Abstract
Background and aim of the work:
Patients undergoing elective primary total hip replacement and spinal anesthesia may encounter significant hemodynamic instability.
Objective:
The study is aimed at observing the haemodynamic modifications after spinal anaesthesia during total hip replacement in patients managed to “preload independence” with goal directed fluid therapy (GDFT) and monitored non-invasively with Clearsight.
Methods:
Thirty patients, aged 50-80 years, with an American Society of Anaesthesiologists’ (ASA) score II-III, undergoing elective primary total hip replacement and spinal anaesthesia were enrolled. Patients were monitored with the EV1000 platform and the Clearsight finger-cuff and managed with a goal directed fluid therapy.
Results:
The 79% of the population showed preload independence at baseline. After spinal, the 93% did not show a significant mean arterial pressure reduction. In our population, 79% reported a decrease >10% of the stroke volume during surgery, while 66% in the Recovery Room. Patients showed an improvement in mean arterial pressure, systemic vascular resistances indexed (SVRI), stroke volume (SV) and stroke volume indexed (SVI) at spinal resolution compared to baseline.
Conclusions:
Our cohort population showed hemodynamic stability throughout the study period, with increased SV and decreased SVRI at spinal resolution compared to basal values. Further randomized prospective studies are advocated in the same setting. (www.actabiomedica.it)
Keywords: hemodynamic, monitoring, intraoperative, anesthesia, spinal, arthroplasty, replacement, hip, blood pressure monitors, stroke volume
List of abbreviations:
- GDFT:
goal directed fluid therapy
- ASA:
American Society of Anesthesiologists
- SV:
stroke volume
- SVI:
stroke volume indexed
- SVRI:
systemic vascular resistances indexed
- CO:
cardiac output
- CI:
cardiac index
- SpO2:
pulse oxymetry
- ECG:
electrocardiography
- NIBP:
non-invasive blood pressure
- SAP:
systolic arterial pressure
- MAP:
mean arterial pressure
- DAP:
dyastolic arterial pressure
- HR:
heart rate
- SVbas, SVIbas, HRbas, CObas, CIbas, SVRIbas:
basal values
- SVopt, SVIopt, HRopt, COopt, CIopt, SVRIopt:
values at maximal optimization
- SVlt1, SVIt1, HRt1, COt1, CIt1, SVRIt1:
values immediately before maximal optimization
- SVlt2, SVIt2, HRt2, COt2, CIt2, SVRIt2:
trigger values after spinal
- RBC:
red blood cells
- RR:
recovery room
1. Introduction
In the perioperative period, patients undergoing elective primary total hip replacement (THR) may encounter significant blood losses and therefore hemodynamic instability (1). A targeted Goal Directed Fluid Therapy (GDFT) has been demonstrated to reduce the incidence of perioperative complications in orthopaedic surgery (2, 3). Non invasive hemodynamic monitoring represents a validated alternative to invasive methods of blood pressure curve monitoring and analysis which, though rare, are not exempt from complications (4, 7). The Clearsight (Edwards Lifesciences LLC One Edwards Way Irvine, CA, USA) registers continuously the patient’s arterial blood pressure curve by an inflatable cuff around the middle phalanx adopting the “volume clamp mehod” (8). The pulsating digital arteries are clamped to a constant volume by applying a varying counter pressure equivalent to the arterial pressure using a built-in photoelectric plethysmograph and an automatic algorithm (Physiocal). The resulting finger arterial pressure waveform is reconstructed into a brachial artery pressure waveform by a generalized algorithm. Cardiac Output (CO) is calculated by a pulse contour method using the measured systolic pressure time integral and the heart’s afterload determined from the Windkessel model (9). To our knowledge, very few studies investigating non-invasive hemodynamic monitoring in patients undergoing orthopedic surgery in regional anesthesia have been published.
The present study aims at observing the variations of principal hemodynamic parameters in patients undergoing spinal anesthesia for total hip replacement when treated with a goal directed fluid therapy algorythm and monitored with Clearsight.
2. Methods
The Local Research Ethics Committee approved this prospective, cohort study in February 2016. In the present study we have followed the Strobe guidelines for observational studies.
After written informed consent, we enrolled thirty patients with the following inclusion criteria: age between 50 and 80 years, an American Society of Anaesthesiologists’ (ASA) score 2 or 3, elective primary total hip arthroplasty to be performed under spinal anaesthesia. Exclusion criteria were contraindications to regional anaesthesia, peripheral vascular abnormalities (severe vascular diseases, Raynaud’s disease, arteriovenous fistula for hemodialysis, previous vascular surgery of the upper arms, advanced diabetes with neuropathy or vascular disease), atrial fibrillation, aortic regurgitation, ASA classes 4 and 5 and patients’ refusal.
All patients were transferred to the operating room between 7 AM and 4 PM after fasting both from fluids and food since midnight. At patient arrival in the operating room, vital signs were monitored [pulse oximetry (SpO2), three-lead electrocardiography (ECG) and non-invasive blood pressure (NIBP)]. Then, after selection of the appropriate size, the Clearsight finger cuff (Edwards Lifesciences, Milan, Italy) was applied to the II, III or IV middle phalanx of the hand controlateral to surgery. After initial calibration, we registered the basal values of systolic, diastolic and mean arterial blood pressure (SAP, DAP, MAP), stroke volume (SVbas) and stroke volume index (SVIbas), heart rate (HRbas), cardiac output (CObas), cardiac index (CIbas) and peripheral vascular resistances (SVRIbas) assuming a central venous pressure of 0 mmHg. After iv cannulation with a 16G cannula, we administered midazolam 0.05 mg/Kg of ideal weight [height (cm) - 100 (males); height (cm) - 110 (females)] and a 4 L/min flow of oxygen with ventimask.
Then we followed the algorithm described in Figure 1: a first rapid fluid challenge of 200 ml ringer acetate was infused in less than 5 minute and parameters were observed for 5 minutes after bolus completion to identify any significant SV increase from the basal value at patient’s arrival in the operating room (considered as an arbitrary SV increase ≥10%). In case of a significant raise in SV value ≥10%, the patient was considered fluid responder and another equivalent fluid challenge was administered until we did not encounter any further increase in SV value (fluid independency).
Figure 1.
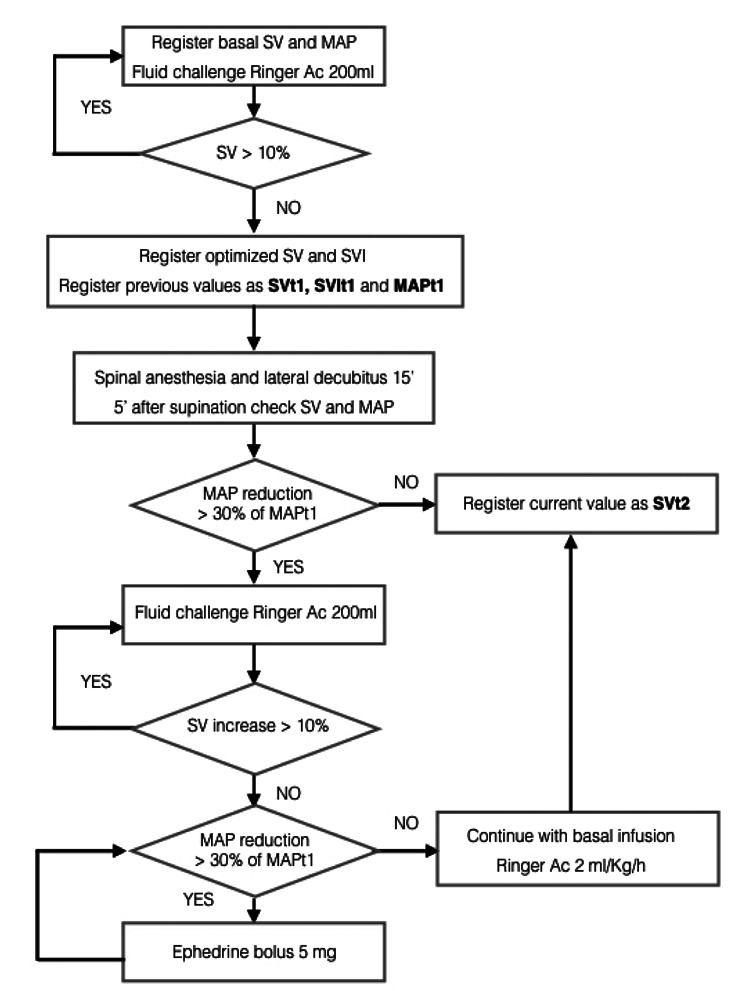
Patients were optimized before spinal anesthesia. Then target values “t1” and “t2” were registered before and after levobupivacaine 0.5% 0.25 mg/Kg (ideal weight). A basal infusion of Ringer Acetate was maintained at 2 ml/Kg/h
At this point, we registered the hemodynamic variables as optimized values. The stroke volume, stroke volume index and medium blood pressure values recorded before the last crystalloid intravenous bolus were therefore considered as target 1 (SVt1, SVIt1 and MAPt1). A basal continuous infusion of ringer acetate at 2 ml/Kg/h (ideal weight) was maintained throughout the entire protocol.
After that the patient was placed in lateral decubitus. Spinal anaesthesia was performed at the L3-L4 level with 0.25 mg/Kg (ideal weight) of 0.5% isobaric levobupivacaine. Hemodynamic variables before and after spinal were measured via the Clearsight fingercuff and registered.
Twenty minutes after spinal anaesthesia, once in supine position, if MAP did not decrease more than 30% from MAPt1, new observed values were named as “target 2” (MAPt2, HRt2, SVt2, SVIt2, CIt2, COt2, SVRIt2). In the case of a significant reduction of MAP below 30% of baseline, the SV and SVI were instead optimized as described in Figure 1.
Patients were then managed as described in Figure 2. Crystalloid infusions were managed according to the SV value: a decrease of more than 10% of the SVt2 triggered a 200ml ringer acetate bolus (Fig. 2). In cases of hypotension and fluid independency, then 5 mg of ephedrine were given.
Figure 2.
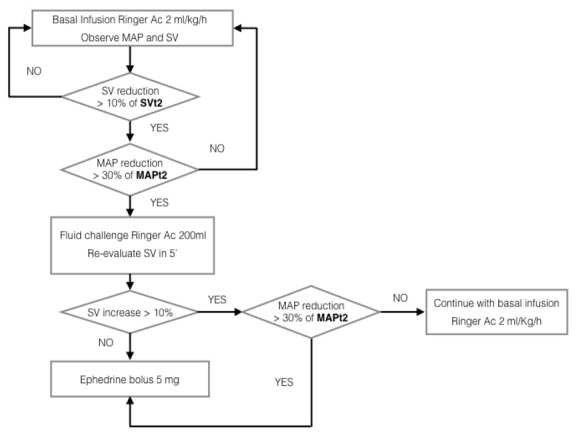
Intraoperative algorythm. Trigger for fluid challenges was SVt2 in cases of significant hypotension (MAP reduction > 30% of MAPt2)
At the end of surgery all patients were transferred to the Recovery Room where the Clearsight monitoring was continued and the algorithm in Fig. 2 applied.
A Pinprick test was administered every 15 minutes in Recovery Room to each patient and the time to resolution of sensory block to S1 level was registered. At the complete resolution of the lower limb motor block, evaluated with the Bromage’s scale, fluid therapy was managed as described in Figure 3. Patients’ final hemodynamic state was compared with values registered before spinal anesthesia.
Figure 3.
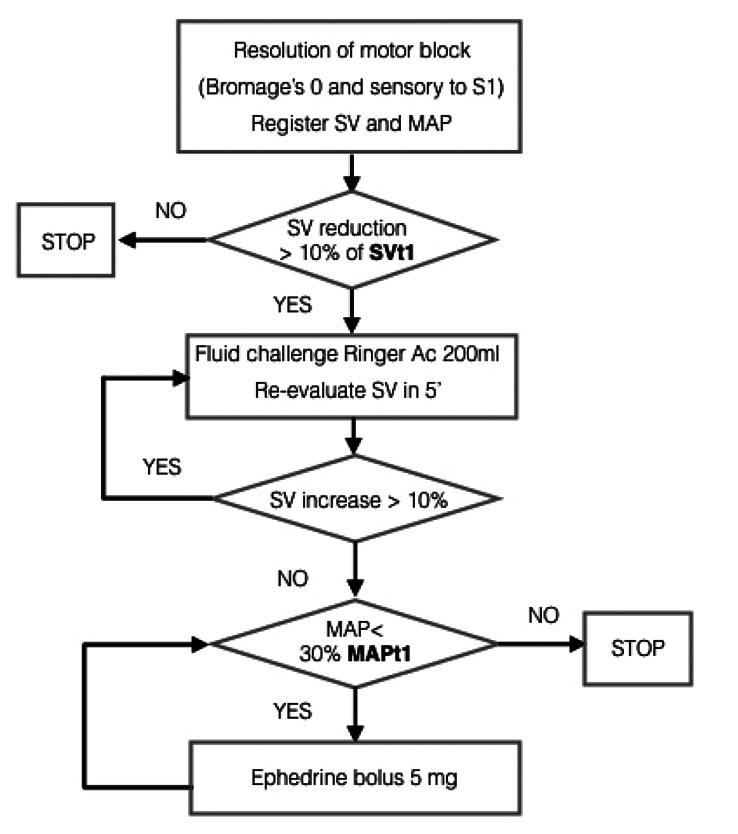
At resolution of spinal block, current SV was compared with SVt1 and treated accordingly.
At S1 sensory block resolution and before Recovery Room discharge, each patient bladder volume was scanned with ultrasound: a volume greater than 600 ml without spontaneous micturition was deemed indication to bladder catheterization. At sensory and motor block resolution (pinprick sensibility restored at S1 and Bromage’s 0) and fulfilment of discharge criteria from the Recovery Room all patients were transferred to the floor and the study completed.
A hematocrit ≤24% was considered trigger for transfusion of one unit of Red Blood Cells (RBC) and the event was registered.
At study completion we collected and stored each patient data from the EV1000 platform (Edwards Lifesciences, Milan, Italy) for further data analysis.
2.1 Statistical analysis
The primary endpoint of the study is the observation of SV variation at spinal anesthesia resolution compared to baseline. At spinal resolution, we hypothezised a reduction in SV >10% in 50% of the general population after primary elective hip arthroplasty under spinal anesthesia. Since we hypothezised a reduction of SV >10% at spinal resolution in 25% of patients undergoing our goal directed fluid protocol, considering a power of 0.80 and an alfa error of 0.05, we established to conduct our study in 29 patients. We enrolled one more patients to account for drop outs.
The Results were analyzed using Excel and SPSS 17.0 softwares for Windows. Kolmogorov-Smirnov test was used to confirm normality of distribution among the continuous variables. Data were compared by using repeated measures ANOVA with Bonferroni correction and are reported as mean ± standard deviation (SD). Continuous variables not normally distributed are represented with median (IQR). P ≤0.05 was set as statistically significant. We performed a per-protocol analysis of observed data.
3. Results
Demographic characteristics are summarised in Table 1. Basal hemodynamic data are presented in Table 2. The mean basal infusion of i.v. crystalloids was 122 (80-180) ml/h, while the dose of sedation before spinal anesthesia was 3 (2-4) mg of midazolam i.v., both calculated on ideal body weight.
Table 1.
Anthropometric and demographic characteristics of the study cohort
| Women [n (%)] Men [n (%)] |
19 (66%) 10 (34%) |
| Age, years [mean (range)] | 68 (50-80) |
| Weight, Kg [mean (range)] | 73 (42-104) |
| Height, cm [mean (range)] | 165 (150-183) |
| BMI, Kg/m2 [mean (range)] | 27 (16-37) |
| ASA II [n (%)] ASA III [n (%)] |
2 (83%) 3 (17%) |
Table 2.
Basal arterial pressure and oximetry in the cohort population
| Systolic pressure, mmHg [mean (range)] | 146 (117-199) |
| Diastolic pressure, mmHg [mean (range)] | 80 (54-95) |
| Mean pressure, mmHg [mean (range)] | 106 (85-140) |
| SpO2, % [mean (range)] | 97 (90-100) |
After registration of basal hemodynamic values, we administered the first fluid challenge: 23 patients (79%) did not respond to the first 200 ml of crystalloid infusion and were therefore judged as preload-independent. Among the remaining 6 patients (21%), 5 needed a second fluid challenge of 200 ml and 1 patient needed two extra boluses (total of 400 ml bolus). No patient required ephedrine. Data after optimization and comparison with basal hemodynamic values are reported in Table 3.
Table 3.
Hemodynamic variables in the study population at baseline (B), after initial optimization (O), 20 minutes after spinal anesthesia in supine position (S), at entrance in the Recovery Room (RR), at the end of the study (F): p (O vs B) = significativity of the comparison between optimized and basal values; p (S vs O) = significativity of the comparison of values after spinal versus after optimization; p (RR vs O) = significativity of the comparison of values between Recovery Room admission and optimization; p (F vs B) = significativity of the comparison between the end of the study and baseline
| Basal (B) | Optimized (O) | Supine (S) | Recovery (RR) | Final (F) |
p (O vs B) |
p (S vs O) |
p (RR vs O) |
p (F vs B) |
|
| MAP, mmHg | 106±12 | 109±15 | 94±15 | 88±14 | 90±14 | 1.000 | < 0.0001 | < 0.0001 | < 0.0001 |
| FC, bpm | 70±11 | 72±11 | 69±12 | 66±11 | 71±11 | 1.000 | 0.558 | 0.086 | 1.000 |
| SV, ml | 65±13 | 68±15 | 65±16 | 63±16 | 70±16 | 0.163 | 0.492 | 0.358 | 0.038 |
| SVI, ml/m2 | 36±5 | 37±7 | 35±7 | 35±8 | 38±7 | 0.063 | 0.013 | 0.897 | 0.022 |
| CI, L/min/m2 | 2.6±0.7 | 2.8±1.3 | 2±0.6 | 1.9±0.7 | 2.4±0.8 | 1.000 | 0.264 | 0.131 | 0.713 |
| CO, L/min = | 4.7±1.3 | 4.7±1.2 | 3.9±1.2 | 5.1±7.8 | 4.6±1.4 | 1.000 | 0.263 | 1.000 | 1.000 |
| SVRI, dyn*s*m-2*cm-5 | 3627±1156 | 3533±1022 | 3307±768 | 3312±1111 | 2893±986 | 1.000 | 0.346 | 0.646 | 0.003 |
Patients received a mean dose of 15 (11.5 20) mg of isobaric levobupivacaine 0.5% for spinal anesthesia in lateral decubitus, as per protocol. After performance of spinal, patients were turned supine. Hemodynamic variables in supine position after spinal anesthesia and comparison with values after optimization are presented in Table 3. The maximum dermatomeric level of spinal anesthesia evaluated 20 minutes after spinal was T10 (T8-L1).
After spinal, 27 patients (93%) did not show a significant MAP reduction (>30% compared to baseline) and did not require further crystalloid boluses. Their SV and SVI values were set as SVt2 and SVIt2 at this timepoint (20 minutes after spinal injection). No patient needed ephedrine.
During surgery, 23 patients (79%) reported a reduction greater than 10% of SV when compared to SVt2 value, versus 6 patients who did not (21%). The mean intraoperative volume of infused crystalloid was 508 (92-957) ml. Due to hypotension beyond 30% of basal value, 2 patients (6.9%) who did not respond to fluid challenge required 5 mg of ephedrine and 2 patients (6.9%) required 10 mg of ephedrine intraoperatively.
Hemodynamic variables at Recovery Room entrance and at the end of the study in comparison with both basal values and those optimized are reported in Table 3.
During patient stay in the Recovery Room, 66% of patients showed a decrease in SV >10% when compared to SVt2. One bolus of ephedrine (5 mg) was necessary in 10% of cases, while one patient required 3 ephedrine boluses (total dose of 15 mg) in Recovery Room. The mean total volume of fluids administered during the entire study period was 1705 (700-4000) ml.
Spinal anesthesia had a mean duration of 5.6 (4.1-8.9) hours. At motor block resolution (Bromage=0), 23 patients (79%) did not show a reduction in SV greater than 10% of SVt1. Mean MAP value at resolution of spinal block (MAPf) was 90±14 mmHg versus 106±12 mmHg at baseline (p<0.0001). SV at the end of spinal block was increased compared to baseline SV (70±16 ml vs 65±13 ml, p=0.038). SVRI were significanlty reduced at the resolution of spinal block compared to basal value (2893±986 vs 3627±1156 dyn*s*m-2*cm-5, p=0.003). Patients’ mean blood loss collected in the surgical drainage at the end of study (resolution of motor block and sensory block to S1) was 290 (20-750) ml. Two patients (7%) received one unit of RBC in the Recovery Room for hematocrit values ≤24%. Five patients (17%) required a bladder catheter in Recovery Room.
4. Discussion
The present study shows comparable values of SV throughout the protocol in patients monitored non-invasively with Clearsight and treated with a GDFT protocol during elective primary hip replacement and spinal anesthesia. The values of SV and SVI significantly improved at spinal block resolution when compared to baseline values.
Some other interesting observations can be drawn from this cohort population. First of all, at the arrival in the anesthesia induction room, 79% of patients were fluid non-responder, while 17% needed 400 ml and 4% needed 600 ml for optimization. These results are consistent with current literature: most of patients scheduled for elective surgery are still normovolemic after fasting over night, while about 15% of patients may present a bigger deficit (≥400 ml) (10, 11).
Secondly, SVRI were higher than the physiological range 1970 - 2390 dyn*s*m-2*cm-5 both before and after spinal anaesthesia (mean values SVRIba =3627±1156 and SVRIsup=3307±768 dyn*s*m-2*cm-5, respectively), maybe due to relative hypothermia after being transferred from the floor and to the sympathetic hyperactivity caused by stress in patients scheduled for surgery. The mean value of SVRI decreased significantly at complete resolution of motor block and regression of sensory block to S1, when compared to baseline (2893±986 versus 3627±1156 dyn*s*m-2*cm-5, p=0.003). The significant reduction of SVRI could be a consequence of patient warming throughout the study period and reduced stress when compared to preoperative condition, although a residual sympathetic block cannot be excluded.
At arrival in Recovery Room as well as at spinal resolution we observed a significant reduction of MAP when compared to optimized values, while other hemodynamic variables were comparable. However, at spinal resolution the mean MAP value returned within its physiological range, below 100 mmHg showing therefore an improvement if compared to baseline. The intraoperative algorythm described in Figure 2 allowed therefore to maintain a good stability of hemodynamic variables throughout the surgical procedure.
The intraoperative volume of crystalloids was 508 ml (range 92-957) ml with a medium basal infusion of 121 ml/h (range 80-180). The wide range of volume administered during the procedure indicates a goal-directed approach rather than a simply restrictive fluid administration. Two patients (7%) received one unit of RBC in the Recovery Room for hematocrit values ≤ 24%. A prospective, randomized study will be necessary to compare the incidence of transfusion in our cohort versus cases of liberal fluid administration based on MAP values.
The study presents some limitations. First of all, it is based on the observation of a limited number of patients. A randomized controlled trial would have had a stronger impact, although the paucity of previous data in literature in the same setting required a first investigation to assess the hemodynamic response to the goal directed fluid algorythm here applied in spinal anesthesia. The second major pitfall is that we did not report protocol compliance, although all 30 cases were conducted by the same anesthesia team (D.G., M.G.) in a short period of time (March-May 2016), helping in reducing variability in the reproduction of the study protocol. We also set specific alarms on the EV1000 monitor for each patient to make sure that every reduction of at least 10% in the SV, compared to the SVt2, triggered a fluid challenge as requested by the protocol. The third major study limitation is that although we accurately monitored hemodynamic variables throughout the study period, we did not report eventual intraoperative acute blood losses, so that it was not possible to correlate hypotension or SV reduction with associated events such as hemorrhage. Nevertheless, blood losses were modest and no acute event of severe entity was observed in our 30 patient population. Surgical procedures were all performed by the same surgical team with a minimally invasive approach to have a more reproducible surgical technique with reduced blood losses. Another limitation may be that our main results were not unexpected. On the other hand, very few studies have been conducted in the same setting applying the Clearsight monitoring and specific goal directed approach in elective major surgery performed under spinal anesthesia.
In our opinion, the interesting result of the study is that patients returned to the floor with a better hemodynamic status than the preoperative, after a major orthopedic procedure which is currently performed worldwide also in fragile patients. This hemodynamic stability in ASA II and III patients has encouraged us to design a prospective randomized trial in a larger sample comparing the algorythm here applied with a liberal fluid conduct.
Another strength point is that an invasive hemodynamic monitoring is not commonly applied in this surgical population, although surgery may be complex, blood losses may be significant and therefore patients’ hemodynamic may be unstable. The opportunity to monitor the patient in a non-invasive fashion allowed us to observe the hemodynamic variables in a reliable and reproducible manner: results were expected but still interesting and not fully described before in the same minimally invasive and accurate way.
In conclusion, following the GDFT algorythms described in Figure 1, 2 and 3 and guided by non-invasive hemodynamic monitoring, we observed a good hemodynamic stability in our cohort population throughout the study period with a significant improvement in MAP, SVRI, SV and SVI at spinal resolution compared to baseline, together with a restrained incidence of blood transfusion. Further randomized prospective studies are advocated to test if SV monitoring and GDFT would improve clinical outcomes when compared to liberal fluid administration guided solely by MAP monitoring in patients undergoing primary elective hip replacement under spinal anesthesia.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Dan M, Liu D, Martos SM, Beller E. Intra-operative blood salvage in total hip and knee arthroplasty. J Orthop Surg. 2016;24:204–8. doi: 10.1177/1602400217. [DOI] [PubMed] [Google Scholar]
- 2.Habicher M, Balzer F, et al. Implementation of goal-directed fluid therapy during hip revision arthroplasty: a matched cohort study. 2016;5:31. doi: 10.1186/s13741-016-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cecconi M, Fasano N, Langiano N, et al. Goal-directed haemodynamic therapy during elective total hip arthroplasty under regional anaesthesia. Crit Care. 2011;15:R132. doi: 10.1186/cc10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gratz I, Deal E, Spitz F, et al. BMC Anesthesiol. 2017;17:48. doi: 10.1186/s12871-017-0337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathur SK, Singh P. Transoesophageal echocardiography related complications. Indian J Anaesth. 2009;53:567–74. [PMC free article] [PubMed] [Google Scholar]
- 6.Brzezinski M, Luisetti T, London MJ. Radial artery cannulation: a comprehensive review of recent anatomic and physiologic investigations. Anesth Analg. 2009;109:1763–81. doi: 10.1213/ANE.0b013e3181bbd416. [DOI] [PubMed] [Google Scholar]
- 7.Bartels K, Esper SA, Thiele RH. Blood pressure monitoring for the anesthesiologist: a practical review. Anesth Analg. 2016;122:1866–79. doi: 10.1213/ANE.0000000000001340. [DOI] [PubMed] [Google Scholar]
- 8.Penaz J, Voigt A, Teichmann W. Contribution to the continuous indirect blood pressure measurement. Z Gesamte Inn Med. 1976;31:1030–3. [PubMed] [Google Scholar]
- 9.Westerhof N, Lankhaar J, Westerhof BE. The arterial Windkessel. Med Biol Eng Comput. 2009;47:131–141. doi: 10.1007/s11517-008-0359-2. [DOI] [PubMed] [Google Scholar]
- 10.Jacob M, Chappell D, Conzen P, Finsterer U, Rehm M. Blood volume is normal after pre-operative overnight fasting. Acta Anaesthiol Scand. 2008;52:522–529. doi: 10.1111/j.1399-6576.2008.01587.x. [DOI] [PubMed] [Google Scholar]
- 11.Bundgaard-Nielsen M, Jørgensen CC, Secher NH, Kehlet H. Functional intravascular volume deficit in patients before surgery. Acta Anaesthesiol Scand. 2010;54:464–9. doi: 10.1111/j.1399-6576.2009.02175.x. [DOI] [PubMed] [Google Scholar]


