Abstract
Background:
Eosinophils can be considered as multifunctional leukocytes that contribute to various physiological and pathological processes depending on their location and activation status. There are emerging eosinophil-related considerations concerning COVID-19. Variable eosinophil counts have been reported during COVID-19. Whether these changes are related to the primary disease process or due to immunomodulation induced by the treatment has not yet been elucidated.
Aim of the study:
To describe changes in the differential leukocyte counts including eosinophils, in a cohort of symptomatic patients with confirmed COVID-19 and to correlate these changes, if any, with the severity of the disease.
Patients and methods:
We recorded the clinical data, lab findings, including inflammatory markers and leukocyte and differential count, course of the disease and severity score in 314 confirmed symptomatic cases of COVID-19.
Results:
Laboratory tests revealed that 28.7 % (n =86) had mild eosinophilia (eosinophil count > 500 <1,500/µL). Thirty-four patients (11.3%) had elevated absolute neutrophil count (ANC) (>8,000/µL), and 7 (2.3%) had decreased ANC (< 1,500/µl). Seven patients (2.3%) had lymphopenia (<1,000/µL) and 4 (4.67%) had lymphocytosis (> 4,000/µL). C-reactive protein (CRP) was elevated in 83 patients (27.6%). Chest X-Ray changes included: increased broncho vascular markings (38%), ground-glass opacity (GGO) pneumonitis (19.3%), lobar consolidation (5%), bronchopneumonia (8.3%), nodular opacity (1%), acute respiratory distress syndrome (ARDS) (2.3%), pleural effusion (1.0%) and other atypical findings (6.6%). Patients with eosinophilia had significantly lower CRP, and lower % of GGO, lobar and bronchopneumonia and ARDS in their chest images compared to patients without eosinophilia (p: <0.05). They also had a lower requirement for a hospital stay, ICU admission, mechanical ventilation, and oxygen supplementation versus patients without eosinophilia (p: <0.05). The eosinophils count was correlated negatively with the duration of ICU admission, mechanical ventilation, and oxygen supplementation and with CRP level (r: - 0.34, -0.32, -0.61 and - 0.39, respectively) (p: < 0.01).
Conclusions:
Our study reports a relatively high prevalence of eosinophilia in symptomatic COVID-19 positive patients. Patients with eosinophilia had a lower level of CRP, milder clinical course and better disease outcomes compared to those without eosinophilia. Our findings indicated a protective role of eosinophils in mitigating the severity of inflammatory diseases through an inhibitory mechanism, as evidenced by lower CRP. This protective role of eosinophils needs to be validated by further prospective studies. (www.actabiomedica.it)
Keywords: Eosinophils, COVID19, C-reactive protein, white blood cells count, severity, outcome
Introduction
Since the discovery of COVID-19 in December 2019 in Wuhan, China (1), many key questions were raised on the potential relationship between various clinical and laboratory parameters and the development and progression of COVID-19. SARS-CoV-2 infection is a viral infection where the primary immune response is cellular. Hematological parameters associated with COVID-19 disease are variable among different reports.
Human eosinophils are effector cells with pro-inflammatory and destructive capabilities. During cellular inflammation, eosinophils are recruited from bone marrow and blood to the sites of the immune response. They were previously considered as end-stage cells involved in host protection against parasite infection and immunopathology in hypersensitivity disease. Accumulating evidence suggests that eosinophils can perform various immune regulatory functions likely through the presentation of antigens and production and release of a range of cytokines and other immunomodulatory molecules. Therefore, now eosinophils can be considered as multifunctional leukocytes that contribute to various physiological and pathological processes depending on their location and activation status (2,3).
The eosinophils count (EC) in the body is normally tightly regulated and accounts for only a small minority of peripheral-blood leukocytes (1-3%) (4). Normal EC ranges from 200x 103 /µL to 520 x 103 /µL. Peripheral blood eosinophilia (≥500 x 103 /µL) may be caused by numerous conditions, including allergic, infectious, inflammatory, and neoplastic disorders (5). Eosinopenia is defined as a reduction of circulating eosinophils <100x 103 /µL (6).
There are emerging eosinophil-related considerations concerning COVID-19. However, current data are limited on the association between COVID-19 infection and eosinophil count. Variable Eosinophil counts have been reported during SARS-CoV-2 infection. Whether these changes are related to the primary disease process or due to immunomodulation by the treatment used has not been clear. In addition, the possible association between eosinophil count and the course and severity of COVID-19 need further elucidation.
This study aimed to describe changes in the differential leukocyte counts including eosinophils, in symptomatic patients with COVID-19 and to correlate these changes, if any, with the severity of the disease.
Materials and Methods
A retrospective study was conducted in several COVID-19 designated facility Centers in Qatar: Hazm Mebaireek General Hospital (HMGH), Communicable Disease Center (CDC), Mesaieed Hospital (MGH), and Ras Laffan Hospital (RLH). All these Centers are run by Hamad Medical Corporation (HMC).
The primary purpose of this study was to evaluate the white cell count and EC among patients with COVID-19 as well as to find any relation between EC and severity of the disease and its outcomes.
All adult symptomatic patients with confirmed COVID-19 admitted to any of the designated facility Centers between January 2, 2020, and May 17, 2020, with no pre-existing eosinopenia or eosinophilia, were studied. All patients were diagnosed based on the WHO recommendations for cases that have a positive PCR test for SARS-CoV-2 (7). WHO guidelines for clinical management were utilized to categorize COVID-19 patients accordingly (1).
We recorded the clinical presentations, lab findings, including inflammatory markers and radiological findings. Recovery was defined as the resolution of clinical symptoms assessed by clinicians, such as no fever for more than three days, improved respiratory symptoms with reduced oxygen requirement, and no further need for hospital care required for any reason. The outcomes (severity of the disease) included mortality, ICU admission, and need for mechanical ventilation and oxygen therapy. The Charlson comorbidity index was calculated for all patients to assess additional risk factors in the course of the disease (8).
In our study, patients followed the treatment protocol officially approved in Qatar for the treatment of all COVID-19 hospitalized patients. The suggested treatment for symptomatic hospitalized COVID-19 adults (9-11) was as follows:
Hydroxychloroquine: 400 mg oral tablets twice daily on day 1, followed by 400 mg tablet daily for a total of 5 days, or
Chloroquine phosphate: 500 mg (300 mg base) oral tablets twice daily for 5 days
Plus, Azithromycin: 500 mg oral or IV once daily for 5 days.
Plus, Oseltamivir: 150 mg oral capsule twice daily for 5 days (if suspected or confirmed H1N1 co-infection).
In the case of confirmed pneumonia, by chest X-ray or CT scan, we added to the therapy Lopinavir: 400 mg-Ritonavir: 100 mg, twice daily for 10 days (Kaletra ®) tablets or syrup, along with Ceftriaxone:2 g/ intravenously (IV), once daily for additional bacterial coverage
antibacterial/antifungal drug was added as per culture results
For critically ill COVID-19 patients with acute respiratory distress syndrome (ARDS) or possible sepsis, in addition to the above-mentioned protocol, we gave empirical antimicrobial coverage as per our local pneumonia guidelines or as per culture results Tocilizumab IV 4-8 mg/kg was added if evidence of cytokine release syndrome. Patients with no signs of improvement received a second dose of Tocilizumab, 12-24 hours later.
This practice was continued in our study patients until there were new and major changes in COVID-19 treatment based on International RCTs, such as Solidarity trail (supported by WHO) and Recover Trial (supported by NHS) after which Chloroquine/Hydroxychloroquine, Azithromycin, and Lopinavir-Ritonavir was replaced in our protocol, with Dexamethasone IV, Remdesivir, and Favipiravir for critically ill patients (9-11).
The possibility that these drugs could mildly affect eosinophilic count during treatment has been reviewed (12) (Table 1).
Table 1.
Drugs used in our patient population that may potentially cause eosinophilia (adapted from Ref. 12)
| Drug | Type of reported reaction | Percentage and severity of eosinophilia |
| Chloroquine and Hydroxychloroquine | Pulmonary and Immunologic | Drug reaction with eosinophilia and systemic symptomsDRESS syndromeSevere based on case reports, frequency not defined |
| Macrolides: Azithromycin | Pulmonary and dermal | Eosinophilia <1%, (severity unclear) |
| Vancomycin | Dermal and renal | Eosinophilia 1-10% (moderate to severe, reversible) |
| Teicoplanin | Dermal and renal | Eosinophilia <1%, (severity unclear) |
| Cephalosporins | Cardiac, pulmonary and dermal | Usually mild and self-limiting |
| Ceftriaxone | Cardiac, pulmonary and dermal | Eosinophilia 6%, (mild) |
| Cefuroxime | Pulmonary and dermal | Eosinophilia 1-7%, (mild) |
| Cefepime | Pulmonary and dermal | Eosinophilia 2%, (mild) |
| Piperacillin tazobactam | Renal and dermal | Eosinophilia frequency not defined, (mild). |
| Sulphonamides | Pulmonary and cardiac | Not usually reported, (could be severe) |
| Trimethoprim-sulfamethoxazole(Co-trimoxazole). | Pulmonary and cardiac | Eosinophilia frequency not defined, (mild to moderate) |
We also studied the correlation between EC and the different parameters of disease severity. Expert radiologists interpreted the chest radiographs (CXR) for all admitted patients.
The study was approved by the Institutional Review Board of Hamad Medical Corporation, Doha Qatar (MRC-01-20-511).
Statistical analysis was performed using Excel statistical Pack software. The non-paired student t-test was used to compare variables between eosinophilic and non-eosinophilic groups when data were normally distributed and Wilcoxon rank-sum test when the data were not normally distributed. The linear regression equation was used to find a possible correlation between clinical and laboratory variables. Statistical significance was accepted with a p-value of less than 0.05.
Results
Three hundred and fourteen confirmed cases of COVID-19 were included in our study (274 were males (87.2%), and 40 (12.8%) were females, which is justified by Qatar’s population distribution pattern, mainly composed of male expatriate laborers. Their nationalities included Indians (31.8%), Bangladeshi (27.7%), Nepalese (21.3%) and 1.2% were Qataris.
Their mean age was 39.7 ± 13 years. Forty-eight (15.2%) had a body mass index (BMI) > 30 kg/m2. The most common comorbidity was diabetes mellitus (DM) in 55 (17.5%) followed by hypertension in 50 (15.9%). Six of them had chronic kidney disease (CKD), and seven had a previous myocardial infarction (MI). Four had a history of stroke and/or transient ischemic attack, and one had residual hemiplegia. Two patients had a history of congestive heart failure (CHD).
Our patients presented with fever, cough, and shortness of breath. 25% had mild to moderate pneumonia, 4.6% severe pneumonia, and 2.3% had adult ARDS. Only 2.3% had a sepsis-like picture. The clinical and hematological changes in COVID-19 positive patients (n = 314) on admission is presented in table 2.
Table 2.
Clinical and hematological changes in COVID-19 positive patients (n = 314)
| Clinical findings | N = | % |
| Pneumonia | 75 | 25.0 |
| Severe Pneumonia | 14 | 4.6 |
| ARDS | 6 | 2.0 |
| Sepsis picture | 7 | 2.3 |
| Lab results | ||
| Eosinophilia | 86 | 28.6 |
| Mild > 500 <1500/µL | 71 | 23.6 |
| Moderate > 1500 /µL | 15 | 5.0 |
| Lymphocytosis > 4000/µL | 14 | 4.6 |
| Lymphopenia <1000/µL | 7 | 2.3 |
| ANC < 1500/µL | 7 | 2.3 |
| ANC > 8000/µL | 34 | 11.3 |
| Platelets > 450 x 103 /µL | 16 | 5.3 |
| Platelets <150 x 103 /µL | 10 | 3.3 |
| High CRP > 10 mg/L | 83 | 27.6 |
| Mortality | 2 | 0.6 |
Legend=ANC: absolute neutrophil count, CPR: C-reactive protein
Laboratory tests revealed that 28.7 % (n =86) had mild eosinophilia (eosinophil count > 500 <1,500/µL). Thirty-four patients (11.3%) had elevated absolute neutrophil count (ANC) (>8,000/µl), and 7 (2.3%) had decreased ANC (< 1,500/µl). Seven patients (2.3%) had lymphopenia (<1,000/µl), 14 (4.6%) had lymphocytosis (> 4,000/µL). Platelets count was elevated (> 450 x 103 /µL) in 16 patients (5.3%) and decreased (<150 x 103 /µL) in 10 cases (3.3%).
C-reactive protein (CRP) was elevated in 83 of patients (27.6%). Eighty three percent of the patients had a CRP level > 10 mg/L at diagnosis. Lymphopenia was seen in 2.3% and thrombocytopenia in 3.3% of the patients.
257 (85.6%) patients had abnormal radiological findings. Chest X-Ray (CXR) changes included increased bronchovascular markings (38%), ground-glass opacity (GGO) pneumonitis (19.3%), lobar consolidation (5%), bronchopneumonia (8.3%), nodular opacity (1%), ARDS (2.3%), pleural effusion (1%) and atypical findings (6.6%).
Table 3 presents CBC findings in patients with eosinophilia versus those without eosinophilia. Patients with eosinophilia had significantly higher CRP and lymphocytic count.
Table 3.
Complete blood count and CPR results in Covid-19 patients with eosinophilia vs. those without eosinophilia
| WBC | Hb | HTC | MCV | MCHC | PLT | LYMP | MONO | EOSIN | BASO | CRP | |
| Patients with eosinophilia | |||||||||||
| Mean | 8.79 | 14.8 | 44.46 | 84.77 | 33.33 | 278.59 | 2.7* | 0.73 | 0.89* | 0.05 | 6.70 |
| SD | 2.82 | 1.56 | 4.03 | 5.81 | 1.36 | 93.47 | 0.91 | 0.29 | 0.50 | 0.03 | 21.2 |
| Patients without eosinophilia | |||||||||||
| Mean | 8.24 | 13.82 | 42.15 | 84.47 | 32.61 | 244.21 | 1.59 | 0.68 | 0.10 | 0.03 | 77.1* |
| SD | 3.27 | 1.88 | 6.00 | 7.41 | 5.36 | 98.2 | 0.91 | 0.43 | 0.14 | 0.04 | 86.35 |
| *p: < 0.05 | |||||||||||
Legend= CPR: C-reactive protein
Table 4 compares CXR findings in patients with eosinophilia versus those without eosinophilia. Patients with eosinophilia had lower % of GGO, lobar and bronchopneumonia and ARDS.
Table 4.
Chest X-Ray (CXR) findings in Covid-19 patients with eosinophilia vs. those without eosinophilia
| Normal CXR | GGO pneumonitis | Lobar pneumonitis | Bronchopneumonia | ARDS | |
|
Patients wth eosinophilia (n = 86) |
|||||
| (%) | 44.1* | 15.1 | 3.23 | 2.69 | 0.00 |
|
Patients without eosinophilia (n = 214) |
|||||
| (%) | 23.4 | 24.1* | 7.1* | 17.9* | 16.0 |
| *p: < 0.05 | |||||
Legend= ARDS: acute respiratory distress syndrome
Table 5 depicts the severity of disease in patients with eosinophilia versus those without eosinophilia. Patients with eosinophilia had a lower requirement for a hospital stay, ICU admission, mechanical ventilation, and oxygen supplementation.
Table 5.
Severity of disease in Covid-19 patients with eosinophilia vs. those without eosinophilia
| Hospitalization (D) |
Required ICU (%) |
ICU stay (D) | Required MV (%) | MV (D) |
O2 requirement |
|
| Patients wth eosinophilia | ||||||
| Mean | 3.00 | 1.08 | 0.07 | 1.12 | 0.04 | 11.24% |
| SD | 5.40 | 0.64 | 0.43 | 0.92 | ||
| Patients without eosinophilia | ||||||
| Mean | 17.1* | 26.8 | 4.3* | 21.6 | 2.2* | 60%* |
| SD | 13.30 | 8.05 | 5.62 | 10.61 | ||
| *p: < 0.05, MV: Mechanical ventilation, D: Days, ICU: Intensive Care Unit, O2: Oxygen. | ||||||
During treatment, the complete blood count showed a significant (p: <0.05) elevation of white blood cells (WBC), platelets, basophils, and eosinophils. A significant drop was observed in hemoglobin (Hb), hematocrit (HCT), and EC on discharge (Table 6).
Table 6.
Complete blood count changes at diagnosis (Dx), during treatment and at the discharge in COVID-19 patients
| WBC | Hb | HCT | MCV | MCHC | RDW | PLT | ANC | LYMP | MONO | EOSIN | BASO | ||
| At Dx of COVID-19 | Mean | 8.57 | 14.46 | 43.61 | 84.69 | 33.06 | 13.98 | 265.15 | 4.93 | 2.29 | 0.71 | 0.59 | 0.05 |
| SD | 3.00 | 1.76 | 4.71 | 6.00 | 1.77 | 14.13 | 95.79 | 2.69 | 1.04 | 0.35 | 0.46 | 0.04 | |
| During treatment | Mean | 9.11* | 13.2* | 40.0* | 84.25 | 33.03 | 13.83 | 333.7* | 5.47* | 2.05 | 0.76 | 0.88* | 0.079* |
| SD | 3.67 | 1.85 | 5.53 | 6.40 | 1.34 | 1.95 | 136.38 | 3.56 | 0.95 | 0.38 | 1.18 | 0.13 | |
| At discharge | Mean | 8.81 | 11.34* | 34.85 | 84.52 | 34.48 | 16.12 | 364.21 | 5.52 | 1.85 | 0.80 | 0.55 | 0.33 |
| SD | 3.33 | 2.03 | 5.32 | 11.41 | 9.67 | 4.40 | 183.74 | 2.96 | 0.74 | 0.34 | 0.37 | 1.07 | |
| * p: < 0.05 | |||||||||||||
The EC was correlated negatively with the duration of ICU admission, mechanical ventilation, and oxygen supplementation as well as with CRP level (r: - 0.34, -0.32, -0.61 and - 0.39, respectively) (p: < 0.01). EC was positively correlated with lymphocytic count (r: 0.45, p: <0.01; table 7).
Table 7.
Correlations between hematological variables and parameters of COVID- 19 severity
| Age | CHARLSON CO-MORBIDITY INDEX | WBC | HB2 | HCT | MCV | MCHC | RDW | PLT | ANC | LYMP | MONO | EOSIN | BASO | CRP | Days of ICU admission | Days on mechanical ventilation | Number of days on oxygen supplementation | |
| Age | 1.00 | |||||||||||||||||
| CHARLSON CO-MORBIDITY INDEX | 0.67 | 1.00 | ||||||||||||||||
| WBC | -0.01 | -0.04 | 1.00 | |||||||||||||||
| HB | -0.18 | -0.33 | 0.10 | 1.00 | ||||||||||||||
| HCT | -0.15 | -0.30 | 0.09 | 0.95 | 1.00 | |||||||||||||
| MCV | 0.03 | 0.05 | 0.02 | 0.23 | 0.13 | 1.00 | ||||||||||||
| MCHC | -0.06 | -0.21 | -0.03 | 0.32 | 0.10 | 0.29 | 1.00 | |||||||||||
| RDW | -0.04 | 0.25 | 0.12 | 0.05 | 0.07 | -0.08 | -0.70 | 1.00 | ||||||||||
| PLT | -0.07 | -0.09 | 0.36 | -0.10 | -0.10 | -0.14 | 0.07 | -0.15 | 1.00 | |||||||||
| ANC | 0.13 | 0.10 | 0.87 | -0.07 | -0.05 | -0.03 | -0.04 | 0.03 | 0.27 | 1.00 | ||||||||
| LYMP | -0.27 | -0.27 | 0.31 | 0.29 | 0.26 | 0.08 | 0.19 | -0.09 | 0.25 | -0.15 | 1.00 | |||||||
| MONO | -0.03 | -0.07 | 0.61 | 0.09 | 0.09 | 0.11 | 0.06 | -0.03 | 0.34 | 0.44 | 0.27 | 1.00 | ||||||
| ESINO | -0.12 | -0.23 | 0.15 | 0.15 | 0.12 | 0.02 | 0.13 | -0.06 | 0.13 | -0.16 | 0.45 | 0.02 | 1.00 | |||||
| BASO | -0.04 | 0.02 | 0.47 | 0.11 | 0.09 | 0.01 | -0.30 | 0.53 | 0.16 | 0.23 | 0.31 | 0.27 | 0.29 | 1.00 | ||||
| CRP2 | 0.30 | 0.28 | 0.23 | -0.25 | -0.22 | -0.07 | -0.15 | 0.16 | 0.04 | 0.51 | -0.45 | 0.03 | -0.39 | -0.15 | 1.00 | |||
| Days of ICU admission | 0.33 | 0.24 | -0.05 | -0.13 | -0.11 | -0.06 | -0.07 | -0.01 | -0.13 | 0.16 | -0.35 | -0.17 | -0.36 | -0.18 | 0.56 | 1.00 | ||
| Days on Mechanical Ventilation | 0.30 | 0.30 | -0.04 | -0.10 | -0.08 | -0.02 | -0.05 | 0.00 | -0.13 | 0.12 | -0.26 | -0.16 | -0.34 | -0.14 | 0.42 | 0.78 | 1.00 | |
| Number of days on oxygen supplementation | 0.35 | 0.32 | -0.07 | -0.17 | -0.15 | -0.04 | -0.07 | -0.01 | -0.14 | 0.17 | -0.39 | -0.19 | -0.61 | -0.20 | 0.61 | 0.92 | 0.68 | 1.00 |
There was a significant positive correlation between CRP and duration of O2 requirements (r: 0.614, p: 0.001; figure 1).
Figure 1.
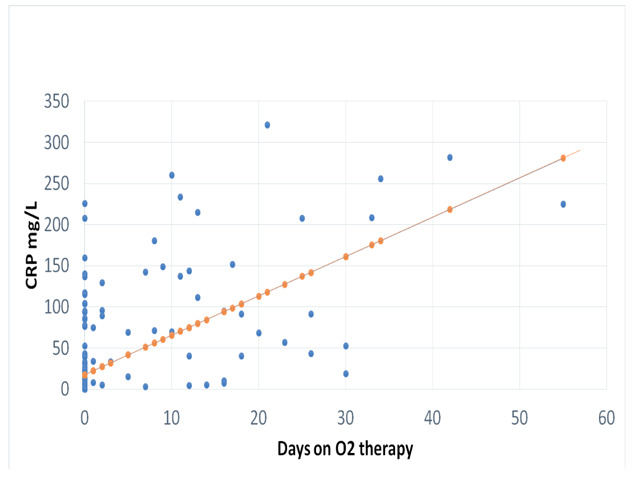
Correlation between CPR (mg/L) and number of days of O2 therapy (r= 0.614, p: 0.001)
Discussion
Normally, eosinophils are present in fewer numbers in peripheral blood and present in mucosal surfaces where viruses can overcome the host defense. Eosinophils have various granules and inflammatory mediators that have antiviral activity. Human eosinophils express several endosomal Toll-like receptors (TLRs), including TLR3, TLR7, and TLR9, that detect viral microbe–associated molecular patterns. TLR7 enables eosinophils to recognize single-stranded RNA viruses such as coronavirus and stimulating this receptor in human eosinophils triggers eosinophil cytokine production, degranulation, superoxide, and nitric oxide (NO) generation, and prolonged cellular survival (13-15). Once they are involved in immune response they start degranulation and superoxide and NO generation have significant anti-viral effects and increase cell resistance to viral infection (13).
In this study, we reported eosinophilia in 28 % of symptomatic patients with COVID-19 on admission.
Eosinophil count increased significantly during the first week of treatment and decreased again on the discharge of the patients (after 2 weeks of treatment). The relative contribution of disease and/or drug treatment in the associated eosinophilia cannot be completely separated; however, eosinophilia is a unusual side effect of these drugs (Table 1), in addition, eosinophilia was present before initiation of drug treatment.
Patients with eosinophilia had a milder clinical course and lower radiological abnormalities compared to those without eosinophilia. Eosinophils count was correlated negatively with the duration of ICU admission, mechanical ventilation, and oxygen supplementation and with CRP (r: -0.34, -0.614 and -0.39, respectively; p: < 0.01). These data indicated that eosinophilia was associated with a milder course and favorable outcome in SARS-CoV-2 infected patients.
In support of our findings, Du et al. (16) reviewed the 85 fatal cases of COVID-19 and noted that 81% of the patients had absolute EC below the normal range (absolute eosinophil counts <0.02 × 109 cells/L) at the time of admission.
In a single-center retrospective study, eosinopenia was observed in more than half of 140 patients (52.9%) with acute respiratory deterioration during COVID-19 (17). Another study showed similar results where 47 COVID-19 patients (51.6%), had eosinopenia (18). Liu et al. (19) showed that a large proportion of patients had low levels of eosinophil in the early stage of SARS-CoV-2 infection, especially during the first week of hospitalization. They also noted an eosinopenia at the time of initial presentation in a small cohort of patients who were treated with lopinavir. In their study, EC improved in all patients before discharge, suggesting that resolution of eosinopenia may be an indicator of improving clinical status (19). These studies, including ours, indicate that eosinopenia is a bad prognostic sign while eosinophilia was a good prognostic sign in patients with COVID-19.
On the contrary, a recent systematic review by Lippi and Henry (20) analyzed three studies from China and concluded that the eosinophil count did not differ between patients with or without severe COVID-19.
CRP is an acute-phase protein produced by the liver during infections and inflammation. The cytokines interleukin (IL)-1 beta, IL-6, and tumor necrosis factor (TNF) are widely reported to induce synthesis of CRP by hepatocytes both in vitro and in vivo. SARS-Cov-2 selectively induces a high level of IL-6. The elevated IL-6 plays a crucial role in the pathologic of COVID-19, including the chemotaxis of neutrophils and lymphocyte necrosis and exhaustion. Human eosinophils release immunomodulatory mediators, notably IL-6, in response to infection with respiratory virus pathogens (21,22). In our COVID-19 patients EC was negatively correlated with CRP level and CRP level was correlated significantly with the severity of the disease (durations of ICU admission and O2 requirement).
These data suggested that eosinophilia was associated with lower inflammatory response and better outcomes in these patients. In support of this view, in 991 asthmatic patients with lower respiratory tract infection (LRT), Cag et al. (23) found that the presence of peripheral blood eosinophilia was associated with significantly lower inflammatory markers (neutrophil-to-lymphocyte ratio (NLR) and CRP) regardless of the presence or absence of an LRT infection. The eosinophil count percentage showed moderate inverse correlations with CRP and NLR (r : −0.20 and −0.34, respectively).
Moreover, a recent concept supported that eosinophils and their secretory mediators can have a role in promoting antiviral host defense. An initial study showed that eosinophil secretory mediators decrease the ability of RSV to infect target host epithelial cells (24). Another later report observed that eosinophils that were induced by allergen sensitization decreased viral loads during parainfluenza virus infection in a guinea pig asthma model. Accelerated clearance of RSV has been demonstrated in the lungs of eosinophil-enriched Cd2-IL-5-transgenic mice, and activated eosinophils protect mice from the lethal sequelae of acute pneumo-virus infections (25-29). These can partially explain the lower lung affection in eosinophilic patients with COVID-19.
Eosinophils also interact with and modulate the functions of other leukocytes. In our study eosinophil count was correlated significantly with the lymphocytic count. The same findings were reported by Zang et al. (17). However, contrary to other studies, our patients did not have significant lymphopenia. The high proportion of eosinophilia and higher lymphocytic count in our patients with eosinophilia may explain the milder course and better prognosis in our COVID-19 patients (30-32).
We also found a notable reduction in hemoglobin levels during the treatment of our patients. This reduction may be related to the severity of the disease secondary to reduced red blood cell production due to the systemic inflammatory response and cytokine release (33).
The most common comorbidities found in our patients were hypertension (15.9%) and DM (17.5%) which conform with other studies from the USA, China, and Italy. Most of our symptomatic patients presented with fever, cough, and dyspnea (17,18).
Conclusion
Our study reported a relatively high prevalence of eosinophilia in symptomatic COVID-19 positive patients. Patients with eosinophilia had a lower level of CRP, milder clinical course and better disease outcomes compared to those without eosinophilia. These findings suggested a protective role of eosinophils in mitigating the severity of inflammatory diseases through an inhibitory mechanism, as evidenced by lower CRP. This protective role of eosinophils needs to be validated by further prospective studies.
Conflict of Interest:
None to declare
Funding:
None.
References
- 1.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus ( 2019-nCoV) infection is suspected: interim guidance, 28 January 2020. 2020, World Health Organization [Google Scholar]
- 2.Kita H. Eosinophils: multifaceted biological properties and roles in health and disease. Immunol Rev. 2011;242:161–177. doi: 10.1111/j.1600-065X.2011.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothenberg ME. Eosinophilia. N Engl J Med. 1998;338:1592–1600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
- 5.Weller PF, Klion AD, Feldweg AM. Approach to the patient with unexplained eosinophilia. In: Mahoney DH, Bochner DS, editors. UpToDate. Waltham (MA): 2014. [Google Scholar]
- 6.Zini G. In: Blood and bone marrow pathology. Churchill Livingstone Philadelphia: 2011. Abnormalities in leukocyte morphology and number; pp. 247–261. [Google Scholar]
- 7.World Health Organization. Coronavirus disease (COVID-19) technical guidance. WHO (Accessed on March 4, 2020). Online Version, 2020 [Google Scholar]
- 8.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 9.Hung IF, Lung KC, Tso EY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Solidarity” clinical trial for COVID-19 treatments. World Health Organization (WHO). Situation reports. Geneva: WHO.[Accessed: 5 Apr 2020]. Available from: https://www. who. int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments , 2020. [Google Scholar]
- 11.Trivedi A, Sharma S, Ashtey B. Investigational treatments for COVID-19. Pharmaceutical J. 23 JUN 2020 https://www.pharmaceutical-journal.com . [Google Scholar]
- 12.Maidment I, Williams C. Drug-induced eosinophilia. Pharmaceutical J. l8 JAN 2000 https://www.pharmaceutical-journal.com . [Google Scholar]
- 13.Drake MG, Bivins-Smith ER, Proskocil BJ, et al. Human and Mouse Eosinophils Have Antiviral Activity against Parainfluenza Virus. Am J Respir Cell Mol Biol. 2016;55:387–394. doi: 10.1165/rcmb.2015-0405OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagase H, Okugawa S, Ota Y, et al. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J Immunol. 2003;171:3977–3982. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- 15.Wong CK, Cheung PF, Ip WK, Lam CW. Intracellular signaling mechanisms regulating toll-like receptor-mediated activation of eosinophils. Am J Respir Cell Mol Biol. 2007;37:85–96. doi: 10.1165/rcmb.2006-0457OC. [DOI] [PubMed] [Google Scholar]
- 16.Du Y, Tu L, Zhu P, et al. Clinical Features of 85 Fatal Cases of COVID-19 from Wuhan. A Retrospective Observational Study. Am J Respir Crit Care Med. 2020;201:1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 18.Qian GQ, Yang NB, Ding F, et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM. 2020;113:474–481. doi: 10.1093/qjmed/hcaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu F, Xu A, Zhang Y, et al. Patients of COVID-19 may benefit from sustained Lopinavir-combined regimen and the increase of Eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis. 2020;95:183–191. doi: 10.1016/j.ijid.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lippi G, Henry BM. Eosinophil count in severe coronavirus disease 2019 (COVID-19) QJM. Int J Med. 2020;13:511–512. doi: 10.1093/qjmed/hcaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheldon J, Riches P, Gooding R, Soni N, Hobbs JR. C-reactive protein and its cytokine mediators in intensive-care patients. Clin Chem. 1993;39:147–150. [PubMed] [Google Scholar]
- 22.Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. Published 2020 Jul 10 doi:10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cag Y, Pacal Y, Gunduz M, et al. The effect of peripheral blood eosinophilia on inflammatory markers in asthmatic patients with lower respiratory tract infections. J Int Med Res. 2019;47:2452–2460. doi: 10.1177/0300060519844859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsh GM. Antagonism of eosinophil accumulation in asthma. Recent Pat Inflamm Allergy Drug Discov. 2010;4:210–213. doi: 10.2174/187221310793564263. [DOI] [PubMed] [Google Scholar]
- 25.Adamko DJ, Yost BL, Gleich GJ, Fryer AD, Jacoby DB. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects. J Exp Med. 1999;190:1465–1478. doi: 10.1084/jem.190.10.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davoine F, Cao M, Wu Y, et al. Virus-induced eosinophil mediator release requires antigen-presenting and CD4+ T cells. J Allergy Clin Immunol. 2008;122:69–77. doi: 10.1016/j.jaci.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 27.Domachowske JB, Dyer KD, Bonville CA, Rosenberg HF. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis. 1998;177:1458–1464. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- 28.Dyer KD, Percopo CM, Fischer ER, Gabryszewski SJ, Rosenberg HF. Pneumoviruses infect eosinophils and elicit MyD88-dependent release of chemoattractant cytokines and interleukin-6. Blood. 2009;114:2649–2656. doi: 10.1182/blood-2009-01-199497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phipps S, Lam CE, Mahalingam S, et al. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110:1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 30.Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollenberg SM, Ahrens TS, Annane D, et al. Practice parameters for hemodynamic support of sepsis in adult patients: 2004 update. Crit Care Med. 2004;32:1928–1948. doi: 10.1097/01.ccm.0000139761.05492.d6. [DOI] [PubMed] [Google Scholar]


