Current guidelines recommend vancomycin and linezolid as first-line agents against methicillin-resistant Staphylococcus aureus (MRSA) nosocomial pneumonia. Telavancin is a potential new therapeutic alternative, specifically in monomicrobial MRSA pneumonia. This study compared the efficacies of telavancin versus linezolid in a porcine model of severe MRSA pneumonia. In 18 mechanically ventilated pigs (32.11 ± 1.18 kg), 75 ml of 106 CFU/ml of MRSA was administered into each pulmonary lobe.
KEYWORDS: telavancin, linezolid, MRSA, severe pneumonia, pig, animal models, mechanical ventilation, porcine model
ABSTRACT
Current guidelines recommend vancomycin and linezolid as first-line agents against methicillin-resistant Staphylococcus aureus (MRSA) nosocomial pneumonia. Telavancin is a potential new therapeutic alternative, specifically in monomicrobial MRSA pneumonia. This study compared the efficacies of telavancin versus linezolid in a porcine model of severe MRSA pneumonia. In 18 mechanically ventilated pigs (32.11 ± 1.18 kg), 75 ml of 106 CFU/ml of MRSA was administered into each pulmonary lobe. After the onset of pneumonia, pigs were randomized into three groups: a control group, a group receiving 22.5 mg/kg of body weight every 24 h (q24h) of telavancin, and a group receiving 10 mg/kg q12h of linezolid intravenously. Tracheal aspirate and bronchoalveolar lavage (BAL) fluids were cultured every 24 h. After 48 h of treatment, tissue samples were collected from the ventral and dorsal sections of each lobe. Microbiological and histopathological analyses were performed. Lung tissue concentrations differed among the groups (P = 0.019), with the lowest MRSA lung burden in the telavancin group (P < 0.05 versus the control). MRSA was detected in 46.7%, 40.0%, and 21.7% of the lung tissue samples from the control, linezolid, and telavancin groups, respectively (P < 0.001). MRSA concentrations differed among the groups in tracheal aspirate fluid (P = 0.011) but not in BAL fluid. Furthermore, there was no increased risk of kidney injury during telavancin use. Thus, telavancin has higher bactericidal efficacy than linezolid during the first 48 h of treatment in a porcine model of severe MRSA pneumonia. However, studies are needed to confirm the benefits of telavancin in treating MRSA nosocomial pneumonia.
INTRODUCTION
Staphylococcus aureus is the most common Gram-positive microorganism responsible for nosocomial pneumonia (NP) (1). Approximately 60% of strains show resistance to methicillin (methicillin-resistant S. aureus [MRSA]) (2). NP includes hospital-acquired pneumonia (HAP), which is the second most frequent nosocomial infection in health care settings, and ventilator-associated pneumonia (VAP) (3). The latest guidelines recommend empirical combination therapy against Gram-negative bacteria and MRSA that are at risk of developing multidrug resistance in patients with HAP/VAP (3, 4). The available therapeutic options against MRSA are currently limited to the glycopeptide vancomycin and the oxazolidinone linezolid (5, 6), which are considered first-line antibiotics for MRSA pneumonia worldwide (3, 4).
In 2016, telavancin was approved by the European Medicines Agency for treating NP and complicated skin and soft tissue infections. A derivate of vancomycin, telavancin is a novel semisynthetic lipoglycopeptide that demonstrates concentration-dependent bactericidal activity and a broad spectrum of antibacterial activity (7). Telavancin inhibits peptidoglycan synthesis and causes pronounced membrane depolarization, exerting excellent activity against S. aureus, MRSA, vancomycin-intermediate S. aureus, and linezolid- or daptomycin-resistant S. aureus (8). The pharmacokinetic profile of telavancin indicates that it can be administered daily at a dose of approximately 10 mg per kg of body weight (9).
As it currently stands, there is ongoing controversy and a lack of comparative studies on the efficacy and safety of telavancin against MRSA pneumonia. Only two studies have compared the drug with linezolid in small-animal models, suggesting that it could be used as an alternative to vancomycin and linezolid in treating MRSA pneumonia (10, 11). To date, no comparative trials in humans have been performed. Furthermore, only clinical appraisals of telavancin versus vancomycin have been published (12–14). In these appraisals, telavancin was associated with higher costs. However, when the indirect costs incurred by longer hospitalizations and vancomycin-related renal failure were included in the analysis, the total economic impacts of the antimicrobial drugs were similar (15).
All of these considerations led us to design a comprehensive study using a large-animal model of MRSA pneumonia. We have previously used an experimental porcine model of MRSA pneumonia to demonstrate an improved pharmacokinetic/pharmacodynamic (PK/PD) profile of linezolid compared to vancomycin as well as increased antibacterial efficacy (16).
To identify the appropriate dosage of telavancin that would simulate human exposure, we conducted a preliminary study. The primary aim of our main study was to assess the bactericidal efficacies of telavancin and linezolid against MRSA in lung tissue. Secondary aims were determining the MRSA concentrations in tracheal aspirates and bronchoalveolar fluids. In addition, we investigated the PK/PD profiles of telavancin and linezolid, assessing their benefits on systemic inflammation and clinical parameters. Potential drug-related side effects were also monitored.
(This work was selected as an oral presentation at the ERS Congress in 2018 [Paris, France] and the ESICM Congress in 2018 [Paris].)
RESULTS
Preliminary study.
A preliminary study was conducted on four large white female Landrace pigs (33.90 ± 2.12 kg) for 30 h.
PK/PD profile of telavancin.
The mean pharmacokinetic parameters of telavancin at two different doses (5 and 25 mg/kg) are summarized in Table S1 in the supplemental material. A confirmatory pharmacokinetic study was performed in an MRSA-infected animal administered 25 mg/kg of telavancin, in which an epithelial lining fluid (ELF) area under the concentration-time curve over the first 24 h (AUC0–24) of 47 mg · h/liter was achieved. This dosage resulted in an ELF AUC0–24/MRSA MIC ratio of 391 (Table S1). We adjusted the dosage to 22.5 mg/kg of telavancin administered every 24 h (q24h) as 1-h infusions for the main study.
Main study.
Eighteen animals (32.11 ± 1.18 kg) completed the 76-h study and were administered a full course of antibiotics.
MRSA lung burden.
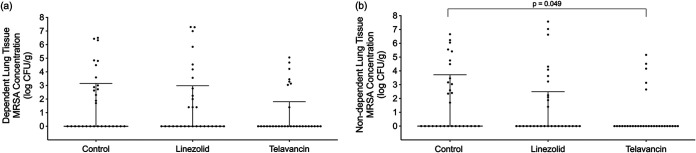
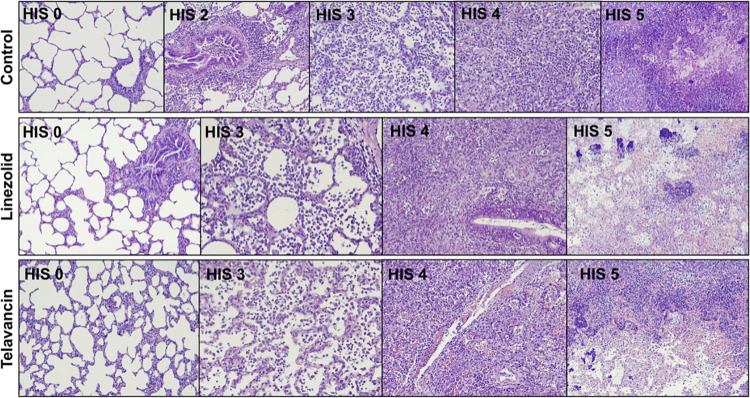
The mean values ± standard deviations (SD) for the MRSA concentrations in lung tissue were 1.85 ± 1.41, 1.58 ± 1.99, and 0.80 ± 1.04 log CFU/g in the control, linezolid, and telavancin (P < 0.05 versus the control by post hoc analysis) groups (P = 0.019), respectively. MRSA was isolated in 28/60 (46.67%), 24/60 (40%), and 13/60 (21.67%) of the lung tissue samples from the control, linezolid, and telavancin groups, respectively (P < 0.001). Moreover, MRSA was not found in one linezolid-treated and two telavancin-treated animals. MRSA was isolated from all the control animals. Figure 1 shows the differences between the groups in MRSA colonization of lung tissue obtained from either the dorsal (nondependent) or ventral (dependent) pulmonary region. In the dorsal pulmonary region, there was a significant difference in MRSA lung burdens among the groups (P = 0.049). Among the five pulmonary lobes, MRSA colonization varied considerably (P < 0.001), with the right middle lobe containing the highest MRSA concentrations (2.83 log CFU/g [interquartile range, 0.00 to 4.43 log CFU/g]), followed by the left upper lobe (0.70 log CFU/g [0.00 to 3.03 log CFU/g]). Figure S1 in the supplemental material shows the gross features of the lungs retrieved during autopsy. The lung/body weight ratios were 1.4 ± 0.4, 1.2 ± 0.2, and 1.4 ± 0.1 in the control, linezolid, and telavancin groups, respectively (P = 0.28). Gross signs of pneumonia were found in 70%, 43%, and 63% of the lobes retrieved from the control, linezolid, and telavancin groups, respectively (P = 0.095). Histopathological analysis was carried out on 180 lung tissue samples from the ventral and dorsal pulmonary regions. Figure 2 shows the histological characteristics of the groups. Pneumonia was confirmed in 58.33% of the histological samples from the control group, 43.33% of the samples from the linezolid group, and 48.33% of the samples from the telavancin group (P = 0.247). Pneumonia with an abscess was observed in 16.67% of the samples from the control group, 8.33% of the samples from the linezolid group, and 13.33% of the samples from the telavancin group (P = 0.43). As shown in Table S2 in the supplemental material, the histological injury score did not differ among the groups (P = 0.64) or between the dependent and nondependent regions (P = 0.92). However, the score varied among the pulmonary lobes (P = 0.002), with the right middle lobe being the most injured lobe.
FIG 1.
Methicillin-resistant Staphylococcus aureus (MRSA) concentrations in lung tissue. Data points indicate MRSA colonization of lung tissue samples in the dependent (ventral) (a) and nondependent (dorsal) (b) pulmonary regions. Solid horizontal lines depict the medians, while error bars show the 95% confidence intervals. MRSA colonization in lung tissue did not differ among the groups in the dependent pulmonary regions (P = 0.260), but it differed significantly in the nondependent sections (P = 0.049).
FIG 2.
Histopathological studies of lung tissue. Control-HIS 0 (histological injury score of 0), normal histology of the lung (n = 24; 40%), showing empty dilated alveolar lumina with thin alveolar walls, an absence of significant inflammation or septal thickening, and a mild degree of alveolar overdistension due to barotrauma; Control-HIS 2, respiratory bronchiolitis (n = 1; 1.67%), presenting focal chronic inflammation of the terminal bronchioles and alveolar ducts, with adjacent focal interstitial inflammation and fibrosis, and histiocytes filling the peribronchiolar alveolar ducts and spaces; Control-HIS 3, pneumonia (n = 21; 35%), with foci of intra-alveolar polymorphonuclear leukocytes preserving the alveolar architecture; Control-HIS 4, confluent pneumonia (n = 4; 6.67%), with multiple and confluent foci of intra-alveolar polymorphonuclear neutrophils filling the alveolar lumina, effacing the alveolar architecture; Control-HIS 5, pneumonia with an abscess (n = 10; 16.67%), showing nodular areas of necrosis with parenchymal disruption surrounded by dense polymorphonuclear infiltrates; Linezolid-HIS 0, normal histology of the lung (n = 34; 56.67%); Linezolid-HIS 3, pneumonia (n = 19; 31.67%); Linezolid-HIS 4, confluent bronchopneumonia (n = 2; 3.33%), accompanied by foci of microabscesses infiltrating the alveolar wall; Linezolid-HIS 5, bronchopneumonia with an abscess (n = 5; 8.33%), with multiple foci of bacterial colonies in the center of necrotic areas; Telavancin-HIS 0, normal histology of the lung (n = 31; 51.67%); Telavancin-HIS 3, pneumonia (n = 18; 30%); Telavancin-HIS 4, confluent pneumonia (n = 3; 5%); Telavancin-HIS 5, pneumonia with an abscess (n = 8; 13.33%).
Microbiological assessments.
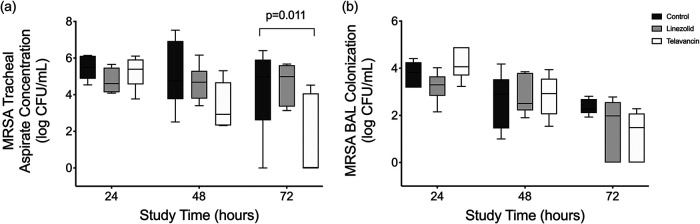
MRSA concentrations in the tracheal secretions differed among the groups throughout the study (P = 0.011; for post hoc comparisons, P < 0.050 for telavancin versus the control). At the end of the study, MRSA concentrations (interquartile ranges) in the tracheal secretions of the control, linezolid, and telavancin groups were 4.97 (2.60 to 5.91), 4.99 (3.35 to 5.61), and 0.00 (0.00 to 4.07) log CFU/ml, respectively (Fig. 3a). In bronchoalveolar lavage (BAL) fluid, MRSA concentrations at 72 h were 4.98 (2.61 to 5.91), 4.47 (3.28 to 5.64), and 1.81 (0.00 to 4.77) log CFU/ml in the control, linezolid, and telavancin groups, respectively (without statistical significance [P = 0.093]) (Fig. 3b). MRSA bacteremia was never observed throughout the study in any of the groups. Adaptive resistance through antimicrobial pressure during treatment was not observed in the linezolid- or telavancin-treated animals. Indeed, the median linezolid MIC remained at 1.0 (1.0 to 1.5) μg/ml, while the median telavancin MIC was 0.12 (0.09 to 0.19) μg/ml among the isolates.
FIG 3.
MRSA concentrations in tracheal secretions and bronchoalveolar fluids among the groups. Box plots show MRSA concentrations in the tracheal secretions and bronchoalveolar lavage (BAL) fluids among the groups. Horizontal bars represent the medians, boxes indicate the interquartile ranges, and whiskers correspond to the ranges. (a) In tracheal secretions, MRSA concentrations varied among the groups (P = 0.011) and time points (P = 0.011). At the end of the study, MRSA concentrations (interquartile ranges) in the tracheal secretions of the control, linezolid, and telavancin groups were 4.97 (2.60 to 5.91), 4.99 (3.35 to 5.61), and 0.00 (0.00 to 4.07) log CFU/ml, respectively (P = 0.011). It should be noted that the production of tracheal secretions at baseline was marginal. Therefore, samples were not obtained and cultured. (b) In BAL fluids, MRSA concentrations (interquartile ranges) at 72 h were 4.98 (2.61 to 5.91), 4.47 (3.28 to 5.64), and 1.81 (0.00 to 4.77) log CFU/ml in the control, linezolid, and telavancin groups, respectively (without statistical significance [P = 0.093]). MRSA concentrations varied among the times of assessments (P < 0.001).
Inflammatory response.
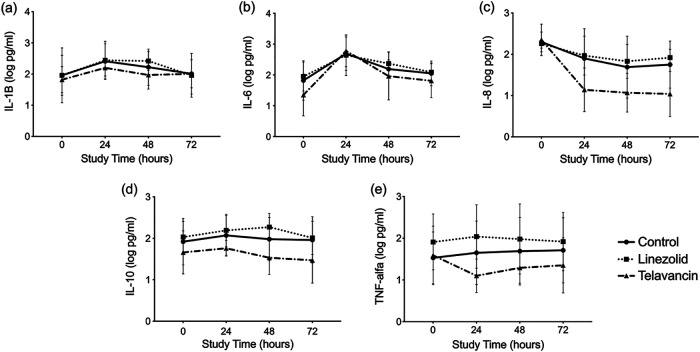
Figure 4 displays the dynamics of the inflammatory markers over time among the groups. Only interleukin-8 (IL-8) showed differences among the groups (P = 0.036), specifically 24 h (P = 0.019) and 72 h (P = 0.030) after bacterial challenge. Telavancin-treated animals showed lower values. The other cytokines studied did not show differences among the groups.
FIG 4.
Systemic cytokine kinetics among the groups. In each graph, the mean cytokine levels and standard deviations are reported per time point among the groups. Only interleukin-8 (IL-8) (c) differed significantly among the groups, particularly 24 and 72 h after the start of the study. TNF, tumor necrosis factor.
Pharmacokinetics.
The pharmacokinetic study showed that the selected regimens simulated exposures in humans, based on the area under the curve for the free, unbound concentration of the drug in ELF over the first 24 h (fAUC0–24), as described above. Linezolid concentrations in blood and BAL fluids were quantified in all the treated animals, while telavancin concentrations were measured in 3 out of the 6 animals treated. The bound drug amount was considered negligible for both telavancin and linezolid. For linezolid, 18 plasma and 12 ELF concentrations were fitted to a two-compartment model, following the best Akaike information criterion (AIC) score (AIC score, 149.29). For telavancin, 11 plasma and 9 ELF concentrations were modeled (AIC score, 130.71). The correlation between the observed and the individually predicted plasma and ELF concentrations for both antimicrobials is provided in Fig. S2. Table 1 displays the plasma and ELF pharmacokinetic profiles of linezolid and telavancin. The median ratios of penetration into the ELF were 92.12% and 7.96% for the linezolid and telavancin models, respectively. The linezolid dosage regimen achieved the pharmacodynamic target fAUC/MIC value of 80 to 120 in both compartments without reaching drug overexposure, which is defined as an fAUC0–24 of ≥400 mg · h/liter (17). Similarly, the telavancin dosage regimen reached the threshold, resulting in at least a 2-log kill based on the immunocompromised murine lung infection model (fAUC/MIC ratio of ≥119) in both the plasma and ELF compartments (18).
TABLE 1.
Pharmacokinetics and pharmacodynamics of linezolid and telavancina
| Parameter | Median value for drug (IQR) |
|
|---|---|---|
| Linezolid (n = 6; 10 mg/kg) | Telavancin (n = 3; 22.5 mg/kg) | |
| Pharmacokinetic parameters | ||
| CL (liters/h) | 1.55 (0.70–3.25) | 1.34 (1.13–2.08) |
| Vc (liters) | 11.76 (8.33–14.76) | 6.73 (5.66–7.59) |
| VELF (liters) | 10.19 (6.80–20.65) | 40.95 (29.63–153.93) |
| Kcp (h−1) | 27.37 (23.65–40.51) | 0.12 (0.10–0.15) |
| Kpc (h−1) | 32.47 (29.97–43.88) | 0.19 (0.05–0.28) |
| Plasma Cmax (mg/liter) | 14.58 (13.21–17.16) | 93.63 (84.49–96.10) |
| Plasma Ctrough (mg/liter) | 6.17 (3.14–9.14) | 3.73 (1.55–5.96) |
| Pharmacodynamic indices | ||
| Plasma fAUC0–24 (mg · h/liter) | 208.96 (177.49–312.95) | 515.57 (316.93–539.78) |
| ELF fAUC0–24 (mg · h/liter) | 202.70 (111.70–366.77) | 42.45 (23.51–60.34) |
| % penetration | 92.12 (50.97–170.42) | 7.96 (4.56–21.53) |
| Plasma fAUC0–24/MIC ratio | 208.96 (177.49–312.95) | 4,296.46 (2,641.11–4,498.19) |
| ELF fAUC0–24/MIC ratio | 202.70 (111.70–366.77) | 353.74 (195.92–503.60) |
Data are reported as the medians and interquartile ranges (IQR) (25th to 75th percentiles). CL, clearance; Vc, volume of distribution of the central compartment; VELF, volume of distribution of the peripheral epithelial lining fluid (ELF) compartment; Kcp, transfer rate constant from the central compartment to the peripheral ELF compartment; Kpc, transfer rate constant from the peripheral ELF compartment to the central compartment; Cmax, peak concentration; Ctrough, lowest concentration; fAUC0–24/MIC ratio, ratio of the free area under the curve to the MIC over the first 24 h. The linezolid MIC is 1 μg/ml, and the telavancin MIC is 0.12 μg/ml.
Safety.
The overall incidence of adverse events is reported in Table 2. No differences were found at baseline and at pneumonia diagnosis, with median values (interquartile ranges) of 1.08 (0.99 to 1.19) and 1.17 (1.06 to 1.28) mg/dl, respectively. The median creatinine levels were significantly higher than the values for the control and telavancin-treated animals at the end of the study (Fig. S3). The median creatinine levels (interquartile ranges) after 48 h of treatment were 1.10 (0.97 to 1.23), 1.48 (1.43 to 1.80), and 1.28 (1.23 to 1.38) mg/dl in the control, linezolid, and telavancin groups, respectively. Indeed, potentially significant increases in serum creatinine levels (>25% increase from that at pneumonia diagnosis or >1.5 mg/dl) were more common in the linezolid group than in the telavancin group. Regarding renal impairment, low urinary output and furosemide administration were more common in the linezolid group. Despite the increases in creatinine levels, the most common abnormalities in both of the treatment groups were platelet reduction and increased alanine aminotransferase (ALT) levels.
TABLE 2.
Safety parameters and laboratory abnormalities upon diagnosis of pneumoniae
| Safety or laboratory parameter | No. (%) of animals |
||
|---|---|---|---|
| Control (n = 6) | Linezolid (n = 6) | Telavancin (n = 6) | |
| Skin rash | 0 (0) | 0 (0) | 0 (0) |
| Bronchospasm | 0 (0) | 0 (0) | 0 (0) |
| Diarrhea | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | 0 (0) | 0 (0) | 0 (0) |
| Hb reductiona | 1 (16.7) | 0 (0) | 0 (0) |
| Platelet count reductiona | 2 (33.7) | 3 (50.0) | 1 (16.7) |
| ALT level > ULN | 1 (16.7) | 2 (33.7) | 1 (16.7) |
| Alkaline phosphatase level > 2× ULN | 1 (16.7) | 1 (16.7) | 0 (0) |
| Abnormal potassium levelb | 1 (16.7) | 1 (16.7) | 0 (0) |
| Creatinine level increasec | 0 (0) | 4 (66.7) | 1 (16.7) |
| Creatinine level of >1.5 mg/dl | 1 (16.7) | 4 (66.7) | 0 (0) |
| Urinary output reductiond | 2 (33.7) | 2 (33.7) | 0 (0) |
| Furosemide administration | 3 (50.0) | 4 (66.7) | 0 (0) |
At least a 30% reduction from the value at pneumonia diagnosis.
Potassium level of <3.5 or >5.5 meq/liter.
Serum creatinine level increase of ≥25% from that at pneumonia diagnosis.
Urinary output of ≤0.5 ml · h/kg.
Hb, hemoglobin; ALT, alanine aminotransferase; ULN, upper limit of normal.
Clinical parameters.
Information on pulmonary mechanics among the groups is shown in Table S3. Pulmonary shunt and ventilatory support requirements increased over the study period, without significant differences among the groups. Interestingly, the worst lung and chest wall elastances were found in the telavancin and linezolid groups, respectively. Table S4 shows the clinical parameters of the groups. After MRSA inoculation, platelet counts drastically increased in the control group, while alanine aminotransferase levels were higher in the two treatment groups. Other signs of infection, such as fever, white blood cell levels, and prothrombin time, were also increased after bacterial inoculation; however, no significant differences in these were observed among the groups. Hemodynamic parameters, excluding heart rate, were not affected by the treatments (Table S5). The mean arterial and pulmonary pressures and the systemic and pulmonary vascular resistances varied significantly over the study period, but they did not show significant differences among the groups.
DISCUSSION
The main finding of this study in pigs with severe multilobar MRSA pneumonia was that human-simulated exposures of telavancin appeared to be more advantageous than linezolid in terms of anti-MRSA efficacy in lung tissue. Similarly, telavancin demonstrated good bactericidal activity in tracheal secretions over the study period, specifically after 48 h of treatment. While telavancin did not clearly improve any other clinically relevant parameters, a daily dosage of 22.5 mg of telavancin did not increase the risk of kidney injury compared to linezolid.
Only two preclinical studies have previously compared the efficacies of telavancin and linezolid against MRSA in lung tissue. The first study, by Reyes et al. in 2005, evaluated the efficacy of telavancin versus linezolid and vancomycin in mice with methicillin-sensitive S. aureus (MSSA) pneumonia. They found greater reductions in lung bacterial burdens and mortality with the use of telavancin (10). In 2008, Hegde et al. assessed the efficacy of telavancin versus linezolid, nafcillin, and vancomycin in a neutropenic murine model of MRSA pneumonia. Compared to the animals treated with linezolid, nafcillin, or vancomycin, those administered telavancin presented a dose-dependent reduction in the MRSA lung burden 48 h after inoculation. In addition, the telavancin and vancomycin groups showed greater survival than the linezolid group (11). Irrespective of these results, it is important to emphasize that these preclinical studies in mice did not reproduce the severity and settings found in critically ill patients. For example, small animals rapidly fail during severe infections (19). Moreover, both of the above-mentioned studies in murine models of lung infection used an equivalent dose that was close to the human plasma AUC. The degree of drug penetration into peripheral compartments may vary among different species (20). Measurement of antimicrobial concentrations at the site of infection (i.e., ELF) is therefore considered desirable to improve the application of animal studies to clinical settings and predict exposure-response relationships (21). In the present study, following dose adjustment, confirmatory pharmacokinetic studies showed that both 10 mg/kg of linezolid and 22.5 mg/kg of telavancin approximated the humanized doses based on ELF exposures. Following a dosage of 600 mg q12h as a 1-h infusion, the linezolid regimen in animals simulated the ELF AUC0–24 of 155 mg · h/liter reached in critically ill patients (22), while the ELF AUC0–24 for the telavancin regimen simulated the ELF AUC profile in humans after a dosage of 10 mg/kg over 1 h (45 mg · h/liter) (Table 1) (23). Both regimens reached the pharmacodynamic threshold targets (see Table S2 in the supplemental material) (18, 24).
Our current study is the first comparative evaluation to report on the efficacy of human-simulated ELF exposure of telavancin versus linezolid in a large-animal model of severe MRSA pneumonia. Given the recent approval of telavancin for the treatment of MRSA nosocomial pneumonia (25), there is a pressing need for confirmatory evidence from preclinical and clinical studies. We used highly regulated laboratory methods in this study to gain further knowledge on this relatively new antibiotic. In our model, MRSA colonization of lung tissue varied among the pulmonary regions, with small differences between gravity-dependent and non-gravity-dependent pulmonary regions.
MRSA clearance by telavancin was confirmed in the tracheal secretions. This could have clinical implications by potentially reducing the risk of cross-contamination between intubated patients in busy intensive care units. These positive findings were not observed in the BAL fluid samples, probably due to the significant bacterial burden of our model and the short-term duration of the antibiotic treatment before euthanasia. As for pulmonary mechanics and lung function, we did not observe any significant improvement with linezolid or telavancin treatment. There was a slight increase in lung elastance with the use of telavancin. There was no increase in the MIC of the MRSA isolates after in vivo exposure to telavancin or linezolid. It has been previously reported that there is an increase in the MIC in response to telavancin treatment although after 50 serial passages (26). Irrespective of these effects, only a marginal reduction in systemic inflammation was found in the animals treated with telavancin.
One of the most feared complications of telavancin treatment is the impairment of kidney function. The ATTAIN clinical trial in patients with HAP confirmed that 10 mg/kg daily of telavancin compared to 1 g q12h of vancomycin for up to 21 days resulted in similar cure rates in patients with MRSA pneumonia but higher cure rates in patients with methicillin-susceptible staphylococcus pneumonia. In contrast, vancomycin showed higher efficacy than telavancin in patients with mixed Gram-positive/Gram-negative infections. However, the clinical trial raised concerns about kidney injury, with 16% of patients in the telavancin group and 10% of patients in the vancomycin group experiencing an increase in creatinine levels (12). A post hoc analysis of the ATTAIN study in a subpopulation of patients that excluded those with severe renal impairment (creatinine clearance of <30 ml/min, such as patients on hemodialysis) or with preexisting acute renal failure at baseline showed impaired kidney function during treatment in 8.8% of the patients in the telavancin group and 6.7% of the patients in the vancomycin group (13). Irrespective of these previous clinical results, there are currently no comparative studies on the risks of renal failure associated with telavancin or linezolid use. Using animals without kidney dysfunction at baseline, we did not find any risk associated with telavancin. Indeed, the AUC0–24 remained below the target AUC0–24 that is associated with acute kidney injury in all animals (i.e., AUC0–24 of ≥763 mg · h/liter) (27). Nevertheless, our findings should be interpreted with caution, as only a short duration of antibiotic treatment was tested.
Regarding the clinical parameters, the only difference found among the groups was in the platelet count, which was lower in the treatment groups. The effects of linezolid on platelets are already known and are thought to occur via a mechanism similar to that of quinine/quinidine-induced, immune-mediated thrombocytopenia (28). In our setting, however, the reduction in platelet counts was clinically irrelevant. Regarding the slight increase in alanine aminotransferase (ALT) levels in response to linezolid and telavancin, both antibiotics have minor effects on hepatic metabolism and do not significantly affect the activity of the microsomal cytochrome P450 enzymes (9). Nevertheless, these findings suggest that further investigations into the metabolism of these drugs, specifically during highly severe infections, should be undertaken. Finally, all the groups in this study showed similar hemodynamic profiles, that is, sepsis-related reductions in the mean arterial pressure and systemic vascular pressure and increases in the mean pulmonary pressure and pulmonary resistance. These findings confirmed the severity of our model of MRSA pneumonia as well as the systemic derangement caused by the infection.
Our study had several strengths. This is the first comparative evaluation of the bactericidal efficacy of telavancin versus linezolid in large animals. The severity of infection in our model of MRSA pneumonia closely simulated that of critically ill patients with MRSA pneumonia who are mechanically ventilated and supported by vasoactive drugs. The extensive clinical, microbiological, and postmortem evaluations should also be highlighted, as they provided a preliminary and thorough picture of the effects and safety of telavancin and linezolid. However, there were some limitations of our study that should be acknowledged. Although we conducted a comprehensive sample size analysis, corroboration of several of the secondary outcomes with our previous work was limited by the small population and the use of only one distinct MRSA strain in this study. Therefore, a direct comparison between telavancin and vancomycin might be inaccurate. We did, however, select an MRSA strain with a high MIC for telavancin (29) when determining telavancin efficacy. Second, as mentioned above, we monitored the effects of treatment for up to 48 h only, potentially reducing the translatability of our findings to the clinical setting. Finally, there are some differences between a porcine model of pneumonia and pneumonia in humans, including comorbidities and drug interactions.
Conclusions.
Telavancin can have high efficacy against MRSA during the first 48 h of treatment in severe nosocomial pneumonia compared to linezolid. In our settings, telavancin use was safe and not associated with an increased risk of kidney injury. Further clinical corroboration of these preliminary findings is crucial to confirm the indication of telavancin instead of linezolid in treatments.
MATERIALS AND METHODS
We conducted a prospective observational study at the Division of Animal Experimentation, Department of Pulmonary and Critical Care Medicine, Hospital Clinic, Barcelona, Spain. The study was approved by the Institutional Review Board and the Ethics Committee of our institution (approval number 344/17). Animal care complied with the Guide for the Care and Use of Laboratory Animals of the U.S. National Institutes of Health (30) and local government guidelines.
Preliminary PK/PD study.
The primary aim of this preliminary study was to identify the appropriate dosage of telavancin that would simulate human exposure (i.e., an ELF AUC0–24 of 45 mg · h/liter) (23). A confirmatory pharmacokinetic study was performed in an MRSA-infected animal. Details on animal preparation, telavancin administration, sample collection, urea assays, and telavancin PK analysis are given in the supplemental material.
Main study design.
We conducted a prospective 76-h randomized study in pigs at the Division of Animal Experimentation, Department of Pulmonary and Critical Care Medicine, Hospital Clinic, Barcelona, Spain. The study was approved by the Institutional Review Board and the Ethics Committee of our institution (approval number 344/17). Animal care complied with Spanish Government regulations. A detailed description of the materials and methods used is provided in the supplemental material.
Aims.
The primary aim was to evaluate the efficacy of telavancin in comparison to linezolid against MRSA in lung tissue. Secondary aims were to study MRSA concentrations in tracheal aspirates and bronchoalveolar fluids. In addition, the pharmacokinetics/pharmacodynamics of telavancin and linezolid were investigated, and their benefits on systemic inflammation and clinical parameters were evaluated. Potential drug-related side effects, such as acute changes in renal function, were also monitored.
Animal preparation and mechanical ventilation.
Eighteen large white Landrace pigs (32.11 ± 1.18 kg) were anesthetized, intubated, and mechanically ventilated for up to 76 h. Antibiotic prophylaxis (50 mg/kg q12h of ceftriaxone administered intravenously [i.v.]) was administered to prevent contamination by endogenous oropharyngeal flora. Esophageal pressure was measured with an esophageal catheter (CareFusion, Yorba Linda, CA, USA). Airway pressure and airflows were also measured, as previously described (31, 32). Pulmonary mechanics were assessed daily. The data were recorded with dedicated software (Colligo; Elekton, Milan, Italy). Hemodynamic monitoring was undertaken by placing a Swan-Ganz catheter into a femoral artery (Swan-Ganz pulmonary artery catheter [PAC]; Edwards Lifesciences, Irvine, CA, USA). An indwelling catheter was surgically positioned. Animal preparation and mechanical ventilation methods were previously described by our group and are provided in detail in the supplemental material (31, 32).
Bacterial inoculation and severe MRSA pneumonia.
After surgical preparation, pigs were placed in the prone position and inoculated with 75 ml (15 ml into each lobe) of approximately 106 CFU/ml of an MRSA strain that is susceptible to linezolid and telavancin (MICs of 1 and 0.12 μg/ml, respectively) with a bronchoscope (Pentax Safe-3000; Ricoh Imaging Deutschland GmbH). A pathogenic Panton-Valentine leukocidin-negative MRSA strain (agr type II and sequence type [ST] 125) isolated from a patient with MRSA pneumonia was used. Its complete antimicrobial resistance profile is displayed in Table S6 in the supplemental material.
At 24 h, clinical diagnosis of pneumonia was confirmed by at least two of the following clinical features: a body temperature of >38.5°C or <36°C, a white blood count of >14,000 cells/mm3 or <4,000 cells/mm3, and purulent secretions. During autopsy, severe MRSA pneumonia was confirmed based on a mean pulmonary histological injury score of ≥3 and a mean pulmonary S. aureus burden of ≥3 log CFU/g in at least 3 lung lobes (16, 31).
Randomization.
Twenty-four hours after MRSA inoculation, pigs were randomized into 3 treatment groups: (i) one that received 100 ml of a 5% glucose solution i.v. every 24 h (n = 6 animals), (ii) one that was administered 22.5 mg/kg of telavancin every 24 h as a 1-h i.v. infusion (n = 6 animals), and (iii) one that received 10 mg/kg of linezolid every 12 h as a 1-h i.v. infusion (n = 6 animals). The human-simulated linezolid regimen in pigs was previously described (16).
Measurements and sampling.
(i) MRSA lung burden. During autopsy, pulmonary biopsy specimens from the dorsal (nondependent) and ventral (dependent) pulmonary regions of each lobe were collected and cultured to quantify MRSA concentrations in lung tissue and corroborate differences among the groups. MRSA concentrations in the dorsal and ventral regions were also compared. Pulmonary biopsy specimens from each region and lobe were collected for histological assessment. Lung histology was evaluated according to a previous study using an injury score (16).
(ii) Microbiological assessments. Quantitative cultures of tracheal aspirates and BAL fluids were collected before bacterial inoculation, prior to antibiotic administration, and then daily. Blood cultures were assessed every day. MRSA resistance to linezolid and telavancin was also assessed.
(iii) Inflammatory responses. Blood was collected prior to bacterial inoculation, at clinical diagnosis of pneumonia, and at 24 and 48 h thereafter to quantify the levels of tumor necrosis factor alpha (TNF-α), IL-1β, IL-6, IL-8, and IL-10 using enzyme-linked immunosorbent assays and specific porcine kits (R&D Systems, Minneapolis, MN, USA), as previously reported by our group (33).
(iv) Pharmacokinetics. In infected, treated animals, linezolid and telavancin concentrations were quantified in plasma and BAL fluids that were obtained before and 1, 2, 6, 12, and 24 h after the administration of the antibiotic. ELF concentrations were determined using the urea concentration as an endogenous marker. Protein binding was assessed in duplicate (34). Linezolid concentrations were measured at the Center for Anti-Infective Research and Development (Hartford, CT, USA) using validated high-performance liquid chromatography (35). Telavancin concentrations were measured at Theravance Inc. (South San Francisco, CA, USA) using validated liquid chromatography-tandem mass spectrometry (23). Briefly, the concentrations obtained for linezolid and telavancin were fitted separately to a two-compartment model using the nonparametric adaptive grid algorithm (36). The linezolid AUC0–24 was calculated as follows: AUC0–24 = 2 × AUC0–12.
(v) Safety. The safety and tolerability of linezolid and telavancin were monitored throughout the course of treatment. Safety was determined by assessing any potential drug-related side effects (i.e., skin rash, bronchospasms, diarrhea, and vomiting) and any clinically significant changes in laboratory values (chemistry, hematology, and liver and renal function tests) during the start and completion of antimicrobial treatment. Serum creatinine levels were measured twice a day, and an increase of 25% from that at pneumonia diagnosis was considered significant.
(vi) Clinical assessments. Respiratory mechanics were assessed daily. Ventilatory settings, arterial blood gases, and hemodynamic parameters were monitored and adjusted every 6 h to maintain clinical stability.
Statistical analysis.
The sample size calculation is reported in the supplemental material. Normally distributed parameters were expressed as means ± standard deviations (SD), whereas nonnormally distributed parameters were expressed as the medians (interquartile ranges). Categorical variables were described as frequencies and percentages. Continuous variables were analyzed using restricted maximum likelihood (REML) analysis based on a repeated-measures approach (PROC MIXED), with times of assessment and pulmonary lobes included as factors. A compound symmetry or univariate (co)variance structure was used to model the within-subject errors. For each continuous variable, the overall F test was first performed to assess significance (P ≤ 0.05). Two-sided comparisons among the groups were also performed, and a given comparison was considered significant if its P value was ≤0.05. Each pairwise comparison was corrected using the Bonferroni method to control for the experiment-wise error rate. We tested the assumption about the normality of the model residuals in PROC MIXED. For the nonnormally distributed residuals, we used the Friedman test. All statistical analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA).
Supplementary Material
ACKNOWLEDGMENTS
Support was provided by the Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) (Barcelona, Spain) and the Centro de Investigación Biomedica en Red de Enfermedades Respiratorias (CIBERES) (Mallorca, Spain). We acknowledge Laura Muñoz, Jordi Vila, and Christina Sutherland for their assistance in conducting the study. We thank Anthony Armenta for assisting with the editing of the manuscript in English.
Theravance Biopharma R&D Inc. (George Town, Cayman Islands) provided funding support for this study. Financial support was provided by IDIBAPS and CIBERES (CB 06/06/0028). A. Motos is the recipient of a long-term research fellowship (LTRF 2017-01-00073) from the European Respiratory Society (ERS) and the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR). G. Li Bassi is the recipient of a postdoctoral grant from the Strategic Plan for Research and Innovation in Health (PERIS), 2017 to 2021. G. Li Bassi is the recipient of a BITRECS fellowship grant from IDIBAPS funded by Horizon 2020 and the La Caixa Foundation. A. Torres is the recipient of the ICREA academy award.
All authors declare no competing interests, except for G. Li Bassi and A. Torres, who received research funding from Theravance Biopharma.
G. Li Bassi and A. Torres participated in protocol development, study design, and study management; D. Battaglini, G. Li Bassi, and A. Motos participated in study management, data collection, statistical analysis, data interpretation, and manuscript writing; H. Yang, F. Pagliara, M. Yang, E. Aguilera Xiol, A. Meli, J. Bobi, G. Frigola, T. Senussi, F. Idone, C. Travierso, C. Chiurazzi, J. Bringue, and L. Guerrero participated in data collection, data interpretation, and the critical review of the first draft of the manuscript; L. Fernandez-Barat, M. Rigol, J. Ramirez, P. Pelosi, D. Chiumello, M. Antonelli, D. P. Nicolau, A. Artigas, D. Soy, and A. Torres participated in data interpretation as well as in the revision and review of the manuscript. All authors have read and approved the final version of the manuscript.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Graffunder EM, Venezia RA. 2002. Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. J Antimicrob Chemother 49:999–1005. doi: 10.1093/jac/dkf009. [DOI] [PubMed] [Google Scholar]
- 2.Fridkin SK, Hill HA, Volkova NV, Edwards JR, Lawton RM, Gaynes RP, McGowan JE, Jr, Intensive Care Antimicrobial Resistance Epidemiology Project Hospitals. 2002. Temporal changes in prevalence of antimicrobial resistance in 23 US hospitals. Emerg Infect Dis 8:697–701. doi: 10.3201/eid0807.010427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, Kollef M, Li Bassi G, Luna CM, Martin-Loeches I, Paiva JA, Read RC, Rigau D, Timsit JF, Welte T, Wunderink R. 2017. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT). Eur Respir J 50:1700582. doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 4.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O’Grady NP, Bartlett JG, Carratala J, El Solh AA, Ewig S, Fey PD, File TM, Jr, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. 2016. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kollef MH. 2007. Limitations of vancomycin in the management of resistant staphylococcal infections. Clin Infect Dis 45(Suppl 3):S191–S195. doi: 10.1086/519470. [DOI] [PubMed] [Google Scholar]
- 6.Wunderink RG, Mendelson MH, Somero MS, Fabian TC, May AK, Bhattacharyya H, Leeper KV, Jr, Solomkin JS. 2008. Early microbiological response to linezolid vs vancomycin in ventilator-associated pneumonia due to methicillin-resistant Staphylococcus aureus. Chest 134:1200–1207. doi: 10.1378/chest.08-0011. [DOI] [PubMed] [Google Scholar]
- 7.Draghi DC, Benton BM, Krause KM, Thornsberry C, Pillar C, Sahm DF. 2008. Comparative surveillance study of telavancin activity against recently collected gram-positive clinical isolates from across the United States. Antimicrob Agents Chemother 52:2383–2388. doi: 10.1128/AAC.01641-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leuthner KD, Cheung CM, Rybak MJ. 2006. Comparative activity of the new lipoglycopeptide telavancin in the presence and absence of serum against 50 glycopeptide non-susceptible staphylococci and three vancomycin-resistant Staphylococcus aureus. J Antimicrob Chemother 58:338–343. doi: 10.1093/jac/dkl235. [DOI] [PubMed] [Google Scholar]
- 9.Shaw JP, Seroogy J, Kaniga K, Higgins DL, Kitt M, Barriere S. 2005. Pharmacokinetics, serum inhibitory and bactericidal activity, and safety of telavancin in healthy subjects. Antimicrob Agents Chemother 49:195–201. doi: 10.1128/AAC.49.1.195-201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reyes N, Skinner R, Kaniga K, Krause KM, Shelton J, Obedencio GP, Gough A, Conner M, Hegde SS. 2005. Efficacy of telavancin (TD-6424), a rapidly bactericidal lipoglycopeptide with multiple mechanisms of action, in a murine model of pneumonia induced by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 49:4344–4346. doi: 10.1128/AAC.49.10.4344-4346.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hegde SS, Reyes N, Skinner R, Difuntorum S. 2008. Efficacy of telavancin in a murine model of pneumonia induced by methicillin-susceptible Staphylococcus aureus. J Antimicrob Chemother 61:169–172. doi: 10.1093/jac/dkm417. [DOI] [PubMed] [Google Scholar]
- 12.Rubinstein E, Lalani T, Corey GR, Kanafani ZA, Nannini EC, Rocha MG, Rahav G, Niederman MS, Kollef MH, Shorr AF, Lee PC, Lentnek AL, Luna CM, Fagon JY, Torres A, Kitt MM, Genter FC, Barriere SL, Friedland HD, Stryjewski ME, ATTAIN Study Group. 2011. Telavancin versus vancomycin for hospital-acquired pneumonia due to gram-positive pathogens. Clin Infect Dis 52:31–40. doi: 10.1093/cid/ciq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres A, Rubinstein E, Corey GR, Stryjewski ME, Barriere SL. 2014. Analysis of phase 3 telavancin nosocomial pneumonia data excluding patients with severe renal impairment and acute renal failure. J Antimicrob Chemother 69:1119–1126. doi: 10.1093/jac/dkt490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corey GR, Kollef MH, Shorr AF, Rubinstein E, Stryjewski ME, Hopkins A, Barriere SL. 2014. Telavancin for hospital-acquired pneumonia: clinical response and 28-day survival. Antimicrob Agents Chemother 58:2030–2037. doi: 10.1128/AAC.02330-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rello J, Bin C. 2016. Cost of nosocomial pneumonia: the example of vancomycin versus linezolid—shorter stay or fewer complications? Int J Infect Dis 51:1–3. doi: 10.1016/j.ijid.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Olondris P, Rigol M, Soy D, Guerrero L, Agusti C, Quera MA, Li Bassi G, Esperatti M, Luque N, Liapikou M, Filella X, Marco F, de la Bellacasa JP, Torres A. 2012. Efficacy of linezolid compared to vancomycin in an experimental model of pneumonia induced by methicillin-resistant Staphylococcus aureus in ventilated pigs. Crit Care Med 40:162–168. doi: 10.1097/CCM.0b013e31822d74a2. [DOI] [PubMed] [Google Scholar]
- 17.Pea F, Viale P, Cojutti P, Del Pin B, Zamparini E, Furlanut M. 2012. Therapeutic drug monitoring may improve safety outcomes of long-term treatment with linezolid in adult patients. J Antimicrob Chemother 67:2034–2042. doi: 10.1093/jac/dks153. [DOI] [PubMed] [Google Scholar]
- 18.Lepak AJ, Zhao M, Andes DR. 2017. Comparative pharmacodynamics of telavancin and vancomycin in the neutropenic murine thigh and lung infection models against Staphylococcus aureus. Antimicrob Agents Chemother 61:e00281-17. doi: 10.1128/AAC.00281-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bielen K, ‘s Jongers B, Malhotra-Kumar S, Jorens PG, Goossens H, Kumar-Singh S. 2017. Animal models of hospital-acquired pneumonia: current practices and future perspectives. Ann Transl Med 5:132. doi: 10.21037/atm.2017.03.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao M, Lepak AJ, Andes DR. 2016. Animal models in the pharmacokinetic/pharmacodynamic evaluation of antimicrobial agents. Bioorg Med Chem 24:6390–6400. doi: 10.1016/j.bmc.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Rodvold KA, George JM, Yoo L. 2011. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents. Clin Pharmacokinet 50:637–664. doi: 10.2165/11594090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Boselli E, Breilh D, Rimmele T, Djabarouti S, Toutain J, Chassard D, Saux MC, Allaouchiche B. 2005. Pharmacokinetics and intrapulmonary concentrations of linezolid administered to critically ill patients with ventilator-associated pneumonia. Crit Care Med 33:1529–1533. doi: 10.1097/01.ccm.0000168206.59873.80. [DOI] [PubMed] [Google Scholar]
- 23.Gotfried MH, Shaw JP, Benton BM, Krause KM, Goldberg MR, Kitt MM, Barriere SL. 2008. Intrapulmonary distribution of intravenous telavancin in healthy subjects and effect of pulmonary surfactant on in vitro activities of telavancin and other antibiotics. Antimicrob Agents Chemother 52:92–97. doi: 10.1128/AAC.00875-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stalker DJ, Jungbluth GL. 2003. Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial. Clin Pharmacokinet 42:1129–1140. doi: 10.2165/00003088-200342130-00004. [DOI] [PubMed] [Google Scholar]
- 25.Anonymous. . 2018. Vibativ (telavancin) prescribing information. Cumberland Pharmaceuticals, Nashville, TN. [Google Scholar]
- 26.Kosowska-Shick K, Clark C, Pankuch GA, McGhee P, Dewasse B, Beachel L, Appelbaum PC. 2009. Activity of telavancin against staphylococci and enterococci determined by MIC and resistance selection studies. Antimicrob Agents Chemother 53:4217–4224. doi: 10.1128/AAC.00742-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel N, Lo A, Barriere S, Lodise T. 2017. Quantitative evaluation of telavancin exposure-response relationships among patients in the assessment of telavancin for treatment of hospital-acquired pneumonia (ATTAIN) trials, abstr AAID LB20. Abstr ASM Microbe 2017, New Orleans, LA. American Society for Microbiology, Washington, DC. [Google Scholar]
- 28.Kalil AC, Murthy MH, Hermsen ED, Neto FK, Sun J, Rupp ME. 2010. Linezolid versus vancomycin or teicoplanin for nosocomial pneumonia: a systematic review and meta-analysis. Crit Care Med 38:1802–1808. doi: 10.1097/CCM.0b013e3181eb3b96. [DOI] [PubMed] [Google Scholar]
- 29.Mendes RE, Sader HS, Flamm RK, Farrell DJ, Jones RN. 2015. Telavancin in vitro activity against a collection of methicillin-resistant Staphylococcus aureus isolates, including resistant subsets, from the United States. Antimicrob Agents Chemother 59:1811–1814. doi: 10.1128/AAC.04616-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Research Council. 1996. Guide for the care and use of laboratory animals. Publication no 85-23, revised 1996. National Academies Press, Washington, DC. [Google Scholar]
- 31.Martinez-Olondris P, Sibila O, Agusti C, Rigol M, Soy D, Esquinas C, Piner R, Luque N, Guerrero L, Quera MA, Marco F, de la Bellacasa JP, Ramirez J, Torres A. 2010. An experimental model of pneumonia induced by methicillin-resistant Staphylococcus aureus in ventilated piglets. Eur Respir J 36:901–906. doi: 10.1183/09031936.00176709. [DOI] [PubMed] [Google Scholar]
- 32.Sibila O, Luna CM, Agusti C, Baquero S, Gando S, Patron JR, Morato JG, Absi R, Bassi N, Torres A. 2008. Effects of glucocorticoids in ventilated piglets with severe pneumonia. Eur Respir J 32:1037–1046. doi: 10.1183/09031936.00009208. [DOI] [PubMed] [Google Scholar]
- 33.Luna CM, Baquero S, Gando S, Patron JR, Morato JG, Sibila O, Absi R, Famiglietti A, Vay CA, Von Stecher F, Agusti C, Torres A. 2007. Experimental severe Pseudomonas aeruginosa pneumonia and antibiotic therapy in piglets receiving mechanical ventilation. Chest 132:523–531. doi: 10.1378/chest.07-0185. [DOI] [PubMed] [Google Scholar]
- 34.Rennard SI, Basset G, Lecossier D, O’Donnell KM, Pinkston P, Martin PG, Crystal RG. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol 60:532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 35.Tobin CM, Sunderland J, White LO, MacGowan AP. 2001. A simple, isocratic high-performance liquid chromatography assay for linezolid in human serum. J Antimicrob Chemother 48:605–608. doi: 10.1093/jac/48.5.605. [DOI] [PubMed] [Google Scholar]
- 36.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467–476. doi: 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.