Abstract
Objective:
To establish a biorepository of clinical, metabolomic, and microbiome samples from adolescents with obesity as they undergo lifestyle modification.
Methods:
We enrolled 223 adolescents aged 10–18 years with Body Mass Index ≥ 95th percentile, along with 71 healthy weight participants. We collected clinical data, fasting serum, and fecal samples at repeated intervals over 6 months. Here we present our study design, data collection methods, and interim analysis, including targeted serum metabolite measurements and fecal 16S rRNA gene amplicon sequencing among adolescents with obesity (n=27) and healthy weight controls (n=27).
Results:
Adolescents with obesity have higher serum alanine aminotransferase, C-reactive protein, and glycated hemoglobin, and lower high-density lipoprotein cholesterol when compared with healthy weight controls. Metabolomics revealed differences in branched chain amino acid-related metabolites. We also observed differential abundance of specific microbial taxa and lower species diversity among adolescents with obesity when compared with the healthy weight group.
Conclusions:
The Duke Pediatric Metabolism and Microbiome Study biorepository is available as a shared resource. Early findings suggest evidence of a metabolic signature of obesity unique to adolescents, along with confirmation of previously reported findings describing metabolic and microbiome markers of obesity.
Clinical Trial Registration:
Biorepository: NCT02959034
Observational Trial: NCT03139877
Keywords: Pediatric, obesity, adolescents, microbiome, metabolism
INTRODUCTION
Despite sustained research into effective treatment strategies, pediatric obesity remains at epidemic levels, and strongly predicts adult obesity, metabolic, and cardiovascular disease (1). Currently, one in three children in the US are classified as overweight or obese, with the highest prevalence among low-income and non-white youth (2). The US Preventative Service Task Force recommends that pediatric providers screen all children aged 6–18 years for obesity annually, using the Centers for Disease Control and Prevention sex- and age-specific Body Mass Index (BMI) curves. Additionally, children with BMI at or above the 95th percentile should be referred to a comprehensive behavioral intervention of medium to high intensity, defined as achieving ≥26 hours of contact over six months (3). However, existing data show that recommended interventions for pediatric obesity result in a heterogeneous response, with most participants showing non-significant BMI reduction (4), with little understanding of the underlying predictive factors for treatment success.
Strong evidence suggests that the intestinal microbiome and its products influence chronic diseases such as obesity, insulin resistance, and heart disease (5). Observational studies in adult humans and stool transplantation experiments in animal models have found novel connections among obesity, insulin resistance, metabolic pathways, inflammation, and intestinal microbiota that may differ between individuals (6). Circulating and tissue metabolites serve as diagnostic, prognostic, and therapeutic targets for metabolic disease, and can be affected by microbiome composition (7). Thus, analyses of the microbiome and metabolome have the potential to provide insights into the host-microbiome pathways and inform personalized and effective treatments.
Adolescents provide a unique opportunity to garner deeper insights into the obesity-associated microbiome and related metabolic pathways during the transition from childhood to adulthood. First, adolescents with severe obesity mimic adult metabolic and cardiovascular risk phenotypes (8, 9). However, unlike adults, adolescents are at early stages of disease, have fewer and less severe comorbid conditions, tend to be treatment-naïve, and have a disease risk that may be reversible. Notably, an Israeli study following approximately 3000 youth from adolescence through mid-life found that if teens with obesity were able to acheieve a normal BMI by adulthood, their risk of diabetes development was reversed so that it was comparable to those adults who never had obesity (10). Second, adolescence is a unique window of development and its associated microbiome and metabolic signatures in health and disease are understudied compared to those signatures in adults (11). Similarly, while significant evidence supports the association between decreased gut microbiome diversity and obesity in adults, this has not been confirmed in a larger and racially diverse group of adolescents (12), even though obesity and its complications are more prevalent in racial and ethnic minorities (13). Third, the prospective longitudinal design of adolescents enrolled in a weight management program will provide insights into metabolomic and microbiome changes in response to treatment and the early biomarkers of improved health.
The Pediatric Obesity Microbiome and Metabolism Study (POMMS) is an NIH-funded prospective observational study with two main objectives: 1) to identify serum metabolic biomarkers or stool microbial signatures in adolescents for obesity severity, obesity-related comorbid disease, and the later development of obesity-related disease; and 2) to identify serum or stool biomarkers that predict response to treatment, precede response to treatment, or that may help to personalize treatment to optimize individual outcomes. We describe herein the POMMS methods and procedures, as well as early findings from an interim analysis of 27 participants with obesity and 27 healthy weight controls.
METHODS
Screening and enrollment
Research coordinators screened patients referred by their primary care physician to the Duke Healthy Lifestyles clinic in Durham, NC for obesity treatment. Study inclusion criteria were age 10–18 years, Sexual Maturity Rating ≥ 2, age- and gender-specific BMI ≥ 95th percentile, ability to communicate in English or Spanish, and plans to stay in weight management for ≥ 6 months. Patients were excluded for recent weight loss of ≥ 5%, antibiotic use in the prior month or anticipated in the next 6 months, immunodeficiency, prior transplantation, Type 1 diabetes mellitus, inborn errors of metabolism, endogenous obesity (i.e. hyperthyroidism, Cushing syndrome, Mc4R mutation), drug-induced obesity (steroids or anti-psychotics), a significant medical or mental health condition, or pregnancy. Healthy weight controls were recruited from the same community health system, aged 10–18 years with a Sexual Maturity Rating ≥ 2, and age- and gender-specific BMI ≥5th and <85th percentile and had the same exclusion criteria as the group with obesity.
Study design
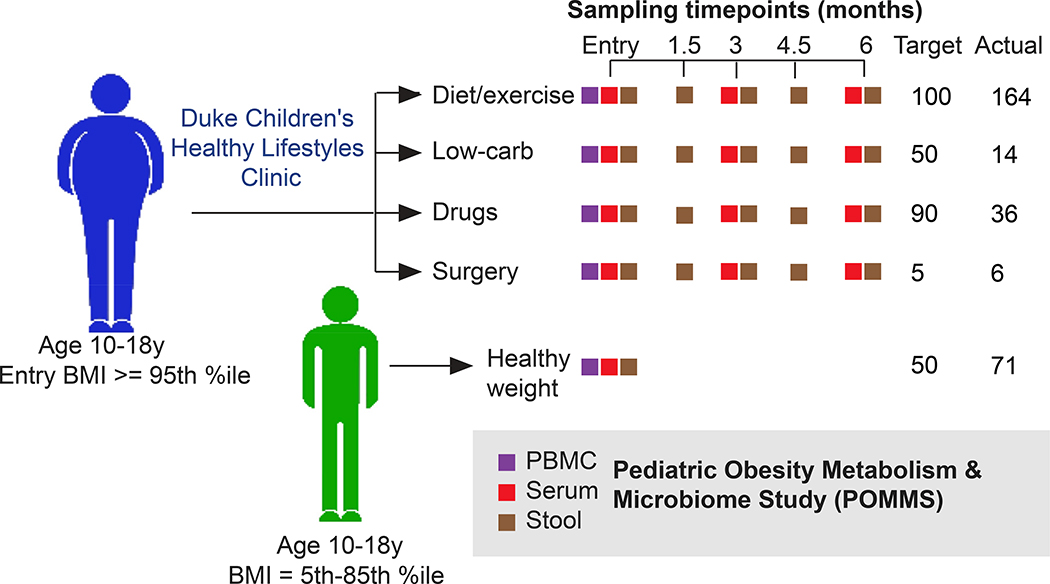
The Duke Institutional Review Board approved the study design and procedures. Eligible participants had parental consent and child assent for the observational study and long-term storage and future use of clinical data and biospecimens. Figure 1 highlights the prospective observational treatment group assignment and study assessments.
Figure 1. POMMS study design.
Adolescents between 10 and 18 with either BMI >5th %ile to < 85th percentile (healthy weight controls, or HWC) or BMI equal to or over the 95th percentile (adolescents with obesity, or OB) were recruited to join the study. Target numbers of recruits for each intervention are indicated, along with actual recruitment numbers. Age matched HWC recruits had clinical measurements of health as well as serum, stool, and PBMCs collected at a single timepoint, while OB recruits were evaluated and offered a treatment arm that was clinically relevant depending on the individual. OB recruits then had clinical measurements of health, blood, PBMC and stool samples taken at baseline, 3 months, and 6 months (or termination) of the study. Stool samples were also collected from OB patients at 1.5 and 4.5 months after the first visit. All samples are stored in replicates in the POMMS Biorepository managed within the Duke Children’s Biobank to be made available for research purposes.
Groups
All participants in the intervention (obese or OB) group are patients in the Healthy Lifestyles program, which includes visits to a multi-disciplinary clinic and membership in a community-based fitness program (described below and in Supplemental Methods). All participants in the comparison (healthy weight or HWC) group received a single measurement at baseline only and no additional intervention.
The Healthy Lifestyles clinic is a tertiary-care weight management program that meets current recommendations for family-based child and adolescent obesity treatment (14). The Healthy Lifestyles clinic sees 800 new patients aged 2–18 annually, and more than 15,000 children have received care to date with demonstrated efficacy (15). Dietary counseling promoted a reduction in sugar-sweetened beverages and processed foods, and encouraged regular consumption of non-starchy vegetables, whole grains, and healthy fats. Participants in the low-carbohydrate group had a recommended limit of <30 grams per day on overall carbohydrate intake. All dietary counseling followed a patient-centered approach and was tailored to individual preferences and family resources. Advanced treatment options included weight-loss medications (e.g. metformin, phentermine, lisdexamphetamine, lorcaserin, and topriamate), and surgery (Roux-en-Y Gastric Bypass or Laparascopic Vertical Sleeve Gastrectomy). The clinical provider determined weight loss medications and dose adjustments based on medication profile, side effect tolerability and treatment response. Shared decision-making between parent, teen, and healthcare provider guided choice of lifestyle, diet, medication or surgical treatment, and treatment decisions were made based on child’s age, obesity severity, comorbidities, and family preference. In addition to clinical care, participants were enrolled in a community-based fitness program, delivered through a local Parks and Recreation Department (see Supplemental Methods for details).
Measures
The primary study outcome was change in BMI at 3 and 6 months and was determined through standardized measures of body weight and height measured at baseline, 3, and 6 months using a digital scale and stadiometer. A large number of children referred to the Healthy Lifestyles clinic have a BMI significantly higher than the 95th percentile. Therefore, as recommended by Flegal et al. (16) we assess and report raw BMI, BMI percentile, and child relative BMI, expressed as a standard deviation score (zBMI) and as a percent of the BMI value at the 95th percentile (%95th BMI) to evaluate and track obesity.
Secondary endpoints were collected at baseline, 3, and 6 months. Cardiorespiratory fitness was assessed by heart rate at the completion of the YMCA submaximal bench-stepping test (17). Blood pressure was measured with a calibrated auscultatory sphygmomanometer in the seated position with an appropriately sized cuff using standard methods (18). Fasting blood samples were obtained for measuring cardiometabolic biomarkers, including lipids, glucose, and transaminases, as described in Supplemental Methods. Body fat percentage was measured using calibrated bioelectrical impedance. Parent BMI was directly measured from parent height and weight using calibrated stadiometers.
Survey data (available in English and Spanish) included weight-specific quality of life, measured using the validated “Sizing Me Up” child obesity-specific instrument at baseline and 6 months (19). Household income, transportation access, parent stress, food insecurity, home food environment, and parental expectations for treatment were also collected at baseline (Supplemental Methods). Factors likely to influence the intestinal microbiome, including birth weight, breastfeeding history, pets in the home, recent or distant antibiotic use, concurrent medication use, and other self-reported measures were recorded.
Serum:
Following a fast of 8–14 hours, blood was collected in anti-coagulant containing tiger top (Becton Dickenson (BD) #367988) and green top (BD #367874) tubes at baseline, 3 and 6 months. Blood samples were processed within 2 hours of collection according to established protocols (20). Serum samples were then snap-frozen in liquid nitrogen and stored at −80°C in the Duke Children’s Biobank.
Stool:
Patients collected stool samples and immediately stored the collection container (Fisher Scientific #02–544-208) in their home freezer. Patients then returned the sample or scheduled a home pickup within 18 hours of stooling. Stool was transported frozen and transferred to a −80°C freezer for long-term storage. Stool samples for analysis were processed by thawing on ice in a biological safety cabinet and opened to the atmosphere for a maximum of 10 minutes while samples were portioned into approximately 2g aliquots. Aliquots were then stored at −80°C in the Duke Children’s Biobank.
Serum and stool samples were organized into a POMMS Biorepository that is available for researchers, with complete clinical data, and fecal and serum longitudinal sample sets from 50 HWC and 140 OB and baseline data and samples from over 200 adolescents with obesity. More information on the repository, sample requests, protocols, recruitment and study completion incentivizing strategies can be found at https://sites.duke.edu/pomms/.
Interim analysis
An interim group of baseline samples in the POMMS Biorepository was randomly selected for an initial analysis of the association between metabolites and microbiome characteristics and adolescent obesity. These interim analysis samples were taken at the study enrollment visit from early study entrants with complete sample sets. They included 27 healthy weight controls (HWC) and 27 adolescents with obesity (OB) matched for puberty status, sex, and ethnicity. Study numbers are in alignmnent with sample size in similar relevant studies (39, 43) and were not defined by power calculations.
Targeted serum metabolite analysis
Sample preparation and data acquisition:
74 amino acids, acylcarnitines and organic acids were analyzed by targeted mass spectrometry methods employing stable isotope dilution for analyte quantitation. Amino acids and acylcarnitine were measured by flow injection tandem mass spectrometry using sample preparation methods described previously (21). The data were acquired using a Waters TQD mass spectrometer equipped with AcquityTM UPLC system and controlled by MassLynx 4.1 operating system (Waters, Milford, MA). Organic acids were quantified as described (22), employing Trace Ultra GC coupled to ISQ MS operating under Xcalibur 2.2 (Thermo Fisher Scientific, Austin, TX).
Statistical analysis:
Levels of all targeted metabolites were log-transformed and tested for association with obesity using a linear model, adjusting for baseline BMI, age, sex, race, and ethnicity and correcting for false discovery rate (23). The complete set of metabolite data can be found as online supporting information.
16S rRNA gene sequencing
DNA purification, amplification, and sequencing:
The Duke Microbiome Shared Resource staff extracted bacterial DNA from stool sample aliquots (~120 mg stool per sample) using a MagAttract PowerSoil DNA EP Kit (Qiagen, 27100–4-EP) and a Retsch MM400 plate shaker. Resulting DNA concentration was assessed using a Qubit dsDNA HS assay kit (ThermoFisher, Q32854). Bacterial community composition in isolated DNA samples was characterized by amplification of the V4 variable region of the 16S rRNA gene by PCR using the barcoded primers 515 forward and 806 reverse following the Earth Microbiome Project protocol (http://www.earthmicrobiome.org/). Equimolar 16S rRNA PCR products from all samples were pooled before sequencing by the Duke Sequencing and Genomic Technologies shared resource on an Illumina MiSeq instrument configured for 250 base-pair paired-end sequencing runs. Raw sequence data can be found at the NIH Sequence Read Archive (submission in progress). Resulting data were processed using the Qiime2 bioinformatics platform (24). Data denoising was conducted with the DADA2 Bioconductor (25) package in the R statistical programming environment. The RAxML algorithm (26) was used to generate the phylogenetic tree with a midpoint root. We used the Silva 132 classifier for taxonomic classification using variable region 4 (99% identity) (27).
Statistical analysis:
Statistical analysis of taxonomic data, including relative abundance plots, alpha and beta diversity comparisons, and PCoA plots, were performed using the R packages Phyloseq v1.26.1, vegan 2.5.5 (28, 29), Phylogenetic Isometric Log-Ratio (PhILR) transformation (30), and LefSe (31). Relevant R code can be found at github (https://github.com/POMMS/methods_baseline_insights).
RESULTS
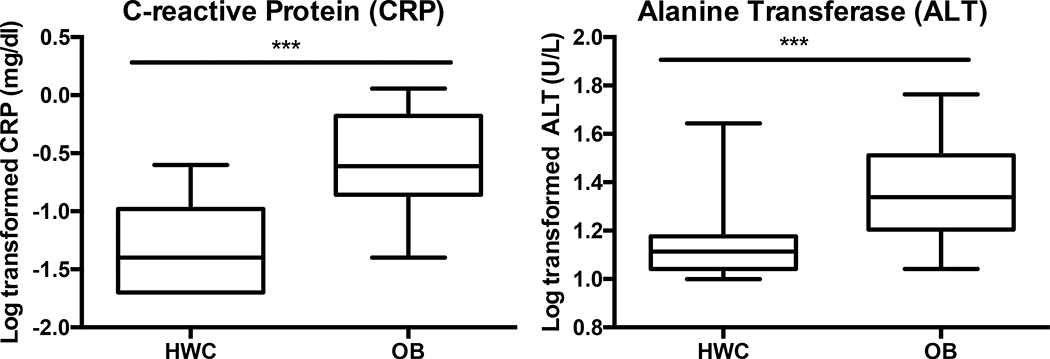
We enrolled 294 adolescents (223 adolescents with obesity, or OB, and 71 healthy weight control adolescents, or HWC) (Table 1) between December 2016 and July 2019. Approximately half (49.8% OB v. 54.9% HWC) of all participants identified as Black, while 19.3% of the OB group and 18.3% of the HWC group identified as Hispanic. The demographic characteristics of the interim analysis sample (27 OB and 27 HWC study participants) are shown in Table 2. Traditional laboratory parameters, including triglycerides, insulin, total cholesterol, blood pressure, and high- and low-density lipoprotein cholesterol (HDL and LDL, respectively) were analyzed and compared between the healthy weight cohort (HWC) and adolescents with obesity (OB) (All parameters collected are described in Supplemental Methods). Triglycerides, fasting glucose, LDL, and total cholesterol were not significantly different between HWC and OB after adjusting for false discovery rates (FDR) (Table 2). We did find higher levels of homeostatic model of insulin resistance (HOMA-IR (32), HDL, glycated hemoglobin (Hb1Ac), C-reactive protein (CRP, Fig. 2), and alanine amino transferase (ALT, Fig. 2) in the OB as compared to the HWC control group, and lower levels of HDL cholesterol (Table 2).
Table 1.
Description of all study participants in the Healthy Weight Control (HWC) and Adolescents with Obesity (OB) cohort.
| Characteristics | HWC | OB |
|---|---|---|
| Participants | 71 | 223 |
| Sex (F/M/NA#) | 38/32/1 (54/45/1%) | 132/88/0 (60/40/0%) |
| Intervention: | ||
| Lifestyle | 167 (74.9%) | |
| Low Carb | 14 (6.3%) | |
| Pharmaceuticals | 36 (16.1%) | |
| Surgery | 6 (2.7%) | |
| Race: | ||
| Black | 39 (54.9%) | 111 (49.8%) |
| White | 26 (36.6%) | 88 (39.5%) |
| Black/White | 2 (2.8%) | 13 (5.8%) |
| Other | 2 (2.8%) | 8 (3.6%) |
| Missing Data | 2 (2.8%) | 0 |
| Ethnicity: | ||
| Hispanic | 13 (18.3%) | 43 (19.3%) |
| Non-Hispanic | 57 (80.3%) | 177 (79.4%) |
| Other | 1 (1.4%) | 0 |
| Average %95th BMI* | 75 ± 7 | 134 ± 32 |
| Average age at entry in years | 15.0 ± 1.8 | 13.5 ± 2.3 |
| Tanner stage 2/3/4/5/NA# | 11/12/23/24/1 (15.5/16.9/32.4/33.8/1.4%) | 61/38/58/59/4 (27.4/17.0/26.0/26.5/1.8%) |
Percentages of total are included in parentheses for numbers describing Race and Ethnicity. Numbers represent actual number of participants or mean result ± standard deviation.
NA, Not Available
percent of the BMI value at the 95th percentile
Table 2.
Demographics and clinical lab results for study participants included in the interim analysis.
| HWC | OB | FDR adj. P value | |
|---|---|---|---|
| Participants | 27 | 27 | |
| Sex (F/M/NA) | 15/12/0 (55/45/0%) | 15/12/0 (55/45/0%) | |
| Race: | |||
| Black | 13 (48.1%) | 12 (44.4%) | |
| White | 12 (44.4%) | 11 (40.1%) | |
| Black/White | 0 | 2 (7.4%) | |
| Other | 2 (7.4%) | 2 (7.4%) | |
| Average %95th BMI* | 75.6 ± 5.1 | 137.8 ± 48.7 | 0.05−11 |
| Average Age at Entry | 15.0 ± 1.7 | 12.6 ± 2.4 | 0.06 |
| Clinical Labs: | |||
| Triglycerides (mg/dl) (mg(mg/dl( | 84.7 ± 35.6 | 113.7 ± 75.2 | 0.14 |
| HDL (mg/dl) | 54.1 ± 14.1 | 45.6 ± 11.1 | 0.02 |
| LDL (mg/dl) | 84.9 ± 30.5 | 100.1 ± 25.3 | 0.06 |
| Glucose (mg/dl) | 84.6 ± 9.9 | 88.9 ± 9.1 | 0.12 |
| HOMA-IR | 2.4 ± 2.8 | 4.0 ± 3.1 | 0.01 |
| HbA1c (IU/dl) | 5.2 ± 0.3 | 5.4 ± 0.4 | 0.01 |
| Cholesterol (mg/dl) | 155.9 ± 38.7 | 163.9 ± 29.0 | 0.41 |
Numbers respresent actual number of participants or mean result ± standard deviation.
percent of the BMI value at the 95th percentile.
NA, Not Available; HDL, high density lipoprotein; LDL, low density lipoprotein; HbA1c, percent acylated (glycated) hemoglobin; HOMA-IR, homeostatic model of insulin resistance; Mg/dl, milligrams/deciliter of blood; IU, International Units
Figure 2. Clinical lab measurements of liver function and inflammation were significantly higher in OB v HWC.
Log transformed serum values of alanine transferase (ALT) and C-reactive protein (CRP) were compared at baseline between healthy weight control (HWC) and adolescents with obesity (OB). Error bars indicate standard deviation of the mean. *** = P< 0.001 following correction for multiple testing. See Methods for details on statistical analysis.
Metabolic Profiling
After FDR adjustment for multiple comparisons, no metabolites were significantly different between the OB and HWC groups. However, several metabolites related to branched chain amino acid (BCAA) metabolism were nominally significantly different (Table 3). Specifically, the BCAA valine was higher in OB vs. HWC, while the ketoacid products of BCAA catabolism, α-ketoisocaproate (KIC) and α-keto-β-methyl valerate (KMV), were lower in OB vs HWC. Serum glycine was also lower in OB when compared with HWC. We also observed higher serum glycerol and serum insulin in the OB vs. HWC group.
Table 3.
Log transformed nominally significant serum metabolites ± standard deviation
| Targeted metabolites | HWC | OB | P Value | FDR adj. P value |
|---|---|---|---|---|
| Glycerol | −0.60 ± 0.41 | −0.11 ± 0.26 | 0.003 | 0.08 |
| Insulin | 2.29 ± 0.37 | 2.63 ± 0.28 | 0.003 | 0.08 |
| Glycine | 2.48 ± 0.09 | 2.34 ± 0.05 | 0.001 | 0.08 |
| KIC | 1.52 ± 0.09 | 1.45 ±0.08 | 0.005 | 0.09 |
| KMV | 1.31 ± 0.09 | 1.23 ± 0.08 | 0.011 | 0.13 |
| Valine | 2.33 ± 0.07 | 2.36 ± 0.07 | 0.024 | 0.20 |
Microbiome Profiling
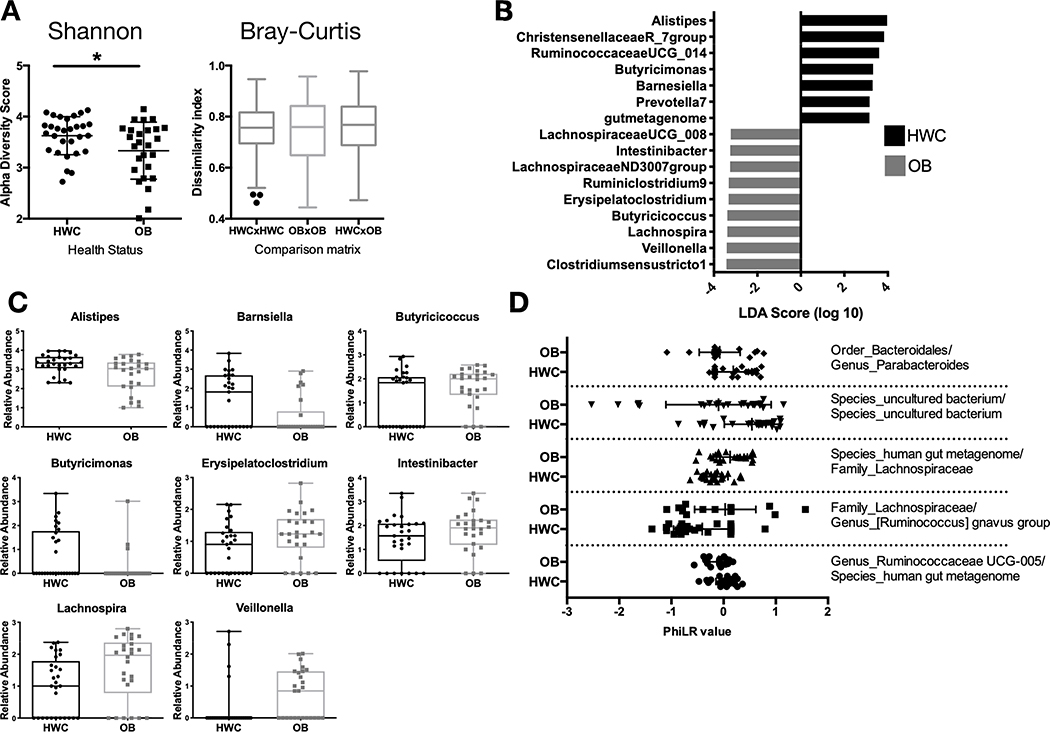
Stool sample 16S rRNA gene sequence analysis revealed significant differences in measurements of alpha and beta diversity between OB and HWC groups (Fig. 3A, and Supplemental Fig. S1). However, we did not detect significant differences in alpha or beta diversity based on sex nor on age at sampling, suggesting that obesity as defined by %95th BMI was the main driver of the observed difference in microbiome diversity between groups (data not shown).
Figure 3. A subset of adolescents with obesity have reduced gut microbial diversity and differences in abundance of specific microbial taxa when compared to their healthy weight counterparts.
(A) Shannon α-diversity and Bray Curtis β-diversity metrics between HWC and OB stool 16S rRNA gene sequences based on identified amplicon sequence variants. (B-C) The Linear discriminant analysis (LDA) effect size tool (LEfSe) was applied to taxonomic data resulting from 16S rRNA gene amplicon sequencing. (B) Relative abundance of taxa with that vary significantly by cohort are displayed. (C) Count data of each taxon with a significantly different abundance between HWC and OB cohorts in the LDA analysis. Each symbol represents abundance in an individual sample. (D) PhiLR Taxa identified by 16S rRNA gene sequencing that were present at a read count of > 3 across at least 10% of patient samples were retained for analysis. PhiLR results are displayed as log relative abundances of opposing clades in a phylogenetic tree and significant balances were chosen on the basis of a simple predictive model. A PhILR value greater than zero indicates greater abundance of the numerator component relative to the denominator component and a negative value indicates the reverse. These balances were most effective at predicting patient membership in either the HWC or OB cohort. See Methods section for details on statistical analyses and access to relevant R code.
There were several bacterial taxa (here defined as a group of one or more populations of an organism or organisms that forms the lowest phylogenenetic unit identifiable by 16S rRNA gene sequencing) detected as differentially abundant in OB vs. HWC groups following analysis using the linear discriminant analysis (LDA) effect size tool (LEfSe) (Fig. 3C–D). Members of the Christensenellaceae and Ruminococcae UCG_14 families, along with an Alistipes species, were more likely to be found in samples from HWC. In contrast, two Lachnospiraceae families and a Lachnospira species were more likely to be associated with OB samples. To identify microbiome community configurations that might be associated with obesity, we examined the phylogenetic relatedness of microbes in each group using 2 different methods. In the first, we applied the Phylogenetic Isometric Log-Ratio (PhILR) transformation (Fig. 3D) to select taxonomic balances which were most effective at predicting patient membership in either the HWC or OB group. The abundance of Eubacterium brachy genera relative to the abundance of the Family XIII AD3011 group was higher in the HWC group as compared with OB. Conversely, the abundance ratio of Lachnospiraceae family members to Ruminococcus gnavus was substantially lower in the OB group as compared to HWC (Fig. 3D).
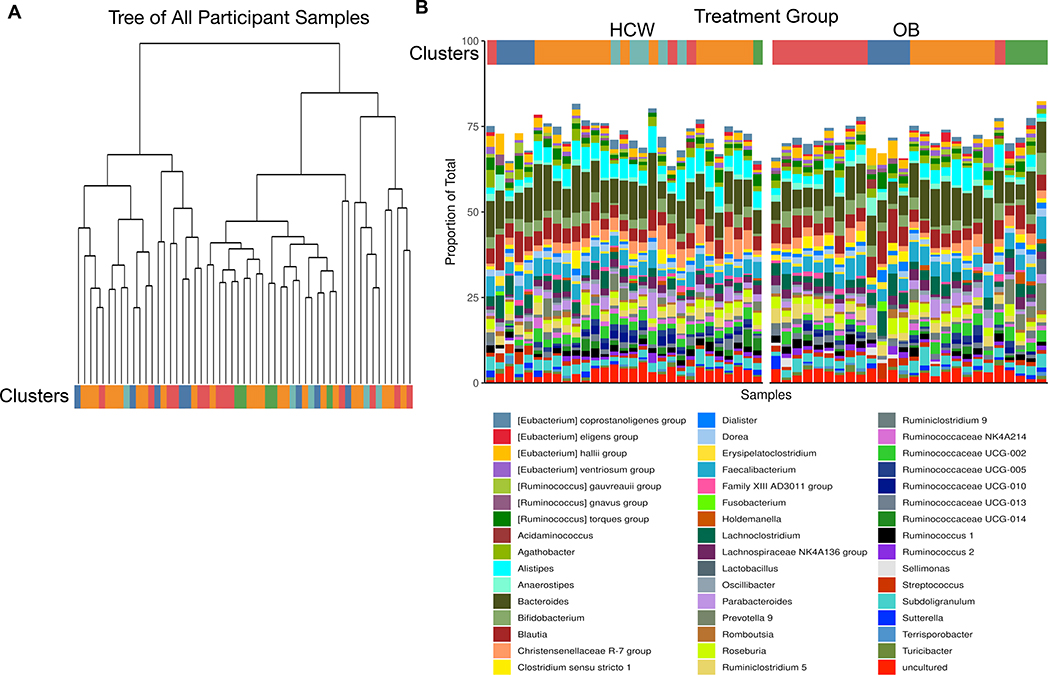
In the second phylogenetic analysis, species or lowest taxa relatedness was determined without regard to weight status (Fig. 4A). The resulting five phylogenetic clusters were then applied to weight status, revealing groups of related organisms that were then color coded and plotted. We found that some clusters were more likely to appear in the HWC cohort than the OB cohort, and vice versa (Fig. 4B). Taxa associated with the red cluster were more likely to be found in the OB group when compared with HWC, and this cluster appears to be driven by the decreased abundance of Bacteroides family members. Increased abundance of several Ruminococcae and specific members of the Provetellaceae was also more likely to be associated with OB (Fig. 4B and Supplemental Fig. S2).
Figure 4: Phylogenetic clustering of gut microbial taxa suggest that genetic relationships predict presence or absence of taxa in OB cohorts.
(A) Taxa identified by 16S rRNA gene amplicon sequencing were subjected to unsupervised clustering into 5 taxonomic clusters and grouped by color and treatment status. Each terminal node of the tree represents results from an individual fecal sample. (B) Clusters were assigned a color and each fecal sample result was grouped by cluster in (A) and then divided by HWC or OB status in order of participant ID number. See methods for links to relevant R code and raw data.
DISCUSSION
In this interim analysis, we demonstrated proof of concept for 1) collection of clinical data, plasma and stool samples from a racially and ethnically diverse sample of adolescents with and without obesity, and 2) for conducting the analyses that may help to untangle the associations between microbiome and metabolome that contribute to health and disease. While representative of a small subset of the POMMS cohort, we observed interesting findings that both support and contradict existing adult data, and deserve further investigation.
Clinical lab measures are not always predictive of obesity
We did not detect significant differences in some laboratory screening measures for obesity comorbidities between participants with or without obesity. This is not surprising, in part because of the small sample size, but also because larger studies have demonstrated that comorbidities in children present most clearly only at the extremes of excess weight (33). Future analysis of the full POMMS cohort will be appropriately powered to confirm or refute this finding. However, prior research has established that obesity in children is a risk factor for cardiometabolic disease, even in the absence of clinical lab abnormalities, as demonstrated by the 2-hour glucose tolerance test, carotid intima-medial thickening, and pulse wave velocity (34). Future studies are needed to define the correlation between these candidate plasma metabolites and “clinically silent” cardiometabolic disease, both for early identification of risk and to monitor response to treatment. The inflammatory marker CRP and the liver function enzyme ALT were significantly higher in adolescents with obesity when compared to the healthy weight cohort. In a recent meta-analysis of pediatric weight-loss trials, a reduction in CRP and ALT scores was significantly correlated with BMI reduction over time (35).
Targeted serum metabolite analysis confirms BCAA metabolites are potential biomarkers of obesity in adolescence.
Analysis of targeted serum metabolite levels between HWC and OB groups revealed that the branched-chain amino acid (BCAA) valine trended higher in the OB group, while ketoacids (BCKA) KMV and KIC were trended lower in OB, when compared to the HWC cohorts (Table 3). In addition, glycine levels trended lower in our OB adolescents, consistent with studies in adults with obesity and rodent models (36). These metabolites were analyzed in our adolescents as they are emerging biomarkers for obesity and future development of type 2 diabetes in adults (11). In fact, BCAA are typically elevated in adults with obesity and are among the strongest baseline predictors of improvements in insulin sensitivity with weight loss (37) as well as later life development of type 2 diabetes in adults (11). However, unlike in adult obesity in humans and animals models, where BCAA and BCKA are both elevated, BCKA were lower in OB subjects from our adolescent cohort. This may be further evidence of an existing hypothesis that there is “metabolic plasticity” in youth protecting against insulin resistance by preventing mitochondrial overload of BCAA metabolites (11). Low plasma glycine levels may also be predictive of type 2 diabetes development, and plasma glycine levels increase with an improvement in insulin sensitivity (38).
Evidence suggests that levels of some metabolites such as circulating BCAA and fatty acid oxidation intermediates correlate with insulin resistance, and higher HOMA-IR scores can be influenced by sex (39). In a recent metabolomic survey of overweight and healthy weight children, BCAA and related metabolites trended as significantly different in obesity until the data were corrected for age, sex, and sexual maturity stage (40). However, in other more extensive studies, serum or plasma BCAA were significantly positively associated with obesity over a broad age range of children and young adults (11). Indeed, in these larger studies with a combined sample size of over 1,000 HWC and OB children, the principal components with the most significant contribution to risk factors of later cardiometabolic disease such as HOMA-IR and BMI were composed of the BCAA and their breakdown products. Thus, our data agree with these prior studies in that elevated BCAA levels were associated with obesity in adolescents, and may provide the rationale for more research into their viability as biomarkers of later life disease development (41). A study examining potential biomarkers of later life disease progression characterized metabolites associated with obesity in 396 girls (mean age, 11.2 years at baseline) over a 7 year period. The authors found that that serum levels of the BCAA leucine and isoleucine at baseline positively correlated with triglyceride levels at age 18, regardless of the triglyceride level at baseline (11).
Elevated glycerol levels in our OB cohort (Table 3) suggest that adolescents with OB have increased levels of lipolysis. Lipolysis is elevated in adult obesity, resulting in higher circulating glycerol levels in adults, and in a recent study, partial inhibition of hormone-sensitive lipase in mice and human adipocytes led to improved insulin resistance and glucose uptake (42).
Adolescents with obesity may have specific microbiome characteristics
We found significant differences in fecal microbial community composition and presence or absence of specific taxa when comparing 16S rRNA gene sequences between our HWC and OB interim cohort. While the majority of studies comparing gut microbes between OB and HWC have examined samples from adults, there are a few examples of studies that compare microbial consortia between obese and HWC children. A recent cross sectional study of 36 HWC and 42 Italian children with obesity aged 6–16 years (43) revealed that several individual microbes identified to their lowest taxonomic identifier correlated significantly either positively or negatively with an increasing BMI z-score (BMI normalized to age and sex), including Bacteroides vulgatus (R = −0.4321), Faecalibacterium praustnitzii (0.3508), and Bacteroides stercoris (−0.3252). A second study that examined differences between 81 HW, 29 overweight, and 80 OB children aged 9–11 years also found that Faeacalibacterium sp. was elevated with obesity, along with members of the Lachnospiraceae family (44), as we also demonstrated here. Further, our study includes a racially and ethnically diverse group of adolescents, which we hope will help eludicate the factors contributing to a higher prevalence of pediatric obesity in nonwhite populations (45). A recent analysis of microbiome data from obese subjects found that while most variation in interindivual microbiomes is unexplained, race and ethnicity are drivers of microbiome diversity in humans, and low representiation of samples from diverse subjects might help explain varied outcomes when describing the microbiome phenotype in obesity (13).
We still lack evidence to connect the adolescent obese microbiome with metabolic changes associated with youth onset of obesity and the related metabolome. The data reported here on the interim set of participants was not sufficiently powered to make these connections, however we will have the ability to analyze microbial contributions to the metabolome in the samples extracted from our full POMMS cohort of nearly 300 individuals. Overall, our preliminary data suggest that adolescents with obesity inhabit a transitional space in which some indicators of health appear normal. In contrast, others may be strongly predictive of later life disease development. Future analyses of microbiota in human and animal studies will help us define targets for new adolescent medicine therapeutics to intervene in what may otherwise be an unhealthy trajectory into adulthood.
CONCLUSION AND IMPACT
Early life obesity increases the lifetime risk for cardiovascular disease and other obesity-related diseases. The POMMS project will move the field of obesity research forward by providing unique developmental insights into the mechanisms of obesity and chronic disease. Importantly, the POMMS cohort of racially and ethnically-diverse adolescents provides a critical opportunity to address the significant disparities in obesity prevalence and outcomes for people of color. Our interim analysis suggests that microbiome and metabolome research in adolescent obesity mirrors some results in adult obesity, but also offers new targets for further research, specifically surrounding BCAA-related metabolism. This clinical-basic science collaboration has resulted in a diverse sample set that will be used to better understand metabolomic-microbiome profiles in the development of obesity.
Supplementary Material
What is already known about this subject?
The intestinal microbiome plays an important role in adult obesity and the regulation of metabolism. Although it is well-established that obesity has its roots in childhood, very little is known about the role of the microbiome in pediatric obesity and how it changes during adolescence.
What are the new findings in your manuscript?
This manuscript provides details of a new shared biorepository including clinical data, stool samples and plasma samples from a diverse cohort of 223 adolescents with obesity followed longitudinally over 6 months during a weight management intervention, as well as 71 adolescents with healthy weight as a comparison group.
Interim analyses suggest that adolescents with obesity have microbiome signatures and metabolite profiles similar to adults, however, key differences in microbial communities and metabolic by-products are identified.
How might your results change the direction of research or focus of clinical practice?
The POMMS biorepository will be available for investigators to use in future research, to elucidate the underlying mechanisms of obesity and related chronic health conditions.
Preliminary data reveal metabolite profiles that provide further evidence that adolescence may be a window of metabolic plasticity and disease reversibility, as specific metabolites trend in a direction opposite to what we expect to see in adults with obesity.
Microbiome and metabolomic signatures suggest potential biomarkers that may serve as prognostic or predictive factors in disease remission, or targets for future therapeutics.
Acknowledgements:
We would like to thank the study participants and their families for their involvement in this study. All raw data sequencing data are available via the NIH Sequence Read Archive (SRA) (submission in progress). Information on how to request access to the clinical sample biorepository and deidentified participant data can be found at https://sites.duke.edu/pomms.
Sources of Funding
National Institutes of Health R24-DK110492
American Heart Association 17SFRN33670990
Footnotes
Disclosures
Svati H. Shah, Christopher B. Newgard: Unlicensed patent on a related finding.
Raphael H. Valdivia is a founder at Bloom Sciences, (San Diego, CA).
All other authors have declared no conflicts of interest.
REFERENCES
- 1.Pollock BD, Stuchlik P, Harville EW, Mills KT, Tang W, Chen W, et al. Life course trajectories of cardiovascular risk: Impact on atherosclerotic and metabolic indicators. Atherosclerosis. 2019;280:21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics. 2018;141(3):e20173459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grossman DC, Bibbins-Domingo K, Curry SJ, Barry MJ, Davidson KW, Doubeni CA, et al. Screening for Obesity in Children and Adolescents: US Preventive Services Task Force Recommendation Statement. Jama. 2017;317(23):2417–26. Epub 2017/06/21. doi: 10.1001/jama.2017.6803. [DOI] [PubMed] [Google Scholar]
- 4.Ryder JR, Kaizer AM, Jenkins TM, Kelly AS, Inge TH, Shaibi GQ. Heterogeneity in Response to Treatment of Adolescents with Severe Obesity: The Need for Precision Obesity Medicine. Obesity. 2019;27(2):288–94. doi: 10.1002/oby.22369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cani PD, Hul MV, Lefort C, Depommier C, Rastelli M, Everard A. Microbial regulation of organismal energy homeostasis. Nature Metabolism. 2019;1(1):34–46. doi: 10.1038/s42255-018-0017-4. [DOI] [PubMed] [Google Scholar]
- 6.Maruvada P, Leone V, Kaplan LM, Chang EB. The Human Microbiome and Obesity: Moving beyond Associations. Cell Host Microbe. 2017;22(5):589–99. Epub 2017/11/10. doi: 10.1016/j.chom.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Koh A, Backhed F. From Association to Causality: the Role of the Gut Microbiota and Its Functional Products on Host Metabolism. Mol Cell. 2020. Epub 2020/04/03. doi: 10.1016/j.molcel.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Freedman DS, Ogden CL, Kit BK. Interrelationships between BMI, skinfold thicknesses, percent body fat, and cardiovascular disease risk factors among U.S. children and adolescents. BMC Pediatr. 2015;15:188. Epub 2015/11/20. doi: 10.1186/s12887-015-0493-6. PubMed PMID: 26582570; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhle S, Tong O, Woolcott C. Association between caesarean section and childhood obesity: a systematic review and meta‐analysis. Obesity Reviews. 2015;16(4):295–303. [DOI] [PubMed] [Google Scholar]
- 10.Twig G, Tirosh A, Leiba A, Levine H, Shor DB, Derazne E, et al. Body Mass Index at Age 17 and Diabetes Mortality in Midlife: A Nationwide Cohort of 2.3 Million Adolescents. Diabetes Care. 2016. Epub 2016/10/14. doi: 10.2337/dc16-1203. [DOI] [PubMed] [Google Scholar]
- 11.Balikcioglu PG, Newgard CB. Metabolomic Signatures and Metabolic Complications in Childhood Obesity. In: Freemark M, editor. Pediatric Obesity: Etiology, Pathogenesis and Treatment: Springer; 2018. [Google Scholar]
- 12.Castaner O, Goday A, Park Y-M, Lee S-H, Magkos F, Shiow S-ATE, et al. The Gut Microbiome Profile in Obesity: A Systematic Review. Int J Endocrinol. 2018;2018. doi: 10.1155/2018/4095789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanislawski MA, Dabelea D, Lange LA, Wagner BD, Lozupone CA. Gut microbiota phenotypes of obesity. npj Biofilms Microbiomes. 2019;5:18. Epub 2019/07/10. doi: 10.1038/s41522-019-0091-8. PubMed PMID: 31285833; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. PEDIATRICS. 2007;120 Suppl 4:S164–92. Epub 2007/12/18. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 15.Dolinsky DH, Armstrong SC, Walter EB, Kemper AR. The effectiveness of a primary care-based pediatric obesity program. Clinical pediatrics. 2012;51(4):345–53. Epub 2011/10/21. doi: 10.1177/0009922811425232. [DOI] [PubMed] [Google Scholar]
- 16.Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. The American journal of clinical nutrition. 2009;90(5):1314–20. Epub 2009/09/25. doi: 10.3945/ajcn.2009.28335. [DOI] [PubMed] [Google Scholar]
- 17.LA G. YMCA Fitness Testing and Assessment Manual, 4th ed. Human Kinetics. 2000;Champaign, IL. [Google Scholar]
- 18.Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128 Suppl 5:S213–56. Epub 2011/11/16. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeller MH, Modi AC. Development and initial validation of an obesity-specific quality-of-life measure for children: sizing me up. Obesity (Silver Spring). 2009;17(6):1171–7. Epub 2009/03/07. doi: 10.1038/oby.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuck MK, Chan DW, Chia D, Godwin AK, Grizzle WE, Krueger KE, et al. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res. 2009;8(1):113–7. Epub 2008/12/17. doi: 10.1021/pr800545q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrara CT, Wang P, Neto EC, Stevens RD, Bain JR, Wenner BR, et al. Genetic Networks of Liver Metabolism Revealed by Integration of Metabolic and Transcriptional Profiling. PLOS Genetics. 2008;4(3):e1000034. doi: 10.1371/journal.pgen.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen MV, Joseph JW, Ilkayeva O, Burgess S, Lu D, Ronnebaum SM, et al. Compensatory Responses to Pyruvate Carboxylase Suppression in Islet β-Cells PRESERVATION OF GLUCOSE-STIMULATED INSULIN SECRETION. J Biol Chem. 2006;281(31):22342–51. doi: 10.1074/jbc.M604350200. [DOI] [PubMed] [Google Scholar]
- 23.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological). 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 24.QIIME 2: Reproducible, interactive, scalable, and extensible microbiome data science. PeerJ. doi: 10.7287/peerj.preprints.27295v2 [DOI] [PubMed] [Google Scholar]
- 25.Huber W, Carey VJ, Gentleman R, Anders S, Carlson M, Carvalho BS, et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nature Methods. 2015;12(2):115–21. doi: 10.1038/nmeth.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stamatakis A RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–3. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41(D1):D590–D6. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oksanen J, Blanchet F, Kindt R, Legendre P, Minchin P, O’hara R, et al. Package ‘vegan’. Community ecology package, version 2. 2013. [Google Scholar]
- 29.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLOS ONE. 2013;8(4):e61217. Epub 2013/05/01. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverman JD, Washburne AD, Mukherjee S, David LA. A phylogenetic transform enhances analysis of compositional microbiota data. Elife. 2017;6. Epub 2017/02/16. doi: 10.7554/eLife.21887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segata N, Huttenhower C. Toward an efficient method of identifying core genes for evolutionary and functional microbial phylogenies. PLOS ONE. 2011;6(9):e24704. Epub 2011/09/21. doi: 10.1371/journal.pone.0024704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. Epub 1985/07/01. doi: 10.1007/bf00280883. [DOI] [PubMed] [Google Scholar]
- 33.Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults. New England Journal of Medicine. 2015;373(14):1307–17. doi: 10.1056/NEJMoa1502821. [DOI] [PubMed] [Google Scholar]
- 34.Mendizabal B, Urbina EM. Subclinical Atherosclerosis in Youth: Relation to Obesity, Insulin Resistance, and Polycystic Ovary Syndrome. The Journal of pediatrics. 2017;190:14–20. Epub 2017/07/18. doi: 10.1016/j.jpeds.2017.06.043. [DOI] [PubMed] [Google Scholar]
- 35.Matson RI, Perry R, Hunt LP, Chong AH, Beynon R, Hamilton-Shield J, et al. Change in obesity-related metabolic abnormalities associated with body mass index improvement through life-style intervention: A meta-regression. Pediatr Diabetes 2020;21(2):173–93. Epub 2019/12/11. doi: 10.1111/pedi.12955. [DOI] [PubMed] [Google Scholar]
- 36.White PJ, Lapworth AL, An J, Wang L, McGarrah RW, Stevens RD, et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol Metab 2016;5(7):538–51. Epub 2016/07/14. doi: 10.1016/j.molmet.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah SH, Crosslin DR, Haynes CS, Nelson S, Turer CB, Stevens RD, et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia. 2012;55(2):321–30. Epub 2011/11/09. doi: 10.1007/s00125-011-2356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adeva-Andany M, Souto-Adeva G, Ameneiros-Rodriguez E, Fernandez-Fernandez C, Donapetry-Garcia C, Dominguez-Montero A. Insulin resistance and glycine metabolism in humans. Amino Acids. 2018;50(1):11–27. Epub 2017/11/03. doi: 10.1007/s00726-017-2508-0. [DOI] [PubMed] [Google Scholar]
- 39.Newbern D, Gumus Balikcioglu P, Balikcioglu M, Bain J, Muehlbauer M, Stevens R, et al. Sex Differences in Biomarkers Associated With Insulin Resistance in Obese Adolescents: Metabolomic Profiling and Principal Components Analysis. J Clin Endocrinol Metab. 2014;99(12):4730–9. doi: 10.1210/jc.2014-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michaliszyn SF, Sjaarda LA, Mihalik SJ, Lee S, Bacha F, Chace DH, et al. Metabolomic Profiling of Amino Acids and β-Cell Function Relative to Insulin Sensitivity in Youth. The Journal of Clinical Endocrinology & Metabolism. 2012;97(11):E2119–E24. doi: 10.1210/jc.2012-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rangel-Huerta OD, Pastor-Villaescusa B, Gil A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics. 2019;15(6):93. Epub 2019/06/15. doi: 10.1007/s11306-019-1553-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Girousse A, Tavernier G, Valle C, Moro C, Mejhert N, Dinel A-L, et al. Partial Inhibition of Adipose Tissue Lipolysis Improves Glucose Metabolism and Insulin Sensitivity Without Alteration of Fat Mass. PLoS Biol. 2013;11(2):e1001485. doi: 10.1371/journal.pbio.1001485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riva A, Borgo F, Lassandro C, Verduci E, Morace G, Borghi E, et al. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in <span style=“font-variant:small-caps;”> F irmicutes populations. Environ Microbiol. 2017;19(1):95–105. doi: 10.1111/1462-2920.13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murugesan S, Ulloa-Martínez M, Martínez-Rojano H, Galván-Rodríguez FM, Miranda-Brito C, Romano MC, et al. Study of the diversity and short-chain fatty acids production by the bacterial community in overweight and obese Mexican children. Eur J Clin Microbiol Infect Dis. 2015;34(7):1337–46. doi: 10.1007/s10096-015-2355-4. [DOI] [PubMed] [Google Scholar]
- 45.Stovitz SD, Schwimmer JB, Martinez H, Story MT. Pediatric obesity: the unique issues in Latino-American male youth. American journal of preventive medicine. 2008;34(2):153–60. Epub 2008/01/19. doi: 10.1016/j.amepre.2007.09.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.