Continuous infusion (CON) of fosfomycin has been proposed as potentially advantageous in certain clinical scenarios. However, no clinical data on the pharmacokinetics (PK) of fosfomycin after CON are available to date. This study aimed to investigate the PK of fosfomycin after CON and compare it with intermittent infusion (INT) of fosfomycin. A randomized two-way crossover study including 8 healthy male volunteers was performed. Each subject received fosfomycin as INT of 8 g over 30 min every 8 h and, separated by a washout period, as CON of 1 g/h preceded by a loading dose of 8 g over 30 min.
KEYWORDS: PK/PD, clinical trials, continuous infusion, fosfomycin, pharmacokinetics
ABSTRACT
Continuous infusion (CON) of fosfomycin has been proposed as potentially advantageous in certain clinical scenarios. However, no clinical data on the pharmacokinetics (PK) of fosfomycin after CON are available to date. This study aimed to investigate the PK of fosfomycin after CON and compare it with intermittent infusion (INT) of fosfomycin. A randomized two-way crossover study including 8 healthy male volunteers was performed. Each subject received fosfomycin as INT of 8 g over 30 min every 8 h and, separated by a washout period, as CON of 1 g/h preceded by a loading dose of 8 g over 30 min. PK sampling was performed for 18 and 24 h in the CON and INT groups, respectively. Fosfomycin was generally well tolerated. However, 2 out of 8 subjects (25%) developed thrombophlebitis at the infusion site following CON, which was prevented in the following subjects with a simultaneous coinfusion of Ringer’s lactate. The steady-state maximum concentration of drug in serum (Cmax) and area under the concentration-time curve from 0 to 24 h at steady state (AUCSS,0–24) of fosfomycin after INT were 551.5 ± 67.8 mg/liter and 3,678.5 ± 601.9 h · mg/liter, respectively. CON led to an average steady-state concentration of 183.8 ± 35.9 mg/liter, resulting in a calculated AUCSS,0–24 of 4,411.2 ± 862.4 h · mg/liter, which was 1.2-fold higher than that with INT. CON resulted in a 100% T>MIC (time during which the drug concentration exceeds the MIC) for MICs of ≤128 mg/liter, whereas the %T>MIC for INT was only 44% for an MIC of 128 mg/liter. CON of fosfomycin led to improved PK and PK/pharmacodynamic (PD) determinants in plasma of healthy volunteers. The clinical relevance of these findings remains to be investigated in patients.
INTRODUCTION
Fosfomycin (FOM) is a bactericidal broad-spectrum antimicrobial drug that acts by inhibition of bacterial cell wall synthesis and was discovered in 1969 (1). It has been shown to be highly active against many Gram-negative and Gram-positive bacteria, including multidrug-resistant (MDR), extensively drug-resistant (XDR), and pan-drug-resistant (PDR) organisms (2, 3). The antimicrobial activity of FOM against highly resistant bacteria can be further improved through coadministration with other antibiotics due to synergistic effects (4–6).
In terms of pharmacokinetics/pharmacodynamics (PK/PD), the index best correlating with clinical efficacy is still subject to debate. Fosfomycin has been associated with a relatively fast development of resistance once the concentration falls below the MIC (7). Therefore, independent from the mode of action of FOM, concentrations above the MIC for the entire dosing interval seem highly desirable.
Continuous infusion (CON) has been propagated for different antibiotics to maximize the time during which the drug concentration exceeds the MIC (T>MIC). Given the chemical in vitro stability of FOM, continuous infusion seems to be a logical step to improve pharmacodynamic action and prevent the development of resistant bacteria.
To date, no PK data on continuous infusion of FOM derived from human subjects have been published. Yet PK/PD simulations have suggested that under certain circumstances, continuous infusion of FOM might be advantageous compared to intermittent infusion (INT), which is the usual mode of administration (8).
The present study sets out to investigate plasma PK of FOM in healthy volunteers, administered as a continuous in comparison with an intermittent infusion.
RESULTS
Pharmacokinetics of intermittent dosing.
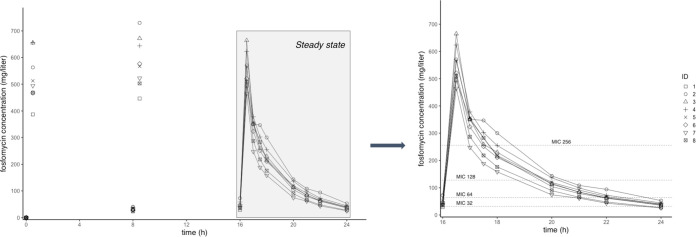
Concentration-time profiles of FOM in plasma of healthy volunteers after intermittent infusion (8 g three times a day [t.i.d.]) are shown in Fig. 1. The simulated steady-state (SS) profile after intermittent dosing is shown in Fig. 2 (left). This concentration-time graph was obtained by assuming that the third dosing interval (from 16 to 24 h) represents the course of the FOM concentration at SS. Different representative MIC values of FOM have been added to the graphs for illustrative purposes. Table 1 shows the PK parameters of FOM after intermittent infusion calculated by noncompartmental analysis. The steady-state maximum concentration of drug in serum (Cmax) and half-life (t1/2) after intermittent infusion were 551.5 ± 67.8 mg/liter and 2.6 ± 0.2 h, respectively. The clearance (CL) and volume of distribution (V) were 6.7 ± 1.2 liters/h and 21.0 ± 2.8 liters, respectively. The calculated area under the concentration-time from 0 to 24 h at SS (AUCSS,0–24) was 3,678.5 ± 601.9 mg · h/ml.
FIG 1.
Concentration-versus-time profile after intermittent infusion of fosfomycin (8 g t.i.d.) over 24 h (left) and after the third dose (assumed steady state) (right).
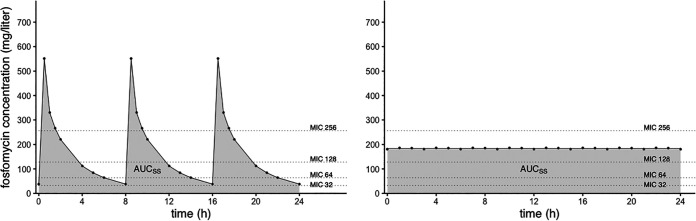
FIG 2.
Simulated concentration-versus-time profiles of fosfomycin at steady state over 24 h for intermittent infusion (left) and continuous infusion (right).
TABLE 1.
PK parameters at steady state after intermittent infusion of fosfomycin (8 g t.i.d.)
| PK parameter | Value for subject |
Mean value ± SD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| t1/2 (h) | 2.59 | 2.83 | 2.52 | 2.47 | 2.48 | 2.69 | 2.53 | 2.35 | 2.56 ± 0.15 |
| Cmax (mg/liter) | 494.14 | 570.44 | 664.43 | 623.55 | 565.17 | 520.68 | 464.32 | 509.07 | 551.48 ± 67.75 |
| AUCSS,0–8 (h · mg/liter) | 1,184.34 | 1,517.22 | 1,293.5 | 1,420.43 | 1,277.68 | 1,198.79 | 901.64 | 1,015.66 | 1,226.16 ± 200.63 |
| AUCSS,0–24 (h · mg/liter) | 3,553.02 | 4,551.66 | 3,880.5 | 4,261.29 | 3,833.04 | 3,596.37 | 2,704.92 | 3,046.98 | 3,678.47 ± 601.89 |
| CL (liters/h) | 6.75 | 5.27 | 6.18 | 5.63 | 6.26 | 6.67 | 8.87 | 7.88 | 6.69 ± 1.18 |
| VSS (liters) | 21.26 | 18.81 | 18.59 | 17.85 | 19.74 | 22.42 | 26.34 | 22.6 | 20.95 ± 2.81 |
Pharmacokinetics of continuous dosing.
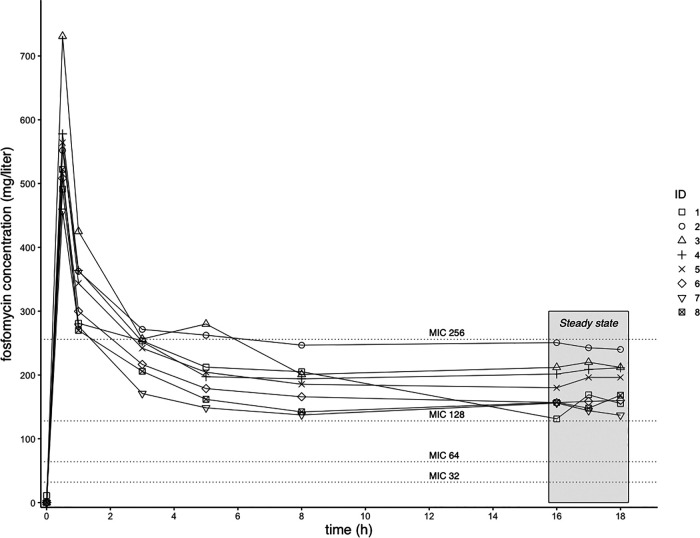
Concentration-time profiles of FOM in plasma of healthy volunteers after continuous infusion (loading dose of 8 g over 30 min and then 1 g/h) are shown in Fig. 3. The simulated SS profile after continuous infusion was obtained by the use of the average concentration of the drug at SS (CSS,av) and is depicted in Fig. 2 (right). Different representative MIC values of FOM have been added to the graphs for illustrative purposes. The calculated PK parameters at SS after continuous infusion are shown in Table 2. The average steady-state concentration was 183.8 ± 35.9 mg/liter, resulting in a calculated AUCSS,0–24 of 4,411.2 ± 862.4 mg · h/ml.
FIG 3.
Concentration-versus-time profile of fosfomycin after a loading dose of 8 g followed by a continuous infusion of 1 g/h.
TABLE 2.
PK parameters at steady state after continuous infusion of fosfomycin (loading dose of 8 g and then 1 g/h)
| PK parameter | Value for subject |
Mean value ± SD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| CSS,av (mg/liter) | 151.7 | 244.5 | 214.6 | 207.1 | 190.7 | 158.4 | 145.6 | 157.7 | 183.8 ± 35.9 |
| AUCSS,0–24 (h · mg/liter) | 3,641.7 | 5,867.1 | 5,151.1 | 4,971.6 | 4,577.4 | 3,801.8 | 3,494.5 | 3,784.2 | 4,411.2 ± 862.4 |
Pharmacokinetics/pharmacodynamics of intermittent versus continuous dosing.
Pharmacokinetic/pharmacodynamic parameters of intermittent and continuous dosing calculated using representative MIC values of FOM are shown in Table 3. The chosen values correspond to common clinical MICs of FOM for Pseudomonas aeruginosa, enterococci, and Staphylococcus aureus (including methicillin-resistant S. aureus [MRSA]) as reported by the European Committee on Antimicrobial Susceptibility Testing (EUCAST). Furthermore, MICs of FOM against clinical Enterobacteriaceae (including KPC-producing Klebsiella pneumoniae) isolates are also covered by the chosen MIC values (9, 10). For Pseudomonas aeruginosa, 95% of the reported MICs were equal to or lower than 128 mg/liter, and 75% of the isolates’ MICs were within the range of 32 to 128 mg/liter. Likewise, the MIC90 for clinical KPC-producing Klebsiella pneumoniae isolates was 128 mg/liter or lower (9, 10). The highest number of enterococcal isolates (86%) showed MICs of 32 or 64 mg/liter, and 94% of the reported MICs were equal to or lower than 64 mg/liter. The chosen representative MICs (16 to 256 mg/liter) cover 97% of the reported MICs for Pseudomonas aeruginosa, 99% for Staphylococcus aureus (including MRSA), and 99% for enterococci as reported by EUCAST.
TABLE 3.
PK/PD parameters at steady state after intermittent and continuous dosing of fosfomycin
| MIC (mg/liter) | Mean AUCSS,0–24/MIC ratio ± SD |
Mean %T>MIC ± SD |
Mean Cmax/MIC ratio ± SD |
|||
|---|---|---|---|---|---|---|
| INT | CON | INT | CON | INT | CON | |
| 16 | 229.90 ± 37.62 | 275.70 ± 53.9 | 100 ± 0 | 100 ± 0 | 34.47 ± 4.23 | 11.49 ± 2.25 |
| 32 | 114.95 ± 18.81 | 137.85 ± 26.95 | 97.79 ± 4.2 | 100 ± 0 | 17.23 ± 2.12 | 5.74 ± 1.12 |
| 64 | 57.48 ± 9.4 | 68.92 ± 13.47 | 74.20 ± 10.09 | 100 ± 0 | 8.62 ± 1.06 | 2.87 ± 0.56 |
| 128 | 28.74 ± 4.7 | 34.46 ± 6.74 | 43.72 ± 7.83 | 100 ± 0 | 4.31 ± 0.53 | 1.44 ± 0.28 |
| 256 | 14.37 ± 2.35 | 17.23 ± 3.37 | 17.66 ± 5.91 | 0 ± 0 | 2.15 ± 0.26 | 0.72 ± 0.14 |
AUCSS,0–24/MIC ratios following continuous infusion were higher than those following intermittent infusion. For an MIC of 64 mg/liter, the AUCSS,0–24/MIC ratio after continuous infusion was 68.9 ± 13.5, compared with 57.5 ± 9.4 for intermittent infusion.
The %T>MIC with continuous infusion was 100% up to an MIC of 128 mg/liter. For intermittent infusion, the %T>MIC was notably lower at 43.7% ± 7.8% for an MIC of 128 mg/liter.
In contrast to the above-described data, intermittent infusion of FOM yielded higher Cmax/MIC values than for continuous infusion. For an MIC of 128 mg/liter, the Cmax/MIC ratios for continuous and intermittent infusions were 1.4 ± 0.3 and 4.3 ± 0.5, respectively.
Demographics and safety analysis.
Subject demographics, including glomerular filtration rate (GFR), albumin, and total protein values, are shown in Table 4. The mean age of the subjects was 34.6 ± 6.1 years, with a mean weight of 77.9 ± 14.3 kg and a mean height of 181 ± 7 cm. All of the subjects had normal renal function (mean GFR of 119.6 ml/min calculated with the Chronic Kidney Disease Epidemiology Collaboration equation).
TABLE 4.
Demographics of study subjects (n = 8), including estimated glomerular filtration rates calculated using the Chronic Kidney Disease Epidemiology Collaboration equation, albumin, and total protein
| Subject | Age (yrs) | Wt (kg) | Ht (cm) | eGFRa (ml/min) | Albumin concn (g/liter) | Total protein concn (g/liter) |
|---|---|---|---|---|---|---|
| 1 | 42 | 93 | 196 | 137 | 43.7 | 66 |
| 2 | 39 | 80 | 182 | 89 | 42.7 | 66.5 |
| 3 | 29 | 53 | 171 | 109 | 44.9 | 73 |
| 4 | 23 | 65 | 178 | 111 | 48.9 | 69.6 |
| 5 | 36 | 67.7 | 175 | 115 | 43.6 | 70.9 |
| 6 | 33 | 87 | 183 | 132 | 44.9 | 71 |
| 7 | 38 | 89 | 182 | 126 | 41.5 | 62.8 |
| 8 | 37 | 88.4 | 180 | 138 | 44.3 | 69.3 |
| Mean ± SD | 34.6 ± 6.1 | 77.9 ± 14.3 | 180.9 ± 7.3 | 119.6 ± 16.8 | 44.3 ± 2.2 | 68.6 ± 3.3 |
eGFR, estimated glomerular filtration rate.
Fosfomycin was generally well tolerated. All adverse events (AEs) subsided without further consequences. The following 4 AEs were classified as possibly related to the study medication and mild: gastritis, hyperbilirubinemia, eosinophilia, and blurred vision (once each). Another 4 AEs were classified as probably related to the study medication and mild: nausea (4 times), abdominal pain (3 times), feeling of tension in the arm (once), swelling at the infusion site (twice), and extravasation in the arm (twice). Two out of eight subjects (25%) developed thrombophlebitis at the infusion site following continuous infusion, which was classified as probably related to the study drug and moderate. Both of them were treated with fondaparinux at 2.5 mg subcutaneously once daily for 6 weeks until occlusion and induration of the veins improved. To improve local tolerability in the following subjects, the continuous infusion of FOM was administered with a simultaneous coinfusion of Ringer’s lactate at 20 ml/h. As a result of this measure, no further thrombophlebitis or similar events occurred in the remaining subjects. One subject reported circumscribed paresthesia of the wrist region ipsilateral to the infusion site, which was most likely ascribable to neural trauma during the insertion of the intravenous cannula. The remaining AEs were not related to the study medication.
DISCUSSION
To our knowledge, our study is the first study to examine the PK of continuous infusion of FOM. The PK parameters measured in our study after intermittent infusion correspond well with the values already reported in the literature for patients with normal renal function (11, 12).
Our study found low interindividual variability (IIV) in FOM plasma concentrations after intermittent and continuous intravenous infusions. As expected, the IIV after intravenous infusion in our study was much lower than reported values after oral administration of FOM (13). In a patient population, a high IIV compared to our data can be expected due to varying renal function and distribution kinetics. This must be kept in mind when transferring our data to real patients.
The only safety issue that occurred during the administration of FOM as a continuous infusion was the development of thrombophlebitis. An analysis of FOM-associated adverse events that included 23 trials with intravenous FOM administration reported phlebitis in 10 out of 1,242 patients (one of whom developed thrombophlebitis) (14). Compared to this, we had a much higher incidence of thrombophlebitis in our study with continuous infusion of FOM.
A recent meta-analysis that included 15,791 patients found an incidence of 30.7% of phlebitis associated with peripheral intravenous catheter use and identified a long dwelling time and antibiotic infusion among the most important risk factors (15). This might explain the finding in our study since we administered an antibiotic and thrombophlebitis occurred, associated with continuous infusion. However, since the simultaneous administration of Ringer’s lactate in the same intravenous catheter solved this issue by diluting the concentration of the FOM preparation at the infusion site, localized vascular irritation by the FOM preparation probably contributed to the development of thrombophlebitis. This finding does not disqualify continuous infusion as a dosing scheme. Yet as a future consideration, the continuous infusion of FOM should always be administered together with a dilution solution (e.g., Ringer’s lactate, saline solution, or glucose solution).
In order to be able to estimate the efficacy of a dosing regimen of an antimicrobial agent, knowledge about the PK/PD index linked with its antimicrobial activity is crucial. However, the relevant PK/PD index of FOM is still subject to debate. Some studies suggest a time-dependent activity of FOM, which implies that the time during which the FOM plasma concentration exceeds a certain threshold concentration should be maximized for optimal clinical efficacy (16). On the other hand, there are data indicating that the antimicrobial activity of FOM is best predicted by the AUC/MIC ratio (7, 17) or the Cmax/MIC ratio (18, 19). To further complicate this, some studies have provided evidence that the PK/PD index associated with efficacy varies from the index associated with the suppression of the occurrence of resistant bacteria (7, 20).
In a hollow-fiber infection model (HFIM) with Pseudomonas aeruginosa, Louie et al. aimed at identifying the most fitting indices for bacterial cell killing as well as the suppression of bacterial resistance. The results of this study indicate that the AUC/MIC ratio is the PK/PD index that correlates best with bacterial cell killing in vitro. Yet the %T>MIC was most closely associated with the suppression of resistant isolates (7).
Lepak et al. investigated the activity of FOM against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa in a neutropenic murine thigh infection model. In parallel to the findings of Louie et al., they identified the AUC/MIC ratio as the PK/PD index with the best correlation to antibacterial efficacy. The mean AUC0–24/MIC ratios that correlated with net stasis were 23.7, 21.1, and 14.6 for Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa, respectively. For 1-log killing, the ratios were 98.9, 21.5, and 28.2 for Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa, respectively (17).
Zykov et al. employed a murine urinary tract infection model to investigate the activity of FOM against extended-spectrum-beta-lactamase (ESBL)-, plasmid-mediated AmpC-, and carbapenemase-producing Escherichia coli. According to those authors, the AUC from 0 to 72 h above the MIC (AUC0–72/MIC ratio) and the Cmax/MIC ratio were the best predictors of the in vivo antimicrobial activity of FOM in urine (19).
Another study that investigated the PK/PD relationship of FOM was performed by Docobo-Pérez et al. and employed the HFIM. For bacterial isolates, they selected three ESBL-producing Escherichia coli strains. The results of their experiments indicate that the AUC/MIC ratio of the unbound drug (fAUC/MIC ratio) is the best predictor of resistance suppression (20).
VanScoy et al. employed an in vivo infection model to explore the PK/PD relationship of FOM against different Escherichia coli isolates. Those authors demonstrated a time-dependent mode of action of FOM. Yet the PK/PD index associated most closely with the efficacy of FOM was the percentage of the dosing interval that FOM concentrations were above the inherent resistance-inhibitory concentration (%T>RIC). This RIC was derived from mutation frequency experiments where the authors found an inherently FOM-resistant subpopulation of Escherichia coli in the inoculum with MICs of 32 to 64 mg/liter. %T>RIC ratios of 11.9 and 20.9 were associated with net bacterial stasis and 1-log killing, respectively (16). It has to be noted that in the studies by Docobo-Pérez et al. and VanScoy et al., the MICs of the tested isolates were relatively low, and therefore, the majority of the %T>MIC values were 100%. Hence, the experiments in these studies did not deliver a convincing assessment of %T>MIC as a PK/PD index of FOM.
Bilal et al. investigated the relevant PK/PD index of FOM against Pseudomonas aeruginosa in a dynamic in vitro model with a dose fractionation design. In their experiments, the fAUC/MIC ratio correlated most closely with the antimicrobial efficacy of FOM. fAUC/MIC ratios of 489 and 1,024 were necessary to achieve 1-log and 2-log bacterial killing, respectively (21).
Recent work by Noel et al. employed a dynamic in vitro model to study the relevant PK/PD index of FOM against Staphylococcus aureus. Those authors found the Cmax/MIC ratio to be the PK/PD index that was best correlated with bacterial killing of Staphylococcus aureus (ATCC 29213), although the fit was not very pronounced (R2 = 0.66 at 24 h). The second-best fit was observed for the AUC/MIC ratio at 24 h (R2 = 0.51). Against different clinical Staphylococcus aureus strains, Cmax/MIC ratios of 3.0 ± 1.7 and 6.6 ± 3.8 led to stasis and 2-log killing after 24 h, respectively. AUC/MIC ratios for stasis and 2-log killing after 24 h were 26.4 ± 11.8 and 66.6 ± 39.1, respectively (18).
In summary, there is disagreement as to which PK/PD index is best suited to predict the antimicrobial activity of FOM and to what extent this index is dependent on the organism. At present, AUC/MIC, %T>MIC, and Cmax/MIC should therefore be considered for the evaluation of a dosing scheme. Further research is needed to clarify which PK/PD target should be aimed at for optimized clinical efficacy.
Of note, the study by Louie et al. clearly demonstrated that after an early bacterial kill, selection of resistant Pseudomonas aeruginosa isolates occurs rapidly when FOM is administered as monotherapy, irrespective of the PK/PD indices. An increase of the T>MIC can only delay this process but not avert it (7). The emergence of resistance with monotherapy of FOM has also been demonstrated by other investigators (20, 21). These findings emphasize that FOM should be administered in combination with a second antimicrobial agent.
With regard to the %T>MIC, our data indicate that continuous infusion is superior to intermittent infusion starting at an MIC of 64 mg/liter. At an MIC of 128 mg/liter, the %T>MIC with intermittent infusion was only 44% compared to 100% with continuous infusion. According to data from EUCAST, significant proportions of clinical Pseudomonas aeruginosa and enterococcal isolates have MICs of 64 mg/liter and higher for FOM. In order to achieve sufficient efficacy against bacteria that are difficult to treat, for antibiotics with time-dependent activity, a %T>MIC target in the range of 70 to 100% is often required. Accordingly, increased activity and reduced occurrence of resistance could be expected with continuous infusion of FOM. The difference between the two dosing schemes was less pronounced with regard to the AUCSS,0–24/MIC ratios. Still, continuous infusion resulted in AUCSS,0–24/MIC ratios that were 1.2 times higher than those for intermittent infusion. Considering the target AUC/MIC ratios for stasis and 1-log killing reported by Louie et al. (14.6 and 28.2, respectively), continuous infusion of FOM was able to achieve target ratios for net stasis and 1-log bacterial killing against Pseudomonas aeruginosa isolates with MICs of up to 256 and 128 mg/liter, respectively. With intermittent infusion, the target values for stasis and 1-log killing were only narrowly achieved up to an MIC of 128 mg/liter. Neither continuous nor intermittent infusion was able to achieve the AUC/MIC target ratios postulated by Bilal et al. for 1- and 2-log bacterial killing against Pseudomonas aeruginosa (489 and 1,024, respectively). Not surprisingly, intermittent infusion was superior to continuous infusion with regard to the Cmax/MIC ratio. The target Cmax/MIC ratios against Staphylococcus aureus as determined by Noel at al. (stasis, 3.0; 2-log killing, 6.6) were achieved with continuous infusion for MICs of up to 32 and 15 mg/liter, respectively, and with intermittent infusion for MICs of up to 128 and 64 mg/liter, respectively.
Based on the data of our study, an improved antimicrobial behavior of continuous infusion of FOM compared to intermittent infusion is hypothesized. This applies in particular to the occurrence of resistance, as the available studies indicate that the T>MIC ratio correlates well with the development of antimicrobial resistance. However, continuous infusion was also able to achieve an increase in the AUC/MIC ratio, which in a number of studies has been linked to the antimicrobial efficacy of FOM.
In conclusion, continuous infusion of FOM led to improved PK and PK/PD determinants in plasma of healthy volunteers. The clinical relevance of these findings remains to be verified in patients and further in vitro models.
MATERIALS AND METHODS
Ethics.
This pharmacokinetics (PK) study was conducted at the Department of Clinical Pharmacology of the Medical University of Vienna (Austria) in accordance with the Declaration of Helsinki and good clinical practice guidelines of the International Conference on Harmonization (https://www.ema.europa.eu/en/ich-e6-r2-good-clinical-practice). The study was approved by the Ethics Committee of the Medical University of Vienna and the Austrian Agency for Health and Food Safety and registered under EudraCT number 2018-000653-45. Before inclusion, all study subjects gave oral and written informed consent for study participation.
Trial design and study population.
The present study was a prospective, open-labeled, single-center, randomized two-way crossover PK study with 8 healthy male volunteers.
At the screening visit, subjects were informed about the objective and the procedures of the study and the risks involved. Inclusion and exclusion criteria as well as demographic data were assessed. Each subject underwent a general physical examination, including medical history, determination of body mass index (BMI), drawing of blood for laboratory screening tests and serological tests (HIV and hepatitis B and C), vital sign recordings (blood pressure and heart rate after 5 min of rest in the supine position), and electrocardiogram (ECG). In addition, urinalysis was performed.
The most relevant inclusion criteria were healthy males aged between 18 and 50 years with a BMI of between 18 and 30 and no regular medication within the last 2 weeks prior to the first study day. The most relevant exclusion criteria were a known allergy or hypersensitivity to the study drug, alcohol or drug abuse, active smoker, abnormalities in ECG, and abnormalities in laboratory tests or physical examination, which were considered clinically relevant by the responsible investigator. Upon signature of the informed consent form and after assessment of the inclusion and exclusion criteria, subjects were enrolled in the study. A final examination was performed up to 7 days after the last administration of FOM and included the same procedures as those for the screening visit.
On the study day, subjects were randomized to receive FOM as an intermittent or a continuous infusion. After a washout period of at least 48 h, subjects were readmitted to the clinical trial unit and received the alternative treatment regimen.
For intravenous infusion of FOM, a plastic cannula (Venflon) was placed into an antecubital vein. Another plastic cannula was inserted into an antecubital vein of the contralateral arm to allow plasma PK sampling at predefined time points.
Fosfomycin infusion solutions were prepared immediately prior to administration. For intermittent infusion and the loading dose of the continuous infusion, 8 g FOM disodium powder for solution for infusion was dissolved in 300 ml of a 5% glucose solution. For continuous infusion, 8 g FOM disodium powder for solution for infusion was dissolved in 50 ml of 5% glucose.
Intermittent infusion was administered as a single dose of 8 g FOM every 8 h (t.i.d.) for a total of three doses, each administered as an intravenous infusion over 30 min. Since steady-state (SS) conditions were expected to be present from the third dose onward, intensive PK sampling was performed at time points from 16 to 24 h after the first dose.
During the continuous infusion period, a loading dose of 8 g FOM was administered as an intravenous infusion over 30 min, followed by a continuous infusion of FOM at an infusion rate of 1 g/h, corresponding to 24 g of FOM per day. Plasma PK sampling in the continuous infusion group was performed from baseline to 18 h after the loading dose.
Sampling time points and handling of samples.
For the intermittent infusion period, sampling of venous blood was performed before and after the end of each FOM infusion. Additionally, after the third infusion, intensive plasma sampling was performed at 1 h, 1.5 h, 2 h, 4 h, 5 h, 6 h, and 8 h postdose (corresponding to 17 h, 17.5 h, 18 h, 20 h, 21 h, 22 h, and 24 h after the first FOM dose).
For the continuous infusion period, plasma sampling was performed before and at the end of the loading dose infusion. During the continuous infusion, plasma was obtained 1 h, 3 h, 5 h, 8 h, 16 h, 17 h, and 18 h after the administration of the loading dose.
At each time point, approximately 4 ml of venous blood was drawn and collected into lithium-heparin tubes (lithium-heparin Vacuette). Blood samples were kept on ice for a maximum of 60 min and then centrifuged at +4°C at 2,600 × g for 10 min to separate the plasma. The plasma samples were snap-frozen at approximately −20°C and stored at approximately −80°C until analysis.
Fosfomycin concentrations were quantified in the ISO 15189-accredited pharmacy laboratory of Erasmus MC using a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method (validated according to FDA guidelines) with a method that is applicable to a concentration range of 0.75 to 375 mg/liter (R2 of 0.9998) encompassing the clinically relevant concentration range based on the susceptibility of possible (uro)pathogens in the clinical setting. The validation results for plasma for all quality control (QC) levels were <3.2% for accuracy, <1.7% for within-day precision, and <3.8% for between-day precision (22).
Trial endpoints and pharmacokinetic analysis.
The primary outcome variable was the area under the plasma concentration-time curve from 0 to 24 h at SS (AUCSS,0–24).
Further pharmacokinetic outcome variables were the maximum concentration (Cmax), half-life (t1/2), total body clearance (CL), and apparent volume of distribution (V). PK/pharmacodynamic (PD) outcome variables included the ratio of the AUCSS,0–24 to the MIC (AUCSS,0–24/MIC ratio), the percentage of the dosing interval during which drug concentrations exceeded the MIC at SS (%T>MIC), and the ratio of the peak concentration to the MIC (Cmax/MIC ratio). For continuous infusion, the Cmax at SS was replaced with the mean steady-state concentration (CSS,av).
For the safety analysis, data on adverse events were collected during the whole study period, and laboratory investigations were performed before and after study drug administration.
Pharmacokinetic data were assessed by noncompartmental analysis using a commercially available software program (Phoenix WinNonlin build 8.0; Certara USA, Inc., Princeton, NJ).
For intermittent dosing, the AUCSS,0–24 was calculated by multiplying the AUC from 0 to 8 h after the third dose (AUCSS,0–8) (assumed SS) by 3.
For continuous dosing, the AUCSS,0–24 was calculated by multiplying the CSS,av by 24. CSS,av was obtained by calculating the mean of the plasma concentrations at assumed SS (16 h, 17 h, and 18 h after the loading dose).
ACKNOWLEDGMENTS
We have no funding to declare.
The medication was provided by Astro Pharma.
REFERENCES
- 1.Kahan FM, Kahan JS, Cassidy PJ, Kropp H. 1974. The mechanism of action of fosfomycin (phosphonomycin). Ann N Y Acad Sci 235:364–386. doi: 10.1111/j.1749-6632.1974.tb43277.x. [DOI] [PubMed] [Google Scholar]
- 2.Beuk C, Hill C, Whitehead S, Blondel-Hill E, Wagner K, Cheeptham N. 2013. Determination of susceptibility to fosfomycin and tigecycline of Enterobacteriaceae, particularly Escherichia coli isolates, producing extended-spectrum β-lactamases from multiple regional Canadian hospitals. Can J Infect Dis Med Microbiol 24:e80–e82. doi: 10.1155/2013/645018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahni RD, Balaji V, Varghese R, John J, Tansarli GS, Falagas ME. 2013. Evaluation of fosfomycin activity against uropathogens in a fosfomycin-naive population in South India: a prospective study. Future Microbiol 8:675–680. doi: 10.2217/fmb.13.31. [DOI] [PubMed] [Google Scholar]
- 4.Qi C, Xu S, Wu M, Zhu S, Liu Y, Huang H, Zhang G, Li J, Huang X. 2019. Pharmacodynamics of linezolid-plus-fosfomycin against vancomycin-susceptible and-resistant enterococci in vitro and in vivo of a Galleria mellonella larval infection model. Infect Drug Resist 12:3497–3505. doi: 10.2147/IDR.S219117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Wafa WMA, Ibrahim YM. 2020. In vitro activity of fosfomycin in double and triple combinations with imipenem, ciprofloxacin and tobramycin against multidrug-resistant Escherichia coli. Curr Microbiol 77:755–761. doi: 10.1007/s00284-019-01871-w. [DOI] [PubMed] [Google Scholar]
- 6.Mihailescu R, Furustrand Tafin U, Corvec S, Oliva A, Betrisey B, Borens O, Trampuz A. 2014. High activity of fosfomycin and rifampin against methicillin-resistant Staphylococcus aureus biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother 58:2547–2553. doi: 10.1128/AAC.02420-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louie A, Maynard M, Duncanson B, Nole J, Vicchiarelli M, Drusano GL. 2018. Determination of the dynamically linked indices of fosfomycin for Pseudomonas aeruginosa in the hollow fiber infection model. Antimicrob Agents Chemother 62:e02627-17. doi: 10.1128/AAC.02627-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodríguez-Gascón A, Canut-Blasco A. 2019. Deciphering pharmacokinetics and pharmacodynamics of fosfomycin. Rev Esp Quimioter 32(Suppl 1):19–24. [PMC free article] [PubMed] [Google Scholar]
- 9.Endimiani A, Patel G, Hujer KM, Swaminathan M, Perez F, Rice LB, Jacobs MR, Bonomo RA. 2010. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother 54:526–529. doi: 10.1128/AAC.01235-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falagas ME, Maraki S, Karageorgopoulos DE, Kastoris AC, Mavromanolakis E, Samonis G. 2010. Antimicrobial susceptibility of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Enterobacteriaceae isolates to fosfomycin. Int J Antimicrob Agents 35:240–243. doi: 10.1016/j.ijantimicag.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Wenzler E, Ellis-Grosse EJ, Rodvold KA. 2017. Pharmacokinetics, safety, and tolerability of single-dose intravenous (ZTI-01) and oral fosfomycin in healthy volunteers. Antimicrob Agents Chemother 61:e00775-17. doi: 10.1128/AAC.00775-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorn C, Petroff D, Neumann N, Kratzer A, El-Najjar N, Dietrich A, Kloft C, Zeitlinger M, Kees MG, Kees F, Wrigge H, Simon P. 2019. Plasma and tissue pharmacokinetics of fosfomycin in morbidly obese and non-obese surgical patients: a controlled clinical trial. J Antimicrob Chemother 74:2473. doi: 10.1093/jac/dkz249. [DOI] [PubMed] [Google Scholar]
- 13.Wijma RA, Koch BCP, van Gelder T, Mouton JW. 2018. High interindividual variability in urinary fosfomycin concentrations in healthy female volunteers. Clin Microbiol Infect 24:528–532. doi: 10.1016/j.cmi.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 14.Iarikov D, Wassel R, Farley J, Nambiar S. 2015. Adverse events associated with fosfomycin use: review of the literature and analyses of the FDA adverse event reporting system database. Infect Dis Ther 4:433–458. doi: 10.1007/s40121-015-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lv L, Zhang J. 2020. The incidence and risk of infusion phlebitis with peripheral intravenous catheters: a meta-analysis. J Vasc Access 21:342–349. doi: 10.1177/1129729819877323. [DOI] [PubMed] [Google Scholar]
- 16.VanScoy BD, McCauley J, Ellis-Grosse EJ, Okusanya OO, Bhavnani SM, Forrest A, Ambrose PG. 2015. Exploration of the pharmacokinetic-pharmacodynamic relationships for fosfomycin efficacy using an in vitro infection model. Antimicrob Agents Chemother 59:7170–7177. doi: 10.1128/AAC.04955-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lepak AJ, Zhao M, Vanscoy B, Taylor DS, Ellis-Grosse E, Ambrose PG, Andes DR. 2017. In vivo pharmacokinetics and pharmacodynamics of ZTI-01 (fosfomycin for injection) in the neutropenic murine thigh infection model against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Antimicrob Agents Chemother 61:e00476-17. doi: 10.1128/AAC.00476-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noel A, Attwood M, Bowker K, MacGowan A. 2020. The pharmacodynamics of fosfomycin against Staphylococcus aureus studied in an in vitro model of infection. Int J Antimicrob Agents 56:105985. doi: 10.1016/j.ijantimicag.2020.105985. [DOI] [PubMed] [Google Scholar]
- 19.Zykov IN, Samuelsen Ø, Jakobsen L, Småbrekke L, Andersson DI, Sundsfjord A, Frimodt-Møller N. 2018. Pharmacokinetics and pharmacodynamics of fosfomycin and its activity against extended-spectrum-β-lactamase-, plasmid-mediated AmpC-, and carbapenemase-producing Escherichia coli in a murine urinary tract infection model. Antimicrob Agents Chemother 62:e02560-17. doi: 10.1128/AAC.02560-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Docobo-Pérez F, Drusano GL, Johnson A, Goodwin J, Whalley S, Ramos-Martín V, Ballestero-Tellez M, Rodriguez-Martinez JM, Conejo MC, Van Guilder M, Rodríguez-Baño J, Pascual A, Hope WW. 2015. Pharmacodynamics of fosfomycin: insights into clinical use for antimicrobial resistance. Antimicrob Agents Chemother 59:5602–5610. doi: 10.1128/AAC.00752-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilal H, Peleg AY, McIntosh MP, Styles IK, Hirsch EB, Landersdorfer CB, Bergen PJ. 2018. Elucidation of the pharmacokinetic/pharmacodynamic determinants of fosfomycin activity against Pseudomonas aeruginosa using a dynamic in vitro model. J Antimicrob Chemother 73:1570–1578. doi: 10.1093/jac/dky045. [DOI] [PubMed] [Google Scholar]
- 22.Wijma RA, Bahmany S, Wilms EB, van Gelder T, Mouton JW, Koch BCP. 2017. A fast and sensitive LC-MS/MS method for the quantification of fosfomycin in human urine and plasma using one sample preparation method and HILIC chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 1061–1062:263–269. doi: 10.1016/j.jchromb.2017.07.036. [DOI] [PubMed] [Google Scholar]