Abstract
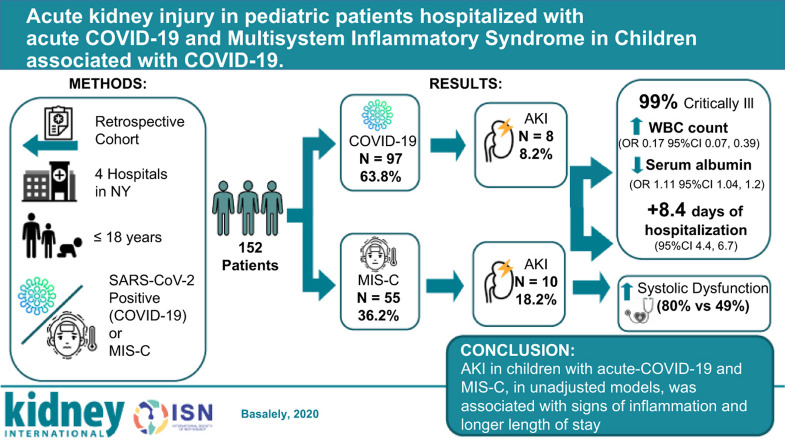
This study describes the incidence, associated clinical characteristics and outcomes of acute kidney injury in a pediatric cohort with COVID-19 and Multisystem Inflammatory Syndrome in Children (MIS-C). We performed a retrospective study of patients 18 years of age and under admitted to four New York hospitals in the Northwell Health System interned during the height of the COVID-19 pandemic, between March 9 and August 13, 2020. Acute kidney injury was defined and staged according to Kidney Disease: Improving Global Outcomes criteria. The cohort included 152 patients; 97 acute-COVID-19 and 55 with MIS-C associated with COVID-19. Acute kidney injury occurred in 8 with acute-COVID-19 and in 10 with MIS-C. Acute kidney injury, in unadjusted models, was associated with a lower serum albumin level (odds ratio 0.17; 95% confidence interval 0.07, 0.39) and higher white blood cell counts (odds ratio 1.11; 95% confidence interval 1.04, 1.2). Patients with MIS-C and acute kidney injury had significantly greater rates of systolic dysfunction, compared to those without (80% vs 49%). In unadjusted models, patients with acute kidney injury had 8.4 days longer hospitalizations compared to patients without acute kidney injury (95% confidence interval, 4.4-6.7). Acute kidney injury in acute-COVID-19 and MIS-C may be related to inflammation and/or dehydration. Further research in larger pediatric cohorts is needed to better characterize risk factors for acute kidney injury in acute-COVID-19 and with MIS-C consequent to COVID-19.
Keywords: acute kidney injury, COVID-19, pediatric nephrology, SARS-CoV-2
Graphical abstract
By October 2020, the United States had >7.5 million cases of coronavirus disease 2019 (COVID-19).1, 2, 3, 4 Initially considered a respiratory illness, COVID-19 has proven to be a complex multisystem illness frequently associated with kidney injury.5, 6, 7, 8
Acute kidney injury (AKI) is a common complication in adults with COVID-19. Initial studies from China and Italy reported AKI rates as high as 29%.6 , 9, 10, 11, 12, 13, 14, 15 A recent study from our health system reported a significantly higher incidence of AKI in adult patients (36.6%) and found AKI to be associated with morbidity and mortality.5 , 16 These incidences and mortality risks were corroborated in subsequent US studies.7 , 8
Although there are several studies describing COVID-19–related AKI in adults, there are limited data describing AKI in pediatric patients with acute COVID-19. A retrospective observational study of 238 pediatric patients admitted to Wuhan Children’s Hospital with COVID-19 reported a 1.2% incidence of AKI.17 Recent studies from the United Kingdom and Saudi Arabia reported an incidence rate of pediatric AKI between 21% and 29%.18 , 19 A preliminary report from a multicenter study evaluating AKI in 106 critically ill children with acute COVID-19, including 32 US sites, reported a point prevalence rate of 44% (N = 47).20
Children, initially thought to be spared from serious effects of COVID-19, are in fact vulnerable to sequelae. In May of 2020, the Centers for Disease Control and Prevention released a public health advisory along with a case definition for multisystem inflammatory syndrome in children (MIS-C) associated with recent COVID-19 infection.21 These children presented with features similar to typical Kawasaki disease or toxic shock syndrome.22 Recent reports of children with MIS-C have highlighted the incidence of AKI in this subset.22 , 23 In a systematic review of 662 patients with MIS-C, 108 (16.3%) developed AKI; however, the definition of AKI differed between centers.23
Although there is early data that AKI develops in pediatric patients with acute COVID-19 and MIS-C, the rates, associated clinical characteristic, and short-term outcomes are not well characterized. Therefore, we aimed to describe the incidence of AKI in these populations, assess associated demographic and clinical factors in those who had AKI, and determine the association of AKI with length of time on mechanical ventilation, length of stay, and mortality.
Methods
Study design
A retrospective chart review of children admitted to the Northwell Health system with acute COVID-19 and MIS-C was conducted. Participating hospitals were within the New York metropolitan area and included Cohen Children’s Medical Center, an academic tertiary children’s hospital, as well as 3 tertiary hospitals: South Shore Hospital, Staten Island University Hospital, and Lenox Hill Hospital. Data from March 9, 2020, through August 13, 2020, were collected retrospectively using the inpatient electronic health record Sunrise Clinical Manager (Allscripts, Chicago, Illinois). This study was approved by the Institutional Review Board of Northwell Health.
Our study included children aged ≤18 years who were admitted for treatment of acute COVID-19 or MIS-C. Patients were considered to have acute COVID-19 if, within 24 hours of admission, they tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by polymerase chain reaction testing (Northwell Health Labs). The Centers for Disease Control and Prevention case definition of MIS-C was utilized: children who presented with fevers, significant evidence of inflammation, evidence of ≥2 organ dysfunction, and tested positive for current or recent SARS-CoV-2 infection or had serologic confirmation of exposure to COVID-19 within 4 weeks of symptom onset.20 Patients who were pregnant, kidney transplant recipients, patients with end-stage kidney disease (estimated glomerular filtration rate <15 ml/min per 1.73 m2 or dialysis), or those transferred from outside of the health system were excluded.
Incidence of AKI
The primary outcome of this study was the incidence rate of AKI. The diagnosis and staging of AKI were conducted in accordance with the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines.24 Only serum creatinine was used to define and stage AKI as the documentation of urine output in the electronic health record was not reliably documented. None of the patients had baseline serum creatinine available (defined as creatinine within 3 months of admission); therefore, as previously descried in the literature, baseline creatinine was estimated by back-calculating from the original Schwartz formula assuming a normal glomerular filtration rate for children (estimated glomerular filtration rate set as 120 ml/min per 1.73 m2).25 If height was not documented, the average height (Centers for Disease Control and Prevention 50th percentile) was imputed for sex and age (N = 8).26
Associations with AKI
Secondary outcomes included demographic and clinical factors associated with development of AKI. The following measures were evaluated: patient demographics, presenting symptoms, and comorbid conditions. Laboratory measurements included serum electrolytes, creatinine, blood urea nitrogen, albumin, d-dimer, inflammatory markers, and hematologic markers. Details regarding hospital stay, such as the usage of vasoactive medications, i.v. Ig, corticosteroids, extracorporeal membrane oxygenation (ECMO), and exposure to nephrotoxic medications, were also collected. In patients with MIS-C, 2-dimensional echocardiographic data were included. Nadir left ventricular systolic ejection fractions were utilized to define lowest left ventricular ejection fraction during hospitalization. Systolic dysfunction was defined as left ventricular ejection fraction <55%. Coronary artery dilation was defined as >2 mm.
Clinical course and outcomes
The impact of AKI (all stages combined and severe, stages 2 and 3) on clinical course and outcomes was also evaluated. Outcomes included mortality, kidney replacement therapy, length of mechanical ventilation, length of hospital stay, and pediatric intensive care unit (PICU) stay.
Statistical analysis
Baseline demographic and clinical characteristics were described according to admission type, acute COVID-19 and MIS-C, respectively. Continuous data were described utilizing medians and interquartile ranges (IQRs), and categorical data were presented as frequencies and proportions. Mann-Whitney U test, χ2 test, Kruskal-Wallis test, and Fisher exact test were utilized to compare baseline characteristics of acute COVID-19 and MIS-C patients with and without AKI.
Logistic regression analysis was performed to identify factors associated with AKI (both MIS-C and acute COVID-19 combined). Because of the low AKI frequency, we were not powered to adjust for confounders in our models. Multiple imputation was used for missing values in regression analysis. Subsequently, simple linear regression analysis was performed to assess the relationship between AKI and continuous outcomes, including PICU and hospital length of stay and length of time on mechanical ventilation. Two-tailed P < 0.05 was set as the level of significance, and SPSS version 26 was used for analysis.
Results
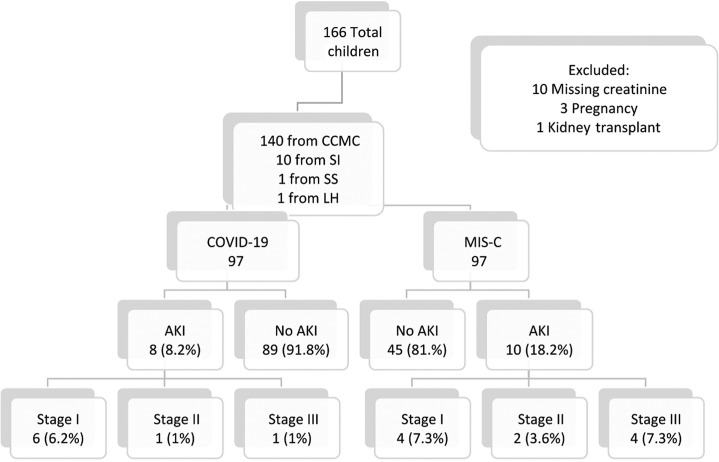
Of the 166 children admitted for acute COVID-19 or MIS-C, 152 met inclusion criteria. Over 63% (N = 97) patients were admitted for acute COVID-19, whereas 55 patients (36.2%) were diagnosed with MIS-C (Figure 1 ). AKI developed in 18 (11.8%) of all patients. Demographic data, presenting symptoms, and baseline laboratory values of those with and without AKI in both groups were compared (Tables 1 and 2 ).
Figure 1.
Flowchart of study population. Acute kidney injury (AKI) was staged by Kidney Disease: Improving Global Outcomes (KDIGO) criteria. CCMC, Cohen Children’s Medical Center; COVID-19, coronavirus disease 2019; LH, Lenox Hill Hospital; MIS-C, multisystem inflammatory syndrome in children; SI, Staten Island University Hospital; SS, Southside Hospital.
Table 1.
Admission characteristics and laboratory values by AKI diagnosis in children with acute COVID-19
| Variables | Overall (N = 97) | AKI (N = 8) | No AKI (N = 89) | P value AKI vs. no AKI |
|---|---|---|---|---|
| Age, yr | 8.2 (1.5 to 13.8) | 6 (0.17 to 13.7) | 7.6 (1.6 to 14.1) | 0.5 |
| Male, n (%) | 50 (51.5) | 5 (63.5) | 45 (50.6) | 0.39 |
| Race, n (%) | 0.49 | |||
| White | 14 (14.4) | 0 (0) | 14 (15.7) | |
| Black | 23 (23.7) | 3 (37.5) | 20 (22.5) | |
| Asian | 10 (10.3) | 2 (25) | 8 (9) | |
| Other | 45 (46.4) | 3 (37.5) | 42 (47.2) | |
| Unknown | 4 (4.1) | 0 (0) | 4 (4.5) | |
| Ethnicity, n (%) | 0.95 | |||
| Hispanic/Latino | 24 (24.7) | 2 (25) | 22 (24.7) | |
| Not Hispanic/Latino | 66 (68) | 6 (75) | 66 (67.4) | |
| Other/unknown | 6 (6.2) | 0 (0) | 6 (6.7) | |
| BMI z-score | 0.46 (–0.46 to 1.96) | 0 (–0.13 to 0.63) | 0.27 (0 to 1.8) | 0.26 |
| Obese, n (%) | 13 (19.1) | 0 (0) | 13 (20.3) | 0.42 |
| Hospital type, n (%) | 0.75 | |||
| Children’s hospital | 85 (87.6) | 8 (100) | 77 (86.5) | |
| Non–children’s hospital | 12 (12.4) | 0 (0) | 12 (13.5) | |
| Presenting symptoms, n (%) | ||||
| Gastrointestinal | 47 (48.5) | 4 (50) | 43 (48.3) | 0.61 |
| Fever | 68 (70.1) | 3 (37.5) | 65 (73) | 0.049 |
| Cough | 21 (21.6) | 1 (12.5) | 20 (22.5) | 0.45 |
| Rash | 16 (16.5) | 1 (12.5) | 15 (16.9) | 0.61 |
| Myalgias/joint aches | 10 (10.3) | 0 (0) | 10 (11.2) | 0.59 |
| Comorbid condition, n (%) | ||||
| Hypertension | 1 (1) | 0 (0) | 1 (1.1) | 0.92 |
| Diabetes mellitus | 1 (1) | 0 (0) | 1 (1.1) | 0.92 |
| Asthma | 7 (7.2) | 0 (0) | 7 (7.9) | 0.54 |
| Cancer | 4 (4.1) | 1 (12.5) | 3 (3.4) | 0.3 |
| Congenital heart disease | 7 (7.2) | 2 (25) | 5 (5.6) | 0.1 |
| Immunosuppressed | 4 (4.1) | 0 (0) | 4 (4.5) | 0.71 |
| Baseline SCr, mg/dla | 0.61 (0.52 to 0.74) | 0.47 (0.25 to 0.83) | 0.55 (0.39 to 0.72) | 0.58 |
| Admission SCr, mg/dl | 0.55 (0.31 to 0.85) | 0.69 (0.31 to 0.95) | 0.36 (0.24 to 0.52) | 0.06 |
| Admission eGFR, ml/min per 1.73 m2 | 145.5 (99.8 to 186,7) | 85.3 (59.4 to 99) | 150 (110.3 to 187.1) | 0.001 |
| Sodium, mEq/L | 134 (131 to 136) | 136.5 (130 to 137) | 137 (135 to 138) | 0.06 |
| Potassium, mEq/L | 3.9 (3.7 to 4.4) | 4.3 (3.9 to 5.4) | 4.1 (3.75 to 4.95) | 0.62 |
| Bicarbonate, mEq/L | 20 (18 to 22) | 19.5 (12.5 to 24) | 21 (19 to 23) | 0.71 |
| Magnesium, mEq/L (N = 65) | 2 (1.8 to 2.2) | 2.3 (2 to 2.5) | 2.1 (1.8 to 2.3) | 0.06 |
| Calcium, mg/dl | 9.2 (8.6 to 9.5) | 8.4 (7.2 to 10.3) | 9.5 (9.1 to 9.95) | 0.047 |
| Albumin, mg/dl | 3.6 (3 to 3.9) | 3.3 (2.5 to 3.95) | 4.1 (3.85 to 4.5) | 0.001 |
| While blood cell count, mm3 | 10.3 (7.5 to 14.3) | 19.6 (8.4 to 38.9) | 7.8 (5.4 to 13.4) | 0.02 |
| Hemoglobin, g/dl | 11.3 (10.5 to 12.1) | 9.1 (6.5 to 14.4) | 11.6 (10.25 to 12.7) | 0.18 |
| Platelets, mm3 | 184 (124 to 276) | 237 (81 to 449) | 264 (187 to 346.5) | 0.66 |
| LDH, U/L (N = 51) | 350 (271 to 523) | 374 (291 to 1800) | 349 (238 to 513) | 0.49 |
| Fibrinogen, mg/dl (N = 53) | 509 (394 to 616) | 480 (397 to 604) | 511 (390 to 623) | 0.69 |
| CRP, μg/ml (N = 61) | 29 (8.5 to 88) | 41.3 (8.9 to 190.3) | 28.4 (7.7 to 88) | 0.67 |
| D-dimer, μg/ml (N = 49) | 575 (372 to 1106) | 2320 (366 to 3789) | 569 (375 to 992) | 0.35 |
| Nephrotoxic medication exposure, n (%) | ||||
| ACE-I/ARB | 1 (1) | 1 (1) | 0 (0) | >0.99 |
| NSAID | 31 (32) | 2 (25) | 29 (33) | >0.99 |
| Vancomycin | 11 (11) | 3 (38) | 8 (9) | 0.046 |
| Gentamicin | 4 (4) | 0 (0) | 4 (5) | >0.99 |
ACE-I, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin receptor blocker; BMI, body mass index; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; LDH, lactate dehydrogenase; NSAID, nonsteroidal anti-inflammatory drug; SCr, serum creatinine.
Data are given as median (interquartile range), unless otherwise indicated.
Baseline SCr was estimated from assumed eGFR 120 ml/min per 1.73 m2 using original Schwartz formula.25
Table 2.
Admission characteristics and laboratory values by AKI diagnosis in children with MIS-C
| Variables | Overall (N = 55) | AKI (N = 10) | No AKI (N = 45) | P value AKI vs. no AKI |
|---|---|---|---|---|
| Age, yr | 7.5 (1.5 to 13.8) | 8.7 (5.4 to 14) | 8.1 (4.2 to 12) | 0.42 |
| Male, n (%) | 35 (63.6) | 6 (60) | 29 (64.4) | 0.79 |
| Race, n (%) | 0.27 | |||
| White | 7 (12.7) | 1 (10) | 6 (13.3) | |
| Black | 14 (25.5) | 5 (50) | 9 (20) | |
| Asian | 6 (10.9) | 1 (10) | 5 (11.1) | |
| Other | 25 (45.5) | 2 (20) | 23 (51.1) | |
| Unknown | 3 (5.5) | 1 (10) | 3 (4.4) | |
| Ethnicity, n (%) | 0.07 | |||
| Hispanic/Latino | 15 (27.3) | 0 (0) | 15 (33.3) | |
| Not Hispanic/Latino | 38 (69.1) | 10 (26.3) | 28 (62.2) | |
| Other/unknown | 2 (4.4) | 0 (0) | 2 (3.6) | |
| BMI z-score | 0.12 (0 to 1.72) | 2 (0.32 to 2.2) | 1.1 (–0.99 to 1.7) | 0.045 |
| Obese, n (%) | 13 (25) | 5 (50) | 8 (19) | 0.057 |
| Hospital type, n (%) | — | |||
| Children’s hospital | 55 (100) | 10 (100) | 45 (100) | |
| Non–children’s hospital | 0 (0) | 0 (0) | 0 (0) | |
| Presenting symptoms, n (%) | ||||
| Gastrointestinal | 49 (89.1) | 10 (100) | 39 (70.9) | 0.28 |
| Fever | 52 (94.5) | 10 (100) | 42 (93.3) | 0.54 |
| Cough | 7 (12.7) | 1 (10) | 6 (13.3) | 0.63 |
| Rash | 27 (49.1) | 6 (60) | 21 (46.7) | 0.34 |
| Myalgias/joint aches | 8 (14.5) | 3 (30) | 5 (11.1) | 0.3 |
| Comorbid condition, n (%) | ||||
| Hypertension | 0 (0) | 0 (0) | 0 (0) | — |
| Diabetes mellitus | 0 (0) | 0 (0) | 0 (0) | — |
| Asthma | 6 (10.9) | 2 (20) | 4 (8.9) | 0.3 |
| Cancer | 0 (0) | 0 (0) | 0 (0) | — |
| Congenital heart disease | 2 (3.6) | 1 (10) | 1 (2.2) | 0.33 |
| Immunosuppressed | 1 (1.8) | 0 (0) | 1 (2.2) | |
| Baseline SCr, mg/dla | 0.54 (0.37 to 0.72) | 0.64 (0.57 to 0.8) | 0.61 (0.48 to 0.73) | <0.0001 |
| Admission SCr, mg/dl | 0.38 (0.24 to 0.57) | 1.58 (0.89 to 2.52) | 0.49 (0.3 to 0.65) | <0.0001 |
| Admission eGFR, ml/min per 1.73 m2 | 144.1 (99.7 to 186) | 54.2 (27.3 to 98.9) | 150.8 (126.6 to 190.6) | <0.001 |
| Sodium, mEq/L | 137 (135 to 138) | 132 (130 to 135) | 134 (132 to 136) | 0.28 |
| Potassium, mEq/L | 4.1 (3.8 to 4.95) | 3.9 (3.4 to 4.4) | 3.9 (3.7 to 4.45) | 0.44 |
| Bicarbonate, mEq/L | 21 (19 to 23) | 18 (14.8 to 20) | 21 (18 to 23) | 0.02 |
| Magnesium, mEq/L | 2.1 (1.8 to 2.3) | 2.1 (1.9 to 2.2) | 2 (1.8 to 2.2) | 0.7 |
| Calcium, mg/dl | 9.5 (8.9 to 10) | 8.9 (8.5 to 9.3) | 9.2 (8.5 to 9.75) | 0.18 |
| Albumin, mg/dl | 4.1 (3.7 to 4.5) | 3.1 (2.6 to 3.3) | 3.7 (3.3 to 4) | 0.004 |
| White blood cell count, mm3 | 8.7 (5.5 to 13.8) | 12.5 (9.9 to 15.7) | 10.1 (7.3 to 13.2) | 0.11 |
| Hemoglobin, g/dl | 11.6 (10 to 12.7) | 11.4 (10.3 to 12.2) | 11.3 (10.5 to 12.1) | 0.18 |
| Platelets, mm3 | 264 (183 to 346) | 143 (99 to 225) | 193 (131 to 286) | 0.66 |
| LDH, U/L (N = 46) | 297 (234 to 376) | 305 (232 to 375) | 296 (235 to 392) | 0.88 |
| Fibrinogen, mg/dl (N = 49) | 719 (633 to 876) | 800 (627 to 1014) | 714 (632 to 829) | 0.26 |
| CRP, μg/ml (N = 54) | 151 (95 to 240) | 312 (206 to 397) | 129 (84 to 191) | <0.0001 |
| D-dimer, μg/ml (N = 48) | 1355 (651 to 2328) | 1999 (1405 to 2384) | 1048 (500 to 2337) | 0.08 |
| Nephrotoxic medication exposure, n (%) | ||||
| ACE-I/ARB | 0 | 0 | 0 | N/A |
| NSAID | 37 (67) | 6 (60) | 31 (69) | 0.71 |
| Vancomycin | 7 (13) | 4 (40) | 3 (7) | 0.016 |
| Gentamicin | 0 | 0 | 0 | N/A |
ACE-I, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin receptor blocker; BMI, body mass index; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; LDH, lactate dehydrogenase; MIS-C, multisystem inflammatory syndrome in children; N/A, not applicable; NSAID, nonsteroidal anti-inflammatory drug; SCr, serum creatinine.
Data are given as median (interquartile range), unless otherwise indicated.
Baseline SCr was estimated from assumed eGFR 120 ml/min per 1.73 m2 using original Schwartz formula.25
Acute COVID-19
The median age of children with acute COVID-19 was 8.2 (IQR, 1.5–13.8) years, and more than half were male. Eight patients (8.2%) developed AKI; 4 presented with AKI on admission (Table 1,25 and Figure 2 ); 6 (6.2%) had stage 1, and 2 had stage 3 (Supplementary Table S1). There were no significant differences in age, sex, race, and body mass index z-score among children with and without AKI. Although there was no significant difference in presenting symptoms, 50% of patients with AKI presented with gastrointestinal symptoms. Patients with AKI presented with a significantly lower serum calcium and albumin (P = 0.047 and P = 0.001, respectively). Baseline white blood cell (WBC) count was significantly higher in those with AKI compared with those without AKI (P = 0.02) (Table 1).
Figure 2.
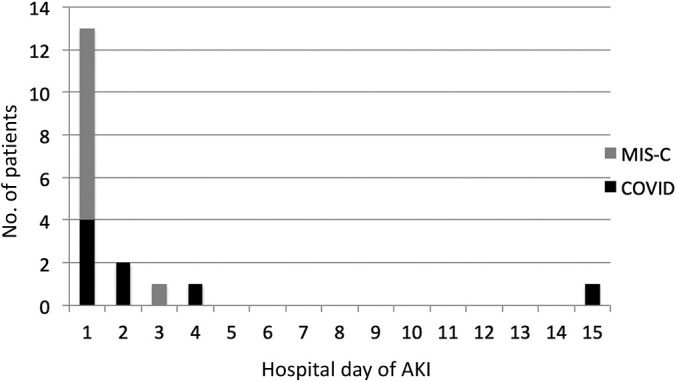
Acute kidney injury (AKI) by day of hospitalization. COVID, acute coronavirus disease 2019; MIS-C, multisystem inflammatory syndrome in children.
Multisystem inflammatory syndrome in children
The median age of children hospitalized with MIS-C was 7.5 (IQR, 1.5–13.8) years, and >60% were male. AKI developed in 10 (18.2%) patients. Eight (80%) of these patients presented with AKI on admission; 4 (7.3%) had stage 1, 2 (3.6%) had stage 2, and 4 (7.3%) had stage 3 (Figure 2 and Supplementary Table S1). There were no significant differences in age, sex, race, or ethnicity between the 2 groups. Those with AKI had a greater median body mass index z-score compared with those who did not develop AKI (P = 0.045). All patients in the MIS-C group were hospitalized in a children’s hospital. Although there was no significant difference of presenting symptoms, all MIS-C patients with AKI presented with gastrointestinal symptoms. Patients with AKI had a lower serum bicarbonate and albumin at presentation (P = 0.02 and P = 0.004, respectively). Baseline C-reactive protein was also significantly elevated among MIS-C patients who developed AKI (P < 0.0001). Although not statistically significant, patients with AKI presented with a higher WBC count (Table 2 25).
Echocardiography was available and analyzed for 89% of MIS-C patients (N = 49). Median left ventricular ejection fraction was lower for those with AKI (49%; IQR, 40%–54%) compared with those without AKI (56%; IQR, 49%–62%) (P = 0.02). Systolic dysfunction occurred in 80% (N = 8) of AKI patients compared with 49% (N = 17) without AKI. Coronary artery dilation (>2 mm) did not differ significantly between the groups (Supplementary Table S2).
Baseline demographic and clinical characteristics associated with AKI
There was no significant difference in age, ethnicity, or presenting symptoms in those with and without AKI (Supplementary Table S3). Those who identified as Black had 2.86 times higher unadjusted odds of AKI compared with those who were not Black (P = 0.042; 95% confidence interval [CI], 1.04–7.93). Of the 60 children who were critically ill and admitted to the PICU, 28% (N = 17) had AKI (Supplementary Table S3).
AKI was associated with higher WBC counts (odds ratio, 1.11; 95% CI, 1.04–1.2) and lower serum albumin levels (odds ratio, 0.17; 95% CI, 0.07–0.39) at admission (Supplementary Table S4). These findings maintained significance in a secondary analysis comparing critically ill patients with AKI with critically ill patients without AKI. There was a modest, but significant, odds of having a higher C-reactive protein at presentation (odds ratio, 1.01; 95% CI, 1.004–1.01). However, these findings may be related to severity of illness as most of the patients with AKI admitted to the intensive care unit had significantly higher C-reactive protein, and these findings were not maintained on secondary analysis comparing critically ill patients with and without AKI (Supplementary Table S2). Among patients who developed AKI after admission (N = 5), exposure to nephrotoxic medications (including angiotensin-converting enzyme inhibitor/angiotensin receptor blockers, nonsteroidal anti-inflammatory drugs, vancomycin, and aminoglycosides) was also associated with the development of AKI (P = 0.018; odds ratio, 5.5; 95% CI, 1.33–22.6).
Clinical course and outcomes
Patients with AKI had higher rates of vasopressor treatment and ionotropic support (Table 3 25). Continuous kidney replacement therapy, utilizing continuous venovenous hemodiafiltration, was needed in 2 children with acute COVID-19, whereas 2 others required ECMO support. There were 2 deaths in the acute COVID-19 cohort, one from bowel perforation and the other from acute hypoxic/hypercapneic respiratory failure. For further details regarding hospital outcomes by KDIGO stage of AKI, see Supplementary Table S2.
Table 3.
Hospital course and outcomes by AKI diagnosis in patients with COVID-19 and MIS-C
|
Variables |
Acute COVID-19 |
MIS-C |
||||
|---|---|---|---|---|---|---|
| No AKI (N = 88) | AKI (N = 8) | P value | No AKI (N = 45) | AKI (N = 10) | P value | |
| Treatment, n (%) | ||||||
| I.v. Ig | 10 (11) | 2 (25) | 0.26 | 40 (89) | 9 (90) | >0.99 |
| Anakinra | 4 (5) | 2 (25) | 0.077 | 3 (7) | 3 (30) | 0.07 |
| Hydroxychloroquine | 14 (16) | 3 (38) | 0.15 | 1 (2) | 1 (10) | 0.33 |
| Remdesivir | 5 (6) | 1 (12) | 0.42 | 0 (0) | 1 (10) | 0.18 |
| Methylprednisolone or prednisone | 14 (16) | 2 (25) | 0.62 | 26 (58) | 9 (90) | 0.29 |
| PICU, n (%) | 18 (20) | 8 (100) | <0.0001 | 25 (55.6) | 9 (90) | 0.070 |
| Length of PICU stay, d | 3.1 (2.1 to 8.7) | 11.9 (3.5 to 17.7) | 0.16 | 3.4 (2.6 to 4.9) | 8.7 (4.5 to 10.1) | 0.025 |
| Inotropes, n (%) | 1 (1) | 3 (38) | 0.002 | 7 (16) | 6 (60) | 0.007 |
| Vasopressors, n (%) | 3 (3) | 5 (32) | <0.001 | 18 (40) | 9 (90) | 0.005 |
| ECMO, n (%) | 1 (1) | 1 (12) | 0.08 | 0 (0) | 0 (0) | — |
| Mechanical ventilation, n (%) | 4 (4.5) | 2 (33.3) | <0.001 | 1 (2) | 4 (40) | 0.001 |
| Length of mechanical ventilation, d | 10 (6 to 14) | 4 (4 to 13) | 0.15 | 2 (2 to 2) | 3.5 (2.5 to 4.5) | 0.28 |
| CKRT, n (%) | 0 (0) | 2 (25) | <0.001 | 0 (0) | 0 (0) | — |
| Length of hospital stay, d | 2.8 (1.6 to 6.2) | 22.2 (13.5 to 29.1) | 0.003 | 4.6 (3.5 to 5.8) | 8.2 (4.5 to 12.5) | 0.04 |
| Serum creatinine, mg/dl | ||||||
| Median | 0.34 (0.23 to 0.5) | 0.69 (0.27 to 0.86) | 0.27 | 0.37 (0.28 to 0.51) | 0.7 (0.65 to 0.76) | <0.001 |
| Peak | 0.39 (0.26 to 0.57) | 1.1 (0.435 to 1.89) | 0.005 | 0.49 (0.31 to 0.7) | 1.7 (1.47 to 2.92) | <0.001 |
| Discharge | 0.33 (0.23 to 0.5) | 0.26 (0.23 to 0.59) | 0.83 | 0.36 (0.26 to 0.47) | 0.475 (0.37 to 0.56) | 0.15 |
| Discharge eGFR, ml/min per 1.73 m2a | 159 (114 to 198) | 149 (90 to 195) | 0.53 | 189 (162 to 211) | 181 (139 to 197) | 0.27 |
| Mortality, n (%) | 1 (1) | 1 (12) | 0.16 | 0 (0) | 0 (0) | — |
AKI, acute kidney injury; CKRT, chronic kidney replacement therapy; COVID-19, coronavirus disease 2019; ECMO, extracorporeal membranous oxygenation; eGFR, estimated glomerular filtration rate; MIS-C, multisystem inflammatory syndrome in children; PICU, pediatric intensive care unit.
Data are given as median (interquartile range), unless otherwise indicated.
Discharge eGFR was based on original Schwartz equation.25
A total of 11 (7.2%) patients required mechanical ventilation. Of the mechanically ventilated patients, 6 (55%) were diagnosed with acute COVID-19, whereas 5 (45%) had MIS-C. There was a greater proportion of mechanical ventilation in children with AKI in both COVID-19 and MIS-C groups compared with those without AKI (P < 0.001 and P = 0.001, respectively).
AKI resolved before discharge in all but 2 patients with acute COVID-19 and 1 patient with MIS-C. There were no significant differences in serum creatinine or estimated glomerular filtration rate on discharge between those with and without AKI.
In unadjusted regression analysis, patients with all stages of AKI had a 5-day significantly increased length of PICU stay (95% CI, 0.86–9; P = 0.025). In addition, patients with AKI had a significantly greater length of hospital stay of 8.4 days compared with patients without AKI (95% CI, 4.4–6.7; P < 0.0001). We did not observe a significant association with mortality or length of mechanical ventilation (Table 4 ).
Table 4.
Association of AKI with hospital outcomes
| Outcome by AKI stage | Hazard ratio (95% CI) | P value |
|---|---|---|
| Mortality | ||
| AKI stage 1 + 2 + 3 | 0.57 (0.03 to 11.8) | 0.72 |
| AKI stage 2 + 3 | — | — |
| Outcome by AKI stage | β (95% CI) | P value |
|---|---|---|
| Length of hospital stay, d | ||
| AKI stage 1 + 2 + 3 | 8.4 (4.4 to 6.7) | <0.0001 |
| AKI stage 2 + 3 | 5.2 (0.07 to 10.2) | 0.047 |
| Length of PICU stay, d | ||
| AKI stage 1 + 2 + 3 | 5 (0.86 to 9.1) | 0.02 |
| AKI stage 2 + 3 | 2.3 (–3.7 to 8.3) | 0.45 |
| Length of mechanical ventilation, d | ||
| AKI stage 1 + 2 + 3 | –3.9 (–9.4 to 12.7) | 0.15 |
| AKI stage 2 + 3 | –1.08 (–7.4 to 5.3) | 0.71 |
AKI, acute kidney injury; CI, confidence interval; PICU, pediatric intensive care unit.
Discussion
In this study, AKI developed in 11.8% of the cohort: 8.2% in acute COVID-19 and 18.2% of cases of MIS-C. Most patients developed AKI on admission, and AKI resolved in almost all cases before discharge. Lower serum albumin and higher WBC count were associated with AKI in children with acute COVID-19 and MIS-C. MIS-C patients with AKI had more systolic dysfunction on echocardiogram compared with those without AKI. AKI, in unadjusted models, was significantly associated with longer PICU and hospital length of stay.
AKI is reported to occur in under half of the adults with COVID-19.5 , 7 , 8 In comparison, 8.2% of acute COVID-19 pediatric patients in our study developed AKI. Recent studies in pediatric cohorts estimate COVID-19–associated AKI to be between 1.3% and 44%. 17, 18, 19, 20 The disparate nature of these rates is likely due to heterogeneity of the populations studied (e.g., hospitalized vs. critically ill) and definitions of AKI. Nonetheless, incidence of AKI in children with acute COVID-19 was consistently demonstrated to be less than adults. Adult patients with COVID-19–related AKI have a higher prevalence of comorbid conditions, such as diabetes, chronic kidney disease, and congestive heart failure.8 In comparison, our study population was relatively healthy, with a low prevalence of comorbid conditions and thus more renal reserve.
AKI rates in children with MIS-C are more prevalent compared with children with acute COVID-19. Case series have estimated AKI occurrence in 15% to 73% of cases.18 , 23 , 27 The incidence rate of AKI in MIS-C patients in this study was >18%. The most common presenting symptoms of our MIS-C cohort were fever, gastrointestinal symptoms, and skin rash, which are consistent with current literature.27, 28, 29, 30 Recent studies have noted a greater prevalence of MIS-C within African American/Afro-Caribbean populations; however, there were no significant differences in race and AKI in our MIS-C cohort.23 This may be due to sample size limitations as there was over double the odds of AKI (MIS-C and acute COVID-19 combined) in patients who identified as Black. This is consistent with disproportionate rates of morbidity and mortality associated with COVID-19 reported in African American children and adults.23 Current reports have highlighted the severity of MIS-C with increased rates of PICU admissions and need for inotropic support and ECMO.22 , 23 , 27, 28, 29, 30 Most MIS-C patients in our cohort were admitted to the PICU and required treatment with inotropes and vasopressors. Although MIS-C patients are reported to have comparable mortality rates to adults with severe COVID-19, there were no deaths in our MIS-C cohort.23
COVID-19–related AKI may be associated with severe disease and acute lung injury. Hirsch et al. found that mechanical ventilation and vasopressors were risk factors for AKI development in adults.5 In this study, all AKI patients were almost always critically ill and had high rates of mechanical ventilation and need for vasoactive support. However, as most patients developed AKI on admission, the temporality of AKI and mechanical ventilation cannot be appreciated. Two patients with acute COVID-19 required continuous kidney replacement therapy, whereas 2 others needed ECMO support. Although there have been reports of increased need of ECMO and continuous kidney replacement therapy in patients with MIS-C, none of the MIS-C patients studied required these treatments. Last, AKI, in unadjusted models, was associated with longer PICU and hospital stays. This association, although to be interpreted with caution, is consistent with previously reported findings of AKI in critically ill pediatric patients.31 , 32
Obesity is associated with severe COVID-19 disease and AKI in adults.33 , 34 This finding was not observed within our pediatric acute COVID-19 cohort; however, those with MIS-C and AKI had higher body mass index z-scores. Reports demonstrate that >50% of patients with MIS-C are noted to be obese. This may be due to the increased baseline inflammatory milieu observed in obesity and/or increased number of comorbidities.23
Most patients developed AKI on the first day of hospitalization. This phenomenon is reported in multiple adult studies where most adult patients developed AKI on admission or on day 1 of hospitalization.5 , 8 Gastrointestinal complaints were the most common presenting symptoms among children with AKI, which may suggest a prerenal etiology due to losses and dehydration. The significant association of lower median left ventricular ejection fraction, systolic dysfunction, and AKI in MIS-C patients may indicate that low cardiac output also contributed to AKI. More important, not all patients with left ventricular dysfunction and/or shock, requiring vasoactives, developed AKI, suggesting multifactorial mechanisms of injury.
Other possible etiologies of AKI in acute COVID-19 include inflammation and exposure to nephrotoxins. Adult COVID-19–related AKI was associated with increased inflammatory markers.7 , 8 The same association was appreciated in this pediatric cohort. Higher WBC count, higher C-reactive protein, and lower serum albumin were associated with AKI in acute COVID-19 and MIS-C. Lower serum albumin and consequently lower serum calcium may have been due to increased capillary permeability secondary to systemic inflammation. Iatrogenic causes, such as nephrotoxic medications, as described at length in the literature, may have induced AKI as well.35 In our study, of the 5 patients who developed AKI after admission, nephrotoxic medication exposure (angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, nonsteroidal anti-inflammatory drugs, vancomycin, and aminoglycosides) was significantly associated with AKI.
There are several limitations to our study. Our study had a small sample size, and most AKI developed at admission, making it difficult to draw causal associations and adjust for confounders. As with any retrospective study, there was potential for ascertainment bias. However, most COVID-19 and MIS-C patients had a basic metabolic panel on presentation and during their hospital stay to assess for AKI. Utilization of serum creatinine without urine output and back-calculation of baseline creatinine values may have also underestimated the incidence of AKI.
Despite these limitations, our study has notable strengths. Our study population consisted of patients in the greater New York City area at the epicenter of the COVID-19 outbreak that represents a diverse racial, ethnic, and socioeconomic pediatric population. In addition, KDIGO AKI definitions were used to uniformly compare our rates with adults and data from other centers.
Conclusions
In conclusion, AKI occurred in 11.8% of children with acute COVID-19 and MIS-C. Children with COVID-19–related AKI had increased WBC counts and lower serum albumin levels on admission, which may reflect the inflammatory cascade’s complex role in development and perpetuation of AKI. In addition, decreased intravascular volume and distributive/cardiogenic shock may have contributed to AKI development in the cohort. Pediatric COVID-19–related AKI, similar to reports in larger AKI studies, was associated with poor outcomes, such as increased PICU and hospital length of stay. Further research in larger cohorts is needed to characterize AKI risk factors in children with acute COVID-19 and MIS-C.
Disclosure
AB has consulted for Impact Communication Partners. KDJ is a consultant for Astex Pharmaceuticals and Natera. All the other authors declared no competing interests.
Acknowledgements
We acknowledge the Northwell COVID-19 Research Consortium for review and contribution to research concept and study design.
Footnotes
see commentary on page 16
Table S1. Hospital course and outcomes by stage of acute kidney injury.
Table S2. Echocardiographic data in MIS-C patients with and without acute kidney injury.
Table S3. Admission characteristics and laboratory values by admission unit: pediatric intensive care unit versus medical/surgical floor.
Table S4. Logistic regression analyses of baseline demographics and clinical variables associated with acute kidney injury in COVID and MIS-C.
Supplementary Material
References
- 1.COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Available at:
- 2.Li Q., Guan X., Wu P., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holshue M.L., DeBolt C., Lindquist S., et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch J.S., Ng J.H., Ross D.W., et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng Y., Luo R., Wang K., et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher M., Neugarten J., Bellin E., et al. AKI in hospitalized patients with and without COVID-19: a comparison study. J Am Soc Nephrol. 2020;31:2145–2157. doi: 10.1681/ASN.2020040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan L., Chaudhary K., Saha A., et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021;32:151–160. doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diao B., Wang C., Wang R., et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv. Accessed October 12, 2020. [DOI] [PMC free article] [PubMed]
- 16.Ng J.H., Hirsch J.S., Hazzan A., et al. Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis. 2021;77:204–215.e1. doi: 10.1053/j.ajkd.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X., Chen X., Tang F., et al. Be aware of acute kidney injury in critically ill children with COVID-19. Pediatr Nephrol. 2021;36:163–169. doi: 10.1007/s00467-020-04715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart D.J., Hartley J.C., Johnson M., et al. Renal dysfunction in hospitalised children with COVID-19. Lancet Child Adolesc Health. 2020;4:e28–e29. doi: 10.1016/S2352-4642(20)30178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kari J.S.M., Albanna A.S., Alahmadi T., et al. Acute kidney injury in children with COVID-19 [preprint] BMC Nephrol. [DOI] [PMC free article] [PubMed]
- 20.Bjornstad E.C., Krallman K.A., Askenazi D., et al. Preliminary assessment of acute kidney injury in critically ill children associated with SARS-CoV-2 infection: a multicenter cross-sectional analysis. Clin J Am Soc Nephrol. 2021;16:446–448. doi: 10.2215/CJN.11470720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19) https://emergency.cdc.gov/han/2020/han00432.asp Available at:
- 22.Chiotos K., Bassiri H., Behrens E.M., et al. Multisystem inflammatory syndrome in children during the coronavirus 2019 pandemic: a case series. J Pediatr Infect Dis Soc. 2020;9:393–398. doi: 10.1093/jpids/piaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed M., Advani S., Moreira A., et al. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine. 2020;26:100527. doi: 10.1016/j.eclinm.2020.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Workgroup KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 25.Zappitelli M., Parikh C.R., Akcan-Arikan A., et al. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008;3:948–954. doi: 10.2215/CJN.05431207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention Growth charts. https://www.cdc.gov/growthcharts/ Available at:
- 27.Whittaker E., Bamford A., Kenny J., et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dufort E.M., Koumans E.H., Chow E.J., et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee P.Y., Day-Lewis M., Henderson L.A., et al. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J Clin Invest. 2020;130:5942–5950. doi: 10.1172/JCI141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diorio C., Henrickson S.E., Vella L.A., et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest. 2020;130:5967–5975. doi: 10.1172/JCI140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kari J.A., Alhasan K.A., Shalaby M.A., et al. Outcome of pediatric acute kidney injury: a multicenter prospective cohort study. Pediatr Nephrol. 2018;33:335–340. doi: 10.1007/s00467-017-3786-1. [DOI] [PubMed] [Google Scholar]
- 32.Kaddourah A., Basu R.K., Bagshaw S.M., et al. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376:11–20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowe B., Cai M., Xie Y., et al. Acute kidney injury in a national cohort of hospitalized US veterans with COVID-19. Clin J Am Soc Nephrol. 2020;16:14–25. doi: 10.2215/CJN.09610620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchis-Gomar F., Lavie C.J., Mehra M.R., et al. Obesity and outcomes in COVID-19: when an epidemic and pandemic collide. Mayo Clin Proc. 2020;95:1445–1453. doi: 10.1016/j.mayocp.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moffett B.S., Goldstein S.L. Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin J Am Soc Nephrol. 2011;6:856–863. doi: 10.2215/CJN.08110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.