ABSTRACT
In the treatment of children and adolescents with cancer, multimodal approaches combining surgery, chemotherapy and radiation can cure most patients, but may cause lifelong health problems in survivors. Current therapies only modestly reflect increased knowledge about the molecular mechanisms of these cancers. Advances in next-generation sequencing have provided unprecedented cataloging of genetic aberrations in tumors, but understanding how these genetic changes drive cellular transformation, and how they can be effectively targeted, will require multidisciplinary collaboration and preclinical models that are truly representative of the in vivo environment. Here, I discuss some of the key challenges in pediatric cancer from my perspective as a physician-scientist, and touch on some promising new approaches that have the potential to transform our understanding of these diseases.
Summary: This Perspective discusses the special features that make it challenging to develop new therapies for pediatric cancers, and the ways in which collaboration centered on improved models can meet these challenges.
Introduction: the challenge of pediatric cancer
Cancer in children makes up ∼3% of the global incidence of cancer, translating to ∼16,500 new cases/year in the United States. Despite these relatively small numbers, pediatric cancer exerts an outsized impact: beyond the physical and emotional toll on children, their families and the community, the number of years of productive life lost to pediatric cancers is proportionately higher than that of adult cancers. While there is much to celebrate in the treatment of pediatric cancers – overall survival rates for children with cancer have improved from less than 10% in the 1960s to around 80% today – it is fair to say that much of this improvement had little to do with better understanding of tumor biology. Instead, progress has largely been based on the empiric use of multimodality therapy (surgery, chemotherapy, radiation) that still serves as first-line treatment for the vast majority of pediatric cancer patients. The cost of this success becomes more and more apparent each year, as the growing number of survivors of childhood cancer face lifelong adverse health effects due to the toxicity of chemotherapy and radiation (Suh et al., 2020). Recognition of the problems associated with non-targeted therapies has spurred efforts to develop alternative approaches for childhood cancers that could be more effective and less toxic than current treatments.
“The cost of [pediatric cancer] success becomes more and more apparent each year, as the growing number of survivors of childhood cancer face lifelong adverse health effects due to the toxicity of chemotherapy and radiation.”
Advances in next-generation sequencing have made possible one such approach, known as precision medicine. This strategy is based on genomic profiling of a given patient's tumor, yielding information that can then be used to select therapies designed to counter the effects of specific driver mutations. The approach has had some notable successes, including the treatment of pediatric cancers with NTRK or ALK gene rearrangements (Butrynski et al., 2010; Drilon et al., 2018; Laetsch et al., 2018). In addition to genomics, a lot of progress has been made in the regulatory environment and the pharmaceutical industry, enabling cooperative trials of precision medicine, such as Pediatric Molecular Analysis for Therapy Choice (MATCH) in the USA, Precision Oncology for Young People (PROFYLE) in Canada and Individualized Therapy for Relapsed Malignancies in Childhood (INFORM) in Europe (Hadjadj et al., 2020). One of the largest such studies to date, the Zero Childhood Cancer (ZERO) initiative in Australia, illustrates both the promise and challenges of the precision medicine approach. In this study, a combination of whole-genome sequencing and RNA sequencing was used to analyze more than 250 tumor specimens, resulting in specific therapeutic recommendations for two-thirds of the patients (Wong et al., 2020). Of 38 evaluable patients in this group with high-risk cancers, 31% exhibited a complete or partial response, comparable to results from larger precision medicine trials in adults (Hazim and Prasad, 2018). While encouraging, the long-term benefits of this ‘sequence tumor, choose agent’ paradigm may ultimately apply only for a small percentage of patients (Marquart et al., 2018). The reasons for the lower impact are several, but accumulating evidence suggests that one major challenge is the difficulty of predicting the biological impact of specific mutations and the efficacy of targeted therapy from genomic data. This is almost certainly due to the complex nature of tumor cell behavior in the in vivo environment, where tumor cell growth, survival and treatment response may depend on multiple genetic and epigenetic features of both cancer cells and normal host cells in the tumor microenvironment. It is likely that, in children, special features of pediatric cancers will create even more difficulties for the precision medicine approach. Below, I discuss these features, as well as some potential strategies to improve the effectiveness of targeted therapies for childhood cancer.
The most common cancers of adults are carcinomas of the lung, breast, colon, prostate and other epithelial tissues; however, these cancers are vanishingly rare in children. Instead, acute leukemias and brain tumors predominate. Another major category of childhood cancer is the so-called embryonal tumors or blastomas, such as neuroblastoma, medulloblastoma, nephroblastoma and hepatoblastoma. This category of tumors, which also includes germ cell tumors and certain sarcomas, is striking because of the resemblance of the tumor cells to the corresponding fetal tissue, albeit with features of aberrant development (Chen et al., 2015; Gojo et al., 2020; Scotting et al., 2005). These histologic clues to the developmental origins of pediatric cancers are supported by several other features. The unique age spectrum associated with most childhood cancers suggests that there are time- and tissue-specific windows of susceptibility to cell transformation (Johnston et al., 2020; Linabery and Ross, 2008). Pediatric cancers exhibit fewer somatic mutations on average than adult cancers (Grobner et al., 2018), and some are driven by features such as transcription factor fusion oncogenes, which have so far been refractory to targeting. Pediatric cancers also disproportionately carry alterations in epigenetic factors (Huether et al., 2014) and developmental signaling pathways such as WNT, Notch, TGF-beta and Hedgehog (Filbin and Monje, 2019), which can represent novel targets but also introduce therapeutic complexity owing to the importance of these factors in normal, developing tissues in children (Chheda and Gutmann, 2017; Gajjar et al., 2013; Morinello et al., 2014; Zwergel et al., 2018). The theme of early development is further apparent in the strong linkage between cancer predisposition and developmental syndromes, such as Noonan and Costello RASopathy syndromes, or overgrowth syndromes such as Beckwith-Wiedemann or Perlman's syndromes (Bharathavikru and Hastie, 2018; Cizmarova et al., 2013). Pediatric cancers arise during a time period of profound changes in tissue patterning and organ development. Any attempt to fully understand the origin of these cancers must therefore take into account not only the spectrum of molecular lesions linked to specific cancer types, but also the particular biological features and developmental stage of the tissue lineage in which the cancers arise.
Modeling developmental mechanisms of childhood cancer
How can developmental biology contribute to better understanding of tumor biology and ultimately to better outcomes for children with cancer? How do we move beyond in silico analyses or studies done with cell lines grown on plastic? Certainly xenografts, especially orthotopic and patient-derived xenografts, hold promise as potentially more accurate models (Aparicio et al., 2015; Hermans and Hulleman, 2020; Zarzosa et al., 2017), although the overall rarity of most pediatric cancers can make it difficult to assemble large cohorts. In vitro, innovative spheroid culture approaches have begun to reveal dramatic changes in drug sensitivity of the same cells cultured in three-dimensional versus two-dimensional environments (Breslin and O'Driscoll, 2016; Fujii et al., 2009; Imamura et al., 2015; Musah-Eroje and Watson, 2019; Polo et al., 2010). Organoid models – self-organizing tissues grown in vitro from stem or progenitor cells, exhibiting lineage-specific cell differentiation and the formation of tissue architecture resembling the relevant organ – can provide great advantages for live imaging (Bolhaqueiro et al., 2018; Srivastava et al., 2020), metabolic studies (Browne et al., 2017) and drug screening (Burkhart et al., 2018; Francies et al., 2019; Jabs et al., 2017). Many pediatric cancers are thought to arise during embryonic or fetal life, meaning that the relevant stem and progenitor cell populations are often unknown or difficult to obtain, complicating efforts to build relevant organoid models. One approach to overcome this barrier involves the generation of induced pluripotent stem cells (iPSCs), which can then be directed to differentiate along lineage-specific trajectories. Introducing oncogenic mutations into the cells, or deriving the iPSCs from donors carrying germline cancer susceptibility mutations, provides a source of progenitors that can be developed into cancer organoids or established as xenografts in immunocompromised mice (Dost et al., 2020; Hwang et al., 2019; Papapetrou, 2016). This method has produced models of pediatric cancers including medulloblastoma (Huang et al., 2019) and retinoblastoma (Saengwimol et al., 2018). For some cancers, such as hepatoblastoma (Saltsman et al., 2020) and Wilms tumor of the kidney (Calandrini et al., 2020), tumor-derived cells have been used to develop organoids exhibiting multilineage potential.

James F. Amatruda, MD, PhD. Head of Basic and Translational Research in the Cancer and Blood Disease Institute and the Division of Pediatric Hematology-Oncology at Children's Hospital Los Angeles; and Professor of Pediatrics and Medicine at the Keck School of Medicine of the University of Southern California.
Model organisms can contribute greatly to understanding cancer pathways, even without directly modeling tumorigenesis. For example, studies of the effects of gene regulatory networks and signaling pathways on early embryonic development have provided critical insight into cancer-relevant phenomena including RAS signaling (Beitel et al., 1990; Han and Sternberg, 1990; Simon et al., 1991), microRNA biology (Lee et al., 1993; Wightman et al., 1993) and programmed cell death (Yuan and Horvitz, 2004). Mutations in mammalian oncogenes and tumor suppressors, when engineered into model organisms, may cause developmental phenotypes that can be used as a platform for testing small molecules or genetic modifiers (Al-Olabi et al., 2018; Anastasaki et al., 2012; Levinson and Cagan, 2016; Liu et al., 2017; van der Hoeven et al., 2020; Vidal et al., 2005).
Further insight can be provided by models that attempt to recapitulate human cancer genetics via regulated expression of gain- and loss-of-function cancer mutations in the relevant organ or tissue. Such models, instituted in genetically engineered mice, worms, fish, flies and other organisms, have perhaps the greatest potential to exhibit the full range of cancer phenotypes encountered in patients, including the role of germline (inherited) variants, tumor initiation and growth, the role of the tumor microenvironment, interaction with the host immune system, metastasis and response to treatment. There are many examples pointing to the success of this strategy for modeling lung cancer, brain tumors, breast cancer, melanoma, leukemias, testicular cancer and other malignancies (Annunziato et al., 2016; Ceol et al., 2011; Johnson et al., 2001; Kohnken et al., 2017; Meuwissen et al., 2003; Milagre et al., 2010; Patton et al., 2005; Pierpont et al., 2017; Read et al., 2009).
In the case of pediatric cancers, the ‘cell-of-origin’ problem discussed above presents a challenge for models, requiring investigators to choose carefully when targeting expression of candidate cancer-driving genetic changes to specific tissues. Here is where model organisms can be especially useful, as they can provide access to a range of developmental time windows and tissue lineages, as well as reflect the changing epigenetic landscape of early development. Targeting these developmental compartments has allowed the generation of animal models of neuroblastoma, rhabdomyosarcoma, medulloblastoma and Wilms tumor, among other childhood cancers (Hackett et al., 2003; Keller et al., 2004; Kendall et al., 2018; Stewart et al., 2010; Urbach et al., 2014; Zurawel et al., 2000). The value of model organisms is especially clear in the case of pediatric brain tumors, where molecular profiling has increasingly revealed distinct epigenetically defined subgroups within tumor types, with important clinical implications. For example, single-cell analysis of tumors, coupled with cross-species transcriptomics of mouse neural development, provides new insight into the distinct developmental origins of medulloblastoma subtypes (Hovestadt et al., 2019; Marino and Gilbertson, 2021). And in a recent review in Disease Models & Mechanisms, Cédric Maurange elegantly lays out the case that fundamental work on temporal patterning in Drosophila neural progenitors can inform our understanding of the origins of a range of pediatric brain tumors (Maurange, 2020).
“[…] model organisms can be especially useful, as they can provide access to a range of developmental time windows and tissue lineages, as well as reflect the changing epigenetic landscape of early development.”
In parallel to these efforts in worms, flies and mice, we and others have turned to zebrafish as a powerful and flexible animal model for human cancer. The strengths of the fish model for genetic modeling, imaging and drug screening have previously been described (Amatruda and Patton, 2008; Casey and Stewart, 2020; Mayrhofer and Mione, 2016; Xie et al., 2015; Yen et al., 2014). In the context of pediatric cancer, one of the most valuable aspects of the fish model is the access it provides to a range of developmental time windows and tissue lineages, some only present during early development and absent at the adult stage. Pioneering work modeling T-cell leukemias in fish (Langenau et al., 2003) enabled use of the system to discover novel leukemia genes such as ARID5B (Leong et al., 2017) and JDP2 (Mansour et al., 2018). A transgenic model of neuroblastoma (Zhu et al., 2012) highlighted the role of developmental apoptosis as an oncogene-induced antitumor response. Models of central nervous system primitive neuroectodermal tumor (PNET) identified oligodendrocyte precursor cells as a cell of origin for this pediatric brain tumor subtype, and provide a platform for drug testing (Modzelewska et al., 2016). Zebrafish melanoma models have probed links between RAS signaling, development and cancer (Anastasaki et al., 2009; Patton et al., 2005), and have identified reactivation of embryonic developmental pathways as a critical event in tumor initiation (Kaufman et al., 2016; White et al., 2011). The role of epigenetic modifiers has been tested in fish models of liver cancer, myelodysplastic syndrome and rhabdomyosarcoma (Albacker et al., 2013; Chernyavskaya et al., 2016; Gjini et al., 2015; Mudbhary et al., 2014). Zebrafish modeling RAS-driven embryonal rhabdomyosarcoma have elegantly probed the cell of origin of this disease (Storer et al., 2013; Tenente et al., 2017). Our own work on alveolar rhabdomyosarcoma, a clinically aggressive tumor driven by oncogenic PAX3–FOXO1 fusion proteins, leveraged developmental assays in zebrafish embryos and adult tumor models to identify HES3 as a cooperating oncogene that impairs muscle differentiation and contributes to poor clinical outcomes (Kendall et al., 2018). Collectively, these studies demonstrate the power of applying a developmental biology approach to generate key insights into the origin and uncontrolled growth of pediatric cancers.
“[…] integrated projects will accommodate multiple scales of speed – including both the slow, meticulous process of building, evaluating and refining models, and in parallel the rapid generation of custom models reflecting an individual patient's genetics, with real-time return of results to the treatment team […]”
Looking forward
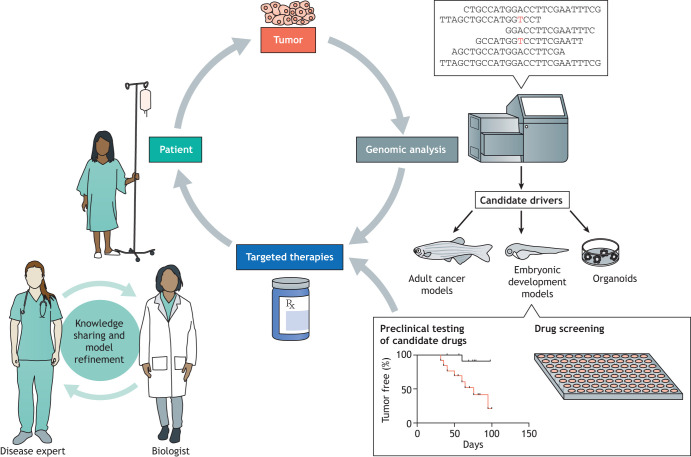
Today, while it is true that most cancers are still treated with combinations of surgery, radiation therapy and chemotherapy, important progress in the development of molecularly targeted and immune-modulating therapies has begun to change this paradigm, albeit slightly. To continue and expand on this progress will take a concerted effort, one in which mechanistically based models will play a crucial role (Fig. 1). Developmental biology, by its nature concerned with understanding gene function in the fuller context of tissue lineage and cell–cell interactions at the organism level, has a lot to contribute to the understanding of how genomic and signaling alterations lead to unrestrained growth of pediatric cancers. To achieve this, more powerful understanding will require new types of collaborations between the cancer and developmental biologists who build these models with pathologists, oncologists and other disease experts. This team approach can serve, through iterative feedback and discussion, not only to define the clinically important problems, but also to ‘credential’ a given model as representative of the human disease – especially important, as we must acknowledge the inherent limitations of all of our models. Ideally these integrated projects will accommodate multiple scales of speed – including both the slow, meticulous process of building, evaluating and refining models, and in parallel the rapid generation of custom models reflecting an individual patient's genetics, with real-time return of results to the treatment team in a time window that can benefit that patient. A recent inspiring example is one in which a zebrafish model of a novel gene variant suspected of causing lymphatic anomaly led to successful treatment of the patient (Li et al., 2019). One can imagine a similar approach being applied for pediatric cancers, including clarifying the pathogenic role of variants of unknown significance.
Fig. 1.
Improving therapy of pediatric cancers through collaboration. Next-generation sequencing of tumor samples may directly identify candidate targeted therapies. In many cases, further investigation is required. Model systems such as organoids or genetically engineered animals can interrogate the function of candidate driver genes in a setting that recapitulates the complexity of the in vivo tumor environment. Such models can support drug screening and preclinical testing of novel therapies. Throughout, collaboration between clinicians and basic scientists is essential to define clinical challenges and to build and refine disease models.
In this effort, it will be especially important for new models to reflect the great heterogeneity of human disease processes, not only intratumoral heterogeneity, but also the effects of gender, race and ethnicity, which may strongly impact disease phenotypes and response to treatment. Involvement of patients and advocates as members of these interdisciplinary teams can further help to prioritize research goals. Moving forward will require not only collaboration but also creativity, finding new ways to recognize the efforts of team members with diverse skillsets, and sustaining funding for disease-focused research without neglecting the fundamental importance of basic research in molecular and developmental biology. While much work is still required to address therapy resistance and metastasis, we may look forward to bringing the formidable power of molecular developmental biology to bear for the benefit of children with cancer and other diseases.
Acknowledgements
I thank Genevieve Kendall, Gaudenz Danuser, Elizabeth Patton and other colleagues for helpful discussions.
Footnotes
Competing interests
The author declares no competing or financial interests.
Funding
The author is supported by the 1 Million 4 Anna Foundation, the St. Baldrick's Foundation, Curing Kids Cancer, and grants 5 P50CA196516-03 and U54CA231649-01-A1 from the National Institutes of Health.
References
- Al-Olabi, L., Polubothu, S., Dowsett, K., Andrews, K. A., Stadnik, P., Joseph, A. P., Knox, R., Pittman, A., Clark, G., Baird, W.et al. (2018). Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J. Clin. Invest. 128, 1496-1508. 10.1172/JCI98589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albacker, C. E., Storer, N. Y., Langdon, E. M., Dibiase, A., Zhou, Y., Langenau, D. M. and Zon, L. I. (2013). The histone methyltransferase SUV39H1 suppresses embryonal rhabdomyosarcoma formation in zebrafish. PLoS ONE 8, e64969. 10.1371/journal.pone.0064969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatruda, J. F. and Patton, E. E. (2008). Genetic models of cancer in zebrafish. Int. Rev. Cell Mol. Biol. 271, 1-34. 10.1016/S1937-6448(08)01201-X [DOI] [PubMed] [Google Scholar]
- Anastasaki, C., Estep, A. L., Marais, R., Rauen, K. A. and Patton, E. E. (2009). Kinase-activating and kinase-impaired cardio-facio-cutaneous syndrome alleles have activity during zebrafish development and are sensitive to small molecule inhibitors. Hum. Mol. Genet. 18, 2543-2554. 10.1093/hmg/ddp186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasaki, C., Rauen, K. A. and Patton, E. E. (2012). Continual low-level MEK inhibition ameliorates cardio-facio-cutaneous phenotypes in zebrafish. Dis. Model Mech. 5, 546-552. 10.1242/dmm.008672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato, S., Kas, S. M., Nethe, M., Yucel, H., Del Bravo, J., Pritchard, C., Bin Ali, R., van Gerwen, B., Siteur, B., Drenth, A. P.et al. (2016). Modeling invasive lobular breast carcinoma by CRISPR/Cas9-mediated somatic genome editing of the mammary gland. Genes Dev. 30, 1470-1480. 10.1101/gad.279190.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio, S., Hidalgo, M. and Kung, A. L. (2015). Examining the utility of patient-derived xenograft mouse models. Nat. Rev. Cancer 15, 311-316. 10.1038/nrc3944 [DOI] [PubMed] [Google Scholar]
- Beitel, G. J., Clark, S. G. and Horvitz, H. R. (1990). Caenorhabditis elegans ras gene let-60 acts as a switch in the pathway of vulval induction. Nature 348, 503-509. 10.1038/348503a0 [DOI] [PubMed] [Google Scholar]
- Bharathavikru, R. and Hastie, N. D. (2018). Overgrowth syndromes and pediatric cancers: how many roads lead to IGF2? Genes Dev. 32, 993-995. 10.1101/gad.317792.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhaqueiro, A. C. F., van Jaarsveld, R. H., Ponsioen, B., Overmeer, R. M., Snippert, H. J. and Kops, G. J. P. L. (2018). Live imaging of cell division in 3D stem-cell organoid cultures. Methods Cell Biol. 145, 91-106. 10.1016/bs.mcb.2018.03.016 [DOI] [PubMed] [Google Scholar]
- Breslin, S. and O'Driscoll, L. (2016). The relevance of using 3D cell cultures, in addition to 2D monolayer cultures, when evaluating breast cancer drug sensitivity and resistance. Oncotarget 7, 45745-45756. 10.18632/oncotarget.9935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne, A. W., Arnesano, C., Harutyunyan, N., Khuu, T., Martinez, J. C., Pollack, H. A., Koos, D. S., Lee, T. C., Fraser, S. E., Moats, R. A.et al. (2017). Structural and functional characterization of human stem-cell-derived retinal organoids by live imaging. Invest. Ophthalmol. Vis. Sci. 58, 3311-3318. 10.1167/iovs.16-20796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhart, R. A., Baker, L. A. and Tiriac, H. (2018). Testing susceptibility of patient-derived organoid cultures to therapies: pharmacotyping. Methods Mol. Biol. 1787, 253-261. 10.1007/978-1-4939-7847-2_19 [DOI] [PubMed] [Google Scholar]
- Butrynski, J. E., D'Adamo, D. R., Hornick, J. L., Dal Cin, P., Antonescu, C. R., Jhanwar, S. C., Ladanyi, M., Capelletti, M., Rodig, S. J., Ramaiya, N.et al. (2010). Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N. Engl. J. Med. 363, 1727-1733. 10.1056/NEJMoa1007056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandrini, C., Schutgens, F., Oka, R., Margaritis, T., Candelli, T., Mathijsen, L., Ammerlaan, C., van Ineveld, R. L., Derakhshan, S., de Haan, S.et al. (2020). An organoid biobank for childhood kidney cancers that captures disease and tissue heterogeneity. Nat. Commun. 11, 1310. 10.1038/s41467-020-15155-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey, M. J. and Stewart, R. A. (2020). Pediatric cancer models in zebrafish. Trends Cancer 6, 407-418. 10.1016/j.trecan.2020.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceol, C. J., Houvras, Y., Jane-Valbuena, J., Bilodeau, S., Orlando, D. A., Battisti, V., Fritsch, L., Lin, W. M., Hollmann, T. J., Ferré, F.et al. (2011). The histone methyltransferase SETDB1 is recurrently amplified in melanoma and accelerates its onset. Nature 471, 513-517. 10.1038/nature09806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X., Pappo, A. and Dyer, M. A. (2015). Pediatric solid tumor genomics and developmental pliancy. Oncogene 34, 5207-5215. 10.1038/onc.2014.474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyavskaya, Y., Kent, B. and Sadler, K. C. (2016). Zebrafish discoveries in cancer epigenetics. Adv. Exp. Med. Biol. 916, 169-197. 10.1007/978-3-319-30654-4_8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chheda, M. G. and Gutmann, D. H. (2017). Using epigenetic reprogramming to treat pediatric brain cancer. Cancer Cell 31, 609-611. 10.1016/j.ccell.2017.04.008 [DOI] [PubMed] [Google Scholar]
- Cizmarova, M., Kostalova, L., Pribilincova, Z., Lasabova, Z., Hlavata, A., Kovacs, L. and Ilencikova, D. (2013). Rasopathies - dysmorphic syndromes with short stature and risk of malignancy. Endocr. Regul. 47, 217-222. 10.4149/endo_2013_04_217 [DOI] [PubMed] [Google Scholar]
- Dost, A. F. M., Moye, A. L., Vedaie, M., Tran, L. M., Fung, E., Heinze, D., Villacorta-Martin, C., Huang, J., Hekman, R., Kwan, J. H.et al. (2020). Organoids model transcriptional hallmarks of oncogenic KRAS activation in lung epithelial progenitor cells. Cell Stem Cell 27, 663-678.e8. 10.1016/j.stem.2020.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drilon, A., Laetsch, T. W., Kummar, S., DuBois, S. G., Lassen, U. N., Demetri, G. D., Nathenson, M., Doebele, R. C., Farago, A. F., Pappo, A. S.et al. (2018). Efficacy of Larotrectinib in TRK fusion–positive cancers in adults and children. N. Engl. J. Med. 378, 731-739. 10.1056/NEJMoa1714448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbin, M. and Monje, M. (2019). Developmental origins and emerging therapeutic opportunities for childhood cancer. Nat. Med. 25, 367-376. 10.1038/s41591-019-0383-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francies, H. E., Barthorpe, A., McLaren-Douglas, A., Barendt, W. J. and Garnett, M. J. (2019). Drug sensitivity assays of human cancer organoid cultures. Methods Mol. Biol. 1576, 339-351. 10.1007/7651_2016_10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii, H., Honoki, K., Tsujiuchi, T., Kido, A., Yoshitani, K. and Takakura, Y. (2009). Sphere-forming stem-like cell populations with drug resistance in human sarcoma cell lines. Int. J. Oncol. 34, 1381-1386. 10.3892/ijo_00000265 [DOI] [PubMed] [Google Scholar]
- Gajjar, A., Stewart, C. F., Ellison, D. W., Kaste, S., Kun, L. E., Packer, R. J., Goldman, S., Chintagumpala, M., Wallace, D., Takebe, N.et al. (2013). Phase I study of vismodegib in children with recurrent or refractory medulloblastoma: a pediatric brain tumor consortium study. Clin. Cancer Res. 19, 6305-6312. 10.1158/1078-0432.CCR-13-1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjini, E., Mansour, M. R., Sander, J. D., Moritz, N., Nguyen, A. T., Kesarsing, M., Gans, E., He, S., Chen, S., Ko, M.et al. (2015). A zebrafish model of myelodysplastic syndrome produced through tet2 genomic editing. Mol. Cell Biol. 35, 789-804. 10.1128/MCB.00971-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gojo, J., Englinger, B., Jiang, L., Hübner, J. M., Shaw, M. L., Hack, O. A., Madlener, S., Kirchhofer, D., Liu, I., Pyrdol, J.et al. (2020). Single-cell RNA-seq reveals cellular hierarchies and impaired developmental trajectories in pediatric ependymoma. Cancer Cell 38, 44-59.e9. 10.1016/j.ccell.2020.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobner, S. N., Worst, B. C., Weischenfeldt, J., Buchhalter, I., Kleinheinz, K., Rudneva, V. A., Johann, P. D., Balasubramanian, G. P., Segura-Wang, M., Brabetz, S.et al. (2018). The landscape of genomic alterations across childhood cancers. Nature 555, 321-327. 10.1038/nature25480 [DOI] [PubMed] [Google Scholar]
- Hackett, C. S., Hodgson, J. G., Law, M. E., Fridlyand, J., Osoegawa, K., de Jong, P. J., Nowak, N. J., Pinkel, D., Albertson, D. G., Jain, A.et al. (2003). Genome-wide array CGH analysis of murine neuroblastoma reveals distinct genomic aberrations which parallel those in human tumors. Cancer Res. 63, 5266-5273. [PubMed] [Google Scholar]
- Hadjadj, D., Deshmukh, S. and Jabado, N. (2020). Entering the era of precision medicine in pediatric oncology. Nat. Med. 26, 1684-1685. 10.1038/s41591-020-1119-6 [DOI] [PubMed] [Google Scholar]
- Han, M. and Sternberg, P. W. (1990). let-60, a gene that specifies cell fates during C. elegans vulval induction, encodes a ras protein. Cell 63, 921-931. 10.1016/0092-8674(90)90495-Z [DOI] [PubMed] [Google Scholar]
- Hazim, A. and Prasad, V. (2018). A pooled analysis of published, basket trials in cancer medicine. Eur. J. Cancer 101, 244-250. 10.1016/j.ejca.2018.06.035 [DOI] [PubMed] [Google Scholar]
- Hermans, E. and Hulleman, E. (2020). Patient-derived orthotopic xenograft models of pediatric brain tumors: in a mature phase or still in its infancy? Front. Oncol. 9, 1418. 10.3389/fonc.2019.01418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovestadt, V., Smith, K. S., Bihannic, L., Filbin, M. G., Shaw, M. L., Baumgartner, A., DeWitt, J. C., Groves, A., Mayr, L., Weisman, H. R.et al. (2019). Resolving medulloblastoma cellular architecture by single-cell genomics. Nature 572, 74-79. 10.1038/s41586-019-1434-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, M., Tailor, J., Zhen, Q., Gillmor, A. H., Miller, M. L., Weishaupt, H., Chen, J., Zheng, T., Nash, E. K., McHenry, L. K.et al. (2019). Engineering genetic predisposition in human neuroepithelial stem cells recapitulates medulloblastoma tumorigenesis. Cell Stem Cell 25, 433-446.e7. 10.1016/j.stem.2019.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huether, R., Dong, L., Chen, X., Wu, G., Parker, M., Wei, L., Ma, J., Edmonson, M. N., Hedlund, E. K., Rusch, M. C.et al. (2014). The landscape of somatic mutations in epigenetic regulators across 1,000 paediatric cancer genomes. Nat. Commun. 5, 3630. 10.1038/ncomms4630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, J. W., Desterke, C., Féraud, O., Richard, S., Ferlicot, S., Verkarre, V., Patard, J. J., Loisel-Duwattez, J., Foudi, A., Griscelli, F.et al. (2019). iPSC-Derived embryoid bodies as models of c-met-mutated hereditary papillary renal cell carcinoma. Int. J. Mol. Sci. 20, 4867. 10.3390/ijms20194867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura, Y., Mukohara, T., Shimono, Y., Funakoshi, Y., Chayahara, N., Toyoda, M., Kiyota, N., Takao, S., Kono, S., Nakatsura, T.et al. (2015). Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 33, 1837-1843. 10.3892/or.2015.3767 [DOI] [PubMed] [Google Scholar]
- Jabs, J., Zickgraf, F. M., Park, J., Wagner, S., Jiang, X., Jechow, K., Kleinheinz, K., Toprak, U. H., Schneider, M. A., Meister, M.et al. (2017). Screening drug effects in patient-derived cancer cells links organoid responses to genome alterations. Mol. Syst. Biol. 13, 955. 10.15252/msb.20177697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, L., Mercer, K., Greenbaum, D., Bronson, R. T., Crowley, D., Tuveson, D. A. and Jacks, T. (2001). Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature 410, 1111-1116. 10.1038/35074129 [DOI] [PubMed] [Google Scholar]
- Johnston, W. T., Erdmann, F., Newton, R., Steliarova-Foucher, E., Schüz, J. and Roman, E. (2020). Childhood cancer: estimating regional and global incidence. Cancer Epidemiol. 101662. 10.1016/j.canep.2019.101662 [DOI] [PubMed] [Google Scholar]
- Kaufman, C. K., Mosimann, C., Fan, Z. P., Yang, S., Thomas, A. J., Ablain, J., Tan, J. L., Fogley, R. D., van Rooijen, E., Hagedorn, E. J.et al. (2016). A zebrafish melanoma model reveals emergence of neural crest identity during melanoma initiation. Science 351, aad2197. 10.1126/science.aad2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, C., Arenkiel, B. R., Coffin, C. M., El-Bardeesy, N., DePinho, R. A. and Capecchi, M. R. (2004). Alveolar rhabdomyosarcomas in conditional Pax3:Fkhr mice: cooperativity of Ink4a/ARF and Trp53 loss of function. Genes Dev. 18, 2614-2626. 10.1101/gad.1244004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall, G. C., Watson, S., Xu, L., LaVigne, C. A., Murchison, W., Rakheja, D., Skapek, S. X., Tirode, F., Delattre, O. and Amatruda, J. F. (2018). PAX3-FOXO1 transgenic zebrafish models identify HES3 as a mediator of rhabdomyosarcoma tumorigenesis. eLife 7, e33800. 10.7554/eLife.33800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohnken, R., Porcu, P. and Mishra, A. (2017). Overview of the use of murine models in leukemia and lymphoma research. Front. Oncol. 7, 22. 10.3389/fonc.2017.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laetsch, T. W., DuBois, S. G., Mascarenhas, L., Turpin, B., Federman, N., Albert, C. M., Nagasubramanian, R., Davis, J. L., Rudzinski, E., Feraco, A. M.et al. (2018). Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 19, 705-714. 10.1016/S1470-2045(18)30119-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenau, D. M., Traver, D., Ferrando, A. A., Kutok, J. L., Aster, J. C., Kanki, J. P., Lin, S., Prochownik, E., Trede, N. S., Zon, L. I.et al. (2003). Myc-induced T cell leukemia in transgenic zebrafish. Science 299, 887-890. 10.1126/science.1080280 [DOI] [PubMed] [Google Scholar]
- Lee, R. C., Feinbaum, R. L. and Ambros, V. (1993). The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843-854. 10.1016/0092-8674(93)90529-Y [DOI] [PubMed] [Google Scholar]
- Leong, W. Z., Tan, S. H., Ngoc, P. C. T., Amanda, S., Yam, A. W. Y., Liau, W.-S., Gong, Z., Lawton, L. N., Tenen, D. G. and Sanda, T. (2017). ARID5B as a critical downstream target of the TAL1 complex that activates the oncogenic transcriptional program and promotes T-cell leukemogenesis. Genes Dev. 31, 2343-2360. 10.1101/gad.302646.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson, S. and Cagan, R. L. (2016). Drosophila cancer models identify functional differences between ret fusions. Cell Rep. 16, 3052-3061. 10.1016/j.celrep.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, D., March, M. E., Gutierrez-Uzquiza, A., Kao, C., Seiler, C., Pinto, E., Matsuoka, L. S., Battig, M. R., Bhoj, E. J., Wenger, T. L.et al. (2019). ARAF recurrent mutation causes central conducting lymphatic anomaly treatable with a MEK inhibitor. Nat. Med. 25, 1116-1122. 10.1038/s41591-019-0479-2 [DOI] [PubMed] [Google Scholar]
- Linabery, A. M. and Ross, J. A. (2008). Trends in childhood cancer incidence in the U.S. (1992-2004). Cancer 112, 416-432. 10.1002/cncr.23169 [DOI] [PubMed] [Google Scholar]
- Liu, H., Dowdle, J. A., Khurshid, S., Sullivan, N. J., Bertos, N., Rambani, K., Mair, M., Daniel, P., Wheeler, E., Tang, X.et al. (2017). Discovery of stromal regulatory networks that suppress ras-sensitized epithelial cell proliferation. Dev. Cell 41, 392-407.e6. 10.1016/j.devcel.2017.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour, M. R., He, S., Li, Z., Lobbardi, R., Abraham, B. J., Hug, C., Rahman, S., Leon, T. E., Kuang, Y.-Y., Zimmerman, M. W.et al. (2018). JDP2: An oncogenic bZIP transcription factor in T cell acute lymphoblastic leukemia. J. Exp. Med. 215, 1929-1945. 10.1084/jem.20170484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino, S. and Gilbertson, R. J. (2021). Harnessing brain development to understand brain tumours. Development 148, dev193342. 10.1242/dev.193342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquart, J., Chen, E. Y. and Prasad, V. (2018). Estimation of the percentage of US patients with cancer who benefit from genome-driven oncology. JAMA Oncol. 4, 1093-1098. 10.1001/jamaoncol.2018.1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurange, C. (2020). Temporal patterning in neural progenitors: from Drosophila development to childhood cancers. Dis. Model Mech. 13, dmm044883. 10.1242/dmm.044883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer, M. and Mione, M. (2016). The toolbox for conditional zebrafish cancer models. Adv. Exp. Med. Biol. 916, 21-59. 10.1007/978-3-319-30654-4_2 [DOI] [PubMed] [Google Scholar]
- Meuwissen, R., Linn, S. C., Linnoila, R. I., Zevenhoven, J., Mooi, W. J. and Berns, A. (2003). Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell 4, 181-189. 10.1016/S1535-6108(03)00220-4 [DOI] [PubMed] [Google Scholar]
- Milagre, C., Dhomen, N., Geyer, F. C., Hayward, R., Lambros, M., Reis-Filho, J. S. and Marais, R. (2010). A mouse model of melanoma driven by oncogenic KRAS. Cancer Res. 70, 5549-5557. 10.1158/0008-5472.CAN-09-4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modzelewska, K., Boer, E. F., Mosbruger, T. L., Picard, D., Anderson, D., Miles, R. R., Kroll, M., Oslund, W., Pysher, T. J., Schiffman, J. D.et al. (2016). MEK inhibitors reverse growth of embryonal brain tumors derived from oligoneural precursor cells. Cell Rep. 17, 1255-1264. 10.1016/j.celrep.2016.09.081 [DOI] [PubMed] [Google Scholar]
- Morinello, E., Pignatello, M., Villabruna, L., Goelzer, P. and Bürgin, H. (2014). Embryofetal development study of vismodegib, a hedgehog pathway inhibitor, in rats. Birth Defects Res. B Dev. Reprod. Toxicol. 101, 135-143. 10.1002/bdrb.21093 [DOI] [PubMed] [Google Scholar]
- Mudbhary, R., Hoshida, Y., Chernyavskaya, Y., Jacob, V., Villanueva, A., Fiel, M. I., Chen, X., Kojima, K., Thung, S., Bronson, R. T.et al. (2014). UHRF1 overexpression drives DNA hypomethylation and hepatocellular carcinoma. Cancer Cell 25, 196-209. 10.1016/j.ccr.2014.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musah-Eroje, A. and Watson, S. (2019). A novel 3D in vitro model of glioblastoma reveals resistance to temozolomide which was potentiated by hypoxia. J. Neurooncol 142, 231-240. 10.1007/s11060-019-03107-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetrou, E. P. (2016). Patient-derived induced pluripotent stem cells in cancer research and precision oncology. Nat. Med. 22, 1392-1401. 10.1038/nm.4238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton, E. E., Widlund, H. R., Kutok, J. L., Kopani, K. R., Amatruda, J. F., Murphey, R. D., Berghmans, S., Mayhall, E. A., Traver, D., Fletcher, C. D. M.et al. (2005). BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr. Biol. 15, 249-254. 10.1016/j.cub.2005.01.031 [DOI] [PubMed] [Google Scholar]
- Pierpont, T. M., Lyndaker, A. M., Anderson, C. M., Jin, Q., Moore, E. S., Roden, J. L., Braxton, A., Bagepalli, L., Kataria, N., Hu, H. Z.et al. (2017). Chemotherapy-induced depletion of OCT4-positive cancer stem cells in a mouse model of malignant testicular cancer. Cell Rep. 21, 1896-1909. 10.1016/j.celrep.2017.10.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo, M. L., Arnoni, M. V., Riggio, M., Wargon, V., Lanari, C. and Novaro, V. (2010). Responsiveness to PI3K and MEK inhibitors in breast cancer. Use of a 3D culture system to study pathways related to hormone independence in mice. PLoS ONE 5, e10786. 10.1371/journal.pone.0010786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read, R. D., Cavenee, W. K., Furnari, F. B. and Thomas, J. B. (2009). A drosophila model for EGFR-Ras and PI3K-dependent human glioma. PLoS Genet. 5, e1000374. 10.1371/journal.pgen.1000374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saengwimol, D., Rojanaporn, D., Chaitankar, V., Chittavanich, P., Aroonroch, R., Boontawon, T., Thammachote, W., Jinawath, N., Hongeng, S. and Kaewkhaw, R. (2018). A three-dimensional organoid model recapitulates tumorigenic aspects and drug responses of advanced human retinoblastoma. Sci. Rep. 8, 15664. 10.1038/s41598-018-34037-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltsman, J. A., Hammond, W. J., Narayan, N. J. C., Requena, D., Gehart, H., Lalazar, G., LaQuaglia, M. P., Clevers, H. and Simon, S. (2020). A human organoid model of aggressive hepatoblastoma for disease modeling and drug testing. Cancers 12, 2668. 10.3390/cancers12092668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotting, P. J., Walker, D. A. and Perilongo, G. (2005). Childhood solid tumours: a developmental disorder. Nat. Rev. Cancer 5, 481-488. 10.1038/nrc1633 [DOI] [PubMed] [Google Scholar]
- Simon, M. A., Bowtell, D. D. L., Dodson, G. S., Laverty, T. R. and Rubin, G. M. (1991). Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell 67, 701-716. 10.1016/0092-8674(91)90065-7 [DOI] [PubMed] [Google Scholar]
- Srivastava, V., Huycke, T. R., Phong, K. T. and Gartner, Z. J. (2020). Organoid models for mammary gland dynamics and breast cancer. Curr. Opin. Cell Biol. 66, 51-58. 10.1016/j.ceb.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, R. A., Lee, J.-S., Lachnit, M., Look, A. T., Kanki, J. P. and Henion, P. D. (2010). Studying peripheral sympathetic nervous system development and neuroblastoma in zebrafish. Methods Cell Biol. 100, 127-152. 10.1016/B978-0-12-384892-5.00005-0 [DOI] [PubMed] [Google Scholar]
- Storer, N. Y., White, R. M., Uong, A., Price, E., Nielsen, G. P., Langenau, D. M. and Zon, L. I. (2013). Zebrafish rhabdomyosarcoma reflects the developmental stage of oncogene expression during myogenesis. Development 140, 3040-3050. 10.1242/dev.087858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh, E., Stratton, K. L., Leisenring, W. M., Nathan, P. C., Ford, J. S., Freyer, D. R., McNeer, J. L., Stock, W., Stovall, M., Krull, K. R.et al. (2020). Late mortality and chronic health conditions in long-term survivors of early-adolescent and young adult cancers: a retrospective cohort analysis from the childhood cancer survivor study. Lancet Oncol. 21, 421-435. 10.1016/S1470-2045(19)30800-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenente, I. M., Hayes, M. N., Ignatius, M. S., McCarthy, K., Yohe, M., Sindiri, S., Gryder, B., Oliveira, M. L., Ramakrishnan, A., Tang, Q.et al. (2017). Myogenic regulatory transcription factors regulate growth in rhabdomyosarcoma. eLife 6, e19214. 10.7554/eLife.19214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbach, A., Yermalovich, A., Zhang, J., Spina, C. S., Zhu, H., Perez-Atayde, A. R., Shukrun, R., Charlton, J., Sebire, N., Mifsud, W.et al. (2014). Lin28 sustains early renal progenitors and induces Wilms tumor. Genes Dev. 28, 971-982. 10.1101/gad.237149.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoeven, D., Truong, T. N. L., Naji, A., Thapa, S., Hancock, J. F. and van der Hoeven, R. (2020). Identification of EGFR and RAS Inhibitors using Caenorhabditis elegans. J. Vis. Exp. 10.3791/61788 [DOI] [PubMed] [Google Scholar]
- Vidal, M., Wells, S., Ryan, A. and Cagan, R. (2005). ZD6474 suppresses oncogenic RET isoforms in a Drosophila model for type 2 multiple endocrine neoplasia syndromes and papillary thyroid carcinoma. Cancer Res. 65, 3538-3541. 10.1158/0008-5472.CAN-04-4561 [DOI] [PubMed] [Google Scholar]
- White, R. M., Cech, J., Ratanasirintrawoot, S., Lin, C. Y., Rahl, P. B., Burke, C. J., Langdon, E., Tomlinson, M. L., Mosher, J., Kaufman, C.et al. (2011). DHODH modulates transcriptional elongation in the neural crest and melanoma. Nature 471, 518-522. 10.1038/nature09882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman, B., Ha, I. and Ruvkun, G. (1993). Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75, 855-862. 10.1016/0092-8674(93)90530-4 [DOI] [PubMed] [Google Scholar]
- Wong, M., Mayoh, C., Lau, L. M. S., Khuong-Quang, D.-A., Pinese, M., Kumar, A., Barahona, P., Wilkie, E. E., Sullivan, P., Bowen-James, R.et al. (2020). Whole genome, transcriptome and methylome profiling enhances actionable target discovery in high-risk pediatric cancer. Nat. Med. 26, 1742-1753. 10.1038/s41591-020-1072-4 [DOI] [PubMed] [Google Scholar]
- Xie, X., Ross, J. L., Cowell, J. K. and Teng, Y. (2015). The promise of zebrafish as a chemical screening tool in cancer therapy. Future Med. Chem. 7, 1395-1405. 10.4155/fmc.15.73 [DOI] [PubMed] [Google Scholar]
- Yen, J., White, R. M. and Stemple, D. L. (2014). Zebrafish models of cancer: progress and future challenges. Curr. Opin. Genet. Dev. 24, 38-45. 10.1016/j.gde.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, J. and Horvitz, H. R. (2004). A first insight into the molecular mechanisms of apoptosis. Cell 116, S53-S56. 10.1016/S0092-8674(04)00028-5 [DOI] [PubMed] [Google Scholar]
- Zarzosa, P., Navarro, N., Giralt, I., Molist, C., Almazan-Moga, A., Vidal, I., Soriano, A., Segura, M. F., Hladun, R., Villanueva, A.et al. (2017). Patient-derived xenografts for childhood solid tumors: a valuable tool to test new drugs and personalize treatments. Clin. Transl. Oncol. 19, 44-50. 10.1007/s12094-016-1557-2 [DOI] [PubMed] [Google Scholar]
- Zhu, S., Lee, J.-S., Guo, F., Shin, J., Perez-Atayde, A. R., Kutok, J. L., Rodig, S. J., Neuberg, D. S., Helman, D., Feng, H.et al. (2012). Activated ALK collaborates with MYCN in neuroblastoma pathogenesis. Cancer Cell 21, 362-373. 10.1016/j.ccr.2012.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawel, R. H., Allen, C., Wechsler-Reya, R., Scott, M. P. and Raffel, C. (2000). Evidence that haploinsufficiency of Ptch leads to medulloblastoma in mice. Genes Chromosomes Cancer 28, 77-81. [DOI] [PubMed] [Google Scholar]
- Zwergel, C., Romanelli, A., Stazi, G., Besharat, Z. M., Catanzaro, G., Tafani, M., Valente, S. and Mai, A. (2018). Application of small epigenetic modulators in pediatric medulloblastoma. Front. Pediatr. 6, 370. 10.3389/fped.2018.00370 [DOI] [PMC free article] [PubMed] [Google Scholar]