
To address the main study objective, mixed analyses of covariance were used to compare the AHI, ODI, and minSpO2 between groups and assess group and sleep stage interactions. These analyses of covariance had 1 within-subjects factor (sleep stages: REM vs NREM) and 1 between-subjects factor, either (1) the main groups (antidepressant vs nonnedicated vs control groups) or (2) the depression subgroups (SSRI vs NDRI vs non-medicated groups). Age, sex, and BMI were included in these analyses of covariance as covariates because of their potential effects on the relationship between medication use, depression status, and sleep disturbances.33,34 We used a similar approach for supplemental analysis to explore the potential influence of depressive states (see the supplemental material (36.9KB, pdf) ).
The BMI, sleep onset latency, and ODI were log-transformed, and AHI and BDI-II were square-root-transformed to improve distribution normality. Statistical significance was set at P < .050. All statistical analyses were carried out using SPSS (SPSS, version 22.0; IBM Corp, Armonk, NY).
RESULTS
Sample characteristics
Sample characteristics and sleep architecture variables for the main groups and for the SSRI and NDRI subgroups are reported in Tables 1 and 2, respectively.
Table 2.
Sleep architecture and descriptive information across depression subgroups.
| Variable | Non-medicated | NDRI | SSRI | F/χ2 | P Value |
|---|---|---|---|---|---|
| n | 117 | 18 | 80 | — | — |
| Sex distribution (% female) | 67.5 | 55.0 | 67.5 | 2.3 | .324 |
| Age (y) | 43.7 ± 13.7 | 37.7 ± 16.2 | 44.7 ± 14.5 | 1.8 | .170 |
| Comorbid anxiety, n (%) | 18 (15.4) | 5 (27.8) | 24 (30.0) | — | — |
| Substance use disorder, n (%) | 5 (4.3) | 4 (22.2) | 4 (5.0) | — | — |
| SSRI subtypes, n (%) | |||||
| Citalopram (Celexa) | — | — | 23 (28.8) | — | — |
| Escitalopram (Cipralex) | — | — | 24 (30.0) | — | — |
| Fluoxetine (Prozac) | — | — | 16 (20.0) | — | — |
| Sertraline (Zoloft) | — | — | 14 (17.5) | — | — |
| Fluvoxamine (Luvox) | — | — | 5 (6.3) | — | — |
| Paroxetine (Paxil) | — | — | 2 (2.5) | — | — |
| NDRI subtypes, n (%) | |||||
| Bupropion (Wellbutrin) | — | 18 (100.0) | — | — | — |
| BMI (kg/m2) | 28.9 ± 6.5 | 26.1 ± 5.9 | 30.3 ± 6.5 | 4.0 | .019 |
| BDI-II score | 23.1 ± 8.6 | 26.9 ± 10.0 | 24.1 ± 7.9 | 1.7 | .177 |
| Sleep architecture (x̅ ± SD) | |||||
| Sleep efficiency (%) | 84.0 ± 11.9 | 86.2 ± 12.1 | 83.5 ± 10.8 | 0.4 | .663 |
| TST (min) | 357.8 ± 75.0 | 363.0 ± 64.3 | 355.9 ± 60.3 | 0.08 | .924 |
| Sleep onset latency (min) | 17.7 ± 19.1 | 20.3 ± 17.4 | 26.3 ± 26.2 | 3.6 | .030 |
| REM sleep latency (min)a | 130.2 ± 74.6 | 112.9 ± 59.7 | 231.3 ± 99.3 | 36.6 | < .001 |
| NREM sleep (%) | 83.2 ± 5.8 | 83.3 ± 7.9 | 87.6 ± 7.3 | 11.3 | < .001 |
| REM sleep (%) | 16.8 ± 5.8 | 16.7 ± 7.9 | 12.4 ± 7.3 | 11.3 | < .001 |
Numbers in brackets are percentages. aThere was no REM sleep noted in 1 individual from the non-medicated group, 1 from the NDRI group, and 7 from the SSRI group. F/χ2 and P values reflect global omnibus statistics (statistics for paired comparisons are reported in the text; please see “Depression subgroups analyses” in the Results section). BDI-II = Beck Depression Inventory-II, SD = standard deviation, TST = total sleep time.
Clinical characteristics
Across the main groups, there were significant differences in age (F [2, 360] = 17.2; P < .001) and sex distribution (χ2[2] = 14.8; P = .001), but there was no difference in BMI (P = .271). Specifically, the antidepressant and non-medicated groups were significantly younger (both P < .001) and had significantly higher proportions of women (both P ≤ .005) than the control group. Across the depression subgroups, there was a significant difference in BMI (F [2, 212] = 4.0; P = .019), wherein the SSRI group had a significantly higher BMI compared with the NDRI group (P = .007). There was no significant difference in sex distribution, age, or BDI-II across the depression subgroups (all P > .050).
Sleep architecture
In unadjusted analyses, significant differences between the main groups were found for sleep onset latency (F [2, 360] = 8.1; P = <.001), sleep efficiency (F [2, 360] = 5.5; P = .004), REM sleep latency (F [2, 351] = 26.2; P < .001), and the percentages of NREM (F [2, 360] = 9.1; P < .001) and REM (F [2, 360] = 9.1; P < .001) sleep. The antidepressant group had a slightly, but statistically significant, higher sleep efficiency compared with the control group (P = .007) but not compared with the non-medicated group (P = .974). The antidepressant group also had significantly longer sleep onset latency and REM sleep onset latency when compared with the nonmedicated group (P = .008 and P < .001, respectively) and the control group (both P < .001). The antidepressant group had a lower percentage of REM sleep and a higher percentage of NREM sleep compared with the non-medicated group (both P < .001).
Among the depression subgroups, significant differences were found for sleep onset latency (F [2, 212] = 3.6; P = .030), REM sleep latency (F [2, 203] = 36.6; P < .001), and the percentages of REM (F [2, 212] = 11.3; P < .001) and NREM (F [2, 212] = 11.3; P < .001) sleep. The SSRI group had a significantly longer sleep onset latency than the non-medicated group (P = .011). Compared with both the non-medicated and NDRI groups, the SSRI group had a significantly longer REM sleep latency, a lower percentage of REM sleep, and a higher percentage of NREM sleep (all P ≤ .014).
Comparison of respiratory measures across groups and sleep stages
Main groups analyses
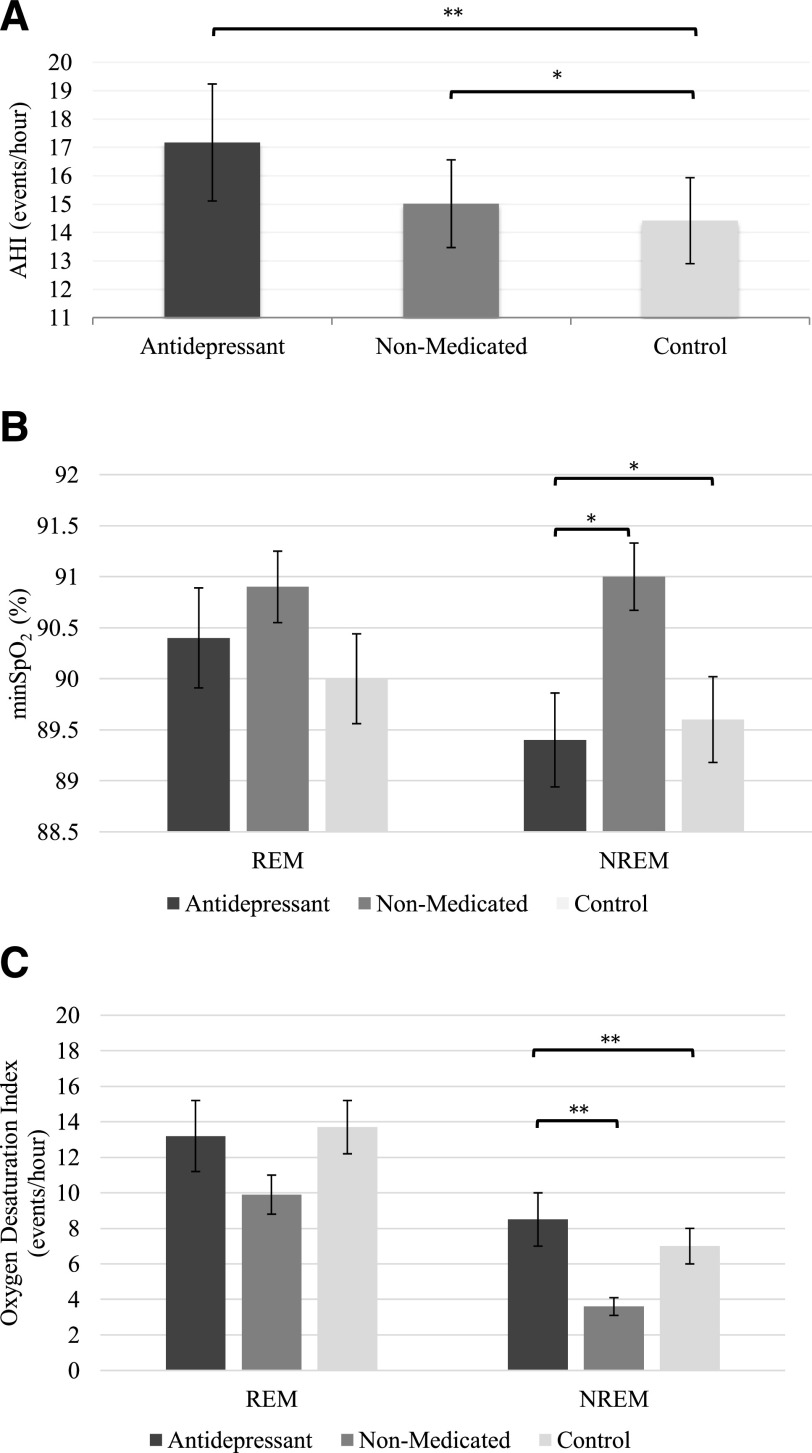
Figure 2 depicts respiratory measures for the antidepressant, non-medicated, and control groups. Detailed respiratory data are reported in Table 3.
Figure 2. Respiratory parameters in the main groups.
(A) AHI (REM and NREM sleep combined), (B) minSpO2, and (C) ODI across REM and NREM sleep. Bars indicate unadjusted mean and error bars indicate the standard errors of the mean. *P ≤ .009, **P ≤ .001. There was no REM sleep noted in 8 individuals in the antidepressant group and 1 in the non-medicated group. Analyses were adjusted for age, sex, and BMI.
Table 3.
Respiratory indices across main groups.
| Control x̅ ± SD | Non-medicated x̅ ± SD | Antidepressant x̅ ± SD | Statistics (F)a | |||
|---|---|---|---|---|---|---|
| Group | Sleep | Interaction | ||||
| AHI (events/h) | 6.1** | 3.7(*) | 2.7(*) | |||
| NREM sleep | 10.9 ± 16.7 | 9.8 ± 13.0 | 13.8 ± 16.8 | |||
| REM sleep | 17.9 ± 20.2 | 20.2 ± 20.3 | 20.5 ± 22.3 | |||
| MinSpO2 (%) | 2.0 | 2.4 | 4.1* | |||
| NREM sleep | 89.6 ± 5.2 | 91.0 ± 3.5 | 89.4 ± 4.3 | |||
| REM sleep | 90.0 ± 5.3 | 90.9 ± 3.7 | 90.4 ± 4.6 | |||
| ODI (events/h) | 2.9(*) | 15.3** | 3.5* | |||
| NREM sleep | 7.0 ± 12.7 | 3.6 ± 5.6 | 8.5 ± 14.3 | |||
| REM sleep | 13.7 ± 17.9 | 9.9 ± 11.7 | 13.2 ± 18.8 | |||
aAnalyses were adjusted for age, sex, and BMI. F values are reported for main group effects (differences between mentally healthy control patients, non-medicated patients with depression, and medicated patients with depression), main effects of sleep stages (NREM and REM sleep), and interactions between main groups and sleep stages. Eight individuals in the antidepressant group and 1 in the non-medicated group had no REM sleep. *P ≤ .032, **P ≤ .003, (*)P ≤ .067 (ie, trends). SD = standard deviation.
There was no significant group by sleep-stage interaction or main effect of sleep stages for AHI. A significant main effect of group (F [2, 348] = 6.1; P = .003) showed that the antidepressant (P = .001) and non-medicated (P = .007) groups had a slight but statistically significant higher total AHI compared with the control group. Although the antidepressant group had a marginally higher AHI than the non-medicated group, this difference was not statistically significant (P = .493).
Significant interactions between groups and sleep stages were found for minSpO2 (F [2, 348] = 4.1; P = .018) and ODI (F [2, 348] = 3.5; P = .032). During NREM sleep, the antidepressant group had a significantly lower minSpO2 and a higher ODI than both the control (both P ≤ .009) and the non-medicated (both P ≤ .007) groups. The NREM sleep minSpO2, and ODI did not differ significantly between the control and non-medicated groups. There was no significant group difference in these variables during REM sleep.
Depression subgroups analyses
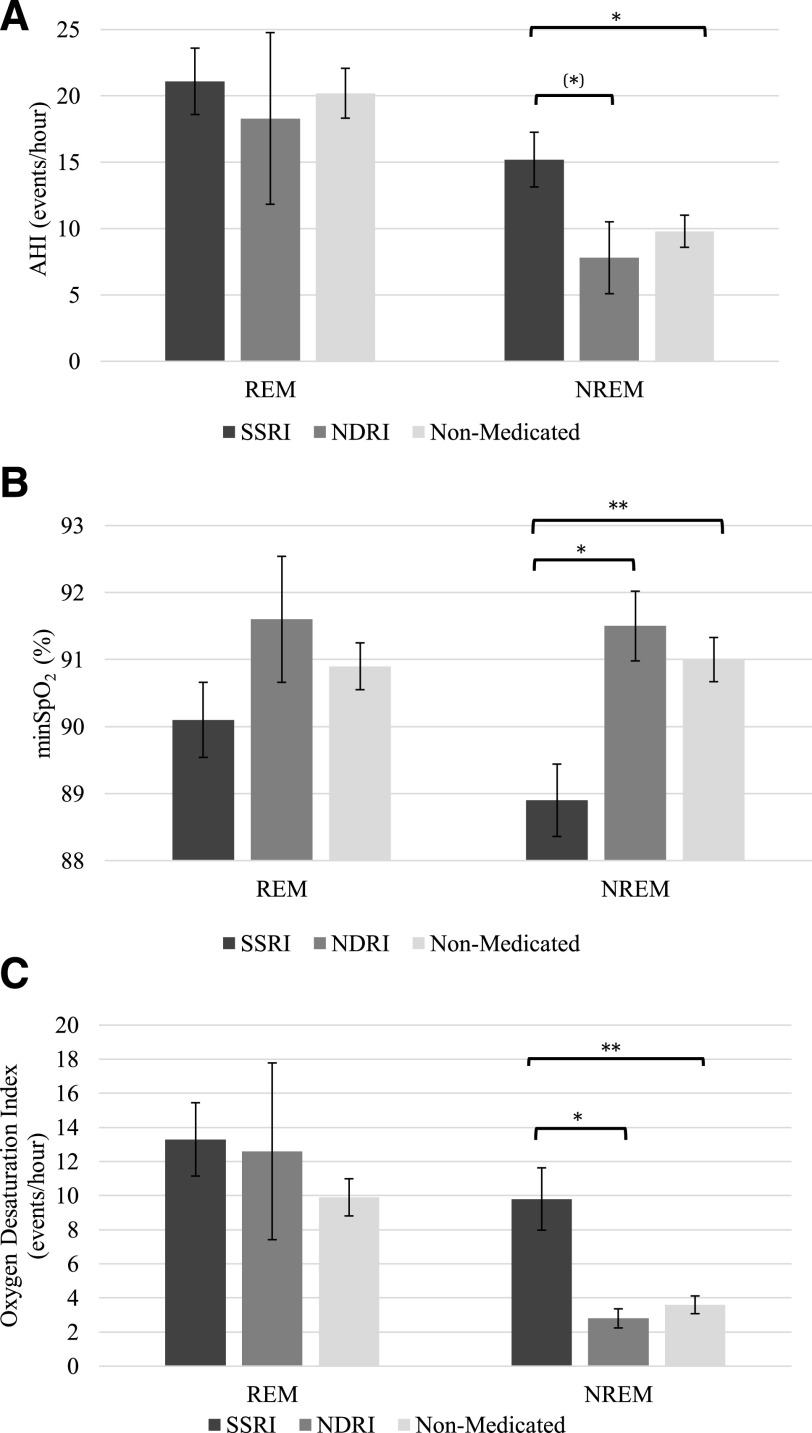
Figure 3 depicts respiratory measures for the SSRI, NDRI, and non-medicated groups. Detailed respiratory data are reported in Table 4.
Figure 3. Respiratory parameters during REM and NREM sleep in the depression subgroups.
(A) AHI, (B) minSpO2, and (C) ODI. Bars indicate unadjusted mean and error bars indicate the standard errors of the mean. *P ≤ .045, (*)P ≤ .069, **P ≤ .001. There was no REM sleep noted in 1 individual in the non-medicated group, 1 in the NDRI group, and 7 in the SSRI group. Analyses were adjusted for age, sex, and BMI.
Table 4.
Respiratory indices across depression subgroups.
| Non-medicated x̅ ± SD | NDRI x̅ ± SD | SSRI x̅ ± SD | Statistics (F)a | |||
|---|---|---|---|---|---|---|
| Group | Sleep | Interaction | ||||
| AHI (events/h) | 0.4 | 1.8 | 4.3* | |||
| NREM sleep | 9.8 ± 13.0 | 7.8 ± 11.2 | 15.2 ± 17.6 | |||
| REM sleep | 20.2 ± 20.3 | 18.3 ± 26.7 | 21.1 ± 21.4 | |||
| MinSpO2 (%) | 3.2* | 0.4 | 4.8** | |||
| NREM sleep | 91.0 ± 3.5 | 91.5 ± 2.2 | 88.9 ± 4.6 | |||
| REM sleep | 90.9 ± 3.7 | 91.6 ± 3.9 | 90.1 ± 4.8 | |||
| ODI (events/h) | 2.8 | 9.3** | 7.2*** | |||
| NREM sleep | 3.6 ± 5.6 | 2.8 ± 2.3 | 9.8 ± 15.6 | |||
| REM sleep | 9.9 ± 11.7 | 12.6 ± 21.3 | 13.3 ± 18.3 | |||
aAnalyses were adjusted for age, sex, and BMI. F values are reported for main group effects (differences between patients with depression taking SSRIs, patients taking NDRIs, and non-medicated patients with depression), main effects of sleep stages (NREM and REM sleep), and interactions between main groups and sleep stages. There was no REM sleep noted in 1 individual in the non-medicated group, 1 in the NDRI group, and 7 in the SSRI group. *P ≤ .041, **P ≤ .009, ***P ≤ .001.
Significant interactions between antidepressant subtypes and sleep stages were found for AHI (F [2, 200] = 4.3; P = .015), minSpO2 (F [2, 200] = 4.8; P = .009), and ODI (F [2, 200] = 7.2; P = .001). During NREM sleep, the SSRI group had a significantly lower minSpO2 and higher ODI compared with both the non-medicated (both P < .001) and NDRI (both P ≤ .045) groups. The SSRI group also had a significantly higher AHI than the non-medicated group (P = .014) and tended to have a higher AHI than the NDRI group (P = .069) during NREM sleep. The NREM sleep AHI, minSpO2, and ODI did not differ significantly between the NDRI and non-medicated groups. There was no significant group difference in these variables during REM sleep. Results from supplemental analyses on the influence of depressive states are presented in the supplemental material (36.9KB, pdf) .
DISCUSSION
In this sample of people who were referred for a sleep disorder assessment and underwent a diagnostic sleep study, individuals with depression had significantly more frequent sleep-related breathing disruptions than nondepressed individuals, which echoes previous findings.35–37 The present study is the first to report that these breathing disruptions, and their effect on nocturnal blood oxygenation, may be worse in individuals with depression who are taking SSRIs, the most commonly prescribed medications for depression.38–40
In the current study, individuals using SSRIs had more frequent apneas and hypopneas during NREM sleep than non-medicated individuals with depression, with a similar trend when compared with those taking non-serotonergic antidepressants (ie, NDRIs). The differences in NREM sleep AHI and ODI were clinically meaningful according to established standards.41 Notably, the overall rates of apneas and hypopneas were 1.6–1.9 times higher in the SSRI group than in the other groups. Supplemental analyses suggest that this effect may be dependent on depressive state, taking place in individuals currently symptomatic for depression but not in those with a history of depression who were no longer symptomatic. Symptomatic individuals using SSRIs also had lower blood oxygen saturation and more frequent desaturations during NREM sleep when compared to non-medicated individuals with depression or to those using non-serotonergic antidepressants. Although these findings need to be further investigated with prospective studies, this is the first empirical evidence in humans suggesting that the use of SSRIs in people with depression and sleep complaints may be associated with worse breathing during sleep. The hypothesis that this condition may operate via alterations in 5-HT-mediated respiratory functions is reinforced by the observation in our study that breathing disturbances were not significantly elevated in people taking non-serotonergic antidepressants.
Previous work indicates that in the central nervous system, the excitatory effects of 5-HT on respiration centers are mostly mediated by 5-HT1A, 5-HT2A, and 5-HT2C receptors.16 For instance, 5-HT in the brainstem increases the activation of upper-airway dilators,29,30 which facilitates respiration. Conversely, in the periphery, 5-HT has mostly inhibitory effects on respiration that are mediated by 5-HT2A, 5-HT2C, and 5-HT3 receptors. Hence, although the stimulation of 5-HT2A and 5-HT2C receptors may have opposite effects on respiration in different parts of the nervous system, 5-HT1A and 5-HT3 receptors may induce more consistent excitatory and inhibitory modulation, respectively. Although this remains to be formally investigated, depression-related reduction in 5-HT1A receptor activity11,42 and potential increases in 5-HT3 receptor activity31 may worsen individual vulnerability to the suppressing effects of SSRIs on respiration.
In line with the well-known REM sleep–suppressing effects of SSRIs,1 the SSRI group was found to have significantly reduced amounts of REM sleep and increased REM sleep onset latency compared with the non-medicated and NDRI groups. Considering that there is increased risk for upper-airway collapse during REM sleep, it has previously been suggested that antidepressant medications may reduce sleep apneas by suppressing REM sleep.43 However, in the present study, significant differences in AHI and blood oxygen desaturation persisted despite the reduction in REM sleep and occurred most prominently during NREM sleep. This result suggests that the effects of SSRIs on nocturnal respiratory function in people with depression may not solely stem from changes in sleep architecture. These findings may have important clinical implications, considering the potential for SRBDs to worsen depression.44,45 Researchers have suggested that SRBDs negatively impact mood, notably via the effects of chronic hypoxia on the brain.45,46 If SSRI use in people with depression worsens not only sleep apneas/hypopneas but also oxygen desaturation, then in the long term, their use may induce progressive mood worsening.
This study has several limitations. Because it was based on retrospective data, unmeasured confounders may have resulted in biased exposure effect estimates. For example, we were unable to control for factors such as medication dosage, duration of treatment, central obesity, and smoking status. Because of the cross-sectional nature of this study, causation cannot be inferred. The sample included individuals referred to a sleep clinic, so the findings may not generalize to people with depression without marked sleep problems. Generalizability is further limited by a single-center study design. Only information about medications taken on the night of PSG were available. As such, we were unable to control for other medications that could have been taken around the same period but that may have been skipped on that specific night. Although individuals taking any type of psychotropic medications aside from the ones defining the drug classes under study were systematically excluded, other types of medications (eg, antihypertensives) may have impacted sleep. The sample size of the group taking NDRIs was fairly small. Larger comparative and interventional studies are required to investigate the specificity of the effects of different serotonergic agents as compared with those of non-serotonergic antidepressants on respiratory functions during sleep. There is also a need to assess potential sex differences in these effects.
Although they are limited by the fact that they are based on a retrospective clinical sample, the current study suggests that individuals with depression may have worse SRBDs than mentally healthy individuals. Independent of depression severity, sleep-related respiratory disturbances during NREM sleep may be more prominent in people using SSRIs as opposed to those using NDRIs, possibly because of interactions between depression neuropathophysiology and the effects of exogenous 5-HT manipulation on the respiratory system. Prospective studies are required to determine whether 5-HT agents may actively exacerbate SRBDs in individuals with depression and whether this increase may be modulated by 5-HT receptor expression and/or binding abnormalities linked to depression. Such studies may unveil a new and previously unsuspected adverse effect of one of the most heavily prescribed classes of psychotropic medication. This potential effect would have serious implications for the clinical management of depression because SRBDs can have adverse impacts on physical health and depressive symptoms.
DISCLOSURE STATEMENT
All authors have read and approved the manuscript. This study was funded by the Canadian Institutes of Health Research (Frederick Banting and Charles Best Canada Graduate Scholarship, for MS) and the Royal’s Institute of Mental Health Research (The Emerging Research Innovators in Mental Health Program, for RR). The authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
The authors thank the following individuals for their invaluable help building the Royal Ottawa Mental Health Centre retrospective database: Alexandre Lafrenière, Stephanie Lalande, Ashley Nixon, Katharine Welch, Antoine Benoit, Emily Jerome, Reuben Bong, Joshua McArdle, Holly Shannon, Claude Richard-Malenfant, Meenakshie Bradley-Garcia, Julia Lagoutina, Laura Hum, and Dylan Price. Grateful appreciation is also directed to Dr. Georg Northoff and Dr. Heather Tulloch for their insightful comments.
ABBREVIATIONS
- 5-HT
serotonin
- BDI
Beck Depression Inventory
- BMI
body mass index
- minSpO2
minimum blood oxygen saturation
- NDRI
norepinephrine-dopamine reuptake inhibitor
- ODI
oxygen desaturation index
- SRBDs
sleep-related breathing disturbances
- SSRI
selective serotonin reuptake inhibitor
REFERENCES
- 1.Armitage R. The effects of antidepressants on sleep in patients with depression. Can J Psychiatry. 2000;45(9):803–809. 10.1177/070674370004500903 [DOI] [PubMed] [Google Scholar]
- 2.Jovaisas B. Compendium of Pharmaceuticals and Specialties. Ottawa, ON: Canadian Pharmacists Association; 2015. [Google Scholar]
- 3.Kraiczi H, Hedner J, Dahlöf P, Ejnell H, Carlson J. Effect of serotonin uptake inhibition on breathing during sleep and daytime symptoms in obstructive sleep apnea. Sleep. 1999;22(1):61–67. [PubMed] [Google Scholar]
- 4.Prasad B, Radulovacki M, Olopade C, Herdegen JJ, Logan T, Carley DW. Prospective trial of efficacy and safety of ondansetron and fluoxetine in patients with obstructive sleep apnea syndrome. Sleep. 2010;33(7):982–989. 10.1093/sleep/33.7.982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carley DW, Radulovacki M. Mirtazapine, a mixed-profile serotonin agonist/antagonist, suppresses sleep apnea in the rat. Am J Respir Crit Care Med. 1999;160(6):1824–1829. 10.1164/ajrccm.160.6.9902090 [DOI] [PubMed] [Google Scholar]
- 6.BaHammam AS, Kendzerska T, Gupta R, et al. Comorbid depression in obstructive sleep apnea: an under-recognized association. Sleep Breath. 2016;20(2):447–456. 10.1007/s11325-015-1223-x [DOI] [PubMed] [Google Scholar]
- 7.Coppen A, Eccleston E, Craft I, Bye P. Letter: total and free plasma-tryptophan concentration and oral contraception. Lancet. 1973;2(7844):1498. 10.1016/S0140-6736(73)92762-1 [DOI] [PubMed] [Google Scholar]
- 8.Cowen PJ, Parry-Billings M, Newsholme EA. Decreased plasma tryptophan levels in major depression. J Affect Disord. 1989;16(1):27–31. 10.1016/0165-0327(89)90051-7 [DOI] [PubMed] [Google Scholar]
- 9.Asberg M, Thorén P, Träskman L, Bertilsson L, Ringberger V. “Serotonin depression”—a biochemical subgroup within the affective disorders? Science. 1976;191(4226):478–480. 10.1126/science.1246632 [DOI] [PubMed] [Google Scholar]
- 10.Sargent PA, Kjaer KH, Bench CJ, et al. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57(2):174–180. 10.1001/archpsyc.57.2.174 [DOI] [PubMed] [Google Scholar]
- 11.Drevets WC, Frank E, Price JC, et al. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46(10):1375–1387. 10.1016/S0006-3223(99)00189-4 [DOI] [PubMed] [Google Scholar]
- 12.Malison RT, Price LH, Berman R, et al. Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl) tropane and single photon emission computed tomography. Biol Psychiatry. 1998;44(11):1090–1098. 10.1016/S0006-3223(98)00272-8 [DOI] [PubMed] [Google Scholar]
- 13.Alvarenga RM, Pires JGP, Futuro Neto HA. Functional mapping of the cardiorespiratory effects of dorsal and median raphe nuclei in the rat. Braz J Med Biol Res. 2005;38(11):1719–1727. 10.1590/S0100-879X2005001100022 [DOI] [PubMed] [Google Scholar]
- 14.Kumaido K. Studies on the respiratory control mechanism of medullary raphe nuclei and their serotonergic system. Article in Japanese. No To Shinkei. 1988;40(10):929–938. [PubMed] [Google Scholar]
- 15.Adrien J. Neurobiological bases for the relation between sleep and depression. Sleep Med Rev. 2002;6(5):341–351. 10.1053/smrv.2001.0200 [DOI] [PubMed] [Google Scholar]
- 16.Veasey SC. Serotonin agonists and antagonists in obstructive sleep apnea: therapeutic potential. Am J Respir Med. 2003;2(1):21–29. 10.1007/BF03256636 [DOI] [PubMed] [Google Scholar]
- 17.Heym J, Steinfels GF, Jacobs BL. Activity of serotonin-containing neurons in the nucleus raphe pallidus of freely moving cats. Brain Res. 1982;251(2):259–276. 10.1016/0006-8993(82)90743-0 [DOI] [PubMed] [Google Scholar]
- 18.Real C, Popa D, Seif I, et al. Sleep apneas are increased in mice lacking monoamine oxidase A. Sleep. 2007;30(10):1295–1302. 10.1093/sleep/30.10.1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veasey SC, Fenik P, Panckeri K, Pack AI, Hendricks JC. The effects of trazodone with L-tryptophan on sleep-disordered breathing in the English bulldog. Am J Respir Crit Care Med. 1999;160(5 Part 1):1659–1667. 10.1164/ajrccm.160.5.9812007 [DOI] [PubMed] [Google Scholar]
- 20.Reid G, Rand M. Physiological actions of the partially purified serum vasoconstrictor (serotonin). Aust J Exp Biol Med Sci. 1951;29(6):401–415. 10.1038/icb.1951.46 [DOI] [PubMed] [Google Scholar]
- 21.Carley DW, Radulovacki M. Role of peripheral serotonin in the regulation of central sleep apneas in rats. Chest. 1999;115(5):1397–1401. 10.1378/chest.115.5.1397 [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto S. Effects of carotid body chemoreceptor stimulation by 5-HT on phrenic nerve activity and ventilation in the rabbit. Arch Int Pharmacodyn Ther. 1981;254(2):282–292. [PubMed] [Google Scholar]
- 23.Radulovacki M, Trbovic SM, Carley DW. Serotonin 5-HT3-receptor antagonist GR 38032F suppresses sleep apneas in rats. Sleep. 1998;21(2):131–136. 10.1093/sleep/21.2.131 [DOI] [PubMed] [Google Scholar]
- 24.Hanzel DA, Proia NG, Hudgel DW. Response of obstructive sleep apnea to fluoxetine and protriptyline. Chest. 1991;100(2):416–421. 10.1378/chest.100.2.416 [DOI] [PubMed] [Google Scholar]
- 25.Berry RB, Yamaura EM, Gill K, Reist C. Acute effects of paroxetine on genioglossus activity in obstructive sleep apnea. Sleep. 1999;22(8):1087–1092. 10.1093/sleep/22.8.1087 [DOI] [PubMed] [Google Scholar]
- 26.Manzke T, Guenther U, Ponimaskin EG, et al. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science. 2003;301(5630):226–229. 10.1126/science.1084674 [DOI] [PubMed] [Google Scholar]
- 27.Manzke T, Dutschmann M, Schlaf G, et al. Serotonin targets inhibitory synapses to induce modulation of network functions. Philos Trans R Soc Lond B Biol Sci. 2009;364(1529):2589–2602. 10.1098/rstb.2009.0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carley DW, Depoortere H, Radulovacki M. R-zacopride, a 5-HT3 antagonist/5-HT4 agonist, reduces sleep apneas in rats. Pharmacol Biochem Behav. 2001;69(1–2):283–289. 10.1016/S0091-3057(01)00535-4 [DOI] [PubMed] [Google Scholar]
- 29.Fenik P, Veasey SC. Pharmacological characterization of serotonergic receptor activity in the hypoglossal nucleus. Am J Respir Crit Care Med. 2003;167(4):563–569. 10.1164/rccm.200202-107OC [DOI] [PubMed] [Google Scholar]
- 30.Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci Lett. 1992;139(2):243–248. 10.1016/0304-3940(92)90563-M [DOI] [PubMed] [Google Scholar]
- 31.Rajkumar R, Mahesh R. The auspicious role of the 5-HT3 receptor in depression: a probable neuronal target? J Psychopharmacol. 2010;24(4):455–469. 10.1177/0269881109348161 [DOI] [PubMed] [Google Scholar]
- 32.Silber MH, Ancoli-Israel S, Bonnet MH, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3(2):121–131. 10.5664/jcsm.26814 [DOI] [PubMed] [Google Scholar]
- 33.Mokhlesi B, Ham SA, Gozal D. The effect of sex and age on the comorbidity burden of OSA: an observational analysis from a large nationwide US health claims database. Eur Respir J. 2016;47(4):1162–1169. 10.1183/13993003.01618-2015 [DOI] [PubMed] [Google Scholar]
- 34.LaGrotte C, Fernandez-Mendoza J, Calhoun SL, Liao D, Bixler EO, Vgontzas AN. The relative association of obstructive sleep apnea, obesity and excessive daytime sleepiness with incident depression: a longitudinal, population-based study. Int J Obes Lond. 2016;40(9):1397–1404. 10.1038/ijo.2016.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds CF III, Kupfer DJ, Taska LS, et al. Sleep apnea in Alzheimer’s dementia: correlation with mental deterioration. J Clin Psychiatry. 1985;46(7):257–261. [PubMed] [Google Scholar]
- 36.Ohayon MM. The effects of breathing-related sleep disorders on mood disturbances in the general population. J Clin Psychiatry. 2003;64(10):1195–1200. 10.4088/JCP.v64n1009 [DOI] [PubMed] [Google Scholar]
- 37.Farney RJ, Lugo A, Jensen RL, Walker JM, Cloward TV. Simultaneous use of antidepressant and antihypertensive medications increases likelihood of diagnosis of obstructive sleep apnea syndrome. Chest. 2004;125(4):1279–1285. 10.1378/chest.125.4.1279 [DOI] [PubMed] [Google Scholar]
- 38.Trifirò G, Barbui C, Spina E, et al. Antidepressant drugs: prevalence, incidence and indication of use in general practice of Southern Italy during the years 2003-2004. Pharmacoepidemiol Drug Saf. 2007;16(5):552–559. 10.1002/pds.1303 [DOI] [PubMed] [Google Scholar]
- 39.Raymond CB, Morgan SG, Caetano PA. Antidepressant utilization in British Columbia from 1996 to 2004: increasing prevalence but not incidence. Psychiatr Serv. 2007;58(1):79–84. 10.1176/ps.2007.58.1.79 [DOI] [PubMed] [Google Scholar]
- 40.Wu CS, Shau WY, Chan HY, Lee YC, Lai YJ, Lai MS. Utilization of antidepressants in Taiwan: a nationwide population-based survey from 2000 to 2009. Pharmacoepidemiol Drug Saf. 2012;21(9):980–988. 10.1002/pds.3255 [DOI] [PubMed] [Google Scholar]
- 41.Tran K, Kim J, Tsoi B, et al. Interventions for the Treatment of Obstructive Sleep Apnea in Adults: A Health Technology Assessment—Project Protocol. Canadian Agency for Drugs and Technologies in Health optimal use report number 6.1a. Canadian Agency for Drugs and Technologies in Health; 2016. [PubMed]
- 42.Drevets WC, Thase ME, Moses-Kolko EL, et al. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl. Med. Biol. 2007;34(7):865–877. 10.1016/j.nucmedbio.2007.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeMartinis NA, Winokur A. Effects of psychiatric medications on sleep and sleep disorders. CNS Neurol Disord Drug Targets. 2007;6(1):17–29. 10.2174/187152707779940835 [DOI] [PubMed] [Google Scholar]
- 44.Robillard R, Chase T, Courtney D, Ward M, De Koninck J, Lee EK. Sleep-related breathing disturbances in adolescents with treatment resistant depression. Sleep Med. 2019;56:47–51. 10.1016/j.sleep.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 45.Ejaz SM, Khawaja IS, Bhatia S, Hurwitz TD. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci. 2011;8(8):17–25. [PMC free article] [PubMed] [Google Scholar]
- 46.Ishman SL, Cavey RM, Mettel TL, Gourin CG. Depression, sleepiness, and disease severity in patients with obstructive sleep apnea. Laryngoscope. 2010;120(11):2331–2335. 10.1002/lary.21111 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.