Abstract
Background and aim of the work:
since 2003, a series of so called ‘micro-posterior’ approaches have been developed in the orthopaedic surgery to perform total hip replacement. These techniques present several theoretical advantages compared to the classic postero-lateral approach: reduction in blood loss, post-operative pain, and length of stay but also improving functional outcomes. In our hypothesis these goals could also be obtained in patients with femoral neck fractures, especially in the elderly with several comorbidities.
Methods:
In our series we performed 50 consecutive cemented hemiarthroplasties through SuperPATH approach. At the latest follow-up (FU) 41 patients were included in the study. Clinical and radiological evaluation was performed before the surgery and at the latest FU using VAS score, Harris Hip Score (HHS) and conventional AP and LL X-rays.
Results:
We didn’t report any intra-operative complication (i.e. periprosthetic fractures, vasculo-nervous injuries or dislocations) or any case with a dysmetria greater than 1 cm. Mean blood transfusion units were 0.8 during surgery (0 – 4) and 1,6 after surgery (0-4). We reported a mean length of stay of 9.7 days (range 7-15) mainly due to our patients’ high comorbidity rate. At the last one-year FU no cases of dislocation, infection and clinical or radiographical signs of prosthetic loosening were recorded. All our patients returned to the same activity level before femoral fracture occurred, according to HHS.
Conclusion:
The SuperPATH is a real minimally invasive approach with a low rate of intra- and post-operative complications. It is related to a reduced dislocation rate and potentially to a reduced infection rate compared to the conventional surgical approaches, allowing, furthermore, a faster functional recovery. According to our experience the advantages of this type of approach can be obtained in the trauma field too. (www.actabiomedica.it)
Keywords: femoral neck fracture, hip replacement, hemiarthroplasty, superpath, percutaneously assisted total hip
Introduction
In developed countries, the number of hip replacements has rapidly increased throughout the 21th century. The mean hip replacement rate increased by 35% between 2000 and 2013. [1] Worldwide it has been estimated that every year 959,000 between primary and revision total hip procedures are performed. Total Hip Arthroplasty (THA) has an incidence of 131 procedures per 100,000 population, with a mean revision rate of 12.9% [2]. This trend is mainly due to the population ageing and, according to the lengthening of life expectancy, we await an exponential increase of primary and revision THA in the next future. [3]
Over the years, the surgical technique has considerably evolved, looking for new surgical accesses designed to provide specific advantages in different clinical scenarios. The trans-trochanteric approach, as first described in literature, facilitates joint exposure, but it is also related with several intra- and post- operative complications including greater trochanter non-union. For this reason, this approach was gradually replaced by other more conservative and tissue-sparing ones, such as the anterior, antero-lateral, lateral and postero-lateral approaches. Nowadays the best Surgical approach in THA is debated among orthopedic surgeons, and any of the aforementioned approaches presents its advantages and disadvantages. High-quality clinical reports about these approaches are lacking in current literature, so most of the surgeons use to perform the approach they consider to be the most confidential. [4]
In the last 15 years, several minimally invasive surgical techniques have been described with controversial results. Most of them present a limited skin incision without an actual tissue sparing, having a negative impact on the global results. It is important to distinguish ‘skin minimally invasive’ approaches, an evolution of the traditional approaches with less incision extension, from ‘true minimally invasive’ ones, performed without major sacrifice of soft tissues and, in some cases, bone. [5]
The rational and the main features of Tissue Sparing Surgery (TSS) concept are maximum respect of anatomy, restoration of joint biomechanics and removal of degenerated tissues, preserving the healthy ones. So, the prosthesis should just ‘integrate’ the joint instead of substitute it. The purposes of these techniques are to reduce blood loss, post-operative pain and hospital length of stay while improving rehab and functional results. [6,7]
The most TSS approaches reported in literature are the anterior approach (Smith-Petersen), Watson-Jones approach (modified by Rottinger), MIS-2 approach according to Berger (modified by Irving) and micro-posterior approaches (i.e. PATH, SuperCap, SuperPath).
The “micro-posterior approaches” have been developed since 2003 and they include the Percutaneously Assisted Total Hip (PATH) approach [8], the Supercapsular (SuperCap) approach [9] and the hybrid Supercapsular Percutaneously Assisted Total Hip (SuperPATH) approach. [10] They represent a continuum from a standard posterior-lateral approach to a ‘mini posterior approach’ (external rotators sacrificing) to a ‘micro posterior approach’ (external rotators sparing). This feature should keep the surgeon within his comfort zone during the procedure’s learning curve, leaving more options for complicated reconstructions with the possibility to convert the micro-posterior to a more confident classic posterior one. [10]
In this study we analyzed our experience performing hemiarthroplasties using the SuperPATH technique in the treatment of femoral neck fractures.
Methods
This single-center study was approved by our local ethical committee and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All patients gave written consent.
Fifty consecutive patients affected by medial proximal femur fracture were treated with SuperPATH surgical approach and all the procedures were performed by the same expert orthopedic surgeon (MS) without any intra-operative navigation.
Most of the patients included in this study were female (80%) with a mean age at surgery of 86 years. The mean BMI at surgery was 25.5 (range 21.5-34.8). Fracture site was subcapital in 68%, transcervical in 22% (fig. 1), basicervical in 8% and pertrochanteric in one case. Cement fixation was performed in every replacement, as suggested by NICE guidelines. [11] All the patients were treated with the same prosthetic device (Microport PROFEMUR® Gladiator with modular neck and bipolar head).
Fig. 1.
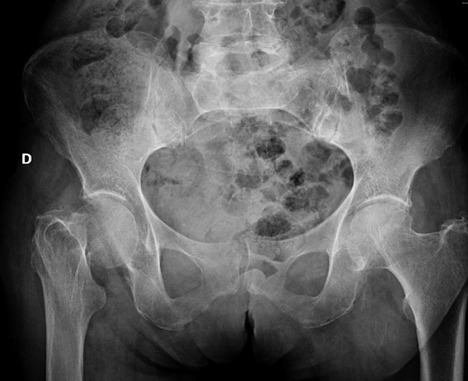
Femoral neck fracture at pelvis x-ray
Blood units administered to our patients during and after surgery were recorded, as well as surgical time, peri-operative complications, hospital length of stay, leg length discrepancy, and x-ray measurements.
Clinical and radiological evaluations were performed at 1, 3, 6 and 12 months after surgery. (fig. 2). At the latest FU, 41 Patients were included in the study because 9 patients died for other comorbidities. Clinical evaluation was performed using VAS score, Harris Hip Score (HHS), patient satisfaction and also evaluating the presence of complications.
Fig. 2.
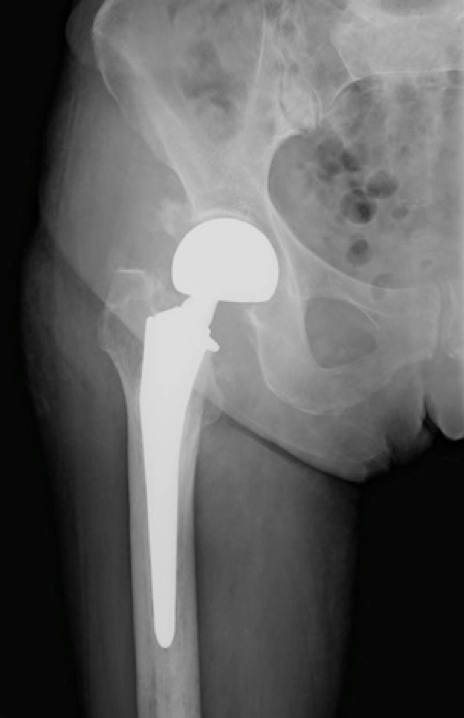
Radiographic follow-up at 6 months after surgery
AP and LL views x-rays were performed for instrumental FU.
Surgical Technique
Digital pre-operative surgical planning is performed to determine stem size, neck length and femoral head size before the surgery.
Patient is placed in a lateral decubitus position, with the operative hip at 45° of flexion, 15° of internal rotation and in maximal adduction.
Skin incision is performed from the tip of the greater trochanter and extended proximally in line with femur shaft axis for a length of 6 to 8 cm. (fig. 3). Generally, a longer exposure is required for overweight patients. The muscular fascia is opened in line with skin incision to expose the gluteus maximus that is carefully splitted in line with its fibers to expose the underlying gluteus medius. The gluteus medius muslce is then retracted anteriorly to show both piriformis tendon and gluteus minimus. Subsequently, the piriformis is spared. Using the interval between the gluteus minimus and the piriformis, a Hohmann retractor is placed under the gluteus minimus, over the anterior edge of the acetabulum. Slightly lifting the knee to reduce the tension on the piriformis, a second retractor is placed beneath the piriformis to protect it. Then the capsule is incised superiorly from the trochanteric fossa proximally to the superior acetabular rim at approximately 12 o’clock position. A blunt Hohmann retractor is placed between the posterior capsule and the posterior femoral neck, and a second Hohmann was disposed between the anterior capsule and the anterior femoral neck; the femoral neck is fully exposed, and the fracture can know be visualized. The femoral head is left in place and the femoral neck fracture is re-aligned in order to have a proper guide for the version of the femoral neck. Moreover, leaving the femoral head and neck in situ during this part of the procedure, we provide stability to the femur during the broaching phase. The femoral canal is opened through the trochanteric fossa with a cylindrical reamer. Then the femur is prepared with progressive broaches. It is broached up to the final size and at this point a one size smaller broach is positioned inside the femoral canal. Using the top of the broach as a cutting guide, the femoral neck osteotomy is completed. The femoral head is then removed using a Shanz Screw and the final cemented stem is placed into the femoral canal. Then a bipolar femoral head is inserted into the acetabulum and the prosthesis implant is reduced. (fig. 4). At this point the capsule is sutured and gluteus minimus and medius are returned to their native positions removing the retractors. Finally, fascia, subcutaneous and skin are sutured.
Fig. 3.
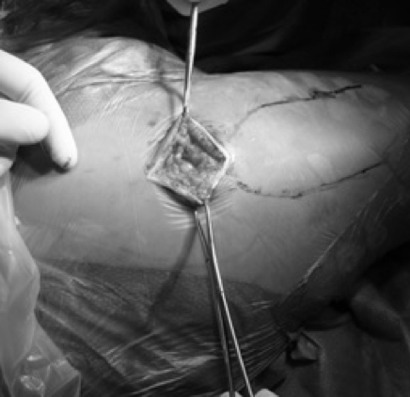
Skin incision from the tip of greater trochanter extended proximally in line with femoral shaft axis
Fig. 4.
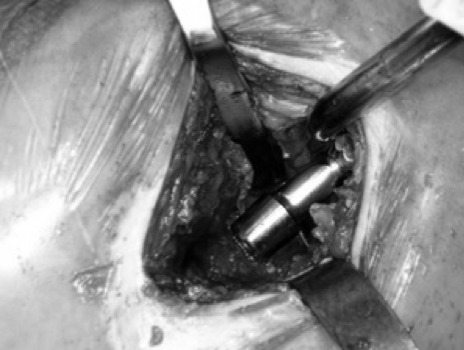
Definitive component positioning
Weight-bearing and active mobilization is allowed the day after surgery. [9]
Results
We didn’t report any case of intra-operative and post-operative complication (i.e. periprosthetic fractures, vasculo-nervous injuries, dislocations).
Femoral head size diameter was 28 mm in 56% of our patients, 36 mm in 26% and 22,25 mm in the remaining 18%. A modular neck was always used, 80% straight and 20% with 8° var/val. We didn’t have any case of lower limb dysmetria (more than 1 cm). Only 56% of our patients underwent surgery within 48 hours from their hospital admission, due to critical comorbidities that required a multidisciplinary clinical evaluation. Subarachnoid anesthesia was provided in the 76% of our cases. The others underwent general anesthesia, or (in one case) spinal anesthesia that was converted in a general one. The mean surgery time was 95 minutes (range:60 – 125). The mean value of blood transfusions was 0.8 units during surgery (0 – 4) and 1,6 after surgery (0-4). The average length of the stay was 9.7 days (range 7-15) due to the patients’ high comorbidity rate. At one year of FU, no cases of dislocation, infections and clinical or radiographical signs of prosthetic loosening were recorded. All patients returned to the their before-fracture activity level according to HHS. Before femoral neck fracture, the mean HHS was 73,4 while 1 year after surgery it was 83,4. As we already referred, at the latest FU we didn’t report any case of hip pain directly related to the surgical procedure - except for one patient who referred occasional mild pain (VAS 3/10) - and there were no cases of dislocation, infection or aseptic loosening.
Discussion
Micro-posterior approaches were originally designed and are generally used for primary elective hip replacement. These techniques present several theoretical advantages compared to the classic postero-lateral approach: blood loss, post-operative pain and length of stay are reduced, while rehab and functional outcomes are increased. In their series Chow et al. reported a mean hospital stay of 1.7 days with a low complications rate and radiographic results comparable to the ones obtained with other standard approaches [10].
Another multicentric study, made of 479 primary THA implanted using the SuperPATH technique, showed a low complications rate, decreased blood loss and lower blood transfusions compared to the conventional approaches. Moreover, authors reported a reduced readmission rate within 30 days from surgery. [12]
Rasuli et al. described relatively low length of stay and complications rate despite surgeons’ learning curve. [13]
In our hypothesis, this muscle-sparing approach could also be used to treat femoral neck fractures performing a hemiarthroplasty. Above all, elderly patients, typically subjected to proximal femur fractures, could be the most advantaged from this surgical approach. It could decrease post-operative pain, blood loss and use of narcotics, improving, on the other hand, the post-operative active mobilization. For these reasons, we may expect a reduction in femoral neck-related complications and hospital length of stay.
These fractures are common in elderly patients and are related to a high mortality rate, up to 29% at 1 year from injury. [14] Surgical complications (i.e. implant failure, surgical site infection, loosening and dislocation) are strictly related to the post-operative mortality risk. [15]
In the minimally invasive approaches, also intraoperative peri-prosthetic fractures represent a potential complication. Arthritic and osteoporotic patients have an additional risk of periprosthetic fracture during stem implantation, mainly due to the limited surgical field visibility and the poor quality of the bone tissue. Broaching the proximal femur with the native neck/head left inside prevents this complication, but when the uncemented definitive stem is impacted, after femoral neck resection, this protective effect is lost. [16] This negative event didn’t occur in our series and we didn’t find a greater risk of femoral fracture compared to more extended approaches.
Implant dislocation represents another reported complication after a hemiarthroplasty. It has been reported a higher dislocation rate in patient with mental dysfunction: in this sub-population the risk increases from the standard 12% to a higher 32%. In these patients, dislocation events should be avoided and prevented, especially considering that severe cognitive dysfunctions are an independent risk factor for poorer overall functional outcomes. [17, 18] Only a few studies reported the use of the SuperPATH approach in the treatment of femoral neck fractures. In 2012 Han et al. reported a low dislocation rate after bipolar hemiarthroplasty via the modified minimally invasive posterior approach [19]. Badrogi et al. reviewed 17 patients with femoral neck fractures treated with this technique. Due to its minimal dissection and lack of hip dislocation, they observed a reduced blood loss, a decreased postoperative pain, and the possibly of a quicker return to patients’ normal activity of daily living. [16] Recently Mitchell et al. described the SuperPATH as a safe and effective technique to treat displaced femoral neck fragility fractures, allowing their patients to early return to function without an increased risk of dislocation. [20] The results reported in our study support these data. We didn’t report any intraoperative complication or dislocation event 1 year after surgery. Our results in terms of blood loss and length of stay are comparable to the ones reported in the recent literature. [21,22]
Regarding HHS, we have to underline how this score was originally designed for the primary THA and not for the hemiarthroplasty in case of femoral neck fractures. Nevertheless, in our series, it was employed as a measure instrument to evaluate patients’ return to their pre-fracture activity level.
The decreased iatrogenic muscular injury due to TSS could potentially reduce patients’ postoperative pain enhancing their post-operative mobilization [16]. The small exposure should also reduce the risk of infection, even if more studies are needed to confirm this hypothesis.
In conclusion the SuperPATH represents a real minimally invasive approach with a low rate of intraoperative bone and neurovascular complications. It is related to a lower dislocation rate, blood transfusions and infection risk compared to the most common surgical approaches. This approach allows a good exposure during the implant positioning and it can be extended, where necessary, into the conventional posterior-lateral approach. Patients are allowed to a progressive weight bearing without any restriction of active and passive movements. Usually, they can walk with crutches just a few hours after surgery. Tissue sparing also allows a faster functional recovery because of capsule integrity and proprioception conservation. In our opinion the SuperPATH approach represents a valid surgical option available in traumatology, with a low rate of complications and surgical morbidity.
Conflict of Interest
Each author declares that he has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- 1.McPherson K, Gon G, Scott M. International variations in a selected number of surgical procedures. OECD Health Working Papers, No. 61. OECD Publishing [Google Scholar]
- 2.Kurtz SM, Roder C, Lau E, et al. International survey of primary and revision total hip replacement. Paper #365. Presented at the 56th Annual Meeting of the Orthopaedic Research Society. March 6-9, 2010. New Orleans [Google Scholar]
- 3.Pabinger C, Geissler A. Utilization rates of hip arthroplasty in OECD countries. Osteoarthritis Cartilage. 2014 Jun;22(6):734–41. doi: 10.1016/j.joca.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Petis S, Howard JL, Lanting BL, Vasarhelyi EM. Surgical approach in primary total hip arthroplasty: anatomy, technique and clinical outcomes. Can J Surg. 2015 Apr;58(2):128–20. doi: 10.1503/cjs.007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Imporzano M, Pierannunzii L. Minimally invasive total hip replacement. J Orthopaed Traumatol. 2006;7:42–50. [Google Scholar]
- 6.Pipino F. Tissue-sparing surgery (TSS) in hip and knee arthroplasty. J Orthop Traumatol. 2006;7:33–35. [Google Scholar]
- 7.Rossi P, Castoldi F, Rossi R, Caranzano F, Baronetti M, Dettoni F. TSS and traditional surgery in hip and knee replacement. J Orthop Traumatol. 2007 Sep;8(3):157–20. [Google Scholar]
- 8.Penenberg BL, Bolling WS, Riley M. Percutaneously assisted total hip arthroplasty (PATH): a preliminary report. J Bone Joint Surg Am. 2008;90(Suppl 4):209–20. doi: 10.2106/JBJS.H.00673. [DOI] [PubMed] [Google Scholar]
- 9.Murphy SB. Tissue –Preserving, minimally invasive total hip arthroplasty using a superior capsulotomy. In: Hozack W, editor. Minimally invasive total hip and knee arthroplasty. Springer-Verlag; 2004. pp. 1001–107. [Google Scholar]
- 10.Chow J, Penenberg B, Murphy S. Modified micro-superior percutaneously-assisted total hip: early experiences & case reports. Curr Rev Musculoskelet Med. 2011;4:146–50. doi: 10.1007/s12178-011-9090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hip fracture: management. NICE guidance (CG124). Available from: http://nice.org.uk/guidance/cg124. Accessed 03 April 2017. [Google Scholar]
- 12.Gofton W, Chow J, Olsen KD, Fitch DA. Thirty-day readmission rate and discharge status following total hip arthroplasty using the supercapsular percutaneously-assisted total hip surgical technique. Int Orthop. 2015 May;39(5):847–20. doi: 10.1007/s00264-014-2587-4. [DOI] [PubMed] [Google Scholar]
- 13.Rasuli KJ, Gofton W. Percutaneously assisted total hip (PATH) and Supercapsular percutaneously assisted total hip (SuperPATH) arthroplasty: learning curves and early outcomes. Ann Transl Med. 2015 Aug;3(13):179. doi: 10.3978/j.issn.2305-5839.2015.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haleem S, Lutchman L, Mayahi R, Grice JE, Parker MJ. Mortality following hip fracture: trends and geographical variations over the last 40 years. Injury. 2008 Oct;39(10):1157–20. doi: 10.1016/j.injury.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Lin JC, Liang W. Mortality and complications after hip fracture among elderly patients undergoing hemodialysis. BMC Nephrol. 2015;16:100. doi: 10.1186/s12882-015-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodrogi AW, Sciortino R, Fitch DA, Gofton W. Use of the supercapsular percutaneously assisted total hip approach for femoral neck fractures: surgical technique and case series. J Orthop Surg Res. 2016;11:113. doi: 10.1186/s13018-016-0446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enocson A, Hedbeck C, Tidermark J, Pettersson H, Ponzer S, Lapidus LJ. Dislocation of total hip replacement in patients with fractures of the femoral neck - A prospective cohort study of 713 consecutive hips. Acta Orthop. 2009 Apr 29;80(2):20–21. doi: 10.3109/17453670902930024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Söderqvist A, Miedel R, Ponzer S, Tidermark J. The influence of cognitive function on outcome after a hip fracture. J Bone Joint Surg (Am) 2006;88(10):2115–23. doi: 10.2106/JBJS.E.01409. [DOI] [PubMed] [Google Scholar]
- 19.Han SK, Kim YS, Kang SH. Treatment of femoral neck fractures with bipolar hemiarthroplasty using a modified minimally invasive posterior approach in patients with neurological disorders. Orthopedics. 2012 May;35(5):e20–21. doi: 10.3928/01477447-20120426-15. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell R, Smith K, Murphy S, Le D. Early results of displaced femoral neck fragility fractures treated with supercapsular percutaneous assisted total hip arthroplasty. Orthopaedic Proceedings. Published 1 March 2017 doi: 10.1016/j.artd.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frihagen F, Nordsletten L, Madsen JE. Hemiarthroplasty or internal fixation for intracapsular displaced femoral neck fractures: randomised controlled trial. BMJ. 2007;335(7632):1251–1254. doi: 10.1136/bmj.39399.456551.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blomfeldt R, Törnkvist H, Eriksson K, Söderqvist A, Ponzer S, Tidermark J. A randomised controlled trial comparing bipolar hemiarthroplasty with total hip replacement for displaced intracapsular fractures of the femoral neck in elderly patients. J Bone Joint Surg Br. 2007 Feb;89(2):160–20. doi: 10.1302/0301-620X.89B2.18576. [DOI] [PubMed] [Google Scholar]


