To the editor:
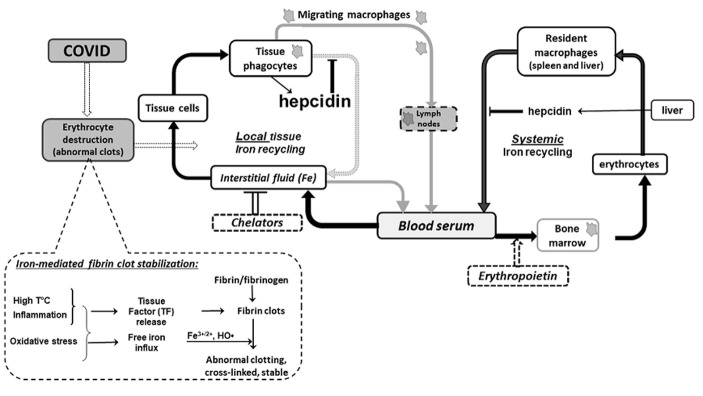
In line with excellent review recently published in Acta Biomedica (1) I would like to support an idea regarding detrimental role of iron overload in COVID19-induced complications versus beneficial role of hepcidin aimed to overcome the ironinduced pathology. A core of COVID19-induced complications (acute respiratory distress syndrome, multiorgan failure, Kawasaki-like disease etc.) is iron-induced abnormal coagulation in small vessels and capillaries (2). Without these complications the COVID-19 infection could be less dangerous than a flu is. The miraculous case described by (3) may be explained already by iron withdrawal from lungs via stimulation of erythropoiesis (4) presumably followed by a removal of excess iron by migrating macrophages. Figure 1 illustrates cause-event relations between body demand to remove iron from the damaged organ and two basic ways of this task solution in which hepcidin is a major player.
Figure 1.
The main function of hepcidin in COVID-induced pathology presumably is to preserve iron-induced abnormal coagulation in affected organ by blocking local iron recycling. Hepcidin temporary locks the accumulated iron inside of iron sequestrating cells thus preventing iron-mediated abnormal fibrine clot stabilization. Two ways of iron removal from damaged organ are presented: (1) transferrin-bound iron via body fluids circulation; (2) iron incorporated into ferritin inside of migrating iron-sequestrating macrophages. Temporal activation of iron withdrawal by either erythropoietin or phlebotomy may support iron turnover between infected organ and systemic iron homeostasis.
Briefly, it is vitally important to keep extracellular iron concentration in safe range as free iron ions are hazard in inflammatory conditions because of production of harmful hydroxyl radical via Fenton chemistry.
For this reason, iron homeostasis (FeH) is tightly regulated (5). Basically, safe concentration of free iron (0-0,3 mkM) is supported by transferrin-binding capacity of body fluids (blood, lymph and interstitial fluid). When iron efflux exceeds the transferrinbinding capacity, an expression of hepcidin, a master regulator of iron homeostasis, is induced locally in macrophages by inflammatory cytokines like IL-6. A physiological role of hepcidin is protecting. Mechanistically, hepcidin down-regulates the only iron exporter ferroportin to lock the iron inside cells, thus, blocking local iron recycling to prevent both tissue injury and abnormal coagulation. In injured tissues phagocytes sequestrate iron in various states (free iron, transferrin-bound iron, damaged erythrocytes, hemoglobin etc), accumulate it safely inside of macrophages in ferritin complex. Therefore, hepcidin does not allow the cells to release iron. These steps must be followed by a removal of the iron overloaded cells from injured tissue via lymphatics to bone nodes where they could be allowed to release the sequestrated iron into circulation.
When both iron-sequestrating capacity of transferrin and resident macrophages are overloaded the iron-induced pathology is enhanced leading to formation of proteolytically stable clots seen as ground glass opacity. Ferritin and hepcidin usually are co-expressed to function in concert.
In conclusion. Hereby, I tried to add additional arguments to the finding presented by (1). Surely, the complications of COVID19-induced pathology are related to disturbance of local FeH in damaged tissue.
Although deregulation of local FeH may be reflected in serological parameters (hepcidin, ferritin, total and free iron, transferrin, transferrin saturation in serum), these parameters measurements locally could be especially beneficial for monitoring disease progression and for prediction of susceptibility to the complication.
Because a disbalance of local FeH results from cytokine storm and results in abnormal coagulation (6), therefore, successful treatment would be possible via modulation of all the three systems (inflammation, coagulation and iron homeostasis) in their interaction via monitoring of dynamic changes and correlation between parameters of the linked physiological systems.
Author declare that she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Banchini F, Vallisa D, Maniscalco P, Capelli P. Iron overload and Hepcidin overexpression could play a key role in COVID infection, and may explain vulnerability in elderly, diabetics, and obese patients. Acta Biomed. 2020;91(3):e2020013. doi: 10.23750/abm.v91i3.9826. doi: 10.23750/abm.v91i3.9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sukhomlin T. Fibrinolysis Shutdown in COVID-19-Infected Patients Can Result from Iron-Induced Stabilization of Fibril Clots. J Am Coll Surg. 2020 doi: 10.1016/j.jamcollsurg.2020.08.170. S1072-7515(20)31499-X. doi: 10.1016/j.jamcollsurg.2020.08.170. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadadi A, Mortezazadeh M, Kolahdouzan K, Alavian G. Does recombinant human Erythropoietin administration in critically ill COVID-19 patients have miraculous therapeutic effects? J Med Virol. 2020;92(7):915–918. doi: 10.1002/jmv.25839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sukhomlin T. Could an acute respiratory distress syndrome in COVID-19 infected patients be calmed down simply by iron withdrawal from lung tissues? J Med Virol. 2020 doi: 10.1002/jmv.26372. doi: 10.1002/jmv.26372. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Coffey R, Ganz T. Iron homeostasis: An anthropocentric perspective. J Biol Chem. 2017;292(31):12727–12734. doi: 10.1074/jbc.R117.781823. doi: 10.1074/jbc.R117.781823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pretorius E, Vermeulen N, Bester J, Lipinski B, Kell DB. A novel method for assessing the role of iron and its functional chelation in fibrin fibril formation: the use of scanning electron microscopy. Toxicol Mech Methods. 2013;23(5):352–9. doi: 10.3109/15376516.2012.762082. [DOI] [PubMed] [Google Scholar]