Clinical Implications.
-
•
In our cohort of 979 children with polymerase chain reaction–confirmed coronavirus disease 2019 (COVID-19), 205 (21%) had asthma, a number consistent with previously published asthma prevalence estimates within our network. Asthma diagnosis was negatively associated with COVID-19–related hospitalization.
Although initial reports of the novel coronavirus disease 2019 (COVID-19) showed limited and nonsevere manifestations in children, more recent reports have begun to describe more serious illness in children, including patient characteristics associated with hospitalization and death.1 , 2 During the early stages of the pandemic, the US Centers for Disease Control and Prevention listed moderate-to-severe asthma as a potential risk factor for severe COVID-19, yet there is little data to support this assertion in children and published data in adults are conflicting.3, 4, 5 The largest cohort of pediatric COVID-19–positive patients in the United States included only descriptive statistics on underlying conditions and severity and did not delineate diagnosis-specific associations with hospitalization.6 To help address these gaps in knowledge, we completed a retrospective cohort study in a large pediatric cohort of patients with polymerase chain reaction (PCR)-confirmed COVID-19 to determine the association between current asthma and hospitalization.
We extracted electronic health records (EHR), including demographics, clinical characteristics, and asthma medications, for all patients aged ≤21 years with a positive PCR test for SARS-CoV-2 at any Children's Hospital of Philadelphia setting (drive-through testing, outpatient, emergency department [ED], or inpatient) between March 17, 2020, and August 26, 2020. The presence of a current asthma diagnosis was determined using an existing EHR asthma registry definition, which required that they met either of the following criteria at the time of testing: (1) encounter diagnosis for asthma (International Classification of Disease, 10th Revision, code J45) within the past 1 year or an active problem list diagnosis for asthma and a prescription for an asthma-specific medication in the last year; or (2) an active persistent asthma diagnosis on the problem list. COVID-19 hospitalizations were defined as any hospitalization within 14 days of a first positive PCR for SARS-CoV-2. To account for potential confounding, multivariable logistic regression was used to calculate adjusted odds ratios (ORs) for COVID-19 hospitalization. Because all hospitalized patients were screened for COVID-19 on admission regardless of symptoms or exposure risk, unlike other care settings, we performed a secondary analysis including only hospitalizations determined to be COVID-19 related on chart review by board-certified pediatricians (KM, SEH, DAH, CCK) to investigate for differential confounding by testing indication. For patients with a current asthma diagnosis, we classified asthma severity based on asthma medications prescribed within the year before a first COVID-19–positive PCR, using tiers modeled from the Global Initiative for Asthma guidelines for asthma severity. The association between asthma controller prescriptions and COVID-19 hospitalization was examined using the Fisher exact test.
From March 17, 2020, to August 26, 2020, 979 patients aged 0 to 21 years were tested positive for COVID-19 within our health system. Of these patients, 205 (21%) had an active asthma diagnosis at the time of testing. One hundred twenty-one patients (12%) were hospitalized with COVID-19, 11 of whom had a current asthma diagnosis (Table I ).
Table I.
Demographic and clinical characteristics of patients with COVID-19 stratified by hospitalization status
| Characteristic, n (%) | Cohort |
||
|---|---|---|---|
| All patients (n = 979) | Non-hospitalized (n = 858) | Hospitalized (n = 121) | |
| Age (y) | |||
| 0-4 | 279 (28) | 232 (27) | 47 (39) |
| 5-11 | 225 (23) | 194 (23) | 31 (26) |
| 12-17 | 328 (34) | 291 (34) | 37 (31) |
| 18-21 | 147 (15) | 141 (16) | 6 (5) |
| Sex | |||
| Male | 504 (51) | 431 (50) | 73 (60) |
| Race | |||
| Black | 421 (43) | 370 (43) | 51 (42) |
| Non-black | 558 (57) | 488 (57) | 70 (58) |
| Ethnicity | |||
| Hispanic or Latino | 145 (15) | 121 (14) | 24 (20) |
| Insurance payer status | |||
| Medicaid | 477 (49) | 399 (47) | 78 (64) |
| Private/unknown | 502 (51) | 459 (53) | 43 (36) |
| Obesity diagnosis | 101 (10) | 91 (11) | 10 (8) |
| Complex chronic conditions | |||
| 0 | 615 (63) | 575 (67) | 40 (33) |
| 1 | 175 (18) | 746 (17) | 29 (24) |
| 2+ | 189 (19) | 137 (16) | 52 (43) |
| Asthma diagnosis | 205 (21) | 194 (23) | 11 (9) |
| Asthma treatment∗ | |||
| SABA only | 117 (57) | 115 (59) | 2 (18) |
| ICS or LM | 58 (28) | 52 (27) | 6 (55) |
| ICS/LABA or ICS + LM | 28 (14) | 25 (13) | 3 (27) |
| Biologic | 2 (1) | 2 (1) | 0 (0) |
| Systemic corticosteroid | 65 (32) | 58 (30) | 7 (64) |
COVID-19, Coronavirus disease 2019; ICS, inhaled corticosteroids; LABA, long-acting β-agonist; LM, leukotriene modifier; SABA, short-acting β-agonist.
Percentages represent the proportion of patients within the asthma cohort who were prescribed asthma medications in the year preceding the positive SARS CoV-2 test. Categories are mutually exclusive with the exception of systemic corticosteroid.
In bivariate modeling, asthma was associated with a lower odds of COVID-19 hospitalization (OR: 0.34; 95% confidence interval [CI]: 0.16-0.65; P = .001). In the adjusted model (Table II ), asthma diagnosis remained associated with a lower odds of COVID-19 hospitalization compared with children without asthma (OR: 0.28; 95% CI: 0.14-0.55; P < .001).
Table II.
Multivariable model results demonstrating the odds of hospitalization by model covariate in (1) main model (all hospitalizations occurring within 14 days of a positive PCR for COVID-19) and (2) sensitivity analysis (COVID-19–related hospitalizations determined by chart review)
| Covariate | Main multivariable model |
Sensitivity analysis model |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Asthma | 0.28 (0.14-0.55) | <.001 | 0.40 (0.19-0.84) | .02 |
| Age | ||||
| 0-4 | 1.65 (0.99-2.76) | .053 | 1.86 (0.99-3.50) | .055 |
| 5-11 | 1.47 (0.86-2.53) | .16 | 1.36 (0.68-2.71) | .39 |
| 12+ (Reference) | – | – | – | – |
| Sex | ||||
| Male | 1.85 (1.21-2.82) | .004 | 2.08 (1.22-3.52) | .007 |
| Race | ||||
| Black | 1.07 (0.67-1.70) | .79 | 1.06 (0.60-1.89) | .83 |
| Ethnicity | ||||
| Hispanic or Latino | 1.11 (0.61-2.01) | .73 | 0.98 (0.47-2.06) | .96 |
| Payer | ||||
| Medicaid | 1.85 (1.17-2.93) | .009 | 2.54 (1.40-4.62) | .002 |
| Obesity diagnosis | 0.76 (0.36-1.63) | .49 | 0.66 (0.24-1.80) | .42 |
| Complex chronic conditions | ||||
| 0 (Reference) | – | – | – | – |
| 1 | 3.58 (2.10-6.12) | <.001 | 3.51 (1.79-6.90) | <.001 |
| 2+ | 6.54 (4.04-10.59) | <.001 | 7.37 (4.05-13.41) | <.001 |
CI, Confidence interval; COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction.
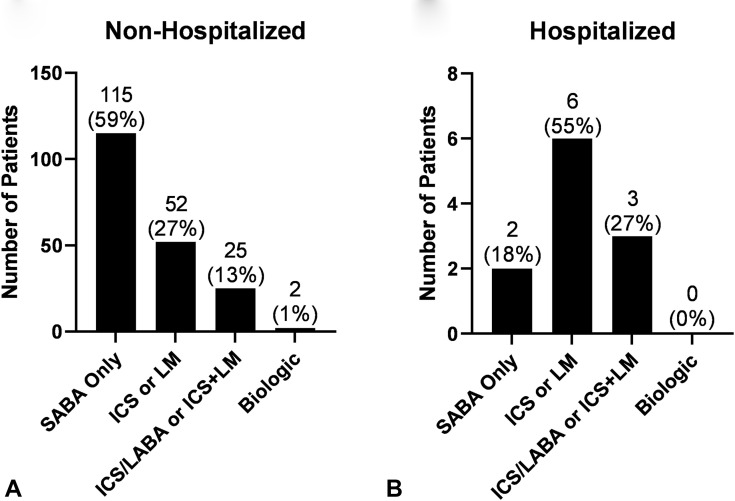
The frequency of patients with an asthma controller medication prescription was higher in the subgroup of patients with COVID-19 hospitalizations compared with those who were not hospitalized (82% vs 41%, respectively, P = .01). Of note, only 2 children were prescribed a biologic for asthma, and neither of these children were hospitalized (Figure E1, available in this article's Online Repository at www.jaci-inpractice.org).
Figure E1.
Number of (A) non-hospitalized and (B) hospitalized patients grouped by 4 different asthma medication severity categories. ICS, Inhaled corticosteroids; LABA, long-acting β-agonist; LM, leukotriene modifier; SABA, short-acting β-agonist.
In the sensitivity analysis, 74 of the 121 hospitalizations (61%) were determined to be COVID related. In the multivariable sensitivity analysis regression, the association between asthma and lower odds of hospitalization remained (OR: 0.40; 95% CI: 0.19-0.84; P = .02) (Table II).
In this study, we report data on asthma prevalence and odds of hospitalization among a cohort of children who tested positive for COVID-19 from a large pediatric health system. Despite an asthma prevalence (21%) in COVID-positive children that is nearly identical to the asthma prevalence within the health system's primary care network, asthma was inversely associated with COVID-19 hospitalization, both in our main analysis and sensitivity analysis. Results from small cohorts of children with COVID-19 have demonstrated a relatively high prevalence of asthma in hospitalized children (16%-24%). However, in addition to smaller sample sizes, these studies either did not describe how they defined asthma1 or used a less specific definition, such as asthma diagnosis in the last 5 years,4 which might inflate the prevalence. In these studies, children with asthma did not have a higher risk of severe hospital outcomes, such as intensive care unit admission or death.1 , 4 Our results add to these previous findings and suggest that asthma may actually be associated with lower hospitalization risk in children.
There are several suggested mechanisms for a potential protective effect of asthma against severe COVID-19. First, concern for COVID-19 causing severe asthma exacerbations may have led to increased adherence to protective behaviors such as physical distancing, mask wearing, and asthma controller medication adherence,7 so children with asthma may have had a lower burden of COVID-19 symptoms when they presented. Second, early in the pandemic, inhaled steroids were hypothesized to protect against severe COVID-19 and thus lower the risk of hospitalization. This relationship, however, is still unclear, and the majority of children in our cohort were not on controller medications.8 Lastly, there is associative evidence that suggests that underlying allergic inflammation may protect against SARS-COV-2 infection or severe COVID-19 outcomes.9
There are several limitations to this analysis. First, given our retrospective study design, we were not able to determine causality. Second, for children who only presented to the ED or inpatient setting, we could not ascertain past asthma encounters to primary care outside of our network and thus asthma in hospitalized children could be undercounted. However, our manual chart review of all hospitalized children did not identify missed asthma diagnoses in the hospitalized cohort. Lastly, our results reflect the experience of a single pediatric health system. Larger, multicenter studies are needed to confirm the observed negative association between asthma diagnosis and COVID-related hospitalization, as well as elucidate how asthma severity may affect COVID-19 outcomes.
Footnotes
This work was supported by the National Institutes of Health awards (grant no. K23HL136842 to C. C. Kenyon, grant no. DK116668 to D. A. Hill, and grant no. K08AI135091 to S. E. Henrickson). S. E. Henrickson's effort was also supported by the Burroughs Wellcome Fund CAMS award. The funders had no influence on the design of the study, interpretation of findings, or the decision to publish.
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
Online Repository. Methods
Data source and identification of coronavirus disease 2019 (COVID-19)
This retrospective cohort study was conducted at the Children's Hospital of Philadelphia (CHOP), a free-standing pediatric hospital with approximately 2500 asthma-related hospitalizations and 6000 emergency department (ED) visits annually. We extracted data for all patients aged ≤21 with a positive polymerase chain reaction (PCR) test for SARS-CoV-2 at any CHOP setting (drive-through testing, outpatient, ED, or inpatient) between March 17 and August 26. Mandatory preprocedural testing was implemented on March 24, and preadmission testing for all hospitalized patients began on April 1. We included patients aged 18 to 21 because the transition to adult care often occurs late for children with chronic conditions at our institution and to allow for internal reliability and validity testing of our cohort definition with institutional data definitions of COVID-positive patients. Testing indications at drive-through, outpatient, and ED sites included patients having symptoms of COVID-19 or having a recent high-risk exposure.
Determination of asthma severity
For those patients with a current asthma diagnosis, we classified asthma severity based on asthma medications prescribed within the year before a first COVID-19–positive PCR. We broke down asthma severity into 4 tiers from low to high based on prescription for: (1) short-acting β-agonist only, (2) inhaled corticosteroids (ICS) or leukotriene modifier, (3) ICS and long-acting β-agonist combination medication (ICS/LABA) or ICS and leukotriene modifier, and (4) asthma biologic. These tiers were modeled from the Global Initiative for Asthma guidelines for asthma severity.
To account for whether patients with asthma had a recent asthma exacerbation, systemic corticosteroids prescribed within the year before a first SARS CoV-2–positive PCR were also included.
Demographics and comorbidities
Demographic characteristics included age category, sex (female/male), race (black/non-black), ethnicity (Hispanic/non-Hispanic), and payer (Medicaid/non-Medicaid). Diagnoses for obesity were identified through International Classification of Disease, 10th Revision (ICD-10) codes. The number of complex chronic conditions were quantified using a validated set of ICD-10 codes.
Statistical analysis
We compared demographics and clinical characteristics between hospitalized and non-hospitalized children using the Fisher exact or χ2 tests as appropriate for categorical variables. Multivariable logistic regression was used to calculate adjusted odds ratios as well as the 95% confidence interval for COVID-19 hospitalization. The multivariable models included variables that have been shown to be associated with both severe COVID-19 and asthma in prior studies (ie, sex, age, race, ethnicity, obesity, medical complexity), as well as additional covariates that were associated with hospitalization in bivariate analysis at a significance level of P < .2. The association between COVID-19 hospitalization and asthma controller prescription (ICS, leukotriene modifier, ICS/LABA, or a biologic in the preceding year) was examined using the Fisher exact test rather than multivariable logistic regression, due to the low prevalence of hospitalization in children with asthma.
We performed a secondary analysis including only hospitalizations determined to be COVID-19 related on chart review to investigate for differential confounding by testing indication. One of 4 board-certified pediatricians (KM, SEH, DAH, CCK) abstracted the charts of children with COVID-19 hospitalization using a standardized template. Hospitalizations were considered COVID-19 related based on treating physician documentation. When relevant documentation was not present, this determination was made by the abstracting physician based on recent symptoms consistent with COVID-19 and a plausible pathologic mechanism. Cases where this distinction was unclear were reviewed by the group, and final consensus was consistent with initial determination in 8 of the 9 cases.
Statistical analyses were completed in STATA 16 (StataCorp LLC, College Station, Texas). The CHOP institutional review board reviewed the study and determined that it did not meet the criteria for human subjects research.
References
- 1.DeBiasi R.L., Song X., Delaney M., Bell M., Smith K., Pershad J., et al. Severe coronavirus disease-2019 in children and young adults in the Washington, DC, Metropolitan Region. J Pediatr. 2020;223:199–203.e1. doi: 10.1016/j.jpeds.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bixler D., Miller A.D., Mattison C.P., Taylor B., Komatsu K., Peterson Pompa X., et al. SARS-CoV-2–associated deaths among persons aged <21 years—United States, February 12–July 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1324–1329. doi: 10.15585/mmwr.mm6937e4. [DOI] [PubMed] [Google Scholar]
- 3.Yang J.M., Koh H.Y., Moon S.Y., Yoo I.K., Ha E.K., You S., et al. Allergic disorders and susceptibility to and severity of COVID-19: a nationwide cohort study. J Allergy Clin Immunol. 2020;146:790–798. doi: 10.1016/j.jaci.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovinsky-Desir S., Deshpande D.R., De A., Murray L., Stingone J.A., Chan A., et al. Asthma among hospitalized patients with COVID-19 and related outcomes. J Allergy Clin Immunol. 2020;146:1027–1034.e4. doi: 10.1016/j.jaci.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chhiba K.D., Patel G.B., Vu T.H.T., Chen M.M., Guo A., Kudlaty E., et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146:307–314.e4. doi: 10.1016/j.jaci.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey L.C., Razzaghi H., Burrows E.K., Bunnell H.T., Camacho P.E.F., Christakis D.A., et al. Assessment of 135 794 pediatric patients tested for severe acute respiratory syndrome coronavirus 2 across the United States. JAMA Pediatr. 2021;175:176–184. doi: 10.1001/jamapediatrics.2020.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taquechel K., Diwadkar A.R., Sayed S., Dudley J.W., Grundmeier R.W., Kenyon C.C., et al. Pediatric asthma health care utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8:3378–3387.e11. doi: 10.1016/j.jaip.2020.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schultze A., Walker A.J., MacKenna B., Morton C.E., Bhaskaran K., Brown J.P., et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8:1106–1120. doi: 10.1016/S2213-2600(20)30415-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson D.J., Busse W.W., Bacharier L.B., Kattan M., O’Connor G.T., Wood R.A., et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol. 2020;146:203–206.e3. doi: 10.1016/j.jaci.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]