The use of the kidney function biomarker cystatin C (cysC) can improve the accuracy of vancomycin dosing for target trough attainment in nonobese patients. It is unknown whether cysC can also improve vancomycin target trough attainment in overweight and obese patients. We conducted a retrospective observational study of overweight or obese hospitalized adults with stable renal function administered intravenous vancomycin between January 2011 and July 2019. Linear regression models were used to predict initial steady-state vancomycin troughs using several factors, including various cysC- and serum creatinine (SCr)-based estimates of kidney function.
KEYWORDS: cystatin C, obesity, vancomycin
ABSTRACT
The use of the kidney function biomarker cystatin C (cysC) can improve the accuracy of vancomycin dosing for target trough attainment in nonobese patients. It is unknown whether cysC can also improve vancomycin target trough attainment in overweight and obese patients. We conducted a retrospective observational study of overweight or obese hospitalized adults with stable renal function administered intravenous vancomycin between January 2011 and July 2019. Linear regression models were used to predict initial steady-state vancomycin troughs using several factors, including various cysC- and serum creatinine (SCr)-based estimates of kidney function. We compared the predicted proportion of patients within the target trough range (10 to 20 mg/liter) using the derived models to that observed from usual care. Of the 200 included patients, the mean trough level was 15 ± 6.3 mg/liter. The optimal model to predict the initial trough included both cysC and SCr (R2 = 0.48) rather than either biomarker alone. This model predicted that 79% (95% confidence interval [CI], 73% to 85%) of troughs could be between 10 and 20 mg/liter compared to the 62% observed in clinical practice (P < 0.001), a 1.3-fold increase. This study is the first to examine the role of cysC in predicting vancomycin levels in an exclusively overweight or obese population. While dosing models based on cysC appear promising in this setting, prospective validation is needed.
INTRODUCTION
As the prevalence of adult obesity in the United States continues to rise, last reported at 40% in 2017 (1), the optimization of drug dosing and monitoring in obese patients has become increasingly important. Vancomycin, a hydrophilic renally eliminated glycopeptide antibiotic used for known or suspected Gram-positive infections, is a prototypical example of a medication that poses unique challenges in obesity. Pharmacokinetic differences in obese patients, including a disproportionate volume of distribution (V) relative to total body weight, and increased kidney-mediated drug clearance each contribute to poor vancomycin pharmacokinetic attainment relative to that observed in nonobese patients (2). Even tailored protocols for obese patients have resulted in as few as one-third of patients reaching their goals (3).
While the literature related to this issue has been dominated by studies exploring the vancomycin volume of distribution and optimal patient weight for dose selection (actual, ideal, or adjusted), little attention is paid to the approach to estimating drug clearance (i.e., kidney function assessment) (3, 4). Kidney function assessment is inherently challenging in obese patients due to poor representation in derivation studies of estimated glomerular filtration rate (eGFR) equations (5). Reliance on serum creatinine (SCr), a by-product of skeletal muscle metabolism that traditionally has been used for medication dose adjustment, also contributes to suboptimal kidney function assessment in the obese (6–8). SCr has many nonrenal determinants that are incompletely accounted for in eGFR-estimating equations (9, 10). Specifically, reduced muscle mass often coexists with obesity, rendering SCr less informative for kidney function assessment in this population (11).
Cystatin C (cysC) has emerged as a viable adjunct or alternative to SCr for the prediction of medication clearance. cysC is a low-molecular-weight protein released from all nucleated cells that is freely filtered at the glomerulus and not systemically reabsorbed or actively secreted in the tubules (12). cysC in combination with SCr better predicts measured GFR than either biomarker alone (5).
Models incorporating cysC have been shown to improve the prediction of vancomycin trough levels compared to those using SCr alone (13–17). We previously demonstrated that an eGFRSCr-cysC-based vancomycin dosing nomogram achieved a 2-fold increase in goal trough attainment compared to usual care with the Cockcroft-Gault estimated creatinine clearance (CG eCrCl) in nonobese critically ill patients (16). No studies to our knowledge have attempted to use cysC to predict vancomycin levels in overweight and obese patients. Algorithms using cysC in obese patients may provide an additional opportunity to optimize vancomycin dosing in this population with challenging pharmacokinetics. The overall goal of the study was to develop cysC-inclusive predictive models for vancomycin troughs in obese and overweight patients. Next, the impact of these models was assessed to determine expected vancomycin target trough achievement compared to the observed target trough achievement in routine practice. An exploratory secondary analysis examined the relationship of the observed and expected area under the curve (AUC) estimates.
RESULTS
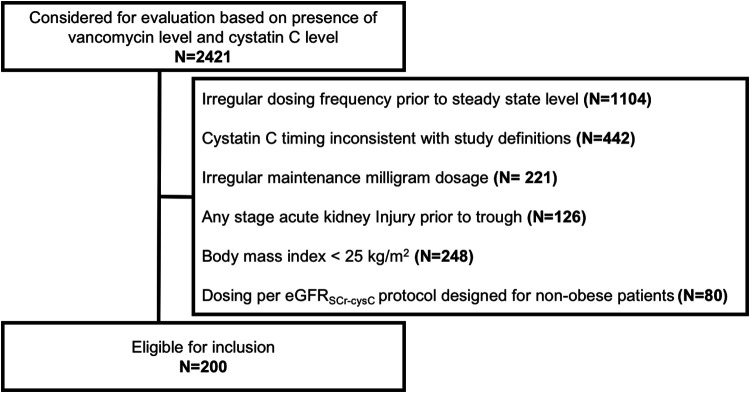
Of the 2,421 subjects electronically screened with available serum vancomycin and cysC concentrations, 200 subjects met the inclusion criteria for the study (Fig. 1). The average body mass index (BMI) ± the standard deviation (SD) was 33 ± 8 kg/m2 (Table 1). The average eGFRcysC and eGFRSCr at vancomycin initiation ± SD were 78 ± 36 ml/min and 102 ± 38 ml/min, respectively, with an intraindividual difference of −24 ml/min (P < 0.001). The mean trough level was 15 ± 6.3 mg/liter, with 62% of subjects with concentrations between 10 and 20 mg/liter. The application of a model derived primarily in nonobese patients (15) to a similarly composed subset of the current cohort resulted in a weak model fit (R2 = 0.38) and poor calibration (see Fig. S1 in the supplemental material).
FIG 1.
Inclusion of subjects (N, number of subjects).
TABLE 1.
Baseline characteristics (n = 200)a
| Parameter | Value |
|---|---|
| Mean age (yrs) ± SD | 59 ± 17 |
| No. (%) of male patients | 119 (60) |
| No. (%) of Caucasian patients | 198 (99) |
| Wt (kg) ± SD | 95 ± 25 |
| Mean body mass index (kg/m2) ± SD | 33 ± 8 |
| No. (%) of patients with body mass index (kg/m2) of: | |
| 25–29.9 | 96 (48) |
| 30–39.9 | 77 (39) |
| 40–49.9 | 18 (9) |
| ≥50 | 9 (4.5) |
| Mean Du Dubois body surface area (m2) ± SD | 2.0 ± 0.3 |
| No. (%) of patients in intensive care unit at initiation | 121 (61) |
| Mean Charlson comorbidity index ± SD | 5.4 ± 3.8 |
| No. (%) of patients on systemic corticosteroids | 34 (17) |
| No. (%) of patients with elevated CRP concentration | 48 (24) |
| No. (%) of patients with low thyroxine concentration | 1 (0.005) |
| No. (%) of patients with elevated thyroxine concentration | 1 (0.005) |
| No. (%) of patients with cancer | 68 (34) |
| Metastatic solid tumor | 30 (15) |
| Renal parameters | |
| Median serum creatinine concentration (mg/dl) (interquartile range) | 0.9 (0.6, 1.1) |
| Mean cystatin C concentration (mg/liter) ± SD | 1.3 ± 0.5 |
| Mean eGFR (ml/min) ± SD | |
| Cockcroft-Gaultb | 108 ± 64 |
| CKD-EPISCr | 102 ± 38 |
| CKD-EPIcysC | 78 ± 36 |
| CKD-EPISCr-cysC | 89 ± 36 |
| Vancomycin | |
| No. (%) of patients with loading dose | 99 (50) |
| Mean maintenance dose ± SD | |
| Non-wt based (mg) | 1,347 ± 304 |
| Wt based (actual) (mg/kg) | 15 ± 2.8 |
| Wt based (adjusted) (mg/kg) | 18 ± 3.4 |
| Wt based (ideal) (mg/kg) | 22 ± 6.8 |
| No. (%) of patients with dosing interval of: | |
| 8 h | 25 (13) |
| 12 h | 139 (70) |
| 24 h | 36 (18) |
| Mean observed trough level (mg/liter) ± SD | 15 ± 6.2 |
| No. (%) of patients with observed trough level (mg/liter) of: | |
| <10 | 40 (20) |
| 10–14.9 | 65 (32.5) |
| 15–19.9 | 59 (29.5) |
| ≥20 | 36 (18) |
| No. (%) of patients with trough level timing | |
| Before dose 4 | 142 (71) |
| Before dose 5 or later | 58 (29) |
Abbreviations: CKD-EPI eGFR, Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate; cysC, cystatin C; SCr, serum creatinine.
Using adjusted body weight.
In the univariate analyses of possible features for a new model, BMI, creatinine, cysC, Cockcroft-Gault eCrCl (using ideal body weight and adjusted body weight), Chronic Kidney Disease Epidemiology Collaborative (CKD-EPI) eGFRSCr, eGFRcysC, and eGFRSCr-cysC, total pretrough dose, and number of doses before the initial steady-state trough level were each significantly associated with vancomycin trough levels (Table 2). A different first dose (i.e., loading dose) was not a significant predictor of the observed trough level in the univariate model beyond its contribution to pretrough total drug exposure.
TABLE 2.
Univariate predictors of vancomycin trough levels in milligrams per litera
| Parameter | Beta value (SE) | P value |
|---|---|---|
| Demographic and anthropometric data | ||
| Age (yr) | 0.01 (0.03) | 0.60 |
| Male | −0.28 (0.90) | 0.76 |
| White race | 5.41 (4.43) | 0.22 |
| Ht (cm) | −0.04 (0.03) | 0.27 |
| Wt (kg) | 0.02 (0.02) | 0.26 |
| BMI (kg/m2) | 0.12 (0.05) | 0.033 |
| Kidney function markers | ||
| Creatinine (mg/dl) | 3.44 (1.13) | 0.003 |
| Cystatin C (mg/dl) | 5.00 (0.81) | <0.001 |
| Equations for estimated GFR (ml/min) | ||
| Cockcroft-Gault (ideal body wt) | −0.01 (0.01) | 0.11 |
| Cockcroft-Gault (adjusted body wt) | −0.01 (0.01) | 0.15 |
| Cockcroft-Gault (total body wt) | −0.01 (0.01) | 0.22 |
| CKD-EPISCr | −0.03 (0.01) | 0.014 |
| CKD-EPIcysC | −0.06 (0.01) | <0.001 |
| CKD-EPISCr-cysC | −0.06 (0.01) | <0.001 |
| Vancomycin parameters | ||
| 1st dose different than maintenance | 0.86 (0.88) | 0.33 |
| No. of doses before level | 1.46 (0.50) | 0.004 |
| Total pretrough dose (g) | 1.11 (0.28) | <0.001 |
| Interval | 0.19 | |
| q8h | Reference | |
| q12h | −1.72 (1.36) | 0.21 |
| q24h | −2.16 (1.63) | 0.19 |
Abbreviations: BMI, body mass index; CKD-EPI eGFR, Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate; cysC, cystatin C; q8h, every 8 h; SCr, serum creatinine; SE, standard error.
Common features of the majority of the tested multivariate models included weight, dosing interval, milligram-per-kilogram dose using adjusted body weight, and number of doses before the trough (Table 3; Table S1). Model 3 (Fig. 2), which contained weight, SCr, cysC, dosing interval, number of doses before the trough, milligram-per-kilogram dose using adjusted body weight, age, and sex, was selected as the optimal model. Elevated C-reactive protein (CRP), abnormal thyroxine, any cancer, metastatic solid tumor, and corticosteroids were assessed for inclusion into model 3, and only abnormal thyroxine (n = 2 total; 1 subtherapeutic and 1 supratherapeutic) was significant (P = 0.047). This feature was ultimately not included in model 3, given the negligible improvement that it would offer (R2 increase of 0.0076 compared to the more parsimonious model).
TABLE 3.
Predictive models for vancomycin trough levels in milligrams per litera
| Model variable | Median beta value (95% CI) | P value | Model fit (R2) | Target trough achievement (%) (95% CI) |
|---|---|---|---|---|
| Model 1 | 0.16 | 68 (62–74) | ||
| Intercept | −5.89 | |||
| Age, per 10 yrs | 0.31 (−0.23, 0.84) | 0.26 | ||
| Male | −1.28 (−3.02, 0.47) | 0.15 | ||
| Wt, per 10 kg | 0.11 (−0.25, 0.47) | 0.55 | ||
| No. of doses prior to level | 1.39 (0.44, 2.33) | 0.005 | ||
| mg/kg dose (AdBW) | 0.50 (0.25, 0.74) | <0.001 | ||
| Every-8-h interval | 2.48 (−0.18, 5.14) | 0.069 | ||
| Every-12-h interval | Reference | |||
| Every-24-h interval | −3.08 (−5.51, −0.65) | 0.014 | ||
| Serum creatinine | 6.08 (3.53, 8.63) | <0.001 | ||
| Model 2 | 0.48 | 77 (71–83) | ||
| Intercept | −15.98 | −15.98 | ||
| Age, per 10 yrs | 0.22 (−0.20, 0.65) | 0.22 | ||
| Male | −0.26 (−1.62, 1.09) | −0.26 | ||
| Wt, per 10 kg | 0.04 (−0.24, 0.31) | 0.04 | ||
| No. of doses prior to level | 1.37 (0.62, 2.12) | 1.37 | ||
| mg/kg dose (AdBW) | 0.70 (0.50, 0.89) | 0.70 | ||
| Every-8-h interval | 4.60 (2.46, 6.74) | 4.60 | ||
| Every-12-h interval | Reference | |||
| Every-24-h interval | −8.39 (−10.54, −6.24) | −8.39 | ||
| Cystatin C | 10.56 (8.86, 12.26) | 10.56 | ||
| Model 3 | 0.48 | 79 (73–85) | ||
| Intercept | −16.13 | |||
| Age, per 10 yrs | 0.22 (−0.21, 0.64) | 0.32 | ||
| Male | −0.44 (−1.83, 0.95) | 0.54 | ||
| Wt, per 10 kg | 0.00 (−0.29, 0.29) | 0.99 | ||
| No. of doses prior to level | 1.36 (0.61, 2.11) | <0.001 | ||
| mg/kg dose (AdBW) | 0.70 (0.50, 0.90) | <0.001 | ||
| Every-8-h interval | 4.69 (2.55, 6.84) | <0.001 | ||
| Every-12-h interval | Reference | |||
| Every-24-h interval | −8.56 (−10.73, −6.39) | <0.001 | ||
| Serum creatinine | 1.19 (−1.02, 3.40) | 0.29 | ||
| Cystatin C | 10.15 (8.29, 12.01) | <0.001 | ||
| Model 4 | 0.09 | 63 (56–70) | ||
| Intercept | 5.89 | |||
| No. of doses prior to level | 1.33 (0.35, 2.31) | 0.008 | ||
| mg/kg dose (AdBW) | 0.36 (0.12, 0.61) | 0.004 | ||
| Every-8-h interval | 2.03 (−0.66, 4.72) | 0.14 | ||
| Every-12-h interval | Reference | |||
| Every-24-h interval | −1.26 (−3.59, 1.06) | 0.29 | ||
| Cockcroft-Gault per 10 ml/min | −0.18 (−0.32, −0.03) | 0.016 | ||
| Model 5 | 0.12 | 64 (57–71) | ||
| Intercept | 9.06 | |||
| No. of doses prior to level | 1.30 (0.34, 2.26) | 0.009 | ||
| mg/kg dose (AdBW) | 0.38 (0.13, 0.62) | 0.003 | ||
| Every-8-h interval | 2.71 (0.05, 5.38) | 0.047 | ||
| Every-12-h interval | Reference | |||
| Every-24-h interval | −2.34 (−4.72, 0.04) | 0.055 | ||
| eGFR with CKD-EPIcreatinine, per 10 ml/min | −0.49 (−0.74, −0.24) | <0.001 | ||
| Model 6 | 0.36 | 73 (67–79) | ||
| Intercept | 11.37 | |||
| No. of doses prior to level | 1.18 (0.36, 2.00) | 0.005 | ||
| mg/kg dose (AdBW) | 0.52 (0.31, 0.73) | <0.001 | ||
| Every-8-h interval | 5.73 (3.35, 8.11) | <0.001 | ||
| Every-12-h interval | Reference | |||
| Every-24-h interval | −5.42 (−7.53, −3.30) | <0.001 | ||
| eGFR with CKD-EPIcystatin C, per 10 ml/min | −1.19 (−1.43, −0.94) | <0.001 | ||
| Model 7 | 0.31 | 72 (66–78) | ||
| Intercept | 12.64 | |||
| No. of doses prior to level | 1.19 (0.34, 2.04) | 0.007 | ||
| mg/kg dose (AdBW) | 0.48 (0.26, 0.70) | <0.001 | ||
| Every-8-h interval | 5.30 (2.84, 7.77) | <0.001 | ||
| Every-12-h interval | Reference | |||
| Every-24-h interval | −5.26 (−7.50, −3.04) | <0.001 | ||
| eGFR with CKD-EPIcreatinine-cystatin C, per 10 ml/min | −1.12 (−1.38, −0.86) | <0.001 | ||
| Model 8 | 0.44 | 80 (74–86) | ||
| Intercept | −0.45 | |||
| Age, per 10 yrs | 0.16 (−0.29, 0.61) | 0.48 | ||
| Male | −1.56 (−3.01, −0.10) | 0.037 | ||
| Wt, per 10 kg | −0.38 (−0.68, −0.08) | 0.015 | ||
| Every-8-h interval | 2.92 (0.61, 5.24) | 0.014 | ||
| Every-12-h interval | Reference | |||
| Every-24-h interval | −7.62 (−9.85, −5.39) | <0.001 | ||
| Cystatin C | 10.18 (8.40, 11.96) | <0.001 | ||
| Steroid positive (yes vs no) | 0.46 (−1.41, 2.32) | 0.63 | ||
| CRP positive (yes vs no) | 0.12 (−1.48, 1.72) | 0.88 | ||
| T4 abnormal | ||||
| Normal | Reference | |||
| Low | −2.74 (−12.50, 7.03) | 0.58 | ||
| High | −9.36 (−18.90, 0.20) | 0.056 | ||
| Metastatic solid tumor (yes vs no) | 2.17 (−0.11, 4.44) | 0.063 | ||
| Cancer (yes vs no) | −1.13 (−2.93, 0.67) | 0.22 | ||
| First dose in ICU (yes vs no) | 0.77 (−0.66, 2.21) | 0.29 | ||
| Difference between baseline and trough SCr levels | 5.61 (0.75, 10.48) | 0.025 | ||
| Vancomycin total dose, per 1,000 g | 1.49 (1.00, 1.99) | <0.001 | ||
| Model 9 | 0.49 | 79 (73–85) | ||
| Intercept | −16.18 | |||
| Age, per 10 yrs | 0.20 (−0.23, 0.62) | 0.37 | ||
| Male | −0.25 (−1.62, 1.12) | 0.72 | ||
| Wt, per 10 kg | 0.06 (−0.22, 0.34) | 0.69 | ||
| No. of doses prior to level | 1.40 (0.65, 2.16) | <0.001 | ||
| mg/kg dose (AdBW) | 0.67 (0.47, 0.87) | <0.001 | ||
| Every-8-h interval | 4.17 (1.91, 6.44) | <0.001 | ||
| Every-12-h interval | Reference | |||
| Every-24-h interval | −8.36 (−10.51, −6.21) | <0.001 | ||
| Cystatin C | 10.93 (9.19, 12.67) | <0.001 | ||
| Steroid positive (yes vs no) | 0.03 (−1.76, 1.82) | 0.97 | ||
| CRP positive (yes vs no) | −0.08 (−1.61, 1.45) | 0.92 | ||
| T4 abnormal | ||||
| Normal | Reference | |||
| Low | −2.88 (−12.22, 6.46) | 0.55 | ||
| High | −7.85 (−16.98, 1.28) | 0.093 | ||
| Metastatic solid tumor (yes vs no) | 2.24 (0.07, 4.41) | 0.044 | ||
| Cancer (yes vs no) | −0.72 (−2.44, 1.00) | 0.42 | ||
| First dose in ICU (yes vs no) | 0.47 (−0.91, 1.85) | 0.50 | ||
| Difference between baseline and trough SCr levels | 4.56 (−0.08, 9.19) | 0.056 | ||
Abbreviations: AdBW, adjusted body weight; CKD-EPI eGFR, Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate; CRP, C-reactive protein; ICU, intensive care unit; SCr, serum creatinine.
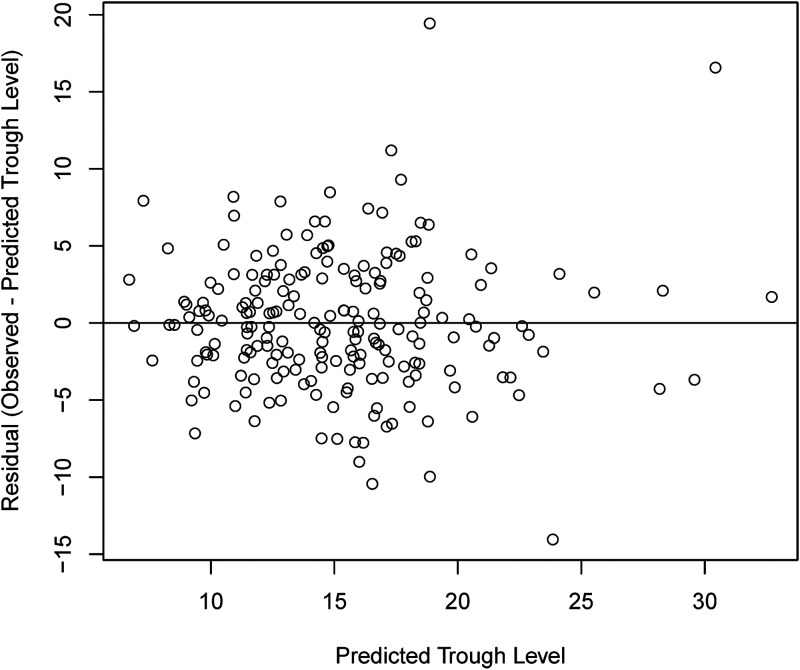
FIG 2.
Residuals of model 3 in relation to the predicted trough level.
Compared to usual care, the model with the best performance (model 3 using cysC and SCr concentrations [R2 = 0.48]) yielded a 1.3-fold increase in the percentage of subjects expected to achieve a vancomycin trough of between 10 and 20 mg/liter (79% [95% confidence interval {CI}, 73% to 85%] in the model versus 62% observed in usual care [P < 0.001]) (Fig. 3 and Table 3). The model fit was stronger in patients with a BMI of ≥40 kg/m2 than in those with a BMI of <40 kg/m2 and in patients with an eGFRSCr-cysC of <60 ml/min than in those with an eGFRSCr-cysC of ≥60 ml/min (Table 4). Cross-validation of model 3 achieved a mean R2 value of 0.51 (range, 0.47 to 0.53).
FIG 3.
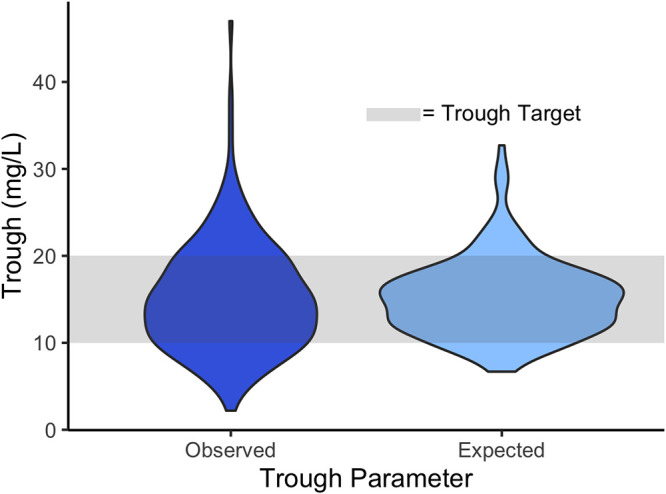
Distribution of observed versus expected troughs using model 3 (n = 200). Compared to usual care, model 3 is expected to achieve an improvement in the percentage of subjects achieving a vancomycin trough of between 10 and 20 mg/liter (79% in the model versus 62% observed in usual care; P < 0.001).
TABLE 4.
Model 3 performance overall and across subgroupsa
| Parameter | R2 | Observed % at 10–20 mg/liter | Expected % if model 3 used 10–20 mg/liter | Pb |
|---|---|---|---|---|
| Overall | 0.48 | 62 | 79 | <0.001 |
| BMI thresholds (kg/m2) | ||||
| ≥40 (n = 27) | 0.54 | 59 | 85 | 0.62 |
| <40 (n = 173) | 0.45 | 62 | 77 | <0.001 |
| ≥32 (n = 76) | 0.68 | 63 | 80 | 0.99 |
| <32 (n = 124) | 0.41 | 61 | 77 | <0.001 |
| eGFRSCr-cysC thresholds (ml/min) | ||||
| ≥60 (n = 158) | 0.4 | 63 | 76 | <0.001 |
| <60 (n = 42) | 0.58 | 60 | 88 | 0.38 |
Abbreviations: BMI, body mass index; CKD-EPI eGFR, Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate; cysC, cystatin C; SCr, serum creatinine.
P values represent the association between the 10- to 20-mg/liter ranges for observed and expected trough levels.
In our exploratory secondary analysis, the mean eAUCobserved (defined in Table 5) ± SD ranged from 575 ± 166 to 805 ± 198 mg · h/liter (using V values of 0.7 mg/liter and 0.3 mg/liter total body weight, respectively). Using the expected trough from the model instead of the observed troughs, the mean eAUCexpected ± SD had a range of 576 ± 122 to 808 ± 151 mg · h/liter (Fig. S2). Compared to 11 to 49% of patients with an eAUCobserved of between 400 and 600 mg · h/liter, 7.5 to 56% of subjects would be expected to fall in the eAUCobserved target using the model.
TABLE 5.
Study definitionsa
| Term(s) | Definition |
|---|---|
| Steady-state vancomycin trough | Vancomycin trough collected no earlier than prior to the 4th dose |
| Trough target range | Vancomycin serum concn of 10–20 mg/liter |
| Observed trough | Steady-state vancomycin trough observed |
| Predicted trough | Steady-state vancomycin trough predicted from the best model |
| Residual | Observed trough − predicted trough |
| Expected trough | 15 (median of goal range) + residual |
| eAUCobserved, eAUCexpected |
Estimated (1-level) 24-h area under the curve using the equation where the inputs include
|
Troughs are in milligrams per liter, and areas under the curve are in milligrams per hour per liter. eAUC, estimated area under the curve.
DISCUSSION
In a cohort of 200 overweight and obese patients, 62% of patients were within the initial vancomycin trough target range of 10 to 20 mg/liter. A model derived in primarily nonobese patients to predict vancomycin trough levels from eGFRSCr-cysC (15) demonstrated poor performance in the present study of overweight and obese patients. To determine whether cysC in conjunction with other clinically available data could be used to predict initial vancomycin trough levels in overweight or obese patient populations, we developed new models using the values for SCr and cysC or as part of the Cockcroft-Gault eCrCl and CKD-EPI eGFR equations. The optimal model used absolute concentrations of SCr and cysC and was expected to achieve target trough concentrations in 79% of cases compared to the 62% observed in clinical practice (P < 0.001). Model performance did not deteriorate at high BMI ranges (≥40 kg/m2) or low eGFRSCr-cysC ranges (<60 ml/min). From the model, a practical set of dosing guidelines tailored to overweight and obese patients could be developed. The present study addresses the unmet need to improve empiric vancomycin dosing precision in obese patients, a subpopulation associated with low rates of target attainment (3) and a correspondingly high risk of elevated trough levels and acute kidney injury (AKI) (4, 18, 19).
While cysC has been used to predict vancomycin levels in nonobese patients, overweight and obese patients warrant their own study due to unique pharmacokinetic differences in obesity (2) along with the known elevation of cysC in obesity independent of the GFR (20). In the present study, models using the eGFR (milliliters per minute) performed poorly relative to models using the absolute concentrations of cysC and SCr. Interestingly, model 2, which mirrored model 3 with the exception that it contained only cysC, had the same R2, 0.48, as model 3. While model 3 was selected due to the assumption that SCr would nearly always be available in practice, the good performance of the cysC concentration as the lone kidney function surrogate in model 2 demonstrates the usefulness of cysC in this setting.
These findings are in contrast to a study of 173 nonobese patients that found that models with eGFRSCr-cysC led to a superior prediction of vancomycin trough levels compared to models using absolute biomarker concentrations (15). The poor performance of GFR-estimating equations for the prediction of measured GFR in obese patients may help explain the suboptimal performance of those models seen for the prediction of vancomycin trough concentrations in the present study (21). We applied the generally accepted principle of reexpressing CKD-EPI eGFR values as milliliters per minute for drug dosing (20). This approach may lead to an overestimation of the eGFR due to disproportionate changes in the glomerular filtration rate with weight increases. In obese patients, a 2-fold increase in body weight results in only a 1.6-fold-higher mean GFR (21). The use of actual weight in GFR estimation equations or the use of a body surface area (BSA) correction could therefore overestimate kidney function. Our finding that eGFR with or without cysC poorly predicts vancomycin levels is consistent with known inaccuracies of eGFR at extremes of weight and reinforces the need to design dosing models specific for the overweight and obese population. The prospective validation of our developed model is warranted.
To our knowledge, no studies analyzing the role of cystatin C to predict vancomycin levels have analyzed common nonrenal factors of cysC, including malignancy, corticosteroids, abnormal thyroxine levels, or elevated CRP levels, as covariates in the model. Given that over two-thirds of hospitalized patients are known to have at least one nonrenal determinant of cysC (22), it is crucial that these factors are considered. We included information about corticosteroids, CRP, thyroxine, and malignancy in our models, noting that the nonrenal determinant of obesity was inherently included given the study cohort. The model with the highest R2 value (model 9) included these factors, but they provided only a limited additional increase in the predictive performance relative to more parsimonious models and thus were excluded in our final determination. A study analyzing the use of cysC for aminoglycoside dosing had similar findings of the negligible impact of these factors (23). There remain many unanswered questions about the effects of nonrenal determinants of cysC in hospitalized patients, and additional studies are needed (22). While not the primary objective of this study, our results provide some reassurance that these factors are unlikely to lead to clinically significant changes in observed drug levels or target attainment.
Our study has various limitations that warrant discussion. Since this was a retrospective study, the timing of cysC and SCr values was not uniform relative to initiation and trough-level timing. We attempted to ensure that SCr and cysC collected at any time during the pretrough period were reflective of stable kidney function by excluding patients with any stage of AKI before the first trough. We also ensured that baseline laboratory measures were collected before the vancomycin trough but no earlier than 36 h before the start of therapy. Most patients had their first trough before the 4th dose, but some patients had their troughs collected at a later point. To account for this variability between patients, the number of doses before the level was included as a covariate within the model.
For feasibility reasons, this study was not restricted to microbiologically confirmed resistant Gram-positive infections (∼10% of treated vancomycin patients [15, 16]). Thus, no conclusions can be drawn about the relationship between the trough level and efficacy, but it is presumed that improved rates of target attainment would offer clinical benefits. While pharmacokinetic target attainment is most critical for efficacy in patients with invasive Gram-positive infections, future application of the model developed in this study could improve safety in all obese patients empirically started on scheduled vancomycin given the known nephrotoxicity risks with supratherapeutic trough levels (4). We considered any trough of between 10 and 20 mg/liter within the target, as this approach has been taken by other studies in obese subjects that have resulted in similar vancomycin target attainment rates (3, 24). Notably, an obesity-tailored algorithm developed from our model could accommodate the traditionally high serum levels targeted in invasive infections (i.e., meningitis, endocarditis, and osteomyelitis) compared to less severe infections (i.e., cellulitis) (25). This approach was taken in a previous algorithm using cysC in nonobese critically ill patients by requiring the clinician to choose the trough goal of either 10 to 15 mg/liter or 15 to 20 mg/liter (16). Since new guideline recommendations discourage target troughs of >15 mg/liter due to the increased risk of nephrotoxicity (4), an algorithm developed from our model could target troughs of 15 mg/liter for invasive infections and slightly lower troughs for less serious infections.
We also acknowledge that the recently released vancomycin guidelines favor AUC-based monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections (4). However, these recommendations do not specifically include evidence in support of this approach for obese patients (4). Notably, troughs are typically necessary to calculate the AUC (26). Since we demonstrated that the addition of cysC to a model improves the accuracy and precision of trough predictions, it is possible that this, by extension, could improve the accuracy and precision of AUC calculations. The recently released vancomycin dosing and monitoring guidelines recommend a two-level method to calculate the AUC, but this was not feasible with the available data (4). Our exploratory secondary analysis to estimate the eAUC using a one-level trough approach provides preliminary data for future study (27). We acknowledge the limitations of using a single serum vancomycin level to estimate the AUC, especially owing to the variability in the vancomycin volume of distribution observed in the obese population, which is a critical parameter in the one-level AUC approach (3). Two plausible volumes of distribution were used (0.3 liters/kg and 0.7 liters/kg total body weight) to approximate a range of clinically relevant eAUC values (3, 4). Future studies could also consider varying the volume of distribution across the level of BMI. This analysis should be viewed as hypothesis generating and expanded upon by using more accurate methods to calculate the AUC, including a two-level equation-based approach or a one- to two-level approach using a Bayesian method validated in obese patients.
Conclusion.
Obese patients have historically experienced low rates of vancomycin pharmacokinetic target attainment (3). cysC is a novel kidney biomarker that has been shown to improve vancomycin target attainment in populations of primarily nonobese patients (13). In an overweight and obese population, we found that the optimal model included cysC and SCr absolute biomarker concentrations rather than the estimated GFR based on a standard equation. This study is the first to our knowledge to examine the role of cysC in predicting vancomycin levels in an exclusively overweight or obese population. While cysC appears promising in this setting, prospective validation is warranted.
MATERIALS AND METHODS
Settings and participants.
This was an observational study of overweight and obese adults (≥18 years of age) who received intravenous vancomycin during hospitalization between 1 January 2011 and 13 July 2019, at Mayo Clinic—Rochester, a 2,059-bed academic medical center. The Mayo Clinic Institutional Review Board approved the study protocol, and the requirement for informed consent was waived. Rapid-turnaround (<3-h) cysC and SCr tests with eGFR reporting were available to be ordered by the care team without restriction throughout the study duration for kidney function assessment (22). cysC is used frequently across inpatient practice settings within our institution, with no specific protocols around use outside the above-mentioned eGFRSCr-cysC-based vancomycin nomogram (22). Included individuals were those with a BMI of ≥25 kg/m2, an available steady-state vancomycin trough concentration, and both SCr and cysC available before the first vancomycin trough and no earlier than 36 h before the first dose. Individuals with an inconsistent vancomycin dose (outside the first dose to allow for loading) or dosing interval were excluded. Also excluded were those with unstable kidney function defined as any stage of AKI during vancomycin therapy but before the drug level based on Kidney Disease Improving Global Outcomes (KDIGO) creatinine criteria (28). Individuals on renal replacement therapy or those who did not authorize the use of their medical records for research were excluded. Patients from the previous derivation and evaluation studies of the eGFRSCr-cysC-based dosing nomogram were excluded (15, 16).
Throughout the study period, vancomycin dosing and monitoring were predominantly managed by pharmacists who were available on patient care units from 0700 to 2230. AUC-based vancomycin dosing was not used during the study period. Rather, doses were adjusted based on trough levels, collected just prior to the 4th dose. Guideline-recommended weight-based dose ranges were followed (25); however, adjusted body weight (0.4 correction factor) was used for dosing instead of total body weight if the BMI was ≥40 kg/m2. The standard institutional protocol used eCrCl to estimate kidney function for dosing interval determination.
Definitions.
Vancomycin parameters are defined in Table 5. The baseline SCr and cysC values for the study were determined by finding the value closest to that at vancomycin initiation that occurred no earlier than 36 h before vancomycin initiation and preceded the initial steady-state trough. An adequately timed steady-state trough level (Table 5) was defined as a trough level collected with a <25% deviation from the prescribed dosing interval and before the 4th or later dose of a regimen with a constant dosage and a constant interval. As an example, if the dosing interval was 12 h, any trough collected between 9 h and 12 h after the preceding dose would be a <25% deviation and considered acceptable. Any trough between 10 and 20 mg/liter was considered within the target trough range.
Data collection.
All data were electronically abstracted from the Mayo Clinic Unified Data Platform (29), with 10% of data manually validated by a member of the study team (H. R. Teaford). Patient demographics (i.e., age, sex, race, height, and weight), level of care (intensive care unit [ICU] versus general care), and comorbidity burden assessment using the Charlson comorbidity index were recorded. Other collected data included vancomycin dosing information (i.e., dose, interval, and duration), laboratory values (i.e., serum vancomycin levels, SCr, and cysC), and information about potential nonrenal confounders of cysC concentrations (i.e., steroid administrations, thyroxine levels, malignancy, and CRP values). The study goal was to make a parsimonious model of factors commonly used when selecting vancomycin doses or readily available nonrenal factors known to directly impact cysC concentrations.
Vancomycin levels were analyzed using the Syva Emit 2000 vancomycin assay (Siemens Healthcare Diagnostics, Inc., Newark, DE, USA). All observed levels in the cohort were detectable. Creatinine measurement was performed by using the standardized, isotope dilution mass spectrometry (IDMS)-traceable, Roche enzymatic creatinine assay (Roche, Basel, Switzerland). cysC was measured using a particle-enhanced turbidimetric assay (Gentian AS, Moss, Norway). This assay is traceable to the same internationally certified cysC reference material (ERM-DA471/IFCC) used to develop the cysC-based CKD-EPI equations (5).
Validation of the prior nonobese model in this obese cohort.
To determine if a separate model for vancomycin troughs is needed in obese versus nonobese patients, our predictive model previously developed in nonobese patients (15) was assessed for its performance in the current cohort. Model features for this analysis included total vancomycin dose (grams), interval, and eGFRSCr-cysC (milliliters per minute) (see Table S2 in the supplemental material). To conform to the design of the nonobese study (15), only patients in the obesity data set who had their trough collected following exactly three doses were included in this validation analysis (n = 142).
Data analysis for new models tailored to overweight/obese populations.
To develop clinically useful tools for drug dosing, linear regression models were fit for the outcome of the initial vancomycin trough concentration using only predictors available to clinicians at drug initiation. Features were selected a priori based upon previous studies (15, 16) and from an assessment of factors that can directly impact cysC levels (corticosteroids, malignancy, elevated CRP, and abnormal thyroxine). Kidney function was estimated in predictive models in several ways. Four previously validated estimation equations were tested, including the CG eCrCl (30) (computed with the actual, ideal, or adjusted [0.4 correction factor] body weights) and the three CKD-EPI eGFR equations (eGFRSCr, eGFRcysC, or eGFRSCr-cysC) (5). The CKD-EPI equations were reexpressed in milliliters per minute by multiplying the result by the body surface area (BSA) derived from the Du Bois formula divided by 1.73 m2 (5, 31). Models including eCrCl or eGFR did not include age and sex since these parameters are already included within the estimating equations. The values for SCr and cysC concentrations were also tested as predictors of the vancomycin trough, separate from the calculated eGFRs from these biomarkers. The other core variables tested for inclusion in models to predict trough concentrations were the number of doses prior to a level (i.e., 3 would indicate a trough prior to the 4th dose), the milligrams-per-kilogram maintenance dose using adjusted body weight, weight, the use of a loading dose, and the dosing interval (15, 16). Also, the presence of an elevated CRP level, abnormal thyroxine levels, or systemic (oral or intravenous) steroid administration in the 3 days prior to the cysC level was evaluated, as these factors have been described as non-GFR determinants of cysC in acutely ill hospitalized patients (22). The impact of the addition of these nonrenal determinants was assessed using the likelihood ratio test. Cross-validation was performed on the optimal model (see Appendix S1 in the supplemental material).
To evaluate the performance of models against a clinically useful reference standard, we sought to determine how well the models would predict vancomycin troughs within a target of 10 to 20 mg/liter compared to usual clinical practice. Model residuals were added to the median of the goal trough range (15 mg/liter) to determine the expected trough achievement with the new model, which was compared to the observed initial trough achievement in the cohort (Table 5). The performance of the optimal model was further tested at various BMI thresholds and eGFR cutoffs.
Given emerging recommendations that favor AUC-guided vancomycin monitoring rather than steady-state trough evaluation, we undertook an exploratory secondary analysis to relate observed and expected troughs to observed and expected estimated AUCs (eAUCs), respectively (4). Methods for calculating the vancomycin AUC traditionally require the use of a Bayesian pharmacokinetic model after attainment of one or two serum levels or the application of an equation-based methodology after obtaining two levels. During the study interval, trough-based monitoring was the standard of care, and thus, it was atypical to have multiple, appropriately timed vancomycin levels per patient to retrospectively calculate the AUC. Therefore, for this study, the AUC was estimated using the one-sample trough-only equation (27). Two population estimates of the volume of distribution (V) (0.3 liters/kg total body weight and 0.7 liters/kg total body weight) were used in the equation to provide an eAUC range due to the known variability of this parameter in obesity (2–4). Observed troughs were used to calculate the eAUCobserved, and the expected troughs from the optimal model were used to calculate the eAUCexpected (Table 5). The proportions of obese patients within the target range of 400 to 600 mg · h/liter were numerically compared between the eAUCobserved and the eAUCexpected.
All analyses were performed with JMP version 9 statistical software (SAS Institute, Inc., Cary, NC, USA) or R version 3.6.0 (2019; R Core Team, R Foundation for Statistical Computing, Vienna, Austria).
Data availability.
Data are available upon request to the corresponding author. The ability to publish data to a public data repository is restricted due to the confidential nature of human subject data.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number K23AI143882 (principal investigator, E.F.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
E.F.B. consults for FAST Biomedical.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Hales CM, Carroll MD, Fryar CD, Ogden CL. 2017. Prevalence of obesity among adults and youth: United States, 2015–2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief 288:1–8. [PubMed] [Google Scholar]
- 2.Grace E. 2012. Altered vancomycin pharmacokinetics in obese and morbidly obese patients: what we have learned over the past 30 years. J Antimicrob Chemother 67:1305–1310. doi: 10.1093/jac/dks066. [DOI] [PubMed] [Google Scholar]
- 3.Durand C, Bylo M, Howard B, Belliveau P. 2018. Vancomycin dosing in obese patients: special considerations and novel dosing strategies. Ann Pharmacother 52:580–590. doi: 10.1177/1060028017750084. [DOI] [PubMed] [Google Scholar]
- 4.Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, Mueller BA, Pai MP, Wong-Beringer A, Rotschafer JC, Rodvold KA, Maples HD, Lomaestro BM. 2020. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 77:835–864. doi: 10.1093/ajhp/zxaa036. [DOI] [PubMed] [Google Scholar]
- 5.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS. 2012. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein SL. 2015. Automated/integrated real-time clinical decision support in acute kidney injury. Curr Opin Crit Care 21:485–489. doi: 10.1097/MCC.0000000000000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tawadrous D, Shariff SZ, Haynes RB, Iansavichus AV, Jain AK, Garg AX. 2011. Use of clinical decision support systems for kidney-related drug prescribing: a systematic review. Am J Kidney Dis 58:903–914. doi: 10.1053/j.ajkd.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Galanter WL, Moja J, Lambert BL. 2010. Using computerized provider order entry and clinical decision support to improve prescribing in patients with decreased GFR. Am J Kidney Dis 56:809–812. doi: 10.1053/j.ajkd.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Kashani K, Rosner MH, Ostermann M. 2020. Creatinine: from physiology to clinical application. Eur J Intern Med 72:9–14. doi: 10.1016/j.ejim.2019.10.025. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Inker LA. 2017. Assessment of glomerular filtration rate in health and disease: a state of the art review. Clin Pharmacol Ther 102:405–419. doi: 10.1002/cpt.729. [DOI] [PubMed] [Google Scholar]
- 11.Kalinkovich A, Livshits G. 2017. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev 35:200–221. doi: 10.1016/j.arr.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Barreto EF, Rule AD, Voils SA, Kane-Gill SL. 2018. Innovative use of novel biomarkers to improve the safety of renally eliminated and nephrotoxic medications. Pharmacotherapy 38:794–803. doi: 10.1002/phar.2149. [DOI] [PubMed] [Google Scholar]
- 13.Barreto EF, Rule AD, Murad MH, Kashani KB, Lieske JC, Erwin PJ, Steckelberg JM, Gajic O, Reid JM, Kane-Gill SL. 2019. Prediction of the renal elimination of drugs with cystatin C vs creatinine: a systematic review. Mayo Clin Proc 94:500–514. doi: 10.1016/j.mayocp.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Chung J-Y, Jin S-J, Yoon J-H, Song Y-G. 2013. Serum cystatin C is a major predictor of vancomycin clearance in a population pharmacokinetic analysis of patients with normal serum creatinine concentrations. J Korean Med Sci 28:48–54. doi: 10.3346/jkms.2013.28.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frazee EN, Rule AD, Herrmann SM, Kashani KB, Leung N, Virk A, Voskoboev N, Lieske JC. 2014. Serum cystatin C predicts vancomycin trough levels better than serum creatinine in hospitalized patients: a cohort study. Crit Care 18:R110. doi: 10.1186/cc13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frazee E, Rule AD, Lieske JC, Kashani KB, Barreto JN, Virk A, Kuper PJ, Dierkhising RA, Leung N. 2017. Cystatin C-guided vancomycin dosing in critically ill patients: a quality improvement project. Am J Kidney Dis 69:658–666. doi: 10.1053/j.ajkd.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 17.DeCarolis DD, Thorson JG, Marraffa RA, Clairmont MA, Kuskowski MA. 2014. Comparison of equations with estimate renal function to predict serum vancomycin concentration in patients with spinal cord injury—does the use of cystatin C improve accuracy? Ther Drug Monit 36:632–639. doi: 10.1097/FTD.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 18.Choi YC, Saw S, Soliman D, Bingham AL, Pontiggia L, Hunter K, Chuang L, Siemianowski LA, Ereshefsky B, Hollands JM. 2017. Intravenous vancomycin associated with the development of nephrotoxicity in patients with class III obesity. Ann Pharmacother 51:937–944. doi: 10.1177/1060028017720946. [DOI] [PubMed] [Google Scholar]
- 19.Zonozi R, Wu A, Shin JI, Secora A, Coresh J, Inker LA, Chang AR, Grams ME. 2019. Elevated vancomycin trough levels in a tertiary health system: frequency, risk factors, and prognosis. Mayo Clin Proc 94:17–26. doi: 10.1016/j.mayocp.2018.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teaford HR, Barreto JN, Vollmer KJ, Rule AD, Barreto EF. 2020. Cystatin C: a primer for pharmacists. Pharmacy 8:35. doi: 10.3390/pharmacy8010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pai MP. 2010. Estimating the glomerular filtration rate in obese adult patients for drug dosing. Adv Chronic Kidney Dis 17:e53–e62. doi: 10.1053/j.ackd.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Teaford HR, Rule AD, Mara KC, Kashani KB, Lieske JC, Schreier DJ, Wieruszewski PM, Barreto EF. 2020. Patterns of cystatin C uptake and use across and within hospitals. Mayo Clin Proc 95:1649–1659. doi: 10.1016/j.mayocp.2020.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chin PKL, Chew-Harris JSC, Florkowski CM, Begg EJ. 2015. The performance of contemporary cystatin C-based GFR equations in predicting gentamicin clearance. Br J Clin Pharmacol 79:268–277. doi: 10.1111/bcp.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds DC, Waite LH, Alexander DP, DeRyke CA. 2012. Performance of a vancomycin dosage regimen developed for obese patients. Am J Health Syst Pharm 69:944–950. doi: 10.2146/ajhp110324. [DOI] [PubMed] [Google Scholar]
- 25.Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Craig W, Billeter M, Dalovisio JR, Levine DP, Reilly C, Rotschafer JC, Lomaestro B, Craig W, Dalovisio JR, Moellering R, Rybak M, Billeter M. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 26.Pai MP, Neely M, Rodvold KA, Lodise TP. 2014. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev 77:50–57. doi: 10.1016/j.addr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Lewis P. 2018. Vancomycin area under the curve simplified. Ther Drug Monit 40:377–380. doi: 10.1097/FTD.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 28.Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, Herzog CA, Joannidis M, Kribben A, Levey AS, MacLeod AM, Mehta RL, Murray PT, Naicker S, Opal SM, Schaefer F, Schetz M, Uchino S. 2012. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138. [Google Scholar]
- 29.Chute CG, Beck SA, Fisk TB, Mohr DN. 2010. The Enterprise Data Trust at Mayo Clinic: a semantically integrated warehouse of biomedical data. J Am Med Inform Assoc 17:131–135. doi: 10.1136/jamia.2009.002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 31.Du Bois D, Du Bois EF. 1916. Clinical calorimetry: tenth paper a formula to estimate the approximate surface area if height and weight be known. Arch Intern Med (Chic) XVII:863–871. doi: 10.1001/archinte.1916.00080130010002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request to the corresponding author. The ability to publish data to a public data repository is restricted due to the confidential nature of human subject data.