Ceftriaxone administered as once-daily high-dose short infusion combined with ampicillin has been proposed for the treatment of Enterococcus faecalis infective endocarditis in outpatient parenteral antibiotic therapy programs (OPAT). This combination requires synergistic activity, but the attainment of ceftriaxone synergic concentration (Cs) with the regimen proposed for OPAT has not been studied. This phase II pharmacokinetic study enrolled healthy adult volunteers who underwent two sequential treatment phases.
KEYWORDS: Enterococcus faecalis, endocarditis, ceftriaxone, pharmacokinetics, OPAT, combination treatment
ABSTRACT
Ceftriaxone administered as once-daily high-dose short infusion combined with ampicillin has been proposed for the treatment of Enterococcus faecalis infective endocarditis in outpatient parenteral antibiotic therapy programs (OPAT). This combination requires synergistic activity, but the attainment of ceftriaxone synergic concentration (Cs) with the regimen proposed for OPAT has not been studied. This phase II pharmacokinetic study enrolled healthy adult volunteers who underwent two sequential treatment phases. During phase A, volunteers received 2 g of ceftriaxone each 12 h during 24 h followed by a 7-day wash-out. Then the participants received phase B, which consisted of a single dose of 4 g of ceftriaxone. Throughout both phases, each volunteer underwent intensive pharmacokinetic (PK) sampling over 24 h. Ceftriaxone total and unbound concentrations were measured. Twelve participants were enrolled and completed both phases. Mean ceftriaxone total and free concentrations 24 h after the administration of 2 g each 12 h were 86.44 ± 25.90 mg/liter and 3.59 ± 1.35 mg/liter, respectively, and after the 4-g single dose were 34.60 ± 11.16 mg/liter and 1.40 ± 0.62 mg/liter, respectively. Only 3 (25%) patients in phase A maintained unbound plasma concentrations superior to the suggested Cs = 5 mg/liter during 24 h, and none (0%) in phase B. No grade 3 to 4 adverse events or laboratory abnormalities were observed. Ceftriaxone optimal exposure combined with ampicillin to achieve maximal synergistic activity against E. faecalis required for the treatment of infective endocarditis remains unknown. However, the administration of a single daily dose of 4 g of ceftriaxone implies a reduction in the time of exposure to the proposed Cs. (This study has been registered in the European Union Drug Regulating Authorities Clinical Trials [EudraCT] database under identifier 2017-003127-29.)
INTRODUCTION
Infective endocarditis (IE) is a relative uncommon life-threatening disease (1, 2). The overall IE incidence rate has remained stable in the past years, although the epidemiology concerning pathogens and patient characteristics has suffered important changes (3–5). Now, enterococcal endocarditis represents 10% of IE episodes and its incidence is growing. Enterococcus faecalis causes 90 to 97% of these infections (1, 6).
Due to the bacteriostatic effect of β-lactam antibiotics against enterococci, relapses after treatment are not infrequent (5, 7) and combination therapy is required to achieve optimal clinical outcomes (6). Aminoglycoside-based regimens combined with a cell-wall-active agent exhibit synergistic activity and have been recommended as a standard therapy (1, 2). The emergence and widespread dissemination of high-level aminoglycoside-resistant (HLAR) strains (8, 9), along with the increase in old and fragile patients with higher numbers of underlying diseases and increased risk of renal toxicity (1, 3, 5, 7), have motivated the investigation of new, less aggressive and nephrotoxic combination regimens (1, 10).
Dual β-lactam therapy has been suggested as an alternative combination therapy (6–9, 11). In particular, ampicillin plus ceftriaxone combination (AC) has been included as a preferential option in recent guidelines for HLAR and no-HLAR E. faecalis IE (1, 2) after showing promising results in large cohort studies in humans (8, 9, 11–13). The employed regimen consisted of 2 g of ceftriaxone given every 12 h plus 2 g of ampicillin given every 4 h and the results showed no differences in effectiveness compared with ampicillin plus gentamicin combination (AG), whereas AC was found less nephrotoxic (9). Dual β-lactam therapy is based on the synergistic effect between two β-lactam antibiotics against E. faecalis investigated in several preclinical studies (6, 14–18). Among dual β-lactam combination, AC is the combination most widely studied (15–18). Galvaldá et al. investigated ceftriaxone exposure required to obtain this effect and suggested that ceftriaxone synergistic concentration (Cs) in vitro was 5 to 10 mg per liter (mg/liter) for most of the isolates (15). Also, based on a humanized experimental model in rabbit, they hypothesized that the administration of a dosing regimen of 2 g each 12 h could efficiently achieve those plasma concentrations (9).
Due to the prolonged antibiotic therapy (4 to 6 weeks) required for E. faecalis EI treatment, AC adaptation for outpatient parenteral antibiotic therapy programs (OPAT) has been suggested for these patients, as risk of complications decreases after 10 to 21 days of hospitalization and, in clinically stable patients, therapy could be completed at home. Early discharge through OPAT is then an efficient and safe option which provides multiple and well-known benefits for both the patients and the health care system (19–22). For this purpose, it has been necessary to introduce subtle changes in the inpatient E. faecalis IE regimen. Particularly, 2 g of ampicillin had been administered each 4 h through an ambulatory programmable infusion pump and 4 g of ceftriaxone had been administered in once-daily short infusion by the nursing OPAT team. Four successfully treated patients had been reported with this scheme by our group, but further research in larger cohorts are warranted (12). Once-daily short infusion avoids OPAT team twice-daily visit or the use of two infusion pumps simultaneously. However, doses lower than 4 g presumably would lead to long periods where ceftriaxone concentrations would be under Cs, which might involve high risk of treatment failure (23), and higher doses are supported by scarce pharmacokinetic data (24).
Given the lack of information regarding ceftriaxone exposure in the AC regimen proposed for OPAT, an examination of ceftriaxone high once-daily dose PK profile seemed necessary. The purpose of this study was to document the PK of ceftriaxone in healthy volunteers receiving 4 g each 24 h and compare it with 2 g each 12 h in terms of minimum plasma concentrations and exposure achieved.
RESULTS
Twelve healthy volunteers were screened and included. All completed both PK phases. All participants were of European (Caucasian) ancestry and 5 participants were female (42%). Median age was 28 years (range 23 to 65), median weight was 71.8 kg (range 49.5 to 93.0), and median body mass index (BMI) was 26.1 kg/m2 (range 18.6 to 30.0).
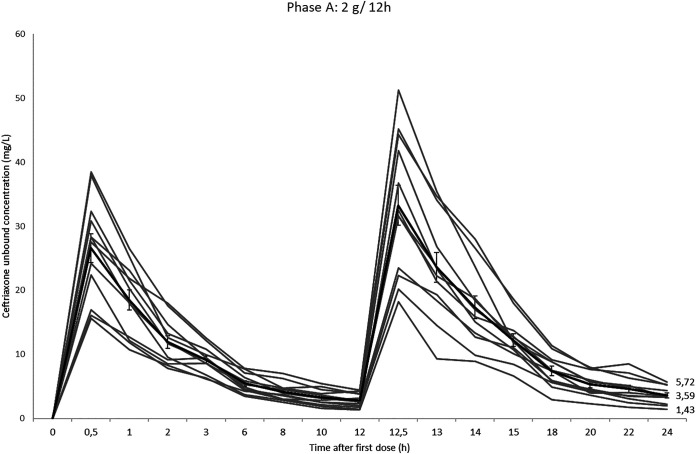
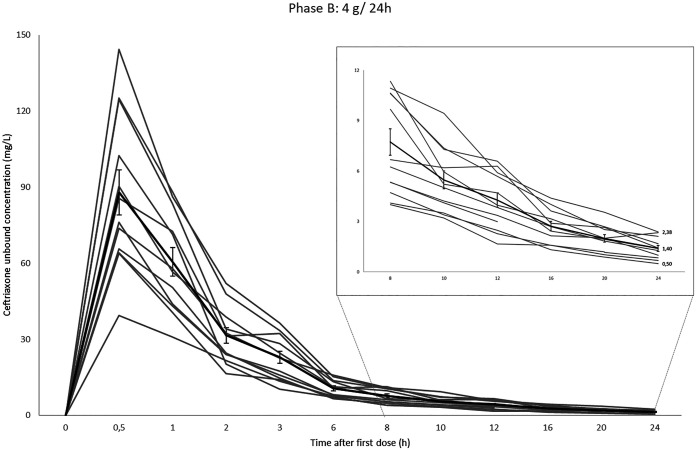
PK parameters for ceftriaxone when administered 2 g/12 h and 4 g/24 h are summarized in Table 1. Mean total concentration 24 h after the first dose (C24h) and mean unbound concentration 24 h after the first dose (uC24h) (geometric mean [GM] ± standard deviation [SD]) were 83.39 ± 25.90 mg/liter (range 47.98 to 135.73) and 3.59 ± 1.35 mg/liter (range 1.43 to 5.72) in phase A and 34.60 ± 11.16 mg/liter (range 18.50 to 51.07) and 1.40 ± 0.62 mg/liter (range 0.5 to 2.38) in phase B, respectively. Above all, ceftriaxone uC24h values higher than the Cs (17) were attained only by 3 patients after 2 g of twice-daily administration. Ninety percent of the volunteers achieved individual uC values similar to the Cs in all time points for 50% (6 h) and 33.3% (8 h) of the interval in phase A and B, respectively. After 20 h, none of the volunteers in phase B and 50% (6/12) in phase A had uC20h values higher than the Cs.
TABLE 1.
Phase A and B mean pharmacokinetic parameters
| PK parametera | Dose regimenb |
||||||
|---|---|---|---|---|---|---|---|
| Phase A: 2 g/12 h |
Phase B: 4 g/24 h |
||||||
| GM ± SD | Range | CV (%) | GM ± SD | Range | CV (%) | P value | |
| C24h (mg/liter) | 83.39 ± 25.90 | 47.98–135.73 | 31.1 | 34.60 ± 11.16 | 18.50–51.07 | 33.7 | <0.001 |
| uC24h (mg/liter) | 3.59 ± 1.35 | 1.43–5.72 | 37.64 | 1.40 ± 0.62 | 0.50–2.38 | 44.53 | <0.001 |
| Cmax (mg/liter) | 306.08 ± 43.93 | 248.63–394.34 | 14.4 | 417.46 ± 68.96 | 322.22–563.65 | 16.5 | <0.001 |
| uCmax (mg/liter) | 33.26 ± 10.82 | 18.24–51.21 | 32.54 | 87.99 ± 30.83 | 63.85–144.36 | 35.04 | <0.001 |
| AUC0–24 (mg · h/liter) | 3,319.6 ± 614.3 | 2,438.6–4,604.0 | 18.5 | 3,035.4 ± 573.3 | 2,056.4–4,306.6 | 18.9 | 0.266 |
| t1/2 (h) | 8.34 ± 2.87 | 4.58–13.52 | 34.4 | 8.19 ± 2.01 | 5.00–12.14 | 24.5 | 0.843 |
| kel (h−1) | 0.092 ± 0.031 | 0.051–0.151 | 33.4 | 0.089 ± 0.022 | 0.057–0.139 | 24.6 | 0.843 |
| CL (liters/h) | 0.97 ± 0.22 | 0.61–1.31 | 22.3 | 1.20 ± 0.26 | 0.82–1.79 | 21.5 | 0.028 |
| V (liters) | 10.91 ± 2.97 | 6.73–16.62 | 27.3 | 14.00 ± 3.90 | 9.35–23.66 | 27.9 | 0.043 |
| Normalized V (liters/kg) | 0.15 ± 0.02 | 0.12–0.20 | 15.5 | 0.20 ± 0.05 | 0.29–0.14 | 22.9 | 0.008 |
C24h, concentration 24 h after the first dose; uC24h, unbound concentration 24 h after the first dose; Cmax, maximum concentration; uCmax, unbound maximum concentration; AUC, area under the concentration-time curve; t1/2, half-life; kel, elimination rate constant; CL, clearance; V, distribution volume; normalized V, distribution volume normalized by weight to liters/kg.
GM, geometric mean; SD, standard deviation; CV, coefficient of variation.
The maximum drug concentrations (Cmax) were higher than those reported (25), ranging from 248.63 to 394.34 mg/liter for the 2 g/12 h phase and from 322.22 to 563.65 mg/liter for the 4 g/24 h phase. As expected, phase A C24h and uC24h mean values were necessarily higher than phase B C24h mean value (P < 0.001). However, ceftriaxone total exposure, measured by the area under the concentration-time curve from 0 to 24 h (AUC0–24), was similar in both phases and mean values were (GM ± SD) 3,319.6 ± 614.3 mg · h/liter in phase A versus 3,035.4 ± 573.3 mg · h/liter in phase B (P = 0.266). Ceftriaxone protein binding varied as a result of changes in total concentration. The mean percentage of protein binding 30 min after the administration of 4 g was 78.46%, and at the end of the study it increased to 95.83%. Mean protein binding when total concentration dropped under 100 mg/liter was (GM ± SD) 95.47 ± 1.14%. Mean ceftriaxone protein binding was 93.38 ± 2.15 and 91.40 ± 5.65 in phase A and B, respectively. Individual and mean ceftriaxone unbound plasma concentrations during phase A and B are shown in Fig. 1 and Fig. 2.
FIG 1.
Individual and median (thicker line) ceftriaxone phase A unbound concentration-time curves with standard error bars over 24 h.
FIG 2.
Individual and median (thicker line) ceftriaxone phase B unbound concentration-time curves with standard error bars over 24 h.
The studied drugs were well tolerated, with no grade 3 to 4 side effects. In total, 7 events were recorded, which were all grade 1 laboratory abnormalities without clinical significance. Drug-related side effects reported were: neutropenia (n = 4), serum creatinine increase (n = 2), and hyperbilirubinemia (n = 1).
DISCUSSION
In this cohort of healthy volunteers, we observed an uC24h after the administration of ceftriaxone at 4 g each 24 h and 2 g each 12 h lower than the proposed Cs for synergistic activity. Ceftriaxone volume of distribution (V), clearance (CL), and half-life (t1/2) were (GM ± SD) 14.00 ± 3.90 liters, 1.20 ± 0.26 liters/h, and 8.19 ± 2.01 h, respectively, when ceftriaxone was administered at 4 g once daily. The differences in ceftriaxone PK parameters between both regimens were expected and there was no variation in total exposure (AUC). Our results reveal that the concentration proposed to guarantee synergistic activity when coadministered with ampicillin for the treatment of E. faecalis IE was not achieved after the administration of ceftriaxone at the 4 g once-daily dose, but neither was it achieved with the gold standard regimen (2 g/12 h).
Based on the encouraging results provided by intrahospital E. faecalis IE series (8, 9, 11), AC is considered a first-option therapy (1, 2). This double β-lactam approach provides a favorable side effect profile and is equally effective as the previous gold standard therapy (1, 2). Also, the negligible cost compared with new antibiotic proposed (26) allows its use despite economic conditions and budget allocations. An attractive proposal for AC adaptation to OPAT has been suggested by Gil Navarro et al (12), with favorable results in a small cohort. However, our study shows that the proposed AC regimen of 4 g/24 h fails to maintain concentrations over the Cs during 24 h. It should be noted that, likewise, the intrahospital AC regimen of 2 g/12 h poorly attains the target proposed, despite the remarkable clinical outcomes reported (8, 9, 11). Several explanations could be hypothesized to explain the discrepancy between pharmacokinetic and clinical outcomes.
First, despite the inherent resistance of E. faecalis to ceftriaxone, synergistic activity of AC combinations against HLAR E. faecalis strains has been demonstrated in vitro, in experimental models and, finally, in patients. (8, 9, 11, 12, 15, 16). Suggested concentrations for ceftriaxone synergistic activity in vitro were 5 to 10 mg/liter. But how long the AC synergistic activity is required to be maintained to achieve the successful results seen in human studies is unknown. Due to ceftriaxone lack of activity, it could be theorized that ampicillin activity should be enhanced during the time necessary to maximize its activity. β-Lactams are time-dependent antibiotics and the efficacy is related to sustained drug concentration above the MIC during the dosing interval. Ampicillin maximal antimicrobial activity requires unbound drug concentrations higher than the MIC for 50 to 60% of the dosing interval (27, 28). Hence ceftriaxone attainment of synergistic concentrations through this period would lead to the greatest bactericidal effect for this combination. In our study, 90% of the patients accomplished this target only in phase A and, despite low uC24h values obtained, proper exposition after the administration of 2 g each 12 h might be considered, but not with the 4 g once-daily dose.
Second, an alternative explanation for the discordance might be based on the lack of studies testing the synergistic activity using lower ceftriaxone concentrations. Only one study (17) has tested concentrations below the initially established Cs. This study showed synergistic activity combining ampicillin at 1 μg/ml and ceftriaxone at 2 μg/ml, but not with ampicillin at 0.5 μg/ml and ceftriaxone at 1 μg/ml in one E. faecalis strain. These favorable results require confirmation in further studies involving different strains and more AC concentrations.
Finally, unknown mechanisms underlying the synergistic interaction between both antibiotics might be having a strong role in the clinical outcomes. For example, the prevention and eradication of biofilm formation may be involved. Thieme et al (29) pointed out that combinations of gentamicin or low concentrations of cephalosporins with ampicillin may be only advantageous for the treatment of persistent bacteremia (i.e., planktonic cells) in a nonmature biofilm. This might explain, in an alternative setting, the basis of the interaction and help us to define new pharmacokinetic targets.
Our study has clear limitations. Subjects were healthy volunteers and thus conclusions cannot be fully extrapolated to E. faecalis IE patients. However, the difficulties inherent in the low prevalence and the other characteristics of patients suffering from IE (i.e., frequently older and with several comorbidities), make it highly unlikely it will be possible to carry out clinical trials in this scenario. Host characteristics derived from age and the presence of comorbidities and increased fragility (i.e., renal or hepatic insufficiency) could lead to changes in pharmacokinetic and pharmacodynamic features. Indeed, ceftriaxone binding to plasma proteins is affected by variations in a patient’s physiopathology and saturation of the binding sites (30). The free AUCs in the geriatric patients were disproportionately higher than in the younger individuals because of the effects of aging on plasma protein binding (31). If the free fraction and protein-binding effects were predominant, the drug could be cleared more rapidly and have a shorter half-life than otherwise expected, and the overall AUC of this drug would be lower because drug was displaced. However, the effect of reduced creatinine clearance in the elderly, lowering the ability to excrete free drug, has been demonstrated to be predominant (31, 32), facilitating higher levels of ceftriaxone at 24 h that could fulfil the desired target.
In conclusion, we investigated the ability of once-daily high-dose ceftriaxone to achieve the suggested exposure needed to ensure the synergistic activity with ampicillin against E. faecalis in comparison with the clinically proven regimen for the treatment of infective endocarditis. We found that the administration of 4 g of ceftriaxone each 24 h implied a reduction in the time of exposure to the proposed Cs. The target exposure goal was not fully reached by either of the studied regimens, although ceftriaxone synergistic concentrations were achieved through 50 to 60% of the dosing interval for the majority of individuals with the two-dose scheme. Therefore, further studies are needed to define new targets for optimal ceftriaxone exposure and to identify others factors affecting clinical outcomes of EI E. faecalis patients.
MATERIALS AND METHODS
Study design and intervention.
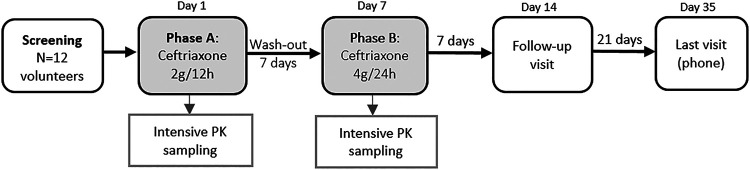
The study design is illustrated in Fig. 3. This was a Phase II, open-label, nonrandomized, crossover, PK study carried out at the Clinical Trial Unit of the Virgen del Rocio University Hospital, Seville, Spain.
FIG 3.
Study design.
At screening, participants had a clinical assessment and routine laboratory investigations were performed. After successful screening, eligible participants received two phases of treatment. Phase A consisted of the administration of 2 g of ceftriaxone infused each 12 h during 24 h, which was followed by a wash-out period (7 days). The same participants received phase B treatment consisting of the administration of a single daily dose of 4 g of ceftriaxone. All doses were infused over 30 min. Each volunteer underwent intensive PK sampling on both phases to measure ceftriaxone plasma concentrations. The first blood sample was taken just before the subject received each treatment phase at the research center (t = 0 h). Phase A samples were collected at fixed time points of 0.5, 1, 2, 3, 6, 8, 10, 12, 12.5, 13, 14, 15, 18, 20, 22, and 24 h and phase B samples at 0.5, 1, 2, 3, 6, 8, 10, 12, 16, 20, and 24 h, and the exact sampling times were recorded. Follow-up visits occurred on days 14 and 28 (phone visit). The day-14 visit included a clinical assessment and routine laboratory investigations. The safety and tolerability of study medications were evaluated throughout the trial (on treatment phases and at follow-up visits). All potentially related side effects were recorded.
Participants.
Eligible participants were healthy volunteers aged older than 18 years, weighing between 40 and 180 kg with a BMI lower than 34 kg/m2. Participants were excluded if they had any of the following: significant acute or chronic medical illness; an abnormal physical examination or clinical laboratory determination; a current infectious disease; treatment with cephalosporins in the 21 days before the trial; active treatment with any drug that could have interactions with ceftriaxone, including over-the-counter medications and herbal preparations; or previous allergy to any of the constituents of the pharmaceuticals administered during the trial. All participants provided written informed consent before their inclusion.
PK sample collection and quantification of plasma ceftriaxone.
Blood samples were collected into EDTA-containing blood tubes at each time point, immediately inverted several times, centrifuged 10 min at 3,000 × g, and then plasma was collected and stored at −80°C until analysis.
Total and unbound plasma concentrations of ceftriaxone were measured by a liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay using methods described elsewhere and adapted to measure unbound concentrations (33). The unbound fraction was isolated by ultrafiltration at 37°C with an Amicon Ultra-0.5 ml 30,000 molecular weight cutoff centrifugal filter device (Millipore, Cork, Ireland). Total and unbound standard curves were highly linear over the range of 5 to 1,000 μg/ml and 0.5 to 200 μg/ml, respectively. The lower limits of quantification were 3 and 0.5 μg/ml for total and unbound ceftriaxone, respectively. The accuracy and precision were 100% ± 15% and <15%, respectively. Validation of the method was performed per the Food and Drug Administration (FDA) guidelines, and the results met the acceptance criteria.
Pharmacokinetics analysis.
For the ceftriaxone PK profiles, the maximum concentration (Cmax) and the concentrations 24 h after the first dose with respect to total (C24h) and unbound (uC24h) ceftriaxone were the observed values. Mean percentage of protein binding was calculated for each time point. All PK parameters were calculated using actual blood sampling time and noncompartmental modeling techniques using the Excel add-in PKSolver from Zhang et al (34). The area under the plasma concentration-time curve from 0 to 24 h (AUC0–24) was calculated using the linear trapezoidal method. Interindividual variability in drug PK parameters and concentrations was expressed as a percentage coefficient of variation (CV, [SD/mean] × 100).
Statistical power.
Descriptive statistics were used to report volunteers’ clinical characteristics and ceftriaxone plasma PK parameters. Continuous variables were expressed as medians (range) or geometric means (GM ± SD), and categorical variables were expressed as numbers of cases and percentages. Drug PK values were calculated in each phase and compared between phases. Continuous variables were compared using the two-tailed t test or Mann-Whitney U test, as appropriate. Associations with P values of <0.05 were considered statistically significant.
Ethics.
This study was approved by the Ethics Committee for Clinical Research of the Virgen del Rocío University Hospital and the Spanish Agency of Medicines and Healthcare Product (AEMPS) and was conducted according to Good Clinical Practice and the Declaration of Helsinki. The study has been registered by the AEMPS, the European Medical Agency and ClinicalTrials.gov (EudraCT number 2017-003127-29).
ACKNOWLEDGMENTS
We would like to pay our gratitude and our respects to our colleague Juan Gálvez Acebal (“In memoriam”) for his contribution and dedication to the E. faecalis infective endocarditis study team and the “Grupo para el Estudio de las Infecciones Cardiovasculares de la Sociedad Andaluza de Enfermedades Infecciosas.” Also, we thank all volunteers and the Clinical Trials Unit of the Virgen del Rocío University Hospital for their dedication to this study.
This work was supported by the Plan Nacional de I+D+i 2013–2016 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía, Industria y Competitividad, Spanish Network for Research in Infectious Diseases (RD16/0016/0009 and RD16/0016/0001), cofinanced by the European Development Regional Fund.
L.F.L.-C. has received unrestricted research funding from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck Sharp, and Dohme (MSD), and ViiV Healthcare, and consultancy fees and lecture fees from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, and ViiV Healthcare, outside the submitted work. L.E.L.-C. has served as scientific advisor for Novartis, and as speaker for MSD, Pfizer, Angelini, and ViiV Healthcare, and has served as trainer for MSD. A.A. has served as scientific advisor for Angellini, Novartis, Roche, and Cook, and speaker for MSD, Pfizer, Angellini, Novartis, Roche, and ViiV Healthcare, and has served as trainer for MSD. The remaining authors have no conflicts of interest to declare.
L.H.-H. wrote the manuscript; M.V.G.-N., R.L.M., L.E.L.-C., A.A., and L.H.-H. designed the research; L.H.-H., M.V.G.-N., and R.L.M. conducted the research; L.H.-H. and A.G.-V. analyzed the data, L.F.L.-C. and A.G.-V. provided analytical tools and all authors reviewed and contributed to the final manuscript.
REFERENCES
- 1.Baddour LM, Wilson WR, Bayer AS, Fowler VG, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O'Gara P, Taubert KA, American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. 2015. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 132:1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 2.Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta J-P, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL, ESC Scientific Document Group. 2015. 2015 ESC Guidelines for the management of infective endocarditis. Eur Heart J 36:3075–3123. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 3.Gouriet F, Chaudet H, Gautret P, Pellegrin L, de Santi VP, Savini H, Texier G, Raoult D, Fournier PE. 2018. Endocarditis in the Mediterranean Basin. New Microbes New Infect 26:S43–S51. doi: 10.1016/j.nmni.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG, Bayer AS, Karchmer A, Olaison L, Pappas PA, Moreillon P, Chambers ST. 2009. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century. Arch Intern Med 169:463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pericàs JM, Llopis J, Muñoz P, Gálvez-Acebal J, Kestler M, Valerio M, Hernández-Meneses M, Goenaga MÁ, Cobo-Belaustegui M, Montejo M, Ojeda-Burgos G, Sousa-Regueiro MD, de Alarcón A, Ramos-Martínez A, Miró JM, Sánchez FF, Noureddine M, Rosas G, de la Torre Lima J, Blanco R, Boado MV, Campaña Lázaro M, Crespo A, Goikoetxea J, Iruretagoyena JR, Irurzun Zuazabal J, López-Soria L, Montejo M, Nieto J, Rodrigo D, Rodríguez R, Vitoria Y, Voces R, García López MV, Georgieva RI, Ojeda G, Rodríguez Bailón I, Ruiz Morales J, María Cuende A, Echeverría T, Fuerte A, Gaminde E, Goenaga MÁ, Idígoras P, Antonio Iribarren J, Izaguirre Yarza A, Kortajarena Urkola X, Reviejo C, Carrasco R, Climent V, GAMES Investigators, et al. 2020. A contemporary picture of enterococcal endocarditis. J Am Coll Cardiol 75:482–494. doi: 10.1016/j.jacc.2019.11.047. [DOI] [PubMed] [Google Scholar]
- 6.Beganovic M, Luther MK, Rice LB, Arias CA, Rybak MJ, Laplante KL. 2018. A review of combination antimicrobial therapy for Enterococcus faecalis bloodstream infections and infective endocarditis. Clin Infect Dis 67:303–309. doi: 10.1093/cid/ciy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Hidalgo N, Escolà-Vergé L, Pericas JM. 2020. Enterococcus faecalis endocarditis : what’s next ? Future Microbiol 15:349–364. doi: 10.2217/fmb-2019-0247. [DOI] [PubMed] [Google Scholar]
- 8.Gavalda J, Len O, Miro JM, Munoz P, Montejo M, Alarcon A, De la Torre-Cisneros JJ, Pena C, Martinez-Lacasa X, Sarria C, Bou G, Aguado JM, Navas E, Romeu J, Marco F, Torres C, Tornos P, Planes A, Falco V, Almirante B, Pahissa A. 2007. Brief communication: treatment of Enterococcus faecalis endocarditis with ampicillin plus ceftriaxone. Ann Intern Med 146:574–579. doi: 10.7326/0003-4819-146-8-200704170-00008. [DOI] [PubMed] [Google Scholar]
- 9.Fernández-Hidalgo N, Almirante B, Gavaldà J, Gurgui MMM, Peña C, De Alarcón A, Ruiz J, Vilacosta I, Montejo M, Vallejo N, López-Medrano F, Plata A, López J, Hidalgo-Tenorio C, Gálvez J, Sáez C, Lomas JM, Falcone M, De La Torre J, Martínez-Lacasa X, Pahissa A. 2013. Ampicillin plus ceftriaxone is as effective as ampicillin plus gentamicin for treating Enterococcus faecalis infective endocarditis. Clin Infect Dis 56:1261–1268. doi: 10.1093/cid/cit052. [DOI] [PubMed] [Google Scholar]
- 10.Ambrosioni J, Hernandez-Meneses M, Téllez A, Pericàs J, Falces C, Tolosana J, Vidal B, Almela M, Quintana E, Llopis J, Moreno A, Miro JM, Hospital Clinic Infective Endocarditis Investigators. 2017. The changing epidemiology of infective endocarditis in the twenty-first century. Curr Infect Dis Rep 19:21. doi: 10.1007/s11908-017-0574-9. [DOI] [PubMed] [Google Scholar]
- 11.Pericas JM, Cervera C, del Rio A, Moreno A, Garcia de la Maria C, Castañeda X, Armero Y, Gatell JM, Miro JM. 2014. Changes in the treatment of Enterococcus faecalis infective endocarditis in Spain in the last 15 years: from ampicillin plus gentamicin to ampicillin plus ceftriaxone. Clin Microbiol Infect 20:O1075–O1083. doi: 10.1111/1469-0691.12756. [DOI] [PubMed] [Google Scholar]
- 12.Gil-Navarro MV, Lopez-Cortes LE, Luque-Marquez R, Galvez-Acebal J, de Alarcon-Gonzalez A. 2018. Outpatient parenteral antimicrobial therapy in Enterococcus faecalis infective endocarditis. J Clin Pharm Ther 43:220–223. doi: 10.1111/jcpt.12635. [DOI] [PubMed] [Google Scholar]
- 13.El Rafei A, DeSimone DC, Narichania AD, Sohail MR, Vikram HR, Li Z, Steckelberg JM, Wilson WR, Baddour LM. 2018. Comparison of Dual β-Lactam therapy to penicillin-aminoglycoside combination in treatment of Enterococcus faecalis infective endocarditis. J Infect 77:398–404. doi: 10.1016/j.jinf.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Mainardi J, Gutmann L, Acar JF, Goldstein FW. 1995. Synergistic effect of amoxicillin and cefotaxime against Enterococcus faecalis. Antimicrob Agents Chemother 39:1984–1987. doi: 10.1128/AAC.39.12.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gavaldà J, Torres C, Tenorio C, Lopez P, Zaragoza M, Capdevila JA, Almirante B, Ruiz F, Borrell N, Gomis X, Pigrau C, Baquero F, Pahissa A. 1999. Efficacy of ampicillin plus ceftriaxone in treatment of experimental endocarditis due to Enterococcus faecalis strains highly resistant to aminoglycosides. Antimicrob Agents Chemother 43:639–646. doi: 10.1128/AAC.43.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavaldà J, Lopez Onrubia P, Gómez Martín MT, Gomis X, Ramirez JL, Len O, Rodríguez D, Crespo M, Ruíz I, Pahissa A. 2003. Efficacy of ampicillin combined with ceftriaxone and gentamicin in the treatment of experimental endocarditis due to Enterococcus faecalis with no high-level resistance to aminoglycosides. J Antimicrob Chemother 52:514–517. doi: 10.1093/jac/dkg360. [DOI] [PubMed] [Google Scholar]
- 17.Liao C-H, Huang Y-T, Tsai H-Y, Hsueh P-R. 2014. In vitro synergy of ampicillin with gentamicin, ceftriaxone and ciprofloxacin against Enterococcus faecalis. Int J Antimicrob Agents 44:85–86. doi: 10.1016/j.ijantimicag.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Lorenzo MP, Kidd JM, Jenkins SG, Nicolau DP, Housman ST. 2019. In vitro activity of ampicillin and ceftriaxone against ampicillin-susceptible Enterococcus faecium. J Antimicrob Chemother 74:2269–2273. doi: 10.1093/jac/dkz173. [DOI] [PubMed] [Google Scholar]
- 19.Candel FJ, Julián-Jiménez A, González J, Castillo -Del JF, González C. 2016. Current status in outpatient parenteral antimicrobial therapy: a practical view. Rev Esp Quimioter 29:55–68. [PubMed] [Google Scholar]
- 20.López-Cortés LE, Luque R, Cisneros JM, DOMUS Outpatient Antimicrobial Therapy Group. 2019. Next step, outpatient antimicrobial therapy programs as a tool of stewardship programs. Clin Infect Dis 68:2155. doi: 10.1093/cid/ciy1052. [DOI] [PubMed] [Google Scholar]
- 21.Shah AB, Norris AH. 2016. Handbook of outpatient parenteral antimicrobial therapy for infectious diseases, 3rd ed, CRG Publishing, a Division of The Curry Rockefeller Group, LLC, and the Infectious Diseases Society of America. [Google Scholar]
- 22.Pericà S JM, Llopis J, González-Ramallo V, Goenaga MÁ, Muñoz P, García-Leoni ME, Fariñas MC, Pajarón M, Ambrosioni J, Luque R, Goikoetxea J, Oteo JA, Carrizo E, Bodro M, Reguera-Iglesias JM, Navas E, Hidalgo-Tenorio C, Miró JM, Spanish Collaboration on Endocarditis-Grupo de Apoyo al Manejo de la Endocarditis Infecciosa en España (GAMES) investigators . 2019. Outpatient parenteral antibiotic treatment for infective endocarditis: a prospective cohort study from the GAMES cohort. Clin Infect Dis 69:1690–1700. doi: 10.1093/cid/ciz030. [DOI] [PubMed] [Google Scholar]
- 23.Pollock AA, Tee PE, Patel IH, Spicehandler J, Simberkoff MS, Rahal JJ. 1982. Pharmacokinetic characteristics of intravenous ceftriaxone in normal adults. Antimicrob Agents Chemother 22:816–823. doi: 10.1128/aac.22.5.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Turnier P, Navas D, Garot D, Guimard T, Bernard L, Tattevin P, Vandamme YM, Hoff J, Chiffoleau A, Dary M, Leclair-Visonneau L, Grégoire M, Pere M, Boutoille D, Sébille V, Dailly E, Asseray N, High-Dose Ceftriaxone CNS Infections Study Group. 2019. Tolerability of high-dose ceftriaxone in CNS infections: a prospective multicentre cohort study. J Antimicrob Chemother 74:1078–1085. doi: 10.1093/jac/dky553. [DOI] [PubMed] [Google Scholar]
- 25.Yuk JH, Nightingale CH, Quintiliani R. 1989. Drug disposition clinical pharmacokinetics of ceftriaxone. Clin Pharmacokinet 17:223–235. doi: 10.2165/00003088-198917040-00002. [DOI] [PubMed] [Google Scholar]
- 26.Gil-Navarro M, Herrera Hidalgo L, Guisado Gil A, Quintero García J, Pérez-Moreno M. 2018. Cost of antimicrobials (In Spanish). Guía PRIOAM 5–9. [Google Scholar]
- 27.Asín-Prieto E, Rodríguez-Gascón A, Isla A. 2015. Applications of the pharmacokinetic/pharmacodynamic (PK/PD) analysis of antimicrobial agents. J Infect Chemother 21:319–329. doi: 10.1016/j.jiac.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Onufrak NJ, Forrest A, Gonzalez D. 2016. Pharmacokinetic and pharmacodynamic principles of anti-infective dosing. Clin Ther 38:1930–1947. doi: 10.1016/j.clinthera.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thieme L, Klinger-Strobel M, Hartung A, Stein C, Makarewicz O, Pletz MW. 2018. In vitro synergism and anti-biofilm activity of ampicillin, gentamicin, ceftaroline and ceftriaxone against Enterococcus faecalis. J Antimicrob Chemother 73:1553–1561. doi: 10.1093/jac/dky051. [DOI] [PubMed] [Google Scholar]
- 30.Hayton WL, Stoeckel K. 1986. Age-associated changes in ceftriaxone pharmacokinetics. Clin Pharmacokinet 11:76–86. doi: 10.2165/00003088-198611010-00005. [DOI] [PubMed] [Google Scholar]
- 31.Luderer JR, Patel IH, Durkin J, Schneck DW, Hershey P, Nutley N. 1984. Age and ceftriaxone kinetics. Clin Pharmacol Ther 35:19–25. doi: 10.1038/clpt.1984.3. [DOI] [PubMed] [Google Scholar]
- 32.Perry TR, Schentag JJ. 2012. Clinical use of ceftriaxone: a pharmacokinetic-pharmacodynamic perspective on the impact of minimum inhibitory concentration and serum protein binding. Clin Pharmacokinet 40:685–694. doi: 10.2165/00003088-200140090-00004. [DOI] [PubMed] [Google Scholar]
- 33.Herrera-Hidalgo L, Gil-Navarro MV, Dilly Penchala S, López-Cortes LE, de Alarcón A, Luque-Márquez R, López-Cortes LF, Gutiérrez-Valencia A. 2020. Ceftriaxone pharmacokinetics by a sensitive and simple LC–MS/MS method: development and application. J Pharm Biomed Anal 189:113484. doi: 10.1016/j.jpba.2020.113484. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Huo M, Zhou J, Xie S. 2010. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed 99:306–314. doi: 10.1016/j.cmpb.2010.01.007. [DOI] [PubMed] [Google Scholar]