This study evaluated the in vitro activity of cefepime-zidebactam in comparison with that of ceftazidime-avibactam and other comparators against clinically significant Gram-negative bacillus isolates. A total of 3,400 nonduplicate Gram-negative clinical isolates were collected from 45 medical centers across China in the CHINET Program in 2018, including Enterobacterales (n = 2,228), Pseudomonas aeruginosa (n = 657), and Acinetobacter baumannii (n = 515).
KEYWORDS: Enterobacterales, Pseudomonas aeruginosa, Acinetobacter baumannii, cefepime-zidebactam, ceftazidime-avibactam, blaKPC, blaNDM, blaOXA-48
ABSTRACT
This study evaluated the in vitro activity of cefepime-zidebactam in comparison with that of ceftazidime-avibactam and other comparators against clinically significant Gram-negative bacillus isolates. A total of 3,400 nonduplicate Gram-negative clinical isolates were collected from 45 medical centers across China in the CHINET Program in 2018, including Enterobacterales (n = 2,228), Pseudomonas aeruginosa (n = 657), and Acinetobacter baumannii (n = 515). The activities of cefepime-zidebactam and 20 comparators were determined by broth microdilution as recommended by the Clinical and Laboratory Standards Institute. Cefepime-zidebactam demonstrated potent activity against almost all Enterobacterales (MIC50/90, 0.125/1 mg/liter) and good activity against P. aeruginosa (MIC50/90, 2/8 mg/liter). Among the 373 carbapenem-resistant Enterobacteriaceae isolates, 57.3% (213/373) and 15.3% (57/373) were positive for blaKPC-2 and blaNDM, respectively. Cefepime-zidebactam showed a MIC of ≤2 mg/liter for 92.0% (196/213) of blaKPC-2 producers and 79.7% (47/59) of blaNDM producers. Ceftazidime-avibactam showed good in vitro activity against Enterobacterales (MIC50/90, 0.25/2 mg/liter; 94.0% susceptible) and P. aeruginosa (MIC50/90, 4/16 mg/liter; 86.9% susceptible). Ceftazidime-avibactam was active against 9.1% of carbapenem-resistant Escherichia coli isolates (63.6% were blaNDM producers) and 84.6% of Klebsiella pneumoniae isolates (74.3% were blaKPC producers). Most (90.1%) blaKPC-2 producers were susceptible to ceftazidime-avibactam. Cefepime-zidebactam demonstrated limited activity (MIC50/90, 16/32 mg/liter) against the 515 A. baumannii isolates (79.2% were carbapenem resistant), and ceftazidime-avibactam was less active (MIC50/90, 64/>64 mg/liter). Cefepime-zidebactam was highly active against clinical isolates of Enterobacterales and P. aeruginosa, including blaKPC-2-positive Enterobacterales and blaNDM-positive Enterobacterales and carbapenem-resistant P. aeruginosa. And ceftazidime-avibactam was highly active against blaKPC-2-positive Enterobacterales and carbapenem-resistant P. aeruginosa.
INTRODUCTION
In the past decades, both the CDC and WHO have emphasized that carbapenem-resistant Gram-negative pathogens, including Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter baumannii, were the major health care threats worldwide (1–3). Therapeutic options for such pathogens are limited because of their multidrug-resistant nature, which may result in death of the infected patients. This situation makes the development of novel and active antimicrobial agents against these pathogens a primary priority (4).
Studies on one class of β-lactamase inhibitors, the diazabicyclooctanes, have identified novel compounds termed β-lactam enhancers, for their potent PBP2 affinity in important Gram-negative pathogens. Ceftazidime-avibactam was approved by the U.S. Food and Drug Administration (FDA) for the treatment of complicated urinary tract infections and complicated intra-abdominal infections in February 2015 (5). Avibactam can inhibit the activity of AmpC cephalosporinases, extended-spectrum β-lactamases (ESBLs), KPC carbapenemases, and some Ambler class D β-lactamases (OXA-48), and it is able to restore or enhance the bactericidal activity of ceftazidime against β-lactamase-producing organisms. Zidebactam, another new β-lactamase inhibitor under clinical evaluation, also demonstrates potent activity against a wide spectrum of Gram-negative pathogens possessing ESBLs, AmpC-type β-lactamases, KPC β-lactamases, and metallo-β-lactamases (MBLs) by combining with cefepime. Unlike avibactam, without inherent activity by itself, zidebactam has antibacterial activity against Enterobacterales and P. aeruginosa, including carbapenemase-producing isolates (6).
There have been several reports on the in vitro activity of ceftazidime-avibactam against Enterobacterales and P. aeruginosa in China (7, 8), but no study has been conducted to evaluate cefepime-zidebactam against isolates from Chinese patients. Our continuing surveillance study aims to assess the in vitro activity of cefepime-zidebactam and ceftazidime-avibactam against clinical strains which were recently isolated in 2018 through the China Antimicrobial Surveillance Network (CHINET). These collective susceptibility data on different carbapenemase-producing organisms might improve rational use of these novel β-lactam combinations in clinical practice.
RESULTS
In vitro activity of cefepime-zidebactam, ceftazidime-avibactam, and comparator agents.
Cefepime-zidebactam exhibited potent antibacterial activity against almost all Enterobacterales (MIC50, 0.125 mg/liter). Overall, 86.1% of the carbapenem-resistant Enterobacteriaceae (CRE) strains and 89.8% of carbapenem-resistant Klebsiella pneumoniae strains were inhibited at 2 mg/liter. Ceftazidime-avibactam also demonstrated good antibacterial activity against Enterobacterales clinical isolates, evidenced by inhibiting 94.0% of Enterobacterales at 8 mg/liter, specifically, inhibiting 95.5% of Escherichia coli strains, 94.7% of K. pneumoniae strains, and 85.0% of Enterobacter cloacae strains. And 69.7% of CRE strains were susceptible to ceftazidime-avibactam. The majority (90.9%) of carbapenem-resistant E. coli isolates were resistant to ceftazidime-avibactam, but cefepime-zidebactam showed a 2-mg/liter or lower MIC against 81.8% of carbapenem-resistant E. coli isolates. The distribution of cefepime-zidebactam MICs against CRE is shown in terms of genotype in Fig. S1 in the supplemental material. Cefepime-zidebactam and ceftazidime-avibactam were highly active against E. coli strains (>95% susceptible), similar to polymyxin B and tigecycline. Cefepime-tazobactam, ceftolozane-tazobactam, and piperacillin-tazobactam also displayed potent activity against E. coli, and about 90% of the test strains were susceptible. More than 60% of E. coli isolates were resistant to ceftriaxone, ciprofloxacin, and levofloxacin, and 33.2% were resistant to cefepime, but only 4.6% (33/719) were resistant to carbapenems. More than 80% of the carbapenem-resistant K. pneumoniae strains were susceptible to cefepime-zidebactam, ceftazidime-avibactam, polymyxin B, and tigecycline. The majority of carbapenem-resistant Klebsiella aerogenes and Serratia marcescens isolates were susceptible to ceftazidime-avibactam, but few carbapenem-resistant E. cloacae and Citrobacter freundii isolates were susceptible to ceftazidime-avibactam. Tables 1 and 2 and Fig. 1 provide the MIC frequency distribution of cefepime-zidebactam and ceftazidime-avibactam to Enterobacterales clinical isolates, including carbapenemase-positive and -negative isolates.
TABLE 1.
In vitro activities of cefepime-zidebactam and comparators tested against 719 isolates of E. coli and 33 isolates of carbapenem-resistant E. coli collected in China, 2018a
| Antimicrobial agent |
E. coli (n = 719) |
Carbapenem-resistant E. coli (n = 33) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/liter) |
R, % | S, % | MIC (mg/liter) |
R, % | S, % | |||||
| Range | 50% | 90% | Range | 50% | 90% | |||||
| Cefepime-zidebactam | ≤0.03 to 32 | 0.06 | 0.125 | 0.4 | 99 | 0.06 to 32 | 0.125 | 8 | 9.1 | 81.8 |
| Ceftazidime-avibactam | ≤0.03 to >64 | 0.125 | 0.5 | 4.5 | 95.5 | 0.25 to >64 | >64 | >64 | 90.9 | 9.1 |
| Ceftolozane-tazobactam | ≤0.06 to >128 | 0.25 | 4 | 9 | 89.6 | 4 to >128 | >128 | >128 | 97 | 0 |
| Cefepime-tazobactam | ≤0.03 to >64 | 0.06 | 0.5 | 4.3 | 94.7 | 8 to >64 | >64 | >64 | 90.9 | 0 |
| Piperacillin-tazobactam | ≤2 to >256 | ≤2 | 32 | 10.6 | 86.4 | 128 to >256 | >256 | >256 | 100 | 0 |
| Cefoperazone-sulbactam | ≤1 to >128 | 8 | 64 | 10.4 | 75.2 | 32 to >128 | >128 | >128 | 97 | 0 |
| Cefazolin | 1 to >32 | >32 | >32 | 71.9 | 15.4 | >32 to >32 | >32 | >32 | 100 | 0 |
| Cefuroxime | ≤0.5 to >32 | >32 | >32 | 62.4 | 33.7 | >32 to >32 | >32 | >32 | 100 | 0 |
| Ceftriaxone | 0.5 to >32 | >32 | >32 | 61.3 | 38.2 | >32 to >32 | >32 | >32 | 100 | 0 |
| Ceftazidime | ≤0.25 to >32 | 2 | >32 | 26.8 | 63.3 | 8 to >32 | >32 | >32 | 97 | 0 |
| Cefepime | ≤0.06 to >128 | 4 | 128 | 33.2 | 45.1 | 16 to >128 | >128 | >128 | 100 | 0 |
| Moxalactam | ≤0.06 to >128 | 0.5 | 16 | 63.1 | 36.5 | 8 to >128 | >128 | >128 | 100 | 0 |
| Aztreonam | ≤1 to >128 | 8 | 64 | 41.4 | 47.6 | ≤1 to >128 | 64 | >128 | 75.8 | 21.2 |
| Imipenem | ≤0.06 to >128 | 0.125 | 0.5 | 4.3 | 94.9 | 2 to >128 | 16 | 64 | 93.9 | 0 |
| Meropenem | ≤0.03 to >64 | ≤0.03 | ≤0.03 | 4.2 | 95.3 | 2 to >64 | 16 | 64 | 90.9 | 0 |
| Amikacin | ≤1 to >128 | 2 | 8 | 5.1 | 88.3 | ≤1 to >128 | 4 | >128 | 30.3 | 57.6 |
| Ciprofloxacin | ≤0.06 to >8 | >8 | >8 | 64.3 | 26.4 | 0.25 to >8 | >8 | >8 | 93.9 | 6.1 |
| Levofloxacin | ≤0.125 to >16 | 8 | 32 | 60.2 | 33.5 | 0.5 to >16 | 16 | 32 | 90.9 | 6.1 |
| Trimethoprim-sulfamethoxazole | ≤0.25 to >32 | >32 | >32 | 64.8 | 35.2 | ≤0.25 to >32 | >32 | >32 | 84.8 | 15.2 |
| Polymyxin B | ≤0.125 to >16 | 0.5 | 1 | 0.8 | 98.5 | 0.25 to >16 | 0.5 | 16 | 12.1 | 87.9 |
| Tigecycline | ≤0.06 to 4 | 0.125 | 0.25 | 0 | 99.7 | 0.125 to 4 | 0.25 | 1 | 0 | 97 |
R, resistant; S, susceptible.
TABLE 2.
In vitro activities of cefepime-zidebactam and comparators tested against 788 isolates of K. pneumoniae and 272 isolates of carbapenem-resistant K. pneumoniae collected in China, 2018
| Antimicrobial agent |
K. pneumoniae (n = 788) |
Carbapenem-resistant K. pneumoniae (n = 272) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/liter) |
R, % | S, % | MIC (mg/liter) |
R, % | S, % | |||||
| Range | 50% | 90% | Range | 50% | 90% | |||||
| Cefepime-zidebactam | ≤0.03 to 32 | 0.125 | 2 | 0.5 | 96.3 | 0.06 to 32 | 1 | 4 | 1.5 | 89.7 |
| Ceftazidime-avibactam | ≤0.03 to >64 | 0.25 | 4 | 5.3 | 94.7 | 0.125 to >64 | 2 | 64 | 15.4 | 84.6 |
| Ceftolozane-tazobactam | ≤0.06 to >128 | 1 | 128 | 39.2 | 58.1 | 0.5 to >128 | 64 | >128 | 97.1 | 1.5 |
| Cefepime-tazobactam | ≤0.03 to >64 | 0.125 | >64 | 32.5 | 65 | 0.06 to >64 | 64 | >64 | 91.5 | 3.3 |
| Piperacillin-tazobactam | ≤2 to >256 | 16 | >256 | 44.4 | 49.7 | ≤2 to >256 | >256 | >256 | 96.3 | 2.9 |
| Cefoperazone-sulbactam | ≤1 to >128 | 16 | >128 | 40.7 | 51 | ≤1 to >128 | >128 | >128 | 94.5 | 3.7 |
| Cefazolin | 1 to >32 | >32 | >32 | 62.8 | 37.2 | 2 to >32 | >32 | >32 | 97.8 | 2.2 |
| Cefuroxime | ≤0.5 to >32 | >32 | >32 | 61.2 | 34.9 | 2 to >32 | >32 | >32 | 98.2 | 1.5 |
| Ceftriaxone | 0.5 to >32 | >32 | >32 | 60.3 | 38.8 | 0.5 to >32 | >32 | >32 | 97.1 | 2.2 |
| Ceftazidime | ≤0.25 to >32 | 8 | >32 | 48.5 | 47.3 | ≤0.25 to >32 | >32 | >32 | 96.3 | 2.2 |
| Cefepime | ≤0.06 to >128 | 8 | >128 | 46.3 | 45.1 | ≤0.06 to >128 | 128 | >128 | 96.7 | 1.5 |
| Aztreonam | ≤1 to >128 | 16 | >128 | 52.4 | 44.8 | ≤1 to >128 | >128 | >128 | 95.6 | 4 |
| Imipenem | ≤0.06 to >128 | 0.5 | 64 | 33.6 | 63.6 | 0.25 to >128 | 32 | 128 | 97.4 | 0.7 |
| Meropenem | ≤0.03 to >64 | ≤0.03 | >64 | 33.1 | 65.7 | ≤0.03 to >64 | 64 | >64 | 96 | 2.6 |
| Amikacin | ≤1 to >128 | ≤1 | >128 | 26.9 | 72.6 | ≤1 to >128 | >128 | >128 | 67.6 | 30.9 |
| Ciprofloxacin | ≤0.06 to >8 | 2 | >8 | 57.4 | 34.1 | ≤0.06 to >8 | >8 | >8 | 95.6 | 4 |
| Levofloxacin | ≤0.125 to >16 | 1 | 32 | 49.9 | 39.8 | ≤0.125 to >16 | >16 | >16 | 92.3 | 5.1 |
| Trimethoprim-sulfamethoxazole | ≤0.25 to >32 | 16 | >32 | 52.4 | 47.6 | ≤0.25 to >32 | >32 | >32 | 72.1 | 27.9 |
| Polymyxin B | ≤0.125 to >16 | 0.5 | 1 | 3 | 96.3 | ≤0.125 to >16 | 0.5 | 1 | 5.9 | 93.8 |
| Tigecycline | ≤0.06 to 16 | 0.5 | 2 | 0.5 | 95.7 | 0.125 to 8 | 1 | 2 | 0.4 | 93.4 |
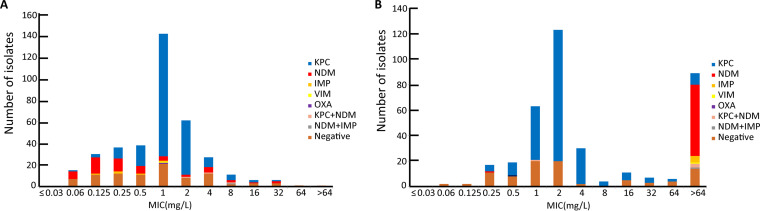
FIG 1.
Distribution of cefepime-zidebactam (A) and ceftazidime-avibactam (B) MIC against carbapenem-resistant Enterobacteriaceae species in terms of carbapenemase genotype.
P. aeruginosa isolates were inhibited by cefepime-zidebactam (MIC50/90, 2/8 mg/liter) at 2 mg/liter (35.6%) and 4 mg/liter (29.7%). The proportions of carbapenem-resistant P. aeruginosa strains inhibited at 4 and 8 mg/liter were 20.5% and 34.5%, respectively. Ceftazidime-avibactam also demonstrated good antibacterial activity against most P. aeruginosa strains, 8 mg/liter of ceftazidime-avibactam could inhibit 88.5% of P. aeruginosa strains, and 64.3% of carbapenem-resistant P. aeruginosa isolates were susceptible to ceftazidime-avibactam. The MIC50/90 values of cefepime-zidebactam and ceftazidime-avibactam against A. baumannii isolates were 16/32 mg/liter and 64/>64 mg/liter, respectively. About 26.0% (171/657) of P. aeruginosa isolates were resistant to carbapenems. P. aeruginosa isolates were mostly susceptible to cefepime-zidebactam (97.4%), ceftazidime-avibactam (86.9%), ceftolozane-tazobactam (89.5%), and polymyxin B (85.4%) (Table 3). As for carbapenem-resistant P. aeruginosa, 34.5% and 32.2% of isolates were susceptible to ceftazidime and cefepime and 64.3% and 93.0% were susceptible to ceftazidime-avibactam and cefepime-zidebactam, respectively. Overall, 79.2% (408/515) of A. baumannii isolates were resistant to carbapenems. Polymyxin B and tigecycline were the only agents showing relatively low MICs and susceptibility higher than 90% (Table 4).
TABLE 3.
In vitro activities of cefepime-zidebactam and comparators tested against 657 isolates of P. aeruginosa and 171 isolates of carbapenem-resistant P. aeruginosa collected in China, 2018
| Antimicrobial agent |
P. aeruginosa (n = 657) |
Carbapenem-resistant P. aeruginosa (n = 171) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/liter) |
R, % | S, % | MIC (mg/liter) |
R, % | S, % | |||||
| Range | 50% | 90% | Range | 50% | 90% | |||||
| Cefepime-zidebactam | 0.06 to 64 | 2 | 8 | 1.1 | 97.4 | 1 to 64 | 4 | 8 | 2.3 | 93 |
| Ceftazidime-avibactam | 0.125 to >64 | 4 | 16 | 13.1 | 86.9 | 1 to >64 | 8 | 64 | 35.7 | 64.3 |
| Ceftolozane-tazobactam | 0.25 to >128 | 1 | 8 | 7.5 | 89.5 | 0.5 to >128 | 2 | 128 | 23.4 | 69.6 |
| Cefepime-tazobactam | ≤0.03 to >64 | 4 | 32 | 14.8 | 72.3 | 2 to >64 | 16 | >64 | 36.3 | 38.6 |
| Piperacillin-tazobactam | ≤2 to >256 | 16 | 256 | 42.9 | 41.1 | ≤2 to >256 | 64 | >256 | 78.4 | 9.4 |
| Cefoperazone-sulbactam | ≤1 to >128 | 16 | 128 | 23.9 | 56.8 | ≤1 to >128 | 64 | >128 | 50.9 | 18.1 |
| Cefazolin | 32 to >32 | >32 | >32 | 100 | 0 | >32 to >32 | >32 | >32 | 100 | 0 |
| Cefuroxime | 16 to >32 | >32 | >32 | 99.8 | 0 | >32 to >32 | >32 | >32 | 100 | 0 |
| Ceftriaxone | 1 to >32 | >32 | >32 | 82 | 1.5 | 16 to >32 | >32 | >32 | 95.3 | 0 |
| Ceftazidime | ≤0.25 to >32 | 8 | >32 | 26 | 63 | 2 to >32 | 32 | >32 | 50.9 | 34.5 |
| Cefepime | 0.125 to >128 | 8 | 32 | 19.6 | 67.6 | 4 to >128 | 16 | >128 | 45 | 32.2 |
| Aztreonam | ≤1 to >128 | 8 | 64 | 33.6 | 51.3 | ≤1 to >128 | 32 | >128 | 73.1 | 16.4 |
| Meropenem | ≤0.03 to >64 | 2 | 16 | 26 | 60.1 | 8 to >64 | 16 | 64 | 100 | 0 |
| Amikacin | ≤1 to >128 | 4 | 16 | 10.8 | 64.1 | ≤1 to >128 | 8 | 64 | 23.4 | 45 |
| Ciprofloxacin | ≤0.06 to >8 | 0.25 | 8 | 22.4 | 67 | ≤0.06 to >8 | 1 | >8 | 46.8 | 36.3 |
| Levofloxacin | ≤0.125 to >16 | 1 | 16 | 27.2 | 62.3 | 0.25 to >16 | 4 | 32 | 56.7 | 27.5 |
| Trimethoprim-sulfamethoxazole | ≤0.25 to >32 | 16 | >32 | 96.7 | 3.3 | ≤0.25 to >32 | 16 | >32 | 96.5 | 3.5 |
| Polymyxin B | 0.25 to >16 | 2 | 4 | 3.8 | 85.4 | 0.25 to >16 | 2 | 4 | 5.3 | 86 |
| Tigecycline | ≤0.06 to >32 | 8 | 16 | NAa | NA | 0.125 to >32 | 16 | 32 | NA | NA |
NA, not available; R, resistant; S, susceptible.
TABLE 4.
In vitro activities of cefepime-zidebactam and comparators tested against 515 isolates of A. baumannii and 408 isolates of carbapenem-resistant A. baumannii collected in China, 2018a
| Antimicrobial agent |
A. baumannii (n = 515) |
Carbapenem-resistant A. baumannii (n = 408) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/liter) |
R, % | S, % | MIC (mg/liter) |
R, % | S, % | |||||
| Range | 50% | 90% | Range | 50% | 90% | |||||
| Cefepime-zidebactam | ≤0.03 to >64 | 16 | 32 | 36.5 | 26 | 2 to >64 | 16 | 32 | 46.1 | 8.8 |
| Ceftazidime-avibactam | 0.125 to >64 | 64 | >64 | 84.9 | 15.1 | 4 to >64 | 64 | >64 | 98.8 | 1.2 |
| Ceftolozane-tazobactam | ≤0.06 to >128 | 32 | 128 | 80.6 | 17.9 | 0.5 to >128 | 64 | 128 | 99 | 0.7 |
| Cefepime-tazobactam | ≤0.03 to >64 | 64 | >64 | 78.1 | 20 | 2 to >64 | >64 | >64 | 97.8 | 1 |
| Piperacillin-tazobactam | ≤2 to >256 | >256 | >256 | 81.4 | 16.3 | 8 to >256 | >256 | >256 | 98.5 | 1 |
| Cefoperazone-sulbactam | ≤1 to >128 | 128 | >128 | 79.8 | 18.3 | 4 to >128 | 128 | >128 | 96.8 | 1.5 |
| Cefazolin | 4 to >32 | >32 | >32 | 99.6 | 0 | >32 to >32 | >32 | >32 | 100 | 0 |
| Cefuroxime | 4 to >32 | >32 | >32 | 95.1 | 1.2 | 8 to >32 | >32 | >32 | 99.8 | 0.2 |
| Ceftriaxone | 0.5 to >32 | >32 | >32 | 82.3 | 8.5 | 8 to >32 | >32 | >32 | 98.8 | 0.2 |
| Ceftazidime | ≤0.25 to >32 | >32 | >32 | 81.9 | 17.3 | 2 to >32 | >32 | >32 | 98.3 | 1.2 |
| Cefepime | ≤0.06 to >128 | 128 | >128 | 79.8 | 18.4 | 2 to >128 | 128 | >128 | 98.3 | 0.7 |
| Aztreonam | ≤1 to >128 | 64 | 128 | 86.8 | 4.7 | 8 to >128 | 64 | 128 | 97.5 | 0.2 |
| Imipenem | 0.125 to >128 | 64 | 128 | 78.8 | 20 | 4 to >128 | 64 | 128 | 99.5 | 0 |
| Meropenem | ≤0.03 to >64 | 64 | >64 | 78.8 | 19.8 | 2 to >64 | 64 | >64 | 99.5 | 0.2 |
| Amikacin | ≤1 to >128 | >128 | >128 | 76.1 | 23.5 | ≤1 to >128 | >128 | >128 | 92.2 | 7.6 |
| Ciprofloxacin | ≤0.06 to >8 | >8 | >8 | 82.5 | 17.5 | 0.125 to >8 | >8 | >8 | 98.3 | 1.7 |
| Levofloxacin | ≤0.125 to >16 | 8 | 32 | 78.3 | 17.9 | ≤0.125 to >16 | 8 | 32 | 94.6 | 1.5 |
| Trimethoprim-sulfamethoxazole | ≤0.25 to >32 | >32 | >32 | 69.3 | 30.7 | ≤0.25 to >32 | >32 | >32 | 82.4 | 17.6 |
| Polymyxin B | 0.25 to >16 | 1 | 2 | 2.5 | 97.5 | 0.25 to >16 | 1 | 2 | 3.2 | 96.8 |
| Tigecycline | ≤0.06 to 16 | 1 | 2 | NA | NA | 0.125 to 16 | 1 | 2 | NA | NA |
NA, not available; R, resistant; S, susceptible.
Detection of carbapenemase genes.
In this study, 16.7% (373/2228) of the Enterobacterales strains were carbapenem resistant, including K. pneumoniae (72.9% [272/373]), E. coli (8.8% [33/373]), and E. cloacae (5.9% [22/373]). Most (74.3% [277/373]) CRE strains had a single carbapenemase, 1.3% (5/373) were positive for dual carbapenemases, and 23.9% (89/373) were negative for all five common carbapenemase genes. More than half (57.1% [213/373]) of the CRE strains were blaKPC-2 positive, while 6.7% (25/373) and 8.1% (30/372) were blaNDM-1 and blaNDM-5 positive, respectively. Four isolates were positive for both blaKPC-2 and blaNDM. For other metallo-β-lactamases, five isolates were positive for blaIMP-4, one isolate was positive for blaVIM-1, and another isolate was positive for both blaNDM-1 and blaIMP-1. Additionally, blaOXA-232 was detected in only one isolate, and blaKPC-2 was mostly detected in K. pneumoniae (94.8% [202/213]). The prevalences of blaNDM were 36.8% (21/57) in E. coli isolates, 22.8% (13/57) in K. pneumoniae isolates, and 22.8% (13/57) in E. cloacae isolates. The results of antimicrobial susceptibility testing indicated that 92% of blaKPC-positive and 82.5% of blaNDM-positive Enterobacterales were susceptible to cefepime-zidebactam. However, 90.1% of blaKPC-positive Enterobacterales were susceptible, while 98.2% of blaNDM-positive Enterobacterales were resistant, to ceftazidime-avibactam.
DISCUSSION
The spread of drug-resistant Gram-negative bacteria, especially CRE strains, P. aeruginosa, and A. baumannii, has substantially increased morbidity and mortality rates worldwide. There is an urgent need to develop new antimicrobial agents for clinical use (4, 9). Results from the CHINET Antimicrobial Surveillance Network showed that more than 25% of the K. pneumoniae strains isolated from 44 hospitals across China were resistant to imipenem and meropenem (10), nearly a 10-fold increase since 2005 (http://www.chinets.com/Data/GermYear). In China, KPC-2, NDM, and OXA-48-like carbapenemases were predominant among clinical CRE isolates. The most prevalent carbapenemase gene was blaKPC-2 among the carbapenem-resistant K. pneumoniae isolates from adult patients, whereas blaNDM, blaKPC-2, and blaOXA-48 were the predominant carbapenemase genes among the carbapenem-resistant K. pneumoniae isolates from pediatric patients. The predominant carbapenemase gene was blaNDM in the carbapenem-resistant E. coli isolates from both adults and children (11).
Currently, tigecycline, polymyxins (including polymyxin B and colistin), and ceftazidime-avibactam are available for the treatment of infections caused by carbapenem-resistant Gram-negative bacilli. In this study, more than 87.9% of the carbapenem-resistant E. coli, carbapenem-resistant K. pneumoniae, and carbapenem-resistant A. baumannii strains tested were susceptible to tigecycline and polymyxin B. Additionally, 86% of carbapenem-resistant P. aeruginosa strains were susceptible to polymyxin B. Ceftazidime-avibactam has been approved by the Center for Drug Evaluation in China for the treatment of infections caused by multidrug-resistant or extensively drug-resistant Gram-negative bacilli, including blaKPC- or blaOXA-48-positive strains. The prevalence of CRE strains was 13.9% in the present study, which was similar to that in the CHINET 2017 study (7) and higher than another national surveillance study of isolates collected in 2012 to 2014 (8). The blaKPC-positive isolates still showed a low (<10%) rate of resistance to ceftazidime-avibactam, but the majority (98.2%) of the isolates possessing blaNDM were resistant to ceftazidime-avibactam. As reported previously (12, 13), a novel L169P mutation in KPC-2 and D179Y/T243M mutation in KPC-3 confer reduced susceptibility to ceftazidime-avibactam.
Unlike avibactam, without inherent antimicrobial activity and with no effect on metallo-β-lactamase, zidebactam has antibacterial activity against Enterobacterales and P. aeruginosa, including carbapenemase-producing isolates (6). Previous studies have demonstrated that the new antibacterial combination cefepime-zidebactam is active against multidrug-resistant Gram-negative pathogens, especially metallo-β-lactamase-producing Enterobacterales, P. aeruginosa, and OXA-carbapenemase-positive A. baumannii (6, 14, 15). According to a study by Khan et al. (16), cefepime-zidebactam had a MIC of ≤2 mg/liter against more than 99% of E. coli, K. pneumoniae, and Enterobacter strains. In the present study, 99% of the E. coli and 96.3% of K. pneumoniae isolates were inhibited by cefepime-zidebactam at the same breakpoint (≤2 mg/liter). Furthermore, 92% of blaKPC-positive and 82.5% of blaNDM-positive Enterobacterales were susceptible to cefepime-zidebactam. Additionally, cefepime-zidebactam could inhibit 97.4% of P. aeruginosa and 93.0% of carbapenem-resistant P. aeruginosa isolates (MIC ≤ 8 mg/liter), consistent with the study by Khan et al. (16), in which 98% of P. aeruginosa isolates and 78% of carbapenem-resistant isolates were inhibited by cefepime-zidebactam at ≤8 mg/liter. Only 26% of the A. baumannii isolates and 8.8% of the carbapenem-resistant isolates were inhibited by cefepime-zidebactam at ≤8 mg/liter. Cefepime-zidebactam did not show good in vitro activity against A. baumannii, though in the neutropenic mouse thigh and lung infection models (17), considerable activity was still demonstrable for cefepime-zidebactam (MIC range of 16 to 64 mg/liter) against carbapenem-resistant A. baumannii. Our study showed MICs comparable to those in several other studies (14, 15, 18), and the clinical role of cefepime-zidebactam would be determined on breakpoints based on further clinical investigations.
In conclusion, this study demonstrated that both cefepime-zidebactam and ceftazidime-avibactam show excellent in vitro antibacterial activity against recent clinical isolates of Enterobacterales and P. aeruginosa. Ceftazidime-avibactam exhibited activity against blaKPC-2-producing strains, and cefepime-zidebactam showed lower MICs against both blaKPC- and blaMBL-producing strains. Diazabicyclooctane β-lactamase inhibitors provide a new therapeutic alternative for the infections caused by carbapenem-resistant Enterobacterales.
MATERIALS AND METHODS
Clinical strains.
A total of 3,400 nonduplicate sequential isolates of Gram-negative bacilli were collected from 45 medical centers in 28 provinces or cities across China in 2018, including Klebsiella pneumoniae (n = 788), Escherichia coli (n = 719), P. aeruginosa (n = 657), A. baumannii (n = 515), Enterobacter cloacae (n = 140), Proteus mirabilis (n = 134), Serratia marcescens (n = 110), Klebsiella aerogenes (n = 106), Morganella morganii (n = 92), Citrobacter freundii (n = 85), and Proteus vulgaris (n = 54). Among the 3,400 clinical strains, 41.8% were isolated from the respiratory tract, 20.6% from the urinary tract, and 11.2% and 6.9% from blood and wounds, respectively. Species identification was performed at each participating site and confirmed by the central laboratory using the Vitek-2 compact system (bioMérieux, France) or matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (Vitek MS; bioMérieux).
Antimicrobial susceptibility testing.
MICs were determined by the reference broth microdilution method recommended by the Clinical and Laboratory Standards Institute (CLSI) (19). Cefepime-zidebactam, ceftazidime-avibactam, ceftolozane-tazobactam, cefepime-tazobactam, cefoperazone-sulbactam, piperacillin-tazobactam, cefepime, ceftazidime, ceftriaxone, cefuroxime, cefazolin, aztreonam, imipenem, meropenem, amikacin, ciprofloxacin, levofloxacin, trimethoprim-sulfamethoxazole, polymyxin B, and tigecycline were tested using a dried customized commercially prepared microdilution panel (Sensititre; Thermo Fisher Scientific). E. coli ATCC 25922 and ATCC 35218, K. pneumoniae ATCC 700603, and P. aeruginosa ATCC 27853 were used as the quality control strains in antimicrobial susceptibility testing. Quality control and interpretation of the results were based on 2019 CLSI breakpoints (20) for all the antimicrobial agents with the exception of cefepime-zidebactam and tigecycline, for which CLSI criteria are not available. Tigecycline MICs were interpreted using U.S. FDA MIC breakpoints for Enterobacterales (21). Cefepime-zidebactam MICs were interpreted using CLSI breakpoints for cefepime for comparison purposes only.
CRE definition and carbapenemase detection.
As defined by the Centers for Disease Control and Prevention (CDC), the Enterobacterales isolates that test resistant to at least one of the carbapenems (ertapenem, meropenem, doripenem, or imipenem) or produce a carbapenemase are called carbapenem-resistant Enterobacteriaceae (CRE) (https://www.cdc.gov/hai/organisms/cre/technical-info.html#Definition). Some Enterobacterales (e.g., Proteus spp., Morganella spp., and Providencia spp.) have intrinsic elevated MICs to imipenem. In such cases, the MIC results of meropenem were used to determine if these organisms meet the CRE definition. The presence of the five most common carbapenemase genes (blaKPC, blaNDM, blaIMP, blaVIM, and blaOXA-48) were confirmed for all the CRE strains by PCR with specific primers and DNA sequencing, as described previously (7).
Study approval.
The study protocol was approved by the Institutional Review Board of Huashan Hospital, Fudan University (no. 2018-408).
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the contribution of the members of CHINET for collection of the isolates in this study, including the following: Yingchun Xu and Xiaojiang Zhang from Peking Union Medical College Hospital; Zhaoxia Zhang and Ping Ji from the First Affiliated Hospital of Xinjiang Medical University; Mei Kang and Chao He from West China Hospital, Sichuan University; Chuanqing Wang and Leiyan He from Children’s Hospital of Fudan University; Yuanhong Xu and Ying Huang from the First Affiliated Hospital of Anhui Medical University; Zhongju Chen and Ziyong Sun from Tongji Hospital, Tongji Medical College, Huazhong University of Science & Technology; Yuxing Ni and Jingyong Sun from Ruijin Hospital, Shanghai Jiaotong University School of Medicine; Yunzhuo Chu and Sufei Tian from the First Affiliated Hospital of China Medical University; Zhidong Hu and Jin Li from Tianjin Medical University General Hospital; Yunsong Yu and Jie Lin from Sir Run Shaw Hospital, Zhejiang University School of Medicine; Bin Shan and Yan Du from the First Affiliated Hospital of Kunming Medical University; Sufang Guo and Yanyan Wang from the First Affiliated Hospital of Inner Mongolia Medical University; Lianhua Wei and Xin Wang from Gansu Provincial Hospital; Hong Zhang and Chun Wang from Children’s Hospital of Shanghai; Yunjian Hu and Xiaoman Ai from Beijing Hospital; Chao Zhuo and Danhong Su from the First Affiliated Hospital of Guangzhou Medical University; Ruizhong Wang and Hua Fang from Pudong New Area People’s Hospital; Bixia Yu from Zhejiang Ningbo Zhenhai Longsai Hospital; Ping Gong and Miao Song from the People’s Hospital of Zigui, Hubei Province; Dawen Guo and Jinying Zhao from the First Affiliated Hospital of Harbin Medical University; Wen’en Liu and Yanming Li from Xiangya Hospital, Central South University; Yan Jin and Yueling Wang from Shandong Provincial Hospital; Kaizhen Weng and Yirong Zhang from Jinjiang Municipal Hospital; Xuesong Xu and Chao Yan from China-Japan Union Hospital, Jilin University; Xiangning Huang and Hua Yu from Sichuan Provincial People’s Hospital; Yi Li and Shanmei Wang from Henan Provincial People’s Hospital; Lixia Zhang and Juan Ma from Shaanxi Provincial People’s Hospital; Shuping Zhou and Jiangwei Ke from Jiangxi Provincial Children’s Hospital; Lei Zhu and Jinhua Meng from Children’s Hospital of Shanxi; Wenqi Song and Fang Dong from Beijing Children’s Hospital, Capital Medical University; Han Shen and Wanqing Zhou from Nanjing Drum Tower Hospital, Affiliated Hospital of Nanjing; Gang Li and Wei Jia from General Hospital of Ningxia Medical University; Jinsong Wu and Yuemei Lu from Shenzhen People’s Hospital; Jihong Li from the Second Hospital of Hebei Medical University; Jiangshan Liu from Jinchang Hospital of Integrated Traditional Chinese and Western Medicine; Longfeng Liao from the People’s Hospital of Ganxian; Hongqin Gu from Guangrao County People’s Hospital; Lin Jiang from the People’s Hospital of Huixian, Henan Province; Wen He from Central Hospital of Yingkou Development Zone, Liaoning Province; Shunhong Xue from Huzhu County People’s Hospital, Qinghai Province; Jiao Feng from the People’s Hospital of Linshui, Sichuan Province; Rui Dou from Lixin County People’s Hospital; and Chunlei Yue from Jiutai People’s Hospital.
This work was supported by National Mega-project for Innovative Drugs (2019ZX09721001-006-004), the National Natural Science Foundation of China (81871690, 81902100, and 81902101), the Science and Technology Innovation Action Plan of Shanghai Science and Technology Committee (17DZ1910403), Shanghai Antimicrobial Surveillance Network (3030231003), and the China Antimicrobial Surveillance Network (WI207259).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We declare that we have no conflict of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2019. AR threats report. https://www.cdc.gov/drugresistance/biggest-threats.html#carp.
- 2.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, WHO Pathogens Priority List Working Group. 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 3.Hornsey M, Phee L, Stubbings W, Wareham DW. 2013. In vitro activity of the novel monosulfactam BAL30072 alone and in combination with meropenem versus a diverse collection of important Gram-negative pathogens. Int J Antimicrob Agents 42:343–346. doi: 10.1016/j.ijantimicag.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Jean S-S, Gould IM, Lee W-S, Hsueh P-R, International Society of Antimicrobial Chemotherapy. 2019. New drugs for multidrug-resistant Gram-negative organisms: time for stewardship. Drugs 79:705–714. doi: 10.1007/s40265-019-01112-1. [DOI] [PubMed] [Google Scholar]
- 5.Actavis. 2015. AVYCAZ (ceftazidime-avibactam) for injection, for intravenous use. Initial U.S. approval: 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206494s000lbl.pdf.
- 6.Sader HS, Rhomberg PR, Flamm RK, Jones RN, Castanheira M. 2017. WCK 5222 (cefepime/zidebactam) antimicrobial activity tested against Gram-negative organisms producing clinically relevant beta-lactamases. J Antimicrob Chemother 72:1696–1703. doi: 10.1093/jac/dkx050. [DOI] [PubMed] [Google Scholar]
- 7.Yin D, Wu S, Yang Y, Shi Q, Dong D, Zhu D, Hu F, China Antimicrobial Surveillance Network Study Group. 2019. Results from the China Antimicrobial Surveillance Network (CHINET) in 2017 of the in vitro activities of ceftazidime-avibactam and ceftolozane-tazobactam against clinical isolates of Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 63:e02431-18. doi: 10.1128/AAC.02431-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou M, Chen J, Liu Y, Hu Y, Liu Y, Lu J, Zhang S, Yu Y, Huang X, Yang Q, Liao K, Jin Y, Huang W, Feng X, Yang Q, Xu Y. 2018. In vitro activities of ceftaroline/avibactam, ceftazidime/avibactam, and other comparators against pathogens from various complicated infections in China. Clin Infect Dis 67:S206–S216. doi: 10.1093/cid/ciy659. [DOI] [PubMed] [Google Scholar]
- 9.Norrby SR, Nord CE, Finch R, European Society of Clinical Microbiology and Infectious Diseases. 2005. Lack of development of new antimicrobial drugs: a potential serious threat to public health. Lancet Infect Dis 5:115–119. doi: 10.1016/S1473-3099(05)70086-4. [DOI] [PubMed] [Google Scholar]
- 10.Hu F, Guo Y, Yang Y, Zheng Y, Wu S, Jiang X, Zhu D, Wang F, China Antimicrobial Surveillance Network Study G, China Antimicrobial Surveillance Network (CHINET) Study Group. 2019. Resistance reported from China antimicrobial surveillance network (CHINET) in 2018. Eur J Clin Microbiol Infect Dis 38:2275–2281. doi: 10.1007/s10096-019-03673-1. [DOI] [PubMed] [Google Scholar]
- 11.Han R, Shi Q, Wu S, Yin D, Peng M, Dong D, Zheng Y, Guo Y, Zhang R, Hu F, China Antimicrobial Surveillance Network (CHINET) Study Group. 2020. Dissemination of carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) among carbapenem-resistant Enterobacteriaceae isolated from adult and children patients in China. Front Cell Infect Microbiol 10:314. doi: 10.3389/fcimb.2020.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemarajata P, Humphries RM. 2019. Ceftazidime/avibactam resistance associated with L169P mutation in the omega loop of KPC-2. J Antimicrob Chemother 74:1241–1243. doi: 10.1093/jac/dkz026. [DOI] [PubMed] [Google Scholar]
- 13.Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, Pandey R, Doi Y, Kreiswirth BN, Nguyen MH, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 61:e02097-16. doi: 10.1128/AAC.02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sader HS, Castanheira M, Huband M, Jones RN, Flamm RK. 2017. WCK 5222 (cefepime-zidebactam) antimicrobial activity against clinical isolates of Gram-negative bacteria collected worldwide in 2015. Antimicrob Agents Chemother 61:e00072-17. doi: 10.1128/AAC.00072-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livermore DM, Mushtaq S, Warner M, Vickers A, Woodford N. 2017. In vitro activity of cefepime/zidebactam (WCK 5222) against Gram-negative bacteria. J Antimicrob Chemother 72:1373–1385. doi: 10.1093/jac/dkw593. [DOI] [PubMed] [Google Scholar]
- 16.Khan Z, Iregui A, Landman D, Quale J. 2019. Activity of cefepime/zidebactam (WCK 5222) against Enterobacteriaceae, Pseudomonas aeruginosa and Acinetobacter baumannii endemic to New York City medical centres. J Antimicrob Chemother 74:2938–2942. doi: 10.1093/jac/dkz294. [DOI] [PubMed] [Google Scholar]
- 17.Almarzoky Abuhussain SS, Avery LM, Abdelraouf K, Nicolau DP. 2018. In vivo efficacy of humanized WCK 5222 (cefepime-zidebactam) exposures against carbapenem-resistant Acinetobacter baumannii in the neutropenic thigh model. Antimicrob Agents Chemother 63:e01931-18. doi: 10.1128/AAC.01931-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomson KS, AbdelGhani S, Snyder JW, Thomson GK. 2019. Activity of cefepime-zidebactam against multidrug-resistant (MDR) Gram-negative pathogens. Antibiotics 8:32. doi: 10.3390/antibiotics8010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. 2018. Methods for dilution antimicrobial susceptibility testing of bacteria that grow aerobically, M07-A11. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. 2019. Performance standards for antimicrobial susceptibility testing, M100, 29th ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 21.FDA. 2019. Tigecycline-injection products. https://www.fda.gov/drugs/development-resources/tigecycline-injection-products.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.