Ventriculostomy-associated infections in critically ill patients remain therapeutically challenging because of drug- and disease-related factors that contribute to suboptimal antibiotic concentrations in cerebrospinal fluid. Optimal antibiotic dosing for the treatment and prevention of such infections should be based on robust and contextually specific pharmacokinetic data. The objects of this study were to describe and critically appraise studies with reported antibiotic concentrations or pharmacokinetic data in cerebrospinal fluid of critically ill patients without meningeal inflammation.
KEYWORDS: antibiotics, beta-lactams, cephalosporins, aminoglycosides, fluoroquinolones, oxazolidinones, polymyxins, lipopeptides, glycopeptides, fosfomycin, metronidazole, rifampicin, pharmacokinetics, pharmacodynamics, pharmacokinetic/pharmacodynamic, ventriculitis, ventriculostomy-associated infection, external ventricular drain, critical illness, intensive care unit
ABSTRACT
Ventriculostomy-associated infections in critically ill patients remain therapeutically challenging because of drug- and disease-related factors that contribute to suboptimal antibiotic concentrations in cerebrospinal fluid. Optimal antibiotic dosing for the treatment and prevention of such infections should be based on robust and contextually specific pharmacokinetic data. The objects of this study were to describe and critically appraise studies with reported antibiotic concentrations or pharmacokinetic data in cerebrospinal fluid of critically ill patients without meningeal inflammation. We systematically reviewed the literature to identify published reports and studies describing antibiotic concentrations, pharmacokinetics, and pharmacokinetics/pharmacodynamics in cerebrospinal fluid of critically ill patients with uninflamed meninges. Fifty-eight articles met the inclusion criteria. There was significant heterogeneity in methodologies and results. When available, antibiotic pharmacokinetic parameters displayed large intersubject variability. Intraventricular dosing achieved substantially higher antibiotic concentrations in cerebrospinal fluid than did intravenous doses. Few studies conducted a robust pharmacokinetic analysis and described relevant clinical pharmacokinetic/pharmacodynamic indices and exposure targets in cerebrospinal fluid. Robust and clinically relevant antibiotic pharmacokinetic data describing antibiotic disposition in cerebrospinal fluid are necessary. Such studies should use a standardized approach to accurately describe pharmacokinetic variability. These data should ideally be tied to clinical outcomes whereby therapeutic targets in the cerebrospinal fluid can be better defined. Altered dosing strategies, in conjunction with exploring the utility of therapeutic drug monitoring, can then be developed to optimize antibiotic exposure with the goal of improving outcomes in this difficult-to-treat patient group.
INTRODUCTION
Management of nosocomial central nervous system (CNS) infections in critically ill patients remains challenging. Of these, ventriculostomy-associated infection (VAI), including ventriculitis, is the most common and a serious complication associated with the surgical insertion of external ventricular drains (EVDs) (1). Non-EVD-associated ventriculitis can also occur secondary to local trauma associated with neurosurgical intervention or contiguous spread from the ears. Conditions necessitating EVD placement include increased intracranial pressure secondary to intracranial hemorrhage, space-occupying lesions, or head trauma (2).
VAIs lead to significant morbidities such as neurological sequelae (including permanent disability), lengthened intensive care unit (ICU) and hospital admission, and increased health care expenditures (3, 4). The diagnostic difficulty characteristic of VAI is a major contributor to the considerable variation in incidence seen in the literature, reported to be anywhere between 0 and 45% (2, 5–9). Additionally, there is a lack of consensus on the risk factors associated with these infections.
Antibiotic use in the treatment and prevention of VAI can be complicated by disease- and drug-related factors as well as critical illness itself. Variable meningeal inflammation in the context of VAI can result in an unpredictable and potentially decreased penetration of some antibiotics across the blood-cerebrospinal fluid (CSF) barrier (10). Hence, current antibiotic dosing regimens that have been derived from the treatment of infections with a high degree of meningeal inflammation, such as meningitis, may not be generalizable in the setting of VAI.
The physicochemical properties of antibiotics (including lipophilicity, molecular size, and degree of protein binding) influence key pharmacokinetic (PK) parameters (such as the volume of distribution [V]) and, thus, their distribution into CSF and need to be considered when deriving dosing strategies for VAI treatment and prophylaxis (10). Similarly, the PK changes seen in critical illness may also alter CSF antibiotic concentrations and further complicate dose optimization strategies (11). Dosing strategies should be based on contextually specific knowledge of antibiotic PK parameters and their variability in CSF.
Additionally, an understanding of antibiotic PK/pharmacodynamic (PD) relationships in CSF is also important to optimize dosing regimens. For example, the PK/PD index describing the bactericidal efficacy of the concentration-dependent aminoglycosides is the ratio of the maximum free drug concentration to the pathogen MIC (fCmax/MIC) (11). The bactericidal activity of time-dependent beta-lactams is described by the time that free drug concentrations remain above the MIC (fT>MIC) (11). The bactericidal activity of vancomycin, which is both concentration and time dependent, is related to the ratio of the area under the concentration-time curve for the free, unbound fraction of the drug to the MIC (fAUC/MIC) (11). Pharmacokinetic/pharmacodynamic targets in plasma have been widely reported but may not necessarily be directly relevant to CSF in the setting of ventriculitis.
The aim of this systematic review was to describe and critically appraise studies with antibiotic PK data in the CSF of critically ill patients with uninflamed meninges during the treatment and prophylaxis of VAI and the treatment of extracerebral infections. Where applicable, this systematic review also summarized PK/PD data that were reported in the included studies.
RESULTS
Studies identified.
The results of the search strategy are detailed in Fig. 1. Numerous articles were identified through searches of referenced papers. After discussion between the two reviewers, 58 studies were included in the systematic review (Table 1).
FIG 1.
PRISMA flow diagram illustrating the screening and selection of the included studies.
TABLE 1.
Summary of studies retrieved with dosing and sampling regimens, methods of PK analysis, antibiotic concentrations in CSF, and percent CNS penetrationa
| Antibiotic (reference) | Dosing practices (PK/PD modeling reference) | No. of subjects | Population | CSF sampling | PK analysis | CSF concn (mg/liter)b (range) | % CNS penetrationc [mean ± SD, median (range), or range] |
|---|---|---|---|---|---|---|---|
| Aminoglycosides | |||||||
| Amikacin (12) | i.v. 1 g Q24h | 1 | VAI treatment | Single sample 1 h post-1st IVT dose | None | >100 | NA |
| IVT 40 mg Q24h, EVD clamped for 1 h after | |||||||
| Gentamicin (13) | i.v. 100 mg Q8h | 1 | VAI treatment | Single sample 24 h post-1st IVT dose, 6th i.v. dose | None | 1.59 | NA |
| IVT 5 mg Q24h | |||||||
| Gentamicin (14) | i.v. 80-mg single dose | 19d | VAI prophylaxis | 1–2.5 h postdose | None | 0.1–0.2 | NA |
| Netilmicin (15) | i.v. 400-mg single dose over 30 min | 6 | Extracerebral infections | Predose, immediately postdose, 10 min postdose, and 0.5, 1, 2, 4, 7, 10, 13, 16, 20, and 2 h postdose | Noncompartmental | 0.13–0.45 | 7.4–29.1 |
| Beta-lactams | |||||||
| Meropenem (20) | i.v. 1 g Q12h over 2 h | 10 | Extracerebral infections | Predose and 0.5, 1, 2, 3, 4, 6, 8, and 12 h post-1st dose | Noncompartmental | 5.05–8.22 (Cmax) | 52.22 |
| Meropenem (21) | i.v. 1-g single dose over 2 h | 6 | Unclear | Predose and 0.5, 1, 2, 3, 4, 6, and 12 h postdose | Noncompartmental | 5.59–6.87 (Cmax) | 52.27 |
| Meropenem (16) | i.v. 8–15-g/day continuous infusion | 1 | VAI treatment | Daily from days 26–31 of treatment | None | 31.3–64.0 | NA |
| Meropenem (17) | i.v. 1–2 g Q8h over 4 h | 21 | VAI treatment | Immediately predose and 4 h postdose at steady state | Population | <0.2–6.2 | 9 (3–16) |
| Meropenem (18) | i.v. 2 g Q6–8h over 30 min | 1 | VAI treatment | Daily on days 5–7 of treatment | None | <1.0–3.0 | NA |
| Meropenem (22) | i.v. 1–2 g Q8h over 30 min (19) | 10 | Extracerebral infections | NA | Population | NA | NA |
| Meropenem (19) | i.v. 1–2 g Q8h over 30 min | 10 | Extracerebral infections | Pre-1st dose, immediately post-1st dose, and 0.5, 1, 2, 4, 7, 10, 13, and 16 h post-1st dose | Noncompartmental | 0.13–1.13 | 4.7 ± 2.2 |
| Piperacillin-tazobactam (23) | i.v. 6.5-g single dose over 30 min | 9 | Extracerebral infections | Predose, immediately postdose, 10 min postdose, and 0.5, 1, 2, 4, 7, 10, 13, and 16 h postdose | Noncompartmental | Piperacillin, 0.75–6.03 | Piperacillin, 5.1 ± 3.5 |
| Tazobactam, 0.1–0.9 | Tazobactam, 1.7 ± 2.32 | ||||||
| Temocillin (82) | i.v. 2 g Q12h | 2 | Unclear | Variable; 4–8 samples between 30 min and 8 h after 2nd, 4th, and 8th doses | None | <0.5–8.5 | NA |
| Cephalosporins | |||||||
| Cefazolin (24) | i.v. continuous infusion of 8–10 g/day | 1 | VAI treatment | Q24–48h from day 2 | None | 6.1–12.0 | NA |
| Cefepime (36) | i.v. 2 g Q12h (25) | 7 | Extracerebral infections | NA | Population | NA | NA |
| Cefepime (25) | i.v. 2 g Q12h over 30 min | 7 | Extracerebral infections | 0.5, 5–7, and ∼8 h after 4th dose | Compartmental | 0.1–2.5 | 8 (4–34) |
| Cefepime (35) | i.v. 2 g Q12h (25) | 7 | Extracerebral infections | NA | Population | NA | 23 ± 57 |
| Simulated doses, i.v. 2 g Q12h, i.v. 2 g Q8h, and i.v. 2-g load followed by 250 mg/h | |||||||
| Cefepime (13) | i.v. 2 g Q8h over 30 min | 1 | VAI treatment | 1 and 8 h after 6th dose | None | 2.18–2.88 | NA |
| Cefoperazone/sulbactam (37) | i.v. 3-g single dose over 2 h | 5 | Unclear | 0, 0.5, 1, 2, 3, 4, 6, 8, and 12 h postdose | Noncompartmental | 53.15–55.78 (Cmax) | 28.6–37.4 |
| Cefotaxime (26) | i.v. 2 g Q8h | 3e | Extracerebral infections | Variable and unclear | None | <0.10–1.03 | NA |
| Pt 2, 4.75 h post-1st dose and then 2, 4, 6, and 8 h after 2nd dose | |||||||
| Pt 5, predose and 2, 4, and 6 h post-2nd dose | |||||||
| Pt 6, 2 h post-1st dose | |||||||
| Cefotaxime (27) | i.v. 2 g Q8h | 1 | Extracerebral infections | 2, 3, 4, 5, 6, and 7 h postdose; unclear how many previous doses | None | 1.42–3.81 | NA |
| Cefotaxime (28) | i.v. 2-g single dose over 30 min | 6 | Extracerebral infections | 5 min, 15 min, and 0.5, 1, 2, 4, 7, 10, 13, 16, and 24 h postdose | Noncompartmental/compartmental | 0.14–1.81 | 3–21 |
| Ceftaroline (29) | i.v. 600 mg Q6h | 1 | VAI treatment | Immediately predose on days 9 and 13 | None | 0.4–0.5 | NA |
| Ceftaroline (30) | i.v. 600-mg single dose over 1 h | 9 | VAI prophylaxis | Predose and 0.5, 1, 3, 6, 12, and 24 h postdose | Compartmental | 0.01–0.9 | 6.4 |
| Ceftaroline (31) | i.v. 300–600 mg Q12h (dependent on CrCL) over 1 h | 5 | VAI prophylaxis | 2, 6, and 12 h after 4th dose | Noncompartmental | 0.04–0.07 (mean Cmin–mean Cmax) | 1.1 (mean) |
| Ceftazidime (22) | i.v. 3-g single dose over 30 min (32) | 8 | Extracerebral infections | NA | Population | NA | NA |
| Ceftazidime (32) | i.v. 3-g single dose over 30 min | 8 | Extracerebral infections | 10 min and 0.5, 1, 2, 4, 7, 10, 13, and 16 h postdose | Noncompartmental | 0.73–2.8 (Cmax range) | 5.7 ± 3.1 |
| Ceftazidime (38) | i.v. 2 g Q8h over 30 min | 11 | VAI prophylaxis | Pre-6th dose and 1, 3, and 7 h post-6th dose | Compartmental | 0–25.5 | 14.6 (median) |
| Ceftazidime (33) | i.v. 2 g Q8h over 30 min | 3 | VAI prophylaxis | Pre-3rd dose and 0.25, 1, 2, 3, 4, 6, and 8 h post-3rd dose | Compartmental | 0.27–6.00 | 1–13 |
| Ceftizoxime (14) | i.v. 2-g single dose | 19d | VAI prophylaxis | 1–2.5 h postdose | None | 0.3–5.2 | NA |
| Ceftriaxone (36) | i.v. 2-g single dose (28) | 7 | Extracerebral infections | NA | Population | NA | NA |
| Ceftriaxone (28) | i.v. 2-g single dose | 7 | Extracerebral infections | 5 min, 15 min, and 0.5, 1, 2, 4, 7, 10, 13, 16, and 24 h postdose | Noncompartmental/compartmental | 0.18–1.04 | 0.6–1.8 |
| Cefuroxime (34) | i.v. 1.5 g Q8h | 4 | VAI prophylaxis | On days 2, 4, and 6 of therapy at 1.5 and 6 h postdose | None | 0.35–2.03 | NA |
| Polymyxins | |||||||
| Colistin (39) | i.v. 720 mg/day | 1 | VAI treatment | Unclear, but multiple samples taken pre- and 1 h post-IVT dose | None | 6.0–15.6 | NA |
| IVT 10–20 mg/day | |||||||
| Colistin (42) | i.v. 75–225 mg daily over 30 min | 1 | VAI treatment | Predose and 10 min, 1 h, 2 h, 6 h, and 8 h postdose after 2 days of therapy | Noncompartmental | 0.47–0.90 | 5.1 |
| Colistin (40) | Group 1, i.v. 90 mg daily | Group 1, 5 | Group 1, extracerebral infections | Group 1, day 1 (1, 4, and 8 h), day 3 (1 and 8 h), and day 5 (1 h) | None | Group 1, 0.65–1.70 | NA |
| Group 2, i.v. 90 mg daily | Group 2, 3 | Group 2, VAI treatment | Group 2, day 1 (1, 4, and 8 h), day 3 (1 and 8 h), and day 5 (1 and 8 h) | Group 2, 1.03–2.75 | |||
| Group 3, i.v. 90 mg daily + IVT 10 mg daily | Group 3, 4 | Group 3, VAI treatment | Group 3, day 1 (1, 4, and 8 h), day 3 (1 h), and day 5 (1 and 8 h) | Group 3, 5.48–15.73 | |||
| Colistin (41) | IVT 2.61–10.44 mg per day; EVD clamped for 1 h postdose | 9 | VAI treatment | After 2 days of therapy, 0.5, 1, 2, 4, 8, and 16 h postdose | Population | 0.99–22.1 | NA |
| Fluoroquinolones | |||||||
| Ciprofloxacin (43) | i.v. 200 mg Q12h over 30 min | 9 | Extracerebral infections | 60 and 600 min post-1st dose in 3 Pts and post-3rd dose in 6 Pts | Noncompartmental | 0.042–0.317 | 21.3–33.5 |
| Levofloxacin (44) | i.v. 500 mg Q12h over 1 h | 10 | Extracerebral infections | Pre-6th dose and 0.5, 1, 2, 4, 6, 8, and 11 h post-6th dose | Compartmental | 2.80–5.32 | 71 ± 7 |
| Ofloxacin (45) | i.v. 400 mg daily over 30 min | 6 | Extracerebral infections | Pre-1st dose and 10 min, 30 min, and 1, 2, 4, 7, 10, 13, 16, and 20 h post-1st dose | Noncompartmental | 1.00–2.85 | 65 ± 8 |
| Pefloxacin (46) | i.v. 400-mg single dose over 1 h | 9 | Extracerebral infections | 1, 2.5, 4, 5, 6, 7, 8, 10, 12, 16, 20, 24, 28, 36, and 48 h | Noncompartmental | 0.9–3.0 | NA |
| Fosfomycin (47) | i.v. 8 g Q8h | 6 | VAI treatment | Q1–2h post-1st dose and then Q4h after steady state | Noncompartmental | 23–100 | 27 ± 8 |
| Glycopeptides | |||||||
| Vancomycin (51) | i.v. infusion over 4 h Q12h (daily dose of 500–4,000 mg) | 21 | VAI treatment | 1st dose and steady state, predose, and 4 h postdose | Population | <0.24–3.95 | 3 (1–18) |
| Vancomycin (52) | i.v. 1-g loading dose followed by continuous infusion at 125 mg/h | 16 | Not mentioned | Pre-1st dose and 1.08, 1.25, 1.5, 2, 3, 5, 7, 9, 13, 17, 21, 25, 33, 41, 49, 57, 65, and 73 h post-1st dose | Population | NA | NA |
| Vancomycin (18) | i.v. 2-g loading dose and then 3.5–6 g/day of continuous infusion | 1 | VAI treatment | Daily on days 5–6 of treatment | None | 3–6 | NA |
| Vancomycin (77) | i.v. single dose of 1 g over 1 h | 9 | VAI prophylaxis | Immediately post-1st dose and 0.25, 0.5, 1, 2, 4, 6, 8, and 12 h post-1st dose | None | 2.55–6.24 (mean Cmin–Cmax) | NA |
| Vancomycin (48) | i.v. continuous infusion of 4 g/day | 1 | VAI treatment | Daily; exact timing unclear | None | i.v., 2.0–3.3 | NA |
| IVT 5 mg Q24h | IVT, 10.0–25.4 | ||||||
| Vancomycin (50) | i.v. 1 g Q12h | 1 | VAI treatment | Q2–3d from 1st dose | None | 25.6–192.5 | NA |
| IVT 20–40 mg/day | |||||||
| Vancomycin (49) | i.v. 500 mg Q6h | i.v., 5 | VAI treatment | 1, 5, 9, 13, 17, and 21 h post-1st dose | None | i.v., 0.08–5.15 (mean Cmin–Cmax) | NA |
| IVT 10 mg daily | IVT, 5 | IVT, 3.74–1,158.60 (mean Cmin–Cmax) | |||||
| Vancomycin (83) | i.v. 15-mg/kg of body wt load and then continuous infusion of 50–60 mg/kg/day | 6 | VAI treatment | Unclear | Noncompartmental | 2.4–4.9 | NA |
| Vancomycin (79) | IVT 10 mg daily | 3 | VAI treatment | 1 h and then q2h post-1st dose | None | 7.6–292.9 (mean Cmin–Cmax) | NA |
| Vancomycin (80) | IVT 10 mg daily | 1 | VAI treatment | Unclear | None | 16–606 | NA |
| Vancomycin (81) | IVT 50 mg | 1 | VAI treatment | 12 and 24 h post-1st dose | Noncompartmental | 19–60 | NA |
| Vancomycin (14) | i.v. single dose of 1 g over 1 h | 19d | VAI prophylaxis | 1–2.5 h postdose | None | 0–0.9 | NA |
| Vancomycin (78) | i.v. 500 g Q6h | 1 | VAI treatment | After 7 doses, Q15m for 2 h (8 samples of plasma + CSF) and then daily | None | i.v., 5 | NA |
| IVT 20 mg/day | IVT, 5–50 | ||||||
| Lipopeptides | |||||||
| Daptomycin (53) | i.v. 10 mg/kg daily | 1 | VAI treatment | Only 2 samples, peak (day 10 after start of IVT therapy) and trough (day 11 after start of IVT therapy) | None | 1.39–6.30 | NA |
| IVT, 10 mg daily for 2 days and then 10 mg every other day | |||||||
| Daptomycin (55) | IVT 5 mg daily into R EVD | 1 | VAI treatment | Unclear | None | R, 1.32–112.2 | NA |
| L, 0.37–37.4 | |||||||
| Daptomycin (54) | i.v. 1 g daily | 1 | VAI treatment | Pre- and 30 min postdose; unclear how many doses before sampling | None | IVT 10 mg, 23–483 | NA |
| IVT 10 mg/3 days, reduced to 5 mg/3 days | IVT 5 mg, 9.9–139 | ||||||
| Oxazolidinones | |||||||
| Linezolid (56) | i.v. 600 mg Q12h over 30 min | 7 | VAI prophylaxis | For 1st + 5th doses, predose and 1, 2, 4, 6, 8, 10, and 12 h postdose | Noncompartmental | 0.11–12.6 | 56.5 |
| Linezolid (57) | i.v. 600 mg Q12h over 30 min | 5 | VAI treatment | For single and multiple doses, 1, 4, 8, and 12 h postdose | Noncompartmental | 0.8–8.6 | 80 ± 30 |
| Linezolid (58) | i.v. 600 mg Q12h | 2 | Pt 1, VAI | Pt 1, after 1st and 4th doses | None | Pt 1, 1.4–4.2 | NA |
| Pt 2, cerebral abscess | Pt 2, daily after day 10, 0.5–3 h postdose | Pt 2, ND–3.9 | |||||
| Linezolid (59) | i.v. 600 mg Q12h over 1 h | 14 | VAI treatment/prophylaxis | Steady state, predose, and 1.5, 2.5, 4, 6, 9, and 12 h postdose | Noncompartmental | 1.2–20.4 | 70 ± 10 |
| Linezolid (60) | i.v. 600 mg Q12h over 1 h | 11 | VAI treatment | Steady state, predose, 1 h postdose (in 7 Pts), and 3, 5, 8, and 12 h postdose | Population | ∼0.1–7.1 | 77 (58–87) |
| Metronidazole (62) | i.v. 500 mg Q8h over 30 min | 4 | Extracerebral infections | Steady state, predose, and then 8–12 samples over 8 h postdose (exact timing unclear) | Noncompartmental | 2–5 | 86 ± 16 |
| Rifampicin (63) | i.v. 200-mg single dose over 3 h | 7 | Extracerebral infections | 1, 2, and 3 h after start and then 0.5, 1, 2, 4, 6, 10, 12, 14, and 22 h after end of infusion | Noncompartmental | 0.57–1.24 | 22 (13–42) |
CSF, cerebrospinal fluid; PK, pharmacokinetic; CNS, central nervous system; SD, standard deviation; i.v., intravenous; Q24h, every 24 h; IVT, intraventricular; VAI, ventriculostomy-associated infection; NA, not available; Cmax, maximum concentration; PK/PD, pharmacokinetic/pharmacodynamic; CrCL, creatinine clearance; Pt, patient; Cmin, minimum concentration; ND, not detectable. Q2–3d, every 2 to 3 days; Q15m, every 15 min; R, right; L, left.
Reported concentrations achieved over the sampling period.
CSF/plasma AUC ratios.
Unclear how many patients received each antibiotic with CSF data in this study.
Only 3 patients without meningitis and EVD in situ.
Quality of included studies.
A comparison of how well the retrieved papers met the 24-item checklist of the ClinPK statement for clinical PK studies can be found in Table S1 in the supplemental material. There were some notable deficiencies in study quality as assessed by the ClinPK reporting criteria. Importantly, only 27/58 (47%) studies described known PK data relevant to study antibiotics (checklist item 3), and 39/58 (67%) studies provided specific hypotheses and objectives (checklist item 5). Only 29/58 studies (50%) described PK modeling methods and software used (checklist item 11), while 10/58 (17%) studies provided relevant variables that may explain PK variability (checklist item 18), and only 28/58 (48%) studies reported the results of PK analyses with appropriate measures of precision (checklist item 19). Only 8/58 (14%) studies performed a population PK analysis (checklist item 12) of measured concentration data to more robustly characterize variability in antibiotic PK parameters; only 6 of these studies specifically identified covariates. Furthermore, only 16/58 (30%) studies met reporting criteria for describing study limitations (checklist item 22).
Overview of antibiotic pharmacokinetics and pharmacokinetics/pharmacodynamics in cerebrospinal fluid.
A full summary of reported antibiotic PK parameters in CSF and plasma, with patient renal function, can be found in the supplemental material (see Table S2 for CSF data and Table S3 for plasma data). There was substantial variability in reported antibiotic PK parameters.
(i) Aminoglycosides.
Aminoglycoside CSF concentrations were measured during the treatment of ventriculitis in two case reports (12, 13). Intraventricular administration resulted in higher CSF concentrations than with systemic dosing, with amikacin concentrations above 100 mg/liter in one report (12). The intravenous (i.v.) route did not achieve CSF concentrations above 0.5 mg/liter in two studies, one of which used gentamicin for VAI prophylaxis (14), while the other study used netilmicin for the treatment of extracerebral infections (15). Pharmacokinetic analysis of measured netilmicin concentrations demonstrated a mean CSF penetration of 20% in six patients, but no PK/PD relationships were described (15).
(ii) Beta-lactams.
Pharmacokinetic parameters of beta-lactams in CSF exhibited intersubject and interstudy variability. No studies describing intraventricular administration were found.
In a case report, high-dose (8 to 15 g/day) meropenem as a continuous infusion for the treatment of ventriculitis achieved uniformly higher CSF concentrations (16) than standard doses of 3 to 6 g/day (17–19). CNS penetration was highly variable (16, 17, 19–21) as well as directly proportional to the level of meningeal inflammation (19). In one study, meropenem clearance (CL) from CSF was higher than its rate of CSF penetration (17). In one analysis, the simulated probabilities of achieving ≥40%, ≥60%, ≥80%, and 100% T>MIC in CSF after a dose of 1 g meropenem every 12 h as a 2-h infusion were calculated for MICs of 1 mg/liter (91.52%, 75.55%, 53.89%, and 34.13%, respectively), 2 mg/liter (35.78%, 17.11%, 7.26%, and 3.05%, respectively), 4 mg/liter (1.81%, 0.46%, 0.13%, and 0%, respectively), and 8 mg/liter (0% for all targets) (20). Another simulation analysis demonstrated a 53% probability of exceeding CSF trough concentrations of 2 mg/liter with standard doses of 1 to 2 g every 8 h (17). The meropenem CSF T>MIC for highly susceptible organisms (MIC ≤ 0.125 mg/liter) was 100% for the dosing interval but below the MIC of moderately susceptible organisms (0.5 mg/liter) for the entire dosing interval in 60% of subjects when standard doses were utilized (19). Lodise et al. conducted a simulated PK/PD analysis (22) of previously reported data (19), calculating the probability of achieving 50% and 100% T>MIC in CSF for MICs of 0.125 mg/liter (>80% and >80%, respectively), 0.25 mg/liter (>80% and >60%, respectively), 0.5 mg/liter (∼60% and >40%, respectively), 1 mg/liter (>20% and >20%, respectively), 2 mg/liter (<20% and <10%, respectively), 4 mg/liter (0% for both targets), and 8 mg/liter (0% for both targets).
None of these CSF simulations were accompanied by clinical outcome data in patients with VAI.
A single dose of 6.5 g of i.v. piperacillin-tazobactam for the treatment of extracerebral infections among 9 subjects achieved variable CSF concentrations, exposures, and CNS penetration (23). A limited PK analysis was performed (Table S2), and quantification of PK/PD relationships was lacking.
Cephalosporins were the beta-lactam antibiotic class with the most CSF PK data, achieving a wide range of concentrations in CSF (see Fig. 2 for a comparison of cephalosporin CSF concentrations).
FIG 2.
Forest plot of cephalosporin concentrations in cerebrospinal fluid (13, 14, 24–34, 37, 38).
In a case report of ventriculitis, high-dose cefazolin (8 to 10 g/day) via continuous infusion attained relatively higher CSF concentrations (24) than other cephalosporins (13, 14, 25–34), with 100% T>MIC for an MIC of 2 mg/liter. This finding was associated with clinical and bacteriological cure in one patient (24).
Standard doses of cefepime (4 to 6 g/day) were unable to achieve steady-state CSF concentrations of >3 mg/liter (13, 25), with CNS penetration ranging from 8 to 34% (25, 35) and clearance from CSF being higher than the rate of CSF penetration in one study (25). A dosing regimen of cefepime at 2 g every 12 h achieved 100% T>MIC against a highly susceptible (MIC ≤ 0.125 mg/liter) Enterobacter cloacae isolate in one patient, supported by clinical outcome data (13). In a separate study, simulated cefepime doses of 4 to 6 g/day were unable to attain CSF PK/PD targets of 50 to 100% T>MIC for >90% of patients for MICs of ≥0.5 mg/liter and for >80% of patients for MICs of ≥1 mg/liter (35). However, a simulated front-loading dose of 2 g followed by a continuous infusion of 250 mg/h achieved a 76% probability of maintaining 50 to 100% CSF T>MIC above an MIC of 1 mg/liter for the duration of the infusion (35). In another simulation study involving cefepime (36), the calculated probabilities of achieving a target of 100% T>MIC were >80% for MICs of 0.25 mg/liter and 0.5 mg/liter and >60%, >40%, >20%, and >20% for MICs of 1 mg/liter, 2 mg/liter, 4 mg/liter, and 8 mg/liter, respectively. However, in these studies, no outcome data were provided.
A single 3-g dose of cefoperazone/sulbactam achieved remarkably high CSF concentrations (53 to 55 mg/liter) compared to other cephalosporins, with CNS penetration of 28 to 37% (37). No other CSF PK or PK/PD data were reported.
Cefotaxime CSF PK data were reported in patients receiving treatment for extracerebral infections (Table S2), with measured CSF concentrations remaining below 4 mg/liter (26–28). When patients were randomized to receive cefotaxime or ceftriaxone, the CNS penetration of ceftriaxone was 10-fold lower (1%) than that of cefotaxime (12%), but because of the markedly different plasma concentrations achieved, similar concentrations and AUCs were reported in CSF (28). A simulated PK/PD study (36) based on previously reported ceftriaxone PK data (28) calculated an <80% probability of achieving 50% and 100% T>MIC for MIC values of >0.06 mg/liter and >0.015 mg/liter, respectively.
Intravenous ceftaroline used for VAI treatment (29), prophylaxis (30), and treatment of extracerebral infections (31) resulted in CSF concentrations of <1 mg/liter, with similarly low CSF AUCs and CNS penetration ranging from 1% (31) to 6% when corrected for protein binding (30). Ceftaroline CSF penetration was inversely proportional to glycorrhachia, and the rate of distribution into CSF was lower than the rate of clearance from CSF in one study (30). No PK/PD data were described, but a PK/PD assessment based on in vitro analysis of the time-kill curve for bacterial isolates was conducted. Bactericidal activity in CSF, determined by inoculating collected CSF samples with Streptococcus colonies and measuring the MIC, was observed only against a strain of highly susceptible Streptococcus pneumoniae (MIC = 0.023 mg/liter) (31).
Ceftazidime demonstrated wide interpatient variability in CSF concentrations measured in patients receiving 2 g every 8 h for prophylaxis against VAI in one study (38). In another study of extracerebral infections, more uniform concentrations were attained after a single i.v. dose of 3 g, achieving 100% and approximately 25% CSF T>MIC for pathogens with MICs of 0.25 mg/liter and 2 mg/liter, respectively (32). Nevertheless, CNS penetration was below 15% in the majority of patients (32, 33, 38), and PK/PD simulations in CSF were not supported by clinical outcome data (32).
Cerebrospinal fluid concentrations of ceftizoxime were variable (14). The cefuroxime Cmax in CSF was reported to be slightly above 2 mg/liter (34), with no other CSF PK or PK/PD data reported.
(iii) Polymyxins.
Intraventricular (IVT) colistin, as both an adjunct (39, 40) and an alternative (41) to i.v. therapy for VAI, achieved higher CSF concentrations and 100-fold-increased AUCs with lower doses than with i.v. administration alone, which was unable to attain concentrations above 2.75 mg/liter in CSF (40, 42) (Table S2). Combined dosing (39) achieved more uniform concentrations than IVT dosing alone (41). A population PK analysis of IVT administered colistin positively correlated colistin clearance from CSF with the EVD output volume (41). In the same study, assuming an MIC of 2 mg/liter, CSF AUC/MIC ratios were 36.2 h, 55.5 to 146.6 h, and 74.6 to 141.5 h with daily dosing regimens of 2.61 mg, 5.22 mg, and 10.44 mg, respectively, and supported by clinical outcome data (41).
(iv) Fluoroquinolones.
Fluoroquinolone PK in CSF have been repeatedly analyzed during treatment of extracerebral infections (43–46) (Table S2). In general, fluoroquinolones demonstrated relatively high CNS penetration, e.g., a mean of 71% for levofloxacin (44). Ciprofloxacin attained the lowest CSF concentrations compared with other quinolones, which remained below 0.5 mg/liter over the sampling period (43). Two studies sought to characterize PK/PD relationships in CSF (44, 45), with Pea and colleagues extrapolating plasma PK/PD targets for levofloxacin to determine MIC thresholds in CSF using standard dosing regimens. These investigators used a priori targets of AUC/MIC and Cmax/MIC ratios of 125 h and 12.2, respectively, and found that the mean MICs of pathogens would need to be less than 0.53 mg/liter and 0.33 mg/liter, respectively, if these targets were to be met in CSF (44). With a dosing regimen of 400 mg i.v. daily and assuming an MIC of 0.5 mg/liter, Nau et al. calculated ofloxacin PK/PD indices in CSF of AUC/MIC and Cmax/MIC ratios of 49.1 h and 4.1, respectively (45). In both studies, no outcome data were provided to clinically support these simulations.
(v) Fosfomycin.
Fosfomycin PK in CSF were analyzed during treatment of VAI, achieving uniformly high CSF concentrations (up to 100 mg/liter), along with increasing concentrations and AUCs after multiple doses (47). Cerebrospinal fluid penetration ranged from 19 to 35%, and based on an i.v. dosing regimen of 8 g every 8 h, the calculated T>MIC values in CSF were 98%, 92%, and 61% for pathogens with MIC values of 8 mg/liter, 16 mg/liter, and 32 mg/liter, respectively. Clinical outcome data were equivocal, but all patients tolerated therapy.
(vi) Glycopeptides.
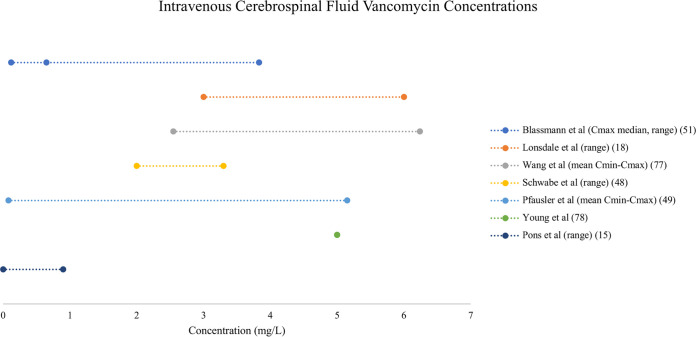
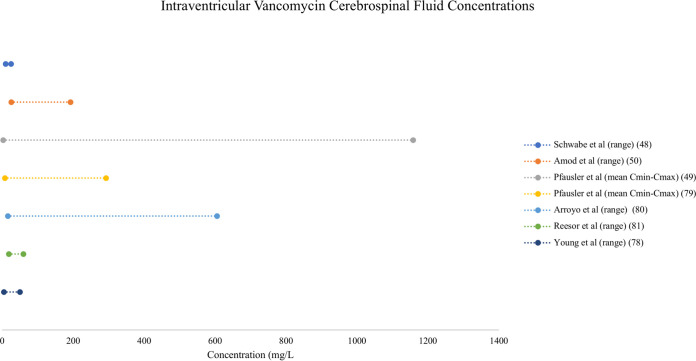
Both i.v. and IVT administration of vancomycin resulted in variable CSF concentrations (see Fig. 3 and 4 for a comparison of vancomycin CSF concentrations resulting from i.v. and IVT dosing, respectively). Consistently higher concentrations were achieved with IVT doses, as both an adjunct and an alternative to i.v. therapy. In one case report, when IVT therapy was added to a continuous i.v. infusion, trough CSF concentrations increased from 2 to 10 mg/liter, and the Cmax increased from 3 to 25 mg/liter (48). Similarly, the mean Cmax surpassed 1,000 mg/liter in a group randomized to receive IVT vancomycin compared to approximately 5 mg/liter in the i.v. group (49). Combined dosing (48, 50) achieved higher CSF trough concentrations than IVT dosing alone (49). Central nervous system penetration ranged from 1 to 18%, and a population PK analysis did not demonstrate a relationship between plasma and CSF AUCs (51). A separate population PK analysis demonstrated increased vancomycin penetration into CSF when CSF albumin concentrations (as a marker of blood-CSF barrier dysfunction) were increased (52). No PK/PD relationships were described.
FIG 3.
Forest plot of vancomycin concentrations in cerebrospinal fluid after intravenous dosing (14, 18, 48, 49, 51, 77, 78).
FIG 4.
Forest plot of vancomycin cerebrospinal fluid concentrations after intraventricular dosing (48–50, 78–81).
(vii) Lipopeptides.
Cerebrospinal fluid concentrations of daptomycin were measured in case reports of ventriculitis treatment with IVT dosing, both in conjunction with (53, 54) and as an alternative to (55) i.v. therapy. Dosing practices varied, and consequently, variable CSF concentrations were achieved, reaching a maximum concentration of nearly 500 mg/liter in one report (54). Cerebrospinal fluid concentrations were not measured when using i.v. treatment alone. In one study, a patient with two in situ EVDs was administered daptomycin, and CSF sampling was conducted over 7 days. The dose of daptomycin was administered into the right EVD, and CSF was collected from both EVDs. Throughout the sampling period of one dosing interval, findings showed consistently lower daptomycin concentrations in samples taken from the left EVD than in samples taken from the right EVD (55).
(viii) Oxazolidinones.
Pharmacokinetic parameters of linezolid in CSF were described during treatment and prevention of ventriculitis (56–60) (Table S2). There was significant interstudy variability in reported CSF concentrations and AUCs, but linezolid manifested high CSF penetration compared to the other antibiotics studied (56–60). One PK analysis demonstrated a statistically significant positive correlation between plasma and CSF concentrations throughout a time-exposure curve (59), while in another analysis, there was a correlation only between trough concentrations in plasma and CSF (60). In the latter population PK analysis, no biological covariates explained the observed PK variability (60). Studies varied on the CSF PK/PD index reported for linezolid, with Luque et al. using the AUC from 0 to 24 h (AUC0–24)/MIC ratio with a target of 80 to 120 h based on previously defined plasma targets (60, 61). The reported CSF AUC0–24/MIC ratios were 42.2 h, 21.1 h, and 10.6 h for pathogens with MICs of 1 mg/liter, 2 mg/liter, and 4 mg/liter, respectively (60). Beer and colleagues reported both AUC0–24/MIC and %T>MIC values. The calculated AUC0–24/MIC ratios were 75.3 and 37.7 h for MICs of 2 mg/liter and 4 mg/liter, respectively. For these same MIC values, the calculated %T>MIC values were 99.8% and 57%, respectively (57). These values were validated with clinical outcome data in patients with VAI. For an MIC of 4 mg/liter, Myrianthefs et al. described an AUC/MIC ratio of 50.8 h, supported by clinical and bacteriological outcome data in two patients with ventriculitis (59). Conversely, Viaggi et al. used %T>MIC as their PK/PD index and calculated T>MIC values of 80%, 68%, and 46% for MICs of 0.5 mg/liter, 1 mg/liter, and 2 mg/liter, respectively (56). This study was conducted in patients who received linezolid for prophylaxis against VAI, so no data on clinical or bacteriological cure could be reported.
(ix) Metronidazole.
Cerebrospinal fluid PK of i.v. metronidazole administered for the treatment of extracerebral infections were analyzed in one study, with CSF concentrations not exceeding 5 mg/liter over the dosing interval but with high CNS penetration (mean of 86%) (62). No PK/PD data were included.
(x) Rifampicin.
Single-dose PK of i.v. rifampicin in CSF were analyzed during treatment of extracerebral infections in one study, demonstrating variable CNS penetration (13 to 42%) and CSF concentrations remaining below 1.25 mg/liter throughout the dosing interval (63). No PK/PD relationships were quantified.
DISCUSSION
Limitations of current data.
There are few robust studies of antibiotic CSF PK data in critically ill patients with uninflamed meninges. The quality of studies could be greatly improved in the future by more closely adhering to the ClinPK criteria for reporting PK studies. In general, sample sizes are relatively small, ranging from case reports to sample sizes of 21. Such small sample sizes may not be sufficiently powered to describe the true PK variability inherent in the critically ill population (64). Bodily fluid sampling is also generally nonstandardized and varies in terms of the sampling timing, method (e.g., lumbar puncture in some patients versus ventricular drainage in others), and number of samples taken. Pharmacokinetic analyses of measured concentration data use heterogeneous approaches (i.e., noncompartmental versus population), and there is a paucity of data, especially in CSF.
The majority of studies report only antibiotic concentrations and no other PK parameters. Of studies that report additional PK parameters, many do not describe the CSF AUC, V, CL, and elimination rate constant (kel), which are important for dose selection. Critically, the movement of drugs between the peripheral, central, and/or CSF compartments is not characterized in most studies. Moreover, key covariates that may explain antibiotic PK variability in CSF, including renal function, organ failure scores, and coadministered medications (e.g., vasopressors or steroids), are not specifically identified or discussed in a large proportion of studies. Population PK modeling to robustly describe PK variability has been performed in only eight studies (17, 22, 35, 36, 41, 51, 52, 60). This modeling approach simultaneously analyzes data at both the population and individual levels to better describe antibiotic PK variability (65). This information can then be combined with identified PK/PD targets and MICs of etiologic pathogens using in silico dosing simulations to determine the dosing regimens most likely to attain target exposures (66). Quantifications of such PK/PD relationships in CSF are currently lacking in the majority of studies. Significantly, very few PK/PD targets have been derived during the treatment of ventriculitis (41, 57, 59, 60), and there remains uncertainty on what is the optimal antibiotic exposure at the site of infection. Importantly, only four studies supported the probability of attaining CSF PK/PD targets with clinical outcome data in patients with ventriculitis (13, 24, 57, 59).
Intravenous dosing.
Standard i.v. doses of most antibiotics, especially hydrophilic agents, may be inadequate for the treatment of ventriculitis. For example, CSF aminoglycoside concentrations are too low to be clinically relevant (14, 15). The relatively narrow therapeutic index of aminoglycosides also precludes increasing systemic doses in order to achieve therapeutic concentrations in CSF. Similarly, standard doses of beta-lactams and cephalosporins may result in theoretically inadequate CSF concentrations maintained throughout the dosing interval, especially against less susceptible organisms (MIC ≥ 0.5 mg/liter) commonly seen in ICU patients (17, 19, 20, 22, 31, 32, 35, 36). However, the dearth of outcome data in patients with ventriculitis makes it difficult to describe a consistent exposure-effect relationship in CSF. Notably, the rates of penetration of meropenem, cefepime, and ceftaroline into CSF may be lower than their clearance rates, further hindering the achievement of therapeutic CSF concentrations (17, 25, 30). This may also lead to unrecognized accumulation in CSF, with potential for CNS toxicity. Antibiotic clearance from CSF comprises two components: diffusion back into blood through the blood-CSF/blood-brain barrier and bulk flow of CSF and brain interstitial fluid into venous blood (10). In the absence of active transport, diffusion into and out of CSF are approximately equal, and the total CSF clearance rate will be higher than the rate of penetration into CSF because of the bulk flow of CSF and brain interstitial fluid into venous blood (10). The extent of drug binding to plasma proteins also seems to play an integral role in CNS penetration and the accumulation of sufficient concentrations at the site of infection. For example, the 10-fold-lower CNS penetration of ceftriaxone than of cefotaxime (28) is likely due to differences in protein binding, e.g., 90 to 95% for ceftriaxone versus 40% for cefotaxime (67). Interestingly, both antibiotics display similar concentration-time profiles in CSF. Higher doses, including the use of front-loading doses with continuous infusions, may be necessary to overcome these limitations (16, 24, 35). Therapeutic drug monitoring (TDM) in CSF may also be useful given the uncertainties in antibiotic concentrations. However, this is possible routinely only in patients with EVDs in situ. Furthermore, until there is a better-described relationship between antibiotic CSF exposure and clinical outcome, TDM may have limited utility.
Intravenous colistin attains inappropriately low concentrations and AUCs in CSF, primarily the result of poor CNS penetration (42). In contrast, fluoroquinolones display comparatively good CNS penetration, but nonetheless, CSF concentrations and AUCs may remain theoretically inadequate (based on PK/PD targets extrapolated from plasma targets in patients with non-CNS infections) for bacteria with even moderately raised MICs. This can be explained by the increased V and reduced AUC observed in plasma due to the high lipophilicity of fluoroquinolones (43–46). Based on theoretical PD targets in CSF extrapolated from plasma targets, higher i.v. doses of fluoroquinolones may be necessary for less susceptible organisms (44, 45). This illustrates the fact that relatively good CSF penetration may be insufficient to guarantee high CSF concentrations or AUCs in ICU patients, as plasma concentrations may be insufficient after unadjusted i.v. dosing. Intravenous fosfomycin, on the other hand, displays reduced penetration compared to fluoroquinolones, but with higher dosing for critical illness (i.e., 8 g every 8 h), it attains considerably higher and clinically relevant concentrations in the CSF of critically ill patients, sufficient against even less susceptible organisms (47). This is likely due to the fact that higher doses (i.e., 8 g every 8 h) produce similarly increased plasma concentrations. Pharmacodynamic indices of fosfomycin in CSF require further validation along with clinical outcome data.
Vancomycin achieves inadequate CSF concentrations after i.v. doses (51), with dose increases being required especially in the setting of augmented renal clearance (18). Intravenous linezolid demonstrates good CNS penetration with moderately high CSF concentrations but with considerable interpatient variability. Although standard i.v. doses (600 mg every 12 h) have resulted in clinical cure of ventriculitis (57, 59), to achieve extrapolated PK/PD targets in CSF, even for susceptible organisms, higher doses may be necessary (56–60). Given that linezolid CSF concentrations correlate with plasma concentrations (59), there may be scope for TDM in plasma as a useful surrogate for measuring CSF concentrations. However, optimal exposure targets in CSF, tied to outcome data, will need to be identified for this approach to have the desired utility. Metronidazole displays CSF penetration similar to that of linezolid (62). Therefore, standard doses of metronidazole are likely appropriate, but no detailed PK/PD analysis has been performed in the study population. Rifampicin achieves similarly poor CSF penetration compared to beta-lactams (63) despite being more lipophilic, likely because of its higher protein binding (up to 90%) (68). Pharmacokinetic/pharmacodynamic relationships of rifampicin in CSF are currently not quantified, and so it remains unclear if standard doses are adequate in treating VAI in critically ill patients.
Intraventricular dosing.
Intraventricular dosing of certain antibiotics, such as aminoglycosides, colistin, vancomycin, and daptomycin, is likely necessary for the treatment of ventriculitis given their generally low CNS penetration and CSF concentrations achieved after systemic i.v. administration. Notably, the IVT route has yet to be studied for use with beta-lactam antibiotics, possibly due to concerns regarding neurotoxicity and seizures. Generally, compared with the i.v. route, very low doses are used for IVT administration. Intraventricular aminoglycosides have been used in case reports and small case series for the treatment of CNS infections (12, 13, 69), achieving high and clinically relevant CSF concentrations. Nonetheless, dosing practices remain inconsistent, and more data are necessary. Intraventricular colistin, as either an adjunct or an alternative to i.v. dosing, attains considerably higher CSF concentrations than with the i.v. route alone (39–42). Combined dosing achieves more uniform concentrations over the dosing interval (39, 40) and may be required if higher volumes of CSF are drained from the EVD (41). A cerebrospinal fluid colistin AUC/MIC ratio of 74.6 to 141.5 h may be an appropriate PK/PD target, as supported by preliminary clinical outcome data in patients with ventriculitis receiving IVT doses recommended by the Infectious Diseases Society of America (41). However, this exposure target will likely need to be validated using more patient outcome data.
Vancomycin concentrations in CSF are at least partially dependent on the level of meningeal inflammation (52, 70), with relatively low CNS penetration overall and substantially variable CSF concentrations following systemic dosing alone (51, 70). Intraventricular vancomycin for VAI appears to be an efficacious modality capable of achieving substantially higher concentrations (49, 71). Cerebrospinal fluid exposures with the IVT route should also be less dependent on patient renal function and dynamic fluid shifts typical of critically ill patients in the acute phase. Combined dosing may be useful to maintain higher trough concentrations in CSF (48, 50). Interestingly, a systematic review by Beach et al. demonstrated no relationship between the overall CSF exposure of vancomycin and clinical/microbiological cure of ventriculitis (70). Hence, a PK/PD relationship in CSF and established targets for optimal exposure have yet to be described for vancomycin. The CNS penetration of daptomycin is low regardless of the level of meningeal inflammation (72), and therefore, the i.v. route is inappropriate. Intraventricular dosing attains substantially higher concentrations, but dosing practices and reported CSF concentrations achieved differ (53–55), and more data are required to determine optimal targets and dosing strategies.
Antibiotic PK/PD considerations in ventriculitis.
Heterogeneous and multisystem pathophysiological derangements are responsible for widely variable antibiotic PK in critically ill patients. A dysregulated host immune response causes widespread endothelial damage, capillary permeability, and changes in fluid distribution, which aggressive fluid resuscitation may amplify (65). This may result in an increased V and, consequently, low antibiotic concentrations in CSF, especially during i.v. administration of hydrophilic antibiotics such as vancomycin and the beta-lactams (65). Hypoalbuminemia can increase unbound plasma concentrations (and, hence, renal clearance) of highly protein-bound antibiotics such as ceftriaxone (73), further impacting CSF concentrations. In some patients, renal dysfunction may reduce antibiotic clearance, potentially resulting in accumulation and toxicity (74). In others, with neurotrauma or subarachnoid hemorrhage, augmented renal clearance, defined as a measured creatinine clearance rate of ≥130 ml/min/1.73 m2 (75), may further reduce CSF antibiotic concentrations and necessitate increased doses (18).
Cerebrospinal fluid dynamics and the function of the blood-CSF/blood-brain barrier in ventriculitis are also important factors influencing antibiotic PK. During meningitis, tight junctions between endothelial cells open (increasing the CSF penetration of hydrophilic antibiotics), proinflammatory cytokines inhibit the activity of efflux pumps such as P-glycoprotein (retaining lipophilic antibiotics such as fluoroquinolones in CSF), and there is decreased CSF outflow, reducing antibiotic elimination from CSF (10). These mechanisms may be less pronounced during VAI, contributing to suboptimal antibiotic exposures at the site of infection. The EVD itself may also remove antibiotics from CSF (41). Moreover, the ventricular CSF may not be uniform, as suggested by significant differences between measured antibiotic concentrations in contralateral EVDs (55). There may be marked variability in individual CSF dynamics, and characterizing sources of PK variability through robust PK analysis is an important step to individualize dosing in patients with ventriculitis.
Another significant consideration is to accurately describe the kill characteristics of antibiotics in CSF to determine the PK/PD relationship and exposure targets for each drug class. At best, studies have used plasma-based PK/PD targets as theoretical targets for CSF antibiotic exposure. Further work is needed to validate whether this extrapolation is justified; for this, outcome data linked to CSF antibiotic exposure in patients with CNS infections such as ventriculitis are required.
A major shortcoming of this review is that it has focused only on antibiotic PK and PK/PD in CSF and has not addressed clinical outcomes or the extent of critical illness that patients were suffering from, in terms of how many patients were in shock or multiple-organ failure, or a summary of their organ failure scores, or whether plasma albumin concentrations were high or low. Moreover, only a qualitative assessment of the available literature has been performed, with data currently being too heterogeneous to provide pooled estimates. Future studies conducted with more homogeneous methods and consistent reporting of PK parameters may provide the means of pooling data to make a quantitative assessment.
Conclusion.
The prevention and treatment of VAI remain challenging, with much uncertainty surrounding therapeutic CSF exposure in treating VAI/CNS infections in the absence of meningeal inflammation. Until PK/PD indices and specific exposure targets can be identified, strategies to optimize dosing remain theoretical. Further research in this area should aim to describe the wide variability in antibiotic PK parameters as well as define contextually specific PK/PD indices in CSF. So that PK studies can be maximally useful for comparison, interpretation, and future PK modeling, reported data should include Cmax, VCSF, intercompartmental rate constants, AUCs, CSF/serum AUC ratios, and CL.
Pharmacokinetic/pharmacodynamic analysis could be used to support alternative dosing strategies such as increased bolus doses, more frequent dosing or continuous infusions, and, importantly, using the IVT route to bypass the blood-CSF barrier to achieve more predictable antibiotic CSF concentrations. Therapeutic drug monitoring in plasma or CSF could potentially be useful for certain antibiotics, where available. However, until research is conducted that establishes optimal antibiotic CSF exposure related to outcome data in critically ill patients with CNS infections, PK/PD analysis remains conceptual, and TDM may have limited scope.
MATERIALS AND METHODS
Search strategy and selection criteria.
The protocol for this systematic review was registered at the PROSPERO database (protocol number CRD42018081372; registered on 9 January 2018). Keyword searches of PubMed and Embase for all years to August 2020 were conducted to identify suitable data for inclusion in the systematic review. Search terms included ([antibiotics OR antimicrobials OR antibacterials OR beta-lactams OR penicillins OR cephalosporins OR carbapenems OR glycopeptides OR aminoglycosides OR fluoroquinolones OR oxazolidinones OR polymyxins OR fosfomycin OR rifamycins OR lipopeptides] AND [cerebral ventriculitis OR {external ventricular drain AND infection}] AND [ICU OR intensive care OR critical illness OR critically ill]) OR ([antibiotics OR antimicrobials OR antibacterials OR beta-lactams OR penicillins OR cephalosporins OR carbapenems OR glycopeptides OR aminoglycosides OR fluoroquinolones OR oxazolidinones OR polymyxins OR fosfomycin OR rifamycins OR lipopeptides] AND [EVD OR ventriculostomy OR external ventricular drain]). The searches were limited to studies published completely in English and Spanish. The resulting outputs were combined, excluding duplicates. After scanning abstracts for relevance, the full text was retrieved for all potentially suitable studies. Pertinent studies from the bibliographies and reference lists of the retrieved articles were also identified and included for review. Conference, congress, and scientific meeting abstracts were not included.
Study content inclusion/exclusion criteria.
The retrieved publications were evaluated with the following inclusion criteria: (i) prospective study; (ii) intensive care setting; (iii) study reporting antibiotic concentrations or PK data in CSF resulting from treatment and/or prophylaxis of VAI or treatment of extracerebral infections; (iv) patients with an in situ device, including an EVD, to collect ventricular CSF; and (v) adult patients.
Exclusion criteria included (i) studies not involving critically ill patients, (ii) the lack of an in situ device to collect ventricular CSF, (iii) an absence of antibiotic concentration or PK data, (iv) patients with meningitis or non-EVD-associated ventriculitis, (v) retrospective studies, (vi) review articles, (vii) meta-analyses, (viii) studies involving pediatric patients, and (ix) studies published in languages other than English or Spanish.
Data extraction and synthesis.
One reviewer (N. Kumta) extracted the following data from each study: number of patients; renal function; reason for antibiotic administration; study antibiotic(s) and dosage practices (including route of administration, infusion times, dose, and frequency); sampling regimen; CSF and plasma PK data, including type of PK analysis, concentrations achieved (including maximum [Cmax] and minimum [Cmin] concentrations and concentration ranges over the dosing/sampling interval); other PK parameters, including the area under the concentration-time curve (AUC), CSF/serum AUC ratio, volume of distribution (V), intercompartmental rate constants (k), clearance (CL), and half-life (t1/2); and PK/PD indices in CSF. Given the heterogeneity of these PK data, there was limited scope to provide pooled and summary data across studies. The quality of retrieved studies was evaluated across six domains, (i) title/abstract, (ii) background, (iii) methods, (iv) results, (v) discussion/conclusion, and (vi) other information, using the 24-item ClinPK checklist for clinical PK studies (Table 2) (76). The quality assessment of CSF PK data was additionally based on how variability in measured CSF PK parameters was described. Cerebrospinal fluid penetration was characterized on the basis of CSF/serum AUC ratios. Cerebrospinal fluid/serum concentration ratios often depend on timing relative to dose (low ratios initially, increasing over the dosing interval) and so were considered to be less accurate. The pharmacodynamic (PD) parameter most commonly used to describe antibiotic potency is the MIC, defined as the lowest antibiotic concentration with no visible bacterial growth after 16 to 20 h of incubation under specifically defined conditions (11). Cerebrospinal fluid PK/PD reporting was assessed and considered acceptable if studies described antibiotic PK/PD indices or the probability of attaining PK/PD targets in CSF using MIC data from either identified organisms or epidemiological cutoff values, preferably with outcome data supporting such targets in CSF. Clinical or bacteriological outcomes are not reported in this review.
TABLE 2.
Clinical pharmacokinetic criteria for clinical PK studies
| Checklist itema |
|---|
| Title |
| 1. Title identifies drug(s) and patient population(s) studied |
| 2. Abstract includes name of drug(s), route of administration, population, results of primary objective, and major clinical PK findings |
| Background |
| 3. PK data that are known and relevant to study drugs are described |
| 4. An explanation of the study rationale is provided |
| 5. Specific objectives or hypotheses are provided |
| Methods |
| 6. Eligibility criteria of study participants are described |
| 7. Coadministration (or lack thereof) of study drug(s) with other potentially interacting drugs or food is described |
| 8. Drug prepn and administration characteristics (including dose, route, formulation, infusion duration, and frequency) are described |
| 9. Body fluid or tissue sampling for quantitative drug measurement is described |
| 10. Validation of quantitative bioanalytical methods used in the study are referenced or described if applicable |
| 11. PK modeling methods and software used are described, including assumptions made regarding no. of compartments and order of kinetics (zero, first, or mixed order) |
| 12. For population PK studies, covariates incorporated into PK models are identified and described |
| 13. Formulas for calculated variables (such as creatinine clearance, body surface area, AUC, and adjusted body wt) are provided or referenced |
| 14. The specific body wt used in drug dosing and PK calculations are reported (i.e., ideal body wt vs actual body wt vs adjusted body wt) |
| 15. Statistical methods, including software used, are described |
| Results |
| 16. Study withdrawals or subjects lost to follow-up (or lack thereof) are reported |
| 17. Quantification of missing or excluded data is provided if applicable |
| 18. All relevant variables that may explain inter- and intrapatient PK variability are provided with appropriate measures of variance |
| 19. Results of PK analyses are reported with appropriate measures of precision (such as ranges or 95% confidence intervals) |
| 20. Studies in patients receiving extracorporeal drug removal (i.e., dialysis) should report the mode of drug removal, type of filters used, duration of therapy, and relevant flow rates |
| 21. In studies of drug bioavailability comparing two formulations of the same drug, F, AUC, Cmax, and Tmax should be reported |
| Discussion |
| 22. Study limitations describing potential sources of bias and imprecision, where relevant, should be described |
| 23. The relevance of study findings (applicability and external validity) is described |
| Other information |
| 24. Funding sources and conflicts of interest for the authors are disclosed |
Tmax, time to maximum concentration of drug in serum.
Supplementary Material
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Ramanan M, Lipman J, Shorr A, Shankar A. 2015. A meta-analysis of ventriculostomy-associated cerebrospinal fluid infections. BMC Infect Dis 15:3. doi: 10.1186/s12879-014-0712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gozal YM, Farley CW, Hanseman DJ, Harwell D, Magner M, Andaluz N, Shutter L. 2014. Ventriculostomy-associated infection: a new, standardized reporting definition and institutional experience. Neurocrit Care 21:147–151. doi: 10.1007/s12028-013-9936-9. [DOI] [PubMed] [Google Scholar]
- 3.Conen A, Fux CA, Vajkoczy P, Trampuz A. 2017. Management of infections associated with neurosurgical implanted devices. Expert Rev Anti Infect Ther 15:241–255. doi: 10.1080/14787210.2017.1267563. [DOI] [PubMed] [Google Scholar]
- 4.Walti LN, Conen A, Coward J, Jost GF, Trampuz A. 2013. Characteristics of infections associated with external ventricular drains of cerebrospinal fluid. J Infect 66:424–431. doi: 10.1016/j.jinf.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Citerio G, Signorini L, Bronco A, Vargiolu A, Rota M, Latronico N, Infezioni LIquorali Catetere Correlate Study Investigators. 2015. External ventricular and lumbar drain device infections in ICU patients. Crit Care Med 43:1630–1637. doi: 10.1097/CCM.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez-Gonzalez R, Boto GR, Perez-Zamarron A. 2012. Cerebrospinal fluid diversion devices and infection. A comprehensive review. Eur J Clin Microbiol Infect Dis 31:889–897. doi: 10.1007/s10096-011-1420-x. [DOI] [PubMed] [Google Scholar]
- 7.Mayhall C, Archer N, Lamb V, Spadora A, Bagget J, Ward J, Narayan R. 1984. Ventriculostomy-related infections: a prospective epidemiologic study. N Engl J Med 310:553–559. doi: 10.1056/NEJM198403013100903. [DOI] [PubMed] [Google Scholar]
- 8.Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES. 2002. Ventriculostomy-related infections: a critical review of the literature. Neurosurgery 51:170–182. doi: 10.1097/00006123-200207000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Bader M, Littlejohns L, Palmer S. 1995. Ventriculostomy and intracranial pressure monitoring: in search of a 0% infection rate. Heart Lung 24:166–172. doi: 10.1016/S0147-9563(05)80012-3. [DOI] [PubMed] [Google Scholar]
- 10.Nau R, Sorgel F, Eiffert H. 2010. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev 23:858–883. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mouton J. 2016. General concepts of pharmacodynamics for anti-infective agents, p 10–13. In Rotschafer J, Andes D, Rodvold K (ed), Antibiotic pharmacodynamics. Springer, New York, NY. [Google Scholar]
- 12.Barrou L, Charra B, Hachimi A, Idali B, Benslama A, Motaouakkil S. 2008. Intrathecal use of amikacin: a case report. Braz J Infect Dis 12:546. doi: 10.1590/s1413-86702008000600022. [DOI] [PubMed] [Google Scholar]
- 13.Barnes BJ, Wiederhold NP, Micek ST, Polish LB, Ritchie DJ. 2003. Enterobacter cloacae ventriculitis successfully treated with cefepime and gentamicin: case report and review of the literature. Pharmacotherapy 23:537–542. doi: 10.1592/phco.23.4.537.32126. [DOI] [PubMed] [Google Scholar]
- 14.Pons VG, Delinger SL, Guglielmo BJ, Octavio J, Flaherty J, Derish PA, Wilson CB. 1993. Ceftizoxime versus vancomycin and gentamicin in neurosurgical prophylaxis: a randomized, prospective, blinded clinical study. Neurosurgery 33:416–423. doi: 10.1227/00006123-199309000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Nau R, Scholz P, Sharifi S, Rohde S, Kolenda H, Prange HW. 1993. Netilmicin cerebrospinal fluid concentrations after an intravenous infusion of 400 mg in patients without meningeal inflammation. J Antimicrob Chemother 32:893–896. doi: 10.1093/jac/32.6.893. [DOI] [PubMed] [Google Scholar]
- 16.Kerz T, von Loewenich FD, Roberts J, Neulen A, Ringel F. 2018. Cerebrospinal fluid penetration of very high-dose meropenem: a case report. Ann Clin Microbiol Antimicrob 17:47. doi: 10.1186/s12941-018-0299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blassmann U, Roehr AC, Frey OR, Vetter-Kerkhoff C, Thon N, Hope W, Briegel J, Huge V. 2016. Cerebrospinal fluid penetration of meropenem in neurocritical care patients with proven or suspected ventriculitis: a prospective observational study. Crit Care 20:343. doi: 10.1186/s13054-016-1523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lonsdale DO, Udy AA, Roberts JA, Lipman J. 2013. Antibacterial therapeutic drug monitoring in cerebrospinal fluid: difficulty in achieving adequate drug concentrations. J Neurosurg 118:297–301. doi: 10.3171/2012.10.JNS12883. [DOI] [PubMed] [Google Scholar]
- 19.Nau R, Lassek C, Kinzig-Schippers M, Thiel A, Prange HW, Sörgel F. 1998. Disposition and elimination of meropenem in cerebrospinal fluid cultures of hydrocephalic patients with external ventriculostomy. Antimicrob Agents Chemother 42:2012–2016. doi: 10.1128/AAC.42.8.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong L, Xu H, Wu C, Kong X, Yu M, Shi Q, Wu X. 2020. Pharmacokinetic/pharmacodynamic analysis of meropenem, by Monte Carlo simulation, in both plasma and cerebrospinal fluid in patients with cerebral hemorrhage after external ventricular drainage. Int J Clin Pharmacol Ther 58:50–56. doi: 10.5414/CP203606. [DOI] [PubMed] [Google Scholar]
- 21.Xu H, Kong L, Wu C, Xu B, Wu X. 2019. Pharmacokinetics of meropenem in plasma and cerebrospinal fluid in patients with intraventricular hemorrhage after lateral ventricle drainage. Eur J Clin Pharmacol 75:595–597. doi: 10.1007/s00228-018-02606-9. [DOI] [PubMed] [Google Scholar]
- 22.Lodise TP, Nau R, Kinzig M, Drusano GL, Jones RN, Sörgel F. 2007. Pharmacodynamics of ceftazidime and meropenem in cerebrospinal fluid: results of population pharmacokinetic modelling and Monte Carlo simulation. J Antimicrob Chemother 60:1038–1044. doi: 10.1093/jac/dkm325. [DOI] [PubMed] [Google Scholar]
- 23.Nau R, Kinzig-Schippers M, Sorgel F, Schinschke S, Rossing R, Muller C, Kolenda H, Prange HW. 1997. Kinetics of piperacillin and tazobactam in ventricular cerebrospinal fluid of hydrocephalic patients. Antimicrob Agents Chemother 41:987–991. doi: 10.1128/AAC.41.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregoire M, Gaborit B, Deschanvres C, Lecomte R, Deslandes G, Dailly E, Ambrosi X, Bellouard R, Asseray N, Lakhal K, Boutoille D. 2019. High-dosage cefazolin achieves sufficient cerebrospinal diffusion to treat an external ventricular drainage-related Staphylococcus aureus ventriculitis. Antimicrob Agents Chemother 63:e01844-18. doi: 10.1128/AAC.01844-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhoney DH, Tam V, Parker D, McKinnon PS, Coplin WM. 2003. Disposition of cefepime in the central nervous system of patients with external ventricular drains. Pharmacotherapy 23:310–314. doi: 10.1592/phco.23.3.310.32108. [DOI] [PubMed] [Google Scholar]
- 26.Bruckner O, Collmann H, Hoffman HG. 1982. Cefotaxime in treatment of meningitis and ventriculitis? Evaluation of drug concentrations in human cerebrospinal fluid. Intensive Care Med 8:33–38. doi: 10.1007/BF01686851. [DOI] [PubMed] [Google Scholar]
- 27.Bruckner O, Collmann H, Borner H. 1983. Cefotaxime levels in ventricular cerebrospinal fluid determined by bioassay and by high-performance liquid chromatography. Chemotherapy 29:237–243. doi: 10.1159/000238204. [DOI] [PubMed] [Google Scholar]
- 28.Nau R, Prange HW, Muth P, Mahr G, Menck S, Kolenda H, Sorgel F. 1993. Passage of cefotaxime and ceftriaxone into cerebrospinal fluid of patients with uninflamed meninges. Antimicrob Agents Chemother 37:1518–1524. doi: 10.1128/aac.37.7.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roujansky A, Martin M, Gomart C, Hulin A, Mounier R. 2020. Multidrug-resistant Staphylococcus epidermidis ventriculostomy-related infection successfully treated by intravenous ceftaroline after failure of daptomycin treatment. World Neurosurg 136:221–225. doi: 10.1016/j.wneu.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Chauzy A, Nadji A, Combes JC, Defrance N, Bouhemad B, Couet W, Chavanet P. 2019. Cerebrospinal fluid pharmacokinetics of ceftaroline in neurosurgical patients with an external ventricular drain. J Antimicrob Chemother 74:675–681. doi: 10.1093/jac/dky489. [DOI] [PubMed] [Google Scholar]
- 31.Stein GE, Yasin F, Smith C, Scharmen A, Havlichek D, Bill C. 2015. A pharmacokinetic/pharmacodynamic analysis of ceftaroline prophylaxis in patients with external ventricular drains. Surg Infect (Larchmt) 16:169–173. doi: 10.1089/sur.2014.098. [DOI] [PubMed] [Google Scholar]
- 32.Nau R, Prange HW, Kinzig M, Frank A, Dressel A, Scholz P, Kolenda H, Sörgel F. 1996. Cerebrospinal fluid ceftazidime kinetics in patients with external ventriculostomies. Antimicrob Agents Chemother 40:763–766. doi: 10.1128/AAC.40.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polk RE, Mayhall CG, Tartaglione T, Baggett J, Patterson P. 1984. Sequential ventricular fluid concentrations of ceftazidime—report of three cases. Drug Intell Clin Pharm 18:984–987. doi: 10.1177/106002808401801209. [DOI] [PubMed] [Google Scholar]
- 34.Kossmann T, Hans V, Stocker R, Imhof H, Joos B, Trentz O, Morganti-Kossmann MC. 1996. Penetration of cefuroxime into the cerebrospinal fluid of patients with traumatic brain injury. J Antimicrob Chemother 37:161–167. doi: 10.1093/jac/37.1.161. [DOI] [PubMed] [Google Scholar]
- 35.Lodise TP, Jr, Rhoney DH, Tam VH, McKinnon PS, Drusano GL. 2006. Pharmacodynamic profiling of cefepime in plasma and cerebrospinal fluid of hospitalized patients with external ventriculostomies. Diagn Microbiol Infect Dis 54:223–230. doi: 10.1016/j.diagmicrobio.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Lodise TP, Jr, Nau R, Kinzig M, Jones RN, Drusano GL, Sörgel F. 2007. Comparison of the probability of target attainment between ceftriaxone and cefepime in the cerebrospinal fluid and serum against Streptococcus pneumoniae. Diagn Microbiol Infect Dis 58:445–452. doi: 10.1016/j.diagmicrobio.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Meng L, Kong L, Wu C, Qi B, Wu X. 2020. Pharmacokinetics of cefoperazone/sulbactam in plasma and cerebrospinal fluid in patients with intraventricular hemorrhage after external ventricular drains. Eur J Clin Pharmacol 76:127–129. doi: 10.1007/s00228-019-02773-3. [DOI] [PubMed] [Google Scholar]
- 38.Fuhr U, Harder S, Hafner S, Rosenthal D, Lorenz R, Staib A. 1989. CSF pharmacokinetics of ceftazidime in neurosurgical patients with an external ventriculostomy. Infection 17:15–16. doi: 10.1007/BF01643492. [DOI] [PubMed] [Google Scholar]
- 39.Perier F, Couffin S, Martin M, Bardon J, Cook F, Mounier R. 2019. Multidrug-resistant Acinetobacter baumannii ventriculostomy-related infection, treated by a colistin, tigecycline, and intraventricular fibrinolysis. World Neurosurg 121:111–116. doi: 10.1016/j.wneu.2018.09.218. [DOI] [PubMed] [Google Scholar]
- 40.Ziaka M, Markantonis SL, Fousteri M, Zygoulis P, Panidis D, Karvouniaris M, Makris D, Zakynthinos E. 2013. Combined intravenous and intraventricular administration of colistin methanesulfonate in critically ill patients with central nervous system infection. Antimicrob Agents Chemother 57:1938–1940. doi: 10.1128/AAC.01461-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imberti R, Cusato M, Accetta G, Marino V, Procaccio F, Del Gaudio A, Iotti GA, Regazzi M. 2012. Pharmacokinetics of colistin in cerebrospinal fluid after intraventricular administration of colistin methanesulfonate. Antimicrob Agents Chemother 56:4416–4421. doi: 10.1128/AAC.00231-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markantonis SL, Markou N, Fousteri M, Sakellaridis N, Karatzas S, Alamanos I, Dimopoulou E, Baltopoulos G. 2009. Penetration of colistin into cerebrospinal fluid. Antimicrob Agents Chemother 53:4907–4910. doi: 10.1128/AAC.00345-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nau R, Prange HW, Martell J, Sharifi S, Kolenda H, Bircher J. 1990. Penetration of ciprofloxacin into the cerebrospinal fluid of patients with uninflamed meninges. J Antimicrob Chemother 25:965–973. doi: 10.1093/jac/25.6.965. [DOI] [PubMed] [Google Scholar]
- 44.Pea F, Pavan F, Nascimben E, Benetton C, Scotton PG, Vaglia A, Furlanut M. 2003. Levofloxacin disposition in cerebrospinal fluid in patients with external ventriculostomy. Antimicrob Agents Chemother 47:3104–3108. doi: 10.1128/aac.47.10.3104-3108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nau R, Kinzig M, Dreyhaupt T, Kolenda H, Sörgel F, Prange HW. 1994. Kinetics of ofloxacin and its metabolites in cerebrospinal fluid after a single intravenous infusion of 400 milligrams of ofloxacin. Antimicrob Agents Chemother 38:1849–1853. doi: 10.1128/aac.38.8.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dow J, Chazal J, Frydman A, Janny P, Woehrle R, Djebbar F, Gaillot J. 1986. Transfer kinetics of pefloxacin into cerebro-spinal fluid after one hour i.v. infusion of 400 mg in man. J Antimicrob Chemother 17:81–87. doi: 10.1093/jac/17.suppl_B.81. [DOI] [PubMed] [Google Scholar]
- 47.Pfausler B, Spiss H, Dittrich P, Zeitlinger M, Schmutzhard E, Joukhadar C. 2004. Concentrations of fosfomycin in the cerebrospinal fluid of neurointensive care patients with ventriculostomy-associated ventriculitis. J Antimicrob Chemother 53:848–852. doi: 10.1093/jac/dkh158. [DOI] [PubMed] [Google Scholar]
- 48.Schwabe M, Juttner E, Blaich A, Potthoff K, Pisarski P, Waller CF. 2007. Cure of ventriculitis and central nervous system shunt infection by Staphylococcus epidermidis with vancomycin by intraventricular injection in a liver transplant recipient. Transpl Infect Dis 9:46–50. doi: 10.1111/j.1399-3062.2006.00157.x. [DOI] [PubMed] [Google Scholar]
- 49.Pfausler B, Spiss H, Beer R, Kampfl A, Engelhardt K, Schober M, Schmutzhard E. 2003. Treatment of staphylococcal ventriculitis associated with external cerebrospinal fluid drains: a prospective randomized trial of intravenous compared with intraventricular vancomycin therapy. J Neurosurg 98:1040–1044. doi: 10.3171/jns.2003.98.5.1040. [DOI] [PubMed] [Google Scholar]
- 50.Amod F, Moodley I, Peer AK, Sunderland J, Lovering A, Wootton M, Nadvi S, Vawda F. 2005. Ventriculitis due to a hetero strain of vancomycin intermediate Staphylococcus aureus (hVISA): successful treatment with linezolid in combination with intraventricular vancomycin. J Infect 50:252–257. doi: 10.1016/j.jinf.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 51.Blassmann U, Hope W, Roehr AC, Frey OR, Vetter-Kerkhoff C, Thon N, Briegel J, Huge V. 2019. CSF penetration of vancomycin in critical care patients with proven or suspected ventriculitis: a prospective observational study. J Antimicrob Chemother 74:991–996. doi: 10.1093/jac/dky543. [DOI] [PubMed] [Google Scholar]
- 52.Li X, Wu Y, Sun S, Mei S, Wang J, Wang Q, Zhao Z. 2015. Population pharmacokinetics of vancomycin in postoperative neurosurgical patients. J Pharm Sci 104:3960–3967. doi: 10.1002/jps.24604. [DOI] [PubMed] [Google Scholar]
- 53.Erritouni M, Ktaich N, Rahal JJ, Figueroa D, Nieto J, Urban C, Mariano N, Eisinger F, Abayev J, Nicolau D, Rubin D, Raifu M, Segal-Maurer S. 2012. Use of daptomycin for the treatment of methicillin-resistant coagulase-negative staphylococcal ventriculitis. Case Rep Med 2012:593578. doi: 10.1155/2012/593578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elvy J, Porter D, Brown E. 2008. Treatment of external ventricular drain-associated ventriculitis caused by Enterococcus faecalis with intraventricular daptomycin. J Antimicrob Chemother 61:461–462. doi: 10.1093/jac/dkm501. [DOI] [PubMed] [Google Scholar]
- 55.Mueller SW, Kiser TH, Anderson TA, Neumann RT. 2012. Intraventricular daptomycin and intravenous linezolid for the treatment of external ventricular-drain-associated ventriculitis due to vancomycin-resistant Enterococcus faecium. Ann Pharmacother 46:e35. doi: 10.1345/aph.1R412. [DOI] [PubMed] [Google Scholar]
- 56.Viaggi B, Paolo AD, Danesi R, Polillo M, Ciofi L, Del Tacca M, Malacarne P. 2011. Linezolid in the central nervous system: comparison between cerebrospinal fluid and plasma pharmacokinetics. Scand J Infect Dis 43:721–727. doi: 10.3109/00365548.2011.582140. [DOI] [PubMed] [Google Scholar]
- 57.Beer R, Engelhardt KW, Pfausler B, Broessner G, Helbok R, Lackner P, Brenneis C, Kaehler ST, Georgopoulos A, Schmutzhard E. 2007. Pharmacokinetics of intravenous linezolid in cerebrospinal fluid and plasma in neurointensive care patients with staphylococcal ventriculitis associated with external ventricular drains. Antimicrob Agents Chemother 51:379–382. doi: 10.1128/AAC.00515-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malacarne P, Viaggi B, Di Paolo A, Danesi R, Del Tacca M. 2007. Linezolid cerebrospinal fluid concentration in central nervous system infection. J Chemother 19:90–93. doi: 10.1179/joc.2007.19.1.90. [DOI] [PubMed] [Google Scholar]
- 59.Myrianthefs P, Markantonis SL, Vlachos K, Anagnostaki M, Boutzouka E, Panidis D, Baltopoulos G. 2006. Serum and cerebrospinal fluid concentrations of linezolid in neurosurgical patients. Antimicrob Agents Chemother 50:3971–3976. doi: 10.1128/AAC.00051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luque S, Grau S, Alvarez-Lerma F, Ferrández O, Campillo N, Horcajada JP, Basas M, Lipman J, Roberts JA. 2014. Plasma and cerebrospinal fluid concentrations of linezolid in neurosurgical critically ill patients with proven or suspected central nervous system infections. Int J Antimicrob Agents 44:409–415. doi: 10.1016/j.ijantimicag.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 61.Rayner CR, Forrest A, Meagher AK, Birmingham MC, Schentag JJ. 2003. Clinical pharmacodynamics of linezolid in seriously ill patients treated in a compassionate use programme. Clin Pharmacokinet 42:1411–1423. doi: 10.2165/00003088-200342150-00007. [DOI] [PubMed] [Google Scholar]
- 62.Frasca D, Dahyot-Fizelier C, Adier C, Mimoz O, Debaene B, Couet W, Marchand S. 2014. Metronidazole and hydroxymetronidazole central nervous system distribution. 2. Cerebrospinal fluid concentration measurements in patients with external ventricular drain. Antimicrob Agents Chemother 58:1024–1027. doi: 10.1128/AAC.01762-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nau R, Prange HW, Menck S, Kolenda H, Visser K, Seydel J. 1992. Penetration of rifampicin into the cerebrospinal fluid of adults with uninflamed meninges. J Antimicrob Chemother 29:719–724. doi: 10.1093/jac/29.6.719. [DOI] [PubMed] [Google Scholar]
- 64.Owen JS, Fielder-Kelly J. 2014. Introduction to population pharmacokinetic/pharmacodynamic analysis with nonlinear mixed effects models, p 1–8. John Wiley & Sons, Hoboken, NJ. 10.1002/9781118784860.ch1. [DOI] [Google Scholar]
- 65.Patel K, Kirkpatrick CM. 2018. Basic pharmacokinetic principles, p 1–16. In Udy A, Roberts J, Lipman J (ed), Antibiotic pharmacokinetic/pharmacodynamic considerations in the critically ill. Adis, Singapore. 10.1007/978-981-10-5336-8_1. [DOI] [Google Scholar]
- 66.Lodise TP, Butterfield J. 2011. Use of pharmacodynamic principles to inform beta-lactam dosing: “S” does not always mean success. J Hosp Med 6:S16–S23. doi: 10.1002/jhm.869. [DOI] [PubMed] [Google Scholar]
- 67.Cherubin C, Eng R, Norrby R, Modai J, Humbert G, Overturf G. 1989. Penetration of newer cephalosporins into cerebrospinal fluid. Rev Infect Dis 11:526–548. doi: 10.1093/clinids/11.4.526. [DOI] [PubMed] [Google Scholar]
- 68.Litjens CHC, Aarnoutse RE, van Ewijk-Beneken Kolmer EWJ, Svensson EM, Colbers A, Burger DM, Boeree MJ, Te Brake LHM, PanACEA-MAMS-TB-01 Team. 2019. Protein binding of rifampicin is not saturated when using high-dose rifampicin. J Antimicrob Chemother 74:986–990. doi: 10.1093/jac/dky527. [DOI] [PubMed] [Google Scholar]
- 69.LeBras M, Chow I, Mabasa VH, Ensom MHH. 2016. Systematic review of efficacy, pharmacokinetics, and administration of intraventricular aminoglycosides in adults. Neurocrit Care 25:492–507. doi: 10.1007/s12028-016-0269-3. [DOI] [PubMed] [Google Scholar]
- 70.Beach J, Perrot J, Turgeon R, Ensom MH. 2017. Penetration of vancomycin into the cerebrospinal fluid: a systematic review. Clin Pharmacokinet 56:1479–1490. doi: 10.1007/s40262-017-0548-y. [DOI] [PubMed] [Google Scholar]
- 71.Ng K, Mabasa VH, Chow I, Ensom MH. 2014. Systematic review of efficacy, pharmacokinetics, and administration of intraventricular vancomycin in adults. Neurocrit Care 20:158–171. doi: 10.1007/s12028-012-9784-z. [DOI] [PubMed] [Google Scholar]
- 72.Riser M, Bland C, Rudisil C, Bookstaver B. 2010. Cerebrospinal fluid penetration of high-dose daptomycin in suspected Staphylococcus aureus meningitis. Ann Pharmacother 44:1832–1835. doi: 10.1345/aph.1P307. [DOI] [PubMed] [Google Scholar]
- 73.Joynt GM, Lipman J, Gomersall CD, Young RJ, Wong ELY, Gin T. 2001. The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J Antimicrob Chemother 47:421–429. doi: 10.1093/jac/47.4.421. [DOI] [PubMed] [Google Scholar]
- 74.Roberts JA, Lipman J. 2009. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med 37:840–851; quiz, 859. doi: 10.1097/CCM.0b013e3181961bff. [DOI] [PubMed] [Google Scholar]
- 75.Udy AA, Jarrett P, Lassig-Smith M, Stuart J, Starr T, Dunlop R, Deans R, Roberts JA, Senthuran S, Boots R, Bisht K, Bulmer AC, Lipman J. 2017. Augmented renal clearance in traumatic brain injury: a single-center observational study of atrial natriuretic peptide, cardiac output, and creatinine clearance. J Neurotrauma 34:137–144. doi: 10.1089/neu.2015.4328. [DOI] [PubMed] [Google Scholar]
- 76.Kanji S, Hayes M, Ling A, Shamseer L, Chant C, Edwards DJ, Edwards S, Ensom MH, Foster D, Hardy B, Kiser TH, La Porte C, Roberts J, Shulman R, Walker S, Zelenitsky S, Moher D. 2015. Reporting guidelines for clinical pharmacokinetic studies: the ClinPK statement. Clin Pharmacokinet 54:783–795. doi: 10.1007/s40262-015-0236-8. [DOI] [PubMed] [Google Scholar]
- 77.Wang Q, Shi Z, Wang J, Shi G, Wang S, Zhou J. 2008. Postoperatively administered vancomycin reaches therapeutic concentration in the cerebral spinal fluid of neurosurgical patients. Surg Neurol 69:126–129. doi: 10.1016/j.surneu.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 78.Young EJ, Ratner RE, Clarridge JE. 1981. Staphylococcal ventriculitis treated with vancomycin. South Med J 74:1014–1015. doi: 10.1097/00007611-198108000-00039. [DOI] [PubMed] [Google Scholar]
- 79.Pfausler B, Haring H, Kampfl A, Wissel J, Schober M, Schmutzhard E. 1997. Cerebrospinal fluid (CSF) pharmacokinetics of intraventricular vancomycin in patients with staphylococcal ventriculitis associated with external CSF drainage. Clin Infect Dis 25:733–735. doi: 10.1086/513756. [DOI] [PubMed] [Google Scholar]
- 80.Arroyo CJ, Quindlen EA. 1983. Accumulation of vancomycin after intraventricular infusions. South Med J 76:1554–1555. doi: 10.1097/00007611-198312000-00023. [DOI] [PubMed] [Google Scholar]
- 81.Reesor C, Chow AW, Kureishi A, Jewesson PJ. 1988. Kinetics of intraventricular vancomycin in infections of cerebrospinal fluid shunts. J Infect Dis 158:1142–1143. doi: 10.1093/infdis/158.5.1142. [DOI] [PubMed] [Google Scholar]
- 82.Brückner O, Trautmann M, Borner K. 1985. A study of the penetration of temocillin in the cerebrospinal fluid. Drugs 29(Suppl 5):162–166. doi: 10.2165/00003495-198500295-00033. [DOI] [PubMed] [Google Scholar]
- 83.Albanèse J, Léone M, Bruguerolle B, Ayem M-L, Lacarelle B, Martin C. 2000. Cerebrospinal fluid penetration and pharmacokinetics of vancomycin administered by continuous infusion to mechanically ventilated patients in an intensive care unit. Antimicrob Agents Chemother 44:1356–1358. doi: 10.1128/AAC.44.5.1356-1358.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.