Abstract
Study Objectives:
Population based estimates of obstructive sleep apnea (OSA) frequency and health impact are incomplete. The aim of this study was to determine the prevalence of risk factors for physician and sleep study diagnosed OSA among individuals in a state-based surveillance program
Methods:
Using questions inserted into the 2016 (n = 5,564) and 2017 (n = 10,884) South Carolina Behavioral Risk Factor Surveillance System of the Centers for Disease Control and Prevention, we analyzed the prevalence of physician diagnosed OSA and associated comorbidities. The validated STOP-BANG questionnaire without neck circumference (STOP-BAG) defined populations at moderate risk (score 3–4) and high risk (score 5–7). Statistical analysis using weighted prevalence and means and their 95% confidence intervals (CI) thus reflect population estimates of disease burden.
Results:
The population-based prevalence of physician diagnosed OSA in South Carolina was 9.7% (95% CI: 9.0–10.4). However, the populations with moderate risk (18.5%, 95% CI: 17.3–19.8) and high risk (25.5%, 95% CI: 23.9–27.1) for OSA, as determined by the STOP-BAG questionnaire, were much higher. Compared to those at low risk for OSA, those at high risk were more often diagnosed with coronary heart disease, stroke, asthma, skin cancer, other cancers, chronic obstructive pulmonary disease, arthritis, depression, kidney disease, and diabetes (all P < .001).
Conclusions:
OSA is common and strongly associated with major comorbidities. As such, this public health crisis warrants more diagnostic and therapeutic attention. The STOP-BAG questionnaire provides a public health platform to monitor this disease.
Citation:
Strange C, Richard CL, Shan S, et al. A population-based estimate of the health care burden of obstructive sleep apnea using a STOP-BAG questionnaire in South Carolina. J Clin Sleep Med. 2021;17(3):367–374.
Keywords: OSA, sleep apnea, STOP-BAG, obstructive sleep apnea
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea is commonly underdiagnosed. However, to date, there are few public health tools to estimate the population burden of the condition and common comorbidities.
Study Impact: With the Behavioral Risk Factor Surveillance System of the Center for Disease Control and Prevention, a STOP-BAG questionnaire was able to estimate obstructive sleep apnea prevalence. The burden of undiagnosed obstructive sleep apnea and common comorbidities associated with obstructive sleep apnea is large and can be assessed longitudinally with this state-based tool.
INTRODUCTION
Obstructive sleep apnea (OSA) is a very common disorder. Associated with aging and body mass index (BMI), the exact prevalence depends on the populations studied. Older studies show an estimated prevalence of 9–25% in the general adult population,1,2 and newer estimates show a prevalence of greater than 50% of adults aged 30–69 years in some countries in the world.3 The repetitive closing and opening of the upper airway with the accompanying intermittent episodic hypoxemia and sleep fragmentation seen in OSA has been associated with significant adverse health consequences, such as cardiovascular disease,4 cognitive impairment,5 and motor vehicle crash.6
Since the respiratory events in OSA occur during sleep, many patients with OSA may not be aware that they have this condition. Hence, the number of undiagnosed individuals remains unknown.7 OSA can be diagnosed by an overnight in-laboratory polysomnography or by a home sleep apnea test in carefully selected individuals; however, these tests are expensive, have limited availability in some geographic areas, and the precise diagnostic criteria remain somewhat controversial, especially for mild to moderate disease.8
For these reasons, screening tools have been developed to help identify patients at high risk for undiagnosed OSA. Two commonly used questionnaires, STOP9 and STOP-BANG,10 were developed as reliable, concise, and easy-to-use screening tools and have been validated in a variety of surgical and medical populations.11–14 Importantly, the 8-question STOP-BANG has a higher sensitivity and specificity than the 4-question STOP tool, since the validation strategy was to add questions identified in univariate analysis of OSA populations. This makes the STOP-BANG questionnaire clinically very useful in screening populations at risk for OSA. Meta-analysis estimates of the sensitivity of STOP-BANG for mild, moderate, and severe OSA are generally 88–93% with specificities of 35–42% for the different OSA populations.15
In this study, the STOP-BANG instrument and question regarding self-report of provider-diagnosed OSA was applied to an adult population-based cohort derived through the Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System (BRFSS). Our goal was to identify individuals at high-risk for OSA using available information. To this purpose, 4 questions were incorporated into the BRFSS sleep module in addition to the existing BRFSS data (age, hypertension, BMI, and sex) that were designed to capture all the elements of the STOP-BANG screening questionnaire with the exception of neck circumference. The STOP-BANG instrument is validated for a score ≥ 3,9,10 indicating moderate to high risk of OSA. However, neck circumference is not easily captured on a questionnaire. Therefore, we analyzed BRFSS data with a STOP-BANG questionnaire that did not include the neck circumference data, which we called the STOP-BAG questionnaire (Table 1). The STOP-BAG has been used in other patient populations to screen for OSA and has been found to have a high sensitivity and reasonable specificity.16,17
Table 1.
STOP, STOP-BANG, and modified STOP-BAG (without neck circumference) questionnaires.
| STOP | STOP-BANG | STOP-BAG | |
|---|---|---|---|
| Loud snoring | × | × | × |
| Tiredness | × | × | × |
| Observed apnea | × | × | × |
| High blood pressure | × | × | × |
| Body mass index > 35 kg/m2 | – | × | × |
| Age > 50 years | – | × | × |
| Neck circumference > 40 cm | – | × | – |
| Gender male | – | × | × |
| Total score | 4 | 8 | 7 |
The principal goal of this research project was to use the BRFSS health survey data to define the epidemiology of diagnosed and undiagnosed populations with OSA in the state of South Carolina between January 1, 2016 and December 31, 2017. Furthermore, we sought to define the impact of OSA on other health outcomes.
METHODS
In 2016, the South Carolina (SC) BRFSS included 4 sleep disorder-related questions as an optional module, which were asked to adults for the last 6 months of the year. These questions were as follows (responses detailed in Table S1 (532.5KB, pdf) in the supplemental material): “Over the last 2 weeks, how many days have you had trouble falling asleep or staying asleep or sleeping too much?”; “Over the last 2 weeks, how many days did you unintentionally fall asleep during the day?”; “Have you ever been told that you snore loudly?”; and “Has anyone ever observed that you stop breathing during your sleep?”
In 2017, another state-added question was incorporated into the SC BRFSS to obtain the prevalence of diagnosed OSA (“Has a doctor ever diagnosed you with a condition called obstructive sleep apnea (also known as OSA) based on a sleep study?”; Table S1 (532.5KB, pdf) ). The 2016 SC BRFSS data were obtained (n = 11,236), and sample exclusions were applied to remove those with incomplete or missing data that limited obtaining a complete STOP-BAG score (final n = 5,557; Figure 1).
Figure 1. Consort diagram for populations analyzed in the 2016 and 2017 BRFSS data.
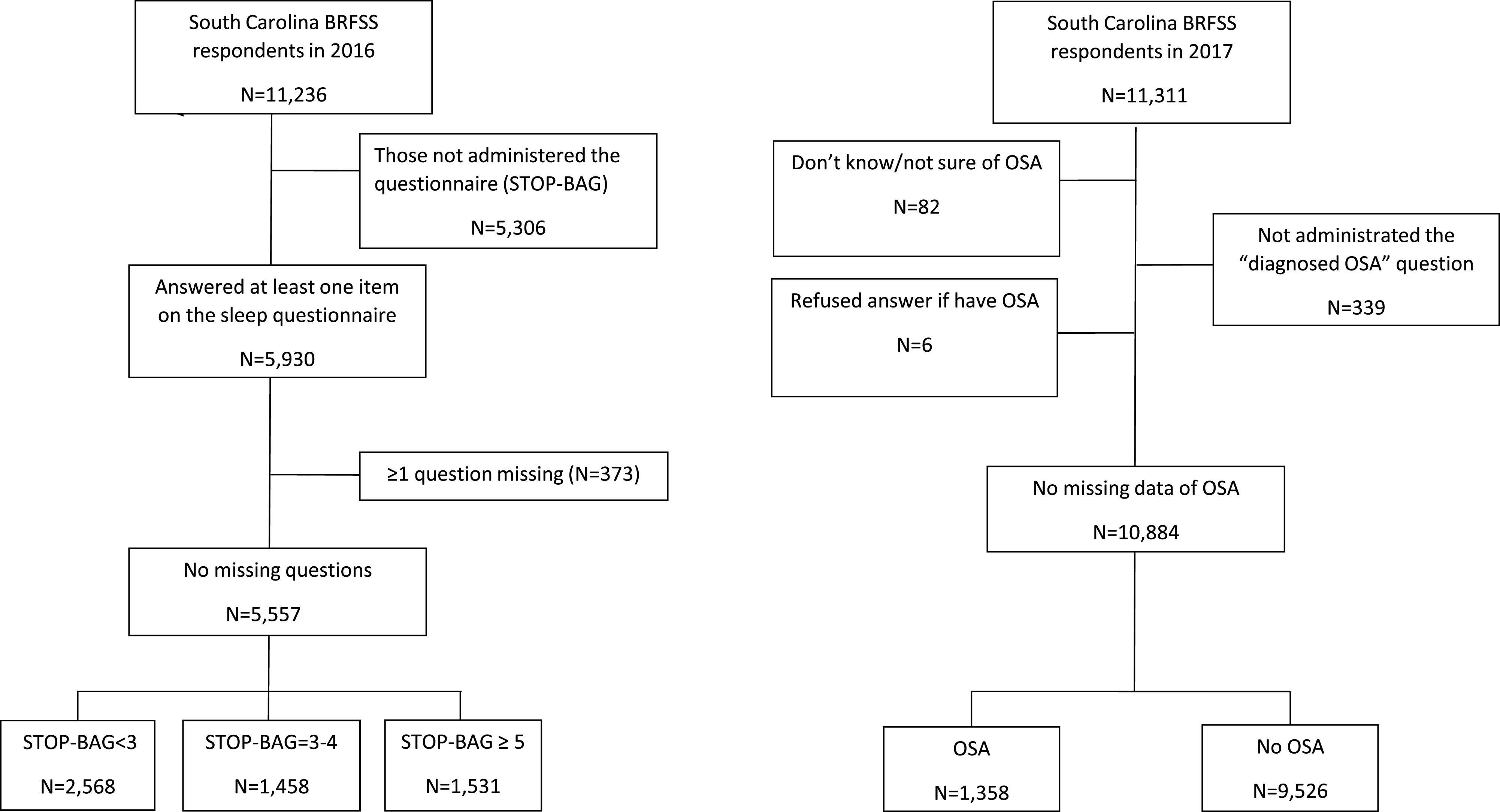
BRFSS = Behavioral Risk Factor Surveillance Survey, OSA = obstructive sleep apnea.
Participants in the 2017 SC BRFSS (n = 11,311) were excluded if they responded, “don’t know/not sure” or “refused” or dropped out of the survey before being asked if they were diagnosed with OSA by a physician (final n = 10,884, Figure 1). Details of the BRFSS and its characteristics are described elsewhere.18
We divided the 2016 cohort into those at low risk for OSA (STOP-BAG < 3), those at moderate risk (STOP-BAG 3–4), and those at high risk for OSA (STOP-BAG ≥ 5) for comparison.
The comorbidity rates of individuals at high risk for OSA (2016) and with diagnosed OSA (2017) were compared to the rates of individuals at low or moderate risk of OSA (2016) and without diagnosed OSA (2017). Comorbidity data included self-report of a physician diagnosis of coronary heart disease, stroke, asthma, skin cancer, any other cancer, COPD, arthritis, depressive disorder, kidney disease, and diabetes.
We compared the impact of at risk OSA and diagnosed OSA on general health (excellent, very good, good, fair, poor), mental health, and physical health.
Statistics
Survey analysis procedures were utilized in SAS 9.4 (SAS Inc., Cary, NC) to account for the complex weighting and sampling of BRFSS. To compare the demographic and health behavior distributions between the levels of STOP-BAG scores, weighted prevalence and mean estimates, along with their 95% confidence intervals (CIs) and chi-square tests for categorical variables, were obtained. The iterative proportional fitting or raking of the data collected allows for multivariate adjustment of outcomes by age, sex, categories of ethnicity, geographic regions within the state, marital status, education level, home ownership, and type of phone ownership. The comparison of STOP-BAG scores and self-reported OSA diagnosis to symptoms and health outcomes used the same variables in modeling. Raw data are also presented in the supplemental material without population weighting to allow an understanding of the general population sampled. ANOVA or chi-square tests with Bonferroni corrections for repeated measures was used in these analyses where referenced. P values < .05 were considered significant.
RESULTS
The 2016 SC BRFSS STOP-BAG estimated a low risk of OSA (STOP-BAG < 3) of 56.0% (95% CI: 54.2–57.9), moderate risk of OSA (STOP-BAG 3–4) of 18.5% (17.3–19.8) and high risk (STOP-BAG ≥ 5) of 25.5% (95% CI: 23.9–27.1) from the population of South Carolina (Table 2). Compared to those at low risk for OSA, those at high risk included a significantly higher proportion of men with hypertension and a higher BMI. However, this is not surprising since these characteristics are components of the STOP-BAG questionnaire. Similarly, those at high risk for OSA had a higher proportion of loud snorers, witnessed apneas, and reported more trouble falling asleep and unintentionally falling asleep during the day in the past 2 weeks compared to those at low risk (Table 3). All of these characteristics suggest that the STOP-BAG is measuring important variables related to OSA, although further validation of this questionnaire will require more rigorous studies.
Table 2.
Population demographics associated with individuals who have low, moderate, or high risk for OSA.
| Variable | Overall Sample | Low Risk For OSA | Moderate Risk For OSA | High Risk For OSA | P Value (chi-square) |
|---|---|---|---|---|---|
| Prevalence (%) | 100.0 | 56.0 (54.2–57.9) | 18.5 (17.3–19.8) | 25.5 (23.9–27.1) | |
| Age (mean years) | 48.3 (47.6–48.9) | 40.8 (40.0–41.7) | 61.8 (60.9–62.8) | 54.7 (53.7–55.8) | < .0001* |
| Sex (% male) | 47.8 (45.8–49.7) | 35.8 (33.0–38.7) | 40.6 (37.1–44.2) | 79.2 (76.6–81.8) | < .0001 |
| Race (%) | < .0001 | ||||
| White | 69.3 (67.5–71.1) | 69.6 (66.9–72.3) | 70.1 (66.8–73.3) | 68.1 (64.9–71.2) | |
| Black | 25.8 (24.1–27.4) | 23.9 (21.5–26.4) | 27.2 (24.0–30.4) | 28.7 (25.6–31.8) | |
| Other | 3.7 (2.7–4.7) | 5.2 (3.5–6.8) | 1.7 (0.9–2.5) | 2.0 (1.2–2.8) | |
| Multiracial | 1.2 (0.9–1.5) | 1.3 (0.8–1.8) | 1.0 (0.5–1.6) | 1.2 (0.8–1.7) | < .0001 |
| Hispanic (Yes %) | 4.5 (3.3–5.6) | 6.8 (4.9–8.8) | 1.2 (0.5–2.0) | 1.5 (0.4–2.6) | < .0001 |
| Income (%) | .0040 | ||||
| < $15,000 | 11.4 (10.1–12.8) | 9.3 (7.4–11.2) | 13.4 (10.9–16.0) | 14.3 (11.6–17.0) | |
| $15,000–25,000 | 19.2 (17.5–20.9) | 18.6 (16.1–21.0) | 20.4 (17.4–23.5) | 19.6 (16.5–22.6) | |
| $25,000–35,000 | 11.0 (9.7–12.2) | 10.4 (8.7–12.1) | 11.8 (9.0–14.7) | 11.5 (9.1–14.0) | |
| $35,000–50,000 | 15.6 (14.1–17.2) | 16.2 (13.8–18.5) | 17.0 (14.0–20.1) | 13.6 (11.1–16.1) | |
| ≥ $50,000 | 42.8 (40.8–44.8) | 45.6 (42.6–48.6) | 37.3 (33.5–41.0) | 41.0 (37.5–44.5) | |
| Marital Status (%) | < .0001 | ||||
| Married | 51.6 (49.7–53.5) | 47.2 (44.4–50.0) | 54.5 (51.0–58.1) | 59.0 (55.6–62.5) | |
| Divorced | 11.3 (10.1–12.5) | 9.5 (7.9–11.0) | 15.3 (12.5–18.1) | 12.4 (10.1–14.8) | |
| Widowed | 7.4 (6.6–8.1) | 5.4 (4.5–6.2) | 14.8 (12.6–17.0) | 6.3 (4.8–7.8) | |
| Separated | 3.4 (2.7–4.1) | 3.4 (2.3–4.5) | 3.0 (1.8–4.2) | 3.5 (2.4–4.7) | |
| Never married | 22.8 (21.0–24.6) | 29.9 (27.1–32.6) | 10.6 (8.2–13.1) | 16.2 (13.3–19.0) | |
| A member of an unmarried couple | 3.5 (2.6–4.5) | 4.6 (3.1–6.1) | 1.7 (0.3–3.2) | 2.5 (1.4–3.7) | |
| Education (%) | .0022 | ||||
| Did not graduate HS | 15.0 (13.4–16.6) | 13.8 (11.3–16.2) | 16.3 (13.4–19.1) | 16.8 (13.9–19.7) | |
| Graduated HS | 29.8 (28.0–31.5) | 27.8 (25.3–30.3) | 31.9 (28.5–35.2) | 32.5 (29.3–35.7) | |
| Attended college or technical school | 32.5 (30.7–34.4) | 33.1 (30.5–35.8) | 31.8 (28.5–35.2) | 31.7 (28.4–34.9) | |
| Graduated from college or technical school | 22.7 (21.3–24.1) | 25.3 (23.1–27.4) | 20.0 (17.7–22.4) | 19.0 (16.7–21.3) | |
| Rural (%) | 16.4 (15.3–17.6) | 15.1 (13.3–16.9) | 19.3 (16.9–21.7) | 17.2 (15.1–19.4) | .0218 |
| Smoker (%) | < .0001 | ||||
| Every day smoker | 12.9 (11.5–14.3) | 11.9 (10.1–13.8) | 13.0 (10.4–15.7) | 14.9 (12.2–17.7) | |
| Some days smoker | 7.3 (6.1–8.4) | 7.4 (5.7–9.2) | 7.8 (5.7–10.0) | 6.4 (4.6–8.2) | |
| Former smoker | 27.0 (25.4–28.6) | 19.9 (17.8–22.0) | 32.3 (29.1–35.6) | 38.8 (35.5–42.1) | |
| Never smoker | 52.8 (50.9–54.7) | 60.7 (57.9–63.5) | 46.8 (43.3–50.3) | 39.9 (36.5–43.2) | |
| BMI (mean kg/m2) | 28.3 (28.0–28.5) | 26.2 (25.9–26.5) | 29.0 (28.5–29.5) | 32.1 (31.6–32.7) | < .0001* |
| Heavy alcohol consumption (Yes %) | 7.3 (6.2–8.3) | 6.8 (5.4–8.2) | 7.6 (5.3–10.0) | 8.1 (5.9–10.3) | .5498 |
| Difficulty concentrating or remembering (Yes %) | 13.7 (12.3–15.0) | 11.7 (9.8–13.6) | 15.1 (12.5–17.7) | 16.8 (14.1–19.6) | .0020 |
| Difficulty walking or climbing stairs (Yes %) | 16.8 (15.5–18.1) | 8.0 (6.7–9.3) | 26.0 (23.0–29.1) | 29.4 (26.3–32.5) | < .0001 |
| Shingles vaccine (Yes %) | 21.3 (19.7–22.9) | 21.2 (18.6–23.9) | 21.7 (18.9–24.4) | 20.9 (18.0–23.8) | .9314 |
| Adult flu shot/spray past 12 months (Yes %) | 36.6 (34.8–38.3) | 31.7 (29.2–34.2) | 46.1 (42.7–49.6) | 40.3 (37.0–43.6) | < .0001 |
| Pneumonia shot ever (Yes %) | 35.8 (34.0–37.7) | 27.8 (25.2–30.3) | 46.7 (43.1–50.2) | 44.4 (40.9–47.9) | < .0001 |
| Days had trouble with sleep (mean days) | 4.2 (4.0–4.4) | 3.8 (3.5–4.1) | 4.3 (3.9–4.7) | 4.9 (4.5–5.3) | < .0001 |
| Sleep days during the day (mean days) | 1.3 (1.1–1.4) | 0.5 (0.4–0.6) | 1.6 (1.4–1.9) | 2.7 (2.4–3.0) | < .0001 |
| Snore loudly (Yes %) | 49.7 (47.8–51.6) | 27.6 (25.1–30.2) | 64.7 (61.4–68.1) | 87.4 (85.2–89.6) | < .0001 |
| Observed stop breathing (Yes %) | 15.8 (14.5–17.1) | 2.1 (1.4–2.9) | 16.3 (13.6–19.0) | 45.6 (42.1–49.0) | < .0001 |
Data are expressed as prevalence or mean with 95% confidence intervals. Low, moderate, and high risk for OSA determined with STOP-BAG questionnaire scores of 1–2, 3–4, and ≥ 5, respectively (SC BRFSS 2016). Bold numbers are significantly different from the high risk group. *Analysis by ANOVA for continuous variables. BMI = body mass index, HS = high school, OSA = obstructive sleep apnea.
Table 3.
Weighted bivariate comparisons of those with OSA and without OSA from the 2017 SC BRFSS comparing demographics and health behaviors of the cohort.
| Variable | Overall Population (n=10,884) | With OSA (n=1,358) | Without OSA (n=9,526) | P Value (chi-square) |
|---|---|---|---|---|
| Prevalence (%) | 100.0 (–) | 9.7 (9.0– 10.4) | 90.3 (89.6– 91.0) | |
| Age (mean years) | 48.6 (48.1–49.1) | 57.9 (56.8– 59.1) | 47.6 (47.1–48.2) | < .0001* |
| Sex (% male) | 47.7 (46.4–49.1) | 56.6 (53.1– 60.1) | 46.8 (45.3–48.2) | < .0001 |
| Race (%) | .0716 | |||
| White | 68.1 (66.8–69.3) | 72.0 (68.9–75.2) | 67.6 (66.2–69.0) | |
| Black | 27.3 (26.1–28.6) | 24.6 (21.5– 27.6) | 27.6 (26.3–29.0) | |
| Other | 3.1 (2.6–3.6) | 2.2 (0.9– 3.4) | 3.2 (2.7–3.8) | |
| Multiracial | 1.5 (1.2–1.8) | 1.2 (0.7–1.7) | 1.5 (1.2–1.9) | |
| Hispanic (Yes %) | 4.8 (4.1–5.5) | 1.8 (0.7–2.9) | 5.1 (4.3–5.9) | .0005 |
| Income (%) | .0456 | |||
| < $15,000 | 11.7 (10.8–12.7) | 11.8 (9.3–14.2) | 11.7 (10.7–12.8) | |
| $15,000–25,000 | 19.8 (18.7–21.0) | 24.3 (20.7–27.9) | 19.3 (18.1–20.6) | |
| $25,000–35,000 | 10.5 (9.6–11.5) | 9.9 (7.6–12.1) | 10.6 (9.6–11.6) | |
| $35,000–50,000 | 15.0 (13.9–16.0) | 12.8 (10.4–15.2) | 15.2 (14.0–16.4) | |
| ≥ $50,000 | 42.9 (41.5–44.4) | 41.2 (37.4–45.1) | 43.1 (41.5–44.7) | |
| Marital Status (%) | < .0001 | |||
| Married | 49.9 (48.5–51.2) | 56.6 (53.0–60.2) | 49.2 (47.7–50.6) | |
| Divorced | 10.6 (9.8–11.4) | 14.9 (12.4–17.5) | 10.1 (9.3–10.9) | |
| Widowed | 8.2 (7.7–8.80 | 11.6 (9.5–13.7) | 7.9 (7.3–8.5) | |
| Separated | 3.7 (3.1–4.2) | 5.0 (3.5–6.6) | 3.5 (3.0–4.1) | |
| Never married | 24.0 (22.7–25.4) | 9.6 (7.1–12.1) | 25.5 (24.1–27.0) | |
| A member of an unmarried couple | 3.6 (3.0–4.2) | 2.2 (0.7–3.7) | 3.7 (3.1–4.4) | |
| Education (%) | .8106 | |||
| Did not graduate HS | 14.5 (13.4–15.5 | 14.8 (11.9– 17.6) | 14.4 (13.3–15.6) | |
| Graduated HS | 30.3 (29.0–31.5) | 29.9 (26.5–33.3) | 30.3 (29.0–31.6) | |
| Attended college or technical school | 31.7 (30.4–33.0) | 30.5 (27.1–33.8) | 31.8 (30.4–33.2) | |
| Graduated from college or technical school | 23.6 (22.6–24.6) | 24.9 (22.0–27.7) | 23.4 (22.4–24.5) | |
| Rural (%) | 16.0 (15.2–16.8) | 16.8 (14.5–19.2) | 15.9 (15.0–16.8) | 0.4623 |
| Smoker (%) | < .0001 | |||
| Every day smoker | 12.8 (11.9–13.8) | 11.2 (8.5–14.0) | 13.0 (12.0–14.1) | |
| Some days smoker | 6.3 (5.6–7.0) | 5.3 (3.4–7.2) | 6.4 (5.6–7.1) | |
| Former smoker | 26.7 (25.5–27.9) | 38.6 (35.1–42.1) | 25.4 (24.2–26.6) | |
| Never smoker | 54.2 (52.8–55.5) | 44.9 (41.3–48.4) | 55.2 (53.7–56.7) | |
| BMI (mean kg/m2) | 28.7 (28.5–28.8) | 33.3 (32.8–33.9) | 28.1 (27.9–28.3) | < .0001* |
| Heavy alcohol consumption (Yes %) | 6.6 (5.8–7.3) | 3.6 (2.2–5.0) | 6.9 (6.1–7.7) | .0012 |
| Difficulty concentrating or remembering (Yes %) | 12.6 (11.7–13.5) | 24.7 (21.4–27.9) | 11.3 (10.3–12.2) | < .0001 |
| Difficulty walking or climbing stairs (Yes %) | 16.9 (16.0–17.8) | 41.3 (37.8–44.9) | 14.2 (13.3–15.1) | < .0001 |
| Shingles vaccine (Yes %) | 25.4 (24.2–26.7) | 29.2 (25.9–32.5) | 24.8 (23.4–26.1) | .0127 |
| Adult flu shot/spray past 12 months (Yes %) | 40.3 (39.0–41.6) | 55.2 (51.5–59.0) | 38.7 (37.2–40.1) | < .0001 |
| Pneumonia shot ever (Yes %) | 39.0 (37.6–40.3) | 56.4 (52.5–60.2) | 36.9 (35.5–38.4) | < .0001 |
Data are expressed as prevalence or mean with 95% confidence intervals comparing those with and without OSA. Bold numbers are significantly different from the not diagnosed group. *Analysis by ANOVA for continuous variables. BMI = body mass index, HS = high school, OSA = obstructive sleep apnea.
Independent of STOP-BAG questions, those at moderate and high risk for OSA more commonly had an annual household income < $15,000, were more commonly married, and were smokers compared to the those in the low-risk group.
Multiple important health outcomes were associated with the STOP-BAG categorical score. Compared to those at low risk for OSA, those at high risk were more often diagnosed with coronary heart disease, stroke, asthma, skin cancer, other cancers, COPD, arthritis, depression, kidney disease, and diabetes (Table S2 (532.5KB, pdf) ). Furthermore, compared to those at low risk for OSA, those at high risk had worse general health, physical health, and mental health and a higher mean number of days in which their physical and mental health was “not good” in the past month. The high-risk group also reported more difficulty concentrating or remembering and more difficulty walking or climbing stairs. Although statistically different, the number of reported sleep hours was not meaningfully different between groups.
The 2017 SC BRFSS included the question of whether OSA had been previously diagnosed by a doctor. From this question, the population-based prevalence of diagnosed OSA was 9.7% (95% CI: 9.0–10.4) (Table 3). In this population were a higher proportion of men and former smokers; BMI and age also were higher. Diagnosed individuals were more commonly diagnosed with hypertension, coronary heart disease, stroke, asthma, skin cancer, other cancers, COPD, arthritis, depression, kidney disease, and diabetes (Table 4). In this cohort of those diagnosed with OSA, there was reported more difficulty concentrating or remembering, more difficulty walking or climbing stairs, more individuals receiving a flu shot in the past year, and more individuals receiving a pneumonia shot ever. The OSA cohort reported more fair-poor general health and more days in the past month in which their physical or mental health was “not good.” The number of days in which their physical or mental health kept them from their normal activities were higher compared to those without OSA. Among those with physician-diagnosed OSA in South Carolina, there was a significantly lower proportion of Hispanics; those identifying as never married, never smokers, heavy alcohol consumers; and those reporting excellent–very good general health compared to those without OSA.
Table 4.
Weighted bivariate comparisons of those with OSA and not diagnosed with OSA showing health behaviors/outcomes from the 2017 SC BRFSS.
| Variable | Overall Population (n=10,884) | With OSA (n=1,358) | Without OSA (n=9,526) | P Value (chi-square) |
|---|---|---|---|---|
| General health (%) | < .0001 | |||
| Excellent | 18.7 (17.6–19.9) | 5.7* (3.8–7.6) | 20.1 (18.9–21.4) | |
| Very good | 30.8 (29.6–32.1) | 21.6* (18.6–24.5) | 31.8 (30.5–33.2) | |
| Good | 30.9 (29.7–32.1) | 33.9 (30.5–37.3) | 30.6 (29.3–31.9) | |
| Fair | 13.4 (12.6–14.3) | 22.1* (19.1–25.1) | 12.5 (11.6–13.4) | |
| Poor | 6.1 (5.5–6.7) | 16.8* (14.1–19.4) | 5.0 (4.3–5.6) | |
| Hypertension (Yes %) | 38.8 (37.5–40.0) | 68.4* (64.9–71.9) | 35.6 (34.3–36.9) | < .0001 |
| Ever diagnosed with CHD (Yes %) | 5.0 (4.5–5.5) | 16.6* (13.8–19.3) | 3.8 (3.3–4.2) | < .0001 |
| Ever diagnosed with stroke (Yes %) | 4.0 (3.5–4.4) | 10.2* (8.0–12.4) | 3.3 (2.9–3.7) | < .0001 |
| Ever diagnosed with asthma (Yes %) | 14.0 (13.1–15.0) | 24.2* (21.1–27.3) | 13.0 (11.9–14.0) | < .0001 |
| Currently have asthma (Yes %) | 9.2 (8.4–10.0) | 19.5* (16.6–22.3) | 8.1 (7.2–8.9) | < .0001 |
| Ever diagnosed with skin cancer (Yes %) | 7.4 (6.9–8.0) | 12.1* (10.0–14.2) | 7.0 (6.4–7.5) | < .0001 |
| Ever diagnosed with other cancer (Yes %) | 7.6 (7.0–8.2) | 12.3* (10.3–14.4) | 7.1 (6.5–7.7) | < .0001 |
| Ever diagnosed with COPD (Yes %) | 7.9 (7.3–8.6) | 21.5* (18.6–24.4) | 6.5 (5.9–7.2) | < .0001 |
| Ever diagnosed with arthritis (Yes %) | 28.8 (27.7–29.9) | 55.9* (52.2–59.5) | 25.9 (24.8–27.0) | < .0001 |
| Ever diagnosed with depressive disorder (Yes %) | 20.4 (19.3–21.4) | 34.7* (31.3–38.1) | 18.8 (17.7–20.0) | < .0001 |
| Ever diagnosed with kidney disease (Yes %) | 3.3 (2.8–3.7) | 9.2* (7.0–11.4) | 2.6 (2.2–3.0) | < .0001 |
| Ever diagnosed with diabetes (Yes %) | 13.8 (13.0–14.6) | 36.6* (33.2–40.0) | 11.3 (10.6–12.1) | < .0001 |
| Physical health not good (mean days) | 4.2 (4.0–4.4) | 9.0* (8.1–9.9) | 3.7 (3.5–3.9) | < .0001* |
| Mental health not good (mean days) | 4.4 (4.1–4.6) | 6.4* (5.6–7.2) | 4.2 (3.9–4.4) | < .0001* |
| Poor physical or mental health (mean days) | 5.4 (5.0–5.7) | 9.7* (8.6–10.7) | 4.8 (4.5–5.2) | < .0001* |
Data are expressed as prevalence or mean with 95% confidence intervals. Bold numbers are significantly different from the not diagnosed group. *Analysis by ANOVA for continuous variables. CHD = coronary heart disease, COPD = chronic obstructive pulmonary disease, OSA = obstructive sleep apnea.
DISCUSSION
The prevalence of diagnosed and undiagnosed OSA in the United States has been the subject of controversy for many years. Using a question of self-report of provider and sleep study-diagnosed OSA inserted into the BRFSS, this study was able to estimate the prevalence of OSA diagnosed by a health care practitioner as 9.7% (95% CI: 9.0–10.4) of the state population. The typical respondent in South Carolina with diagnosed OSA is male, has an annual income > $50,000, and a diagnosis of hypertension. Using a weighting format to overrepresent populations who are difficult to reach by telephone survey, this state-based survey conducted by the Centers for Disease Control and Prevention18 collects self-reported information about chronic conditions and health risk behaviors throughout the year and can provide confidence intervals to inform the true state prevalence.19 This is the first report that describes the use of the BRFSS, the largest ongoing, annual health survey in the United States, to determine the prevalence of OSA and could serve a model to better target this disease using a population-based approach.
However, the prevalence of the population who remains undiagnosed is much harder to define. By using the STOP-BAG instrument, the study found a prevalence of moderate risk as defined by STOP-BAG scores of 3–4 was 18.5%, (95% CI: 17.3–19.8) and high risk as defined by STOP-BAG scores ≥ 5 was 25.5%, (95% CI: 23.9–27.1) of the population. If it is true that 44% of the population has OSA, then this would be a public health emergency of epidemic proportions, since approximately 10% are currently diagnosed with the condition. Much more likely is the possibility that a higher point score on STOP-BAG portends higher risk and more comorbidities. This would suggest that the high-risk STOP-BAG scores ≥ 5 are populations who may derive the most benefit from therapy. However, this estimate of 23.9–27.1% of the entire state population is a public health crisis.
We do not believe that South Carolina is much different from other states in the rise of obesity and thus prevalence of OSA. An analysis of National Health and Nutrition Examination Survey (NHANES) data revealed that the age-adjusted US prevalence of obesity in 2013–2014 was 35.0% in men and 40.4% in women.20 The prevalence of obesity showed significant linear trends for increase between 2005 and 2014 for women; however, there were no significant trends for men. Obesity is linked to several adverse health outcomes, such as cardiovascular disease, diabetes, and also OSA; thus, an increasingly high prevalence of obesity indicates an unhealthy population and a burgeoning national health crisis.21 Moreover the prevalence of obesity has racial differences, with Hispanic and Black populations being disproportionately affected compared to non-Hispanic Whites and Asian adults.22 The baseline examination of the Hispanic Community Health Study/Study of Latinos, a population-based cohort of US Hispanics/Latinos conducted between 2008 and 2011,23 estimated that 37% of men and 43% of women were obese, with a BMI ≥ 30 kg/m2. Since the prevalence of OSA is linear with the prevalence of obesity, one challenge in this study was to define correlates of OSA that were independent of BMI.
Since most patients with OSA remain undiagnosed, it is currently difficult to define how many of the associated comorbidities such as coronary heart disease, stroke, depression, and diabetes are OSA specific and how many are due to obesity and the associated metabolic syndrome. However, these associations in our study are similar to the OSA literature, in which those who screen as high risk for OSA24–26 and those who are diagnosed with OSA27,28 have incredibly high prevalence of these comorbidities. The implication that early and aggressive screening and treatment of OSA may result in overall improvement in general health and well-being of the population is a strategy that health policy makers and clinicians should embrace. Furthermore, if screening is done with newer technology that does not rely on laboratory polysomnography, this would very likely be accompanied by a decreased expenditure on health care. Indeed, aggressive screening and treatment for OSA has been demonstrated to result in health care cost savings in several populations.29,30
We performed this study by adding additional questions to the BRFSS as an optional module, so that the components of the STOP-BAG questionnaire could be captured. Although the 8-item STOP-BANG questionnaire has been demonstrated to have good sensitivity and modest specificity in detecting OSA in many populations,15 the abbreviated STOP-BAG questionnaire without neck circumference could emerge as an important public health tool, since the component questions need not require the presence of a health care practitioner to collect.
We were also able to define aspects of probable health care disparities in our study. In this study, a greater proportion of Hispanics were categorized as being at low risk for OSA and had a lower OSA diagnosis rate compared to Whites. Although published data on US Hispanics is somewhat limited, there is evidence to suggest that the OSA prevalence in Hispanics is quite high. A prevalence survey of sleep-disordered breathing in San Diego adults, which monitored blood oxygen desaturations ≥ 4% with home recording instruments, usually for 3 consecutive nights, found that 16.3% of US Hispanics and racial minorities have ≥ 20 oxygen desaturation events/h of sleep compared to 4.9% of non-Hispanic Whites ages 40–64 years.31 Obesity was the most important demographic predictor of sleep-disordered breathing in this study, followed by age, male sex, and ethnicity. In a cross-sectional analysis of 14,440 individuals from the baseline examination of the Hispanic Community Health Study/Study of Latinos,32 the age-adjusted prevalence of minimal sleep-disordered breathing as assessed with an apnea-hypopnea index (AHI) ≥ 5 events/h, moderate sleep-disordered breathing (AHI ≥ 15), and severe sleep-disordered breathing (AHI ≥ 30) was found to be 25.8%, 9.8%, and 3.9%, respectively. However, only 1.3% of participants reported a sleep apnea diagnosis, which would imply that OSA, although common in Hispanics, was rarely given a clinical diagnosis remembered by the Hispanic BRFSS respondents.
We were perplexed by the increased use of influenza, pneumococcal, and shingles vaccination in the at-risk and diagnosed OSA populations. The best explanation is that these individuals, by way of the comorbidities, have much more contact with the health care community and with more clinic visits have more opportunities to receive these important health interventions. One of the possibilities is that the option of diagnosing OSA was equally discussed and dismissed. Dismissing the opportunity for a sleep study or empiric trial of auto-continuous positive airway pressure can come from many barriers within the medical community, including the lack of effectiveness of therapy in the eyes of many primary care physicians.
There are limitations to this study. There is no doubt that the STOP-BAG needs further validation. As a highly sensitive screening tool, the STOP-BANG parent instrument has modest specificity when measured against the AHI. Furthermore, the STOP-BANG is good at detecting the absence of severe OSA with very low scores.33 However, there are also limitations to using AHI as the gold standard for OSA, since treatment efficacy and comorbidities are more closely aligned with symptoms and the duration of hypoxemia, which may be better captured on questionnaire instruments than by AHI alone.
The 2016 SC BRFSS data included the components of the STOP-BAG questionnaire that were not included in the 2017 data. The 2017 data included a question on diagnosed OSA, which was not included in the 2016 data. Because the BRFSS is designed with weighting to be demographically representative of the population in the entire state, we believe that the populations are similar, but not identical. Other limitations include those that are inherent to all survey-based studies, including self-reporting bias and recall bias. Sleep studies were not available to confirm/refute the diagnosis of OSA in respondents; however, to improve the reliability of self-report of provider-diagnosed OSA, we chose to include history of testing in the prevalence question. The reliability of self-reported diagnoses of chronic conditions between BRFSS and other surveys has been found to be high.19
CONCLUSIONS
Among the adult population of South Carolina, the prevalence of diagnosed OSA is approximately 10% of the state. However, the population at high risk using the STOP-BAG instrument is approximately 25%. This new instrument should be further studied to define the instrument validation metrics, since application to the BRFSS and other large datasets can highlight important health policy applications to better understand OSA prevalence and optimal treatment and improve public health.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Dr. Strange reports grants from Arrowhead, CSL Behring, Grifols, MatRx, Nuvaira, Pulmonx, Shire, and Vertex that are not related to sleep medicine. He has consultancies with AstraZeneca, CSL Behring, Dicerna, GlaxoSmithKline, Uptake Medical, and Vertex that are not related to sleep medicine. Dr. Drummond reports research grants from Boehringer-Ingelheim and consultancies with AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Midmark, and Philips that are not related to sleep medicine. Dr. Lal reports research grants with Jazz Pharmaceuticals and consultancies with Jazz Pharmaceuticals and Cipla Pharmaceuticals that are related to sleep medicine. Prior publication: Abstract submitted at CHEST 2019, New Orleans, LA available at https://doi.org/10.1016/j.chest.2019.08.880
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
The authors thank Frances Chung, MD, for guidance in use of the STOP-BAG questionnaire.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ANOVA
analysis of variance
- BMI
body mass index
- BRFSS
Behavioral Risk Factor Surveillance Survey
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- OSA
obstructive sleep apnea
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med Rev. 2017;34:70–81. 10.1016/j.smrv.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 3.Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. 10.1016/S2213-2600(19)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fava C, Montagnana M, Favaloro EJ, Guidi GC, Lippi G. Obstructive sleep apnea syndrome and cardiovascular diseases. Semin Thromb Hemost. 2011;37(3):280–297. 10.1055/s-0031-1273092 [DOI] [PubMed] [Google Scholar]
- 5.Lal C, Strange C, Bachman D. Neurocognitive impairment in obstructive sleep apnea. Chest. 2012;141(6):1601–1610. 10.1378/chest.11-2214 [DOI] [PubMed] [Google Scholar]
- 6.Tregear S, Reston J, Schoelles K, Phillips B. Continuous positive airway pressure reduces risk of motor vehicle crash among drivers with obstructive sleep apnea: systematic review and meta-analysis. Sleep. 2010;33(10):1373–1380. 10.1093/sleep/33.10.1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20(9):705–706. 10.1093/sleep/20.9.705 [DOI] [PubMed] [Google Scholar]
- 8.Ho V, Crainiceanu CM, Punjabi NM, Redline S, Gottlieb DJ. Calibration Model for Apnea-Hypopnea Indices: Impact of Alternative Criteria for Hypopneas. Sleep. 2015;38(12):1887–1892. 10.5665/sleep.5234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812–821. 10.1097/ALN.0b013e31816d83e4 [DOI] [PubMed] [Google Scholar]
- 10.Nagappa M, Liao P, Wong J, et al. Validation of the STOP-Bang Questionnaire as a Screening Tool for Obstructive Sleep Apnea among Different Populations: A Systematic Review and Meta-Analysis. PLoS One. 2015;10(12):e0143697. 10.1371/journal.pone.0143697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farney RJ, Walker BS, Farney RM, Snow GL, Walker JM. The STOP-Bang equivalent model and prediction of severity of obstructive sleep apnea: relation to polysomnographic measurements of the apnea/hypopnea index. J Clin Sleep Med. 2011;7(5):459–465. 10.5664/JCSM.1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo J, Huang R, Zhong X, Xiao Y, Zhou J. STOP-Bang questionnaire is superior to Epworth sleepiness scales, Berlin questionnaire, and STOP questionnaire in screening obstructive sleep apnea hypopnea syndrome patients. Chin Med J (Engl). 2014;127(17):3065–3070. [PubMed] [Google Scholar]
- 13.Guralnick AS, Pant M, Minhaj M, Sweitzer BJ, Mokhlesi B. CPAP adherence in patients with newly diagnosed obstructive sleep apnea prior to elective surgery. J Clin Sleep Med. 2012;8(5):501–506. 10.5664/jcsm.2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung F, Subramanyam R, Liao P, Sasaki E, Shapiro C, Sun Y. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br J Anaesth. 2012;108(5):768–775. 10.1093/bja/aes022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu HY, Chen PY, Chuang LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: A bivariate meta-analysis. Sleep Med Rev. 2017;36:57–70. 10.1016/j.smrv.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 16.Boulos MI, Colelli DR, Vaccarino SR, Kamra M, Murray BJ, Swartz RH. Using a modified version of the “STOP-BANG” questionnaire and nocturnal oxygen desaturation to predict obstructive sleep apnea after stroke or TIA. Sleep Med. 2019;56:177–183. 10.1016/j.sleep.2018.12.021 [DOI] [PubMed] [Google Scholar]
- 17.Boulos MI, Wan A, Im J, et al. Identifying obstructive sleep apnea after stroke/TIA: evaluating four simple screening tools. Sleep Med. 2016;21:133–139. 10.1016/j.sleep.2015.12.013 [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention . Behavioral Risk Factor Surveillance System. https://www.cdc.gov/brfss/index.html. 2016-17; Accessed November 2019.
- 19.Pierannunzi C, Hu SS, Balluz L. A systematic review of publications assessing reliability and validity of the Behavioral Risk Factor Surveillance System (BRFSS), 2004-2011. BMC Med Res Methodol. 2013;13(1):49. 10.1186/1471-2288-13-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. 10.1001/jama.2016.6458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell NS, Catenacci VA, Wyatt HR, Hill JO. Obesity: overview of an epidemic. Psychiatr Clin North Am. 2011;34(4):717–732. 10.1016/j.psc.2011.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011-2014. NCHS Data Brief. 2015:Nov(219):1–8. [PubMed] [Google Scholar]
- 23.Daviglus ML, Talavera GA, Avilés-Santa ML, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308(17):1775–1784. 10.1001/jama.2012.14517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sunwoo JS, Hwangbo Y, Kim WJ, Chu MK, Yun CH, Yang KI. Prevalence, sleep characteristics, and comorbidities in a population at high risk for obstructive sleep apnea: A nationwide questionnaire study in South Korea. PLoS One. 2018;13(2):e0193549. 10.1371/journal.pone.0193549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan MS, Bawany FI, Khan A, Hussain M, Ali SS, Shah SR, Lashari MN. Risk assessment for obstructive sleep apnea and anxiety in a Pakistani population with coronary artery disease. Sleep Breath. 2015;19(1):291–296. 10.1007/s11325-014-1018-5 [DOI] [PubMed] [Google Scholar]
- 26.Martinez D, da Silva RP, Klein C, et al. High risk for sleep apnea in the Berlin questionnaire and coronary artery disease. Sleep Breath. 2012;16(1):89–94. 10.1007/s11325-010-0460-2 [DOI] [PubMed] [Google Scholar]
- 27.Fox H, Purucker HC, Holzhacker I, et al. Prevalence of sleep-disordered breathing and patient characteristics in a coronary artery disease cohort undergoing cardiovascular rehabilitation. J Cardiopulm Rehabil Prev. 2016;36(6):421–429. 10.1097/HCR.0000000000000192 [DOI] [PubMed] [Google Scholar]
- 28.Hou H, Zhao Y, Yu W, et al. Association of obstructive sleep apnea with hypertension: A systematic review and meta-analysis. J Glob Health. 2018;8(1):010405. 10.7189/jogh.08.010405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burks SV, Anderson JE, Panda B, et al. Employer-mandated obstructive sleep apnea treatment and healthcare cost savings among truckers. Sleep. 2019;43(4):zsz262. 10.1093/sleep/zsz262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wickwire EM, Albrecht JS, Towe MM, et al. The impact of treatments for OSA on monetized health economic outcomes: a systematic review. Chest. 2019;155(5):947–961. 10.1016/j.chest.2019.01.009 [DOI] [PubMed] [Google Scholar]
- 31.Kripke DF, Ancoli-Israel S, Klauber MR, Wingard DL, Mason WJ, Mullaney DJ. Prevalence of sleep-disordered breathing in ages 40-64 years: a population-based survey. Sleep. 1997;20(1):65–76. 10.1093/sleep/20.1.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redline S, Sotres-Alvarez D, Loredo J, et al.The Hispanic Community Health Study/Study of Latinos . Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. Am J Respir Crit Care Med. 2014;189(3):335–344. 10.1164/rccm.201309-1735OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuczyński W, Mokros Ł, Stolarz A, Białasiewicz P. The utility of STOP-BANG questionnaire in the sleep-lab setting. Sci Rep. 2019;9(1):6676. 10.1038/s41598-019-43199-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


