Here, we characterize the fosA genes from three Escherichia coli clinical isolates recovered from Canadian patients. Each fosA sequence was individually overexpressed in E. coli BW25113, and antimicrobial susceptibility testing was performed to assess their role in fosfomycin resistance. The findings from this study identify and functionally characterize FosA3, FosA8, and novel FosA7 members and highlight the importance of phenotypic characterization of fosA genes.
KEYWORDS: fosfomycin, fosA7, beta-lactamase, Escherichia coli, novel fosA
ABSTRACT
Here, we characterize the fosA genes from three Escherichia coli clinical isolates recovered from Canadian patients. Each fosA sequence was individually overexpressed in E. coli BW25113, and antimicrobial susceptibility testing was performed to assess their role in fosfomycin resistance. The findings from this study identify and functionally characterize FosA3, FosA8, and novel FosA7 members and highlight the importance of phenotypic characterization of fosA genes.
INTRODUCTION
Escherichia coli is a common urinary tract pathogen. Treatment of infections caused by extended-spectrum beta-lactamase (ESBL)-producing or multidrug-resistant (MDR) E. coli can be problematic, as therapeutic options may be limited. Due to the increasing prevalence of ESBL and MDR E. coli, there has been renewed interest in the use of fosfomycin (1–4). Fosfomycin is a phosphoenolpyruvate analogue that disrupts bacterial cell wall synthesis by inhibiting UDP-N-acetylglucosamine-3-enolpyruvyl transferase (MurA), an enzyme involved in synthesis of N-acetylmuramic acid (5). Resistance to fosfomycin occurs by three main mechanisms: (i) alteration of fosfomycin drug uptake transporter genes (glpT and uhpT), (ii) modification or overexpression of murA, or (iii) acquisition of a fosfomycin-inactivating (fos) enzyme (5). Fos enzymes are of the greatest concern, since the fos genes that encode them can be found on plasmids, allowing for their dissemination by horizontal gene transfer (4, 5). In Canada, fosfomycin resistance and fos gene detection among E. coli clinical isolates are rare and have not been well described to date (6).
In this study, we characterize the fosA genes from three fosfomycin-resistant E. coli isolates (two from urine and one from blood) from a Canadian collection of clinical strains and describe a novel FosA7.5 variant within the FosA7 group. We also discuss the phylogenetic relationships among previously identified FosA1-A12 members.
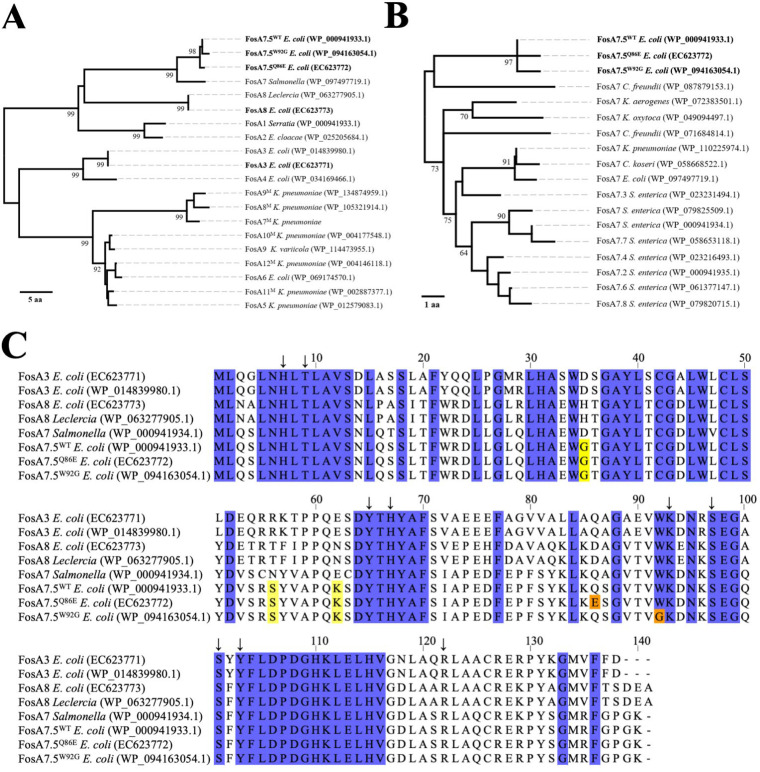
Whole-genome sequencing of the three Canadian isolates (EC623771 [GenBank BioSample no. SAMN13659120], EC623772 [GenBank BioSample no. SAMN13659121], and EC623773 [GenBank BioSample no. SAMN13659122]) was performed on an Illumina MiSeq system using Nextera XT DNA libraries. Contigs were assembled and annotated using the IRIDA version 19.09 assembly and annotation pipeline, which combines Shovill-based assembly and QUAST quality assessment with Prokka annotation (7). Sequence analysis revealed fosA genes in all three genomes (see Table S1 in the supplemental material). The E. coli EC623771 fosA gene was 100% identical to fosA3 (8), and the E. coli EC623773 fosA gene was 100% identical to fosA8 (9) (Fig. 1). The E. coli EC623772 fosA gene was not identical to any previously characterized fosA sequence but demonstrated >99% sequence identity to two publicly deposited sequences, a fosA gene from a canine isolate of E. coli (WP_094163054.1) and a reference sequence annotated as fosA7.5 (WP_000941933.1) (Table S2). We decided to name the novel E. coli EC623772 fosA variant and the E. coli WP_094163054.1 variant fosA7.5, following the numbering convention that has been previously used for annotating fosA7 genes in the NCBI Bacterial Antimicrobial Resistance Reference Gene Database. Neighbor-joining analysis was performed to further investigate the relationship of the FosA variants from our clinical isolates with previously described FosA1 to FosA12 sequences. (Fig. 1A and B) (8–17). This confirmed the similarity of the three FosA7.5 sequences.
FIG 1.
Phylogenetic and sequence analysis of clinically isolated E. coli FosA sequences and their comparison to previously identified FosA variants. (A) Phylogenetic analysis of FosA1 to FosA12 protein sequences using the neighbor-joining distance-based method. Branch lengths represent amino acid differences as distance (scale bar). (B) Phylogenetic comparison of FosA7 family protein sequences using the same method as described in panel A. (C) Multiple sequence alignment of FosA3, FosA8, and FosA7 protein sequence variants. Blue coloring in the alignment indicates conserved residues identified among FosA1 to 12 family members. Amino acid differences that distinguish the FosA7.5 group from FosA7 are shown in yellow. Differences among FosA7.5 sequences are highlighted in orange. Arrows indicate active site residues. The alignment was generated using Jalview v2.10.5 (21).
Relative to FosA7.5 from E. coli WP_000941933.1, FosA7.5 from E. coli EC623772 has a Q86E change, whereas the FosA7.5 from E. coli WP_094163054.1 shows a W92G change at a highly conserved amino acid position. Hence, we refer to the “wild-type” fosA7.5 sequence as fosA7.5WT, the novel E. coli EC623772 fosA variant as fosA7.5Q86E, and the canine WP_094163054.1 variant as fosA7.5W92G. Despite its name, FosA7.5 is distinct from the canonical FosA7 sequence and differs from other FosA7 members at amino acid sites G35, S56, and K62. FosA7 was originally identified in Salmonella enterica serovar Heidelberg (15), and most closely related variants are associated with S. enterica, whereas FosA7.5, along with the EC623772 and canine variants, is restricted to E. coli. These sequences are also distinct from FosA7-like sequences from Klebsiella spp. and Citrobacter spp. (Fig. S1, Table S2).
Twelve FosA variants (FosA1 to FosA12) were previously described in peer-reviewed publications (8–17). It should be noted that there is currently some inconsistency in the FosA literature regarding the naming of FosA enzyme variants. Notably, FosA7, FosA8, FosA9, and FosA10 have each been used twice to describe different variants (9, 15–18). After the published description of FosA7 in Salmonella Heidelberg, Mathur et al. described six FosA variants in Klebsiella pneumoniae and named them FosA7 through FosA12 (referred to in Fig. 1, Table 1, and the following text as FosA7M to FosA12M) (17). Phylogenetic analysis suggests that FosA7M, FosA8M, and FosA9M may make a distinct branch of FosA enzymes, but it is unclear if three separate designations are warranted (Fig. 1A). More recently, E. coli FosA8, FosA9, and FosA10 genes were described in three papers (9, 16, 18). Based on our phylogenetic and sequence analyses in Fig. 1, FosA10M, FosA11M, and FosA12M are very similar to one another as well as to FosA5, FosA6, and the newer FosA9 allele (16). Notably, the original descriptions of FosA5 (13), FosA6 (14), and FosA9 (16) indicate that they were mobilized to E. coli from Klebsiella. Thus, all of these alleles may represent a family of genes derived from Klebsiella.
TABLE 1.
Sequences, strains, and plasmids examined in this study
| Strains and plasmids | Characteristicsa | Reference or source |
|---|---|---|
| Strains | ||
| E. coli EC623771 | Fosfomycin-resistant isolate | CANWARD |
| E. coli EC623772 | Fosfomycin-resistant isolate | CANWARD |
| E. coli EC623773 | Fosfomycin-resistant isolate | CANWARD |
| E. coli BW25113 | F–, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ–, rph-1, Δ(rhaD-rhaB)568, hsdR514 | NBRPb |
| E. coli pMS119EH | E. coli BW25113 transformed with pMS119EH | This study |
| E. coli FosA3 | E. coli BW25113 transformed with pMS-FosA3 | This study |
| E. coli FosA8 | E. coli BW25113 transformed with pMS-FosA8 | This study |
| E. coli FosA7.5WT | E. coli BW25113 transformed with pMS-FosA7.5WT | This study |
| E. coli FosA7.5Q86E | E. coli BW25113 transformed with pMS-FosA7.5Q86E | This study |
| E. coli FosA7.5W92G | E. coli BW25113 transformed with pMS-FosA7.5W92G | This study |
| Plasmids | ||
| pMS119EH | NruI-NdeI deletion of pJF119EH vector | 22 |
| pMS-FosA3 | C-terminus His6-tagged fosA3 EC623771 gene cloned in pMS119EH | This study |
| pMS-FosA8 | C-terminus His6-tagged fosA8 EC623773 gene cloned in pMS119EH | This study |
| pMS-FosA7.5WT | C-terminus His6-tagged fosA7.5 WP_000941933.1 gene cloned in pMS119EH | This study |
| pMS-FosA7.5Q86E | C-terminus His6-tagged fosA7.5 EC623772 gene cloned in pMS119EH | This study |
| pMS-FosA7.5W92G | C-terminus His6-tagged fosA7.5 WP_094163054.1 gene cloned in pMS119EH | This study |
His6, hexahistidine; C-terminus, carboxy-terminus.
National BioResource Project, Keio Collection (23).
Consistent with previous reports, the fosA3 (EC623771) and fosA8 (EC623773) genes identified in our isolates are associated with plasmid sequences. The fosA7.5Q86E allele in EC623772 is flanked on both sides by insertion sequences that confounded our initial attempts to determine the location of this gene. Available genome assemblies (e.g., E. coli Ec40743 [CP041919.1] and E. coli 210205630 [CP015912]) suggest that the fosA7.5WT allele is located on the E. coli chromosome. However, resequencing of E. coli EC623772 with a MinION system (Oxford Nanopore Technologies) and assembly with Flye version 2.8.1 (19) revealed that fosA7.5Q86E is located on a 103-kb plasmid. Sequence comparison using publicly available databases shows that the backbone of this 103-kb plasmid, excluding the fosA7.5Q86E region, is conserved with other plasmids from E. coli, Salmonella, and Klebsiella (e.g., GenBank plasmids CP044142.1, JN983043.1, and MF582638.1).
To verify that the genes from the three Canadian clinical isolates conferred resistance to fosfomycin, fosA3 (EC623771), fosA8 (EC623773), and the three E. coli fosA7.5 sequences were gene synthesized by Bio Basic, Inc. (Canada), and individually cloned into the low copy expression vector pMS119EH. All fosA genes were cloned with a C-terminal hexahistidine affinity tag (His6-tag) and then overexpressed in the E. coli K-12 strain BW25113 with isopropyl β-d-1-thiogalactopyranoside (IPTG) induction (Table 1). Western blotting demonstrated successful FosA protein expression and accumulation of each E. coli transformant (Fig. S2). Each transformant underwent fosfomycin antimicrobial susceptibility testing using agar dilution and disk diffusion according to CLSI standards and an Etest, and the results are shown in Table 2. The fosA3, fosA8, and fosA7.5Q86E genes cloned from the Canadian clinical isolates, as well as the wild-type fosA7.5WT allele, all conferred resistance to fosfomycin (MIC values of >512 μg/ml and >1,024 μg/ml for agar dilution and Etest, respectively). The only exception was E. coli transformed with the fosA7.5W92G variant, which remained susceptible to fosfomycin at MIC values of 32 μg/ml and 2 μg/ml using the agar dilution and Etest methods, respectively.
TABLE 2.
Fosfomycin susceptibility testing results for E. coli transformants
| E. coli plasmid transformantc | Agar dilution MIC (μg/ml) | Disk diffusion zone diam (mm) | Etest MIC (μg/ml) | Result |
|---|---|---|---|---|
| FosA3 | >512 | 6a | >1,024 | Resistant |
| FosA8 | >512 | 6 | >1,024 | Resistant |
| FosA7.5WT | >512 | 6 | >1,024 | Resistant |
| FosA7.5Q86E | >512 | 6 | >1,024 | Resistant |
| FosA7.5W92G | 32 | 30b | 2 | Susceptible |
| pMS119EH | 2–4 | 30 | 0.5 | Susceptible |
6 mm is equivalent to no zone diameter.
CLSI, 30 mm; EUCAST, 36 mm.
All transformants were induced with 1 mM IPTG.
As we noted key amino acid differences between FosA7.5 members, we generated homology models of FosA3, FosA8, and the three FosA7.5 variants using the I-TASSER Web server (20) to determine if any protein structural alterations impacting the FosA active site may explain why the fosA7.5W92G transformant was susceptible to fosfomycin (Fig. S3). Dimeric FosA protein homology models were generated from the FosA1 Serratia marcescens (PDB: 1nbp) crystal structure to model the complete active site spanning the dimer interface. All FosA7.5 variant models demonstrated tight overall alignment to previously characterized FosA3 and FosA8 based on lowest root mean square deviation (RMSD) values (1.722 to 2.011 Å). The W92G amino acid change in FosA7.5W92G (GenBank accession number WP_094163054.1) appeared to generate a larger pocket near the fosfomycin binding site when aligned to other FosA7.5 models, suggesting that the replacement of tryptophan by a smaller glycine residue may reduce the enzymatic activity of this variant. FosA7.5W92G may allow greater substrate movement or amino acid flexibility within the enzyme’s active site by replacing this conserved tryptophan that we observed in FosA alignments at this residue position (Fig. S1).
In conclusion, we identified three fosA genes in three E. coli clinical isolates (EC623771 to EC623773) recovered from Canadian patients. In addition to confirming the role of fosA3 and fosA8 as determinants of fosfomycin resistance (8, 9), we identified and characterized multiple variants of fosA7.5. Unlike other fosA7 alleles, which are associated with Salmonella, distribution of fosA7.5 is primarily restricted to E. coli. Ongoing surveillance for fosfomycin resistance is crucial to ensure that this antimicrobial remains effective as a first-line therapy for urinary tract infections.
Supplementary Material
ACKNOWLEDGMENTS
We thank the participating centers, investigators, and laboratory site staff that contributed to the CANWARD study. We also thank the medical laboratory technologists at Cadham Provincial Laboratory (Winnipeg MB, Canada) for assistance with Illumina sequencing and A. D. S. Cameron’s laboratory at the University of Regina (Regina, SK, Canada) for assistance with Nanopore sequencing.
This research was supported in part by the University of Manitoba and Shared Health.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Hooton TM. 2012. Uncomplicated urinary tract infection. N Engl J Med 366:1028–1037. doi: 10.1056/NEJMcp1104429. [DOI] [PubMed] [Google Scholar]
- 2.Denisuik AJ, Lagacé-Wiens PRS, Pitout JD, Mulvey MR, Simner PJ, Tailor F, Karlowsky JA, Hoban DJ, Adam HJ, Zhanel GG, Canadian Antimicrobial Resistance Alliance. 2013. Molecular epidemiology of extended-spectrum β-lactamase-, AmpC β-lactamase- and carbapenemase-producing Escherichia coli and Klebsiella pneumoniae isolated from Canadian hospitals over a 5 year period: CANWARD 2007–11. J Antimicrob Chemother 68:57–65. doi: 10.1093/jac/dkt027. [DOI] [PubMed] [Google Scholar]
- 3.McDanel J, Schweizer M, Crabb V, Nelson R, Samore M, Khader K, Blevins AE, Diekema D, Chiang HY, Nair R, Perencevich E. 2017. Incidence of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella infections in the United States: a systematic literature review. Infect Control Hosp Epidemiol 38:1209–1215. doi: 10.1017/ice.2017.156. [DOI] [PubMed] [Google Scholar]
- 4.Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. 2016. Fosfomycin. Clin Microbiol Rev 29:321–347. doi: 10.1128/CMR.00068-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhanel GG, Walkty AJ, Karlowsky JA. 2016. Fosfomycin: a first-line oral therapy for acute uncomplicated cystitis. Can J Infect Dis Med Microbiol 2016:1–10. doi: 10.1155/2016/2082693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walkty A, Karlowsky JA, Baxter MR, Adam HJ, Alexander D, Bay DC, Boyd D, McCracken M, Mulvey MR, Zhanel GG. 2019. Fosfomycin resistance mediated by fos genes remains rare among extended-spectrum beta-lactamase-producing Escherichia coli clinical isolates recovered from the urine of patients evaluated at Canadian hospitals (CANWARD, 2007–2017). Diagn Microbiol Infect Dis 96:1–3. doi: 10.1016/j.diagmicrobio.2019.114962. [DOI] [PubMed] [Google Scholar]
- 7.Matthews TC, Bristow FR, Griffiths EJ, Petkau A, Adam J, Dooley D, Kruczkiewicz P, Curatcha J, Cabral J, Fornika D, Winsor GL, Courtot M, Bertelli C, Roudgar A, Feijao P, Mabon P, Enns E, Thiessen J, Keddy A, Isaac-Renton J, Gardy JL, Tang P, Carriço JA, Chindelevitch L, Chauve C, Graham MR, McArthur AG, Taboada EN, Beiko RG, Brinkman FS, Hsiao WW, Van Domselaar G, The IRIDA Consortium. 2018. The Integrated Rapid Infectious Disease Analysis (IRIDA) platform. BioRxiv doi: 10.1101/381830. [DOI]
- 8.Wachino JI, Yamane K, Suzuki S, Kimura K, Arakawa Y. 2010. Prevalence of fosfomycin resistance among CTX-M-producing Escherichia coli clinical isolates in Japan and identification of novel plasmid-mediated fosfomycin-modifying enzymes. Antimicrob Agents Chemother 54:3061–3064. doi: 10.1128/AAC.01834-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poirel L, Vuillemin X, Kieffer N, Mueller L, Descombes M-C, Nordmann P. 2019. Identification of FosA8, a plasmid-encoded fosfomycin resistance determinant from Escherichia coli, and Its origin in Leclercia adecarboxylata. Antimicrob Agents Chemother 63:1–7. doi: 10.1128/AAC.01403-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navas J, Leon J, Arroyo M, Garcia Lobo JM. 1990. Nucleotide sequence and intracellular location of the product of the fosfomycin resistance gene from transposon Tn2921. Antimicrob Agents Chemother 34:2016–2018. doi: 10.1128/aac.34.10.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu H, Miao V, Kwong W, Xia R, Davies J. 2011. Identification of a novel fosfomycin resistance gene (fosA2) in Enterobacter cloacae from the Salmon River, Canada. Lett Appl Microbiol 52:427–429. doi: 10.1111/j.1472-765X.2011.03016.x. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez MM, Ghiglione B, Power P, Naas T, Gutkind G. 2018. Proposing Kluyvera georgiana as the origin of the plasmid-mediated resistance gene fosA4. Antimicrob Agents Chemother 62:1–5. doi: 10.1128/AAC.00710-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Y, Xu X, Guo Q, Wang P, Wang W, Wang M. 2015. Characterization of fosA5, a new plasmid-mediated fosfomycin resistance gene in Escherichia coli. Lett Appl Microbiol 60:259–264. doi: 10.1111/lam.12366. [DOI] [PubMed] [Google Scholar]
- 14.Guo Q, Tomich AD, McElheny CL, Cooper VS, Stoesser N, Wang M, Sluis-Cremer N, Doi Y. 2016. Glutathione-S-transferase FosA6 of Klebsiella pneumoniae origin conferring fosfomycin resistance in ESBL-producing Escherichia coli. J Antimicrob Chemother 71:2460–2465. doi: 10.1093/jac/dkw177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rehman MA, Yin X, Persaud-Lachhman MG, Diarra MS. 2017. First detection of a fosfomycin resistance gene, fosA7, in Salmonella enterica serovar Heidelberg isolated from broiler chickens. Antimicrob Agents Chemother 61:1–6. doi: 10.1128/AAC.00410-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ten Doesschate T, Abbott IJ, Willems RJL, Top J, Rogers MRC, Bonten MM, Paganelli FL. 2019. In vivo acquisition of fosfomycin resistance in Escherichia coli by fosA transmission from commensal flora. J Antimicrob Chemother 74:3630–3632. doi: 10.1093/jac/dkz380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathur P, Veeraraghavan B, Devanga Ragupathi NK, Inbanathan FY, Khurana S, Bhardwaj N, Kumar S, Sagar S, Gupta A. 2018. Multiple mutations in lipid-A modification pathway & novel fosA variants in colistin-resistant Klebsiella pneumoniae. Future Sci OA 4:FSO319. doi: 10.4155/fsoa-2018-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y, Lin Q, Zhou Q, Lv L, Wan M, Gao X, Wang C, Liu JH. 2020. Identification of fosA10, a novel plasmid-mediated fosfomycin resistance gene of Klebsiella pneumoniae origin, in Escherichia coli. Infect Drug Resist 13:1273–1279. doi: 10.2147/IDR.S251360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolmogorov M, Yuan J, Lin Y, Pevzner PA. 2019. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol 37:540–546. doi: 10.1038/s41587-019-0072-8. [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y. 2015. The I-TASSER suite: protein structure and function prediction. Nat Methods 12:7–8. doi: 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. 2009. Jalview version 2: a multiple sequence alignment editor and analysis workbench. Bioinformatics 25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strack B, Lessl M, Calendar R, Lanka E. 1992. A common sequence motif, -E-G-Y-A-T-A-, identified within the primase domains of plasmid-encoded I- and P-type DNA primases and the α protein of the Escherichia coli satellite phage P4. J Biol Chem 267:13062–13072. [PubMed] [Google Scholar]
- 23.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:1–11. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.