Abstract
Severe coronavirus disease 2019 (COVID-19) is often associated with features of the hypercoagulable state which can manifest as venous thromboembolism (VTE) and/or microthrombosis. Given the high risk of VTE in critically ill COVID-19 patients, appropriate VTE prophylaxis seems to be an important part of managing these patients. Although many protocols regarding venous thromboembolism (VTE) prophylaxis or therapeutic (full-dose) anticoagulation have been conducted worldwide, primarily in hospitalised adult patients, details on paediatric patients, if included, are limited or incomplete. The current evidence suggests that anticoagulation therapy with low molecular weight heparins (LMWH) appears to be associated with better prognosis in patients with moderate to severe COVID-19 induced coagulopathies or elevated D-dimer levels. Our recommendations are intended to offer guidance for anticoagulation prophylaxis and treatment in COVID-19 children and adolescent patients and not intend to supersede the clinician’s judgment. We are also conscious that several clinical questions deserve further studies and clarifications because this area is rapidly evolving. (www.actabiomedica.it)
Keywords: SARS-Corona-Virus-2, COVID-19, venous thromboembolism, anticoagulation chemoprophylaxis, pediatric
Introduction
Increasing numbers of confirmed cases and mortality rates of coronavirus disease 2019 (COVID-19) are occurring in several countries and continents. Up to September 10th 2020, COVID-19 has led to 899,916 deaths (1). The mortality rate varies from country to country and depends on the capacity and performance of the health care systems.
The clinical spectrum of infection with the novel SARS-CoV-2 ranges from the absence of any symptoms to fatal septic shock. Of all COVID-19 reported cases in adults and children, 81% had a mild illness, 14% severe illness (e.g. dyspnoea or oxygen saturation ≤93%) and 5% critical illness (e.g. respiratory failure or septic shock) (2).
From the beginning of the pandemic, Iran was and still is considered a high-risk area with a mortality rate significantly higher compared to most studies from China (3-5). Older age and comorbidities such as cardiovascular disease, diabetes, hypertension, and lung disease were identified as risk factors for severe illness and mortality. An overall 8% mortality rate was reported, the majority were among the ICU admitted patients (5/55.6%) (3).
The transition from mild to severe in patients with COVID-19 may be caused by cytokine storms and increased hypercoagulability with a significant risk of thromboembolic complications, mainly affecting the venous, but also the arterial vascular system (6). To describe the coagulation changes in COVID-19 patients, the term CAC (COVID-19-associated coagulopathy) has been introduced (7). In critically ill patients, the range of thromboembolism was 25%-27% for venous thrombosis and approximately 4% for arterial thrombosis (6). Disseminated intravascular coagulation (DIC) is observed most often in severe cases of COVID-19 (about 2% of all hospitalized patients) and indicates a poor prognosis. The characteristic laboratory findings of CAC, elevated levels of D-dimer and fibrin degradation products indicate a highly thrombotic state with high fibrin turnover. However, other markers of disseminated intravascular coagulation remain relatively unchanged.
Although many protocols regarding venous thromboembolism (VTE) prophylaxis or therapeutic (full-dose) anticoagulation have been conducted worldwide (8-17), primarily in hospitalised adult patients, details on paediatric patients, if included, are limited or incomplete (18-20).
We propose a practical approach to CAC in patients with COVID-19, based on current literature knowledge and local institutional experience.
Search strategy
We searched through Scholar, PubMed, Scopus, ISI, Wanfang database, and guideline-relevant websites, such as SIGN, WHO, and GIN from February to August 2020 with the following keywords: “COVID-19,” “SARS-CoV-2,” “SARS-coronavirus-2,” “Iran,” “coagulopathy,” “thrombosis,” “pediatric,” “adolescents,” “thrombotic guideline,” ”epidemiology,” “management,” and “phenotype,” “Severity,” “asymptomatic,” “mild,” “moderate,” “severe,” “critical,” and “arterial thrombosis,” “venous thrombosis,” “venous thromboembolism,” “enoxaparin,” and “D-dimer,” “ferritin,” “fibrinogen,” “C-reactive protein,”. Registered guidelines relevant to COVID-19 and VTE, printed in English, and published in peer-reviewed journals or on websites were also included. The references were assessed by our analyst using the reference management software (Endnote X9). Data extraction and cross-checking was also performed by our main editor accordingly. The literature search was updated until August 2020.
Research ethics and patient consent
The Ethics Committee approved the study protocol at Hematology Research Center, Shiraz University of Medical Sciences, Shiraz. The study protocol was conformed to the ethical guidelines of the 1975 Helsinki Declaration. The patient’s consent was not required by the Ethics Committee for the study development.
Pathophysiology of hypercoagulable state in COVID-19
The mechanisms that activate coagulation in SARS-CoV-2 infection is complex and multifactorial.
In general, microorganisms and viruses can activate monocytes, tissue macrophages, and endothelial cells, thus triggering the production of proinflammatory cytokines and activation of the coagulation protease cascade. Both, inflammation, and coagulation, are synchronous responses of the host’s defence aimed at containing invading pathogens. These complex processes are referred to as thrombo-inflammation or immunothrombosis. The loss of normal antithrombotic and anti-inflammatory functions of endothelial cells lead to dysregulation of coagulation, platelet activation, and leukocyte recruitment in the microvasculature, with complement activation likely playing an important role in the context of COVID-19-associated complications (21). Furtherly, hypoxia can predispose to thrombosis by increasing blood viscosity and via a hypoxia-inducible transcription factor-dependent signalling pathway (22) and an immune-mediated damage by antiphospholipid antibodies may contribute to thrombosis, as speculated by Zhang et al. (23).
Stages of CAHA (COVID-19 associated hemostatic abnormalities) and laboratory patients’ monitoring
In stage 1 CAHA, the patient has non-severe symptoms and may be cared for at home or in the hospital. Pulmonary microthrombi may be missed at this stage on computed tomography, if performed for respiratory symptoms (24).
In stage 2 CAHA, the patient may develop more severe symptoms and require critical care support. These patients may have filling defects noted on CT imaging due to pulmonary thrombi or emboli. They may also have asymptomatic DVT in the lower limbs signifying extensive coagulation activation (24).
In stage 3 CAHA, the patient is worsening clinically, requiring higher-level critical care support. Extensive pulmonary thrombi and systemic thrombosis, including disseminated intravascular coagulation in some patients (24).
Therefore, there is a need to identify the increased risk of thrombotic events at an early stage and to prevent thrombotic events and organ damage as far as possible.
Based on the currently available literature, the International Society on Thrombosis and Hemostasis (ISTH) recommends measuring D-dimers, prothrombin time (PT), platelet count, and fibrinogen in all patients who present with SARS-Cov-2 infection (25).
D-dimer levels of 0.5 μg/mL or higher were found in 59.6%% of patients with severe disease vs 43.2% of those with mild disease (26). High levels also correlated with the need for intensive care and death. A prolongation time of PT > 3 seconds or of PTT > 5 seconds are an independent risk factor for thrombosis. Thrombocytopenia at presentation is associated with an increased risk of severe disease and death, with a weighted mean difference of 31 × 109/L in the platelet count between those a severe and non-severe disease (27). Fibrinogen levels, which are elevated in the initial phase, drop late in the course of disease in non-survivors and may signal impending death (28).
These measures of coagulopathy may help in stratifying patients requiring hospital admission and to monitor their general clinical condition for critical care support (25).
Pharmacological thromboprophylaxis of COVID-19-associated coagulopathy in adults
To the best of our knowledge, all published studies regarding VTE prophylaxis in patients with COVID-19 have been conducted mainly in adult critically ill patients.
Histopathologic studies reveal diffuse alveolar damage with profound inflammation, thrombosis, and thrombotic microangiopathy of small vessels and capillaries of the lung. Endothelial cell injury and diffuse microvascular thrombosis suggestive of thrombotic microangiopathy have also been reported in extrapulmonary organs, which may explain the acute onset of multiorgan failure (29-31).
As per the current recommendations, patients in stage 1 CAHA should receive prophylactic low-molecular weight (LMW) heparin in the absence of contraindications (24,29). For stage 2 CAHA there are several trials currently underway to determine whether full dose compared to prophylactic dose anticoagulation may help to prevent worsening of the coagulopathy and ischemia in the extrapulmonary circulation. Once thrombi are detected, it is standard practice to treat such patients with therapeutic (full dose) anticoagulation (24,25,29). Intensification of antithrombotic therapy (double dose LMWH prophylaxis) as per stage 2 in combination with several other experimental measures (thrombolysis) may sometimes be effective in stage 3 CAHA (24).
In summary, the present recommendations aim to provide a guide for frontline clinicians caring for patients with COVID-19 and/or patients with chronic thrombotic conditions requiring ongoing management in the era of the COVID-19 pandemic. Anticoagulant strategies to prevent or treat VTE in ambulatory and hospitalized patients with proven SARS-CoV-2 infection have gained tremendous attention, over the last weeks and months, to prevent or treat thrombosis.
How should VTE prophylaxis be administered in pediatric and adolescent patients with COVID-19?
According to Dong et al. (28) report, 94 (4.4%), 1088 (51.0%), and 826 (38.7%) pediatric and adolescent COVID-19 cases were diagnosed as asymptomatic, mild, or moderate (32). Rare paediatric deaths have been reported globally and, as in adults, the risk of severe illness and death was higher in those with underlying comorbidities (33). Consequently, the pediatric experience caring for infants and children with COVID-19 in hospitals is limited.
Therefore, we prepared the following general institutional recommendations in the knowledge that several clinical questions deserve further study and clarification. At first, we divided the children and adolescent patients with COVID-19 in two groups according to the disease severity (Figure 1).
Figure 1.
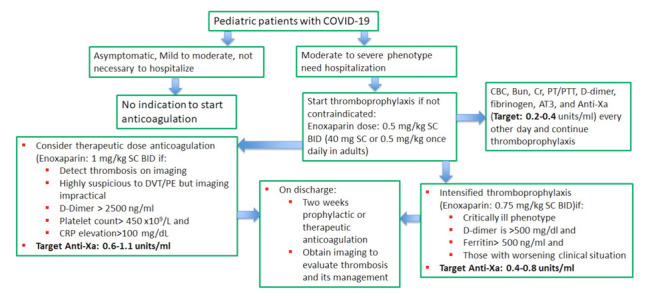
The suggested alogorithmic approach for anticoagulation therapy in COVID-19 patients ≤ 18 years
The mild severity represents those with mild symptoms without radiographic features. Patients with moderate phenotype present with fever, respiratory symptoms, and radiographic features. Patients with severe phenotype meet one of three criteria: (a) dyspnoea, a respiratory rate greater than 30/min, (b) oxygen saturation less than 94% in ambient air, and (c) PaO2/FiO2 less than 300 mmHg. Critically ill patients meet one of three criteria: (a) respiratory failure, (b) septic shock, and (c) multiple organ failure (34).
Outpatients with mild COVID-19 should not receive pharmacological thromboprophylaxis, but increased mobility and appropriate hydration, in the presence of fever or vomiting should be encouraged. Given the rapid deterioration reported in many adult patients with mild symptoms, regular monitoring of D-dimer, fibrinogen, platelet count, PT, and PTT every 48 hours, for 5-7 days can be advised, along with a high index of suspicion for thrombosis. Patients with D-dimer levels > 300 ng/mL (>3 µg/mL) should receive prophylaxis with low molecular weight heparin (LMWH) and should be assessed for deep vein thrombosis.
Patients with moderate COVID-19 who need hospitalization should receive anticoagulation therapy with LMWH prophylactic dose. In the case of severe COVID-19 patients, if D-dimer is >500 ng/mL (>5 µg/mL) and serum ferritin is >500 ng/mL, and in those with worsening clinical situation, anticoagulation therapy intensification is recommended, associated to ultrasonography screening (Table 1).
Table 1.
Anticoagulation chemoprophylaxis with enoxaparin for patients with moderate, severe, and critical COVID-19 who need hospitalization*
| Profilactic dose | Profilactic intensified dose | Therapeutic dose | ||
| Target anti-Xa** | 0.2-0.4 units/mL |
0.4-0.8 units/mL |
0.6-1.1 units/mL |
|
| ≤ 2 months | 0.75 mg/kg/dose SC q12 h |
1 mg/kg/dose SC q12 h |
1.5 mg/kg/dose SC q12 h |
|
| >2 months to ≤ 18 years old | ||||
| <40 kg | 0.5 mg/kg/dose SC q12 h |
0.75 mg/kg/dose SC q12 h |
1 mg/kg/dose SC q12 h |
|
| >40 kg | 40 mg SC qd |
40-60 mg SC qd** |
40 mg q12 h |
|
* Normal renal function and no contraindications including active bleeding, acute stroke, require an invasive procedure within the next 24 hours and platelet count <25×109/L
** It should be noted that dose escalation/de-escalation is warranted to obtain recommended target anti-Xa
In critically ill COVID-19 patients, intensified anticoagulation with therapeutic dose is suggested if D-dimer >2500 ng/mL (> 25 µg/mL), platelet count > 450 x109/L and C-reactive protein (CRP) >100 mg/dL (Table 1).
During treatment, the coagulation index should be closely monitored to prevent excessive anticoagulation and bleeding complications. Anti-factor Xa levels and APTT ratios are good ways to determine the efficacy of LMWH and unfractionated heparin regimens in patients with confirmed VTE. Once the desired level is reached, the test can be repeated every 6-7 days. If an invasive procedure is required, skipping two doses of LMWH before the intervention is advised (20).
In patients with renal impairment (Cr Cl < 30 ml/min), unfractionated heparin (UFH) is the anticoagulant of choice with a dose of 75 Units/kg intravenous infusion over 10 minutes (max 5,000 Units) as a loading dose. It should be followed by a maintenance continuous IV infusion (maximum initial rate of 1,300 Units/hour). The recommended infusion rates are 28 Units/kg/hour, 20 Units/kg/hour, and 18 Units/kg/hour in infants < 12 months, children 1 to 15 years, and children 16 years and older, respectively.
For patients already on anticoagulant therapy, the suggested algorithm approach is illustrated in figure 2.
Figure 2.
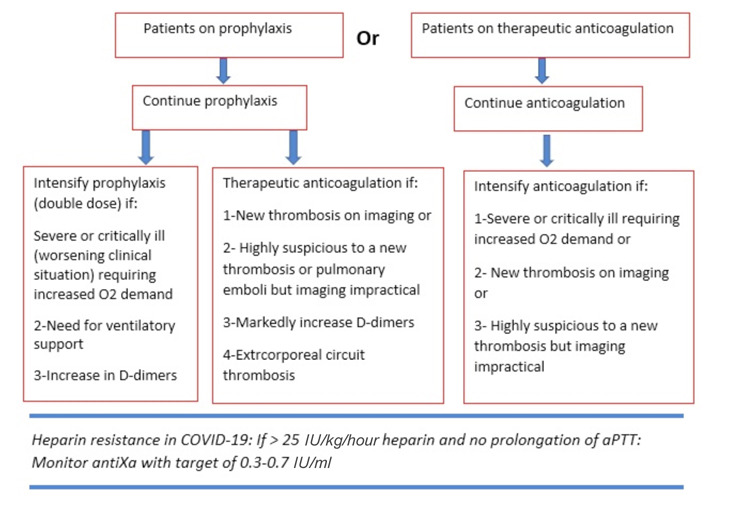
The suggested alogorithmic approach of anticoagulation administration in COVID patients ≤ 18 years old who have been already on anticoagulation
Transitions from the hospital to the outpatient setting are important timepoints to re-assess therapies and ensure adequate communication between clinicians, the patient, and families or caregivers. The use of extended thromboprophylaxis in patients with COVID-19 after hospital discharge should be considered if the VTE risk stratification indicates persistently elevated VTE risk due to comorbidities as well as D-dimer concentrations higher than twice the upper reference range.
However, there are no specific data available on the efficacy and safety of such a strategy.
There are some studies suggesting thromboprophylaxis or therapeutic anticoagulation in post-hospitalization discharge patients (35).
Based on our experience, we recommend at least two weeks of anticoagulation therapy on discharge and imaging studies to evaluate thrombosis, and an extended anticoagulation treatment if necessary.
Conclusion
Thrombotic complications are reported frequently in adults as a COVID-19 complication. The literature describing SARS-CoV-2 infection leading to severe COVID-19 in children and adolescents is limited, but it is growing. Based on our gained experience with COVID-19 patients, we prepared some recommendations to guide clinicians caring children and adolescent patients with COVID-19. We are conscious that several clinical questions deserve further studies and clarifications because this area is rapidly evolving. We believe that there is also an urgent need for multicentre international guidelines in order to provide a better understanding of the specific features, needs, and challenges of critically ill children with SARS-COV-2 infection, especially in those with pre-existing medical conditions.
Acknowledgements
We would like to thank our patients, without whom we would not have been able to complete this study.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard: WHO; 2020 [updated Data last updated: 2020/9/10. Available from: https://covid19.who.int/ [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Shahriarirad R, Khodamoradi Z, Erfani A, et al. Epidemiological and clinical features of 2019 novel coronavirus diseases (COVID-19) in the South of Iran. BMC Infect Dis. 2020 Jun 18;20(1):427. doi: 10.1186/s12879-020-05128-x. doi: 10.1186/s12879-020-05128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian S, Hu N, Lou J, wt al. Characteristics of COVID-19 infection in Beijing. J Inf Secur. 2020;80:401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18:1559–1561. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bikdeli B, Madhavan MV, Jimenez D. the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llau JV, Ferrandis R, Sierra P, et al. SEDAR-SEMICYUC consensus recommendations on the management of haemostasis disorders in severely ill patients with COVID-19 infection. Rev Esp Anestesiol Reanim. 2020;67:391–399. doi: 10.1016/j.redar.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casini A, Alberio L, Angelillo-Scherrer A, et al. Thromboprophylaxis and laboratory monitoring for in-hospital patients with COVID-19 - a Swiss consensus statement by the Working Party Hemostasis. Swiss Med Wkly. 2020 Apr 11;150:w20247. doi: 10.4414/smw.2020.20247. doi: 10.4414/smw.2020.20247. PMID: 32277760. [DOI] [PubMed] [Google Scholar]
- 11.Vazquez FJ, Korin J, Baldessari EM, et al. Recommendations for the use of thromboprophylaxis in hospitalized patients with COVID-19 in Argentina. Medicina (B Aires) 2020;80(Suppl 3):65–66. [PubMed] [Google Scholar]
- 12.Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramacciotti E, Macedo AS, Biagioni RB, et al. Evidence-Based Practical Guidance for the Antithrombotic Management in Patients With Coronavirus Disease (COVID-19) in 2020. Clin Appl Thromb Hemost. 2020 Jan-Dec;26 doi: 10.1177/1076029620936350. 1076029620936350. doi: 10.1177/1076029620936350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American Society of Hematology. https://www.hematology.org/covid-19/covid-19-and-coagulopathy . http://www.hematology.orgcovidcovid-and-coagulopathy . Accessed May 5, 2020. [Google Scholar]
- 15.Song J-C, Wang G, Zhang W, Zhang Y, Li W-Q, Zhou Z. Chinese expert consensus on diagnosis and treatment of coagulation dysfunction in COVID-19. Mil Med Res. 2020;7(1):19. doi: 10.1186/s40779-020-00247-7. doi: 10.1186/s40779-020-00247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. BSH Haemostatis and Thrombosis Task Force. https://b-s-h.org.uk/ media/18206/dic-score-in-covid-19-pneumonia_01-04-2020.pdf . Accessed May 5, 2020:1-2. [Google Scholar]
- 17.Alhazzani W, Al-Suwaidan FA, Al Aseri ZA, et al. The Saudi Critical Care Society Clinical Practice Guidelines on the management of COVID-19 patients in the intensive care unit. Saudi Crit Care J. 2020;4:27–44. [Google Scholar]
- 18.Loi M, Branchford B, Kim J, Self C, Nuss R. COVID-19 anticoagulation recommendations in children. Pediatr Blood Cancer. 2020 Jun 18:e28485. doi: 10.1002/pbc.28485. doi: 10.1002/pbc.28485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50:72–81. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ávila-Castro D, Ortiz-Torres G, Sánchez-Jara B, et al. Proposal for the management of COVID-19-associated coagulopathy in children. Gac Med Mex. 2020;156:344–353. doi: 10.24875/GMM.20000418. [DOI] [PubMed] [Google Scholar]
- 21.Oudkerk M, Buller HR, Kuijpers D, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297:E216–E222. doi: 10.1148/radiol.2020201629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta N, Wish JB. Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors: a Potential New Treatment for Anemia in Patients With CKD. Am J Kidney Dis. 2017;69:815–26. doi: 10.1053/j.ajkd.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Wang CY, Zhou P, Yue H, Du R. Histopathologic Changes and SARS-CoV-2 Immunostaining in the Lung of a Patient With COVID-19. Ann Intern Med. 2020 Aug 18;173(4):324. doi: 10.7326/L20-0895. doi: 10.7326/L20-0895. [DOI] [PubMed] [Google Scholar]
- 24.Thachil J, Cushman M, Srivastava A. A proposal for staging COVID-19 coagulopathy. Res Pract Thromb Haemost. 2020;4:731–736. doi: 10.1002/rth2.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID- 19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020 Jun 16;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ. Vander Heide RS. Pulmonary and Cardiac Pathology in Covid-19: The First Autopsy Series from New Orleans. medRxiv. doi: 10.1016/S2213-2600(20)30243-5. 2020.04.06.20050575; doi: https://doi.org/10.1101/2020.04.06.20050575 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S. Epidemiology of COVID-19 Among Children in China. Pediatrics. 2020 Jun;145(6):e20200702. doi: 10.1542/peds.2020-0702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 33.Chen Z, Fu J, Shu Q, Chen Y, et al. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr. 2020;16:240–246. doi: 10.1007/s12519-020-00345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu L, Wang J, Huang R, et al. Clinical characteristics of a case series of children with coronavirus disease 2019. Pediatr Pulmonol. 2020;55:1430–1432. doi: 10.1002/ppul.24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moores LK, Tritschler T, Brosnahan S, et al. Prevention, Diagnosis, and Treatment of VTE in Patients With COVID-19: CHEST Guideline and Expert Panel Report. Chest. 2020;158:1143–116. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]


