Abstract
Background
Schizophrenia (SCZ) is a neurodevelopmental disorder with a progressive, prolonged course. Early prevention for SCZ is promising, but overall lacks support from preclinical evidence. Previous studies have tested environmental enrichment (EE) in certain models of SCZ and discovered a broadly beneficial effect in preventing behavioral abnormalities relevant, yet not specific, to the disorder. Nonetheless, whether EE can prevent dopamine (DA) dysregulation, a hallmark of psychosis and SCZ, had not been tested
Methods
Using the methylazoxymethanol acetate (MAM) rat model of schizophrenia and saline-treated controls (SAL) we investigated the long-term electrophysiological effects of prepubertal (postnatal day 21–40) EE on DA neurons, pyramidal neurons in the ventral hippocampus (vHipp), and projection neurons in the basolateral amygdala (BLA). Anxiety-related behaviors in the elevated plus maze and locomotor responses to amphetamine were also analyzed.
Results
Prepubertal EE prevented the increased population activity of DA neurons and the associated increase in locomotor response to amphetamine. Prepubertal EE also prevented the hyperactivity in the vHipp, but did not prevent the hyperactivity in the BLA. Anxiety-like behaviors in MAM rats were not ameliorated by prepubertal exposure to EE.
Conclusions
20-day prepubertal EE is sufficient to prevent DA hyperresponsivity in the MAM model, measured by electrophysiological recordings and locomotor response to stimulant. This effect is potentially mediated by normalizing excessive firing in the vHipp without affecting anxiety-like behaviors and BLA firing. This study identified EE as a useful preventative approach which may protect against the pathophysiological development of SCZ.
Keywords: schizophrenia, environmental enrichment, dopamine, ventral tegmental area, ventral hippocampus, amygdala
Introduction
Schizophrenia (SCZ) has long been proposed to be a neurodevelopmental disorder (1, 2), and its etiology involves genetic predisposition (3) and environmental factors (4), which collectively interfere with brain development (5–7). As a core feature of SCZ, psychosis typically manifests relatively late in the clinical course, often preceded by an extended prodromal stage in adolescence (8, 9). Several meta-analyses have concluded that the risk of transition to psychosis can be reduced by active intervention during the prodromal phase, including antipsychotic medication, nutritional support, cognitive behavioral therapy, psychoeducation, and exercise (8, 10–12), although not all have proven effective upon replication. In addition to the plausible preventative efficacy against positive symptoms, recent studies also suggest that early intervention may also reduce the risks for the emergence of other symptom domains (13).
Whereas clinical evidence suggests that both early-life pharmacological and environmental interventions are potentially effective in preventing SCZ, the former is relatively disadvantageous due to ethical limitations, economical infeasibility, and potential side effects given that most individuals will never transition to psychosis (13, 14). Early exposure to environmental enrichment (EE) is a form of behavioral intervention that is broadly beneficial to a wide range of neuropsychiatric conditions, including SCZ (15–18). For example, Raine et al. reported that a 2-year environmental intervention at age 3–5 might protect against behavioral manifestation of SCZ in early adulthood (19). In terms of preclinical research, several studies suggested that early EE can prevent selective behavioral abnormalities relevant, but not specific, to SCZ, such as locomotor hyperactivity, social cognition, and sensorimotor gating in SCZ models involving drug treatment (20–22), genetic manipulation (23, 24), and lesion (25).
However, key preclinical evidence supporting the preventative efficacy of EE is still lacking, because beyond its beneficial effects on SCZ-relevant behavioral endpoints, whether EE can prevent other functional deficits of SCZ is largely unknown. A recent study attempted to address this knowledge gap by testing EE in methylazoxymethanol (MAM) GD17 model (26) of SCZ, in which Bator et al. (27) found that 7-day juvenile exposure to EE not only prevented SCZ-related behavioral deficits, but also the decrease in the expression of glutamate decarboxylase 67. Although this recent study extended the current scope of EE research by indicating EE’s efficacy against GABAergic pathologies implicated in the pathogenesis of SCZ (28), several key questions remain unanswered. In particular, whether EE can prevent dopamine (DA) dysfunction and hippocampal hyperactivity, core features of the pathophysiology of SCZ (5, 29), is still unknown (30).
Using the MAM model, the main goal of this study is to understand the long-term electrophysiological impacts of prepubertal EE on the regulation of the DA system of adult animals. Given that hippocampal hyperactivity and the associated DA dysregulation are robust features of SCZ (31), we also examined the impact of EE on neuronal firing in the ventral hippocampus (vHipp), an upstream regulator of the midbrain DA system (32, 33). Given the high comorbidity between anxiety and SCZ (34) and its implication in the pathogenesis and pathophysiology (35–38), we also determined whether prepubertal EE can ameliorate anxiety and the hyperactivity in the basolateral amygdala (BLA) in adult MAM rats (39).
Methods
Animals.
Timed pregnant Sprague–Dawley rats (Envigo) were obtained at gestational day (GD) 14. At GD17, pregnant rats were injected with 0.9% saline or MAM (20mg/kg, i.p.; MRI Global). Animals were housed in a temperature- (22°C) and humidity-controlled (47%) environment (12-hr light/dark cycle; lights on at 7 am) with ad libitum access to food and water. To avoid litter effects, individual experimental groups were formed by animals from at least 3 litters (range: 3–6) and counterbalanced across all experiments. Experiments were conducted according to the guidelines established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Experimental design.
At postnatal day (PD) 21, male offspring prenatally exposed to saline (SAL) or MAM were randomly assigned into a regular housing environment (RE) throughout the study or an enriched environment (EE) for 20 days until PD40. EE rats were returned to RE on PD41, and stayed in RE until the end of experiments. At PD65–71, rats were tested for performance in the elevated plus maze (EPM, PD65–66) and amphetamine-induced hyperlocomotion (AIH, PD69–71). Following a week of recovery (>PD77), animals were randomly assigned into in vivo single-unit recording experiments (Figure 1). Separate cohorts of rats were also enriched during adulthood (PD65–84), juvenility (PD21–30), and adolescence (PD31–40) to assess possible age-dependence of EE effects. Timeline is shown in Figure 3.
Figure 1. Schematic depiction of the postnatal environmental enrichment protocol for rats treated prenatally with either SAL or MAM at gestational day 17 (GD17).
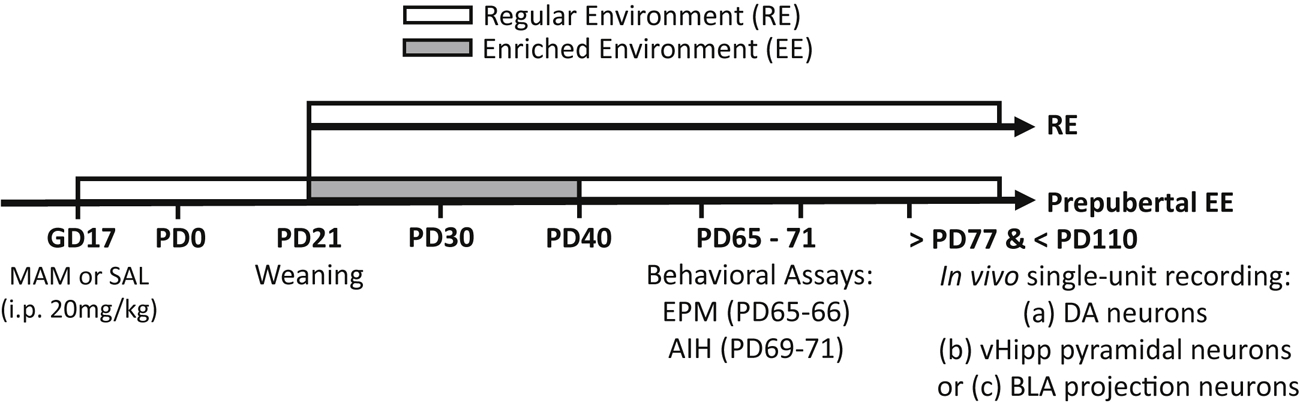
Animals were weaned at PD21 followed by rearing in RE or EE for 20-days. Behavioral assays were performed in PD65–71, and rats were then randomly assigned into three recording experiments.
Figure 3. The effects of 10-day prepubertal and 20-day adult environmental enrichment on DA hyperactivity in MAM rats.
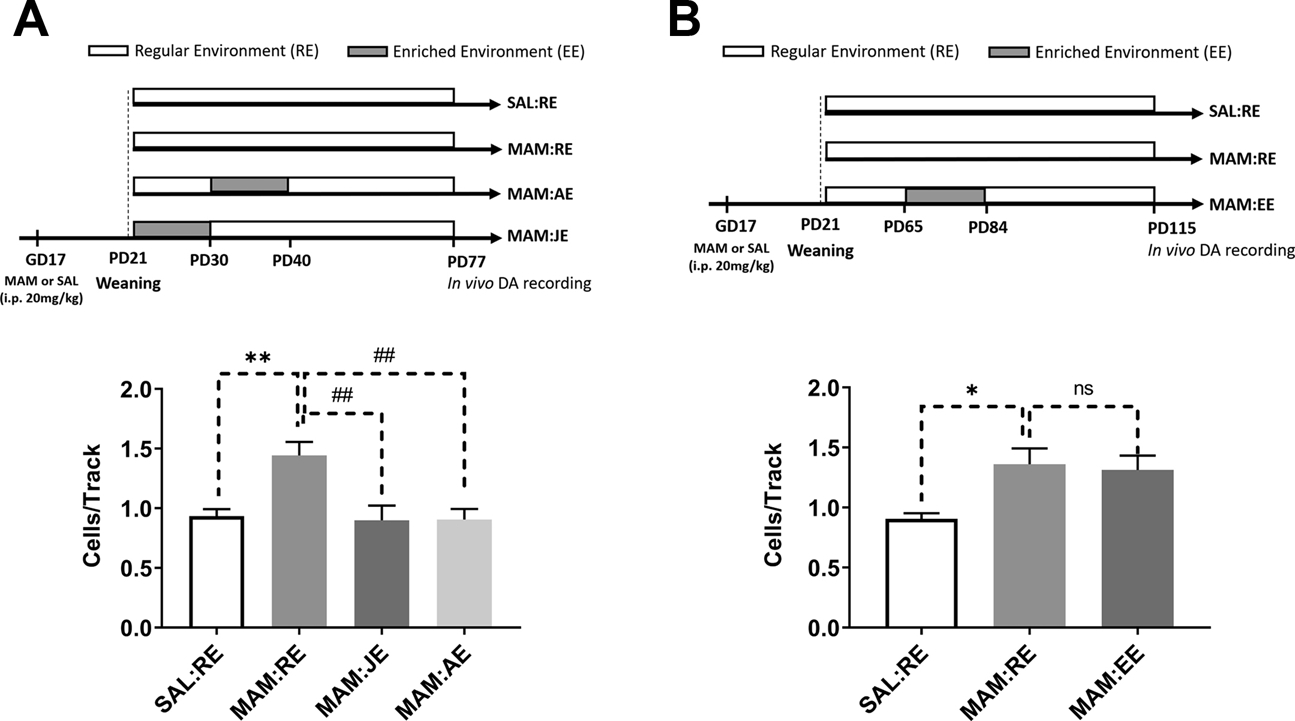
(A, top) A separate cohort of rats (n=10) was exposed to early EE during juvenility (JE, PD21–30) and adolescence (AE, PD31–40). (A, bottom) VTA DA neurons population activity was significantly affected by developmental conditions (one-way ANOVA, F(3,36)=7.110, p<0.001), such that DA hyperactivity induced by prenatal MAM treatment (Tukey’s post hoc test, MAM:RE vs. SAL:RE, p<0.01) was prevented by both JE (p<0.01) and AE (p<0.01). (B, top) To validate the age-dependent effect of EE, a separate cohort of rats (n=4–6) was enriched during adulthood (PD65–84). (B, bottom) A main effect was detected for developmental conditions [F(2,12)=5.203, p<0.05], and MAM:RE rats displayed increased population activity (Tukey’s post hoc test, p<0.05), which was not prevented by adult EE (p>0.05, MAM:RE vs. MAM:EE). Data are presented as mean ± SEM. *p<0.05; **p or ##p<0.01; ns: not significant (p>0.05). * indicated MAM:RE vs. SAL:RE; # indicates effects of enrichment groups.
Environmental conditions.
Animals in RE were pair- or triple-housed in typical rodent cages (L38cm×W26cm×H18cm). The EE paradigm was modified from previously established protocols (40, 41). Briefly, rats were group-housed in five large plastic tubs (L93cm×W53.3cm×H49.5cm) containing objects including toys and tunnels of different shapes, running wheels, chewing materials, and plastic ladders attached to a metal platform. The enrichment boxes also contained increased nesting materials and an increased amount of beddings, which were changed twice a week. The orientation and the color of the objects were altered three times a week.
Behavioral Experiments.
Experiments were conducted during the dark cycle (7:00pm – 7:00am).
Elevated plus maze (EPM).
Animals were first habituated to the testing room for 90 minutes. Rats were introduced to the central area facing an open arm, and their movements were recorded for 5 min. Time spent in open arms and the number of open arm entries were measured to index anxiety-like behaviors.
Amphetamine-induced hyperlocomotion.
Locomotor activity was assessed in open-field chambers (Coulbourn Instruments) with ambulatory movement in the x-y plane recorded for 30 minutes. Rats were next injected with d-amphetamine sulfate (0.75 mg/kg, i.p.; Sigma), followed by the recording of locomotion for 60 minutes. Data were computed in 5-min bins for time-course analysis, and the total distance traveled post-amphetamine was calculated.
In vivo electrophysiology.
Rats were anesthetized with chloral hydrate (400 mg/kg, i.p.; Sigma) and mounted on a stereotaxic frame (Kopf; Tujunga, CA). See Supplement for details.
Glass electrodes were lowered through six to nine vertical tracks in a predetermined pattern within VTA (A/P: −5.4mm from bregma, M/L: ±0.6mm, and D/V: 6.5–9.0mm from brain surface (42, 43)). Spontaneously active DA neurons were identified based on well-established criteria (Figure S1A–B) (44).
Spontaneously active neurons were recorded in the vHipp by four to six tracks (A/P: −5.5 to −5.9mm, M/L: ±4.6–4.8mm from Bregma, D/V: −5.5 to −8.5mm from brain surface). Putative pyramidal neurons were identified based on published criteria of firing rate <2 Hz (45–47), which was also validated in this study. Fast-spiking vHipp interneurons were defined by average firing rate >4 Hz (47) and spike duration <1.0ms, from peak to valley. For BLA recording, single-unit activities were recorded by four to six vertical tracks (A/P: −3.0 to −3.4 mm, M/L: ±4.6–4.8 mm from Bregma, D/V: −6.0mm to −8.5 mm from brain surface). Putative projection neurons were identified based on previously published criteria (see Supplement).
Statistical analysis.
All results are presented as mean ± SEM, and statistical calculations were performed using GraphPad Prism 8. Data were tested for normal distribution (Kolmogorov-Smirnov normality test) and subsequently analyzed with two-way ANOVA with treatment (SAL or MAM) and environment (RE or EE) as main factors, or two-way repeated measure ANOVA (in AIH experiment) with the developmental condition (SAL:RE, SAL:EE, MAM:RE, or MAM:EE) as a main factor and time/bin as a repeated measure factor. Tukey’s post hoc test was used when a significant main effect or interaction was detected. Differences were considered significant at p<0.05.
Results:
Prepubertal EE prevented the electrophysiological and behavioral phenotypes of DA dysregulation in adult MAM rats.
Consistent with previous reports (48, 49), SAL:RE (n=8, 62 neurons) rats displayed an average of 1.0±0.1 spontaneously active DA neurons per electrode track (Figure 2A). A two-way ANOVA revealed a significant main effect of EE [Fenvironment(1,29)=12.91, p<0.01] and a significant interaction between treatment and environment [Finteraction(1,29)=6.913, p<0.05]. Tukey’s post hoc tests revealed that MAM:RE group (n=9, 94 neurons) showed greater population activity (1.4±0.1 cells/track, p<0.05). Compared to MAM:RE group, MAM:EE group (n=8 rats, 47 neurons) showed significantly lower population activity (0.8±0.1 cells/track; p<0.001). Furthermore, population activity in MAM:EE vs SAL:RE groups were not significantly different (p>0.05). Prepubertal EE did not have significant effects in SAL animals, as SAL:EE group (n=8, 52 neurons) showed an average of 0.9±0.1 cells/track, which was not significantly different from SAL:RE group (p>0.05). SAL:RE rats displayed an average firing rate of 3.5±0.3 Hz, with 26.7±3.5% spikes fired in bursts, consistent with previous reports (48, 49). The firing rate (3.5±0.3, 3.4±0.2, and 3.1±0.3 Hz) and %SIB (21.1±3.0, 26.1±2.6, and 20.2±3.7%, in SAL:EE, MAM:RE, and MAM-EE groups, respectively) did not differ significantly (Figure S1C–D, p>0.05; two-way ANOVA). Further analysis of population activity throughout the mediolateral VTA divisions revealed that the increased DA population activity in MAM:RE rats was confined to the medial and the lateral VTA (Figure 2B), consistent with our previous report (50). We found the EE was effective in reducing MAM-induced DA hyperactivity across all subregions (MAM:EE vs. MAM:RE, p<0.01 in medial VTA; p<0.05 in central and lateral VTA; Tukey’s post hoc tests following two-way ANOVA).
Figure 2. Prepubertal environmental enrichment prevented dopamine hyperactivity in adult MAM rats.
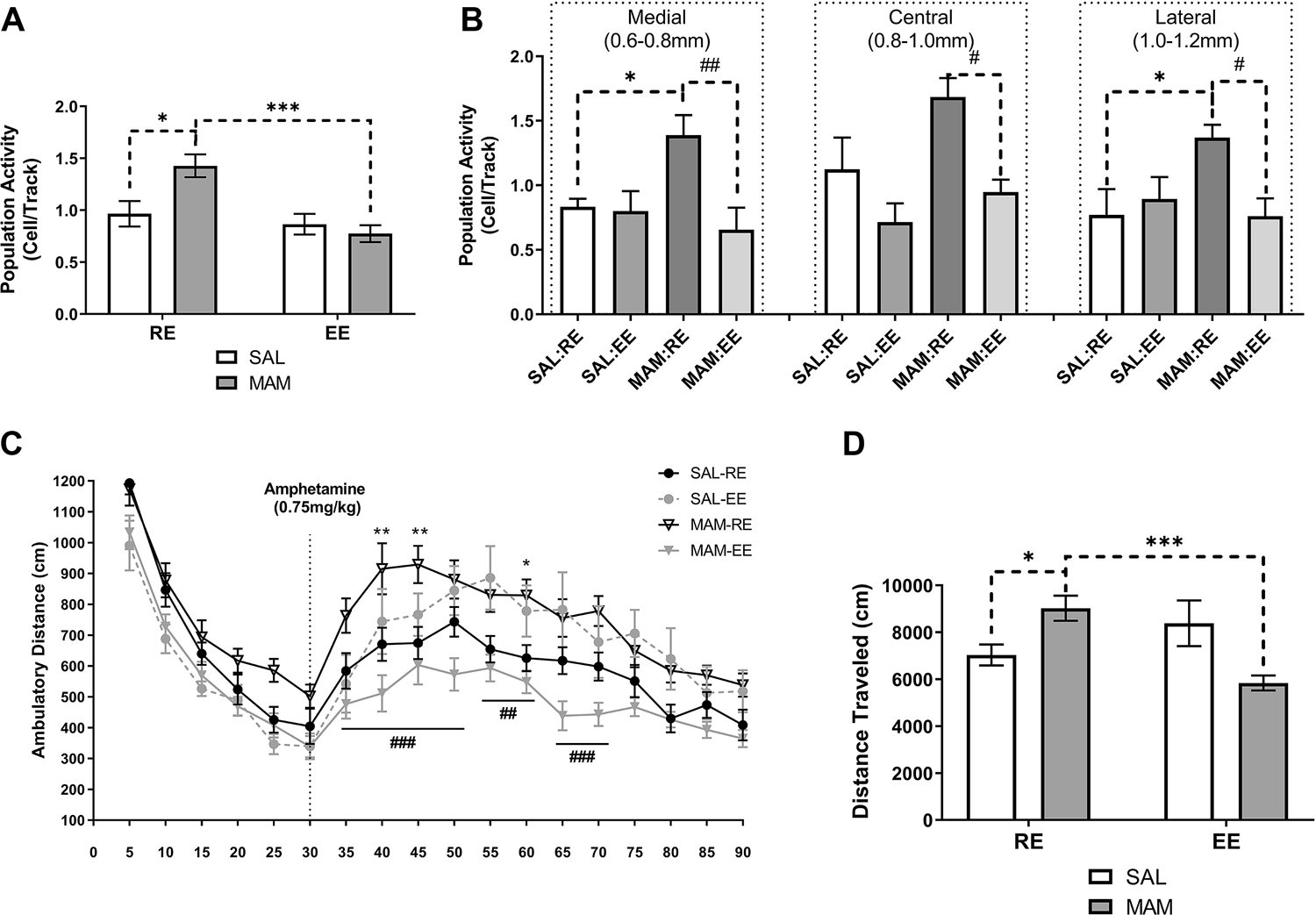
(A) MAM:RE rats displayed an increased number of spontaneously active VTA DA neurons comparing to SAL:RE rats, and prepubertal (PD21–40) exposure to EE prevented the heightened population activity DA neurons (n=8–9). (B) In MAM:RE, the increased in the number of spontaneously active DA neurons was confined to medial and lateral VTA, which were normalized by exposure to EE. (C) The behavioral manifestation of DA hyperresponsivity was measured by AIH, and MAM:RE rats had an augmented locomotor response to amphetamine (0.75 mg/kg; i.p. injection indicated by the dashed line), resulting in (D) increased total distance traveled after amphetamine injection. These changes were prevented by prepubertal EE. (n=8–14). Data are presented as mean ± SEM. *p or #p<0.05; **p or ##p<0.01; ***p or ###p<0.001; * indicated MAM:RE vs. SAL:RE; # indicates MAM:RE vs. MAM:EE
Locomotor response to amphetamine is heightened in adult MAM rats (48, 49, 51, 52). Consistently, MAM:RE (n=12) group showed significantly higher levels of locomotor activity in response to amphetamine administration (0.75 mg/kg; i.p.) compared to SAL:RE group (n=13) [Figure 2C; p<0.01 at 40- and 45-min, p<0.05 at 60-min; Tukey’s post hoc test following repeated measures two-way ANOVA on post-amphetamine movement; interaction of developmental condition*time: F(1,44)=17.46, p<0.001]. In contrast, MAM:EE rats (n=13) showed a significantly lower level of amphetamine-stimulated locomotion compared with MAM:RE rats (p<0.001 at 35–50 and 65–70 min; p<0.01 at 55–60 min), and were not significantly different from SAL-RE rats (p>0.05 at all post-amphetamine timepoints). Furthermore, prepubertal EE in SAL rats did not affect amphetamine-stimulated locomotion (SAL:RE vs SAL:EE rats, n=8, p>0.05 at all post-amphetamine timepoints). The total movement post-amphetamine administration revealed a similar result (Figure 2D), as two-way ANOVA indicated a significant interaction between prenatal MAM treatment and prepubertal environment [Finteraction(1,44)=17.46, p<0.001]. MAM:RE rats showed a significantly higher total movement compared with SAL:RE (p<0.01) and MAM:EE rats (p<0.001).
To examine the age-dependence of EE, separate sets of MAM rats were enriched during PD21–30 or PD31–40 (Figure 3A). Similar to the 20-day EE paradigm, 10-day EE paradigms were found to be effective in normalizing the electrophysiological phenotypes of DA hyperresponsivity (Figure 3A), although no effect of 10-day prepubertal EE paradigms was observed in AIH or EPM (Figure S2B–C). In contrast, in adult rats the 20-day enrichment paradigm (Figure 3B) did not impact DA population activity. Based on these results, subsequent experiments on BLA and vHipp activities were only conducted with animals exposed to 20-day prepubertal EE.
Prepubertal EE prevented vHipp hyperactivity in adult MAM rats.
An increase in vHipp activity is proposed to underlie the DA system hyperresponsivity in SCZ and animal models (53). We therefore performed recordings from identified pyramidal and putative fast-spiking interneurons in the vHipp (Figure 4A). In total, 50 neurons/5 rats in the vHipp of SAL:RE group were recorded (Figure 4B), which were divided into two populations each with a normal distribution (Kolmogorov-Smirnov test of normality, p>0.1) based on previously reported firing rate cut-off of 2 Hz (45). A significant difference in the mean firing rate in the pyramidal vs. non-pyramidal neurons was detected (Figure 3B; t=12.26, Welch’s correction, df=23.61, p<0.001), confirming the applicability of this identification criterion.
Figure 4. Prepubertal environmental enrichment prevented hyperactivity in vHipp pyramidal neurons.
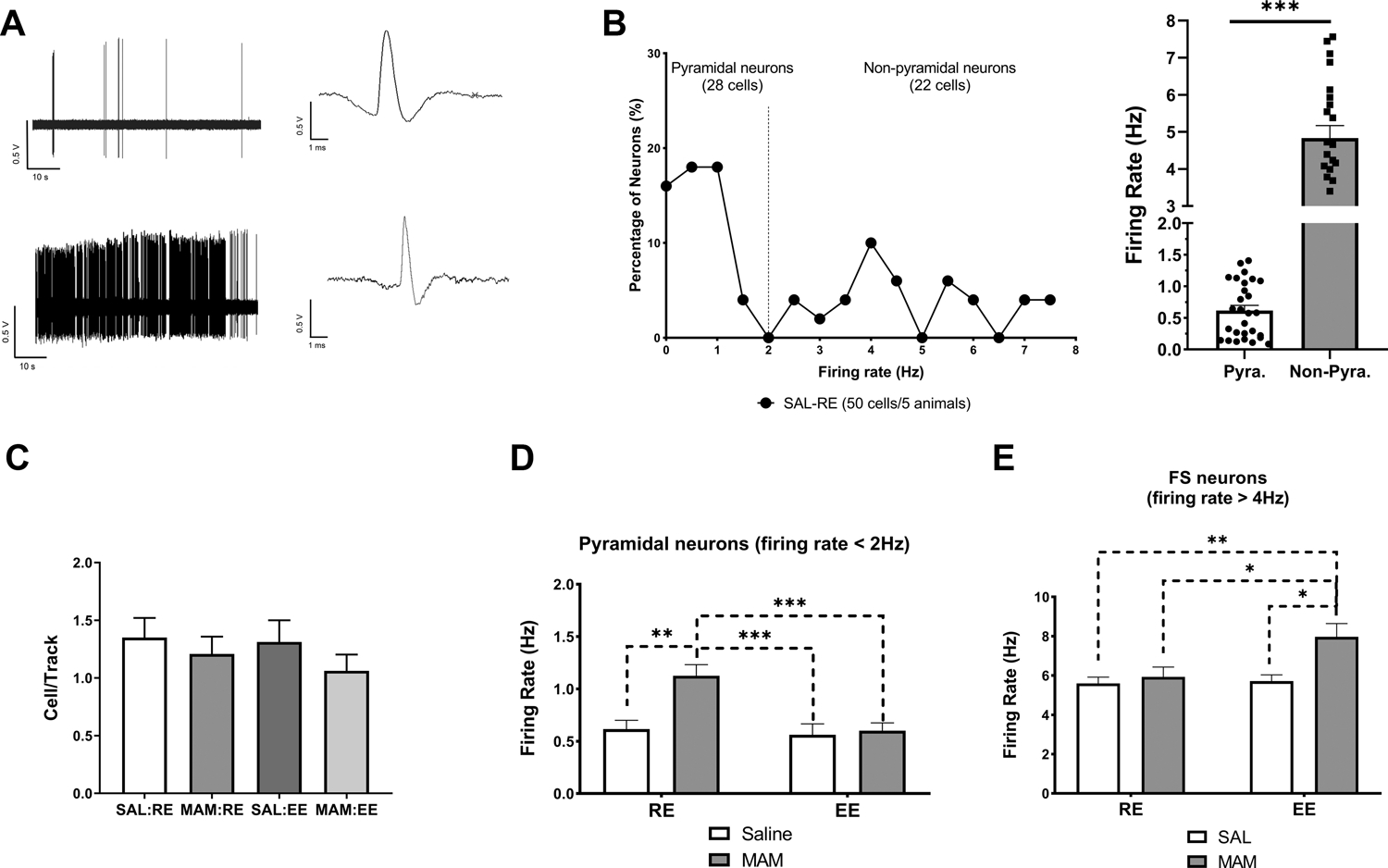
(A) 1-min segments of spontaneous activity and representative waveform of a putative pyramidal neuron (top) and a putative fast-spiking neuron (bottom) in the vHipp. (B) Neuronal firing rate distribution of neurons detected in SAL:RE rats supports the presence of two populations of vHipp neurons putatively following bimodal distribution that can be separated into two normal distributions (with a 2Hz-cutoff). (C) No change was found in the number of spontaneously active pyramidal neurons per track was detected across groups (n = 4–8 rats). (D) MAM:RE rats displayed increased firing rates of pyramidal neurons in the vHipp, which was prevented by prepubertal EE (25–48 cells/4–8 rats). (E) Of the fast-spiking (firing rate > 4 Hz) non-pyramidal neurons (n=11–19 cells/3–7 rats), a main effect of EE was detected (two-way ANOVA, F(1,54)=5.500, p<0.05), and MAM:EE rats displayed increased firing rate in fast-spiking cells (Tukey’s post hoc test). Data are presented as mean ± SEM. *p<0.05; **p<0.01; ***p<0.001. pyra.: putative pyramidal neurons; FS: fast-spiking neurons.
No changes in the number of spontaneously active pyramidal neurons detected per track across groups were observed (Figure 4C). Consistent with previous data (45, 49), vHipp pyramidal neurons in SAL:RE (28 cells/5 rats) group displayed a mean firing rate of 0.62±0.08 Hz (Figure 4D). A two-way ANOVA revealed a significant effect of MAM [Ftreatment(1,125)=8.675, p<0.01], enrichment [Fenvironment(1,125)=9.717, p<0.01], and their interaction [Finteraction(1,125)=6.396, p<0.05]. Tukey’s post hoc tests revealed that MAM:RE group (28 cells/6 rats) displayed significantly higher firing rates (1.13±0.11 Hz; p<0.01, vs. SAL:RE), which were prevented by prepubertal exposure to EE (MAM:EE, 48 cells/8 rats; 0.60±0.07 Hz; p<0.001, vs. MAM:EE). Furthermore, in SAL rats, we did not observe any change associated with EE (SAL:EE, 25 cells/4 rats; p>0.05, vs. SAL:RE).
Of the identified non-pyramidal cells (i.e. firing rate >2 Hz), we further separated neurons at relatively higher firing rate (>4 Hz) and with shorter spike duration (<1.0ms, from peak to valley) (Figure 4E). These operationally defined non-pyramidal neurons putatively represent fast-spiking neurons (47, 54). A two-way ANOVA revealed a significant effect of enrichment [Fenvironment(1,54)=5.50, p<0.05]. Tukey’s post hoc tests revealed that MAM:EE group (19 cells/7 rats) displayed a significantly higher firing rate (7.97±0.68 Hz), compared to MAM:RE (13 cells/5 rats; 5.93±0.50 Hz; p<0.05), SAL:RE (15 cells/4 rats; 5.60±0.32 Hz; p<0.01), and SAL:EE (11 cells/3 rats; 5.72±0.32 Hz; p<0.05) groups.
Prepubertal EE did not prevent anxiety-like behaviors in EPM and BLA hyperactivity in adult MAM rats.
Heightened anxiety and stress sensitivity are proposed to contribute to the onset of DA dysregulation in MAM rats (39, 55). Confirming previous findings (39, 48), MAM:RE (n=9) rats display higher level of anxiety-like behaviors in EPM, indexed by decreased time spent in open arms [Figure 5A; Ftreatment(1,42)=12.84, p<0.001; two-way ANOVA] and decreased percent entries into open arms [Figure 5B; Ftreatment(1,42)=11.85, p<0.01; two-way ANOVA]. Post hoc Tukey’s tests revealed that MAM:RE rats spent less time in open arms (p<0.05) and had lower percent entries into open arms (p<0.05), compared to SAL:RE (n=11) rats. Prepubertal EE did not rescue anxiety-like behaviors in MAM rats [open arm time: Figure 5A; Fenvironment (1,42) = 5.251, p< 0.05; Tukey’s test, MAM:RE vs. MAM:EE (n=16), p=0.577 or percent open arm entries [Figure 5B; Fenvironment(1,42)=0.1856, p=0.669]. Overall locomotion, indexed by total arm entries, was not different among groups [Figure 5C; Finteraction(1, 42)=0.3923, p=0.53]. To assess the effect of EE on anxiety-like behaviors during prepuberty, in a separate group of rats we measured the EPM responses at PD43–44 (Figure S3A). While we observed a main effect of MAM in both open arm time [Figure S3B, Ftreatment(1,16)=5.576, p<0.05] and percent open arm entries [Figure S3C, Ftreatment(1,16)=5.745, p<0.05], these measures were not different between MAM:RE vs. MAM:EE group in post hoc tests, suggesting EE’s inability to rescue MAM-induced anxiety starts early in development.
Figure 5. Prepubertal environmental enrichment did not prevent anxiety-like responses in the elevated plus maze or BLA hyperactivity in adult MAM rats.
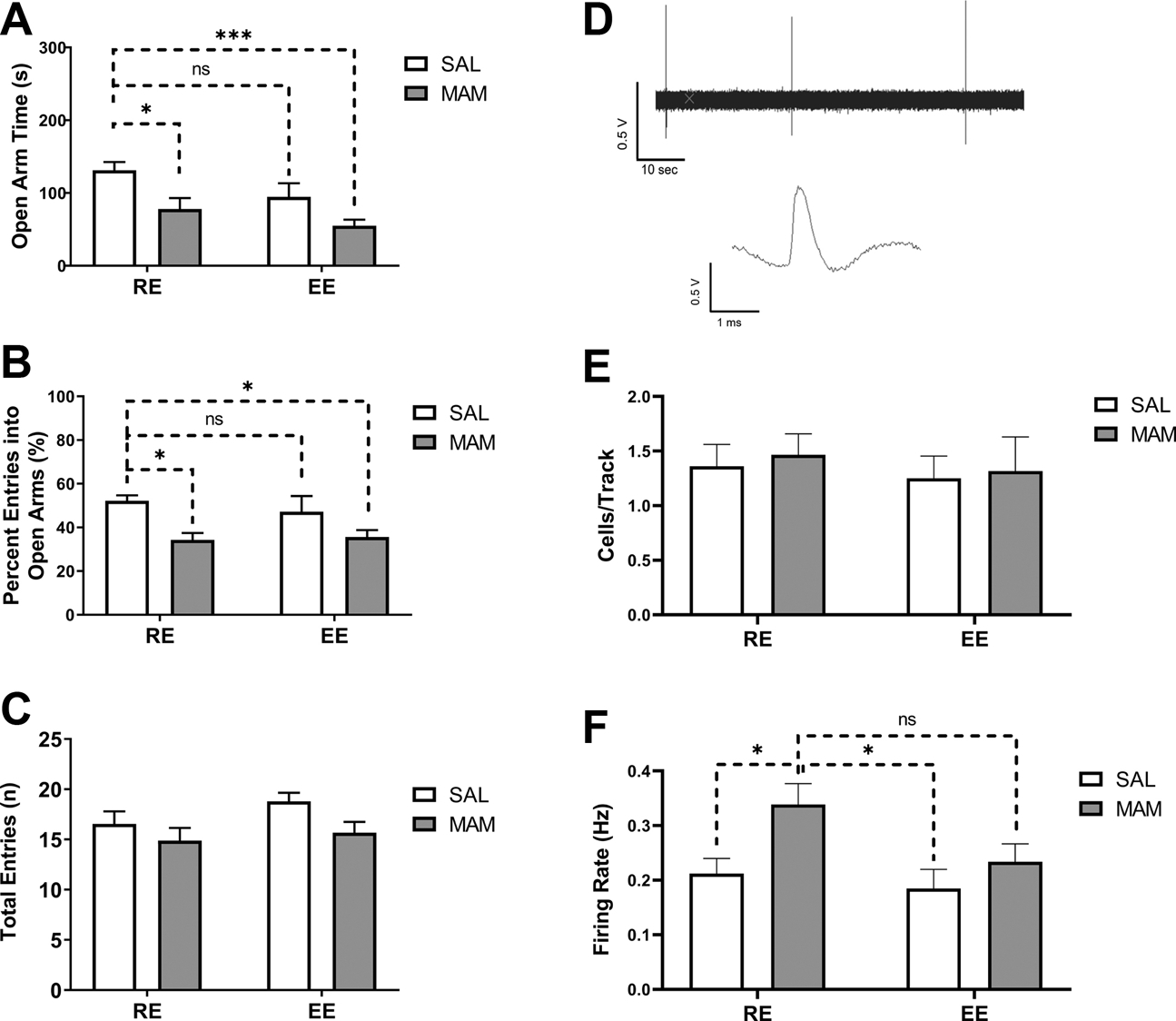
(A-C) Adult MAM:RE rats, compared to SAL:RE rats, exhibited (A) less time spent in open arms, (B) lower percent of entries into open arms, but (C) no difference in the total number of entries, consistent with increased anxiety. Prepubertal EE did not prevent these changes in MAM rats. (n = 9–16 rats). (D-F) the effect of prepubertal EE on BLA neuronal firing. (D) Representative recording of 1-min spontaneous activity of identified putative BLA projection neurons and its waveform. (E) No change was found in the number of spontaneously active projection neurons detected per electrode track. (F) The hyperactivity of BLA neurons in MAM:RE rats was not prevented by prepubertal exposure to EE (n = 20–45/4–6 rats). Data are presented as mean ± SEM. *p<0.05; ***p<0.001; ns: not significant.
We recorded from putative projection neurons in the BLA, a key region involved in the regulation of fear and anxiety-like behaviors (56). Projection neurons were identified based on previously published criteria (39, 57): (1) bi- or triphasic waveforms, (2) long spike duration (>2 ms), and (3) slow firing rate (<1 Hz) (Figure 5D). No changes in the number of spontaneously active neurons in each track were observed across groups (Figure 5E). For firing rate (Figure 5F), confirming previous results (57), the average firing rate of the recorded putative projection neurons was very low (SAL:RE, n=45 cells/6 rats, 0.21±0.03 Hz). A two-way ANOVA revealed a significant main effect of MAM [Ftreatment(1,124)=6.220, p<0.05], but only a trend toward significance for enrichment [Fenvironment(1,124)=3.504, p=0.0636]. Tukey’s post hoc analysis revealed that, compared to SAL:RE and SAL:EE (20 cells/4 rats, 0.1849±0.03 Hz) groups, MAM:RE group (36 cells/5 rats) displayed significantly higher BLA projection neuron firing (0.34±0.04 Hz, p<0.05), which was not significantly different from that of MAM:EE rats (28 cells/5 rats, 0.23±0.03 Hz, p=0.19), consistent with the behavioral measures in the EPM. Altogether these data suggest prepubertal EE is not effective in preventing anxiety in terms of EPM or BLA activity in adult MAM rats.
Discussion:
In this study, we examined prepubertal EE for the prevention of psychosis-related dopamine dysregulation in an animal model. Rearing MAM offspring in an enriched environment prepubertally prevented the emergence of DA system hyperresponsivity and vHipp hyperactivity, without affecting anxiety-like responses and the firing of BLA projection neurons. Altogether, these results indicate that early EE might be effective in reducing psychosis-related electrophysiological changes predisposed by early-life risk factors.
We used the MAM GD17 model (26), in which a single injection of a neurospecific, short-acting mitotoxin (58) during late gestation selectively disrupts neurodevelopment (51, 59), resulting in phenotypes in the offspring consistent with SCZ (53, 60). This model is effective in screening preventative strategies due to its ability to recapitulate certain prodromal aspects of SCZ, specifically increased stress susceptibility, anxiety-like response, and hippocampal pathology during prepuberty (39, 48, 61, 62), before the manifestation of DA-related behavioral abnormalities in adults (51).
Previous research on EE effects in SCZ-relevant models tends to focus on behavioral phenotypes, including sensorimotor gating deficits, social interaction impairment, hyperactivity, and memory deficits (27, 63–65). To our knowledge, the present study represents the first examination of whether EE can protect against psychosis-related dopamine dysregulation, and hence provides novel insight for early intervention. We showed that prepubertal EE is sufficient to prevent the vHipp-driven DA hyperactivity across VTA subregions of adult MAM rats. Noteworthy, the ability of prepubertal EE to prevent DA hyperresponsivity in the lateral VTA (Figure 2B) is strongly consistent with the DA dysfunction in human SCZ, which is most prominent in this region and its target (i.e. associative striatum) (66).
DA hyperresponsivity in SCZ likely originates in its afferent regulators (67), such as the limbic hippocampus which can potently regulate DA system responsivity via a polysynaptic, disinhibitory circuit (33, 68). Thus, aberrant activity in vHipp will induce DA hyperresponsivity through ventral pallidum mediated disinhibition (49). Consistent with this model, anterior hippocampal hyperactivity is reported in SCZ patients (69, 70), which may in turn drive the DA dysfunction (29) that underlies psychosis (71). Moreover, studies of at-risk groups posited a pathogenic role for hippocampal dysregulation in psychotic conversion (72, 73). Importantly, hippocampal parvalbumin (PV) interneuron deficits have been implicated in the pathophysiology of SCZ (74–77). Closely recapitulating this clinical feature, MAM rats display similar abnormalities in the vHipp (a functional equivalent of anterior hippocampus in primates (78, 79)), which leads to VTA DA hyperactivity (49, 51, 80). Altogether, these findings suggest that the hyperdopaminergic state in SCZ might be a consequence of excessive hippocampal activity, driven by the loss of PV or PV interneurons (28, 55).
We found prepubertal EE prevented pyramidal neuron hyperactivity in the vHipp of MAM rats (Figure 4), as well as the DA hyper-responsivity in the VTA (Figure 2). These results are consistent with previous reports using pharmacological (49), genetic (81), and stem-cell-based (45) approaches to modulate vHipp activity and hence to reduce DA hyperactivity. Previous research also indicated that EE attenuates the decrease of the PV interneurons in the hippocampus of MAM rats (82), which could be the structural basis underlying the observed functional prevention. During prepuberty, vHipp interneurons undergo significant development and maturation, characterized by an increase in PV expression and enhanced wrapping of the perineuronal nets (PNNs) (42, 83). PNNs have diverse functions (84), and the protection of PV neurons against cellular damage and oxidative stress is likely relevant to this study. Post-mortem studies have linked PNN loss to the pathogenesis of SCZ (85). Given the preclinical evidence that early EE can increase PNN expression (86, 87), the observed prevention of DA dysregulation might be mediated by positive modulation of PNNs in the vHipp, thus enhancing the resilience of the PV interneurons to increased oxidative stress known to be present in MAM rats (88). Protection of PV interneurons in the MAM vHipp would enhance local inhibition of pyramidal neurons, consistent with the present data in vHipp recording (Figure 4D, E). In contrast, EE in adulthood (PD65–84) was unable to modulate abnormal DA activity in MAM rats (Figure 3B). Since the PV loss in MAM rats emerges early (62), this failure of adult EE to prevent DA dysregulation suggests that the enrichment paradigm is indeed a preventive rather than therapeutic approach, addressing a need for future research to focus on the translation of EE by identifying the optimal window to apply this early intervention (8, 13). Furthermore, given the important sex differences in SCZ (89) and the sexually dimorphic behavioral effects of EE (90, 91), it is likely that female MAM rats will show significant developmental stage-specific changes that differ from males particularly with respect to the impact of stress (92). Further studies are required to investigate if the findings described here in males would be observed in females as well.
During prepuberty, MAM rats display increased stress susceptibility, heightened anxiety-like responses, decreased adaptability to stress, and increased BLA firing (48, 61). Furthermore, treating anxiety in MAM rats with diazepam during prepuberty prevents the adult DA dysregulation (48). These findings and the epidemiologic evidence on the pathogenic role of stress (93, 94) suggest that abnormal stress vulnerability during critical developmental stages, such as adolescence (95, 96), may lead to later-onset of SCZ-related pathophysiology (55). Previous studies suggest that EE can mitigate the negative behavioral effects of stress by enhancing resilience and stress adaptability (97–99). Furthermore, previous studies have also found that EE can mitigate anxiety and the associated maladaptive structural and molecular plasticity in the BLA, examined shortly after EE (100, 101). However, whether EE can chronically modulate BLA activity is unknown. Intriguingly, PD21–40 EE in MAM rats was not sufficient to prevent anxiety-like responses in the EPM and hyperactivity in identified BLA projection neurons (Figure 5A–C and E). These data point to dissociable behavioral and neurophysiological benefits of early EE in the MAM model. Speculatively, these observed negative results in EPM and BLA-related measurements could be related to the anxiogenic effect of enrichment loss (i.e. returning to RE on PD41), which would be consistent with a previous study reporting a rapid loss of the effect of EE on anxiety in open field test when animals return to home cages (102). This explanation would also be supported by the EPM data measured at PD43–44 (Figure S3), during which we found that EE did not rescue anxiety-like behavior even shortly (i.e. 3–4 days) after the termination of the paradigm. Alternatively, these data also suggest possible sensitive periods for EE to produce long-lasting behavioral and/or neurophysiological benefits, which may partially explain the differential effects of 10-day EE starting PD21 or 31 (Figure 3A and S2). A recent study reported that pre-weaning (PD2–21) enrichment can positively modulate anxiety-related behaviors, spine density, and brain-derived neurotrophic factor in the BLA (103). Thus, the full beneficial effects of developmental EE on adult MAM-related phenotypes is possibly achieved via a sensitive-period-like, sequential mechanism, with prepubertal enrichment selectively targeting vHipp-related pathophysiology and DA dysfunction.
Although many effective enrichment paradigms incorporate an early post-weaning phase, the exact outcomes can vary dramatically depending on EE procedures, with onset, duration, and continuity of EE being critical variables (90).
The generalization of rodent enrichment protocols to humans is challenging, as modifications of rodent housing are not directly comparable to treatments in humans (104). Thus, one should carefully consider control groups when interpreting the EE effects in animals. The RE cages here are similar to a typical rodent housing environment, characterized by reduced and unvarying environmental stimuli. This could be construed as relatively impoverished mainly for a lack of cognitive stimulation, an element provided by most EE cages (90). One interpretation of the present study could be that the observed EE effects against SCZ-relevant changes might be generalizable selectively to a population raised in an impoverished environment, such as individuals with low socioeconomic status (SES), a known risk factor for SCZ (105, 106). Lack of cognitive stimulation is a critical mediator of the low SES effects, which can act synergistically with other prenatal factors to negatively affect neurodevelopment (107). According, while the effect of MAM has been largely attributed to the in utero neurodevelopmental disruption (i.e. the “MAM-phenotypes” (26)), current data and the emerging evidence (55) raised the possibility that the adult SCZ-relevant phenotypes of MAM rats might also arise from the impoverished postnatal environment. Whether early EE can specifically counteract the influence of direct neurodevelopmental disruption and/or the impoverished environment in MAM rats warrants future studies.
In summary, the present study supports prepubertal environmental enrichment as a useful preventative approach against the pathophysiological development of SCZ. Although prepubertal EE did not fully prevent abnormal pathophysiology in the adult (such as anxiety-like response and BLA hyperactivity), our results indicate that prepubertal EE is sufficient to prevent DA dysregulation and vHipp hyperactivity in the MAM model.
Supplementary Material
Key Resource Table
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| STERILITE Storage Box | Reference: PMID: 27091967 | Mfr. Model # 19453V04 | EE box | |
| Combo Chew | EE toys | |||
| Running Wheel | MPN:100533403 | EE toys | ||
| Ladders | Penn Plax | MPN: BA243 | EE toys | |
| Play ball pack | Marshall Pet Products | MPN: 572017 | EE toys | |
| Lattice Balls with Bells | MPN: BA-511 | EE toys | ||
| Color Play Dangly | MPN: 2951 | EE toys | ||
| Atomic Ball | Ware | MPN: 089348 | EE toys | |
| Fintronics | WDR420 | amplifier - for eletrophysiology | ||
| Adinstruments Powerlab 8/30 recording unit | Adinstruments | interface - for eletrophysiology | ||
| Lab Chart 8 | Adinstruments | data acquisition software - for eletrophysiology | ||
| GraphPad Prism | GraphPad | data analysis | ||
| WPI Glass Capillaries | WPI | 1B200F-4 | glass eletrode | |
| Med Associates video plus maze - rat | Med associates | ENV-664 | for EPM behaviors | |
| True Scan photobeam sensor | Coulbourn Instruments | E63–22 | for AIH behaviors |
Acknowledgments
This work is supported by National Institutes of Health (NIH MH57440 to AAG).
We thank Niki MacMurdo and Christy Smolak for the technical assistance. We also thank Kaetlyn Conner, Madhura Leninakan, and Junhao Xu for the blinding and data analysis for behavioral experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
AAG has received funds from Lundbeck, Pfizer, Otsuka, Lilly, Roche, Asubio, Abbott, Autofony, Janssen, Alkermes, Newron, Takeda and Minerva. XZ reports no biomedical financial interests or potential conflicts of interest.
References
- 1.Murray RM, Lewis SW (1988): Is schizophrenia a neurodevelopmental disorder? British Medical Journal (Clinical research ed). 296:63–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinberger DR (1987): Implications of normal brain development for the pathogenesis of schizophrenia. Archives of general psychiatry. 44:660–669. [DOI] [PubMed] [Google Scholar]
- 3.Owen MJ, O’Donovan MC (2017): Schizophrenia and the neurodevelopmental continuum:evidence from genomics. World psychiatry : official journal of the World Psychiatric Association. 16:227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Os J, Kenis G, Rutten BP (2010): The environment and schizophrenia. Nature. 468:203–212. [DOI] [PubMed] [Google Scholar]
- 5.McCutcheon RA, Reis Marques T, Howes OD (2019): Schizophrenia—An Overview. JAMA psychiatry.1–10. [DOI] [PubMed] [Google Scholar]
- 6.Van Os J, Rutten BP, Poulton R (2008): Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophrenia bulletin. 34:1066–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rapoport JL, Giedd JN, Gogtay N (2012): Neurodevelopmental model of schizophrenia: update 2012. Molecular psychiatry. 17:1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Millan MJ, Andrieux A, Bartzokis G, Cadenhead K, Dazzan P, Fusar-Poli P, et al. (2016): Altering the course of schizophrenia: progress and perspectives. Nature Reviews Drug Discovery. 15:485. [DOI] [PubMed] [Google Scholar]
- 9.Wood SJ, Yung AR, McGorry PD, Pantelis C (2011): Neuroimaging and Treatment Evidence for Clinical Staging in Psychotic Disorders: From the At-Risk Mental State to Chronic Schizophrenia. Biological psychiatry. 70:619–625. [DOI] [PubMed] [Google Scholar]
- 10.de Koning MB, Bloemen OJ, van Amelsvoort TA, Becker HE, Nieman DH, van der Gaag M, et al. (2009): Early intervention in patients at ultra high risk of psychosis: benefits and risks. Acta psychiatrica Scandinavica. 119:426–442. [DOI] [PubMed] [Google Scholar]
- 11.Stafford MR, Jackson H, Mayo-Wilson E, Morrison AP, Kendall T (2013): Early interventions to prevent psychosis: systematic review and meta-analysis. Bmj. 346:f185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, et al. (2012): Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Archives of general psychiatry. 69:220–229. [DOI] [PubMed] [Google Scholar]
- 13.Sommer IE, Bearden CE, Van Dellen E, Breetvelt EJ, Duijff SN, Maijer K, et al. (2016): Early interventions in risk groups for schizophrenia: what are we waiting for? NPJ schizophrenia. 2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nithianantharajah J, Hannan AJ (2006): Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nature Reviews Neuroscience. 7:697–709. [DOI] [PubMed] [Google Scholar]
- 15.Kentner AC, Khoury A, Lima Queiroz E, MacRae M (2016): Environmental enrichment rescues the effects of early life inflammation on markers of synaptic transmission and plasticity. Brain, behavior, and immunity. 57:151–160. [DOI] [PubMed] [Google Scholar]
- 16.Kentner AC, Lambert KG, Hannan AJ, Donaldson ST (2019): Editorial: Environmental Enrichment: Enhancing Neural Plasticity, Resilience, and Repair. Frontiers in behavioral neuroscience. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Praag H, Kempermann G, Gage FH (2000): Neural consequences of environmental enrichment. Nature reviews Neuroscience. 1:191–198. [DOI] [PubMed] [Google Scholar]
- 18.McFarlane WR, Levin B, Travis L, Lucas FL, Lynch S, Verdi M, et al. (2015): Clinical and functional outcomes after 2 years in the early detection and intervention for the prevention of psychosis multisite effectiveness trial. Schizophrenia bulletin. 41:30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raine A, Mellingen K, Liu J, Venables P, Mednick SA (2003): Effects of environmental enrichment at ages 3–5 years on schizotypal personality and antisocial behavior at ages 17 and 23 years. The American journal of psychiatry. 160:1627–1635. [DOI] [PubMed] [Google Scholar]
- 20.Nozari M, Shabani M, Farhangi AM, Mazhari S, Atapour N (2015): Sex-specific restoration of MK-801-induced sensorimotor gating deficit by environmental enrichment. Neuroscience. 299:28–34. [DOI] [PubMed] [Google Scholar]
- 21.Akillioglu K, Babar Melik E, Melik E, Kocahan S (2012): The investigation of neonatal MK-801 administration and physical environmental enrichment on emotional and cognitive functions in adult Balb/c mice. Pharmacology, biochemistry, and behavior. 102:407–414. [DOI] [PubMed] [Google Scholar]
- 22.Murueta-Goyena A, Ortuzar N, Lafuente JV, Bengoetxea H (2020): Enriched Environment Reverts Somatostatin Interneuron Loss in MK-801 Model of Schizophrenia. Molecular neurobiology. 57:125–134. [DOI] [PubMed] [Google Scholar]
- 23.Burrows EL, McOmish CE, Buret LS, Van den Buuse M, Hannan AJ (2015): Environmental Enrichment Ameliorates Behavioral Impairments Modeling Schizophrenia in Mice Lacking Metabotropic Glutamate Receptor 5. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 40:1947–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McOmish CE, Burrows E, Howard M, Scarr E, Kim D, Shin HS, et al. (2008): Phospholipase C-beta1 knockout mice exhibit endophenotypes modeling schizophrenia which are rescued by environmental enrichment and clozapine administration. Molecular psychiatry. 13:661–672. [DOI] [PubMed] [Google Scholar]
- 25.Lee H, Dvorak D, Kao H-Y, Duffy ÁM, Scharfman HE, Fenton AA (2012): Early cognitive experience prevents adult deficits in a neurodevelopmental schizophrenia model. Neuron. 75:714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lodge DJ (2013): The MAM rodent model of schizophrenia. Current protocols in neuroscience. Chapter 9:Unit9.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bator E, Latusz J, Wedzony K, Mackowiak M (2018): Adolescent environmental enrichment prevents the emergence of schizophrenia-like abnormalities in a neurodevelopmental model of schizophrenia. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 28:97–108. [DOI] [PubMed] [Google Scholar]
- 28.Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, et al. (2012): GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 62:1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCutcheon RA, Abi-Dargham A, Howes OD (2019): Schizophrenia, Dopamine and the Striatum: From Biology to Symptoms. Trends in neurosciences. 42:205–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howes OD, McDonald C, Cannon M, Arseneault L, Boydell J, Murray RM (2004): Pathways to schizophrenia: the impact of environmental factors. The international journal of neuropsychopharmacology. 7 Suppl 1:S7–S13. [DOI] [PubMed] [Google Scholar]
- 31.Lodge DJ, Grace AA (2011): Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends in pharmacological sciences. 32:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Floresco SB, Todd CL, Grace AA (2001): Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 21:4915–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Floresco SB, West AR, Ash B, Moore H, Grace AA (2003): Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nature neuroscience. 6:968–973. [DOI] [PubMed] [Google Scholar]
- 34.Achim AM, Maziade M, Raymond E, Olivier D, Mérette C, Roy MA (2011): How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophrenia bulletin. 37:811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devylder JE, Ben-David S, Schobel SA, Kimhy D, Malaspina D, Corcoran CM (2013): Temporal association of stress sensitivity and symptoms in individuals at clinical high risk for psychosis. Psychological medicine. 43:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benes FM (2010): Amygdalocortical circuitry in schizophrenia: from circuits to molecules. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 35:239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Owens DG, Miller P, Lawrie SM, Johnstone EC (2005): Pathogenesis of schizophrenia: a psychopathological perspective. The British journal of psychiatry : the journal of mental science. 186:386–393. [DOI] [PubMed] [Google Scholar]
- 38.Johnstone EC, Ebmeier KP, Miller P, Owens DG, Lawrie SM (2005): Predicting schizophrenia: findings from the Edinburgh high-risk study. The British Journal of Psychiatry. 186:18–25. [DOI] [PubMed] [Google Scholar]
- 39.Du Y, Grace AA (2016): Amygdala Hyperactivity in MAM Model of Schizophrenia is Normalized by Peripubertal Diazepam Administration. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 41:2455–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma Y-Y, Wang X, Huang Y, Marie H, Nestler EJ, Schlüter OM, et al. (2016): Re-silencing of silent synapses unmasks anti-relapse effects of environmental enrichment. Proceedings of the National Academy of Sciences. 113:5089–5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai L-H (2007): Recovery of learning and memory is associated with chromatin remodelling. Nature. 447:178–182. [DOI] [PubMed] [Google Scholar]
- 42.Gomes FV, Zhu X, Grace AA (2019): The pathophysiological impact of stress on the dopamine system is dependent on the state of the critical period of vulnerability. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomes FV, Grace AA (2017): Prefrontal cortex dysfunction increases susceptibility to schizophrenia-like changes induced by adolescent stress exposure. Schizophrenia bulletin. 43:592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ungless MA, Grace AA (2012): Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends in neurosciences. 35:422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perez SM, Lodge DJ (2013): Hippocampal interneuron transplants reverse aberrant dopamine system function and behavior in a rodent model of schizophrenia. Molecular psychiatry. 18:1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ranck JB Jr (1973): Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats: Part I. Behavioral correlates and firing repertoires. Experimental neurology. 41:462–531. [DOI] [PubMed] [Google Scholar]
- 47.Van Der Meer MA, Redish AD (2011): Theta phase precession in rat ventral striatum links place and reward information. Journal of neuroscience. 31:2843–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du Y, Grace AA (2013): Peripubertal diazepam administration prevents the emergence of dopamine system hyperresponsivity in the MAM developmental disruption model of schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 38:1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lodge DJ, Grace AA (2007): Aberrant Hippocampal Activity Underlies the Dopamine Dysregulation in an Animal Model of Schizophrenia. The Journal of Neuroscience. 27:11424–11430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lodge DJ, Grace AA (2012): Divergent activation of ventromedial and ventrolateral dopamine systems in animal models of amphetamine sensitization and schizophrenia. International Journal of Neuropsychopharmacology. 15:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA (2006): A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 60:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flagstad P, Mørk A, Glenthøj BY, Van Beek J, Michael-Titus AT, Didriksen M (2004): Disruption of neurogenesis on gestational day 17 in the rat causes behavioral changes relevant to positive and negative schizophrenia symptoms and alters amphetamine-induced dopamine release in nucleus accumbens. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 29:2052–2064. [DOI] [PubMed] [Google Scholar]
- 53.Modinos G, Allen P, Grace AA, McGuire P (2015): Translating the MAM model of psychosis to humans. Trends in neurosciences. 38:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Csicsvari J, Hirase H, Czurkó A, Mamiya A, Buzsáki G (1999): Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. Journal of Neuroscience. 19:274–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gomes FV, Zhu X, Grace AA (2019): Stress during critical periods of development and risk for schizophrenia. Schizophrenia research. 213:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romeo RD, McEWEN BS (2006): Stress and the adolescent brain. Annals of the New York Academy of Sciences. 1094:202–214. [DOI] [PubMed] [Google Scholar]
- 57.Rosenkranz JA, Grace AA (1999): Modulation of Basolateral Amygdala Neuronal Firing and Afferent Drive by Dopamine Receptor Activation In Vivo. The Journal of Neuroscience. 19:11027–11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cattabeni F, Di Luca M (1997): Developmental models of brain dysfunctions induced by targeted cellular ablations with methylazoxymethanol. Physiological reviews. 77:199–215. [DOI] [PubMed] [Google Scholar]
- 59.Penschuck S, Flagstad P, Didriksen M, Leist M, Michael-Titus AT (2006): Decrease in parvalbumin-expressing neurons in the hippocampus and increased phencyclidine-induced locomotor activity in the rat methylazoxymethanol (MAM) model of schizophrenia. European Journal of Neuroscience. 23:279–284. [DOI] [PubMed] [Google Scholar]
- 60.Jones CA, Watson DJ, Fone KC (2011): Animal models of schizophrenia. British journal of pharmacology. 164:1162–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zimmerman EC, Bellaire M, Ewing SG, Grace AA (2013): Abnormal Stress Responsivity in a Rodent Developmental Disruption Model of Schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 38:2131–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gill KM, Grace AA (2014): Corresponding decrease in neuronal markers signals progressive parvalbumin neuron loss in MAM schizophrenia model. The international journal of neuropsychopharmacology. 17:1609–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Melik E, Babar E, Kocahan S, Guven M, Akillioglu K (2014): Enriched environment has limited capacity for the correction of hippocampal memory-dependent schizoid behaviors in rats with early postnatal NMDAR dysfunction. International Journal of Developmental Neuroscience. 33:22–28. [DOI] [PubMed] [Google Scholar]
- 64.Ishihama T, Ago Y, Shintani N, Hashimoto H, Baba A, Takuma K, et al. (2010): Environmental factors during early developmental period influence psychobehavioral abnormalities in adult PACAP-deficient mice. Behavioural brain research. 209:274–280. [DOI] [PubMed] [Google Scholar]
- 65.McOmish CE, Burrows E, Howard M, Scarr E, Kim D, Shin H-S, et al. (2008): Phospholipase C-β 1 knockout mice exhibit endophenotypes modeling schizophrenia which are rescued by environmental enrichment and clozapine administration. Molecular psychiatry. 13:661–672. [DOI] [PubMed] [Google Scholar]
- 66.McCutcheon R, Beck K, Jauhar S, Howes OD (2018): Defining the Locus of Dopaminergic Dysfunction in Schizophrenia: A Meta-analysis and Test of the Mesolimbic Hypothesis. Schizophrenia bulletin. 44:1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grace AA (2016): Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nature Reviews Neuroscience. 17:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grace AA, Floresco SB, Goto Y, Lodge DJ (2007): Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends in neurosciences. 30:220–227. [DOI] [PubMed] [Google Scholar]
- 69.Heckers S (2001): Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 11:520–528. [DOI] [PubMed] [Google Scholar]
- 70.Medoff DR, Holcomb HH, Lahti AC, Tamminga CA (2001): Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 11:543–550. [DOI] [PubMed] [Google Scholar]
- 71.Silbersweig DA, Stern E, Frith C, Cahill C, Holmes A, Grootoonk S, et al. (1995): A functional neuroanatomy of hallucinations in schizophrenia. Nature. 378:176–179. [DOI] [PubMed] [Google Scholar]
- 72.Lieberman JA, Girgis RR, Brucato G, Moore H, Provenzano F, Kegeles L, et al. (2018): Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Molecular psychiatry. 23:1764–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Provenzano FA, Guo J, Wall MM, Feng X, Sigmon HC, Brucato G, et al. (2020): Hippocampal Pathology in Clinical High-Risk Patients and the Onset of Schizophrenia. Biological psychiatry. 87:234–242. [DOI] [PubMed] [Google Scholar]
- 74.Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, et al. (2011): Hippocampal interneurons are abnormal in schizophrenia. Schizophrenia research. 131:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang ZJ, Reynolds GP (2002): A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophrenia research. 55:1–10. [DOI] [PubMed] [Google Scholar]
- 76.Eyles D, McGrath J, Reynolds G (2002): Neuronal calcium-binding proteins and schizophrenia. Schizophrenia research. 57:27–34. [DOI] [PubMed] [Google Scholar]
- 77.Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB (2005): Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biological psychiatry. 57:252–260. [DOI] [PubMed] [Google Scholar]
- 78.Fanselow MS, Dong HW (2010): Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 65:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grace AA (2012): Dopamine system dysregulation by the hippocampus: implications for the pathophysiology and treatment of schizophrenia. Neuropharmacology. 62:1342–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lodge DJ, Behrens MM, Grace AA (2009): A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. Journal of Neuroscience. 29:2344–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perez SM, Boley A, Lodge DJ (2019): Region specific knockdown of Parvalbumin or Somatostatin produces neuronal and behavioral deficits consistent with those observed in schizophrenia. Translational psychiatry. 9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hernan AE, Mahoney JM, Curry W, Richard G, Lucas MM, Massey A, et al. (2018): Environmental enrichment normalizes hippocampal timing coding in a malformed hippocampus. PloS one. 13:e0191488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caballero A, Diah KC, Tseng KY (2013): Region-specific upregulation of parvalbumin-, but not calretinin-positive cells in the ventral hippocampus during adolescence. Hippocampus. 23:1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wen TH, Binder DK, Ethell IM, Razak KA (2018): The Perineuronal ‘Safety’ Net? Perineuronal Net Abnormalities in Neurological Disorders. Frontiers in Molecular Neuroscience. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bitanihirwe BK, Woo TU (2014): Perineuronal nets and schizophrenia: the importance of neuronal coatings. Neuroscience and biobehavioral reviews. 45:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carstens KE, Phillips ML, Pozzo-Miller L, Weinberg RJ, Dudek SM (2016): Perineuronal nets suppress plasticity of excitatory synapses on CA2 pyramidal neurons. Journal of Neuroscience. 36:6312–6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O’Connor AM, Burton TJ, Mansuri H, Hand GR, Leamey CA, Sawatari A (2019): Environmental Enrichment From Birth Impacts Parvalbumin Expressing Cells and Wisteria Floribunda Agglutinin Labelled Peri-Neuronal Nets Within the Developing Murine Striatum. Frontiers in neuroanatomy. 13:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steullet P, Cabungcal JH, Coyle J, Didriksen M, Gill K, Grace AA, et al. (2017): Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Molecular psychiatry. 22:936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mendrek A, Mancini-Marïe A (2016): Sex/gender differences in the brain and cognition in schizophrenia. Neuroscience & Biobehavioral Reviews. 67:57–78. [DOI] [PubMed] [Google Scholar]
- 90.Simpson J, Kelly JP (2011): The impact of environmental enrichment in laboratory rats—behavioural and neurochemical aspects. Behavioural brain research. 222:246–264. [DOI] [PubMed] [Google Scholar]
- 91.Lin E-JD, Choi E, Liu X, Martin A, During MJ (2011): Environmental enrichment exerts sex-specific effects on emotionality in C57BL/6J mice. Behavioural brain research. 216:349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Klinger K, Gomes FV, Rincón-Cortés M, Grace AA (2019): Female rats are resistant to the long-lasting neurobehavioral changes induced by adolescent stress exposure. European Neuropsychopharmacology. 29:1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walker E, Mittal V, Tessner K (2008): Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 4:189–216. [DOI] [PubMed] [Google Scholar]
- 94.Van Winkel R, Stefanis NC, Myin-Germeys I (2008): Psychosocial stress and psychosis. A review of the neurobiological mechanisms and the evidence for gene-stress interaction. Schizophrenia bulletin. 34:1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Spear LP (2000): The adolescent brain and age-related behavioral manifestations. Neuroscience & biobehavioral reviews. 24:417–463. [DOI] [PubMed] [Google Scholar]
- 96.Paus T, Keshavan M, Giedd JN (2008): Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 9:947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M (2010): Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Molecular psychiatry. 15:1152–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lehmann ML, Herkenham M (2011): Environmental Enrichment Confers Stress Resiliency to Social Defeat through an Infralimbic Cortex-Dependent Neuroanatomical Pathway. The Journal of Neuroscience. 31:6159–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Southwick SM, Vythilingam M, Charney DS (2005): The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol. 1:255–291. [DOI] [PubMed] [Google Scholar]
- 100.Novaes LS, dos Santos NB, Perfetto JG, Goosens KA, Munhoz CD (2018): Environmental enrichment prevents acute restraint stress-induced anxiety-related behavior but not changes in basolateral amygdala spine density. Psychoneuroendocrinology. 98:6–10. [DOI] [PubMed] [Google Scholar]
- 101.Ashokan A, Hegde A, Mitra R (2016): Short-term environmental enrichment is sufficient to counter stress-induced anxiety and associated structural and molecular plasticity in basolateral amygdala. Psychoneuroendocrinology. 69:189–196. [DOI] [PubMed] [Google Scholar]
- 102.Nader J, Chauvet C, Rawas RE, Favot L, Jaber M, Thiriet N, et al. (2012): Loss of environmental enrichment increases vulnerability to cocaine addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 37:1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hegde A, Suresh S, Mitra R (2020): Early-life short-term environmental enrichment counteracts the effects of stress on anxiety-like behavior, brain-derived neurotrophic factor and nuclear translocation of glucocorticoid receptors in the basolateral amygdala. Scientific reports. 10:14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ball NJ, Mercado E 3rd, Orduña I (2019): Enriched Environments as a Potential Treatment for Developmental Disorders: A Critical Assessment. Frontiers in psychology. 10:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Werner S, Malaspina D, Rabinowitz J (2007): Socioeconomic status at birth is associated with risk of schizophrenia: population-based multilevel study. Schizophrenia bulletin. 33:1373–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Agerbo E, Sullivan PF, Vilhjalmsson BJ, Pedersen CB, Mors O, Børglum AD, et al. (2015): Polygenic risk score, parental socioeconomic status, family history of psychiatric disorders, and the risk for schizophrenia: a Danish population-based study and meta-analysis. JAMA psychiatry. 72:635–641. [DOI] [PubMed] [Google Scholar]
- 107.Hackman DA, Farah MJ, Meaney MJ (2010): Socioeconomic status and the brain: mechanistic insights from human and animal research. Nature reviews Neuroscience. 11:651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


