Abstract
The current pandemic caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has led people to implement preventive measures, including surface disinfection and use of alcohol-based hand gel, in order to avoid viral transmission via fomites. However, the role of surface transmission is still debated. The present systematic review aims to summarize all the evidence on surface survival of coronaviruses infecting humans. The analysis of 18 studies showed the longest coronavirus survival time is 28 days at room temperature (RT) on different surfaces: polymer banknotes, vinyl, steel, glass, and paper banknotes. Concerning SARS-CoV-2 human infection from contaminated surfaces, dangerous viral load on surfaces for up to 21 days was determined on polymer banknotes, steel, glass and paper banknotes. For viruses other than SARS-CoV-2, the longest period of survival was 14 days, recorded on glass. Environmental conditions can affect virus survival, and indeed, low temperatures and low humidity support prolonged survival of viruses on contaminated surfaces independently of surface type. Furthermore, it has been shown that exposure to sunlight significantly reduces the risk of surface transmission. Although studies are increasingly investigating the topic of coronavirus survival, it is difficult to compare them, given the methodology differences. For this reason, it is advisable to define a reference working protocol for virus survival trials, but, as an immediate measure, there is also a need for further investigations of coronavirus survival on surfaces.
Keywords: COVID-19, Inanimate surfaces, Sunlight, Persistence, Health care
Graphical abstract
1. Introduction
The current pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for health and social issues, and economic losses worldwide. The World Health Organization (WHO) declared SARS-CoV-2 a pandemic on 11th March 2020, and as at 3rd November 2020, there have been over 47 million confirmed cases with more than 1 million global deaths (John Hopkins University, 2020). Since 2002, Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) and Middle East Respiratory Syndrome coronavirus (MersCoV) were the only two lethal coronaviruses infecting humans (Drosten et al., 2003; WHO, 2020). Before 2002, human coronaviruses (HCoV) were not considered as serious public health threats but only as nuisance viruses (Ashour et al., 2020).
The current evidence suggests the main route of SARS-CoV-2 transmission is direct contact mediated by respiratory droplets and aerosols, and therefore, the most effective preventive measures are face masks and social distancing (Stadnytskyi et al., 2020; Zhang et al., 2020). Surface and environment disinfection is another measure necessary for SARS-CoV-2 control, since in hospital settings, the possibility of coronavirus transmission from contaminated dry surfaces to individuals exists (Dowell et al., 2004; Otter et al., 2016). Evidence of viral transmission from contaminated surfaces has been shown in the case of other pathogens, such as enteric viruses (Boone and Gerba, 2007), but in contrast, evidence specifically referring to coronaviruses, especially SARS-CoV, is scarce.
The potential for a virus to be transmitted via fomites depends on its characteristics. For example, the presence of a viral envelope is associated with relatively low virus resistance on surfaces (Howie et al., 2008). Environmental factors such as temperature, moisture, exposure to UV, and surface characteristics also affect virus survival on surfaces (Boone and Gerba, 2007). To date, there are at least nine published reviews dealing with the role of inanimate surfaces on coronavirus transmission (Fernández-Raga et al., 2021; Fiorillo et al., 2020; Geller et al., 2012; Kampf et al., 2020; Kaul, 2020; Kramer et al., 2006; Mohan et al., 2021; Noorimotlagh et al., 2020; Ren et al., 2020). However, not all of these are systematic, and among systematic reviews, only one seems to have enrolled all of the existing evidence (Kampf et al., 2020). However, even the systematic review by Kampf et al. (2020), given the increasing number of published studies dealing with this context, needs to be updated. Also, SARS-CoV-2 survival under different environmental conditions, such as sunlight exposure, relative humidity (RH) and temperature, should be considered.
The present systematic review aims to collect and synthesize available evidence on the survival of coronaviruses on inanimate surfaces. In particular, data on survival of HCoV, SARS-CoV, MersCoV and SARS-CoV-2 were summarized, considering materials, methods, and environmental conditions.
2. Materials and methods
The present systematic review was carried out using a specific, non-extensive approach in order to rapidly retrieve and screen relevant records from multiple databases.
The review question was: “How long can coronaviruses survive on different surfaces?”
Population: coronaviruses (particularly SARS-CoV), Outcome: survival time (days).
We considered all studies published in peer-reviewed journals in English language. No time limits were imposed. We searched PUBMED and EMBASE (Title/Abstract and Title, Abstract, Author keywords, respectively) with the search terms reported in Table 1 . The last date searched was November 6th, 2020. To implement the search process, we used the final list of studies to carry out a backward reference search in order to identify potential missing evidence. Several criteria were used to select eligible studies: (1) the study had to be in English; (2) reported data had to belong to primary research; (3) the study had to deal with a coronavirus capable of infecting humans; (4) the study had to be carried out through experimental contamination of inanimate surfaces; (5) the study had to report results about viral survival under normal ambient conditions.
Table 1.
Keywords employed to retrieve relevant studies on coronavirus survival.
| Keywords (Title/Abstract) | ||||
|---|---|---|---|---|
| Coronavirus OR coronaviruses OR cov OR sars OR mers OR sars-cov OR mers-cov OR sars-cov-2 | AND | Surface OR surfaces OR environment OR environmental OR packaging OR packages OR package OR food OR skin OR hand OR hands | AND | Stability OR survival OR resistance OR viability OR inactivation OR disinfection OR biocide OR elimination OR removal OR persistence |
The screening process was carried out using EPPI-4 Reviewer software (Thomas et al., 2010).
In the case of a poorly explicative abstract or in the case of doubt about the available data, the study was included and evaluated at full-text level. Thereafter, two reviewers (SB, FM) screened all studies obtained via the initial literature search according to Title, Abstract, and Full text, independently (parallel method). Disagreements were resolved through consensus. One reviewer collected data from relevant studies and a second reviewer checked the collected data against the original studies (sequential method). All studies were coded according to the previously chosen parameters, and data were recorded.
Data were collected in pre-defined forms for the following variables useful to describe the study design: surface type, surface description, virus, strain/isolate, viral titer on the surface, surface contamination mode, dehydration mode, temperature, relative humidity, virus detection mode, limit of detection, survival (last detection-no detection).
The risk of bias in individual studies can be due to methodological issues such as the presence of replicates and also the use of positive and negative process controls. We extracted data on quality to ensure our systematic review methods are as transparent as possible, but we did not exclude any studies due to quality issues.
2.1. Synthesis of results
The number of days of survival was chosen as effect size. In particular, we reported two values, one corresponding to the last day in which the virus was viable and the second corresponding to the first day in which viability was lost. In addition, we considered studies without our chosen effect size in the results' textual section; these were studies with other effect sizes (log reduction or half-life), studies providing a qualitative assessment, or studies comparing virus survival in different environmental conditions (darkness and sunlight).
Test conditions, such as environmental conditions (temperature, RH, UV or sunlight), inoculum viral titer, surface contamination mode (droplet, aerosol, type of suspension media), dehydration mode (environmental conditions, laminar flow hood drying, time of drying), virus detection methodology, and detection time points, were widely variable among included studies and could potentially be sources of bias across studies. For this reason, a statistical synthesis of the results was not carried out.
3. Results
3.1. Study selection and characteristics
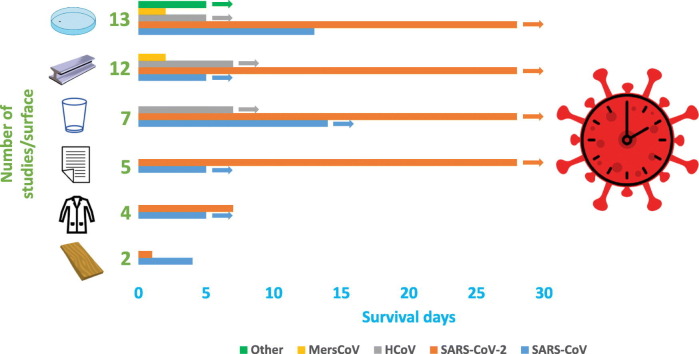
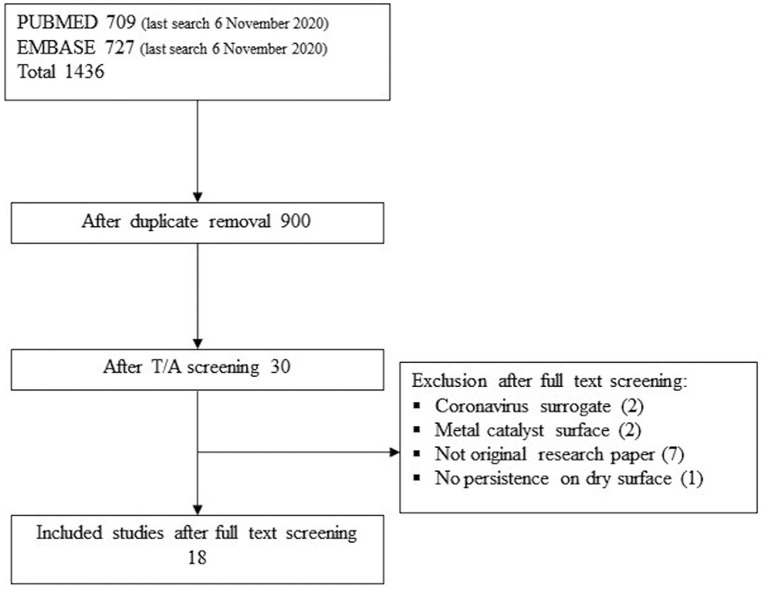
In total, 18 studies investigating the survival of coronavirus on inanimate surfaces were included after screening (Fig. 1 ). Twelve studies investigated the survival of coronaviruses different from SARS-CoV-2 (6 SARS-CoV, 5 HCov, 1 MersCoV, 1 Alphacoronavirus), whereas eight studies assessed SARS-CoV-2 survival on surfaces (Table 2 ). Coronavirus survival was assessed on different materials as follows: polymer, metals, glass, paper, fabric, wood, face mask, sterile sponge, ceramic, banknotes, mosaic, and soil. Thirteen studies reported data on the survival of coronaviruses on polymeric surfaces: plastic, PVC, Teflon, silicon rubber, disposable gloves, polymer banknotes, and vinyl (Table 3 ). Twelve studies were retrieved dealing with metals: steel, aluminum, copper, and non-specified metals (Table 4 ), while seven studies concerned glass surfaces (Table 5 ). Five studies assessed virus survival on different kinds of paper: classic paper, tissue paper, press paper, filter paper, and cardboard, while two studies dealt with wood surfaces (Table 6 ). Also, the survival of virus on fabrics was assessed by four studies, which employed cloth, disposable gown, and cotton gown as inanimate surfaces. Single studies evaluated virus survival on other surfaces: face mask, sterile sponge, ceramic, soil, and mosaic (Table 6). Survival on banknotes was assessed on generic, plastic and paper banknotes. Plastic and paper banknotes are included in Table 3, Table 6, respectively, according to the material.
Fig. 1.
Flow diagram reporting the number of retrieved studies from two databases (PUBMED and EMBASE) and after title/abstract (T/A) screening. After full-text screening, 18 studies were included and 12 studies were excluded for the provided reasons.
Table 2.
Type of tested coronavirus, number of replicates, and process controls conducted during experiments for studies included in the present review.
| Reference | Tested coronavirus |
Quality control |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hu | SARS | MERS | SARS2 | Other | Material replicates |
Titration replicates | NC | PC | NR | |||
| X 2 | X 3 | X 4 | ||||||||||
| Sizun et al., 2000 | • | • | • | |||||||||
| Duan et al., 2003 | • | • | ||||||||||
| Rabenau et al., 2005 | • | • | • | |||||||||
| Lai et al., 2005 | • | • | • | |||||||||
| Müller et al., 2008 | • | • | ||||||||||
| Chan et al., 2011 | • | • | ||||||||||
| van Doremalen et al., 2013 | • | • | ||||||||||
| Warnes et al., 2015 | • | • | ||||||||||
| Bonny et al., 2018 | • | • | ||||||||||
| Behzadinasab et al., 2020 | • | • | • | |||||||||
| Biryukov et al., 2020 | • | • | ||||||||||
| Chan et al., 2020 | • | • | • | • | ||||||||
| Chin et al., 2020 | • | • | ||||||||||
| Kratzel et al., 2020 | • | • | ||||||||||
| Malenovská, 2020 | • | • | • | • | ||||||||
| Ratnesar-Shumate et al., 2020 | • | • | • | |||||||||
| Riddell et al., 2020 | • | • | • | |||||||||
| van Doremalen et al., 2020 | • | • | • | |||||||||
| Tot. | 5 | 6 | 1 | 8 | 1 | 1 | 11 | 1 | 5 | 3 | 1 | 4 |
Hu:HCoV; SARS:SARS-CoV; MERS: MersCoV; SARS2: SARS-CoV-2; NC: negative control; PC: positive control; NR: not reported.
Table 3.
Survival of coronaviruses on polymeric surfaces.
| Surface description | Virus | Viral titer onto the surface | T (°C) | Survivala | Limit of detection | Reference |
|---|---|---|---|---|---|---|
| Plastic | HCoV | 5 × 106 TCID50 | RT | 2–3 db | 10–100 TCID50/ml | (Rabenau et al., 2005) |
| 5 × 106 TCID50 | RT | 1–2 dc | 10–100 TCID50/ml | (Rabenau et al., 2005) | ||
| 2 × 104 PFU | 24d | > 7 de | NR | (Bonny et al., 2018) | ||
| SARS-CoV | 106 TCID50 | RT | 4–5 d | NR | (Duan et al., 2003) | |
| 107 TCID50 | RT | 6–9 d | 10–100 TCID50/ml | (Rabenau et al., 2005) | ||
| 105 TCID50 | 22–25 | 13–21 df | NR | (Chan et al., 2011) | ||
| 2.5 × 103 TCID50 | 21–23 | 3–4 d | 3.2 TCID50/ml | (van Doremalen et al., 2020) | ||
| MersCoV | 106 TCID50 | 20 | 2–3 d | NR | (van Doremalen et al., 2013) | |
| 106 TCID50 | 30 | 1–2 d | NR | (van Doremalen et al., 2013) | ||
| 106 TCID50 | 30 | 8 h - 1 d | NR | (van Doremalen et al., 2013) | ||
| SARS-CoV-2 | 5 × 103 TCID50 | 21–23 | 3–4 d | 3.2 TCID50/ml | (van Doremalen et al., 2020) | |
| 6.3 × 105 TCID50 | 22 | 4–7 d | 102 TCID50/ml | (Chin et al., 2020) | ||
| αcoronavirus 1 | 1.2 × 107 TCID50 | 4 | > 5 d | 6.3 × 102 TCID50/ml | (Malenovská, 2020) | |
| PVC/Teflon | HCoV | 103 PFU | 21 | > 5 d | NR | (Warnes et al., 2015) |
| Silicon rubber | HCoV | 103 PFU | 21 | 3–5 d | NR | (Warnes et al., 2015) |
| Disposable gloves | HCoV | 5 × 103 TCID50 | 21 | 3–6 hg | NR | (Sizun et al., 2000) |
| 5 × 103 TCID50 | 21 | < 1 hh | NR | (Sizun et al., 2000) | ||
| Polymer banknotes | SARS-CoV-2 | 3.38 × 105 TCID50 | 20 | > 28 d | 6.3 TCID50/ml | (Riddell et al., 2020) |
| 3.38 × 105 TCID50 | 30 | 7–14 d | 6.3 TCID50/ml | (Riddell et al., 2020) | ||
| 3.38 × 105 TCID50 | 40 | 1–2 d | 6.3 TCID50/ml | (Riddell et al., 2020) | ||
| Vinyl | SARS-CoV-2 | 3.38 × 105 TCID50 | 20 | > 28 d | 6.3 TCID50/ml | (Riddell et al., 2020) |
| 3.38 × 105 TCID50 | 30 | 3–7 d | 6.3 TCID50/ml | (Riddell et al., 2020) | ||
| 3.38 × 105 TCID50 | 40 | 1–2 d | 6.3 TCID50/ml | (Riddell et al., 2020) |
Last detection time- no detection time.
Virus suspension without FCS.
Virus suspension with FCS.
14 h/d of fluorescent light.
2.5 log reduction approx in 7 days.
1 log reduction in the first 5 days.
Strain 229E.
Strain OC43.
Table 4.
Survival of coronaviruses on metal surfaces.
| Surface description | Virus | Viral titer onto the surface | T (°C) | Survivala | Limit of detection | Reference |
|---|---|---|---|---|---|---|
| Unspecified metal | SARS-CoV | 106 TCID50 | RT | >5 d | NR | (Duan et al., 2003) |
| SARS-CoV-2 | 9.63 × 104 TCID50 | 4 | 8–9 d | 102 TCID50/ml | (Kratzel et al., 2020) | |
| 9.63 × 104 TCID50 | RT | 5–6 d | 102 TCID50/ml | (Kratzel et al., 2020) | ||
| 9.63 × 104 TCID50 | 30 | > 9 d | 102 TCID50/ml | (Kratzel et al., 2020) | ||
| Steel | HCoV | 103 PFU | 21b | > 5 d | NR | (Warnes et al., 2015) |
| 2 × 104 PFU | 24b | > 7 dc | NR | (Bonny et al., 2018) | ||
| SARS-CoV | 5 × 103 TCID50 | 21–23 | 2–3 d | 3.2 TCID50/ml | (van Doremalen et al., 2020) | |
| MersCoV | 106 TCID50 | 20 | 2–3 d | NR | (van Doremalen et al., 2013) | |
| 106 TCID50 | 30 | 1–2 d | NR | (van Doremalen et al., 2013) | ||
| 106 TCID50 | 30 | 8 h – 1 d | NR | (van Doremalen et al., 2013) | ||
| SARS-CoV-2 | 5 × 103 TCID50 | 21–23 | 3–4 d | 3.2 TCID50/ml | (van Doremalen et al., 2020) | |
| 6.3 × 105 TCID50 | 22 | 4–7 d | 102 TCID50/ml | (Chin et al., 2020) | ||
| 3.2 × 105 TCID50 | 22–23 | > 1 d | 90 TCID50/ml | (Behzadinasab et al., 2020) | ||
| 3.38 × 105 TCID50 | 20 | > 28 d | 6.3 TCID50/ml | (Riddell et al., 2020) | ||
| 3.38 × 105 TCID50 | 30 | 7–14 d | 6.3 TCID50/ml | (Riddell et al., 2020) | ||
| 3.38 × 105 TCID50 | 40 | 1–2 d | 6.3 TCID50/ml | (Riddell et al., 2020) | ||
| Aluminum | HCoV | 5 × 103 TCID50 | 21 | 6–12 h | NR | (Sizun et al., 2000) |
| 5 × 103 TCID50 | 21 | 2–3 h | NR | (Sizun et al., 2000) | ||
| Copper | SARS-CoV | 103-104 TCID50 | 21–23 | 8 h – 1 d | 31.6 TCID50/ml | (van Doremalen et al., 2020) |
| SARS-CoV-2 | 103–104 TCID50 | 21–23 | 4–8 h | 31.6 TCID50/ml | (van Doremalen et al., 2020) |
Last detection- no detection).
14 h/d of fluorescent light.
2.5 log reduction approx in 7 days.
Table 5.
Survival of coronaviruses on glass surfaces.
| Virus | Viral titer onto the surface | T (°C) | Survivala | Limit of detection | Reference |
|---|---|---|---|---|---|
| HCoV | 103 PFU | 21 | >5 d | NR | (Warnes et al., 2015) |
| 2 × 104 PFU | 24b, a | >7 dc | NR | (Bonny et al., 2018) | |
| SARS-CoV | 106 TCID50 | RT | 4–5 d | NR | (Duan et al., 2003) |
| 105 TCID50 | 4 | >14 d | NR | (Chan et al., 2020) | |
| 105 TCID50 | 20–25 | 7–14 d | NR | (Chan et al., 2020) | |
| 105 TCID50 | 30 | 1–3 d | NR | (Chan et al., 2020) | |
| 105 TCID50 | 37 | 1–3 d | NR | (Chan et al., 2020) | |
| SARS-CoV-2 | 3.2 × 105 TCID50 | 22–23 | >1 d | 90 TCID50/ml | (Behzadinasab et al., 2020) |
| 3.2 × 104 TCID50 | 4 | >14 d | NR | (Chan et al., 2020) | |
| 3.2 × 104 TCID50 | 20–25 | 3–5 d | NR | (Chan et al., 2020) | |
| 3.2 × 104 TCID50 | 30 | 1–3 d | NR | (Chan et al., 2020) | |
| 3.2 × 104 TCID50 | 37 | 0–1 d | NR | (Chan et al., 2020) | |
| 6.3 × 105 TCID50 | 22 | 2–4 d | 102 TCID50/ml | (Chin et al., 2020) | |
| 3.38 × 105 TCID50 | 20 | >28 d | 6.3 TCID50/ml | (Riddell et al., 2020) | |
| 3.38 × 105 TCID50 | 30 | 7–14 d | 6.3 TCID50/ml | (Riddell et al., 2020) | |
| 3.38 × 105 TCID50 | 40 | 1–2 d | 6.3 TCID50/ml | (Riddell et al., 2020) |
Last detection- no detection.
14 h/d of fluorescent light.
2.5 log reduction approx in 7 days.
Table 6.
Survival of coronaviruses on various surfaces.
| Surface type | Surface description | Virus | Viral titer onto the surface | T (°C) | Survivala | Limit of detection | Reference |
|---|---|---|---|---|---|---|---|
| Paper | General paper | SARS-CoV | 5 × 103 TCID50 | RT | 6 h - 1d | NR | (Lai et al., 2005) |
| 5 × 102 TCID50 | RT | 2–3 h | NR | (Lai et al., 2005) | |||
| 50 TCID50 | RT | < 5 min | NR | (Lai et al., 2005) | |||
| SARS-CoV-2 | 6.3 × 104 TCID50 | 22 | 30 min–3 h | 102 TCID50/ml | (Chin et al., 2020) | ||
| Tissue paper | SARS-CoV-2 | 3.2 × 105 TCID50 | 22 | 30 min–3 h | 102 TCID50/ml | (Chin et al., 2020) | |
| Press paper | SARS-CoV | 106 TCID50 | RT | 4–5 d | NR | (Duan et al., 2003) | |
| Filter paper | SARS-CoV | 106 TCID50 | RT | > 5 d | NR | (Duan et al., 2003) | |
| Cardboard | SARS-CoV | 103–104 TCID50 | 21–23 | 8 h – 1 d | 3.2 TCID50/ml | (van Doremalen et al., 2020) | |
| SARS-CoV-2 | 103–104 TCID50 | 21–23 | 1–2 d | 3.2 TCID50/ml | (van Doremalen et al., 2020) | ||
| Paper banknotes | SARS-CoV-2 | 3.38 × 105 TCID50 | 20 | > 28 d | 6.3 TCID50/ml | (Riddell et al., 2020) | |
| 3.38 × 105 TCID50 | 30 | 21–28 d | 6.3 TCID50/ml | (Riddell et al., 2020 | |||
| 3.38 × 105 TCID50 | 40 | 1–2 d | 6.3 TCID50/ml | (Riddell et al., 2020 | |||
| Fabric | Cloth | SARS-CoV | 106 TCID50 | RT | > 5 d | NR | (Duan et al., 2003) |
| SARS-CoV-2 | 6.3 × 104 TCID50 | 22 | 1–2 d | 102 TCID50/ml | (Chin et al., 2020) | ||
| Disposable gown | SARS-CoV | 5 × 103 TCID50 | RT | 1–2 d | NR | (Lai et al., 2005) | |
| 5 × 102 TCID50 | RT | 6 h–1 d | NR | (Lai et al., 2005) | |||
| 50 TCID50 | RT | 5 min–1 h | NR | (Lai et al., 2005) | |||
| Cotton gown | SARS-CoV | 5 × 103 TCID50 | RT | 6 h–1 d | NR | (Lai et al., 2005) | |
| 5 × 102 TCID50 | RT | 5 min–1 h | NR | (Lai et al., 2005) | |||
| 50 TCID50 | RT | 0–5 min | NR | (Lai et al., 2005) | |||
| Cotton cloth | SARS-CoV-2 | 3.38 × 105 TCID50 | 20 | 7–14 d | 6.3 TCID50/ml | (Riddell et al., 2020) | |
| 3.38 × 105 TCID50 | 30 | 3–7 d | 6.3 TCID50/ml | (Riddell et al., 2020) | |||
| 3.38 × 105 TCID50 | 40 | 1 h–1 d | 6.3 TCID50/ml | (Riddell et al., 2020) | |||
| Wood | SARS-CoV | 106 TCID50 | RT | 4–5 d | NR | (Duan et al., 2003) | |
| SARS-CoV-2 | 5 × 105 TCID50 | 22 | 1–2 d | 102 TCID50/ml | (Chin et al., 2020) | ||
| Face mask | Inner layer | SARS-CoV-2 | 7.9 × 105 TCID50 | 22 | 4–7 d | 102 TCID50/ml | (Chin et al., 2020) |
| Outer layer | SARS-CoV-2 | 5.3 × 105 TCID50 | 22 | >7 d | 102 TCID50/ml | (Chin et al., 2020) | |
| Sponge | HCoV | 5 × 103 TCID50 | 21 | 6–12 hb | NR | (Sizun et al., 2000) | |
| 5 × 103 TCID50 | 21 | <1 hc | NR | (Sizun et al., 2000) | |||
| Ceramic | HCoV | 103 PFU | 21 | >5 d | NR | (Warnes et al., 2015) | |
| Banknotes | SARS-CoV-2 | 1.3 × 106 TCID50 | 22 | 2–4 d | 102 TCID50/ml | (Chin et al., 2020) | |
| Soil | SARS-CoV | 106 TCID50 | RT | 4–5 d | NR | (Duan et al., 2003) | |
| Mosaic | SARS-CoV | 106 TCID50 | RT | 3–4 d | NR | (Duan et al., 2003) |
Last detection- no detection.
Strain 229E.
Strain OC43.
Full details about characteristics of included studies (virus strain, surface contamination mode, dehydration mode, RH, virus detection mode) are reported in Supplementary Table 1. Survival data concerning three studies are only described in Section 3.3, since they reported data other than days of survival, as follows: half-life (Biryukov et al., 2020); qualitative result of virus inactivation (Müller et al., 2008), and; comparison of virus log reduction between a dark environment and sunlight exposure (Ratnesar-Shumate et al., 2020).
3.2. Risk of bias within studies (quality evaluation)
Some sources of bias within studies were identified and described. The number of replicates for each experiment is shown in Table 2; thirteen studies included two or more replicates. Four studies describe their use of process controls. Five studies did not report any information about replicates or process controls.
3.3. Results of individual studies
Concerning polymer surfaces, SARS-CoV was demonstrated to survive for 13 days, becoming unculturable after 21 days (Chan et al., 2011). On the other hand, SARS-CoV-2 was demonstrated to survive for at least 28 days on polymer banknotes at 20 °C under laboratory conditions (Riddell et al., 2020). Data from one study (Müller et al., 2008) are not reported in Table 3 since that study gave only qualitative results, reporting the rapid inactivation of HCoV on polymer surfaces.
The longest period of survival on metal was observed for SARS-CoV-2, which was detected for up to 28 days (Riddell et al., 2020). Data from Ratnesar-Shumate et al. (2020) are not listed in Table 4 since they compared darkness and artificial sunlight survival of SARS-CoV-2 during a 1-hour period. In darkness, the virus survived for 1 h, but exposure to artificial sunlight inactivated the virus in less than 1 h. Concerning both polymers and metals, Biryukov et al. (2020) assessed SARS-CoV-2 survival on these surfaces, concluding that droplet volume and surface type did not play a role in decay rate. Furthermore, at RT, virus half-life ranged from 6.3 to 18.6 h depending on the RH, but was reduced from 1.0 to 8.9 h when the temperature was increased to 35 °C. On glass surfaces at 20 °C, coronaviruses other than SARS-CoV-2 survived up to 14 days (Chan et al., 2020), while viable SARS-CoV-2 was detected for up to 28 days on both glass and paper (Riddell et al., 2020). Regarding fabric, SARS-CoV-2 was the most persistent of the viruses examined on this surface, surviving for 7 days on cotton cloth (Riddell et al., 2020), while SARS-CoV was found to be more resistant than SARS-CoV-2 on wood (Duan et al., 2003). Survival of SARS-CoV-2 on face mask was observed up to 7 days (Chin et al., 2020), on common banknotes up to 4 days (Chin et al., 2020) and up to 28 days on both polymer and plastic banknotes (Riddell et al., 2020). Survival of HCoV on sponge did not exceed 1 day (Sizun et al., 2000), while on ceramic it survived up to 5 days (Warnes et al., 2015). On mosaic and soil, SARS-CoV survived 3 and 4 days, respectively (Duan et al., 2003).
3.4. Risk of bias across studies — study differences
Bias across studies occurs due to the variables related to test conditions, shown in Supplementary Table 1. Concerning temperature, the most commonly employed temperature was RT, from 20 to 25 °C. As regards RH, the fluctuation among studies is from 40 to 80% RH. Differences in initial viral titer were also observed, so viral concentration deposited onto the surface ranged from 50 to 1.2 × 107 TCID50. In order to address the latter point, Fig. 2 shows the influence of viral titer on coronavirus survival on the three most commonly investigated surfaces: polymer, metal and glass. Light radiation was employed only in two studies: Bonny et al. (2018) exposed dried HCoV to fluorescent light for 14 h, while Ratnesar-Shumate et al. (2020) exposed SARS-CoV-2 to different levels of UV light in comparison to darkness. Data concerning the latter study are not reported in Table 4 since the study only assessed virus survival for a short time (1 h) and compared survival in darkness and artificial sunlight exposure.
Fig. 2.
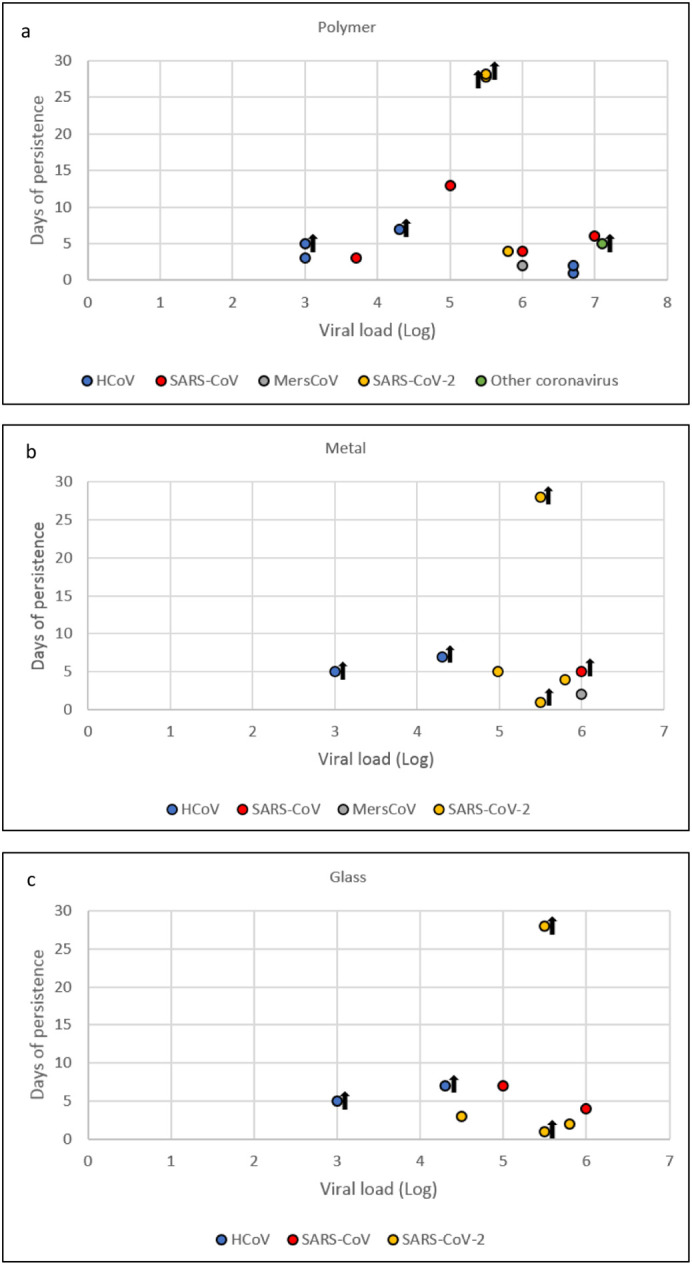
Influence of initial virus titer (TCID50) on coronavirus survival on plastic (a), metal (b) and glass (c) surfaces at room temperature (20–25 °C). Survival <1 day and aerosol-contaminated surfaces are not included. Black arrows mean virus was still detected at the last experimental time point.
As the contamination method, in all studies except one (van Doremalen et al., 2020), droplets containing a specific volume of virus culture were spotted onto the chosen surface. Concerning detection method, all studies assessed the viability or cell culture infectivity of virus dried on surfaces. Titration on cell culture (TCID50) was employed as the main method, while some studies employed immunoperoxidase assay (IPA) (Sizun et al., 2000) or plaque assay (Bonny et al., 2018; Sizun et al., 2000; Warnes et al., 2015). Drying of viral inoculum was performed in different ways. Four studies specified laminar flow hood dehydration (Malenovská, 2020; Riddell et al., 2020; Sizun et al., 2000), with the drying time varying from 15 to 30 min to 2 h. Another important issue preventing a full comparison of results was the choice of time points for analysis. The intervals between time points were very different between studies and, in some cases, too wide to provide accurate comparisons.
4. Discussion
Among the retrieved studies in the present review, the first evaluating human-derived coronavirus survival was performed in 2000 on two HCoV strains (229E, OC43) responsible for common colds (Sizun et al., 2000). Considering the included studies, it has been shown in experimental conditions that some coronaviruses can survive on inanimate surfaces for at least 28 days at RT (Riddell et al., 2020), with SARS-CoV-2 being the most resistant coronavirus in comparison to HCoV, SARS-CoV and MersCoV. Considerable SARS-CoV-2 survival was also determined on personal protective equipment, face mask, on which it survived for 7 days (Chin et al., 2020). It was demonstrated that highly contaminated surfaces of polymer and paper banknotes facilitate SARS-CoV-2 survival for at least 28 days at RT (Riddell et al., 2020). However, we note the considerable discrepancy between Riddell et al. (2020) and another study, which determined survival on glass was only for 2 days despite 0.3 log higher initial virus titer and 15% higher RH being used (Chin et al., 2020). Environmental conditions can affect the survival of viruses in fomites. Indeed, it was demonstrated that high temperature and high RH have a synergistic effect on inactivation of SARS-CoV and SARS-CoV-2 viability, while low temperatures and low RH support prolonged survival of these viruses on contaminated surfaces, independently of surface type (Biryukov et al., 2020; Chan et al., 2020; Chan et al., 2011; van Doremalen et al., 2013).
As stated in Section 3.4, different concentrations of initial virus titer were employed in different studies. To assess its impact, Fig. 2 shows the influence of virus titer on coronaviruses' survival on the three most commonly investigated surfaces: polymer, metal and glass. On these surfaces, the longest survival time was 28 days for SARS-CoV-2, which was assessed using an initial titer of 5.5 log TCID50/ml (Riddell et al., 2020). However, higher virus concentration did not induce longer virus survival on any of the three surfaces. In fact, a virus titer of 5.8 log allowed SARS-CoV-2 to survive a maximum of 4 days on both polymer and metal, and a maximum of 2 on glass (Chin et al., 2020). Referring to SARS-CoV survival on polymer, 6 log and 7 log initial titer allowed it to survive 4 (Duan et al., 2003) and six 6 days (Rabenau et al., 2005), respectively. On glass, SARS-CoV survived 4 days with an initial virus titer of 6 log. Contamination with HCoV starting from 6.7 log virus titer showed a maximum survival of 2 days (Rabenau et al., 2005). The highest initial viral titer was employed by Malenovská (2020) with Alphacoronavirus 1, but the viability assessment stopped after five days of analysis. Some studies stopped the viability assessment before viral inactivation occurred, so it was not possible to correlate their initial virus titer with survival time.
Concerning the titration methods employed in the analyzed studies for detection of viral infectivity on cell culture, when reported, the limit of detection (LOD) was very variable, ranging from 10 to 102.8 TCID50/ ml. These data suggest that even if infective virus is not detected, this does not exclude the possibility that some virus particles survive for a longer time on the surfaces, and thus, virus survival could be underestimated. On the other hand, methods with a very low LOD could reveal virus presence at titers lower than the human infectious dose, with an overestimation of contamination risk. To date, the minimum infectious dose of SARS-CoV-2 in humans is uncertain. Two pre-print studies estimate that only a few hundreds of SARS-CoV-2 virus particles are sufficient to establish an infection in a susceptible individual (Basu, 2020; Karimzadeh et al., 2020). If this infectious dose is confirmed, to assess the infectiousness of SARS-CoV-2 via surfaces, it will be important to set a viral titer threshold of 102 TCID50/ml. With this adjustment, the virus would retain its infectious ability for 21 days on polymer banknotes, steel, glass and paper banknotes, 3 days on cotton cloth (Riddell et al., 2020), 1 day on wood and 2 days on common banknotes (Chin et al., 2020).
Considering irradiation, solar or artificial UV rays can play a role in coronavirus inactivation, as was previously demonstrated with other respiratory viruses (Deford et al., 2019; McDevitt et al., 2012). Exposure of SARS-CoV-2 to artificial UVB, simulating sunlight, produced rapid inactivation, in particular for virus on stainless steel, suggesting that fomite transmission through outdoor surfaces exposed to direct sunlight is very unlikely, especially during the summer season at 40°N latitude (Ratnesar-Shumate et al., 2020). On the other hand, greater attention to fomite transmission should be paid to contaminated surfaces not exposed to sunlight.
Despite the present review taking into account the survival of SARS-CoV on surfaces, it is important to underline that the high chance of fomite transmission is actually related to the presence of droplets before their desiccation. Indeed, the likelihood of virus survival inside a droplet increases about five-fold compared with non-droplet virus, especially under humid conditions as compared to dry conditions (Bhardwaj and Agrawal, 2020).
Our review was unable to show a link between survival and surface characteristics, because of the low number of studies carried out in the same conditions. Despite it being reported that the nature of fomites could influence the survival of viruses, with better persistence on non-porous surfaces (Boone and Gerba, 2007), other studies showed conflicting results, depending on virus type (Alidjinou et al., 2019). Considering porous cotton fabric, the drying process can reduce the recovery rate of the virus with respect to non-porous surfaces. Virus recovery from cotton fabric is also reduced due to the adherence of the virus to the fabric fiber (Riddell et al., 2020). Also, exposure to ion-generating surfaces, such as copper and copper alloy, can compromise the envelope and nucleoprotein in coronavirus more rapidly than in the non-enveloped viruses, such as norovirus (Warnes et al., 2015).
Our systematic review has some limitations. One possible limitation refers to the search strategy that was specific and not extensive, to accelerate the process. We addressed this potential issue, recurring to forward and backward reference searches of both the included studies and of relevant reviews. Another limitation is due to the exclusion of gray literature and of pre-print databases, and this could account for publication bias due to the file drawer effect. However, for quality purposes, we decided to base our investigation on peer-reviewed papers. In addition, due to the rapidly growing body of knowledge referring to the review question, it is possible that in the next period other data will become available, so this topic would require further updates. To reduce this effect, we updated the literature search continuously, with the last date searched being on 6th November 2020. Finally, the methodological heterogeneity, in particular related to initial viral concentration, detection time point, temperature, and viral titration tests, impaired meaningful statistical synthesis of study results.
5. Conclusions
The longest SARS-CoV-2 survival demonstrated under laboratory conditions at RT is 28 days on glass, steel and both polymer and paper banknotes, while coronaviruses different from SARS-CoV-2 can survive 14 days on glass. The survival of SARS-CoV-2 would be reduced to 21 days when a survival threshold of 102 TCID50/ml was measured. However, low temperature and moisture can increase virus survival, while UV light and sunlight can substantially decrease virus survival on exposed surfaces. Nevertheless, the strictly controlled laboratory conditions applied during experiments may not reflect the real resistance of SARS-CoV-2, and therefore, studies better simulating real-life conditions should be performed. In addition, no correlation was found between a higher initial viral titer and a longer survival period. Despite higher initial virus titer being used on glass, Chin et al. (2020) assessed SARS-CoV-2 survival for 26 days less than was reported by Riddell et al. (2020), for instance.
To guarantee data uniformity and allow comparison of virus survival studies, we recommend the definition of a reference study protocol for all laboratories investigating this topic. As an example, it is important to assess viral viability until it is completely inactivated. Further, more homogeneous studies assessing coronavirus survival on fomites are needed to fill data gaps.
Although prolonged survival of SARS-CoV-2 on surfaces has been proved, evidence of transmission from contaminated dry surfaces is still needed, while direct person-to-person transmission remains the main confirmed route.
The following are the supplementary data related to this article.
Survival of coronaviruses on different types of surfaces. Other study characteristics are listed: strain/isolate, viral titer onto the surface, surface contamination and dehydration mode, temperature (T) and relative humidity (RH) during survival test, virus detection mode and limit of detection. NR: not reported.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors have no competing interests to declare.
Edited by: Ewa Korzeniewska
References
- Alidjinou E.K., Sane F., Firquet S., Lobert P.E., Hober D. Resistance of enteric viruses on fomites. Intervirology. 2019;61:205–213. doi: 10.1159/000448807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour H.M., Elkhatib W.F., Rahman M.M., Elshabrawy H.A. Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks. Pathogens. 2020;9:186. doi: 10.3390/pathogens9030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu, S., 2020. Computational characterization of inhaled droplet transport in the upper airway leading to SARS-CoV-2 infection. medRxiv. doi: 10.1101/2020.07.27.20162362. [DOI]
- Behzadinasab S., Chin A., Hosseini M., Poon L., Ducker W.A. A surface coating that rapidly inactivates SARS-CoV-2. ACS Appl. Mater. Interfaces. 2020;12:34723–34727. doi: 10.1021/acsami.0c11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj R., Agrawal A. Likelihood of survival of coronavirus in a respiratory droplet deposited on a solid surface. Phys. Fluids. 2020;32 doi: 10.1063/5.0012009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biryukov, J., Boydston, J.A., Dunning, R.A., Yeager, J.J., Wood, S., Reese, A.L., Ferris, A., Miller, D., Weaver, W., Zeitouni, N.E., Phillips, A., Freeburger, D., Hooper, I., Ratnesar-Shumate, S., Yolitz, J., Krause, M., Williams, G., Dawson, D.G., Herzog, A., Dabisch, P., Wahl, V., Hevey, M.C., Altamura, L.A., 2020. Increasing temperature and relative humidity accelerates inactivation of SARS-COV-2 on surfaces. mSphere 5. doi: 10.1128/mSphere.00441-20. [DOI] [PMC free article] [PubMed]
- Bonny T.S., Yezli S., Lednicky J.A. Isolation and identification of human coronavirus 229E from frequently touched environmental surfaces of a university classroom that is cleaned daily. Am. J. Infect. Control. 2018;46:105–107. doi: 10.1016/j.ajic.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone S.A., Gerba C.P. Significance of fomites in the spread of respiratory and enteric viral disease. Appl. Environ. Microbiol. 2007;73:1687. doi: 10.1128/AEM.02051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.H., Peiris J.S.M., Lam S.Y., Poon L.L.M., Yuen K.Y., Seto W.H. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv. Virol. 2011;2011 doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.-H., Sridhar S., Zhang R.R., Chu H., Fung A.Y.-F., Chan G., Chan J.F.-W., To K.K.-W., Hung I.F.-N., Cheng V.C.-C., Yuen K.-Y. Factors affecting stability and infectivity of SARS-CoV-2. J. Hosp. Infect. 2020;106:226–231. doi: 10.1016/j.jhin.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W., Peiris M., Poon L.L.M. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe. 2020;1 doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deford D.M., Nosek J.M., Castiglia K.R., Hasik E.F., Franke M.E., Nick B.C., Abdelnour A.M., Haas C.E., Junod N.A., Latsko K.N., Moore M.L., Berthrong S.T., Rostad C.A., Stobart C.C. Evaluation of the role of respiratory syncytial virus surface glycoproteins F and G on viral stability and replication: implications for future vaccine design. J. Gen. Virol. 2019;100:1112–1122. doi: 10.1099/jgv.0.001287. [DOI] [PubMed] [Google Scholar]
- Dowell S.F., Simmerman J.M., Erdman D.D., Wu J.-S.J., Chaovavanich A., Javadi M., Yang J.-Y., Anderson L.J., Tong S., Ho M.S. Severe acute respiratory syndrome coronavirus on hospital surfaces. Clin. Infect. Dis. 2004;39:652–657. doi: 10.1086/422652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Günther S., Preiser W., van der Werf S., Brodt H.-R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A.M., Berger A., Burguière A.-M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.-C., Müller S., Rickerts V., Stürmer M., Vieth S., Klenk H.-D., Osterhaus A.D.M.E., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/nejmoa030747. [DOI] [PubMed] [Google Scholar]
- Duan S., Zhao X., Wen R., Huang J., Pi G., Zhang S., Han J., Bi S., Ruan L., Dong X. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed. Environ. Sci. 2003;16:246–255. [PubMed] [Google Scholar]
- Fernández-Raga M., Díaz-Marugán L., García Escolano M., Bort C., Fanjul V. SARS-CoV-2 viability under different meteorological conditions, surfaces, fluids and transmission between animals. Environ. Res. 2021 doi: 10.1016/j.envres.2020.110293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo L., Cervino G., Matarese M., D’Amico C., Surace G., Paduano V., Fiorillo M.T., Moschella A., La Bruna A., Romano G.L., Laudicella R., Baldari S., Cicciù M. COVID-19 surface persistence: a recent data summary and its importance for medical and dental settings. Int. J. Environ. Res. Public Heal. 2020;17 doi: 10.3390/IJERPH17093132. 2020. Page 3132 17, 3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller C., Varbanov M., Duval R.E. Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses. 2012;4:3044–3068. doi: 10.3390/v4113044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie R., Alfa M.J., Coombs K. Survival of enveloped and non-enveloped viruses on surfaces compared with other micro-organisms and impact of suboptimal disinfectant exposure. J. Hosp. Infect. 2008;69:368–376. doi: 10.1016/j.jhin.2008.04.024. [DOI] [PubMed] [Google Scholar]
- John Hopkins University, 2020. Coronavirus Resource Center. URL: https://coronavirus.jhu.edu/map.html (accessed 1.4.20).
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimzadeh, S., Bhopal, R., Huy, N.T., 2020. Review of infective dose, routes of transmission, and outcome of COVID-19 caused by the SARS-COV2 virus: comparison with other respiratory viruses. Running title: COVID-19 infective dose review. Preprints.
- Kaul D. An overview of coronaviruses including the SARS-2 coronavirus – molecular biology, epidemiology and clinical implications. Curr. Med. Res. Pract. 2020;10:54–64. doi: 10.1016/j.cmrp.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A., Schwebke I., Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratzel A., Steiner S., Todt D., Brueggemann Y., Steinmann J., Steinmann E., Thiel V., Pfaender S. Temperature-dependent surface stability of SARS-CoV-2. J. Infect. 2020;81:452–482. doi: 10.1016/j.jinf.2020.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.Y.Y., Cheng P.K.C., Lim W.W.L. Survival of severe acute respiratory syndrome coronavirus. Clin. Infect. Dis. 2005;41:e67–e71. doi: 10.1086/433186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenovská H. Coronavirus persistence on a plastic carrier under refrigeration conditions and its reduction using wet wiping technique, with respect to food safety. Food Environ. Virol. 2020 doi: 10.1007/s12560-020-09447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt J.J., Rudnick S.N., Radonovich L.J. Aerosol susceptibility of influenza virus to UV-C light. Appl. Environ. Microbiol. 2012;78:1666–1669. doi: 10.1128/AEM.06960-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan S.V., Hemalatha M., Kopperi H., Ranjith I., Kumar A.K. SARS-CoV-2 in environmental perspective: occurrence, persistence, surveillance, inactivation and challenges. Chem. Eng. J. 2021;405:126893. doi: 10.1016/j.cej.2020.126893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A., Tillmann R.L., Müller A., Simon A., Schildgen O. Stability of human metapneumovirus and human coronavirus NL63 on medical instruments and in the patient environment. J. Hosp. Infect. 2008 doi: 10.1016/j.jhin.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorimotlagh Z., Mirzaee S.A., Jaafarzadeh N., Maleki M., Kalvandi G., Karami C. A systematic review of emerging human coronavirus (SARS-CoV-2) outbreak: focus on disinfection methods, environmental survival, and control and prevention strategies. Environ. Sci. Pollut. Res. 2020 doi: 10.1007/s11356-020-11060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter J.A., Donskey C., Yezli S., Douthwaite S., Goldenberg S.D., Weber D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J. Hosp. Infect. 2016;92:235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenau H.F., Cinatl J., Morgenstern B., Bauer G., Preiser W., Doerr H.W. Stability and inactivation of SARS coronavirus. Med. Microbiol. Immunol. 2005;194:1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnesar-Shumate S., Williams G., Green B., Krause M., Holland B., Wood S., Bohannon J., Boydston J., Freeburger D., Hooper I., Beck K., Yeager J., Altamura L.A., Biryukov J., Yolitz J., Schuit M., Wahl V., Hevey M., Dabisch P. Simulated sunlight rapidly inactivates SARS-CoV-2 on surfaces. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren S.-Y., Wang W.-B., Hao Y.-G., Zhang H.-R., Wang Z.-C., Chen Y.-L., Gao R.-D. Stability and infectivity of coronaviruses in inanimate environments. World J. Clin. cases. 2020;8:1391–1399. doi: 10.12998/wjcc.v8.i8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell S., Goldie S., Hill A., Eagles D., Drew T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020;17:145. doi: 10.1186/s12985-020-01418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizun J., Yu M.W., Talbot P. Survival of human coronaviruses 229E and OC43 in suspension and after drying onsurfaces: a possible source of hospital-acquired infections. J. Hosp. Infect. 2000;46:55–60. doi: 10.1053/jhin.2000.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadnytskyi V., Bax C.E., Bax A., Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc. Natl. Acad. Sci. U. S. A. 2020;117:11875–11877. doi: 10.1073/pnas.2006874117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J., Brunton J., Graziosi S. 2010. EPPI-Reviewer 4: Software for Research Synthesis. [Google Scholar]
- van Doremalen N., Bushmaker T., Munster V. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Eurosurveillance. 2013;18:20590. doi: 10.2807/1560-7917.ES2013.18.38.20590. [DOI] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnes S.L., Little Z.R., Keevil C.W. Human coronavirus 229E remains infectious on common touch surface materials. MBio. 2015;6 doi: 10.1128/mBio.01697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Middle East Respiratory Syndrome: MERS Situation Update. [Google Scholar]
- Zhang R., Li Y., Zhang A.L., Wang Y., Molina M.J. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc. Natl. Acad. Sci. U. S. A. 2020;117:14857–14863. doi: 10.1073/pnas.2009637117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survival of coronaviruses on different types of surfaces. Other study characteristics are listed: strain/isolate, viral titer onto the surface, surface contamination and dehydration mode, temperature (T) and relative humidity (RH) during survival test, virus detection mode and limit of detection. NR: not reported.