Abstract
Background:
Polymethylmethacrylate (PMMA) bone cement is commonly used in orthopedic surgery for implant fixation and local antibiotic delivery following surgical debridement. The incidence of nephrotoxicity necessitates the balance of antiinfective properties with the potential for toxicity. Thus, understanding antibiotic elution characteristics of different PMMA formulations is essential. We sought to address this by assessing elution of vancomycin, daptomycin, and tobramycin from Palacos LV (Palacos), Stryker Surgical Simplex P (Simplex), BIOMET Cobalt HV (Cobalt), and Zimmer Biomet Bone Cement R (Zimmer) radiopaque bone cements.
Methods:
Antibiotics were mixed with each cement formulation, and molds were used to produce beads of cement. Beads were incubated in phosphate-buffered saline at 37°C, and antibiotic elution was measured daily for 10 days with vancomycin and 5 days with daptomycin and tobramycin. Active antibiotic was quantified by serial dilution and comparison to the minimum inhibitory concentration.
Results:
The elution profiles of Simplex were significantly lower than all other cements with all antibiotics (P < .00093). Palacos exhibited a significantly higher vancomycin elution profile than all other cements (P < .00001). The difference in daptomycin elution profiles for Cobalt and Palacos was not significant (P > .43), but both were significantly higher than Zimmer (P < .0006).
Conclusion:
Overall, Stryker Surgical Simplex P exhibits a significantly lower elution profile than all other cements tested. In general, Palacos LV exhibits an increased elution profile compared with other cements. This elution information may assist the surgeon in choosing different cement formulations for the local delivery of antibiotics.
Keywords: Polymethylmethacrylate, bone cement, antibiotic, infection, Staphylococcus aureus
Polymethylmethacrylate (PMMA) bone cement is used as a means of targeted antibiotic delivery directly to the site of infection following surgical debridement, particularly in the treatment of infected total joint arthroplasty. Owing to the intrinsically resistant nature of these types of infections, achieving concentrations of antibiotics at the sites of infection sufficient to eradicate viable bacteria is a primary concern. However, dosage of antibiotic in the spacer is complicated by the simultaneous use of intravenous antibiotics and the potential for systemic toxicity and adverse effects. Nephrotoxicity has been shown to be a relatively common side-effect when antibiotic-loaded cement is used for spacer placement [1,2]. In a recent prospective study, renal failure was found in 10 of 37 (27%) patients treated for an infected joint replacement with an antibiotic-loaded spacer [3]. Decisions pertaining to antibiotic selection and dosage are further complicated by the availability of alternative PMMA formulations with differing porosities [4], and these differences have been shown to impact antibiotic elution [5–7]. Reports have demonstrated higher rates of toxicity with cements exhibiting higher antibiotic elution rates [1].
A recent emphasis on cutting costs in joint replacement has led to the use of a wider variety of cement types [8]. Elution data are not available for Biomet Cobalt HV cement and the newly released Zimmer Biomet Bone Cement R. This is despite one report of nephrotoxicity with the use of Cobalt cement [9]. The goal of our study was to assess the elution characteristics of these newer bone cement formulations in comparison to previously studied formulations using 3 commonly used antibiotics (vancomycin, daptomycin, and tobramycin).
Materials and Methods
Four different formulations of PMMA bone cement were compared based on antibiotic elution profiles. These were Palacos LV (Palacos) [Heraeus Medical LLC, Yardley, PA], Stryker Surgical Simplex P (Simplex) [Stryker, Kalamazoo, MI], BIOMET Cobalt HV (Cobalt) [DJO Surgical, Austin, TX], and Zimmer Biomet Bone Cement R (Zimmer) [Zimmer Biomet, Warsaw, IN] radiopaque bone cements. Vancomycin was chosen for our primary comparisons because of its prevalent use in this clinical context and its activity against methicillin-resistant Staphylococcus aureus, which is the single most concerning cause of osteomyelitis and implant-associated infection. For these initial studies, 1.0 g of vancomycin was mixed with the powder component (prepolymerized PMMA) for each cement formulation for 5 minutes, at which point a homogenous mixture was achieved. The liquid monomer was then added to the powder-vancomycin mixtures and the cements mixed according to the manufacturers’ protocols using Stryker MixEvac 3BoneCementMixers (Stryker). Spherical beads with diameters of 7 mm and 11mm were created for each PMMA formulation using silicone molds and were allowed to dry for 1 hour at 25°C prior to elution studies.
Elution studies were carried out as previously described with minor modifications [10]. Briefly, 5 beads (11 mm) or 6 beads (7 mm) were placed in 50 mL flasks with 12 mL of sterile phosphate-buffered saline (PBS), a volume sufficient for complete submersion of all beads. These numbers of beads were chosen because it allowed for testing a roughly equal mass of each bead size. Each experiment was performed in triplicate with 2 biological replicates. Samples were held at 37°C for 10 days with PBS being removed in its entirety at 24 hour intervals and replaced with fresh PBS. Eluents were then assayed for the concentration of eluted antibiotic using bioassays as previously described [10]. Briefly, fresh preparation of vancomycin was carried out in sterile PBS on each analysis day. This stock solution was used to prepare 2-fold dilutions in a 96-well microtiter plate. A 2-fold dilution series also was prepared with each experimental eluent sample. Dilutions were prepared in tryptic soy broth. Each well was inoculated subsequently with 104 colony-forming units of a strain of Staphylococcus aureus (UAMS-1) that is sensitive to all of the antibiotics tested in this study. After incubation at 37°C for 24 hours, the concentration of active antibiotic in each experimental sample was assessed by comparing the minimum inhibitory concentration of each eluent sample to the minimum inhibitory concentration obtained in the same assay with the vancomycin standard. Comparisons were made to assess differences between different PMMA formulations and each PMMA formulation as a function of bead diameter.
Following the initial studies assessing the elution of vancomycin, experiments were performed with additional antibiotics to determine whether any observed trends were consistent or vancomycin-dependent. Daptomycin was chosen based on its activity against methicillin-resistant Staphylococcus aureus and previous studies indicating its relative efficacy in the context of an established biofilm [11–13]. Tobramycin was also chosen because of its prevalent use in bone cement for the treatment of prosthetic joint infection [14]. These studies were performed as those with vancomycin. However, because the purpose of these experiments was to determine the consistency of antibiotic elution trends observed with vancomycin, a single bead size was chosen (11 mm), and elution studies were only carried out to 5 days. Antibiotic-mixed bone cement was prepared as described previously using 500 mg of daptomycin or 1.2 g of tobramycin.
Elution profiles of the cement types were compared separately for each antibiotic. Nonparametric longitudinal analysis methods were used to compare elution profiles [15]. P values less than 0.05 were considered statistically significant. No adjustments for multiple comparisons were made.
Results
Comparison of vancomycin elution profiles for each PMMA formulation as a function of bead size demonstrated no significant difference in vancomycin elution between 7 mm or 11 mm beads for a given formulation (data not shown). Thus, for analysis of vancomycin elution from different PMMA formulations, comparisons were made for 11 mm beads only. The results demonstrate that the vancomycin elution profile of Palacos was significantly higher than those of all other cements tested (P < .00001 for all comparisons), while the elution profile of Simplex was significantly lower than those of all other cements (P < .00093, Fig. 1). No differences were observed between vancomycin elution profiles from Cobalt and Zimmer (P > .33). The amount of vancomycin eluted from Simplex beads fell below the limit of detection of the assay by day 9 in all groups, a phenomenon not observed with any other PMMA formulation.
Fig. 1.
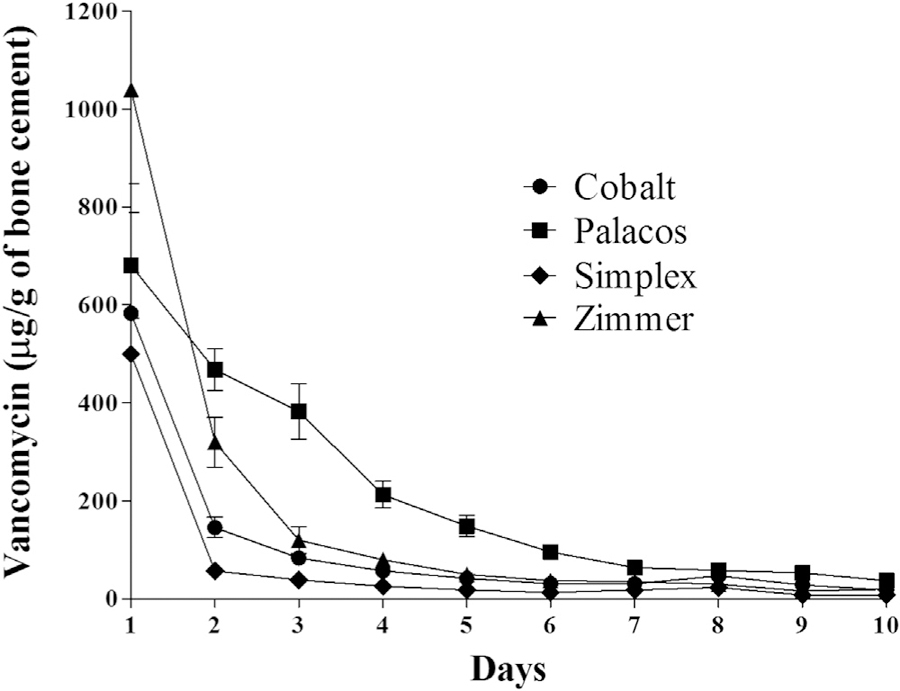
Relative elution of vancomycin from Cobalt (circles), Palacos (squares), Simplex (diamonds), and Zimmer (triangles) measured in μg of antibiotic eluted per g of bone cement. Error bars represent the standard error of the mean.
Assessment of daptomycin elution yielded similar but not identical results (Fig. 2). Specifically, the elution profile from Simplex was significantly lower than those for the other formulations tested (P < .00001), and the elution profile from Zimmer was lower than the profiles from Cobalt and Palacos (P < .00060). Unlike the results observed with the elution of vancomycin, there were no statistically significant differences observed between the elution profiles from Cobalt and Palacos (P > .43). All of these comparisons were made based on the measurement of daptomycin elution from PMMA over the course of 5 days.
Fig. 2.
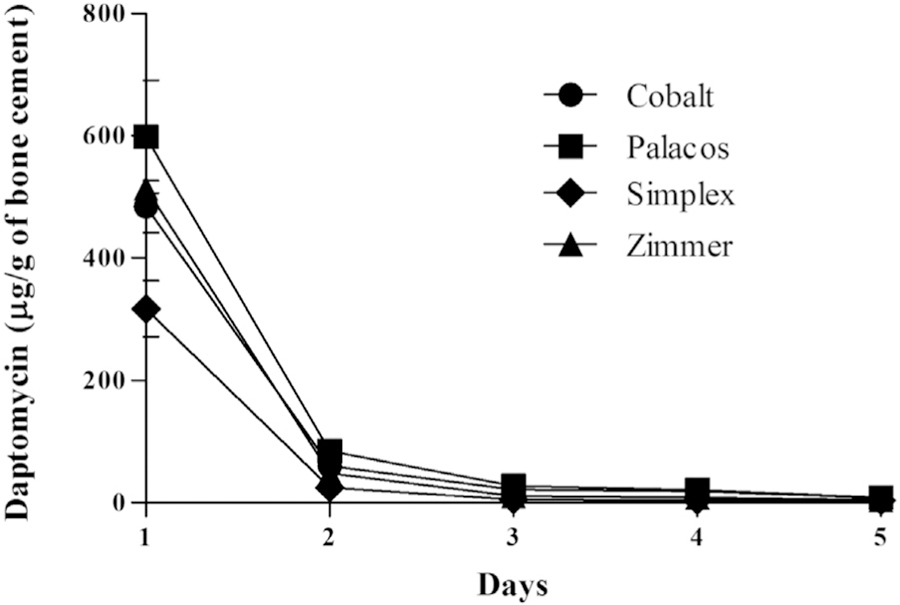
Relative elution of daptomycin from Cobalt (circles), Palacos (squares), Simplex (diamonds), and Zimmer (triangles) measured in μg of antibiotic eluted per g of bone cement. Error bars represent the standard error of the mean.
The results of experiments assessing the elution of tobramycin were the least consistent by comparison to studies using vancomycin or daptomycin. Specifically, the only significant difference observed was a significantly lower elution profile from Simplex by comparison to the other formulations tested (P < .00001, Fig. 3). No significant differences were observed between tobramycin elution profiles for the other PMMA formulations (P > .43).
Fig. 3.

Relative elution of tobramycin from Cobalt (circles), Palacos (squares), Simplex (diamonds), and Zimmer (triangles) measured in μg of antibiotic eluted per g of bone cement. Error bars represent the standard error of the mean.
Discussion
Our study has evaluated elution profiles of 3 commonly used antibiotics in 4 different types of cement. Our goal was to assess the elution profiles of 2 cements, Biomet Cobalt HV cement and Zimmer Biomet Bone Cement R, that have not been previously reported. As in other studies, Palacos cement had the highest rates of elution and Simplex had the lowest elution rates for vancomycin. No cement exhibited greater elution profiles than those of Palacos with any of the antibiotics tested. In contrast, Simplex was found to exhibit reduced elution profiles by comparison to all other cements with all antibiotics. These results are consistent with previous studies comparing antibiotic elution profiles from Palacos and Simplex cements [6]. In general, Cobalt and Zimmer exhibited elution profiles that were similar to Palacos or intermediate by comparison to Palacos and Simplex, depending on the antibiotic.
Overall, these results suggest that Palacos cement offers therapeutic advantages in the context of preventing or eradicating infection. However, this possibility must be balanced with the consideration of potential toxicity. Nephrotoxicity is common with spacers and care should be taken in patients with a history of renal impairment [1]. Indeed, nephrotoxicity with a Cobalt cement spacer has been reported previously [9]. Such reports suggest that the potential advantage of higher eluting cements in the context of infection must be evaluated in the context of renal function and potential nephrotoxicity. This ultimately requires the consideration of both the total dose of antibiotic eluted from a given cement formulation as well as significant bolus release, particularly early in the elution process. For example, Zimmer was observed to elute a significantly large bolus of vancomycin on days 1 and 2 of elution, which resulted in vancomycin concentrations significantly greater than from any other cement formulation tested (Fig. 1). Although the total amount of vancomycin eluted from Palacos was found to be significantly greater than that from Zimmer over the course of 10 days, the peak vancomycin concentration reached was much higher with Zimmer than with Palacos. Thus, the results we report may help guide surgeons when dosing antibiotics in different formulations of PMMA bone cement.
Our study does have some limitations. For instance, clinicians may choose to mix different amounts of antibiotic with bone cement, and this may change overall elution characteristics. Similarly, clinicians often use mixtures of several antibiotics. Our study also evaluated antibiotic elution using an in vitro method and a standardized size of cement bead, while antibiotic-loaded spacers are larger and more irregularly shaped. All of these factors can impact elution characteristics [14]. Finally, our study does not address the impact of incorporating antibiotics on the structural stability of different PMMA formulations. This is potentially relevant in that higher dosages of antibiotics have been shown to weaken cement strength characteristics [6,16]. This is a particularly important consideration in the context of revision arthroplasty but is also relevant in the context of bead-based antibiotic delivery following trauma or surgical debridement in which it is often necessary to remove the beads prior to reconstructive surgery.
Conclusion
In conclusion, we have shown that antibiotic elution profiles of commonly used bone cement formulations can differ significantly depending in part on the antibiotic used. As in previous studies, we demonstrated that Palacos exhibits a greater vancomycin elution profile than that of Simplex. Cobalt and Zimmer cements have not been previously investigated and, in general, our results demonstrate that they exhibit elution profiles that are intermediate by comparison to these 2 other cements. Clinicians may use this information when using these products to make cement spacers for the treatment of prosthetic joint infection to help in the avoidance of nephrotoxicity, while maximizing antimicrobial effects.
Acknowledgments
The authors wish to gratefully acknowledge Mr. Horace Spencer for statistical analysis of the results presented in is work. Research support is provided to the MSS laboratory by grants from the Department of Defense Peer-Reviewed Orthopaedic Research Program (W81XWH-15-1-0716), the National Institute of Allergy and Infectious Diseases, United States (R01-AI119380), and generous support from the Arkansas Research Alliance and the Texas Hip and Knee Society. Additional support was provided by core facilities supported by the Center for Microbial Pathogenesis and Host Inflammatory Responses (P20-GM103450) and the Translational Research Institute (UL1TR000039) at the University of Arkansas for Medical Sciences.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to https://doi.org/10.1016/j.arth.2019.03.008.
References
- [1].Luu A, Syed F, Raman G, Bhalla A, Muldoon E, Hadley S, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control. J Arthroplasty 2013;28:1490e8. [DOI] [PubMed] [Google Scholar]
- [2].Salim SA, Everitt J, Schwartz A, Agarwal M, Castenada J, Fülӧp T, et al. Aminoglycoside impregnated cement spacer precipitating acute kidney injury requiring hemodialysis. Semin Dial 2018;31:88e93. [DOI] [PubMed] [Google Scholar]
- [3].Edelstein AI, Okroj KT, Rogers T, Della Valle CJ, Sporer SM. Nephrotoxicity after the treatment of periprosthetic joint infection with antibiotic-loaded cement spacers. J Arthroplasty 2018;33:2225e9. [DOI] [PubMed] [Google Scholar]
- [4].Arora M, Chan EKS, Gupta S, Diwan AD. Polymethylmethacrylate bone cements and additives: a review of the literature. World J Orthop 2013;4:67e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Stevens CM, Tetsworth KD, Calhoun JH, Mader JT. An articulated antibiotic spacer used for infected total knee arthroplasty: a comparative in vitro elution study of Simplex and Palacos bone cements. J Orthop Res 2015;23:27e33. [DOI] [PubMed] [Google Scholar]
- [6].Lee SH, Tai CL, Chen SY, Chang CH, Chang YH, Hsieh PH. Elution and mechanical strength of vancomycin-loaded bone cement: in vitro study of the influence of brand combination. PLoS One 2016;11:e0166545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].van de Belt H, Neut D, Uges DR, Schenk W, van Horn JR, van der Mei HC, et al. Surface roughness,porosity and wettability of gentamicin-loaded bone cements and their antibiotic release. Biomaterials 2000;21:1981e7. [DOI] [PubMed] [Google Scholar]
- [8].Kee JR, Mears SC, Edwards PK, Bushmiaer M, Barnes CL. Standardization of acrylic bone cement mixing protocols for total knee arthroplasty results in cost savings. Orthopedics 2018;41:e671e5. [DOI] [PubMed] [Google Scholar]
- [9].Menge TJ, Koethe JR, Jenkins CA, Wright PW, Shinar AA, Miller GG, et al. Acute kidney injury after placement of an antibiotic-impregnated cement spacer during revision total knee arthroplasty. J Arthroplasty 2012;27:1221e7. [DOI] [PubMed] [Google Scholar]
- [10].Weiss BD, Weiss EC, Haggard WO, Evans RP, McLaren SG, Smeltzer MS. Optimized elution of daptomycin from polymethylmethacrylate beads. Antimicrob Agents Chemother 2009;53:264e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Weiss EC, Spencer HJ, Daily SJ, Weiss BD, Smeltzer MS. Impact of sarA on antibiotic susceptibility of Staphylococcus aureus in a catheter-associated in vitro model of biofilm formation. Antimicrob Agents Chemother 2009;53:2475e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Weiss EC, Zielinska A, Beenken KE, Spencer HJ, Daily SJ, Smeltzer MS. Impact of sarA on daptomycin susceptibility of Staphylococcus aureus biofilms in vivo. Antimicrob Agents Chemother 2009;53:4096e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Meeker DG, Beenken KE, Mills WB, Loughran AJ, Spencer HJ, Lynn WB, et al. Evaluation of antibiotics active against methicillin-resistant Staphylococcus aureus based on activity in an established biofilm. Antimicrob Agents Chemother 2016;60:5688e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Anagnostakos K, Meyer C. Antibiotic elution from Hip and knee acrylic bone cement spacers: a systematic review. Biomed Res Int 2017:4657874. [DOI] [PMC free article] [PubMed]
- [15].Brunner E, Domhof S, Langer F. Nonparametric Analysis of Longitudinal Data inFactorial Experiments. New York: John Wiley & Sons; 2002. [Google Scholar]
- [16].Bishop AR, Kim S, Squire MW, Rose WE, Ploeg HL. Vancomycin elution, activity and impact on mechanical properties when added to orthopedic bone cement. J Mech Behav Biomed Mater 2018;87:80e6. [DOI] [PubMed] [Google Scholar]


