Abstract
Background:
There is a scarcity of data regarding the effect of hypertension on the clinical presentation and outcome of symptomatic patients with COVID-19 infection in comparison with non-hypertensive patients.
Aim of the study:
To describe the clinical presentation, radiological and hematological data of a cohort of symptomatic COVID-19 positive hypertensive patients (n = 50) in comparison with another cohort of normotensive symptomatic COVID-19 positive patients (n = 250) diagnosed at the same time and managed in the same health facilities (from January 2020 to May 2020). Associated comorbidities were assessed, and the Charlson Comorbidity Index was calculated. The outcomes, including duration of hospitalization, length of intensive care unit (ICU) stay, duration of mechanical ventilation, and duration of O2 supplementation, were also assessed.
Results:
The prevalence of hypertension in symptomatic COVID-19 positive patients was 50/300 (16%; the prevalence of hypertension in Qatari adults is ~ 30%). Hypertensive patients had a higher prevalence of diabetes mellitus (DM), chronic kidney disease (CKD), and cardiac dysfunction [(coronary heart disease (CHD) and, congestive heart failure (CHF)] compared to normotensive patients (p: < 0.01). They had a higher Charlson Co-morbidity score (2.3 ± 1.8) compared to the normotensive patients (0.4 ± 0.9) (p: < 0.01). Clinically and radiologically, hypertensive patients had significantly higher percentage of pneumonia, severe pneumonia, and acute respiratory distress syndrome (ARDS) versus normotensive patients (p: < 0.01). Complete blood count (CBC) and differential white cell count (WBC) did not differ between hypertensive and normotensive patients. Hypertensive patients had significantly higher C-reactive protein (CRP) (58.5 ± 84), compared to normotensive patients (28 ± 59) (p: < 0.01). Furthermore, a longer duration of hospitalization, intensive care unit (ICU) stay, mechanical ventilation and oxygen therapy versus normotensive patients was also observed. CRP was correlated significantly with the duration of stay in the ICU and the duration for oxygen supplementation (r = 0.56 and 0.61, respectively; p: <0.01).
Conclusions:
Hypertensive patients with COVID-19 had a higher inflammatory response (higher CRP levels), a significant increase of comorbidities, and a more aggressive course of the disease necessitating a higher rate of ICU admission, longer requirement for hospitalization and oxygen use compared to normotensive patients. (www.actabiomedica.it)
Keywords: COVID-19, hypertension, prevalence, clinical manifestations, biochemical data, outcome
Introduction
Coronavirus disease 2019 (COVID-19), a highly infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that was firstly reported in Wuhan, Hubei
Province, China, in December 2019 and rapidly spread to worldwide (1). It belongs to a large family of viruses that are common in many animal species like bats, camels, and cattle (2-6).
The total number of people infected since the beginning of the pandemic in Qatar is 108,638, with a mortality of 164 patients to date (7).
Advanced age and different comorbidities have been associated with poor prognosis in hospitalized COVID-19 patients (8). Emerging data showed that hypertension (HTN), diabetes mellitus (DM), chronic kidney disease (CKD) and cardiac dysfunction [coronary heart disease (CHD), congestive heart failure (CHF)], were significant risk factors that may worsen the clinical course and outcome in patients with COVID-19 (8-12).
The aim of this study was to assess and compare the clinical presentation, radiological changes, and hematological data in symptomatic COVID-19 patients with hypertension with those of normotensive COVID-19 patients.
Patients and Methods
We performed a retrospective analysis of adult patients with symptomatic COVID-19 infection (positive PCR tests for SRAS-CoV-2). Disease severity was classified based on the WHO classification (12). These patients were admitted to one of the COVID-19 designated facilities Centers of Doha (Qatar), including Hazm Mebaireek General Hospital (HMGH), Mesaieed General Hospital (MGH), Communicable Disease Center (CDC) and Ras Laffan Hospital (RLH).
A total of 300 patients have been admitted to Qatar ‘s designated COVID-19 health care facilities Centers between January 2020 and May 17, 2020. These patients were divided into two groups: hypertensive and normotensive, according to the definition of the American Heart Association. Patients with systolic blood pressure (SBP) ≥130 mmHg and diastolic blood pressure (DBP) ≥80 mmHg) and those on oral antihypertensive agents were considered hypertensive (n =50). The normotensive group (n-250) included all the other patients with normal blood pressure on admission and no history of taking antihypertensive medication at home. Inclusion criteria comprised all COVID- 19 positive symptomatic adult patients > 18 years who required hospitalization. Younger patients <18 years, pregnant women, and those with malignancy were excluded.
Clinical presentations, lab findings, including inflammatory markers and radiological findings were recorded. Recovery was defined as the resolution of clinical symptoms assessed by clinicians, such as no fever for more than three days, improvement of respiratory symptoms with reduced oxygen requirement, and no further needs for hospitalization care.
The outcomes included: mortality, severity of the disease, intensive care unit (ICU) admission, and need for mechanical ventilation. The Charlson comorbidity index score was calculated for all patients to assess additional risk factors in the course of the disease (13).
The study was approved by the Institutional Review Board of Hamad Medical Corporation, Doha Qatar (MRC-01-20-511).
Statistical analysis was performed using Excel statistical Pack software package. The non-paired student t -test was used to compare variables between hypertensive and normotensive groups when data were normally distributed and Wilcoxon rank-sum test when the data were not normally distributed. Linear regression equation was used to find a possible correlation between clinical and laboratory variables. Statistical significance was accepted with a p-value < 0.05.
Results
In our symptomatic COVID-19 positive patients, hypertension was diagnosed in 50/300 patients (16%). Hypertensive patients were older and had a higher prevalence of DM, CKD, and cardiac dysfunction (CHD and CHF) (p:< 0.01) compared to normotensive patients. They had also a higher Charlson Comorbidity score compared to the normotensive patients (2.3 ±1.8 and 0.4 ± 0.9, respectively) (p: < 0.01) (Table 1).
Table 1.
Clinical characteristics of COVID- 19 positive in hypertensive patients (HTN) vs no hypertensive patients (No HTN)
| HTN | No HTN | ||||
| Clinical Characteristics of Covid-19 patients | n = 50 | n = 250 | |||
| Mean | SD | Mean | SD | ||
| Age | 55.1 | 11.3 | 36.1 | 11.1 | |
| Weight | 75.9 | 21.6 | 72.1 | 13.1 | |
| Systolic BP | 148.7* | 25.1 | 122.8 | 13.0 | |
| Diastolic BP | 88.1* | 13.8 | 76.5 | 9.3 | |
| Myocardial Infarction | % | 8* | 1.00 | ||
| Heart Failure | % | 2* | 0.00 | ||
| CVA/TIA | % | 8* | 0.00 | ||
| Diabetes Mellitus | % | 63* | 9.70 | ||
| Chronic Kidney Disease | % | 10* | 0.00 | ||
| Charlson Comorbidity Index (CCI; §) | 2.3* | 1.8 | 0.4 | 0.9 | |
(§) no comorbidity (CCI = 0), moderate comorbidity (CCI = 1–5) or severe comorbidity (CCI ≥ 6); (*) p: < 0.05
Clinically and radiologically, hypertensive patients had a significantly higher proportion and severity of pneumonia, and acute respiratory distress syndrome (ARDS) versus normotensive patients (p: < 0.01).
Complete blood count (CBC) and differential white cell count (WBC) did not differ between hypertensive and normotensive patients. Hypertensive patients had significantly higher C-reactive protein (CRP) (58.5 ± 84) compared to normotensive (28 ± 59) (p: < 0.01; table 2).
Table 2.
Laboratory findings in patients with (HTN) and without hypertension (No HTN), and COVID-19
| Patient Lab. findings | HTN | No HTN | ||
| n = 50 | n = 250 | |||
| Mean | SD | Mean | SD | |
| WBC | 9.04 | 3.41 | 8.49 | 2.92 |
| Hb | 13.92 | 2.05 | 14.57 | 1.68 |
| Htc | 42.05 | 5.78 | 43.93 | 4.42 |
| MCV | 85.90 | 4.33 | 84.44 | 6.27 |
| MCHC | 33.09 | 1.13 | 33.04 | 1.87 |
| RDW | 13.56 | 1.39 | 14.07 | 15.49 |
| PLT | 245.31 | 94.55 | 269.94 | 95.32 |
| ANC | 5.80 | 2.98 | 4.77 | 2.60 |
| LYMP | 2.05 | 1.04 | 2.35 | 1.02 |
| MONO | 0.77 | 0.53 | 0.70 | 0.30 |
| ESINO | 0.41 | 0.73 | 0.62 | 0.51 |
| BASO | 0.05 | 0.03 | 0.05 | 0.04 |
| CRP | 58.57* | 84.49 | 28.08 | 59.32 |
| * p < 0.05 | ||||
Hypertensive patients with and without other co-morbidities had a longer duration of hospitalization, ICU stay, mechanical ventilation, and oxygen therapy versus normotensive patients (Table 3).
Table 3.
Outcome of COVID-19 positive hypertensive (HTN) vs. normotensive patients (No HTN) with COVID-19
| Outcome of patients | HTN | No HTN | |||
| n = 50 | n = 250 | ||||
| Mean | SD | Mean | SD | ||
| Days of hospitalization | 15.8* | 12.15 | 7.1 | 11.1 | |
| ICU admission | % | 24* | 8.0 | ||
| Days of ICU admission | 2.7* | 5.06 | 1.4 | 5.3 | |
| Mech. ventilation | % | 22* | 6.0 | ||
| Days of mech. ventilation | 1.2* | 2.77 | 0.7 | 3.6 | |
| Number of days on oxygen supplementation | 4.8* | 8.53 | 1.8 | 6.7 | |
| Days before CRP returned negative | 8.2 | 11.48 | 11.4* | 15.4 | |
| Mortality | % | 0 | 1.3 | ||
* p < 0.05
Hypertensive patients without any other co-morbidity (n =16) had a longer duration of hospitalization, ICU stay, mechanical ventilation, and oxygen therapy versus normotensive patients (Table 4).
Table 4.
Outcome of COVID 19 positive with hypertension only (HTN) and no other comorbidity) vs. no hypertensive patients (No HTN)
| Charlson Co-Morbidity Index | Days of hospitalization | % Admitted to ICU | Days of ICU admission | Mech Vent | Days on Mechanical Ventilation | Days on oxygen supplemention | ||
| HTN | Mean | 0.72* | 10.17* | 19 %* | 2.27* | 13%* | 1.07 | 3.93* |
| n = 16 | SD | 0.75 | 13.58 | 5.60 | 3.09 | 8.12 | ||
| No HTN | Mean | 0.40 | 7.12 | 8.5% | 1.42 | 6.25% | 0.77 | 1.89 |
| n = 250 | SD | 0.92 | 11.16 | 5.30 | 3.67 | 6.75 |
*p: <0.05
CRP was correlated significantly with the duration of stay in the ICU (r : 0.56; p: <0.001) and the duration of oxygen therapy (r : 0.61; p: < 0.001; figure 1).
Figure 1.
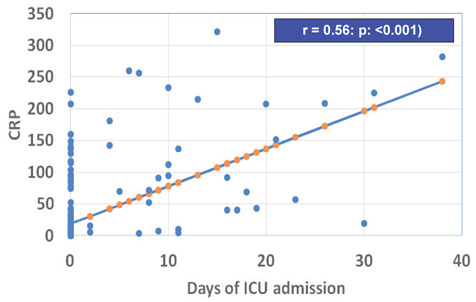
Correlation between C-reactive protein (CRP) and duration (days) of Intensive Care Unit (ICU) admission
Discussion
In a meta-analysis that included seven studies, hypertension was the most reported comorbidity in COVID- 19 patients (15) with a prevalence of 16.7 % (7-8). However, in other studies, the prevalence varied from 11 to 34% (16-19). In our study, 16.7 % of
symptomatic COVID- 19 adults were hypertensive. This ratio was less than the general prevalence of hypertension in adults Qatari population (30%) (14), probably due to a predominantly younger population in our study cohort.
Our hypertensive patients with COVID-19 had higher associated comorbidities, including DM, CKD, and cardiac dysfunction (CHD and CHF) compared to normotensive patients. They had also a significantly longer duration of hospitalization, ICU stay, mechanical ventilation, and oxygen therapy. Fortunately, we had no mortality in the hypertensive group.
This association was also reported in other studies from Asia. In concert with our findings, Guan et al. (16), reported hypertension as the most common comorbidity found among 1.099 patients with confirmed COVID-19. It was also the single highest risk factor (15%) of infection among 173 patients who developed the severe disease (23.7%) and among patients requiring ICU admission, mechanical ventilation, or death (35.8%). Moreover, systematic reviews and meta-analysis on the effect of hypertension on the important outcomes in patients with COVID-19 noticed that hypertension was more frequent in severe (47.6%) and fatal (47.9%) cases compared to total cases (14.3%) (20).
These combined comorbidities can explain, in part, the higher severity of the disease in hypertensive patients. Accumulating evidence suggested that comorbidities in patients with COVID-19 have been associated with worse outcomes such as, severe disease, and increased requirement of ICU admissions (21,22).
A study on 1.590 patients by Guan et al. (16) reported that the presence of any comorbidity resulted in poor outcomes among patients with confirmed COVID-19, and the greater the number of comorbidities, the poorer was the outcome. A meta-analysis supported an increased risk of severe illness and ICU admission in the presence of comorbidities (23). Another meta-analysis of 12 studies with cumulative 2.389 patients showed higher disease severity in COVID-19 patients with hypertension compared to normotensives patients (OR: 2.27, 95% CI: 1.80–2.86). Their meta-analysis on mortality data showed that COVID-19 patients with hypertension were more likely to die than normotensive patients with COVID-19 (OR 3.48 95% CI: 1.72–7.08) (24).
In a systematic review, Tian et al. (25) studied the predictors of mortality in hospitalized patients with COVID-19 and found that comorbidities which were associated with a higher risk of mortality were hypertension (odds ratio 2.5; p:< 0.00001), coronary heart disease (OR, 3.8; p:< 0.00001), and DM (OR, 2.0; p:< 0.00001). Chinese Centre for Disease Control and Prevention reported a case fatality rate (CFR) of 2.3% in 44.672 confirmed cases. The CFR was found to be significantly higher in patients with comorbidities; 6.0% for hypertension, 7.3% for diabetes, and 10.5% for the presence of CVD (26). In addition, a meta-analysis by Zuin et al. (27), including 419 patients, showed that COVID-19 patients with hypertension had higher mortality than normotensive COVID-19 patients OR 3.36 (CI 1.96-5.74; p: <0.0001).
However, contrary to these previous studies, a multivariate analysis showed that after adjusting for age and sex, hypertension was not significantly correlated with increased
COVID-19 disease severity or mortality (28). This study showed that other comorbidity confounders might interfere in the calculation of hypertension risk in relation to disease severity. Therefore, it is important to further analyse data from large scale studies with adjustment of important co-variants to avoid confounding bias. However, our patients with hypertension without other co-morbidities had a longer duration of hospitalization, ICU stay, mechanical ventilation, and oxygen therapy versus normotensive patients.
The spike protein present on the surface of the SARS-CoV-2 virus binds to the extracellular domain of transmembrane ACE2 receptor, with S protein priming by transmembrane serine protease 2 (TMPRSS2), to gain entry to host cells (29,30). SARS- CoV-2 uses ACE receptors for cell entry. This caused speculations over the use of ACE inhibitors and its relationship and effect on the outcomes of patients with hypertension (HTN) (31). Therefore, the effect of angiotensin-converting enzyme inhibitors (ACE inhibitors) or angiotensin receptor blockers (ARBs) therapy has been evaluated (32-34).
Reynolds et al. (34) looked at the history of antihypertensive usage in 12.594 patients undergoing COVID-19 testing in New York, USA. No association was found between the use of anti-hypertensive agents like ACE inhibitors, ARBs, calcium channel blockers, beta-blockers or thiazide diuretics and the likelihood of having a positive or negative result on COVID-19 testing.
A study by Mehta et al. (33) also failed to show any significant association between a positive COVID-19 test and the use of ACE inhibitors or ARBs. These results were supported by the results from a population case-control study from Italy (32).
CRP, an important inflammatory marker, was significantly elevated in our patients with hypertension compared to normotensive patients and was significantly correlated with disease severity, as previously described in other studies. This finding indicated that hypertensive patients had a higher inflammatory response to COVID-19 (35-37).
Hypertension is a well-recognized risk factor for several conditions like coronary artery disease, stroke, and end-stage renal disease (ESRD). The most common pathophysiologic factor is vascular inflammation (38,39). Recent data showed a potential link between inflammation and hypertension, including CRP, oxidative stress, renin-angiotensin system (RAS), prostaglandin, adaptive immune system. However, it is likely that in hypertensive patients, vascular inflammation and endothelial damage may increase the risk of infection and severity of COVID-19.
Different viruses incorporate cyclophilin A and cyclophilin B in their virions and use it to bind and infect other cells (40). It was found that patients with hypertension had increased expression of cyclophilin A in both the bronchoalveolar lavage (BAL) and blood samples (41). This might explain the possibility of increased risk and susceptibility of hypertensive patients to severe COVID-19 disease and worse outcomes. However, it is evident that further studies would be needed to confirm these findings.
gh other studies have shown various hematologic abnormities like lymphopenia, disturbed neutrophil: lymphocyte ratio, and thrombocytopenia as parameters of disease severity (42), there was no hematological difference between hypertensive and normotensive groups in our study.
The relatively small sample size and the retrospective nature are the main limitations of our report. Nevertheless, the strength of this study is the direct comparison of hypertensive and normotensive COVID-19 patients at the same place and time. Furthermore, it is one of the first studies from the Middle East region studying the outcome in COVID-19 patients.
In conclusion, hypertension is a common comorbidity in patients with COVID-19 and is associated with increased disease severity and higher risk for ICU admission and the need for mechanical ventilation. Clinical assessment of symptomatic hypertensive patients with COVID-19 with and without other comorbidities and those on different antihypertensive medications can demarcate the effect of hypertension on the course of the disease.
Conflicts of Interest:
None to declare
Funding:
Medical Research Centre, Hamad Medical Corporation (HMC), Doha (Qatar).
References
- 1.Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corman VM, Muth D, Niemeyer D, Drosten C. Hosts and Sources of Endemic Human Coronaviruses. Adv. Virus Res. 2018;100:163–188. doi: 10.1016/bs.aivir.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan Y, Chang L, Wang L. Laboratory testing of SARS-CoV, MERS-CoV, and SARS-CoV-2 (2019-nCoV): Current status, challenges, and countermeasures. Rev Med Virol. 2020;30(3):e2106. doi: 10.1002/rmv.2106. doi:10.1002/rmv.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin Y, Yang H, Ji W, et al. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses. 2020;12(4):372. doi: 10.3390/v12040372. Published 2020 Mar 27 doi:10.3390/ v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coronavirus Update (Live): 15,842,118 Cases and 639,892 Deaths from COVID-19 Virus Pandemic - Worldometer. Worldometers.info. 2020 [cited 24 July 2020]. Available from: https://www.worldometers.info/coronavirus . [Google Scholar]
- 7. COVID19 Home [Internet]. Covid19.moph.gov.qa. 2020 [cited 24 July 2020]. Available from: https://covid19.moph.gov.qa/EN/Pages/default.aspx . [Google Scholar]
- 8.Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020;81:e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Hu B, Hu C. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Yang B, Li Q. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu C, Chen X, Cai Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clinical management of COVID-19. Who.int. 2020 [cited 24 July 2020]. Available from: https://www.who.int/publications/i/item/clinical-management-of-covid-19 . [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Bener A, Al-Suwaidi J, Al-Jaber K, Al-Marri S, Dagash MH, Elbagi IE. The prevalence of hypertension and its associated risk factors in a newly developed country. Saudi Med J. 2004;25:918–922. [PubMed] [Google Scholar]
- 15.Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of Underlying Diseases in Hospitalized Patients with COVID-19: a Systematic Review and Meta Analysis. Arch Acad Emerg Med. 2020;8(1):e35. [PMC free article] [PubMed] [Google Scholar]
- 16.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noh J, Kim HC, Shin A, et al. Prevalence of Comorbidity among People with Hypertension: The Korea National Health and Nutrition Examination Survey 2007-2013. Korean Circ J. 2016;46:672–680. doi: 10.4070/kcj.2016.46.5.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Ma JJ, Liu J, Zeng DD, Song C, Cao Z. Prevalence and Risk Factors of Comorbidities among Hypertensive Patients in China. Int J Med Sci. 2017;14:201–212. doi: 10.7150/ijms.16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gold MS, Sehayek D, Gabrielli S, Zhang X, McCusker C, Ben-Shoshan M. COVID-19 and comorbidities: a systematic review and meta- analysis. Postgrad Med. 2020:1–7. doi: 10.1080/00325481.2020.1786964. doi:10.1080/00325481.2020.1786964. [DOI] [PubMed] [Google Scholar]
- 21.Lian J, Jin X, Hao S, et al. Analysis of Epidemiological and Clinical Features in Older Patients With Coronavirus Disease 2019 (COVID-19) Outside Wuhan. Clin Infect Dis. 2020;71:740–747. doi: 10.1093/cid/ciaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tadic M, Cuspidi C, Grassi G, Mancia G. COVID-19 and arterial hypertension: Hypothesis or evidence? J Clin Hypertens (Greenwich) 2020 doi: 10.1111/jch.13925. 10.1111/jch.13925. doi:10.1111/jch.13925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain V, Yuan JM. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: a systematic review and meta-analysis. Int J Public Health. 2020;65:533–546. doi: 10.1007/s00038-020-01390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Wu J, Sun X, et al. Association of hypertension with the severity and fatality of SARS-CoV-2 infection: A meta-analysis. Epidemiol Infect. 2020;148:e106. doi: 10.1017/S095026882000117X. doi:10.1017/S095026882000117X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian W, Jiang W, Yao J, et al. Predictors of mortality in hospitalized COVID-19 patients: A systematic review and meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.26050. 10.1002/jmv.26050. doi:10.1002/jmv.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease Control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. 10.1001/jama.2020.2648. doi:10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 27.Zuin M, Rigatelli G, Zuliani G, Rigatelli A, Mazza A, Roncon L. Arterial hypertension and risk of death in patients with COVID-19 infection: Systematic review and meta-analysis. J Infect. 2020;81:e84–86. doi: 10.1016/j.jinf.2020.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang S, Wang J, Liu F, et al. COVID-19 patients with hypertension have more severe disease: a multicenter retrospective observational study. Hypertens Res. 2020;43:824–831. doi: 10.1038/s41440-020-0485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang P, Zhu L, Cai J, et al. Association of Inpatient Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Mortality Among Patients With Hypertension Hospitalized With COVID-19. Circ Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Xu W, Hu G, et al. Retraction Note to: SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion [retraction of: Cell Mol Immunol. 2020 Apr 7] Cell Mol Immunol. 2020;17(8):894. doi: 10.1038/s41423-020-0498-4. doi: 10.1038/s41423-020-0498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta N, Kalra A, Nowacki AS, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020:e201855. doi: 10.1001/jamacardio.2020.1855. doi:10.1001/ jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reynolds HR, Adhikari S, Pulgarin C, et al. Renin–angiotensin–aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. doi:10. 1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blake GJ, Rifai N, Buring JE, Ridker PM. Blood pressure, C-reactive protein, and risk of future cardiovascular events. Circulation. 2003;108:2993–2999. doi: 10.1161/01.CIR.0000104566.10178.AF. [DOI] [PubMed] [Google Scholar]
- 37.Ogura S, Latapati R, Shimosawa T, Nakayama T. Vascular Inflammation and Hypertension Austin J Cardiovasc Dis Atherosclerosis. 2016;3(1):1017. [Google Scholar]
- 38.Case AJ, Zimmerman MC. Sympathetic-mediated activation versus suppression of the immune system: consequences for hypertension. J Physiol. 2016;594:527–536. doi: 10.1113/JP271516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caillon A, Schiffrin EL. Role of inflammation and immunity in hypertension: recent epidemiological, laboratory, and clinical evidence. Curr Hypertens Rep. 2016;18(3):21. doi: 10.1007/s11906-016-0628-7. doi:10.1007/s11906-016-0628-7. [DOI] [PubMed] [Google Scholar]
- 40.Pushkarsky T, Zybarth G, Dubrovsky L, et al. CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A. Proc Natl Acad Sci U S A. 2001;98:6360–6365. doi: 10.1073/pnas.111583198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radzikowska U, Ding M, Tan G, et al. Distribution of ACE2, CD147, CD26 and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy. 2020 doi: 10.1111/all.14429. 10.1111/all.14429. doi:10.1111/all.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95:834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]


