Isavuconazole is the newest triazole antifungal, and it displays a favorable pharmacokinetic and safety profile. Less is known about its long-term use in immunocompetent hosts. We performed a retrospective service evaluation of isavuconazole therapeutic drug monitoring in patients with chronic pulmonary aspergillosis. Adverse events (AEs) and dose adjustments made during routine clinical practice were recorded, and AEs were classified based on Common Terminology Criteria for Adverse Events v5.0.
KEYWORDS: TDM, aspergillosis, chronic pulmonary aspergillosis, isavuconazole, pharmacokinetics
ABSTRACT
Isavuconazole is the newest triazole antifungal, and it displays a favorable pharmacokinetic and safety profile. Less is known about its long-term use in immunocompetent hosts. We performed a retrospective service evaluation of isavuconazole therapeutic drug monitoring in patients with chronic pulmonary aspergillosis. Adverse events (AEs) and dose adjustments made during routine clinical practice were recorded, and AEs were classified based on Common Terminology Criteria for Adverse Events v5.0. Forty-five patients (mean age, 64 years) had 285 isavuconazole blood drug levels measured (mean level, 4.1 mg/liter). A total of 117 measurements (41%) were performed on patients on a 100-mg daily dose instead of 200 mg, and all had blood levels of >1 mg/liter. Age (P = 0.012) and a daily dose of 200 mg versus 100 mg (P = 0.02) were independent predictors of levels of >6 mg/liter. AEs were recorded for 25 patients (56%). The mean drug level at the first measurement was 5.5 ± 2 mg/liter for patients reporting AEs, compared with 4.2 ± 1.7 mg/liter for those not reporting AEs (P = 0.032). The cutoff threshold best predictive of an AE was 4.6 mg/liter (area under the concentration-time curve, 0.710). Sixteen patients (36%) discontinued isavuconazole therapy due to AEs. Twenty-six patients (58%) continued on isavuconazole beyond 6 months. Asthma (P = 0.022) and a daily dose of 200 mg versus 100 mg (P = 0.048) were associated with AEs of grade 2 or higher. A reduced daily dose (100 mg versus 200 mg) of isavuconazole resulted in satisfactory drug levels in a substantial number of patients; it was better tolerated and enabled continuation of therapy for prolonged periods.
TEXT
It is estimated that about 3 million people globally have chronic pulmonary aspergillosis (CPA), a slowly progressive inflammatory process resulting in destruction of lung tissue, cavitation, pleural thickening, and fibrosis (1). Unlike invasive aspergillosis, CPA typically complicates pulmonary conditions without overt immunodeficiency, such as chronic obstructive pulmonary disease (COPD), tuberculosis (TB), severe bacterial pneumonia, and sarcoidosis (2). The 5-year mortality rate for CPA derived from a number of contemporary series is 50 to 85% (3). Treatment results in improvement of functional status and prevention of progressive tissue destruction and fibrosis but is complex due to comorbidities and drug interactions, toxicities, and intolerances (1, 4, 5).
The triazole antifungals are the cornerstone of CPA treatment, with itraconazole and voriconazole as first-line therapy and posaconazole as salvage therapy (6, 7). Isavuconazole is the newest triazole antifungal; it was approved by the Food and Drug Administration and the European Medicines Agency in 2015 for the treatment of invasive aspergillosis and mucormycosis. The pharmacokinetics (PK) of isavuconazole are very predictable, with high oral bioavailability (98%). Isavuconazole was found to be noninferior to voriconazole for treatment of invasive fungal disease and better tolerated (8). Less is known about its safety in CPA, although a retrospective observational study revealed a significantly lower rate of adverse events (AEs) with isavuconazole, compared to voriconazole (9). In addition, around one-quarter of patients who did not tolerate other azoles were able to tolerate isavuconazole (9).
Due to the favorable PK and lack of evidence linking plasma concentrations with efficacy and toxicity, therapeutic drug monitoring (TDM) is not currently recommended for isavuconazole (10). However, real-world data may differ from premarketing evaluations. Andes et al. showed that real-world concentrations were significantly lower in samples obtained during routine use, compared to clinical trials (mean concentration, 2.98 mg/liter versus 3.30 mg/liter [P = 0.014]) (11). Furfaro et al. reported a higher mean concentration of 4.32 mg/liter with a median treatment duration of 90 days (12). In contrast to other azoles, no exposure-toxicity relationship has been demonstrated for isavuconazole, although Furfaro et al. suggested a cutoff threshold for toxicity of 5.13 mg/liter (12). In addition, most published data apply to severely immunocompromised patients, and no information is available regarding the clinical interpretation of TDM data for immunocompetent patients such as those with CPA. Safety data are needed for patients who receive isavuconazole for prolonged periods, e.g., beyond 6 months.
The National Aspergillosis Centre (NAC) (Manchester, UK) sees more than 100 new patients with CPA each year, and isavuconazole is used as a second-line option, usually for prolonged periods. The diagnosis of CPA is made according to the European Respiratory Society guidelines (1). Treatment is normally started with 200 mg of isavuconazole orally once daily following a loading dose of 200 mg every 8 h for 48 h. Based on clinical judgement, some patients may be started on a lower daily dose of 100 mg; this group may include elderly patients, those with a low body mass index, and those with high drug levels with a prior azole. Treatment is typically started in the outpatient setting.
Patients are reviewed for treatment efficacy and safety at 4 weeks after commencement of therapy and then every 3 months. Random drug blood level measurements, full blood counts, and hepatic and renal function tests are repeated at each visit and between visits as required. Patients are encouraged to contact a specialist nurse by telephone in the case of AEs. Isavuconazole blood levels are measured by mass spectrometry in the NHS Antimicrobial Reference Laboratory (Bristol, UK). The reference laboratory advises that levels of 2 to 4 mg/liter are “normal,” but it does not make any recommendations regarding toxic or therapeutic levels. The NAC guidelines advise that levels of >6 mg/liter should lead to a reduction in dose, e.g., from 200 mg daily to 200 mg and 100 mg on alternate days or to 100 mg daily, depending on the presence of AEs considered by the clinician to be attributable to isavuconazole. Treatment is discontinued if the AEs are considered medically significant or debilitating. Levels of <1 mg/liter lead to assessment of compliance and an evaluation of drug interactions. In this study, we describe the PK and tolerability of isavuconazole in a CPA patient cohort during long-term administration.
RESULTS
Patient characteristics.
Forty-five patients had isavuconazole TDM performed and were included in the analyses. Of these, 29 (64%) were male; the mean age was 64 years (range, 38 to 85 years), and the mean weight was 64 kg (range, 35 to 110 kg). All patients had previously been on at least one other oral mold-active triazole. Nineteen patients (44%) had COPD, 16 (36%) had bronchiectasis, 7 (16%) had asthma, 7 (16%) had previous TB, 6 (13%) had sarcoidosis, and 4 (9%) had a history of nontuberculous mycobacterial infection. No one was receiving concomitant antimycobacterial treatment.
Isavuconazole dosing and TDM.
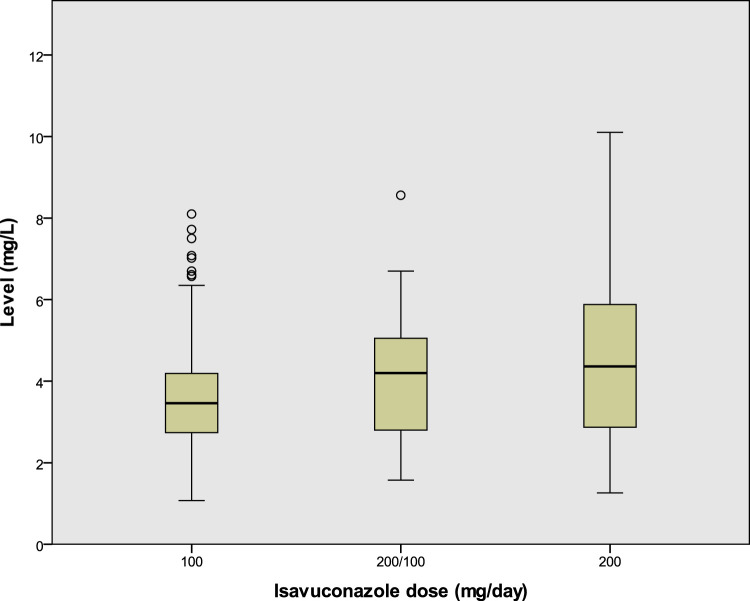
Isavuconazole was administered for a mean of 408.5 days (range, 18 to 1,473 days); 38 patients (86%) were started on 200 mg daily, after a loading regimen. All patients received an oral formulation. A total of 285 measurements (mean, 6.3 measurements per patient [range, 1 to 17 measurements per patient]) were recorded. Of these, 117 (41.1%) were for patients on 100 mg daily, 110 (39%) were for patients on 200 mg daily, 42 (15%) were for patients on 200 mg/100 mg on alternate days, 12 (4%) were for patients on dosing less frequent than 100 mg daily, and 4 (1%) were for patients on 300 mg daily. The overall mean isavuconazole level was 4.1 mg/liter (range, 1.1 to 10.1 mg/liter). Mean ± standard deviation (SD) levels were 4.6 ± 1.4 mg/liter for patients taking 200 mg daily, 4.1 ± 1.5 mg/liter for patients taking 200 mg/100 mg on alternate days, and 3.7 ± 1.4 mg/liter for patients taking 100 mg daily (Fig. 1). No patient had an isavuconazole level of <1 mg/liter. Among the measurements for patients on the lowest recorded dose (100 mg every 48 h), the mean level was 3.7 mg/liter (range, 1.8 to 5.2 mg/liter).
FIG 1.
Boxplot of isavuconazole levels according to dosing regimen. 200/100, 200 mg and 100 mg on alternate days. P is <0.001 for the comparison of 100 mg versus 200 mg, P equals 0.093 for the comparison of 200/100 versus 200 mg, and P equals 0.169 for the comparison of 200/100 versus 100 mg.
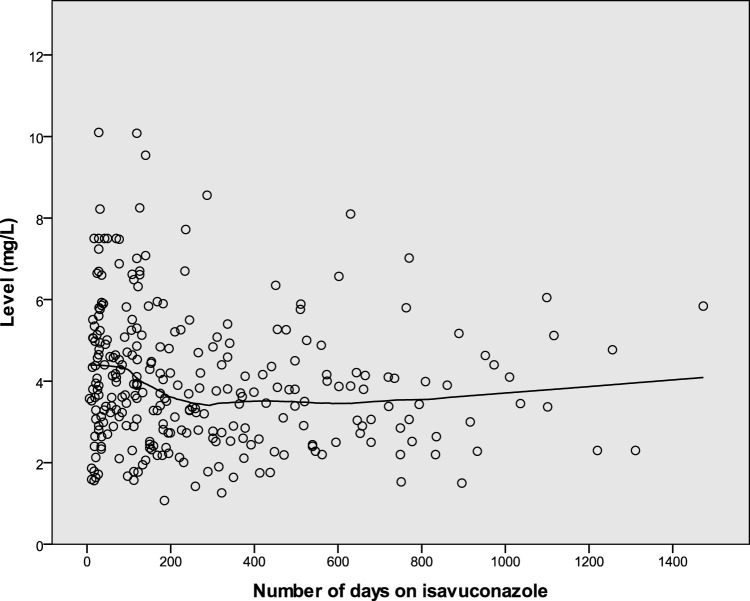
Levels of >6 mg/liter were recorded in 36 cases (13%), i.e., for 24 patients (22%) on 200 mg daily, 2 patients (5%) on 200 mg/100 mg on alternate days, and 10 patients (9%) on 100 mg daily (P = 0.002). The median time from starting treatment to developing a level of >6 mg/liter was 108 days (range, 17 to 1,099 days). Twenty-six male patients (14%) and 10 female patients (10%) had levels of >6 mg/liter (P = 0.353). Older patients were more likely to have high levels (66.6 versus 62.2 years [P = 0.013]). Weights were similar for patients with levels of >6 mg/liter, compared to other patients (62.8 versus 62.1 kg [P = 0.88]). In multivariate analysis, both age (P = 0.012) and daily dose (P = 0.02) remained independent predictors of levels of >6 mg/liter. High levels were more common in the first 4 weeks of treatment (10 [23%] of 43 measurements versus 25 [11%] of 239 measurements [P = 0.04]) and in the first 3 months of treatment (17 [19%] of 90 measurements versus 18 [9%] of 192 measurements [P = 0.032]), compared with subsequent measurements. Figure 2 shows the distribution of levels over time.
FIG 2.
Isavuconazole levels over time. The locally estimated scatterplot smoothing (LOESS) line illustrates the trend over time.
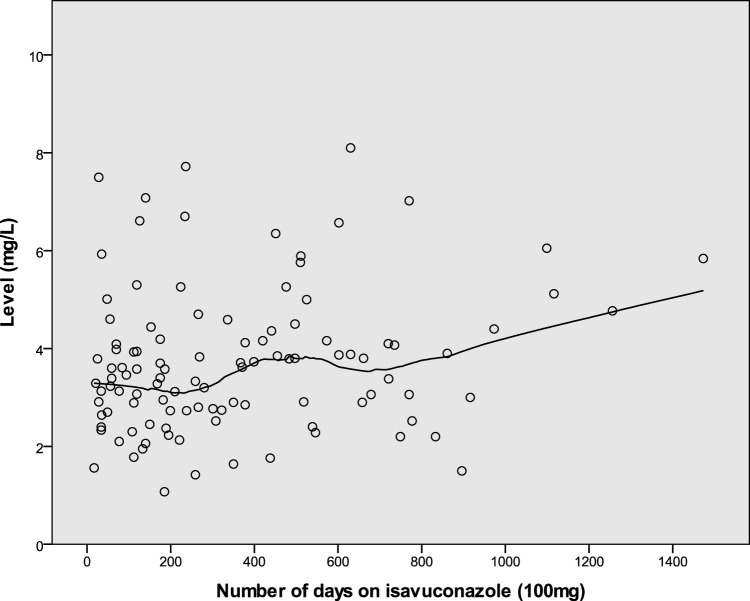
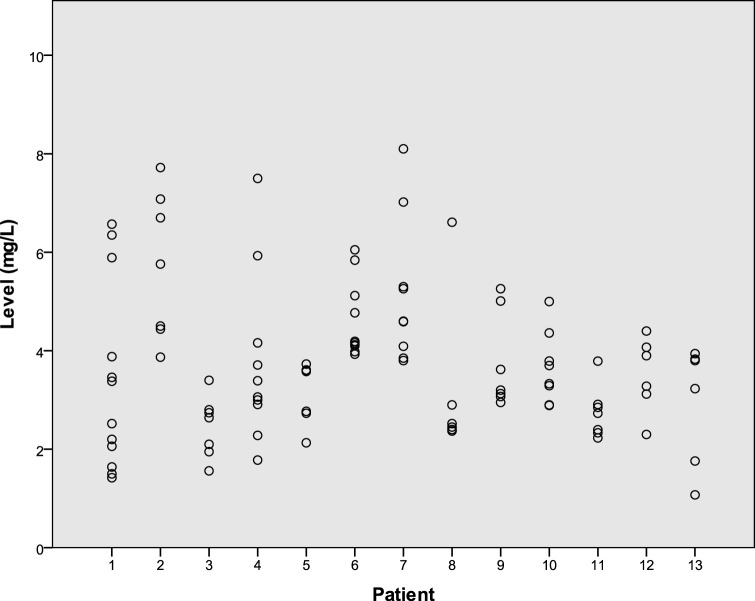
Thirteen patients had 6 or more measurements at the dose of 100 mg; the mean coefficient of variance (CV) was 0.30 (range, 0.17 to 0.56). Figure 3 shows the distribution and trend in levels for these patients. Figure 4 shows the range of measurements for these 13 patients. Four patients had 6 or more measurements at the dose of 200 mg; the mean CV was 0.20 (range, 0.10 to 0.29).
FIG 3.
Isavuconazole levels in patients with six or more drug blood level measurements on the same 100-mg once-daily dose. The LOESS line illustrates the trend over time.
FIG 4.
Isavuconazole levels for each patient among the 13 patients with six or more measurements on a 100-mg once-daily dose.
AEs.
AEs were reported for 25 patients (56%), with 13 patients reporting grade 2 AEs and 2 reporting grade 3 AEs. The mean drug levels at the first TDM measurements after starting isavuconazole were 5.5 ± 2 mg/liter for patients reporting AEs and 4.2 ± 1.7 mg/liter for those not reporting AEs (P = 0.032). In receiver operating characteristic (ROC) analysis, the best predictive cutoff value for the presence of AE was 4.6 mg/liter (area under the concentration-time curve [AUC], 0.710; Youden’s J statistic, 0.486). When all subsequent measurements were included in the analysis, the levels were 4.5 ± 2.1 mg/liter for patients reporting AEs and 3.9 ± 1.6 mg/liter for those not reporting AEs (P = 0.123).
Sixteen patients (36%) discontinued isavuconazole therapy due to AEs; of those patients, 5 discontinued therapy within 4 weeks. The AEs, as reported in the case notes, leading to discontinuation of therapy were hepatotoxicity (4 cases), neuropathy (3 cases), headache (2 cases), malaise (2 cases), weight loss (1 case), confusion (1 case), nausea (1 case), photosensitivity (1 case), and dysgeusia (1 case). Isavuconazole was discontinued due to AEs at a mean of 157 days (range, 21 to 525 days). Twenty-six patients continued isavuconazole beyond 6 months; of those, 5 (19%) discontinued therapy due to AEs, namely, hepatotoxicity (2 cases), weight loss (1 case), nausea (1 case), and neuropathy (1 case).
Of the 4 patients who discontinued treatment due to liver toxicity, 1 had grade 2 toxicity. For all patients, liver function test results returned to normal after discontinuation of therapy. Levels for these patients were 2.85, 3.29, 5.90, and 7.48 mg/liter. Tables 1 and 2 show factors associated with development of AEs. In multivariate analysis, asthma (odds ratio [OR], 3.47 [95% confidence interval [CI], 1.20 to 10.02; P = 0.022]) and a dose of 200 mg versus 100 mg daily (OR, 3.86 [95% CI, 1.02 to 14.65; P = 0.048]) were associated with AEs of grade 2 or higher.
TABLE 1.
Factors associated with AEs of grade 2 or higher with isavuconazole among patients with CPA
| Factor | Data for: |
P | OR (95% CI) | |
|---|---|---|---|---|
| AE grade of <2 (n = 262a) | AE grade of ≥2 (n = 21) | |||
| First TDM measurement (mg/liter) (mean ± SD) | 4.5 ± 1.9 | 4.8 ± 2.1 | 0.766 | |
| TDM measurement at any time (mg/liter) (mean ± SD) | 4.0 ± 1.7 | 4.6 ± 2.2 | 0.144 | |
| Level (no. [%]) | 0.321 | 1.69 (0.53–5.34) | ||
| ≥6 mg/liter | 32 (88.9) | 4 (11.1) | ||
| <6 mg/liter | 230 (93.1) | 17 (6.9) | ||
| Dose (no. [%]) | ||||
| 200 mg/day | 94 (87.9) | 13 (12.1) | 0.011 | 5.26 (1.45–19) |
| 200/100 mg/dayb | 38 (90.5) | 4 (9.5) | 0.078 | 4 (0.86–18.7) |
| 100 mg/day | 114 (97.4) | 3 (2.6) | Reference | |
| Age (yr) (mean ± SD) | 62.8 ± 9.9 | 62.6 ± 12.6 | 0.917 | |
| Weight (kg) (mean ± SD) | 62.3 ± 16.6 | 63.6 ± 16.7 | 0.747 | |
| Sex (no. [%]) | 0.345 | 0.60 (0.24–1.45) | ||
| Male | 170 (93.9) | 11 (6.1) | ||
| Female | 92 (90.2) | 10 (9.6) | ||
| COPD (no. [%]) | 0.073 | 0.42 (0.17–1.08) | ||
| Yes | 142 (95.3) | 7 (4.7) | ||
| No | 120 (89.6) | 14 (10.4) | ||
| TB (no. [%]) | 0.775 | 0.69 (0.20–2.43) | ||
| Yes | 51 (94.4) | 3 (5.6) | ||
| No | 211 (92.1) | 18 (7.9) | ||
| Bronchiectasis (no. [%]) | 0.213 | 1.87 (0.76–4.62) | ||
| Yes | 75 (89.3) | 9 (10.7) | ||
| No | 187 (94) | 12 (6) | ||
| Sarcoidosis (no. [%]) | 1.00 | 0.95 (0.27–3.39) | ||
| Yes | 39 (92.9) | 3 (7.1) | ||
| No | 223 (92.5) | 18 (7.5) | ||
| Asthma (no. [%]) | 0.003 | 5.20 (1.91–14.17) | ||
| Yes | 23 (76.7) | 7 (23.3) | ||
| No | 239 (94.5) | 14 (5.5) | ||
| Steroid inhalers (no. [%]) | 0.462 | 0.67 (0.28–1.73) | ||
| Yes | 184 (93.4) | 13 (6.6) | ||
| No | 78 (90.7) | 8 (9.3) | ||
For two measurements, no information was available on the presence of AEs.
Doses of 200 mg and 100 mg on alternate days.
TABLE 2.
Factors associated with AEs of any severity with isavuconazole among patients with CPA
| Factor | Data for: |
P | OR (95% CI) | |
|---|---|---|---|---|
| No AE | Any AE | |||
| First TDM measurement (mg/liter) | 4.2 ± 1.7 | 5.5 ± 2 | 0.032 | |
| TDM measurement at any time (mg/liter) | 4.0 ± 1.6 | 4.5 ± 2.1 | 0.123 | |
| Level (no. [%]) | 0.111 | 2.03 (0.93–4.43) | ||
| ≥6 mg/liter | 25 (69.4) | 11 (30.6) | ||
| <6 mg/liter | 203 (82.2) | 44 (17.8) | ||
| Dose (no. [%]) | 0.113 | |||
| 200 mg/day | 81 (75.7) | 26 (24.3) | 0.066 | 1.89 (0.96–3.72) |
| 200/100 mg/daya | 31 (73.8) | 11 (26.2) | 0.093 | 2.09 (0.88–4.93) |
| 100 mg/day | 100 (85.5) | 17 (14.5) | Reference | |
| Age (yr) (mean ± SD) | 63.3 ± 10 | 60.9 ± 10.4 | 0.112 | |
| Weight (kg) (mean ± SD) | 62.8 ± 16.6 | 60.7 ± 16.6 | 0.398 | |
| Sex (no. [%]) | 0.349 | 0.74 (0.40–1.35) | ||
| Male | 149 (82.3) | 32 (17.7) | ||
| Female | 79 (77.5) | 23 (22.5) | ||
| COPD (no. [%]) | 0.175 | 0.64 (0.35–1.16) | ||
| Yes | 125 (83.9) | 24 (16.1) | ||
| No | 103 (76.9) | 31 (23.1) | ||
| TB (no. [%]) | 0.849 | 1.08 (0.51–2.25) | ||
| Yes | 43 (79.6) | 11 (20.4) | ||
| No | 185 (80.8) | 44 (19.2) | ||
| Bronchiectasis (no. [%]) | <0.001 | 3.19 (1.73–5.85) | ||
| Yes | 56 (66.7) | 28 (33.3) | ||
| No | 172 (86.4) | 27 (13.6) | ||
| Sarcoidosis (no. [%]) | 0.678 | 1.16 (0.52–2.58) | ||
| Yes | 33 (78.6) | 9 (21.4) | ||
| No | 195 (80.9) | 46 (19.1) | ||
| Asthma (no. [%]) | 0.006 | 3.26 (1.46–7.25) | ||
| Yes | 18 (60) | 12 (40) | ||
| No | 210 (83) | 43 (17) | ||
| Steroid inhalers (no. [%]) | 0.744 | 0.87 (0.47–1.64) | ||
| Yes | 160 (81.2) | 37 (18.8) | ||
| No | 68 (79.1) | 18 (20.9) | ||
Doses of 200 mg and 100 mg on alternate days.
DISCUSSION
We present PK and safety data on the long-term use of isavuconazole in a population of patients without significant immunocompromise. Isavuconazole achieved adequate levels even with lower-than-recommended doses and exhibited a favorable safety profile even with very prolonged use beyond 6 months, exceeding the usual duration of treatment reported in previous studies.
Compared to other real-life studies, the levels seen here are comparable to the results reported by Furfaro et al. but higher than those reported by Andes et al. or those from clinical trials (11–13). Levels were higher in initial measurements, possibly due to of the effect of the loading dose, and reduced thereafter, and this was followed by a trend for rising levels over several months or years. This was also observed for patients with multiple measurements on the same dose. It agrees with the findings of Furfaro et al., who showed a linear increase of 0.032 mg/liter per day of treatment (12). The significance of this finding is not clear, and most patients tolerated isavuconazole over several years of treatment despite a trend for rising levels. We calculated an intrapatient variability of 20% at the 200-mg dose and 30% at the 100-mg daily dose. This is comparable to the value (23.2%) reported from the SECURE trial (14); however, we provide more long-term data, as we included only patients with at least six measurements. Furfaro et al. reported an intrapatient variability of 36% (12). Further studies are needed to confirm intrapatient variability at the lower dose of 100 mg daily.
Interestingly, a dose of 100 mg/day, a lower-than-recommended dose, achieved levels of >1 mg/liter in all cases. In contrast, it was previously reported from clinical trials and real-life studies that approximately 10% of patients may have levels of <1 mg/liter even with the 200-mg dose (11, 13). The patients who achieved levels of >6 mg/liter were older. This is in contrast to population PK studies showing the effect of age on drug levels to be insignificant, although older female patients had lower clearance (15). Drug interactions are unlikely to account for this effect in our population. The majority of elderly patients with CPA are likely to have multiple comorbidities, possibly including hepatic impairment from previous azole use. Therefore, it is possible that clearance of the drug is affected in this population.
Population PK studies have shown that >90% of patients are likely to achieve therapeutic targets with the usual dosing, assuming an MIC of 1 mg/liter (16). However, the target trough isavuconazole level for efficacy has not yet been determined. In our study, the lower doses used (100 mg daily or 100 mg/200 mg on alternate days) resulted in lower levels than the 200-mg daily dose, but whether this is significant in terms of predicted efficacy is unknown. The mean concentration reported from clinic trials was 3.3 mg/liter with a SD of 2.18 mg/liter, whereas that reported from real-world clinical use was 2.98 mg/liter with a SD of 1.91 mg/liter (11). For the 100-mg dose, we observed a higher mean concentration and a lower SD, compared to both of the aforementioned data sets, suggesting adequate exposures with this dose. Therefore, the 100-mg daily dose of isavuconazole may be adequate for this population of patients with chronic lung disease, who may be older or have lower BMI than patients with invasive fungal disease, and may not be extrapolated to other populations.
Levels were predictive of AEs but only at the time of the first TDM measurement; this effect was not seen when subsequent measurements were analyzed. The cutoff value of 4.6 mg/liter that we suggest here is similar to that proposed by Furfaro et al. (12). However, the model had only moderate sensitivity and specificity. The administered daily dose, rather than the level, was predictive of more serious AEs. Therefore, reducing the daily dose may reduce or prevent toxicity, including AEs leading to discontinuation, without resulting in significantly lower levels or compromising efficacy. Finally, certain medical conditions (asthma and bronchiectasis) were more likely to lead to AEs; the significance of this observation is not clear but could be explained by patient frailty, concurrent infections, or drug-drug interactions.
This study has several limitations. The AE profile was recorded retrospectively, possibly leading to some milder events not being documented in the notes. The TDM was performed at a random time, rather than prior to dosing, for practical reasons, because patients attended the clinic at different times. However, the long half-life of isavuconazole (exceeding 100 h) should permit this approach (16). In addition, we could not evaluate TDM results in relation to treatment efficacy. This is challenging for patients with chronic lung disease, who often remain on long-term antifungal therapy to prevent relapse. In a small number of measurements, the levels were reported as >7.5 mg/liter; however, we thought that we should include those cases because they would provide additional information regarding high levels and toxicity. Finally, we used weight rather than BMI because that information was not available.
In summary, isavuconazole was reasonably well tolerated over long periods of up to several years, even though levels appeared to rise over time. A lower isavuconazole dose was better tolerated and resulted in adequate levels, a finding that has the potential to result in significant cost savings. Isavuconazole offers an attractive treatment option for patients requiring prolonged or long-term antifungal therapy, such as those with allergic or chronic fungal disease.
MATERIALS AND METHODS
Patients.
Patients for this study were identified from the NAC database using isavuconazole as the search term. All patients diagnosed with CPA and treated with isavuconazole between October 2015 and March 2020 were included. The medical records and laboratory findings were reviewed.
Definitions.
Adverse drug reactions were classified based on Common Terminology Criteria for Adverse Events (CTCAE) v5.0, as follows (17): grade 1, mild; asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated; grade 2, moderate; minimal, local, or noninvasive intervention indicated; limiting age-appropriate instrumental activities of daily living; grade 3, severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care activities of daily living; grade 4, life-threatening consequences; urgent intervention indicated; grade 5, death related to AE.
The Drug-Induced Liver Injury Network (DILIN) 5-point scale was used to describe hepatic abnormalities, as follows (18): 1+, mild; increased serum aminotransferase or alkaline phosphatase levels or both but total serum bilirubin level of <2.5 mg/dl and no coagulopathy (international normalized ratio [INR] of <1.5); 2+, moderate; increased serum aminotransferase or alkaline phosphatase levels or both and total serum bilirubin level of >2.5 mg/dl or coagulopathy (INR of >1.5) without hyperbilirubinemia; 3+, moderate to severe; increased serum aminotransferase or alkaline phosphatase levels and total serum bilirubin level of >2.5 mg/dl and hospitalization (or preexisting hospitalization is prolonged) because of the drug-induced liver injury; 4+, severe; increased serum aminotransferase or alkaline phosphatase levels and serum bilirubin level of >2.5 mg/dl and at least one of the following: prolonged jaundice and symptoms beyond 3 months, signs of hepatic decompensation (INR >1.5, ascites, encephalopathy), or other organ failure thought to be related to drug-induced liver injury; 5+, fatal; death or liver transplantation for drug-induced liver injury.
Data collection.
Recorded data included demographic characteristics, underlying disease, isavuconazole dosing, time of onset and timing of TDM, presence of AEs as recorded in the clinic notes and the presumed association with isavuconazole, and the action taken. In cases in which the isavuconazole level was reported as >7.5 mg/liter with no exact value given, the value of 7.5 mg/liter was used for the analyses.
Statistical analysis.
Data were analyzed using SPSS v23 (IBM Corp., Armonk, NY, USA). Qualitative variables were presented as frequencies; values were presented as mean ± SD for continuous, normally distributed variables or as median for non-normally distributed variables. P values of <0.05 were considered significant. CV (SD/mean) was calculated for patients who had at least six levels measured with the same dose, to assess intrapatient variability. The t test was used to compare continuous parameters and the chi-square test to compare dichotomous variables. Binary logistic regression was used for multivariate analyses for the risk factors associated with high levels (>6 mg/liters) and for the presence of AEs. ROC analysis was performed to assess the optimal cutoff value predictive of toxicity.
Ethics statement.
Informed consent and ethical approval were not required because this was a retrospective service evaluation, according to UK Health Research Authority guidelines (19).
ACKNOWLEDGMENTS
C.K., M.D.R., and R.R.-R. were supported by the NIHR Manchester Biomedical Research Centre.
We thank the NHS Antimicrobial Reference Laboratory (Bristol, UK) for helpful discussions regarding the interpretation of the isavuconazole concentrations.
C.K. has received speaker and travel fees from Astellas. A.O. has no conflicts of interest. C.B.M. has received travel support from Astellas, Pfizer, and Gilead, has been paid for talks on behalf of Pfizer, and has received a research grant paid to the Mycology Reference Centre from Pfizer. R.R.-R. has given lectures for Astellas, Basilea, Gilead, and Pfizer and has received conference attendance support from Astellas and Gilead. M.D.R. has received speaker fees from Gilead, Mylan, Astellas, MSD, and Basilea, acts as a consultant for Gilead, Pulmocide, Pulmatrix, and Pfizer, and is a longstanding member of the ESCMID diagnosis and treatment guidelines writing groups. He is a cofounder and codirector of Richardson Bio-Tech (Guangzhou) Ltd.
REFERENCES
- 1.Denning DW, Cadranel J, Beigelman-Aubry C, Ader F, Chakrabarti A, Blot S, Ullmann AJ, Dimopoulos G, Lange C. 2016. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J 47:45–68. doi: 10.1183/13993003.00583-2015. [DOI] [PubMed] [Google Scholar]
- 2.Smith NL, Denning DW. 2011. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur Respir J 37:865–872. doi: 10.1183/09031936.00054810. [DOI] [PubMed] [Google Scholar]
- 3.Lowes D, Al-Shair K, Newton PJ, Morris J, Harris C, Rautemaa-Richardson R, Denning DW. 2017. Predictors of mortality in chronic pulmonary aspergillosis. Eur Respir J 49:1601062. doi: 10.1183/13993003.01062-2016. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal R, Vishwanath G, Aggarwal AN, Garg M, Gupta D, Chakrabarti A. 2013. Itraconazole in chronic cavitary pulmonary aspergillosis: a randomised controlled trial and systematic review of literature. Mycoses 56:559–570. doi: 10.1111/myc.12075. [DOI] [PubMed] [Google Scholar]
- 5.Felton TW, Baxter C, Moore CB, Roberts SA, Hope WW, Denning DW. 2010. Efficacy and safety of posaconazole for chronic pulmonary aspergillosis. Clin Infect Dis 51:1383–1391. doi: 10.1086/657306. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Goncer I, Harris C, Kosmidis C, Muldoon EG, Newton PJ, Denning DW. 2018. Assessment of posaconazole salvage therapy in chronic pulmonary aspergillosis using predefined response criteria. Int J Antimicrob Agents 52:258–264. doi: 10.1016/j.ijantimicag.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Cadranel J, Philippe B, Hennequin C, Bergeron A, Bergot E, Bourdin A, Cottin V, Jeanfaivre T, Godet C, Pineau M, Germaud P. 2012. Voriconazole for chronic pulmonary aspergillosis: a prospective multicenter trial. Eur J Clin Microbiol Infect Dis 31:3231–3239. doi: 10.1007/s10096-012-1690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, Bow EJ, Rahav G, Neofytos D, Aoun M, Baddley JW, Giladi M, Heinz WJ, Herbrecht R, Hope W, Karthaus M, Lee D-G, Lortholary O, Morrison VA, Oren I, Selleslag D, Shoham S, Thompson GR, Lee M, Maher RM, Schmitt-Hoffmann A-H, Zeiher B, Ullmann AJ. 2016. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet 387:760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 9.Bongomin F, Maguire N, Moore CB, Felton T, Rautemaa-Richardson R. 2019. Isavuconazole and voriconazole for the treatment of chronic pulmonary aspergillosis: a retrospective comparison of rates of adverse events. Mycoses 62:217–222. doi: 10.1111/myc.12885. [DOI] [PubMed] [Google Scholar]
- 10.Patterson TF, Thompson GR, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young J-AH, Bennett JE. 2016. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andes D, Kovanda L, Desai A, Kitt T, Zhao M, Walsh TJ. 2018. Isavuconazole concentration in real-world practice: consistency with results from clinical trials. Antimicrob Agents Chemother 62:e00585-18. doi: 10.1128/AAC.00585-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furfaro E, Signori A, Di Grazia C, Dominietto A, Raiola AM, Aquino S, Ghiggi C, Ghiso A, Ungaro R, Angelucci E, Viscoli C, Mikulska M. 2019. Serial monitoring of isavuconazole blood levels during prolonged antifungal therapy. J Antimicrob Chemother 74:2341–2346. doi: 10.1093/jac/dkz188. [DOI] [PubMed] [Google Scholar]
- 13.Desai AV, Kovanda LL, Hope WW, Andes D, Mouton JW, Kowalski DL, Townsend RW, Mujais S, Bonate PL. 2017. Exposure-response relationships for isavuconazole in patients with invasive aspergillosis and other filamentous fungi. Antimicrob Agents Chemother 61:e01034-17. doi: 10.1128/AAC.01034-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaindl T, Andes D, Engelhardt M, Saulay M, Larger P, Groll AH. 2019. Variability and exposure-response relationships of isavuconazole plasma concentrations in the phase 3 SECURE trial of patients with invasive mould diseases. J Antimicrob Chemother 74:761–767. doi: 10.1093/jac/dky463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desai AV, Han D, Kowalski DL, Lademacher C, Pearlman H, Yamazaki T. 2019. No dose adjustment for isavuconazole based on age or sex. Antimicrob Agents Chemother 63:e02629-18. doi: 10.1128/AAC.02629-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desai A, Kovanda L, Kowalski D, Lu Q, Townsend R, Bonate PL. 2016. Population pharmacokinetics of isavuconazole from phase 1 and phase 3 (SECURE) trials in adults and target attainment in patients with invasive infections due to Aspergillus and other filamentous fungi. Antimicrob Agents Chemother 60:5483–5491. doi: 10.1128/AAC.02819-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services. 2017. Common terminology criteria for adverse events (CTCAE), version 5. 0. U.S. Department of Health and Human Services, Washington, DC. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf. [Google Scholar]
- 18.Fontana RJ, Watkins PB, Bonkovsky HL, Chalasani N, Davern T, Serrano J, Rochon J, DILIN Study Group. 2009. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf 32:55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NHS Research Authority. 2020. “Does my project require review by a Research Ethics Committee?” version 2.0. NHS Research Authority, London, UK. http://www.hra-decisiontools.org.uk/ethics/docs/Algorithm%20-%20Does%20my%20project%20require%20REC%20review%20v2.0%2020200304.pdf. [Google Scholar]