Abstract
Retinitis pigmentosa, age-related macular degeneration, and Parkinson’s disease remain major problems in the field of medicine. Some of the strategies being explored for treatment include replacement of damaged tissue by transplantation of healthy tissues or progenitor cells and delivery of neurotrophins to rescue degenerating tissue. One of the neurotrophins with promise is the ciliary neurotrophic factor (CNTF). In this study, we report the role played by CNTF in retinal cell differentiation and survival in retinal progenitors. We found that CNTF is a survival factor for multipotential human retinal cells and increased cell survival by 50%, over a 7-d period, under serum-free conditions, as determined by apoptotic assays (immunohistochemistry and flow cytometry). This effect is dose dependent with a maximum survival at a CNTF concentration of 20 ng/ml. We also report that CNTF might be a cell commitment factor, directing the differentiation mainly toward large multipolar cells with ganglionic and amacrine phenotype. These cells express tyrosine hydroxylase (amacrine cells) as well as, thy 1.1 and neuron-specific enolase (ganglionic cells). Additionally, there was also an increase in protein kinase C alpha, a protein expressed in rod and cone bipolars as well as cone photoreceptors and calbindin, a protein expressed in cone photoreceptors and horizontal cells. In our studies, CNTF doubled the number of cells with ganglionic phenotypes, and basic fibroblast growth factor doubled the number of cells with photoreceptor phenotype. Additionally, CNTF induced a subset of progenitors to undergo multiple rounds of cell division before acquiring the large multipolar ganglionic phenotype. Our conclusion is that CNTF could be an agent that has therapeutic potential and possibly induces differentiation of large multipolar ganglionic phenotype in a subset of progenitors.
Keywords: Ciliary neurotrophic factor, Survival factor, Human retinal progenitors, Differentiation
Introduction
Ciliary neurotrophic factor (CNTF) is a member of the cytokine family that includes leukemia inhibitory factor (LIF), oncostatin M, cardiotrophin-1 C, interleukin-6, and interleukin-11 (Bazan 1991; Kishmoto et al. 1994; Stahl and Yancopoulos 1994; Pennica et al. 1995). Ciliary neurotrophic factor has biological effects through the activation of a multi-subunit receptor complex, consisting of an extracellular CNTF binding subunit (CNTFα) and two transmembrane signal transduction proteins: glycoprotein gp130 and LIF receptor (Inoue et al. 1996; Ip and Yancopoulos 1996; Segal and Greenberg 1996; Yano and Chao 2000). Ciliary neurotrophic factor was originally purified from chick eye tissue and rat sciatic nerve on the basis of its survival promoting action on chick ciliary ganglionic neurons. Additionally, a multitude of functions and target cells for CNTF have been reported both in vivo and in vitro (for reviews, see Manthorpe et al. 1993; Sendtner et al. 1994). In vitro data support that CNTF is a survival factor for neurons of the peripheral sensory, sympathetic, and ciliary ganglia (Lehwalder et al. 1989). Ciliary neurotrophic factor is also known to induce glial progenitors to astrocytic 2 phenotype in the developing optic nerve culture (Hughes et al. 1988).
Both neurotrophic and neuroprotective effects of CNTF on photoreceptor cells have also been well documented. Intravitreal injection of brain-derived neurotrophic factor (BDNF), basic fibroblast growth factor (bFGF), and CNTF were reported to reduce retinal injury in response to ischemia and light-induced damage to photoreceptors in albino rats (LaVail et al. 1998). Specifically, CNTF is effective in retarding retinal degeneration in at least 13 different animal models of retinitis pigmentosa (RP; Unoki et al. 1994; Cayouette et al. 1998; Chong et al. 1999; Liang et al. 2001; Bok et al. 2002; Tao et al. 2002). Additionally, the first phase one trials in humans in which encapsulated CNTF-secreting cells were transplanted into seven late stage RP patients seem promising (Sieving et al. 2006).
Ciliary neurotrophic factor exhibits multiple effects during vertebrate retinogenesis, including regulation of photoreceptor cell differentiation. Opposing effects of CNTF on rod photoreceptor development in rodents and chickens have been reported (Kirsch et al. 1996). In rat retina, CNTF treatment results in a dramatic decrease in the number of rod photoreceptors with concomitant increase in the number of bipolar cells (Ezzeddine et al. 1997). Bhattacharya et al. (2004) in their model further provide extensive data to support CNTF’s role in bipolar cell differentiation. Similarly, Zahir et al. (2005) report that CNTF may increase the number of bipolar and glial cells when applied to multipotential retinal progenitors. Rhee et al. (2004) report that CNTF regulates transcription factor in photoreceptor precursors and regulates rod differentiation in mice. Similarly, CNTF induces an increase in Rho4D2 immunoreactive rod photoreceptors in chick retinal cultures (Kirsch et al. 1997). Xie and Adler (2000), however, report that Rho4D2 antibody, although rhodopsin specific in bovine retina, recognizes both rhodopsin and green cone pigment in chick retina, thus suggesting that cells labeled in chick retina could be both rods and green cones.
Expression of CNTF, RNA, and protein are developmentally regulated during retinal development and Muller glia have been identified as a source of CNTF (Wahlin et al. 2000). Also CNTF-specific α subunit of the CNTF receptor (CNTFRα) is expressed in retina (Kirsch et al. 1997; Fuhrmann et al. 1998). Localization of CNTFRα is highly regulated during retinal development, and the CNTF receptor expressing cell types include ganglion cells, subpopulation of amacrine, horizontal cells, and photoreceptors (Heller et al. 1995; Kirsch et al. 1997). Additionally, CNTFRα has been localized to glia and RPE. Ciliary neurotrophic factor supports survival, growth, and/or differentiation in a multitude of retinal cells.
We report in this study that CNTF (1) is a survival factor for multipotential retinal progenitors, (2) induces a subset of progenitors to acquire multipolar ganglionic phenotype, and (3) exposure induces an increase in several retinal-specific proteins: TH, thy 1.1, NSE, protein kinase C alpha (PKCα), calbindin, and rod transcription factor NR2E3. This implies that, at least in our system, there is an increase in the number of more than one cell type. The ability of CNTF to increase survival of retinal progenitors and its ability to induce differentiation could have significant therapeutic potential.
Material and Methods
Cell culture.
Retinal progenitor cell lines established by us have been well characterized (Dutt et al. 1994, 1996). For this study, retinal cells in 25–30 passages were cultured in serum-free defined medium comprising Dulbecco’s Modified Eagle’s Medium: Ham’s F-12 Nutrient Mixture 1:1, supplemented with 2 mM L-glutamine, 100 U/ml penicillin plus 100 μg/ml streptomycin (In vitrogen, Carlsbad, CA); 8.8 ng/ml putrescine (Collaborative Research, Waltham, MA), 2 mM HEPES buffer, 5 μg/ml insulin, 10 μg/ml insulin, 10 μg/ml transfin, 6.3 ng/ml progesterone, and 4 ng/ml sodium selenite (Sigma, St. Louis, MO). Recombinant human CNTF was from Chemicon (Temecula, CA).
Evaluation of mitogenic potential by measurement of DNA synthesis.
Retinal cells grown in serum-free defined medium were exposed to 20 ng/ml bFGF and 20 ng/ml CNTF. Tritiated thymidine was added at a concentration of 4 μCi/well to measure DNA synthesis. Trichloroacetic acid precipitated counts were determined at appropriate times, and the protein content was measured by Lowry’s method. Counts are expressed as counts per min per milligram protein, and each value represents an average of three to five counts+SD. DNA synthesis is directly correlated to mitogenic activity. The experiment was repeated five times. Each experiment consisted of an average of three to five independent samples.
Dose response to CNTF.
Cells grown in defined medium were exposed to different doses of CNTF (5–20 ng/ml) and monitored for 3 to 6 d. Photographs were taken at various times during the culture period. They are identified as untreated (A) cells, cells treated with 5 ng/ml CNTF (B, C), cells treated with 10 ng/ml CNTF (D, E), and cells treated with 20 ng/ml CNTF (F, G). Data presented is on day 6.
CNTF-induced survival.
To determine the survival potential of CNTF, retinal cells grown in defined medium+ CNTF (20 ng/ml) were processed by immunocytochemistry for double labeling with annexin V protein, expressed in apoptotic cells (green) and nuclear staining propidium iodide (red), and processed on days 4–6.
Detection of apoptosis by immunocytochemistry.
To determine the effect of CNTF on cell death by apoptosis, cells in the 27th passage were plated on eight-well glass chamber slides in serum containing medium for 4 h. They were then switched to defined medium. The cells were equilibrated overnight in defined medium and exposed to CNTF (20 ng/ml). On the sixth day after exposure, the cells were stained and analyzed. The cells were washed in phosphate-buffered saline (PBS) and fixed with 100% acetone. The cells were first stained with annexin V–FITC diluted 1:20 in binding buffer (10 mM Hepes/NaOH, pH 7.4, 140 mM NaCI, 2.5 mM CaCI2) for 30 min at 37°C. The cells were washed and stained with propidium iodide diluted 1:20 with the same binding buffer and incubated under the same conditions. Cells were mounted in 90% glycerol and viewed under a fluorescent microscope.
Fluorescence-activated cell sorting analysis of stained cells.
To quantitate survival potential of CNTF, retinal cells grown in defined medium+CNTF for 72 h were double labeled with annexin V–FITC and propidium iodide, before subjecting to fluorescence-activated cell sorting (FACS) analysis. Controls were cells grown in serum-free medium with vehicle only. Note the greater area of overlap (blue–annexin V, over red–propidium iodide) in control vs. cells treated with CNTF.
Immunohistochemistry.
Immunohistochemistry was performed according to our previously published technique to determine the various cell types generated in CNTF treated progenitors (Dutt et al. 1994). Cells were then exposed to CNTF or vehicle and were fixed with either acetone at −20° C for 20 min or 2% ice-cold paraformaldehyde in PBS for 20–60 min. To block nonspecific binding to antibodies, cells were preincubated with nonspecific antisera for 2 h. Cells were incubated with primary antibodies against neuron-specific enolase (NSE), glial fibrillary acidic protein (GFAP; 1:500), thy 1.1 (1:200), Th (1:200), PKCα (1:1,000), D2D3 (1–500), and calbindin (1:200) at room temperature for 1 h to overnight. After appropriate washes, the cells were reacted with appropriate fluorescent secondary antibody, goat anti-rabbit (1–5,000) and donkey anti-goat (1–5,000) at room temperature for 1 h. Washed slides were mounted in glycerol and visualized under Zeiss epifluorescent microscope and photographed. In each experiment, controls were included, with omission of the first antibody or the secondary antibody.
Western blot analysis.
At appropriate times, cells exposed to CNTF and controls were washed three times in cold PBS. Cells were lysed in lysis buffer (50 mM Tris, pH 7.4, 150 M NaC1, 1 mM DTT, 0.5% Triton X-100, 0.2 mM AEBSF, 0.5 mM benzamidine, 2 μg/ml aprotinin, 2 μg/ml chymostatin, 0.5 mM leupetin, 5 mg/ml pepstatin A, and 0.25 mg/ml soybean tyrosine inhibitor). The resulting supernatant was used for Western blot analysis. Whole-cell extracts containing 50 μg proteins were separated by electrophoresis on 10% sodium dodecyl sulfate (SDS) polyacrylamide gels and electrophoretically transferred to a polyvinylidene difluoride membrane (Immobilon P; Millipore Corp., Temecula, CA) in Tris–glycine buffer containing methanol and SDS. The nonspecific binding sites were blocked by immersing the membrane in 5% (w/v) nonfat dry milk in TBS-T (25 mM Tris–HC1, pH 7.6, 150 mM NaC1, and 0.05% Tween-20) for 2 h at room temperature on an orbital shaker. Membranes were rinsed with two changes of washing buffer. The membranes were treated with specific primary antibodies to determine the presence of specific retinal proteins. Antibodies for tyrosine hydroxylase, D2, D3, and D4 receptor protein, calbindin, and PKCα and thy 1.1 were procured from Chemicon Inc., and the antibody for neuron-specific enolase was from Zymed Corporation (San Francisco, CA). After reaction of the membranes with appropriate primary antibodies (dilution 1–200 to 1–1,000), the membranes were reacted with horseradish peroxidase-conjugated appropriate secondary antibodies. Detection was done with chemiluminescent substrates and X-ray film exposure.
Results
CNTF as a proliferative factor.
To test the effect of CNTF on progenitors, cells were plated at medium density 3×104/cm2 on tissue culture plastic in serum-containing medium. After the cell attachment and two washes in Hank’s balanced salt solution, with 1-h incubation in between at 37°C, cells were incubated in defined medium overnight for acclimatization and switched to fresh defined medium and incubated with 20 ng/ml CNTF 1 μCu/ml of 3HTdr for 24 h. 3HTdr incorporation is determined after TCA precipitation. Counts are expressed as counts per minute per milligram protein vehicle-treated cells and exposure to bFGF served as an internal control. Ciliary neurotrophic factor did not stimulate DNA synthesis; 3HTdr incorporation in CNTF treated cultures was similar to bFGF (20 ng/ml) and vehicle treated cultures. Figure 1 represents a mean of five samples. The experiment was repeated three times. There was no difference in bFGF or CNTF’s ability to induce cell proliferation as compared to vehicle-treated cells.
Figure 1.
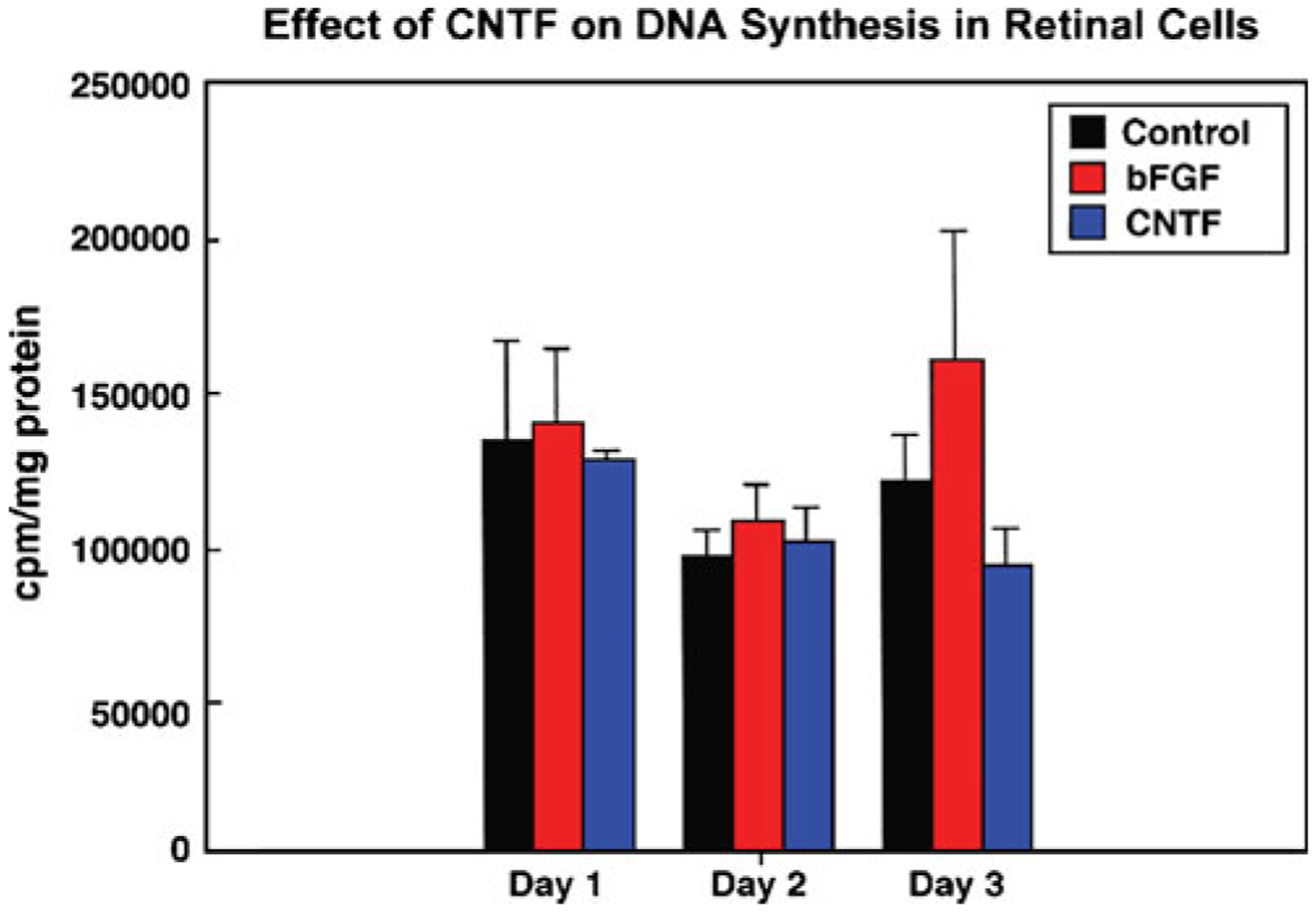
Evaluation of mitogenic potential of CNTF. Retinal cells grown in serum-free-defined medium were exposed to 20 ng/ml CNTF and 20 ng/ml bFGF (internal control). Tritiated thymidine at a concentration of 4 μCi/well was also included in culture medium; trichloroacetic acid precipitated counts were determined on days 1–3. Counts were expressed as counts per min per milligram protein. The data is an average of five samples ± SD. Note, there is no significant difference in DNA synthesis between the controls vs. bFGF and CNTF.
CNTF: a survival factor.
To determine if CNTF acts as a survival factor, cells plated in defined medium at a density of 3×104 cells/cm2 were exposed to 5, 10, and 20 ng/ml of CNTF, and cells were evaluated both morphologically and for cell survival determined by annexin V binding and flow cytometry between 3 and 6 d. Figure 2 depicts the morphology of the cells treated with 5, 10, and 20 ng/ml of CNTF. Figure 2A shows cells that were treated with the vehicle only; note the cells undergoing apoptosis with fragmented nuclei (arrows). Similarly, note in Fig. 2B, C cells exposed to 5 ng/ml of CNTF; note the heterochromatic nuclear band in cells undergoing apoptosis (arrows). Arrowheads in Fig. 2B point to apoptotic bodies. In Fig. 2D, E, note the cells exposed to 10 ng/ml of CNTF; there is a significant drop in the number of cells undergoing apoptosis. In cells exposed to 20 ng/ml of CNTF, indicated in Fig. 2F, G, there is a further significant decrease in apoptotic cells; very few cells with heterochromatic nuclear bands were seen. Data presented is on day 6.
Figure 2.
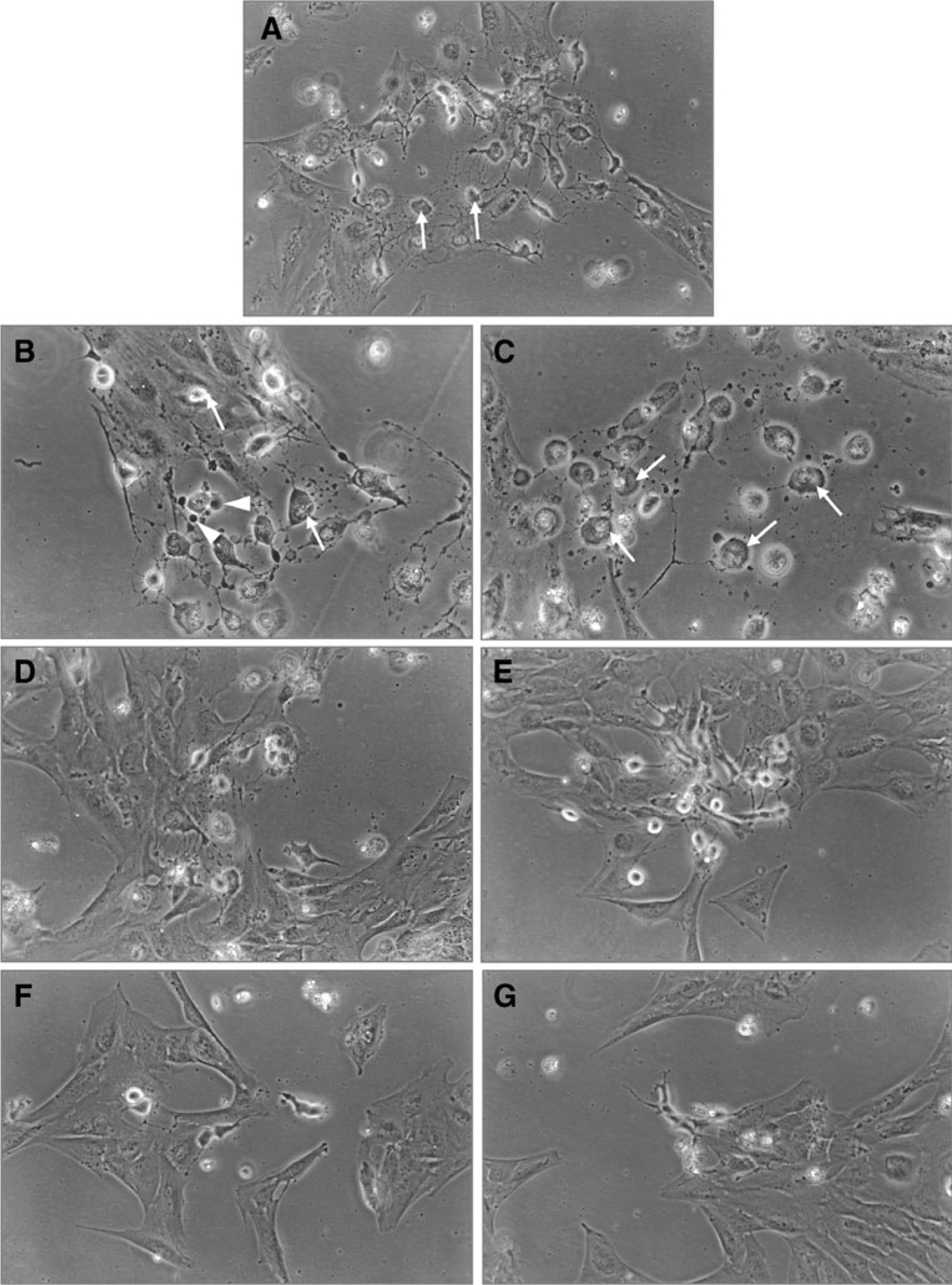
CNTF as survival factor: dose response curve. Cells grown in defined medium were exposed to different doses of CNTF (5–20 ng/ml) and monitored for 3 to 6 d. Photographs were taken on day 6. Untreated cells (A), note cells undergoing apoptosis (arrows). Cells treated with 5 ng/ml CNTF (B, C), note in (B), cells with apoptotic bodies (arrowheads), cells with heterochromatic nuclear band (arrows), cells treated with 10 ng/ml CNTF (D, E), and cells treated with 20 ng/ml CNTF (F, G). Note the increased survival of cells as the concentration of CNTF increased from 5 to 20 ng/ml (apoptotic cells marked by arrows).
Determination of cell survival by apoptotic assay.
When cells exposed to 20 ng/ml CNTF (concentration that gave maximum survival) were assessed by annexin V binding, it was found that increasing the concentration of CNTF beyond 20 ng/ml did not result in a concomitant increase in cell survival. Cells treated with 20 ng/ml CNTF were processed by immunohistochemistry for double labeling with annexin V protein, expressed in apoptotic cells (green/yellow) and nuclear staining with propidium iodide (red). Data are from cells treated with CNTF 20 ng/ml on day 6. Cultures were supplemented with fresh medium and CNTF every second day. Similar results were seen on day 4; data not included. Note: about 50% more cells are labeled with annexin V in untreated cultures (Fig. 3A, B-greenish yellow color). Also note the significant drop in the green/yellow label in cells labeled with annexin V (Fig. 3C, D).
Figure 3.
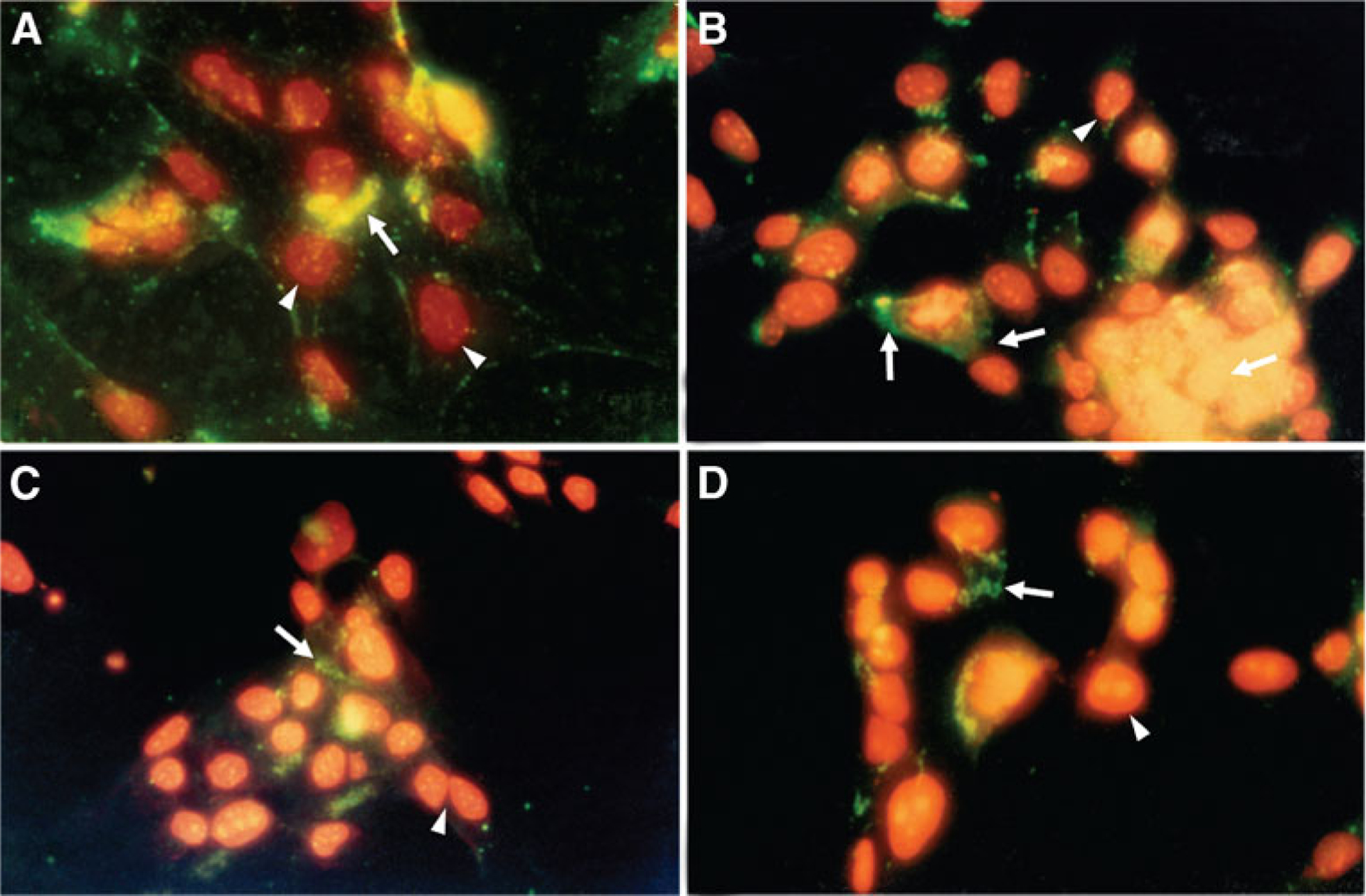
To determine the survival potential of CNTF, retinal cells grown in defined medium +CNTF (20 ng/ml) were processed by immunocytochemistry for double labeling with annexin V protein, expressed in apoptotic cells (green/yellow arrows), and nuclear staining propidium iodide (red arrowheads), processed on day 6. Note the increased staining (about 50%) with annexin V in untreated cells (A, B) vs. cells treated with CNTF (C, D); the cells are labeled red propidium iodide. Also, note a significant drop in annexin V positive cells (green color).
To quantify the number of viable cells in CNTF-treated cultures.
Cells double labeled for annexin V and propidium iodide were assessed by flow cytometry. Note the greater overlap of blue–annexin V over red propidium iodide in the control untreated cultures vs. the treated cultures on day 6. The blue line overlapping the red propidium (dead) cells is reduced by 50% in treated cultures and has moved toward the right, reflecting the increase in number of viable cells. Similar results were noted when cells labeled with annexin V binding were counted under a fluorescent microscope. Similar results were noted in three separate experiments, Fig. 4 represents one such experiment.
Figure 4.
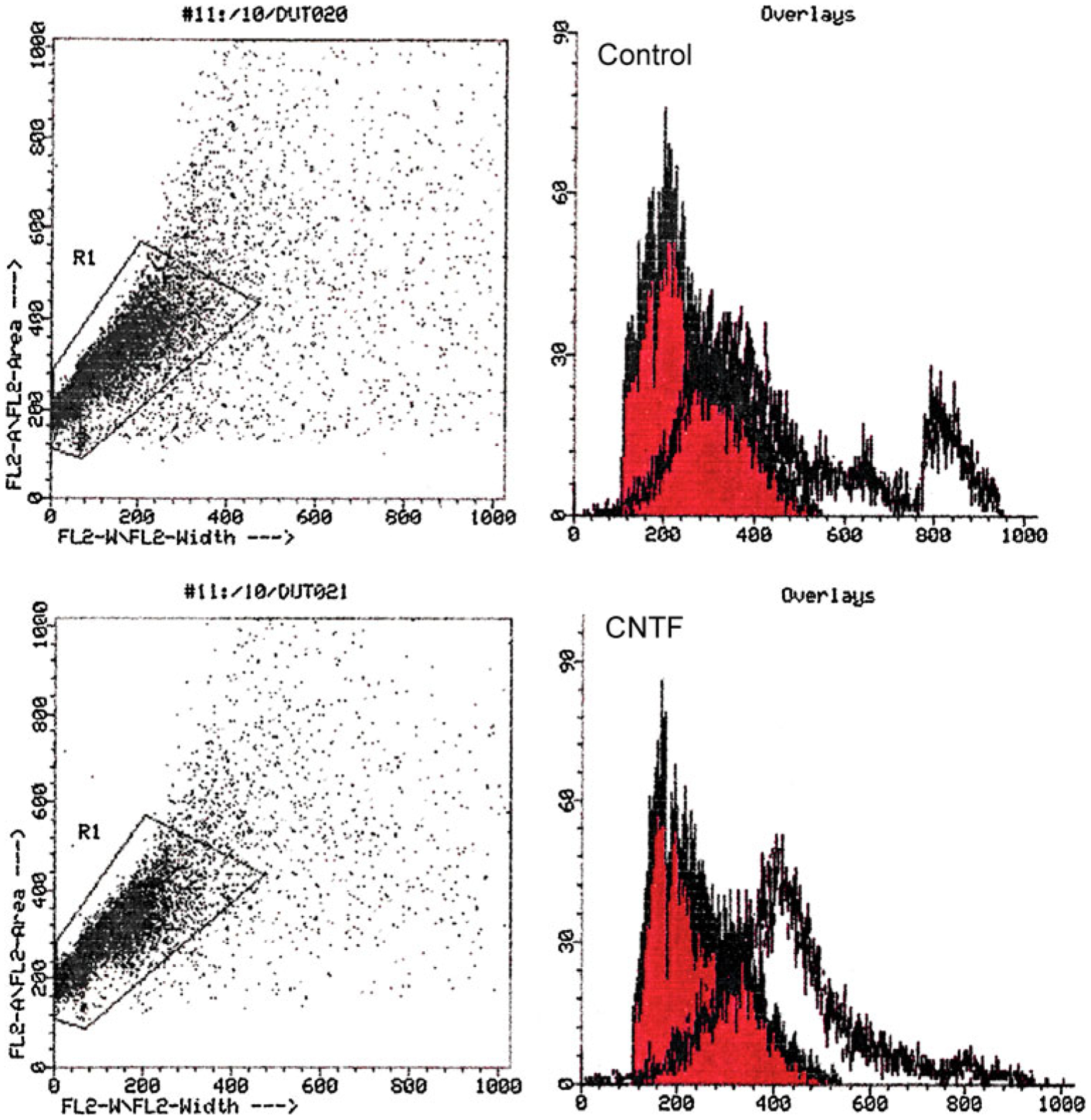
To quantify the survival potential of CNTF, retinal cells grown in defined medium +CNTF for 72 h were double labeled with annexin V–FITC and propidium iodide, before subjecting to FACS analysis on day 6. Note the greater area of overlap (blue annexin V over red propidium iodide) in the control vs. cells treated with CNTF; in the lower panel, note that the blue line has moved to the right, reflecting an increase in the number of viable cells.
Differentiation-inducing effect of CNTF and bFGF.
To test if CNTF induces specific neuronal phenotype in progenitors, progenitor cells grown in defined medium were exposed to CNTF (20 ng/ml) and bFGF (100 ng/ml), our internal control. Basic fibroblast growth factor induces photoreceptor phenotype in progenitor cells (in dose/density dependent manner; Ezeonu et al. 2003). Cells exposed to CNTF increased the number of cells with large multipolar ganglionic phenotype. Cells exposed to CNTF were simultaneously exposed to (Brdu) to determine cell proliferation. We saw that CNTF induces a subset of progenitors to undergo multiple rounds of DNA synthesis and acquire large ganglionic cell phenotype (cells with large cell body and multiple axons). Cells exposed to CNTF were immunophenotyped by different antibodies to assess neuronal phenotype generated in response to CNTF. Fifty to 100 immunophenotyped cells were counted in three different experiments. The summary of the data from a single experiment is presented in Table 1. Antibodies used to assess various neuronal phenotypes are ganglion cells (thy1.1 and neuron-specific enolase), PKCα (rod, cone, bipolars, and some blue cones), rhodopsin antibody Rho4D2, 1D4 (photoreceptors), and Muller glial cells (GFAP). The data from a typical experiment is tabulated in Table 1. The percentage of neurons generated in cells grown in serum-free media serves as the control, and bFGF treatment (internal control for photoreceptor differentiation) doubled the number of cells expressing rhodopsin. Similarly, CNTF treatment resulted in an increase in the number of cells with large multipolar ganglionic phenotype that were positive for thy 1.1. When cells were exposed for the first 3 h to CNTF, followed by 33-h exposure to bFGF, there was still a slight increase in the number of multipolar neurons. Basic fibroblast growth factor treatment for 3 h followed by CNTF resulted in a slight increase in the number of cells with photoreceptor phenotypes at 36 h. Figure 5A, E, I control, note in panel 1, Fig. 5B, C, D cells exposed first to bFGF for 3 h and CNTF for 33 h, there is an increase in the number of cells with photoreceptor phenotype at 24 (Fig. 5B), 48 (Fig. 5C), and 72 (Fig. 5D) h (arrows). A similar increase in large multipolar neurons was seen in cells exposed to CNTF for 3 h followed by bFGF exposure for 33 h at 24 (Fig. 5F), 48 (Fig. 5G), and 72 (Fig. 5H) h (panel 2). When both factors were added, no clear-cut distinction could be seen in panel 3, other than the arrows showing a subset of precursors undergoing multiple rounds of DNA synthesis and acquiring large ganglionic cell phenotype (Fig. 5J, K, L). In Fig. 5N, note a precursor undergoing multiple rounds of DNA synthesis (Brdu labeled) and a cluster of differentiating neurons from Fig. 5N mostly multipolars (Fig. 5O) arrows.
Table 1.
CNTF, bFGF-mediated cell differentiation in retinal cell line
| Cell type | Untreated | bFGF | CNTF | bFGF 3 h+CNTF 33 h | CNTF 3 h+bFGF 33 h |
|---|---|---|---|---|---|
| Ganglion | 17.3±1.8 | 12.2±1.5 | 31.2±5.2 | 19.5±2.5 | 24.8±4.0 |
| Bipolar | 26.9±3.3 | 13.2±3.7 | 8.6±2.6 | 16.6±4.0 | 12.4±0.6 |
| Rods | 13.3±2.5 | 22.9±3.1 | 5.4±1.3 | 24.6±2.8 | 8.9±2.6 |
| Muller/glia | 0 | 0 | 0 | 0 | 0 |
Neuronal cell types in culture were determined by immunolabeling using various antibodies specific for neuronal/glial cells, GFAP, (Muller/glia); thy 1.1 (ganglions); PKCα, (bipolar and cones); and Rho 4D2, ID4 (rod photoreceptors). Counts were obtained by viewing labeled cells under the fluorescent microscope. The values shown are percentages obtained from eight to ten different fields of view and a count of 100–200 cells.
Figure 5.
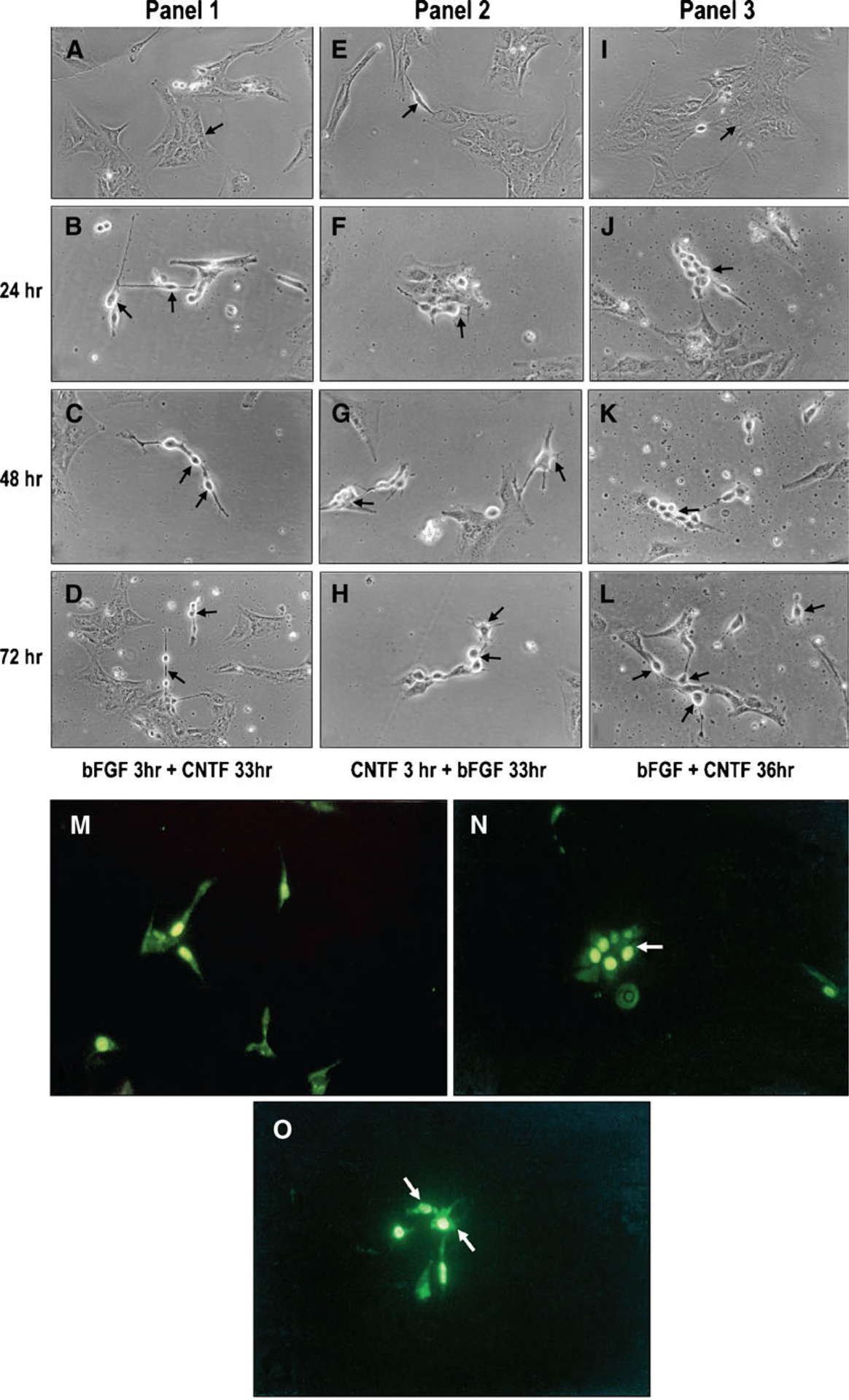
Ciliary neurotrophic-mediated differentiation phase contrast microscopy. Retinal cells exposed to CNTF (20 ng/ml) in defined medium were photographed at various times. Untreated cells (A, E, I). Panel 1, cells treated with bFGF 3 h+CNTF 24 h (b). Note the photoreceptor-like structures generated in response to bFGF (arrows). Cells treated with bFGF followed by CNTF (C, D). Neurons are mostly rods and cones at 48 and 72 h (arrows). Panel 2, cells treated with CNTF 3 h+bFGF 24 h (F). Note the multipolar neurons generated in response to CNTF. Cells treated with CNTF followed by bFGF (G, H)—mostly multipolar neurons are seen at 48 and 72 h. Panel 3, cells treated simultaneously with bFGF and CNTF (J, K, L). Cells seem to undergo multiple rounds of division before differentiating into multipolar neurons (arrows). Control cells labeled with BrdU for 3 h (M). Cells treated with CNTF (N). Note the cluster of cells undergoing multiple rounds of division. Cluster of dividing cells in (N) differentiating into multipolar neurons (O) arrows.
Immunohistochemistry and western blot analysis.
Figure 6 is a composite showing the expression of various retinal specific proteins in CNTF-treated cells. Cells treated with CNTF express thy1.1 (Fig. 6A), a protein expressed in ganglionic cells; CNTF-treated cells also express tyrosine hydroxylase (TH; Fig. 6B), D2, and D3 receptors (Fig. 6C). This suggests that cells with large cell bodies could be ganglionic (thy 1.1) and amacrine (TH). Cells expressing PKCα, D2, D3, and calbindin are possibly bipolars, rods, and cone photoreceptors (Fig. 6D, E).
Figure 6.
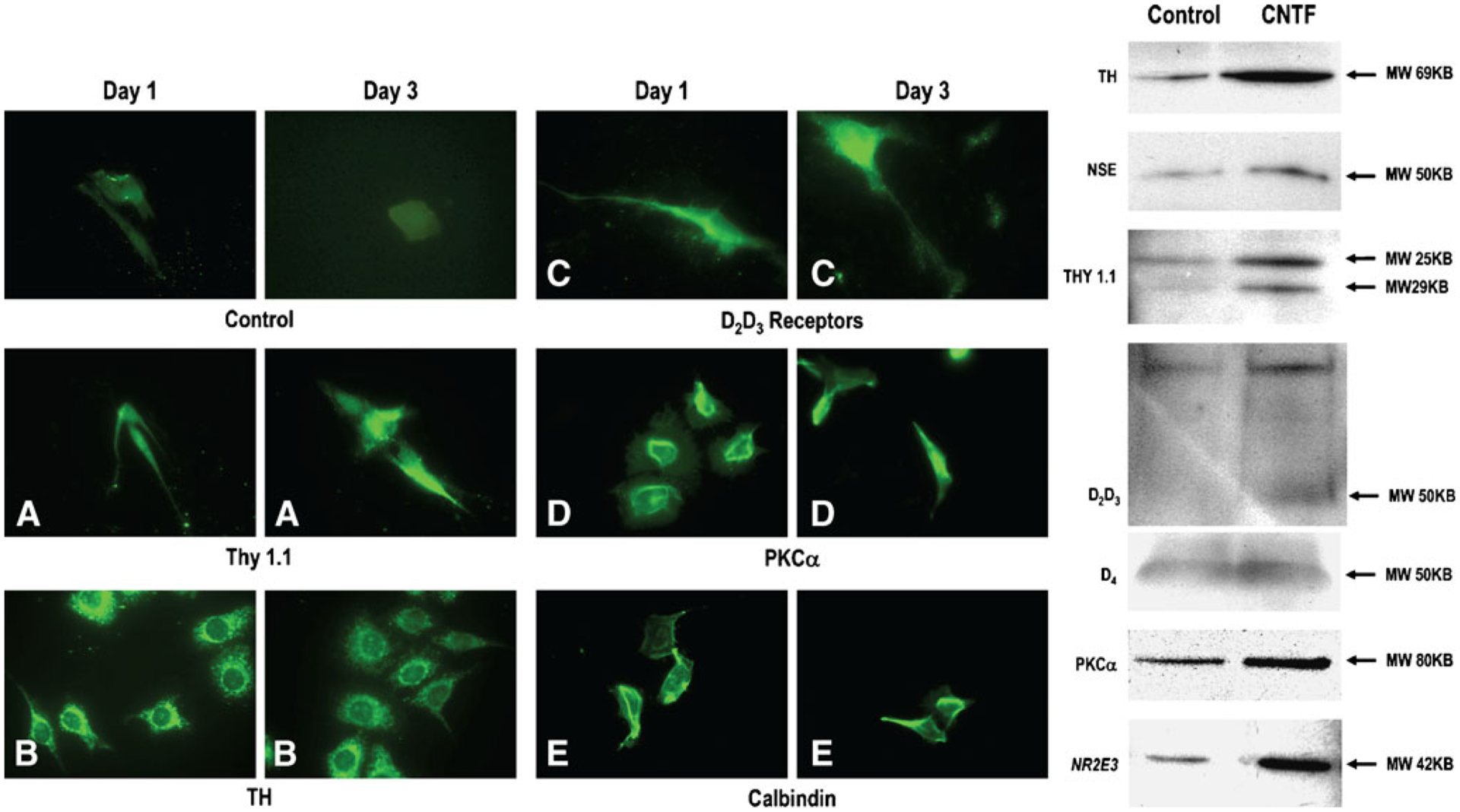
Immunohistochemical characterization of cells treated with CNTF. Retinal cells plated on coverslips and grown in defined medium were exposed to 20 ng/ml CNTF and immunolabeled with primary antibodies: thy 1.1 (ganglion cells), tyrosine hydroxylase (amacrine cells), D2, D3, D4, receptor (photoreceptors and other cell types), PKCα (rod, cone, and bipolars), and calbindin (cone photoreceptors and other cells). The secondary antibody is fluorescein conjugated. Note on day 3, the expression of thy 1.1, TH, PKCα, calbindin, and several other retinal marker proteins. Western blot analysis to characterize CNTF-mediated differentiation on day 3. The composite above depicts increased expression of thy 1.1, TH, PKCα, D2, D3, and NR2E3 in CNTF-treated cells suggesting that CNTF enhances differentiation into a number of retinal cell types.
To further confirm CNTF-induced differentiation in progenitors, we also performed western blot analysis to assess the expression of specific retinal proteins. The composite is represented in Fig. 6. Note in treated CNTF vs. control cultures, a significant increase in tyrosine hydroxylase suggesting that some of the large multipolar neurons are amacrine cells. We saw a significant increase in NSE and thy 1.1 representing a possible increase in the number of ganglions. There was also an increase in cells expressing PKCα (bipolars) and rod transcription factor NR2E3 (Fig. 6). A slight increase in D2, D3, and D4 receptors was also noted. Data shown are on day 3, similar results noted on day 6.
Discussion
The role of neurotrophic proteins in retinal development is quite complex. It includes the regulation of early developmental processes such as cell proliferation, determination of cell fate, and neural plasticity (Lewin and Barde 1996). Lineage tracing studies have confirmed that all classes of retinal cells (six types of neurons and Mueller glia) are generated from multipotential progenitors and that cell fate is determined during or shortly after the final mitosis (Turner and Cepko 1987; Holt et al. 1988; Wetts and Fraser 1988; Reh 1992).
It has been suggested that cell fate is determined by extrinsic signals from the environment (Watanabe and Raff 1992), and these signals could be developmentally regulated (Altshuler and Cepko 1992; Lillien 1998; Belecky-Adams et al. 1999). However, the importance of intrinsic genetic program in cell fate decision in developing retina cannot be ignored (Cayouette et al. 2003).
The intrinsic default pathway for the photoreceptor development in chick retina has been well documented (Adler and Hatlee 1989). Similar photoreceptor (possibly cone) development has been reported for human retinal progenitors in vitro (Ezeonu et al. 2003). Several lines of evidence reveal that the expression of neurotrophins required for cell commitment is both complex and varied.
It has also been substantiated by considerable accumulated data that endogenous factors involved in cell specification and survival during development are the same factors upregulated in response to a variety of injurious stimuli (genetic models of retinal degeneration) and retinal degeneration models induced by injurious external stimuli (hypoxia, exposure to light; Faktorovich et al. 1992; LaVail et al. 1992; Steinberg 1994; Cao et al. 2001). Similarly differential expression of CNTF and its receptors has been reported in optic nerve transected adult retina by Sarup et al. (2004). Several lines of evidence suggest that endogenous factors that promote photoreceptor survival may themselves be self-protective. Factors most often investigated include bFGF, BDNF, and CNTF (Faktorovich et al. 1992; Steinberg 1994).
One such factor that has been extensively investigated is CNTF, a pleiotropic growth factor with a multitude of potential functions described in developing mammalian systems (Steinberg 1994). Ciliary neurotrophic factor’s effect on photoreceptor survival and rescue is well-documented. Varying degrees of success has been seen in CNTF-treated animal models of diseases (Fuhrmann et al. 1994; Sarup et al. 2004). Sieving et al. (2006) report some success in treatment of late stage RP patients with CNTF. Additionally, it is reported to be a survival factor for cultured retinal ganglions (Meyer-Franke et al. 1995). It is also known to stimulate differentiation of cholinergic amacrine cells in the retinal culture of chick (Meyer-Franke et al. 1995) and postnatal rat (D’Cruz et al. 2000). The developmental importance of CNTF is further supported in genetic experiments. Mice lacking the CNTFRα receptor gene show severe developmental defects and die shortly after birth (Dechiara et al. 1995).
It is also well-known that many classes of retinal cells respond to CNTF in vitro and in vivo (Lehwalder et al. 1989; Ip et al. 1991; Escandnon et al. 1994; Valter et al. 2003). Opposing roles for CNTF in photoreceptor commitment have been reported for rat and chick (Kirsch et al. 1996, 1997; Ezzeddine et al. 1997; Bhattacharya et al. 2004). Ciliary neurotrophic factor mRNA expression in different neural cell types is developmentally regulated (Kirsch et al. 1997; Valter et al. 2003). Although it was initially suggested that the CNTFRα receptor is expressed in ganglion, amacrine, and horizontal cells but not in photoreceptors, more recent studies suggest that the same might not be true (Valter et al. 2003). Kirsch et al. (1997) reported that CNTFRα might be expressed transiently in photoreceptors early in embryonic development in the retina but declines significantly in the adult. However, immunohistochemistry and high resolution confocal microscopy used in studies (Valter et al. 2003) suggest that CNTF and CNTFRα are localized to photoreceptors, especially outer segments, and upregulated in ischemic retina (Ju et al. 2000). Ciliary neurotrophic factor effects the survival of ganglion cells, mature photoreceptors, differentiation of photoreceptors, cholinergic amacrine, and bipolar cells. It is considered that apoptotic cell death might be a common path used in degeneration as seen in many models of retinal degeneration including RP and glaucoma (Ju et al. 2000). It was also well established that exogenously administered CNTF, by injection or virally delivered (adenovirus mediated), rescues degenerating photoreceptors by inhibiting apoptosis (Cayouette and Gravel 1997; Wen et al. 1998; Liang et al. 2001; Huang et al. 2004). Ji et al. (2004) have reported that CNTF also provides neuroprotection for retinal ganglion cells in a rat glaucoma model induced by laser photocoagulation and that the mechanism could be possible upregulation of CNTF (Ji et al. 2004). Similar regeneration of adult retinal axon into peripheral nerve graft in vivo has been reported (Cui et al. 1999).
In this study, we report that in the human retinal progenitor cell line:
CNTF acts as an anti-apoptotic factor and inhibits apoptosis by approximately 50%.
CNTF promotes differentiation of the subset of progenitors toward ganglionic and amacrine cell paths as confirmed by upregulation of thy 1.1, neuron-specific enolase in (ganglions), and tyrosine hydroxylase (amacrine cells). Additionally, it might also enhance bipolar and photoreceptor cell differentiation to a lesser degree (data not included).
CNTF can enhance cell renewal and expansion of the subset of retinal stem cells. A similar role in brain stem cells has been reported (Shimzaki et al. 2001).
Considering the multitude of effects CNTF seems to have on human retinal progenitors (survival, commitment, self renewal), the very promising outcomes were reported in various animal models of RP and the first successful phase 1 trials in humans; further studies are warranted to exploit the neuroprotective role of this very pleiotropic molecule in CNS and retina.
Acknowledgments
These studies were partly reported at the Association for Research in Vision and Ophthalmology meetings, Ft. Lauderdale, FL 1997.
The authors thank Renarder Pressley for the excellent secretarial assistance, Mr. Patrick Abramson for the graphics, and Ms. Suzanne Alexander for the critical reading of the manuscript (Morehouse School of Medicine). We thank Ms. Darlene Kelley, information specialist (Morehouse School of Medicine). We also thank Dr. Myrtle Thierry-Palmer, Dr. Sandra Harris-Hooker, and Dr. Marjorie Smith for their friendship and support. The work was supported by RCMI Grant 5 G12RR03034 (K.D.) NASA NCC-11-112 (K.D.).
References
- Adler R; Hatlee M Plasticity and differentiation of embryonic retinal cells after terminal mitosis. Science 243: 391–393; 1989. doi: 10.1126/science.2911751. [DOI] [PubMed] [Google Scholar]
- Altshuler D; Cepko C A temporally regulated, diffusible activity is required for rod photoreceptor development in vitro. Development 114: 947–957; 1992. [DOI] [PubMed] [Google Scholar]
- Bazan JF Neuropoietic cytokines in the hematopoietic fold. Neuron 7: 197–208; 1991. doi: 10.1016/0896-6273(91)90258-2. [DOI] [PubMed] [Google Scholar]
- Belecky-Adams TL; Scheurer D; Adler R Activin family members in the developing chick retina: expression patterns, protein distribution, and in vitro effects. Dev. Biol 210: 107–123; 1999. doi: 10.1006/dbio.1999.9268. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S; Dooley C; Soto F; Madson J; Das AV; Ahmad I Involvement of Ath3 in CNTF-mediated differentiation of the late retinal progenitors. Mol. Cell. Neurosci 27: 32–43; 2004. doi: 10.1016/j.mcn.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Bok D; Yasumura D; Matthes MT; Ruiz A; Duncan JL; Chappelow AV; Zolutukhin S; Hauswirth W; LaVail MM Effects of adeno-associated virus-vectored ciliary neurotrophic factor on retinal structure and function in mice with a P216L rds/peripherin mutation. Exp. Eye Res 74: 719–735; 2002. doi: 10.1006/exer.2002.1176. [DOI] [PubMed] [Google Scholar]
- Cao W; Li F; Steinberg RH; LaVail MM Development of normal and injury-induced gene expression of aFGF, bFGF, CNTF, BDNF, GFAP, and IGF-I in the rat retina. Exp. Eye Res 72: 591–604; 2001. doi: 10.1006/exer.2001.0990. [DOI] [PubMed] [Google Scholar]
- Cayouette M; Barres BA; Raff M Importance of intrinsic mechanisms in cell fate decisions in the developing rat retina. Neuron 40: 897–904; 2003. doi: 10.1016/S0896-6273(03)00756-6. [DOI] [PubMed] [Google Scholar]
- Cayouette M; Behn D; Sendtner M; Lachapelle P; Gravel C Intraocular gene transfer of ciliary neurotrophic factor prevents death and increases responsiveness of rod photoreceptors in the retinal degeneration slow mouse. J Neurosci 18: 9282–9293; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayouette M; Gravel C Adenovirus-mediated gene transfer of ciliary neurotrophic factor can prevent photoreceptor degeneration in the retinal degeneration (rd) mouse. Hum. Gene Ther 8: 423–430; 1997. doi: 10.1089/hum.1997.8.4-423. [DOI] [PubMed] [Google Scholar]
- Chong NH; Alexander RA; Waters L; Barnett KC; Bird AC; Luthbert PJ Repeated injections of a ciliary neurotrophic factor analogue leading to long-term photoreceptor survival in hereditary retinal degeneration. Invest. Ophthalmol. Vis. Sci 40: 1298; 1999. [PubMed] [Google Scholar]
- Cui Q; Lu Q; So KF; Yip HK CNTF, not other trophic factors, promotes axonal regeneration of axotomized retinal ganglion cells in adult hamsters. Invest. Ophthalmol. Vis. Sci 40: 760–766; 1999. [PubMed] [Google Scholar]
- D’Cruz PM; Yasumura D; Weir J; Matthes MT; Abderrahim H; LaVail MM Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet 9: 645–651; 2000. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- DeChiara TM; Vejsada R; Poueymirou WT; Acheson A; Suri C; Conover JC; Friedman B; McClain J; Pan L; Stahl N; Ip NY; Kato A; Yancopoulos GD Mice lacking the CNTF receptor, unlike mice lacking CNTF, exhibit profound motor neuron deficits at birth. Cell 83: 313–322; 1995. doi: 10.1016/0092-8674(95)90172-8. [DOI] [PubMed] [Google Scholar]
- Dutt K; Ezeonu I; Scott M; Semple E; Srinivasan A Protooncogene expression in cAMP and TPA-mediated neuronal differentiation in a human retinal cell line KGLDMSM. Curr. Eye Res 15: 477–485; 1996. doi: 10.3109/02713689609000759. [DOI] [PubMed] [Google Scholar]
- Dutt K; Scott M; Wang M; Semple E; Sharma GP; Srinivasan A Establishment of a human retinal cell line by transfection of SV40 T antigen gene with potential to undergo neuronal differentiation. DNA Cell Biol 13: 909–921; 1994. [DOI] [PubMed] [Google Scholar]
- Escandnon E; Soppet D; Rosenthal A; Mendoza-Ramirez JL; Szonyi E; Burton LE; Henderson CE; Parade LF; Nikolics K Regulation of neurotrophin receptor expression during embryonic and postnatal development. J. Neurosci 14: 2054–2068; 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeonu I; Wang M; Kumar R; Dutt K Density-dependent differentiation in nontransformed human retinal progenitor cells in response to basic fibroblast growth factor- and transforming growth factor-alpha. DNA Cell Biol 22: 607–620; 2003. doi: 10.1089/104454903770238085. [DOI] [PubMed] [Google Scholar]
- Ezzeddine ZD; Yang X; DeChiara T; Yancopoulos G; Cepko CL Postmitotic cells fated to become rod photoreceptors can be specified by CNTF treatment of the retina. Development 124: 1055–1067; 1997. [DOI] [PubMed] [Google Scholar]
- Faktorovich EG; Steinberg RH; Yasumura D; Matthes MT; LaVail MM Basic fibroblast growth factor and local injury protect photoreceptors from light damage in the rat. J Neurosci 12: 3554–3567; 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann S; Heller H; Rohrer H; Hofmann D A transient role for ciliary neurotrophic factor in chick photoreceptor development. J. Neurobiol 37: 672–683; 1998. doi:. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S; Kirsch M; Hofmann HD Ciliary neurotrophic factor promotes chick photoreceptor development in vitro. Development 121: 2695–2706; 1994. [DOI] [PubMed] [Google Scholar]
- Heller S; Finn TP; Huber J; Nishi R; Geissen M; Puschel AW; Rohrer H Analysts of function and expression of the chick GPA receptor (GPAR alpha) suggests multiple roles in neuronal development. Development 121: 2681–2693; 1995. [DOI] [PubMed] [Google Scholar]
- Holt CE; Bertsch TW; Ellis HM; Harris WA Cellular determination in the xenopus retina is independent of lineage and birth date. Neuron 1: 15–26; 1988. doi: 10.1016/0896-6273(88)90205-X. [DOI] [PubMed] [Google Scholar]
- Huang SP; Lin PK; Liu JH; Khor CN; Lee YJ Intraocular gene transfer of ciliary neurotrophic factor rescues photoreceptor degeneration in RCS rats. J. Biomed. Sci 11: 37–48; 2004. doi: 10.1007/BF02256547. [DOI] [PubMed] [Google Scholar]
- Hughes SM; Lillen LE; Raff MC; Rohrer H; Sendtner M Ciliary neurotrophic factor induces type-2 astrocyte differentiation in culture. Nature 335: 70–73; 1988. doi: 10.1038/335070a0. [DOI] [PubMed] [Google Scholar]
- Inoue M; Nakayama C; Noguchi H Activating mechanism of CNTF and related cytokines. Mol. Neurobiol 12: 195–209; 1996. doi: 10.1007/BF02755588. [DOI] [PubMed] [Google Scholar]
- Ip NY; Li YP; van de Stadt I; Panayotatos N; Alderson RF; Lindsay RM Ciliary neurotrophic factor enhances neuronal survival in embryonic rat Hippocampal cultures. J. Neurosci 11: 3124–3134; 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip NY; Yancopoulos GD The neurotrophins and CNTF: two families of collaborative neurotrophic factors. Ann. Rev. Neurosci 19: 491–515; 1996. doi: 10.1146/annurev.ne.19.030196.002423. [DOI] [PubMed] [Google Scholar]
- Ji JZ; Elyaman W; Yip HK; Lee VW; Yick LW; Hugon J; So KF CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats; the possible involvement of STAT3 pathway. Eur. J. Neurosci 19: 265–272; 2004. doi: 10.1111/j.0953-816X.2003.03107.x. [DOI] [PubMed] [Google Scholar]
- Ju WK; Lee MY; Hofmann HD; Kirsch M; Oh SJ; Chung JW; Chu MH Increased expression of ciliary neurotrophic factor receptor alpha mRNA in the ischemic rat retina. Neurosci. Lett 283: 133–136; 2000. doi: 10.1016/S0304-3940(00)00931-9. [DOI] [PubMed] [Google Scholar]
- Kirsch M; Fuhrmann S; Wiese A; Hofman HD CNTF exerts opposite effects on in vitro development of rat and chick photoreceptors. NeuroReport 7: 697–700; 1996. doi: 10.1097/00001756-199602290-00004. [DOI] [PubMed] [Google Scholar]
- Kirsch M; Lee MY; Meyer V; Weise A; Hofmann HD Evidence for multiple, local functions of ciliary neurotrophic factor (CNTF) in retinal development: expression of CNTF and its receptors and in vitro effects on target cells. J. Neurochem 68: 979–990; 1997. [DOI] [PubMed] [Google Scholar]
- Kishmoto T; Taga T; Akira S Cytokine signal transduction. Cell 76: 253–262; 1994. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- LaVail MM; Unoki K; Yasumura D; Matthes MT; Yancopoulos GD; Steinberg RH Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc. Natl Acad. Sci. USA 89: 11249–11253; 1992. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM; Yasumura D; Matthes MT; Lau-Villacorta C; Unoki K; Sung CH; Steinberg RH Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest. Ophthalmol. Vis. Sci 39: 592–602; 1998. [PubMed] [Google Scholar]
- Lehwalder D; Jeffrey PL; Unsicker K Survival of purified embryonic chick retinal ganglion cells in the presence of neurotrophic factors. J. Neurosci. Res 24: 329–337; 1989. doi: 10.1002/jnr.490240225. [DOI] [PubMed] [Google Scholar]
- Lewin GR; Barde YA Physiology of the neurotrophins. Annu. Rev. Neurosci 19: 289–317; 1996. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Liang FQ; Aleman TS; Dejneka NS; Dudus L; Fisher KJ; Maguire AM; Jacobson SG; Bennett J Long-term protection of retinal structure but not function using RAAV CNTF in animal models of retinitis Pigmentosa. Mol. Ther 4: 461–472; 2001. doi: 10.1006/mthe.2001.0473. [DOI] [PubMed] [Google Scholar]
- Lillien L Neurol progenitors and stem cells: mechanisms of progenitor heterogeneity. Curr Opin Neurolbiol 8: 37–44; 1998. doi: 10.1016/S0959-4388(98)80006-8. [DOI] [PubMed] [Google Scholar]
- Manthorpe M; Louis JC; Hagg T; Varon S Ciliary neurotrophic factor. In: Loughlin SE; Fallon JH (eds) Neurotrophins factors Academic, New York, pp 443–473; 1993. [Google Scholar]
- Meyer-Franke A; Kaplan MR; Pfrieger FW; Barner BA Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron 15: 805–819; 1995. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- Pennica D; Shaw KJ; Swanson TA; Moore MW; Shelton DL; Zioncheck KA; Rosenthal A; Taga T; Paoni NF; Wood WI Cardiotrophin-1. Biological activities and binding to the leukemia inhibitory factor receptor/gp 130 signaling complex. J. Biol. Chem 270: 10915–10922; 1995. doi: 10.1074/jbc.270.18.10915. [DOI] [PubMed] [Google Scholar]
- Reh TA Cellular interactions determine neuronal phenotypes in rodent retinal cultures. J. Neurobiol 23: 1067–1083; 1992. doi: 10.1002/neu.480230811. [DOI] [PubMed] [Google Scholar]
- Rhee KD; Goureau O; Chen S; Yang XJ Cytokine-induced activation of signal transducer and activator of transcription in photoreceptor precursors regulates rod differentiation in the developing mouse retina. J. Neurosco 24: 9779–9788; 2004. doi: 10.1523/JNEUROSCI.1785-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarup V; Patil K; Sharma SC Ciliary neurotrophic factor and its receptors are differentially expressed in the optic nerve transected adult rat retina. Brain Res 1013: 152–158; 2004. doi: 10.1016/j.brainres.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Segal RA; Greenberg ME Intracellular signaling pathways activated by neurotrophic factors. Annu. Rev. Neurosci 19: 463–489; 1996. [DOI] [PubMed] [Google Scholar]
- Sendtner M; Carroll P; Holtmann B; Hughes RA; Thoenen H Ciliary neurotrophic factor. J. Neurobiol 25: 1436–1453; 1994. doi: 10.1002/neu.480251110. [DOI] [PubMed] [Google Scholar]
- Shimzaki T; Shingo T; Weiss S The ciliary neurotrophic factor/leukemia inhibitory factor/gp 130 receptor complex operates in the maintenance of mammalian forebrain neural stem cells. J. Neurosci 21: 7642–7653; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieving PA; Caruso RC; Tao W; Coleman HR; Thompson DJ; Fullmer KR; Bush RA Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc. Natl Acad. Sci. USA 103: 3896–3901; 2006. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl N; Yancopoulos GD The tripartite CNTF receptor complex: activation and signaling involves components shared with other cytokines. J Neurobiol 25: 1454–1466; 1994. doi: 10.1002/neu.480251111. [DOI] [PubMed] [Google Scholar]
- Steinberg RH Survival factors in retinal degenerations. Curr. Opin. Neurobiol 4: 515–524; 1994. doi: 10.1016/0959-4388(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Tao W; Wen R; Goddard B; Sherman SD; O’Rourke PJ; Stabila PF; Bell WJ; Dean BJ; Kauper KA; Budz VA; Tsiaras WG; Acland GM; Pearce-Kelling S; Laties AM; Aguirre GD Encapsulated cell-based delivery of CNTF reduces photoreceptor degeneration in animal models of retinitis Pigmentosa. Invest. Ophthalmol. Vis. Sci 43: 3292–3298; 2002. [PubMed] [Google Scholar]
- Turner DL; Cepko CL A common progenitor for neurons and glia persists in rat retina late in development. Nature 328: 131–136; 1987. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- Unoki K; Ohba N; Arimura H; Murmatsu H; Muramatsu T Rescue of photoreceptors from the damaging effects of constant light by midkine, a retinoic acid-responsive gene product. Invest. Ophthalmol. Vis. Sci 35: 4063–4068; 1994. [PubMed] [Google Scholar]
- Valter K; Bisti S; Stone J Location of CNTFRalpha on outer segments: evidence of the site of action of CNTF in rat retina. Brain Res 985: 169–175; 2003. doi: 10.1016/S0006-8993(03)03130-5. [DOI] [PubMed] [Google Scholar]
- Wahlin KJ; Campochiaro PA; Zack DJ; Adler R Neurotrophic factors cause activation of intracellular signaling pathways in Muller cells and other cells of the inner retina, but not photoreceptors. Invest. Ophthalmol. Vis. Sci 41: 927–936; 2000. [PubMed] [Google Scholar]
- Watanabe T; Raff MC Diffusible rod-promoting signals in the developing rat retina. Development 114: 899–906; 1992. [DOI] [PubMed] [Google Scholar]
- Wen R; Cheng T; Song Y; Matthes MT; Yasamura D; LaVail MM; Steinberg RH Continuous exposure to bright light upregulates bFGF and CNTF expression in the rat. Curr. Eye Res 17: 494–500; 1998. doi: 10.1076/ceyr.17.5.494.5186. [DOI] [PubMed] [Google Scholar]
- Wetts R; Fraser SE Multipotent precursors can give rise to all major cell types of the frog retina. Science 239: 1142–1145; 1988. doi: 10.1126/science.2449732. [DOI] [PubMed] [Google Scholar]
- Xie HQ; Adler R Green cone opsin and rhodopsin regulation by CNTF and staurosporine in cultured chick photoreceptors. Invest. Ophthalmol. Vis. Sci 41: 4317–4323; 2000. [PubMed] [Google Scholar]
- Yano H; Chao MV Neurotrophin receptor structure and interactions. Pharm. Acta Helv 74: 253–260; 2000. doi: 10.1016/S0031-6865(99)00036-9. [DOI] [PubMed] [Google Scholar]
- Zahir T; Klassen H; Young MJ Effects of ciliary neurotrophic factor on differentiation of late retinal progenitor cells. Stem Cells 23: 424–432; 2005. doi: 10.1634/stemcells.2004-0199. [DOI] [PubMed] [Google Scholar]


