Abstract
Background
A strong negative correlation is reported between the Bacille Calmette Guerin (BCG) index and COVID-19 mortality. The present study explored if frequent exposure to strong Th1 antigens like Mycobacteria or Salmonella have any effect on the progression of the disease in COVID-19 patients.
Methods
This prospective comparative study comprised of 3 groups of 20 each of mild or asymptomatic COVID-19 patients (A), severely ill patients (S) and healthy volunteers with a COVID Negative report (H).
Results
QuantiFERON TB Gold (QFT) which is interferon gamma release assay (IGRA) against Mtb antigen was used to quantify immunity status of patients against the tuberculosis. Group S showed positive QFT in only 15% patients as against 50% QFT positive patients in group A and H. All fourteen patients in group S with QFT negative report died while 5 of six survived patients showed positive QFT report either on initial or repeat testing done at 6 weeks. The sixth survived patient was QFT negative but showed high antibody titre against H antigen (TH) on Widal test. All severely ill group S patients showed huge reduction of IGRA even to the mitogen stimulus thus suggesting gross general unresponsiveness of T cells. Presence of BCG scar showed no correlation with prevalence or progression of the disease.
Conclusion
Population in an endemic area of tuberculosis and typhoid with good community exposure to these antigen is likely to withstand COVID -19 better and show reduced mortality following it.
Keywords: COVID-19, Mycobacteria, QuantiFERRON, IGRA, Salmonella
1. Introduction
Corona virus infectious disease-19 (COVID-19) is caused by SAR-CoV-2 virus and presents with symptoms of flu and severe acute respiratory syndrome (SARS).1 It has infected more than 43 million persons and taken 1.16 million lives globally till date.2 Male patients with co-morbidities and above 50 years of age are at higher mortality risk following COVID-19.3 Spread and susceptibility to infection, morbidity and mortality following COVID-19 has been found to vary among different countries.4 Besides the varied capacity and quality of testing and healthcare facilities, ethnicity which is a complex mix of genetic constituency, social and cultural practices, and behavioural patterns has also been reported to affect the epidemiological profile of the disease.3 , 4
BCG vaccine is reported to affect course of many diseases including viral infections, and malignancy etc.5 BCG has shown demonstrable protective effect against viral infections in experimental animals and humans as well due to induction of innate immunity and stimulation of cell mediated and humoral immune responses.6 A cohort study of 49 cases of active tuberculosis and COVID-19 co-infection has suggested increased inter-susceptibility and progression of the two diseases.7 Such contradictory observations can be explained if one understands distinction between the immunological profile of a patient with active tubercular disease and that of a latent infection. Active tubercular disease whether due to fresh infection or reactivation of the existing latent infection is an expression of compromised immunity or probably a Th2 bias status which may actually increase patient's susceptibility to SAR-CoV-2. On the contrary, community exposure to Mtb or any other similar antigen in a healthy individual is likely to boost his immunity and thus can be protective against COVID-19.
Human population lacking in Th1 immunity overexpresses Th2 immunity due to the cross regulation between Th1 and Th2 immunity.8 COVID-19 patients who exhibited Th1 type response9 were seen to follow recovery along with increased neutralizing antibodies, while patients showing Th2 response witnessed fatal outcome of the disease.10 Antigens of Mtb and Salmonella predominantly induce Th1 mediated immune response11 and thus might provide some protection against COVID-19. We, therefore proposed to measure Salmonella antibodies titre and Interferon gamma release assay (IGRA) against Mtb antigen among the study population grouped into healthy volunteers, asymptomatic or mildly symptomatic and severe COVID-19 patients and tried to establish their correlation with the disease progression and mortality associated with COVID-19.
2. Material and methods
The present work was a prospective comparative study conducted at Lok Nayak (LN) Hospital and Maulana Azad Medical College, New Delhi comprising of 60 subjects in three groups of 20 each. Group A consisted of asymptomatic or mildly symptomatic cases admitted in a particular male ward on three consecutive days with a Covid-19 positive test report by reverse transcriptase polymerase chain reaction (RT PCR) from the oro-naso-pharyngeal swab, Group S consisted of severely ill COVID-19 positive cases admitted in the intensive care unit of the hospital while Group H comprised of 20 healthy male volunteers working as health care worker (HCW) at LN Hospital with a COVID-19 negative RT-PCR report. Ease of availability and higher mortality among male population made us enrol only male subjects to participate in study for group A and H. Patients with no sign of viral pneumonia or hypoxia were labelled as mild while severely ill patients were those with respiratory rate of more than 30 breaths/min or SpO2 of less than 90% on room air.
Patients with co-morbid conditions like malignancy, chronic renal failure, cardiomyopathy, chronic respiratory failure with pre-existing dyspnoea, inflammatory diseases like rheumatoid arthritis, inflammatory bowel disease or patients on long term immuno-suppressants were excluded from the study. Approval of the Institutional Ethical Committee was obtained (F1/IEC/MAMC/75/03/2020/76 dated 4.05.20). After an informed consent of all participants, a routine clinical examination for fever, pulse rate, blood pressure and respiratory rate along with any organomegaly was done. The socio-economic status, past illness like diabetes mellitus, hypertension, asthma or other allergic disorder, intake of angiotensin converting enzyme-2 (ACE2) inhibitors or steroids were recorded. A note was also made of the dietary habits as well as religious practices of performing ‘Hawan’ and ‘Arti” which involve sublimating mixture of magnifera indica wood with odoriferous and medicinal herbs of cinnamomum camphora, crocus sativus, eugenia caryophyllus, mesua ferra, etc. in the fire exposing nasal, respiratory and gut mucosa to many irritants of ancient unproven qualities. Any past history of vaccinations including BCG, influenza, hepatitis in the preceding ten years were noted along with the clinical evidence of presence of the scar of BCG vaccine. All participants were subjected to haematological investigations including haemoglobin (Hb), total and differential leucocyte counts (TLC and DLC), absolute neutrophil counts (ANC) along with serum ferritin, triacylglycerol (TAG), D-dimer, QFT and Widal test (anti-TO and anti-TH and AH titre) within 24 hours of their recruitment for the study. QFT was repeated 4 weeks after discharge from the hospital in severe COVID-19 cases with negative test report. All other relevant investigations and medical treatment depending upon the individual clinical needs was instituted. None of patients received any supportive treatment with corticosteroids, convalescent plasma or any immunomodulatory agents like hydroxychloroquine (HCQ) etc.
2.1. Statistical methods
MS Excel spreadsheet program and SPSS v23 (IBM Corp.) were used for data analysis. Group comparisons for continuously distributed data were made using independent sample ‘t’ test when comparing two groups. If data were found to be non-normally distributed, appropriate non-parametric tests in the form of Wilcoxon Test were used. Chi-squared test was used for group comparisons for categorical data. In case the expected frequency in the contingency tables was found to be <5 for >25% of the cells, Fisher's Exact test was used instead. Statistical significance was kept at p < 0.05.
3. Results
There was significant difference between the 3 groups in terms of D-dimer (χ2 = 36.533, p = <0.001, S. Ferritin (χ2 = 19.811, p = <0.001), TAG (χ2 = 12.308, p = <0.001), Hb, TLC, neutrophils, lymphocytes (p = <0.001) and monocyte counts (p = 0.015). S group demonstrated the highest level of S. Ferritin, TLC, ANC and monocyte counts (Table 1 ).
Table 1.
Laboratory and microbiological parameters in severely ill (S), asymptomatic or mild symptomatic (A) COVID-19 patients and healthy Subjects (H).
| All Parameters Mean ± std.dev |
Group |
p value | ||
|---|---|---|---|---|
| S (n = 20) | A (n = 20) | H (n = 20) | ||
| Age (Years)∗∗∗ | 57.94 ± 19.33 | 36.00 ± 10.77 | 36.30 ± 11.43 | <0.0011 |
| Gender∗∗∗ | <0.0012 | |||
| Male | 9 (45.0%) | 20 (100.0%) | 20 (100.0%) | |
| Female | 11 (55.0%) | 0 (0.0%) | 0 (0.0%) | |
| Socio-Economic Status∗∗∗ | <0.0012 | |||
| Good | 3 (15.0%) | 1 (5.0%) | 10 (50.0%) | |
| Medium | 15 (75.0%) | 12 (60.0%) | 10 (50.0%) | |
| Poor | 2 (10.0%) | 7 (35.0%) | 0 (0.0%) | |
| D-Dimer (ng/ml)∗∗∗ | 2527.20 ± 1731.80 | 3681.00 ± 1869.56 | 319.30 ± 258.82 | <0.0011 |
| S. Ferritin∗∗∗ | 405.82 ± 323.64 | 322.04 ± 280.15 | 82.91 ± 57.43 | <0.0011 |
| TAG∗∗∗ | 209.40 ± 85.65 | 127.05 ± 77.56 | 247.25 ± 114.33 | <0.0011 |
| Haemoglobin (g/dL)∗∗∗ | 11.18 ± 2.16 | 13.18 ± 1.87 | 14.49 ± 1.27 | <0.0013 |
| Haematocrit (%)∗∗∗ | 34.59 ± 6.35 | 38.87 ± 5.25 | 39.88 ± 9.27 | 0.0011 |
| TLC (/cu.mm)∗∗∗ | 14572.00 ± 7324.18 | 7565.50 ± 3095.59 | 7170.50 ± 1240.85 | <0.0013 |
| ANC (/cu.mm)∗∗∗ | 12385.25 ± 6752.10 | 5541.00 ± 3015.81 | 4500.55 ± 890.76 | <0.0011 |
| Lymphocytes (/cu.mm)∗∗∗ | 1927.50 ± 1520.88 | 1913.50 ± 648.27 | 2566.65 ± 572.46 | 0.0011 |
| Monocytes (/cu.mm)∗∗∗ | 259.25 ± 221.64 | 111.00 ± 103.32 | 103.30 ± 58.86 | 0.0151 |
| Platelet Count (Lac/cu.mm) | 2.14 ± 1.75 | 1.87 ± 0.74 | 1.76 ± 0.69 | 0.9541 |
| Quantiferon TB Gold∗∗∗ | 0.27 ± 0.86 | 1.80 ± 3.01 | 4.32 ± 5.65 | 0.0011 |
| Quantiferon TB Gold +ve (>0.35 iu/ml) (Positive)∗∗∗ | 3 (15.0%) | 10 (50.0%) | 10 (50.0%) | 0.0324 |
| Mitogen∗∗∗ | 1.33 ± 2.77 | 8.84 ± 5.69 | 14.29 ± 3.51 | <0.0011 |
| TO Antigen | 0.7492 | |||
| <50 | 16 (80.0%) | 18 (90.0%) | 16 (80.0%) | |
| 50–100 | 4 (20.0%) | 2 (10.0%) | 4 (20.0%) | |
| TH Antigen∗∗∗ | 0.0202 | |||
| <50 | 14 (70.0%) | 10 (50.0%) | 17 (85.0%) | |
| 50–100 | 2 (10.0%) | 9 (45.0%) | 2 (10.0%) | |
| >100 | 4 (20.0%) | 1 (5.0%) | 1 (5.0%) | |
| AH Antigen | 0.3102 | |||
| <50 | 17 (85.0%) | 19 (100.0%) | 19 (95.0%) | |
| 50–100 | 3 (15.0%) | 0 (0.0%) | 1 (5.0%) | |
| Influenza Vaccine (Present) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.0004 |
| BCG Scar (Present) | 16 (80.0%) | 16 (80.0%) | 16 (80.0%) | 1.0002 |
| Diabetes (Present) | 3 (15.0%) | 3 (15.0%) | 0 (0.0%) | 0.2282 |
| ACE Inhibitor Use (Present) | 4 (20.0%) | 2 (10.0%) | 1 (5.0%) | 0.4782 |
| Steroid, HCQS, Convalescent plasma Use, (Present) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.0004 |
| Religious Practices (Present) | 19 (95.0%) | 18 (90.0%) | 19 (95.0%) | 1.0002 |
| Outcome | 1.0004 | |||
| Survived | 6 (30.0%) | 0 (NaN%) | 0 (NaN%) | |
| Died | 14 (70.0%) | 0 (NaN%) | 0 (NaN%) | |
∗∗∗Significant at p < 0.05, 1: Kruskal Wallis Test, 2: Fisher's Exact Test, 3: One-Way ANOVA, 4: Chi-Squared Test.
There was a significant difference between the various groups in terms of distribution of positive Mtb-induced IGRA (χ2 = 13.838, p = 0.001) or mitogen induced IGRA (χ2 = 36.946, p = <0.001) which were lowest in S Group. The difference between levels of QFT was statistically significant (p < 0.01) when corrected for lymphocyte count (confounder) by linear regression analysis and also when adjusted for age and sex by ordinal logistic regression (χ2(3) = 49.930, p < 0.001). There was no significant difference between the various groups in terms of distribution of Salmonella TO, TH or AH Antigens or BCG scar.
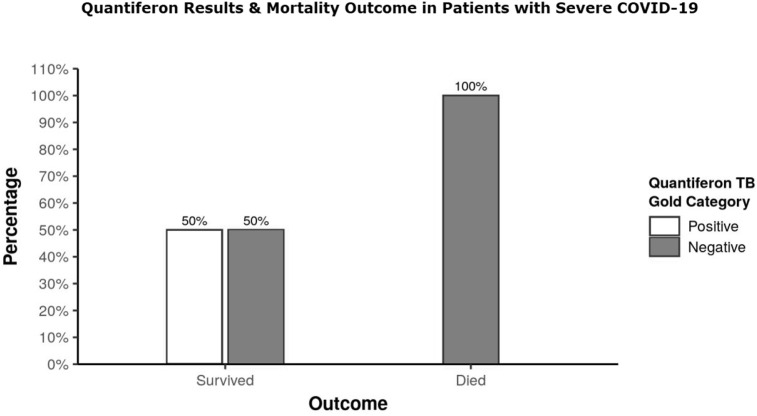
Two subgroups were made based on the outcome measure of “Survived” or “Died” among patients of group S and compared for various parameters. There was a significant difference between the 2 subgroups in terms of TLC (W = 10.000, p = 0.006), positive QFT (χ2 = 8.235, p = 0.018) and mitogen induced IGRA (W = 70.000, p = 0.023) which was also seen between the 2 subgroups made within the total study population (Table 2, Table 3 ). All patients with positive QFT and high mitogen-induced IGRA survived (Fig. 1 ).
Table 2.
Association between laboratory Parameters and Outcome (Death) in Severe COVID-19 cases.
| All Parameters Mean ± std.dev |
Outcome |
p value | |
|---|---|---|---|
| Survived (n = 6) | Died (n = 14) | ||
| Group | 1.0001 | ||
| S | 6 (100.0%) | 14 (100.0%) | |
| A | 0 (0.0%) | 0 (0.0%) | |
| H | 0 (0.0%) | 0 (0.0%) | |
| Age (Years) | 49.67 ± 22.42 | 62.08 ± 17.12 | 0.3982 |
| Gender | 0.1573 | ||
| Male | 1 (16.7%) | 8 (57.1%) | |
| Female | 5 (83.3%) | 6 (42.9%) | |
| Socio-Economic Status | 0.2723 | ||
| Good | 2 (33.3%) | 1 (7.1%) | |
| Medium | 4 (66.7%) | 11 (78.6%) | |
| Poor | 0 (0.0%) | 2 (14.3%) | |
| D-Dimer (ng/ml) | 2158.17 ± 1739.71 | 2685.36 ± 1769.03 | 0.4802 |
| S. Ferritin | 359.83 ± 262.29 | 425.52 ± 353.87 | 0.9672 |
| TAG | 219.50 ± 98.23 | 205.07 ± 83.33 | 0.9342 |
| Haemoglobin (g/dL) | 9.97 ± 2.83 | 11.70 ± 1.66 | 0.2012 |
| Haematocrit (%) | 30.37 ± 7.92 | 36.40 ± 4.80 | 0.0762 |
| TLC (/cu.mm)∗∗∗ | 8735.00 ± 3889.63 | 17073.57 ± 7079.49 | 0.0062 |
| ANC (/cu.mm)∗∗∗ | 6735.67 ± 3831.65 | 14806.50 ± 6319.92 | 0.0032 |
| Lymphocytes (/cu.mm) | 1783.00 ± 678.63 | 1989.43 ± 1785.99 | 0.6802 |
| Monocytes (/cu.mm) | 216.33 ± 271.33 | 277.64 ± 205.59 | 0.3222 |
| Platelet Count (Lac/cu.mm) | 3.06 ± 2.85 | 1.75 ± 0.88 | 0.2742 |
| Quantiferon TB Gold | 0.86 ± 1.48 | 0.02 ± 0.02 | 0.0712 |
| Quantiferon TB Gold +ve (>0.35 iu/ml) (Positive)∗∗∗ | 3 (50.0%) | 0 (0.0%) | 0.0183 |
| Mitogen∗∗∗ | 3.56 ± 4.49 | 0.37 ± 0.43 | 0.0232 |
| TO Antigen | 0.5493 | ||
| <50 | 4 (66.7%) | 12 (85.7%) | |
| 50–100 | 2 (33.3%) | 2 (14.3%) | |
| TH Antigen | 0.4323 | ||
| <50 | 3 (50.0%) | 11 (78.6%) | |
| 50–100 | 1 (16.7%) | 1 (7.1%) | |
| >100 | 2 (33.3%) | 2 (14.3%) | |
| AH Antigen | 0.5213 | ||
| <50 | 6 (100.0%) | 11 (78.6%) | |
| 50–100 | 0 (0.0%) | 3 (21.4%) | |
| Influenza Vaccine (Present) | 0 (0.0%) | 0 (0.0%) | 1.0001 |
| BCG Scar (Present) | 4 (66.7%) | 12 (85.7%) | 0.5493 |
| Diabetes (Present) | 2 (33.3%) | 1 (7.1%) | 0.2023 |
| ACE Inhibitor Use (Present) | 2 (33.3%) | 2 (14.3%) | 0.5493 |
| Steroid, HCQS, Convalescent plasma Use (Present) | 0 (0.0%) | 0 (0.0%) | 1.0001 |
| Religious Practices (Present) | 5 (83.3%) | 14 (100.0%) | 0.3003 |
∗∗∗Significant at p < 0.05, 1: Chi-Squared Test, 2: Wilcoxon-Mann-Whitney U Test, 3: Fisher's Exact Test.
Table 3.
Association between laboratory Parameters and Outcome (Death) in total study population.
| All Parameters Mean ± std.dev |
Outcome (full Sample) |
p value | |
|---|---|---|---|
| Survived (n = 46) | Died (n = 14) | ||
| Group∗∗∗ | <0.0011 | ||
| S | 6 (13.0%) | 14 (100.0%) | |
| A | 20 (43.5%) | 0 (0.0%) | |
| H | 20 (43.5%) | 0 (0.0%) | |
| Age (Years)∗∗∗ | 37.91 ± 13.46 | 62.08 ± 17.12 | <0.0012 |
| Gender∗∗∗ | 0.0141 | ||
| Male | 41 (89.1%) | 8 (57.1%) | |
| Female | 5 (10.9%) | 6 (42.9%) | |
| Socio-Economic Status | 0.2811 | ||
| Good | 13 (28.3%) | 1 (7.1%) | |
| Medium | 26 (56.5%) | 11 (78.6%) | |
| Poor | 7 (15.2%) | 2 (14.3%) | |
| D-Dimer (ng/ml) | 2020.76 ± 2086.76 | 2685.36 ± 1769.03 | 0.0732 |
| S. Ferritin∗∗∗ | 223.00 ± 240.32 | 425.52 ± 353.87 | 0.0482 |
| TAG | 191.37 ± 111.64 | 205.07 ± 83.33 | 0.3972 |
| Haemoglobin (g/dL)∗∗∗ | 13.33 ± 2.27 | 11.70 ± 1.66 | 0.0032 |
| Haematocrit (%) | 38.20 ± 8.03 | 36.40 ± 4.80 | 0.0872 |
| TLC (/cu.mm)∗∗∗ | 7546.30 ± 2574.58 | 17073.57 ± 7079.49 | <0.0012 |
| ANC (/cu.mm)∗∗∗ | 5244.46 ± 2527.45 | 14806.50 ± 6319.92 | <0.0012 |
| Lymphocytes (/cu.mm)∗∗∗ | 2180.46 ± 697.14 | 1989.43 ± 1785.99 | 0.0292 |
| Monocytes (/cu.mm)∗∗∗ | 121.39 ± 124.68 | 277.64 ± 205.59 | 0.0032 |
| Platelet Count (Lac/cu.mm) | 1.98 ± 1.23 | 1.75 ± 0.88 | 0.5122 |
| Quantiferon TB Gold∗∗∗ | 2.78 ± 4.42 | 0.02 ± 0.02 | <0.0012 |
| Quantiferon TB Gold +ve (>0.35 iu/ml) (Positive)∗∗∗ | 23 (50.0%) | 0 (0.0%) | <0.0013 |
| Mitogen∗∗∗ | 10.52 ± 5.93 | 0.37 ± 0.43 | <0.0012 |
| TO Antigen | 1.0001 | ||
| <50 | 38 (82.6%) | 12 (85.7%) | |
| 50–100 | 8 (17.4%) | 2 (14.3%) | |
| TH Antigen | 0.3551 | ||
| <50 | 30 (65.2%) | 11 (78.6%) | |
| 50–100 | 12 (26.1%) | 1 (7.1%) | |
| >100 | 4 (8.7%) | 2 (14.3%) | |
| AH Antigen∗∗∗ | 0.0381 | ||
| <50 | 44 (97.8%) | 11 (78.6%) | |
| 50–100 | 1 (2.2%) | 3 (21.4%) | |
| Influenza Vaccine (Present) | 0 (0.0%) | 0 (0.0%) | 1.0003 |
| BCG Scar (Present) | 36 (78.3%) | 12 (85.7%) | 0.7131 |
| Diabetes (Present) | 5 (10.9%) | 1 (7.1%) | 1.0001 |
| ACE Inhibitor Use (Present) | 5 (10.9%) | 2 (14.3%) | 0.6601 |
| Steroid, HCQS, Convalescent plasma Use (Present) | 0 (0.0%) | 0 (0.0%) | 1.0003 |
| Religious Practices (Present) | 42 (91.3%) | 14 (100.0%) | 0.5641 |
∗∗∗Significant at p < 0.05, 1: Fisher's Exact Test, 2: Wilcoxon-Mann-Whitney U Test, 3: Chi-Squared Test.
Fig. 1.
Bar diagram depicting association between Quantiferon TB Gold and mortality outcome in patients with severe COVID-19.
A gross reversible unresponsiveness of T cells was seen among all patients in S group who showed absolutely no response even to the mitogen stimulus with mean of 1.33 ± 2.77 IU/ml against 8.68 ± 5.50 and 13.90 ± 4.09 in A and H Group respectively. Three out of 6 survived patients had positive QFT. A repeat QFT was done on all survived patients 6 weeks after discharge from the hospital. Two of the remaining 3 survived patients also turned positive on repeat test while the sixth patient still appeared in unresponsive mode with very low response even to mitogen stimulus (1.38 IU/ml). She tested negative on QFT but showed high TH titre on Widal test.
4. Discussion
All severely ill patients showed decreased Hb% and haematocrit, increased TLC, polymorph, and monocyte count along with increased S. ferritin level, thus reflecting acute phase response in viral infection. Lower lymphocyte count in peripheral blood is a consistent finding in patients with severe COVID-191 and was seen in our study as well. Besides lymphocyte depletion, there was a severity dependant significantly decreased IGRA response to mitogen stimulation by lymphocytes (Table 1). Such severe unresponsive state of lymphocytes is known to be invoked through mechanisms like T-cell exhaustion or T cell anergy. T cell exhaustion is elimination of antigen-specific T cells due to excessive and persistent antigenic exposure in patients with viral infections, cancer12 , 13 while T cell anergy is a reversible mechanism of immunological tolerance with failure of T cells to proliferate and to produce cytokines with preference to Th1 cells.14 Poor IGRA even against the non-specific antigen like mitogen and their reversible unresponsiveness indicates T cell anergy probably to be responsible for such dysregulated immune response in COVID-19 patients and requires further studies to substantiate it.
Eighty percent of our study population showed presence of BCG scar implying Mtb antigenic exposure to their immune system during their early childhood but no correlation was found between presence of the BCG scar and prevalence or severity of COVID-19. BCG vaccine is described to have short lived duration of protection which has been attributed to either its failure to induce long lived memory cells or a gradual loss of BCG specific T cells.15 Despite 80% of the study population having BCG scar, positive QFT was seen in 50% patients only of group A and H. BCG is also reported not to confer any protection against tuberculosis in adult population.16 Prevalence of positive QFT in an adult study population therefore cannot be attributed to routine BCG vaccination at birth and is possibly incurred through community exposure to tubercular antigen. Such community exposure to healthy population puts them to risk of some most dreadful complications of tuberculosis but has shown to be advantageous to certain selective patients of COVID-19. The statement is supported by our observations that distribution of QFT positivity was lowest in group S at 15% against 50% in group A and H (Table 1) and that it had close association with increased survival rate in severe COVID-19 patients (Fig. 1). Similar observations have been made by earlier authors also who reported strong correlation between the BCG index, an estimation of the degree of universal BCG vaccination deployment in a country and COVID- 19 mortality in various socially similar European countries.16
India has become the second highest country in the world with maximum number of COVID-19 patients, but continues to shows significantly lower prevalence of the disease, both in terms of disease and the death rate.2 Natural course for most of COVID-19 patients in Th1 predominant population may be to remain asymptomatic or minimally symptomatic which is reflected in the epidemiological reports from India and other similar countries like south America etc.16 Patients with moderate to severe symptoms from such a population, probably have pre-existing low immunity due to malnutrition or other associated co-morbidities. An unforeseen Th2 predominance due to worm infestation, subtle autoimmune or allergic disorder etc. affecting these patients could be another compounding factor and deserves further exploration.
Nothing comes without a cost and so is immunity. Besides the energy requirement to meet the metabolic activities of the immune cells, immunity causes inflammation and damage to local tissue and carry the risk of autoimmune damage too.17 Attenuation or decreased expression of the host cell receptors of innate immunity therefore is an evolutionarily adaptation to minimize immune activity among the population living in relatively immunologically less demanding environment in modern time. Innate immune responses, in addition to providing a first line of defence, plays an instructive role in T cell activation and the Th1/Th2 polarization also.18 Evolution and availability of vaccines against various antigens has been the most accomplished achievement of modern medicine to the humanity, but could be contributing to the adaptive reduction of the innate immunity. Such an evolutionary alteration in innate defence could be the cause for such a periodic and frequent viral outbreaks such as Swine flu, Zika or Ebola. The observation gets corroborated from studies who reported a severely compromised innate immune system in patients with severe COVID-19.19
None of the subject from our study group had ever been administered an influenza vaccine. A poorly compliant society with such low standard of vaccination status could be considered to possess relatively better innate defence than those in developed world. Moreover, frequent affection to common cold, common habit of eating spicy food, lack of access to proper sanitation, hygiene and safe drinking water,20 a common religious practice of performing Hawan and Arti etc. which involves sublimating mixture of wood with odoriferous and medicinal herbs in the fire exposing nasal, respiratory and gut mucosa to many irritants of ancient unproven qualities21 could possibly also be contributing to an enhanced innate immunity among the Indian population. Besides its role in building up of innate immunity, the medicinal smoke from performing Hawan has been found to reduce bacterial counts also by 94% in 60 minutes and was seen to be maintained for 24 hours.22
D-dimer levels have been reported as a reliable prognostic marker for in-hospital mortality in patients admitted for COVID-19. 75% patients in group A in present study had D-dimer levels much above the cut off value of 2000 ng/ml23 qualifying them for initiating an anticoagulant therapy. None of such patients were prescribed with any anticoagulants and had no untoward incident for at least eight weeks after the discharge from the hospital. Interestingly, patients in group S of severely ill patients with 70% mortality also showed raised D-dimer levels but of lesser magnitude than the Group A. It is thus suggested that increased D-dimer level may be an independent risk factor for producing state of hypercoagulability but is likely to benefit select group of patients through T cell independent immune mechanism. Various Th2 cytokines IL-4, IL- 10 and IL-13 have been shown to deactivate monocytes and macrophages and also to downregulate fibrinogen biosynthesis.24 An increased D-dimer blood level therefore may indicate a poor or insufficient Th2 immunity and may prove fatal if lymphocytes in such patients display anergic state of Th1 immunity as well. Most Died patients in group S in present study demonstrated a similar combination of increased D-dimer levels with poor IGRA to mitogen or Mtb. D-dimer is also reported to stimulate peripheral blood monocytes to secrete IL-6 and TNF-α25 and may therefore contribute to building up of protective immunity in asymptomatic or mildly symptomatic COVID-19 patients. However, such an altered pattern of D-dimer levels in present study and its pathogenesis deserves to be investigated with further studies.
5. Conclusions
The present study is constrained by its smaller sample size and lack of a detailed corroborating cytokine profile of all subjects. Other limitations of the study are recruitment of only male patients and HCW in group A and H, and absence of COVID-19 serology for group H subjects to exclude any past infection. Both these shortcomings however do not have much impact on the final outcome of the study. The present work provides sufficient evidence to establish strong correlation between positive QFT and rate of survival among severely ill patients of COVID-19. Grossly deficient IGRA even to mitogen stimulus seen among these patients suggests T cells to be in anergic state in severely ill COVID-19 patients. Community exposure to certain strong antigenic pathogens like Mtb or Salmonella etc. may be a natural safeguard to fight against COVID-19 and should be explored for broader and long-term benefits.
Funding
None except that the Cost of QFT was supported by Systopic Laboratories.
Conflicts of interest
The authors have none to declare
Acknowledgement
Our sincere thanks to Dr Rishi Gupta, Manokalp Clinic, New Delhi for all the statistical work.
References
- 1.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Worldometers. COVID-19 coronavirus pandemic. https://www.worldometers.info/coronavirus Last updated: October 27, 2020.
- 3.Oksanen A., Kaakinen M., Latikka R., Savolainen I., Savela N., Koivula A. Regulation and trust: 3-month follow-up study on COVID-19 mortality in 25 European countries. JMIR Public Health Surveill. 2020;6(2) doi: 10.2196/19218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Udwadia Z.F., Tripathi A.R., Nanda V.J., Joshi S.R. Prognostic factors for adverse outcomes in COVID-19 infection. JAPI. 2020;68:56–60. [PubMed] [Google Scholar]
- 5.Zheng Y.Q., Naguib Y.W., Dong Y., Shi Y.C., Bou S., Cui Z. Applications of bacillus Calmette-Guerin and recombinant bacillus Calmette-Guerin in vaccine development and tumor immunotherapy. Expert Rev Vaccines. 2015;14(9):1255–1275. [PMC free article] [PubMed] [Google Scholar]
- 6.Moorlag S.J.C.F.M., Arts R.J.W., van Crevel R., Netea M.G. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25(12):1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Tadolini M., Codecasa L.R., García-García J.M., et al. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur Respir J. 2020;56(1):2001398. doi: 10.1183/13993003.01398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okada H., Feillet H., Bach J.-F. The “hygiene hypothesis” for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160(1):1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diao B., Wang C., Tan Y., et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020 May 1;11:827. doi: 10.3389/fimmu.2020.00827. PMID: 32425950; PMCID: PMC7205903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C.K., Wu H., Yan H., et al. T cell responses to whole SARS coronavirus in humans. J Immunol. 2008 Oct 15;181(8):5490–5500. doi: 10.4049/jimmunol.181.8.5490. PMID: 18832706; PMCID: PMC2683413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balaram P., Kien P.K., Ismail A. Toll-like receptors and cytokines in immune responses to persistent mycobacterial and Salmonella infections. Int J Med Microbiol. 2009;299(3):177–185. doi: 10.1016/j.ijmm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Brooks D.G., Teyton L., Oldstone M.B., McGavern D.B. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005;79:10514–10527. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wherry E.J., Blattman J.N., Murali-Krishna K., van der Most R., Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebihara M., Hattori M., Yoshida T. Distinctly different sensitivity in the induction and reversal of anergy of Th1 and Th2 cells. Biosci Biotechnol Biochem. 2007;71(1):130–137. doi: 10.1271/bbb.60403. [DOI] [PubMed] [Google Scholar]
- 15.Orme I.M. The Achilles heel of BCG. Tuberculosis. 2010;90:329–332. doi: 10.1016/j.tube.2010.06.002. (Edinb) [DOI] [PubMed] [Google Scholar]
- 16.Escobar L.E., Molina-Cruz A., Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19) PNAS. 2020 doi: 10.1073/pnas.2008410117. www.pnas.org/cgi/doi/10.1073/pnas.2008410117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazzaro B.P. Adenosine signaling and the energetic costs of induced immunity. PLoS Biol. 2015;13(4) doi: 10.1371/journal.pbio.1002136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jankovic D., Liu Z., Gause W. Th1- and Th2-cell commitment during infectious disease: asymmetry in divergent pathways. Trends Immunol. 2001;22(8):450–457. doi: 10.1016/s1471-4906(01)01975-5. [DOI] [PubMed] [Google Scholar]
- 19.Yaqinuddin A., Kashir J. Innate immunity in COVID-19 patients mediated by NKG2A receptors, and potential treatment using Monalizumab, Cholroquine, and antiviral agents. Med Hypotheses. 2020;140:109777. doi: 10.1016/j.mehy.2020.109777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majra J.P., Gur A. India needs a great sanitary awakening. Indian J Occup Environ Med. 2008;12(3):143. doi: 10.4103/0019-5278.44699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bansal P., Kaur R., Gupta V., Kumar S., Kaur Is there any scientific basis of Hawan to be used in epilepsy-prevention/cure? J Epilepsy Res. 2015;5(2):33–45. doi: 10.14581/jer.15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nautiyal C.S., Chauhan P., Nene Y.L. Medicinal smoke reduces airborne bacteria. J Ethnopharmacol. 2008;114(3):4460451. doi: 10.1016/j.jep.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L., Yan X., Fan Q., et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020 Jun;18(6):1324–1329. doi: 10.1111/jth.14859. PMID: 32306492; PMCID: PMC7264730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vasse M., Paysant I., Soria J., Mirshahi S.S., Vannier J.P., Soria C. Down-regulation of fibrinogen biosynthesis by IL-4, IL-10 and IL-13. Br J Haematol. 1996;93:955–961. doi: 10.1046/j.1365-2141.1996.d01-1731.x. [DOI] [PubMed] [Google Scholar]
- 25.Jensen T., Kierulf P., Sandset P.M., et al. Fibrinogen and fibrin induce synthesis of proinflammatory cytokines from isolated peripheral blood mononuclear cells. Thromb Haemost. 2007 May;97(5):822–829. doi: 10.1160/th07-01-0039. PMID: 17479194. [DOI] [PubMed] [Google Scholar]