Abstract
Background and Aims
Evidence is now available in support of using fecal biomarkers to monitor disease activity in inflammatory bowel disease (IBD). Patient adherence is often cited as a barrier to implementation. We assessed patient determinants for using stool tests to monitor disease activity.
Methods
Prospective interview of IBD patients using an analytic hierarchy matrix survey built to understand preferences for choosing between stool testing or colonoscopy for monitoring disease activity, after considering diffferent test criteria (accuracy, preparation, pain, complications). Theoretical thresholds of misclassification were posed to patients to see how they might consider shifting from colonoscopy to stool testing.
Results
A total of 100 patients (n = 51 CD, n = 46 male) were interviewed with median age and disease duration of 44 years (IQR 27–63) and 9 years (IQR 5–21), respectively. Stool-based testing was preferred over colonoscopy by 60% initially; however, a majority of participants changed their choice to colonoscopy after learning more about the diagnostic performance of currently available stool tests for disease monitoring (p < 0.001). Across all sub-groups, accuracy was ranked as the top criterion when choosing between stool-based testing and colonoscopy for disease activity assessments. Most patients were willing to choose stool-based testing over colonoscopy for disease monitoring if the stool test was wrong at most 1 in 20 times (5% misclassification rate).
Discussion
Accuracy is the most important criteria for IBD patients when choosing monitoring strategies, and a high degree of confidence is required of stool test results for patients to choose this strategy.
Keywords: Monitoring, Biomarker, Preferences
Introduction
The achievement of endoscopic improvements in disease activity and endoscopic remission represent current treatment targets in both Crohn’s disease (CD) and ulcerative colitis (UC) given the association between the achievement of these outcomes and reductions in disease-related complications [1–3]. Due to the discordance between symptoms and endoscopic activity, it is recommended that CD and UC patients undergo repeated and frequent endoscopic evaluation of disease activity to accurately guide treatment escalation to achieve these targets (treat-to-target) [4, 5]. Although rationale in conclusion, the practicality, safety, and cost of such approaches need to be considered [6, 7]. A recent claims-based analysis demonstrated that among the highest risk group of CD and UC patients, only 50% had some form of objective assessment with cross-sectional imaging, and/or lower endoscopy within 2 years of starting biologic therapy [8]. Thus, the feasibility of endoscopy-based treat-to-target strategies is clearly limited and alternative noninvasive approaches need to be considered to appropriately guide treatment decisions in the broader population [9].
Fecal calprotectin has been observed to be a potentially reliable surrogate for endoscopic activity in both in CD and UC [10]. The CALM trial recently demonstrated that interim assessments and treatment adjustments with CRP and fecal calprotectin lead to a significantly higher rate of endoscopic remission compared to symptoms alone. In this trial the primary determinate or trigger of treatment adjustment in the biomarker arm was fecal calprotectin [11, 12]. Similar work in UC has demonstrated that fecal calprotectin-based treatment adjustments leads to improvements in disease outcomes, and the combination of symptoms with fecal calprotectin monitoring provides modest diagnostic performance for identifying mucosal inflammation [13, 14]. Although widely available and used in Europe, adherence to fecal calprotectin testing has been estimated to be only 30% [15]. Furthermore, its use in the USA is estimated to be less than 5% [8]. The exact determinants of this variability in utilization remain to be determined; however, patient preferences are likely to be an important consideration given the preparation, storage, and time commitment required to collect these samples. Furthermore, some degree of inaccuracy exists with these tests, and patients need to be willing to accept some degree of uncertainty in test results when making treatment decisions. This may, however, be offset by the inherent risks and preparation required for lower endoscopy and direct comparisons between the two are needed to fully understand patient preferences and determinants of choosing between them for disease monitoring.
To study this, we performed a prospective analytic hierarchy process survey-based cohort study of CD and UC patients with the aim of identifying patient determinants of choosing stool tests versus colonoscopy for disease activity assessments [16, 17]. We anticipate these data will help inform the appropriate integration of fecal biomarker testing into routine practice and provide insights to guide further biomarker discovery and testing.
Methods
Study Design
Prospective, observational, cohort study conducted from October 2018 to February 2019. This study was approved by the University of California San Diego Institutional Review Board, and all patients provided informed consent prior to participation (IRB #180311).
Participants and Study Site
We recruited CD and UC patients from the University of California San Diego Inflammatory Bowel Disease Center, and patients were included if they met the following criteria: (1) confirmed diagnosis of CD or UC based on clinical, endoscopic, radiographic, and/or histologic criteria, (2) at least 18 years of age and able to make independent decisions on treatment choice and disease monitoring, (3) without a contraindication to the performance of colonoscopy as a diagnostic test. Patients were recruited in both the clinic setting and endoscopy setting to ensure broader representation of perceptions and experiences that may influence survey responses.
Data Collection
Data for individual patients were collected at the time of survey administration and through review of the medical record. These variables collected included: (1) patient specific: age, gender, smoking status, co-morbid conditions, (2) disease specific: type of disease (UC or CD), duration, prior treatment exposures (steroids, immunomodulators, biologics), disease course (hospitalizations, surgeries), and disease severity (clinical, endoscopic, and radiographic), and (3) endoscopy specific: number of prior endoscopies in prior 5 years and any prior procedure-related complications or adverse events.
Outcomes
Our primary outcome was to identify the main determinate of patient preference for stool testing versus colonoscopy for the assessment of disease activity. Secondary outcomes included: understanding how patient preferences varied across patient and disease characteristics and explore thresholds of stool test accuracy that might lead to a patient to be willing to undergo evaluation with a stool test in place of a colonoscopy.
Survey Methodology
The choice on how to monitor disease activity for CD and UC patients is a multi-tiered level decision that incorporates patient preferences and thresholds of acceptability. Accordingly, we utilized a widely accepted methodology for addressing multi-tiered level complex decision-making comparisons—the analytical hierarchy process (AHP) decision-making model [16, 17]. Within this model, a hierarchy of criteria was created (Fig. 1) to allow for independent comparisons and pairwise comparisons for each of the chosen alternatives (stool or colonoscopy for disease activity monitoring). According to the AHP model, the problem of how to monitor disease activity is deconstructed into a hierarchy of a goal, criteria, and alternatives or options. The goal for each patient is to choose between options for disease activity assessment, a stool test or a colonoscopy, after considering different criterion that might influence the decision. We focused on accuracy, preparation/discomfort, and complications/inconvenience as the criterion for these options.
Fig. 1.
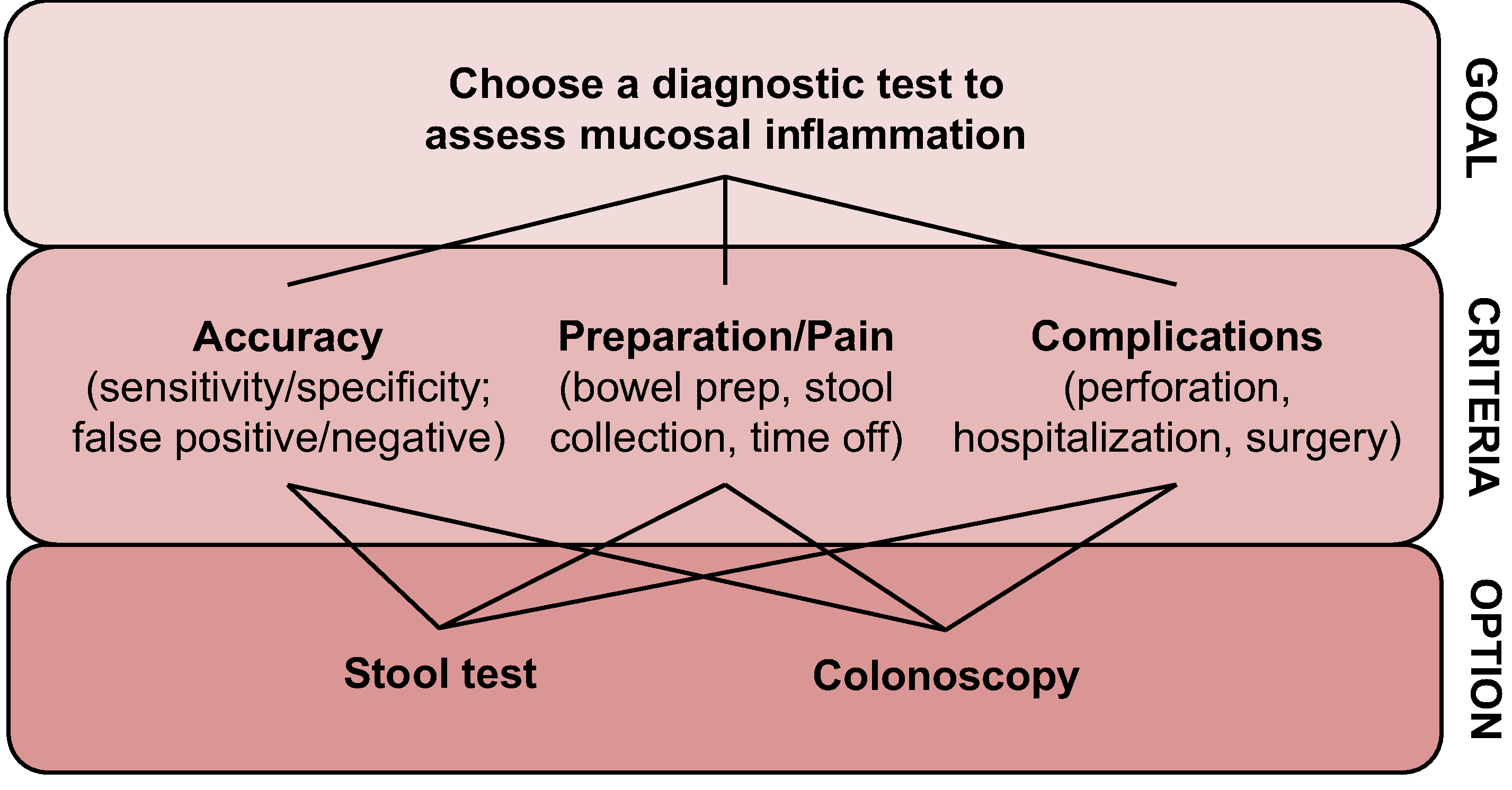
Analytic hierarchy process decision-making model web
The criterion (accuracy, preparation/discomfort, complications/inconvenience) were then converted into a relative importance scale (1–9) and transformed into simple multiple-choice questions for ease of administration (Supplementary material). This approach has been shown to yield the main concerns of a respondent, and consistency exists between the simple multiple-choice survey and traditional AHP survey approaches for identifying the top ranked criterion when making decisions [18]. Inconsistency between the simple multiple-choice survey and traditional AHP survey approaches arises when considering the second alternative, but given we only intended to compare two options (stool test versus colonoscopy) and we were intending to identify the single main determinant of patient preference, this approach of using a simple multiple-choice questionnaire linked back to a relative importance scale was felt to be the best balance between feasibility, patient comprehension, and confidence in survey results.
To identify thresholds of acceptability for accuracy, we posed theoretical thresholds of misclassification to see how patients might consider shifting from colonoscopy to stool testing. If a patient preferred colonoscopy over the stool test by the end of the survey, they were asked if their decision would change if the stool test was wrong at most 10% (1 in 10); if they still preferred colonoscopy, they were asked if their opinion would change if the test was wrong at most 5% of the time (1 in 20). Finally, if the patient still preferred colonoscopy they were asked if their decision would change if the stool test was wrong at most 2% of the time (1 in 50).
Statistical Analysis
Demographic and patient characteristics were reported using means or medians, standard deviations or interquartile ranges (IQR), and overall ranges. Baseline data were then compared using the fisher exact test. The AHP was analyzed through pairwise comparison of the components. The pairwise comparisons result in proportional weights for each criterion and were used to calculate the final priorities for stool test and colonoscopy. The pairwise comparison of criteria was used to determine degree of preference relative to one another and result in three 2 × 2 matrices. On the other hand, pairwise comparison of criterion against the goal determined the importance of the criterion and result in a 3 × 3 matrix. The final priority for each alternative was calculated by multiplying each alternative by the priority of its criterion then adding the resulting relevant proportional weights. The most appropriate decision is the alternative with the highest final priority. Judgments of a group were determined to be consistent within the model using consistency ratio. A true Consistency Ratio is calculated by dividing the Consistency Index for the set of judgments by the Index for the corresponding random matrix. The consistency index is calculated by dividing the minimum amount of change possible for a character: m (m = the number of steps for a character - 1) by s (s = the number of changes in that character observed on the tree). Saaty suggests that if that ratio exceeds 0.1 the set of judgments may be too inconsistent to be reliable. In practice, CRs of more than 0.1 sometimes must be accepted. No formal sample size calculations were performed to guide recruitment.
Results
Patient Demographics
A total of 104 patients were approached for this study, 100 of which consented to participate and completed the survey. Patient demographics are provided in Table 1. Median disease duration was 14 years, with a nearly even split of patients according to gender and disease type. Disease activity at the time of the survey was well distributed with 47% in clinical remission, 24% with mild activity, and 29% with moderate-severe activity. Recruitment occurred in the clinic setting for 65% of patients with the remaining 35% of patients being recruited and surveyed in the endoscopy suite immediately prior to undergoing a colonoscopy.
Table 1.
Baseline demographics of survey cohort
| Gender | |
| Male, n (%) | 54 (54) |
| Female, n (%) | 46 (46) |
| Type of IBD | |
| CD, n (%) | 48 (48) |
| UC, n (%) | 51 (51) |
| Indeterminate colitis, n (%) | 1 (1) |
| Average age | 44.3 (18–80) |
| Average disease duration | 14.2 (0–52) |
| Prior treatments | |
| > 2 prior biologics, n (%) | 49 (49) |
| 1 prior biologic, n (%) | 27 (27) |
| Patients with prior surgeries, n (%) | 33 (33) |
| Average number of colonoscopies in last 5 years | 4.27 (0–12) |
| Disease severity | |
| Remission, n (%) | 47 (47) |
| Mild, n (%) | 24 (24) |
| Moderate, n (%) | 17 (17) |
| Severe, n (%) | 12 (12) |
IBD: Inflammatory bowel disease; CD: Crohn’s disease; UC: ulcerative colitis; Disease severity—categorized based on the Crohn’s Disease Activity Index Patient Reported Outcome sub-scores for Crohn’s disease and the Partial Mayo score for Ulcerative Colitis
Baseline Patient Preference
Stool testing was preferred for disease activity assessment in 60% of patients, with the remainder choosing colonoscopy. Colonoscopy was preferred by 49% of CD patients (n = 25/51) as compared to 31% of UC patients (n = 15/48, p = 0.10). Stool testing was preferred by 66% of patients in clinical remission (n = 31/47) versus 55% of those with active symptoms (n = 29/53, 55%; p = 0.31). Stool testing was preferred by 73% of patients aged 60 years or older (n = 19/27) versus 55% of patients less than 60 years of age (n = 39/71, p = 0.11). Patients who completed the survey in the endoscopy suite after already having undergone bowel prep more often chose colonoscopy as the initial preference (n = 23/35, 66%; vs. n = 28/65, 43%; p = 0.52). No other patient, disease, or colonoscopy specific features were identified to influence the baseline preference for stool test or colonoscopy.
Criterion Priorities
Accuracy was ranked as the most important by patients when choosing a diagnostic test for the assessment of disease activity, followed by complications/inconvenience and pain/preparation. The global priority score was determined to be 55.8% for accuracy, 24.1% for complications/inconvenience, and 19% for pain/preparation. The model consistency ratio was 0.1%, demonstrating reliability in the set of judgments. Global priority scores for key sub-groups are presented in Table 2.
Table 2.
Global priorities for criterion based on key sub-groups
| Accuracy (%) | Complications/inconvenience (%) | Pain/preparation (%) | |
|---|---|---|---|
| No prior surgery | 58 | 22.5 | 19.5 |
| Prior surgery | 53.4 | 27.7 | 18.9 |
| UC | 54.3 | 20.1 | 25.5 |
| CD | 56.7 | 23.8 | 19.5 |
| Remission | 52.8 | 20.4 | 26.7 |
| Active symptoms | 70.1 | 14 | 15.9 |
| No prior hospitalization | 57.2 | 22.1 | 20.8 |
| 1 prior hospitalization | 37 | 41.2 | 21.8 |
| Endoscopy suite survey | 57.4 | 24.3 | 18.3 |
| Clinic survey | 56.2 | 24.1 | 56.2 |
| No prior biologic | 60 | 23.8 | 16.2 |
| Prior biologics | 57.6 | 22 | 20.4 |
UC Ulcerative colitis, CD Crohn’s disease
Post-survey Preferences and Stool Test Thresholds of Accuracy
After being provided with information on the accuracy of stool testing based on current evidence for fecal calprotectin, 25 patients who initially chose stool testing elected to change their preference to colonoscopy (p < 0.001) and at the completion of the survey a total of 65 patients chose colonoscopy for disease activity assessment. If a stool test was wrong at most 1 in 20 times (5%), then 40% of these 65 patients were willing to switch to using stool testing for disease activity assessment. If a stool test was wrong at most 1 in 50 times (2%), 66% of these 65 patients were willing to switch to using stool testing for disease activity assessment.
Conclusion
Many studies have focused on shared decision-making and patient preferences in IBD with regards to treatment decisions, but there have been few studies on IBD patient preferences for a testing modality when assessing disease activity. With the evolving push toward treat-to-target management strategies, patient preferences for testing modalities are an important consideration. We performed a survey in 100 IBD patients to understand determinants of patient preferences for choosing stool testing versus colonoscopy for disease activity assessment and made several key observations. First, the majority of patients preferred stool testing over colonoscopy as the initial test of choice, but this changed to colonoscopy after learning more about the relative di?erences in accuracy. Through our survey, we observed that accuracy was the most important criterion for patients when choosing between stool testing and colonoscopy, and this was consistent across all sub-groups. Finally, we observed that most patients were willing to use stool testing for disease activity monitoring if the test was wrong at most 1 in 20 times, suggesting that the use of stool testing in specific scenarios where the false positive or false negative rate is no more than 5% is likely to be met with support by patients.
Prior literature on patient adherence and preferences for testing modalities is limited and conflicting. A study from Europe suggested that patient adherence to stool testing was only 30% but the authors were unable to comment on reasons for non-compliance [15]. An online survey through the Crohn’s and Colitis Canada website observed that adherence to stool testing for monitoring of disease activity was 97% and comparable to colonoscopy (99% adherence) [19]. Furthermore, patients in this survey felt more comfortable with stool-based testing as compared to colonoscopy and the majority of variation in testing was a result of variation in provider ordering patterns as opposed to patient willingness to use stool-based testing for monitoring of disease activity. In our study we similarly observed that the majority surveyed initially preferred stool-based testing over colonoscopy for monitoring of disease activity. What was notable was that after patients learned more about the currently available stool-based test (fecal calprotectin) and its diagnostic performance in IBD, most changed their preference to colonoscopy. Furthermore, when surveyed about factors that influence their decisions when choosing between stool-based testing and colonoscopy for disease monitoring, accuracy of the test was ranked the top priority across all sub-groups. Taken together, patients are willing to undergo monitoring of disease activity with stool-based tests and may actually prefer this over colonoscopy; however, a shared decision-making approach must be undertaken to identify patients best suited for biomarker-based monitoring strategies and to appropriately inform and guide patients on relative differences in accuracies and allow for a fully informed decision.
In our survey we attempted to identify thresholds at which patients may be willing to switch from colonoscopy to stool-based testing and observed that the majority of patients were willing to accept a 5% misclassification risk. Meaning many patients were comfortable with stool-based testing being incorrect 1 in 20 times and would be willing to choose this over colonoscopy for disease monitoring. A recent study in UC by our group observed that the combination of fecal calprotectin with patient reported outcomes could achieve a misclassification rate of at most 5% in certain clinical scenarios [14]. This analysis used indirect comparisons and evidence, and direct patient level assessments of the combined accuracy for symptoms + fecal calprotectin are still needed, but the work helps to support the potential for achieving thresholds of accuracy that are clinically acceptable and also acceptable to patients.
Our study has several strengths including the use of a survey technique capable of deconstructing a complex decision-making process into a simple survey-based assessment, and the consistency of results across sub-groups and survey analyses. There are however several limitations worth considering. The cohort was overall small with only 100 patients and it was conducted at a single tertiary referral center. This makes it underpowered to fully understand the impact of baseline demographics on patient preferences, and it limits the generalizability to routine practice where variations in disease monitoring are known to exist. The recruitment being limited to a tertiary referral center also limits generalizability due to variation in use of proactive disease monitoring and patient comfort with various testing strategies that likely exists between academic and community centers. Furthermore, the survey model only considered three criteria, but there are likely other criteria to consider such as cost, testing availability, and timeliness of result reporting. Cost may be a more dominant criteria in lower socieoeconomic status populations, testing availability may be a stronger influence when comparing academic versus rural clinical care centers, and timeliness of result reporting likely influences decisions among those with more active symptoms where more immediate decisions need to be made to improve patient well being. All three of these criteria are particularly important in the US where fecal calprotectin is not FDA approved for monitoring of disease activity in IBD and its use for monitoring is off-label, thereby impacting cost, availability, and timeliness of reporting. Future surveys need to be done with pre-defined power calculations to ensure adequate sample size, and where consideration is given to additional criteria and pre-defined sub-group analyses to fully explore and capture these important determinants of decision-making.
In conclusion, we observed that most patients prefer stool-based testing over colonoscopy and are willing to undergo testing with stool-based tests to monitor their disease activity; however, the major determinant of this choice is accuracy. At a threshold misclassification risk of 5%, most patients feel comfortable with choosing stool-based testing over colonoscopy for disease activity monitoring. Further treat-to-target algorithms that incorporate biomarker-based monitoring should consider patient preferences and misclassification rates when determine feasibility of implementation.
Supplementary Material
Acknowledgment
Parambir S. Dulai is supported by an American Gastroenterology Association Research Scholar Award. Brigid Boland is supported by a grant from the NIH/NIDDK (K23DK123406). Siddharth Singh is supported by NIH/NIDDK (K23DK117058), ACG Junior Faculty Development Award and the Crohn’s and Colitis Foundation Career Development Award (#404614). William Sandborn is partially supported by NIDDK-funded San Diego Digestive Diseases Research Center (P30 DK120515).
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10620-020-06568-w) contains supplementary material, which is available to authorized users.
Compliance with Ethical Standards
Conflict of interests PSD: consulting and/or research support from Takeda, Janssen, Pfizer, Abbvie, Polymedco, Buhlmann, Prometheus; BB: consulting from Pfizer and research support from Prometheus; SS: Research grants from Abbvie and Janssen. WJS: research grants from Atlantic Healthcare Limited, Amgen, Genentech, Gilead Sciences, Abbvie, Janssen, Takeda, Lilly, Celgene/Receptos,Pfizer, Prometheus Laboratories (now Prometheus Biosciences); consulting fees from Abbvie, Allergan, Amgen, Arena Pharmaceuticals, Avexegen Therapeutics, BeiGene, Boehringer Ingelheim, Celgene, Celltrion, Conatus, Cosmo, Escalier Biosciences, Ferring, Forbion, Genentech, Gilead Sciences, Gossamer Bio, Incyte, Janssen, Kyowa Kirin Pharmaceutical Research, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pfizer, Progenity, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Reistone, Ritter Pharmaceuticals, Robarts Clinical Trials (owned by Health Academic Research Trust, HART), Series Therapeutics, Shire, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Takeda, Theravance Biopharma, Tigenix, Tillotts Pharma, UCB Pharma, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals; and stock or stock options from BeiGene, Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Progenity, Ritter Pharmaceuticals, Ventyx Biosciences, Vimalan Biosciences. Spouse: Iveric Bio—consultant, stock options; Progenity—consultant, stock; Oppilan Pharma—consultant, stock options; Escalier Biosciences—prior employee, stock options; Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories)—employee, stock options; Ventyx Biosciences—stock options; Vimalan Biosciences—stock options.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affliations.
References
- 1.Ungaro R, Colombel JF, Lissoos T, et al. A treat-to-target update in ulcerative colitis: a systematic review. Am J Gastroenterol. 2019;114:874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colombel JF, D’Haens G, Lee WJ, et al. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2020;14(2):254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dulai PS, Levesque BG, Feagan BG, et al. Assessment of mucosal healing in inflammatory bowel disease: review. Gastrointest Endosc. 2015;82:246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouguen G, Levesque BG, Pola S, et al. Endoscopic assessment and treating to target increase the likelihood of mucosal healing in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:978–985. [DOI] [PubMed] [Google Scholar]
- 5.Bouguen G, Levesque BG, Pola S, et al. Feasibility of endoscopic assessment and treating to target to achieve mucosal healing in ulcerative colitis. Inflamm Bowel Dis. 2014;20:231–239. [DOI] [PubMed] [Google Scholar]
- 6.Navaneethan U, Parasa S, Venkatesh PG, et al. Prevalence and risk factors for colonic perforation during colonoscopy in hospitalized inflammatory bowel disease patients. J Crohns Colitis. 2011;5:189–195. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira J, Akbari M, Gashin L, et al. Prevalence and lifetime risk of endoscopy-related complications among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:1288–1293. [DOI] [PubMed] [Google Scholar]
- 8.Limketkai BN, Singh S, Jairath V, et al. US practice patterns and impact of monitoring for mucosal inflammation after biologic initiation in inflammatory bowel disease. Inflam Bowel Dis. 2019;25:1828–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dulai PS, Jairath V. How do we treat inflammatory bowel diseases to aim for endoscopic remission? Clin Gastroenterol Hepatol 2019. [DOI] [PubMed] [Google Scholar]
- 10.Dulai PS, Peyrin-Biroulet L, Danese S, et al. Approaches to integrating biomarkers into clinical trials and care pathways as targets for the treatment of inflammatory bowel diseases. Gastroenterology. 2019;157:1032–1043. [DOI] [PubMed] [Google Scholar]
- 11.Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390:2779–2789. [DOI] [PubMed] [Google Scholar]
- 12.Panaccione R, Colombel JF, Travis SPL, et al. Tight control for Crohn’s disease with adalimumab-based treatment is cost-effective: an economic assessment of the CALM trial. Gut. 2020;69:658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osterman MT, Aberra FN, Cross R, et al. Mesalamine dose escalation reduces fecal calprotectin in patients with quiescent ulcerative colitis. Clin Gastroenterol Hepatol. 2014;12:1887–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dulai PS, Battat R, Barsky M, et al. Incorporating Fecal Calprotectin Into Clinical Practice for Patients With Moderate-to-Severely Active Ulcerative Colitis Treated With Biologics or Small-Molecule Inhibitors. Am J Gastroenterol. 2020;115(6):885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marechal C, Aimone-Gastin I, Baumann C, et al. Compliance with the faecal calprotectin test in patients with inflammatory bowel disease. U Eur Gastroenterol J. 2017;5:702–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saaty TL. The Analytic Hierarchy Process: Planning, Priority Setting, Resource Allocation. New York, 1980. [Google Scholar]
- 17.Saaty TL. A scaling method for priorities in hierarchical structures. J Math Psychol. 1977;15:234–281. [Google Scholar]
- 18.Sato J. Comparison between multiple-choice and analytic hierarchy process: measuring human perception. Int Trans Oper Res. 2004;11:77–86. [Google Scholar]
- 19.Noiseux I, Veilleux S, Bitton A, et al. Inflammatory bowel disease patient perceptions of diagnostic and monitoring tests and procedures. BMC Gastroenterol. 2019;19:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


