Invasive fungal diseases represent an unmet clinical need that could benefit from novel immunotherapeutic approaches. Host pattern recognition receptors (e.g., Toll-like receptors, C-type lectins, or scavenger receptors) that sense conserved fungal cell wall constituents may provide suitable immunotherapeutic antifungal agents. Thus, we explored the therapeutic potential of the lymphocyte class I scavenger receptor CD5, a nonredundant component of the antifungal host immune response that binds to fungal β-glucans.
KEYWORDS: scavenger receptors, CD5, immunotherapy, β-glucan, fungal infection, Candida albicans, Cryptococcus neoformans, fluconazole, antifungal therapy
ABSTRACT
Invasive fungal diseases represent an unmet clinical need that could benefit from novel immunotherapeutic approaches. Host pattern recognition receptors (e.g., Toll-like receptors, C-type lectins, or scavenger receptors) that sense conserved fungal cell wall constituents may provide suitable immunotherapeutic antifungal agents. Thus, we explored the therapeutic potential of the lymphocyte class I scavenger receptor CD5, a nonredundant component of the antifungal host immune response that binds to fungal β-glucans. Antifungal properties of the soluble ectodomain of human CD5 (shCD5) were assessed in vivo in experimental models of systemic fungal infection induced by pathogenic species (Candida albicans and Cryptococcus neoformans). In vitro mechanistic studies were performed by means of fungus-spleen cell cocultures. shCD5-induced survival of lethally infected mice was dose and time dependent and concomitant with reduced fungal load and increased leukocyte infiltration in the primary target organ. Additive effects were observed in vivo after shCD5 was combined with suboptimal doses of fluconazole. Ex vivo addition of shCD5 to fungus-spleen cell cocultures increased the release of proinflammatory cytokines involved in antifungal defense (tumor necrosis factor alpha and gamma interferon) and reduced the number of viable C. albicans organisms. The results prompt further exploration of the adjunctive therapeutic potential of shCD5 in severe invasive fungal diseases.
INTRODUCTION
The incidence and severity of invasive fungal diseases (IFDs) are especially high among individuals undergoing congenital or acquired immune-debilitating conditions (e.g., neutropenia, stem cell and solid-organ transplantation, corticotherapy, and AIDS) and in the setting of complex multisystem diseases (e.g., diabetes and chronic obstructive pulmonary disease) that are themselves associated with excess morbidity and mortality (1). No significant reductions of the morbidity and mortality of IFDs have been achieved, despite the available antifungals, as a result of inherent or acquired drug resistance, drug interactions, and significant drug-associated toxicities (2). This denotes the importance of developing new therapeutic strategies to improve the clinical outcome of patients undergoing IFDs.
The recent understanding of the molecular and cellular basis of antifungal immunity provides the groundwork for effective antifungal immunotherapies. Fungal recognition by the immune system relies on a series of soluble or membrane-bound receptors (e.g., C-type lectins, scavengers receptors, or Toll-like receptors) expressed by host innate and/or adaptive immune cells that could have immunotherapeutic use (3). This kind of receptor, collectively called pattern recognition receptors (PRRs), targets highly conserved and broadly distributed constituents of fungal cell walls, such as β-glucans (4). Previously reported PRRs for β-glucan include Dectin-1, CD23/FcεRII, complement receptor 3 (CR3 or CD11c/CD18b), ephrin type-A receptor 2 (EphA2), natural cytotoxicity receptor 3 (NCR3/NKp30), and the scavenger receptors SCARF1, CD36, and CD5 (5–11).
Recent work with knockout mice shows that CD5 is a nonredundant β-glucan receptor and an integral component of antifungal defense (12). CD5 is a 67-kDa transmembrane glycoprotein from the class I of scavenger receptors and was found expressed on all T cells, the small B1a cell subset, and certain macrophage (Mϕ) and dendritic cell (DC) subpopulations (13, 14). CD5’s structure consists of an extracellular region composed of three tandem scavenger receptor cysteine-rich (SRCR) domain repeats and an intracytoplasmic region adapted for intracellular signal transduction (14–16). This, together with its physical association with the clonotypic antigen-specific receptor complex, allows CD5 to negatively modulate T and B1a cell differentiation and activation processes (17, 18). To serve such a role, CD5 is presumed to interact with endogenous ligands of controversial nature (14, 16). Recent works showed that CD5 also serves as a PRR for different microbial-associated molecular patterns (MAMPs) from fungi, viruses, or parasites (11, 19, 20). It has been hypothesized that the dual immunomodulatory and PRR function of CD5 would (i) prevent autoimmunity by dampening the activation of low-affinity self-reactive clones and (ii) optimize anti-infectious responses by favoring the expansion of high-affinity non-self-reactive clones (21).
Proteolytic cleavage following lymphocyte activation yields a soluble form of human CD5 (shCD5), which is detected at low levels (picomolar range) in sera from healthy individuals and from patients undergoing inflammation-based disorders (22–25). Based on the immunomodulatory effects of transgenic shCD5 expression in experimental disease models, we speculated that shCD5 contributes to immunomodulation by acting as a decoy with CD5’s natural ligands (26, 27). We have presently explored the immunomodulatory effects of shCD5 infusion in mouse models of fungal infection after our observations that (i) shCD5 binds to and aggregates pathogenic and saprophytic fungal cells through recognition of β-1,3-glucans with an affinity (Kd) in the same range as that of Dectin-1 and (ii) prophylactic infusion of shCD5 protects mice from septic shock-like syndrome induced by zymosan, a non-infective β-glucan-rich particle (11). Our results show the modulatory effect of shCD5 infusion in fungal infections induced by pathogenic species (Candida albicans and Cryptococcus neoformans) and reveal the potential of CD5-based adjunctive immunotherapeutic strategies in IFDs.
RESULTS
Therapeutic effect of shCD5 administration in systemic C. albicans infection.
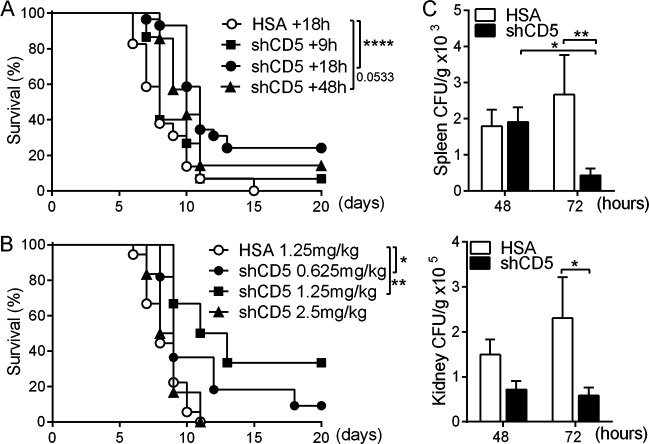
The prophylactic benefits of shCD5 administration in the zymosan-induced generalized inflammation (ZIGI) model led us to explore its therapeutic potential in systemic fungal infection (11). To this end, CD1 mice were intravenously (i.v.) infected with a lethal inoculum of C. albicans (3 × 103 CFU/g) according to previous reports and our own lethality assays (see Fig. S1A in the supplemental material) (28). As illustrated by Fig. 1A, time course experiments showed significant mouse survival following therapeutic infusion of single-dose shCD5 (1.25 mg/kg of body weight, i.v.) at 18 h postinfection. Parallel dose-response experiments also showed increased survival when single 0.625- or 1.25-mg/kg shCD5 doses were administered (i.v.) at 18 h postinfection (Fig. 1B). The benefit of single shCD5 administration (1.25 mg/kg) was maintained when a higher C. albicans inoculum (3 × 104 CFU/g) was used, although optimal survival was observed at an earlier time point (i.e., 9 h postchallenge) (Fig. S1B).
FIG 1.
Time- and dose-dependent effects of shCD5 infusion in C. albicans-infected CD1 mice. (A) Survival percentage over time of CD1 mice infected with C. albicans (3 × 103 CFU/g, i.v.) and treated (i.v.) with a single 1.25-mg/kg dose of HSA (+18 h, n = 29) or shCD5 (+9 h, n = 15; +18 h, n = 29; +48 h, n = 7) at the indicated time points (hours) postinfection. (B) Survival percentage of CD1 mice infected with C. albicans (3 × 103 CFU/g, i.v.) and treated (i.v.) with single-dose HSA (1.25 mg/kg, n = 18) or shCD5 (0.625 mg/kg, n = 11; 1.25 mg/kg, n = 6; 2.5 mg/kg, n = 6) 18 h later. (C) Spleen and kidney fungal burden (CFU/g) at 48 h and 72 h postinfection of CD1 mice with C. albicans (3 × 103 CFU/g, i.v.) and treated (i.v.) with a single 1.25-mg/kg dose of HSA (n = 7) or shCD5 (n = 7) 18 h later. Statistical differences between groups were analyzed by log rank (Mantel-Cox) test (A and B) and Mann-Whitney test (C).*, P ≤ 0.05; **, P ≤ 0.01; ****, P ≤ 0.0001.
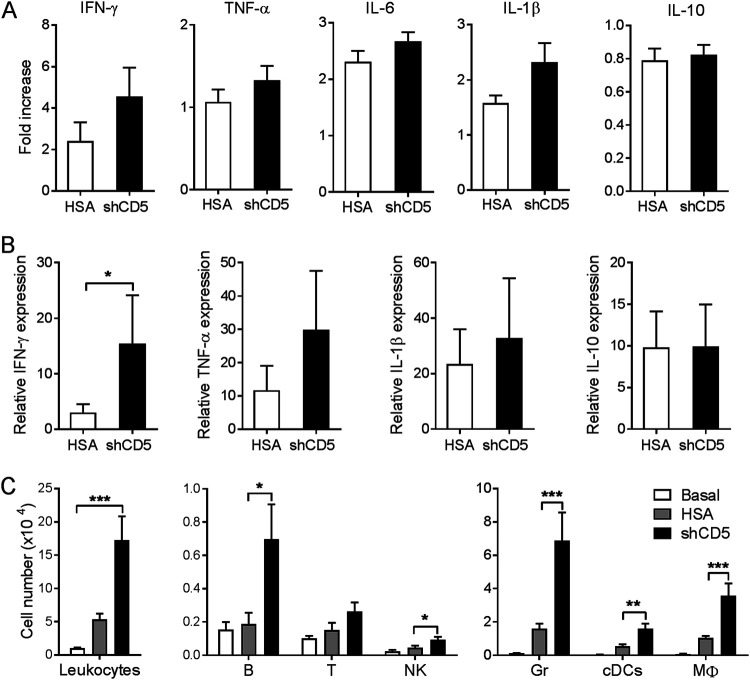
To correlate shCD5-induced survival to reduced fungal loads, the numbers of CFU in spleen and kidneys were assessed at 48 and 72 h after C. albicans infection. As shown in Fig. 1C, shCD5 infusion (1.25 mg/kg; at +18 h) of Candida-infected CD1 mice (3 × 103 CFU/g) significantly reduced CFU counts in both spleen and kidney at 72 h postinfection. Under the same experimental conditions, no differences were observed between control and shCD5-treated groups in serum levels of proinflammatory (gamma interferon [IFN-γ], tumor necrosis factor alpha [TNF-α], interleukin-6 [IL-6], and IL-1β) and anti-inflammatory (IL-10) cytokines (Fig. S2A) as well as numbers of spleen lymphoid (T, B, and NK) and myeloid (granulocyte [Gr], conventional dendritic cell [cDC], and Mϕ) cell subpopulations (Fig. S2B). When similar cytokine analyses were performed in kidney, a primary target organ for C. albicans, again no difference was observed between treatment groups (Fig. 2A). However, kidney cytokine mRNA analyses showed increased IFN-γ expression in shCD5-treated mice compared with that of the controls (Fig. 2B). Moreover, the analysis of kidney leukocyte infiltrates revealed that shCD5-treated mice presented significantly increased total leukocyte cell counts compared to those of controls (Fig. 2C). A more detailed assessment revealed that the increased infiltration involved most of the lymphoid (B and NK) and myeloid (Gr, cDC, and Mϕ) cell types analyzed (Fig. 2C).
FIG 2.
Effect of shCD5 treatment on kidney cytokine and leukocyte infiltration levels of C. albicans-infected CD1 mice. (A) Kidney cytokine levels at 72 h postinfection of CD1 mice infected with C. albicans (3 × 103 CFU/g, i.v.) and treated (i.v.) with a single 1.25-mg/kg dose of HSA (n = 14) or shCD5 (n = 14) 18 h later. Represented are fold inductions with regard to noninfected controls (basal, n = 4). (B) Kidney relative cytokine mRNA expression at 48 h postinfection of CD1 mice infected with C. albicans (3 × 103 CFU/g, i.v.) and treated (i.v.) with a single 1.25-mg/kg dose of HSA (n = 9) or shCD5 (n = 7) 18 h later. Represented is the relative expression with regard to noninfected controls (basal, n = 4). (C) Total leukocyte (CD45+), B (B220+ CD3−), T (CD3+ B220−), natural killer (NK; NK1.1+ CD3−), granulocyte (Gr; Gr-1+ SSChi), conventional dendritic (cDC; CD11c+ B220−), and macrophage (Mϕ; CD11c− Gr-1low SSClow) cell numbers assessed by flow cytometry from the same mice as those depicted in panel A. Statistical differences between groups were analyzed by Mann-Whitney test or Kruskal-Wallis and Dunn’s test. *, P ≤ 0.05; **, P ≤ 0.01.
Therapeutic effect of shCD5 administration in C. neoformans infection.
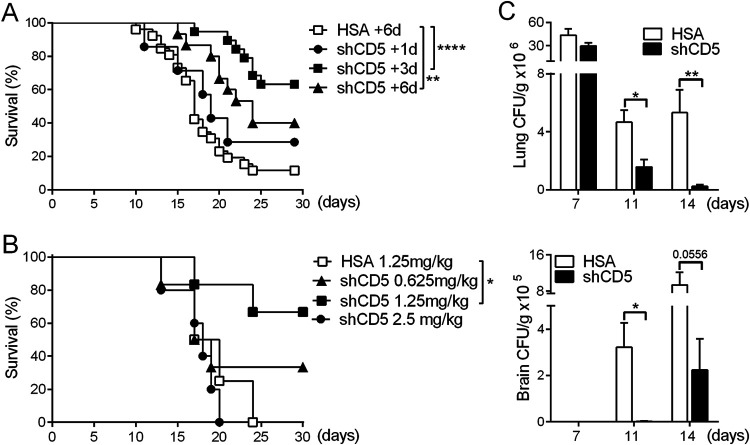
The ability of CD5 to bind to β-1,3-glucan, a broadly distributed fungal MAMP, led us to investigate the possible benefit of shCD5 administration in systemic infections caused by other fungal species (11). To this end, a mouse model of cryptococcosis was used, where CD1 mice are intranasally (i.n.) instilled with C. neoformans (3 × 104 CFU/g) (29, 30). As illustrated by Fig. 3A, time course experiments showed that i.v. infusion of a single 1.25-mg/kg shCD5 dose increased survival rates at all times tested, reaching statistical significance at days 3 and 6 postinfection. Parallel dose-response assays performed at day 3 postinfection showed maximal survival with a single 1.25-mg/kg shCD5 dose (Fig. 3B). Under the optimal therapeutic conditions found (1.25 mg/kg shCD5 at day 3 postinfection), significant reductions in CFU counts were observed at days 11 and 14 postinfection in lung and brain, the main two organs targeted by C. neoformans (Fig. 3C).
FIG 3.
Time- and dose-dependent effects of shCD5 infusion in C. neoformans-infected CD1 mice. (A) Survival percentage over time of CD1 mice infected with C. neoformans (3 × 104 CFU/g, i.n.) and treated (i.v.) with a single 1.25-mg/kg dose of HSA (+6 days, n = 22) or shCD5 (+1 days, n = 7; +3 days, n = 19; +6 days, n = 8) at different time points (days) postinfection. (B) Survival percentage over time of CD1 mice infected with C. neoformans (3 × 104 CFU/g, i.n.) and treated (i.v.) with single-dose HSA (1.25 mg/kg, n = 4) or shCD5 (0.625 mg/kg, n = 6; 1.25 mg/kg, n = 6; 2.5 mg/kg, n = 5) 3 days later. (C) Lung and brain fungal burdens at days 7, 11, and 14 postinfection of CD1 mice with C. neoformans (3 × 104 CFU/g, i.n.) and treated (i.v.) with a single 1.25-mg/kg dose of HSA (n = 5) or shCD5 (n = 5) 3 days later. Statistical differences between groups were analyzed by log-rank (Mantel-Cox) test (A and B) or Mann-Whitney test (C). *, P ≤ 0.05; **, P ≤ 0.01; ****, P ≤ 0.0001.
Ex vivo analysis of shCD5-mediated antifungal effects.
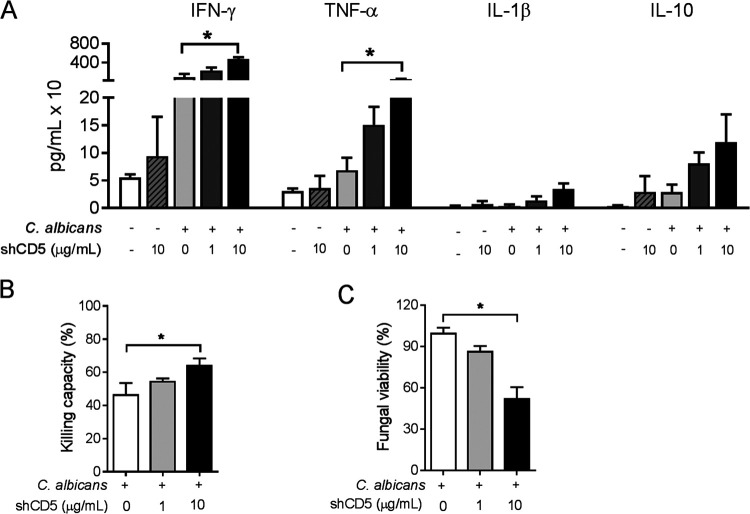
To further understand the shCD5-mediated effects in fungal infection, ex vivo mechanistic studies were performed next with CD1 splenocytes (5 × 106 cells/ml) cocultured for 24 h with heat-killed C. albicans (0.5 × 106 CFU/ml) and shCD5 (1 or 10 μg/ml). As illustrated by Fig. 4A, the assessment of proinflammatory (IFN-γ, TNF-α, and IL-1β) and anti-inflammatory (IL-10) cytokine levels in coculture supernatants showed shCD5-induced dose-dependent increases in IFN-γ and TNF-α production.
FIG 4.
Ex vivo analysis of shCD5-mediated antifungal effects. (A) Cytokine levels in 24-h culture supernatants from CD1 splenocytes (5 × 106 cells/ml) left alone or cocultured with heat-killed C. albicans (0.5 × 106 CFU/ml) in the presence of vehicle or shCD5 (1 or 10 μg/ml). Results are the means ± SEM from n = 5 mice. (B) Percentage of killed C. albicans following 2 h of cocultivation of total splenocytes (106 cells/ml) from CD1 mice with live C. albicans conidia (0.5 × 106 CFU/ml) in the presence of vehicle or shCD5 (1 or 10 μg/ml). Results are the means ± SEM from n = 5 mice. (C) Percentage of viable fungal cells following exposure of live C. albicans conidia (0.5 × 106 CFU/ml) to vehicle or shCD5 (1 or 10 μg/ml) for 2 h. Results are the means ± standard deviations of CFU triplicates. Statistical differences between groups were analyzed by Kruskal-Wallis and Dunn’s test. *, P ≤ 0.05.
Fungal cell killing is an established mechanism for pathogen clearance following recognition by PRRs (3). Thus, it was further investigated whether shCD5 exerted any effect (either direct or indirect) in fungal viability. As shown in Fig. 4B, the addition of shCD5 to cocultures of live C. albicans conidia (0.5 × 106 CFU/ml) with CD1 splenocytes (106 cells/ml) increased fungal killing in a dose-dependent manner. Next, direct effects of shCD5 on fungal viability were assessed by culturing live C. albicans conidia (0.5 × 106 CFU/ml) for 2 h in the presence of increasing amounts of shCD5. As illustrated by Fig. 4C, a dose-dependent reduction of C. albicans viability was directly induced by shCD5.
The assessment of whether direct effects of shCD5 on C. albicans viability observed ex vivo were solely responsible for the beneficial effects observed in in vivo models of C. albicans was further analyzed using immunodeficient NSG (NOD-scid IL-2Rgnull) mice, which lack mature T, B, and NK cells, together with defective function of DC (defective IFN-γ production when cocultured with Listeria monocytogenes antigen) and Mϕ (impaired lipopolysaccharide-induced IL-1 production) (31). To that end, C. albicans inoculum (3 × 102 CFU/g) was adjusted to achieve lethality similar to that induced in immunocompetent CD1 mice (100% lethality at 8 to 12 days postinfection) (Fig. S3). Under these experimental conditions, shCD5 treatment (1.25 mg/kg at 18 h postinfection) did not induce a significant increase in NSG mouse survival (Fig. 5).
FIG 5.
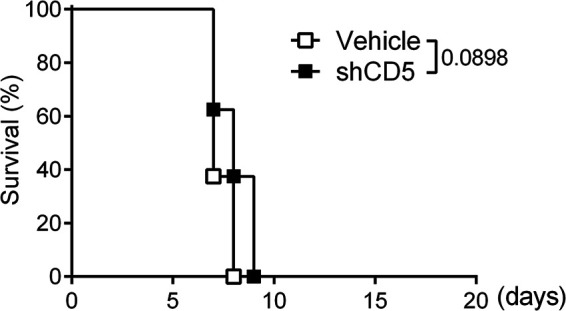
Efficacy of shCD5 infusion in C. albicans-infected immunodeficient NSG mice. Shown is the survival percentage over time of NSG mice infected with a lethal inoculum of C. albicans (3 × 102 CFU/g, i.v.) and treated (i.v.) with vehicle (n = 8) or shCD5 (1.25 mg/kg; n = 8) 18 h later. Statistical differences between groups were analyzed by log rank (Mantel-Cox) test.
Additive therapeutic effects of shCD5 plus fluconazole in C. albicans-induced infection.
Azoles, together with echinocandins and polyenes, are current first-line options for the therapeutic management of systemic fungal infections. Azoles, such as fluconazole (FLC), are cytochrome demethylase system inhibitors, which ultimately inhibit fungal growth without interfering with β-glucan biosynthesis (32). Thus, we tested whether C. albicans-infected mice could benefit from combined FLC plus shCD5 therapy. Based on previous reports (33) and our own FLC dose-response assays (Fig. S4), CD1 mice lethally challenged with C. albicans (3 × 103 CFU/g, i.v.) were treated daily for 1 week (starting at 48 h postinfection) with a suboptimal FLC dose (1 mg/kg, intraperitoneal [i.p.]) alone or in combination with a single shCD5 dose (1.25 mg/kg, i.v.) given at 18 h postinfection. As shown in Fig. 6, the combination of FLC and shCD5 therapies was additive, and survival improved from 40% (FLC) to 80% (FLC plus shCD5).
FIG 6.
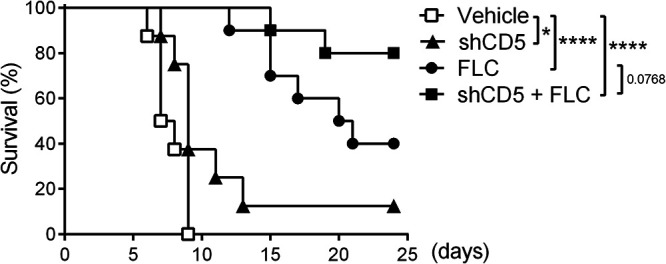
Effect of combined fluconazole plus shCD5 therapy in C. albicans-infected CD1 mice. Shown is the survival percentage over time of CD1 mice infected with C. albicans (3 × 103 CFU/g, i.v.) and either left untreated (vehicle; n = 8) or treated with daily FLC doses (1 mg/kg, i.p.; n = 10) for 1 week (starting at 48 h postinfection), with single-dose shCD5 (1.25 mg/kg, i.v.; n = 8) given at 18 h postinfection, or with a combination of the latter two (shCD5 + FLC; n = 10). Statistical differences between treated and control groups were analyzed by log rank (Mantel-Cox) test. *, P ≤ 0.05; ****, P ≤ 0.0001.
DISCUSSION
The phylogenetic relatedness of mammalian and fungal cells poses restrictions on new antifungal drug developments. This stresses the importance of exploring the host’s own immune response components that may help fight IFDs, resembling immunotherapy strategies in cancer. Research focused on understanding the molecular and cellular basis of antifungal immunity has expanded in recent decades and provides the groundwork for effective antifungal immunotherapies (3, 34, 35). A potential source of such therapies is PRRs involved in sensing fungus-associated molecular patterns (e.g., Toll-like receptors, C-type lectins, and scavenger receptors) and further activation of innate and adaptive immune responses (3). We provide proof-of-concept data on the therapeutic potential of a soluble form of the human class I lymphocytic scavenger receptor CD5 in experimental models of systemic fungal infections by C. albicans and C. neoformans, two of the main species causing IFDs.
Previous work from our group on knockout mice supports CD5 as a nonredundant component of antifungal immune responses, largely due to the ability of its extracellular region to interact with β-1,3-glucans with affinity (Kd) similar to that of Dectin-1, one of the main mammalian β-glucan receptors (11, 12). In vivo benefits of targeting CD5 in IFDs were first evidenced by the prophylactic effects of shCD5 in mice undergoing zymosan-induced generalized inflammation (ZIGI), an experimental model for fungus-induced multiple-organ dysfunction syndrome (MODS) (11, 36). This aseptic inflammation model features only part of the pathophysiology of systemic fungal infections. Our data on C. albicans and C. neoformans-infected CD1 mice reveal the potential of shCD5 in the treatment of life-threatening invasive mycoses. The wide distribution of β-glucans among the fungal phyla, together with the therapeutic effects observed for two different pathogenic fungal species, supports a broad antifungal spectrum for shCD5 and warrants further investigations on other clinically relevant fungal pathogens (e.g., Aspergillus fumigatus).
As for other antifungal agents, the therapeutic effects of shCD5 in lethally infected mice were time and dose dependent (2). Lower mortality of shCD5-treated mice correlated with lower fungal loads, increased IFN-γ mRNA levels, and increased infiltration of leukocytes and target organs (i.e., kidney for C. albicans). The leukocyte infiltrate was increased at the expense of some lymphoid subsets (NK and B, but not T, cells), mainly in involved myeloid cells (cDC, Mϕ, and Gr) with phagocytic activity and deeply involved in the surveillance and elimination of fungal pathogens (37).
Ex vivo analyses showed that the addition of shCD5 to unfractionated CD1 splenocytes cocultured with heat-killed C. albicans increased the production of IFN-γ and TNF-α, two proinflammatory cytokines involved in the activation of antifungal function of macrophages and neutrophils (38). Moreover, shCD5 addition also increased the fungal killing competence of CD1 splenocytes cocultured with viable C. albicans conidia. Such increased fungal killing results, at least in part, from direct effects of shCD5 on C. albicans viability by a mechanism(s) still to be disclosed. However, the lack of shCD5 efficacy in the C. albicans-infected immunodeficient NSG mice supports the notion that an intact immune system is necessary for optimal survival following shCD5 infusion. It is worth mentioning that shCD5 could neither increase the killing capacity of CD1 splenocytes against C. neoformans nor reduce the viability of C. neoformans conidia in vitro (see Fig. S5 in the supplemental material). This may relate to surface composition differences between C. albicans and C. neoformans, where the latter exhibits relatively lower abundance of β-1,3-glucans at the cell wall and a thick exopolysaccharide capsule of glucuronoxylomannan and galactoxylomannan, which masks the inner β-glucan layer (39). Nevertheless, shCD5 efficacy in the mouse model of cryptococcosis suggests other shCD5-mediated mechanisms operating in vivo. We speculate that shCD5 binds to other fungal components, according to the multiligand properties of scavenger receptors in general, including CD5 (19, 20).
The antifungal therapeutic effects we report for shCD5 resemble those of other soluble PRRs, such as galectin 3 (Gal-3) or pentraxin 3 (PTX3). Gal-3 is a soluble lectin known to bind to galactomannans and α- and β-mannans and is active in the immune response against C. albicans and C. neoformans (40, 41). Exogenous Gal-3 increases the in vitro phagocytic activity of neutrophils against C. albicans and C. parapsilosis and exerts a direct lytic effect on C. neoformans extracellular vesicles, while endogenous Gal-3 expression is necessary for TNF-α production by macrophages exposed to C. albicans (40–43). Likewise, the long pentraxin PTX3 binds to galactomannan and is involved in the immune response against A. fumigatus and Paracoccidioides brasiliensis (44). Accordingly, exogenous PTX3 reduces mortality and fungal burden in experimental aspergillosis and increases the phagocytic activity of macrophages against P. brasiliensis (45–47).
Mechanistically, the shCD5-mediated antifungal effects in vivo may result from a combination of its different properties. shCD5 binds to and agglutinates fungal cells (11). Agglutination is a simple innate defensive mechanism used by soluble PRRs to prevent free microbial spread and/or to facilitate phagocytosis. The relevance of particle size in phagocytosis is well recognized. Studies with polystyrene microspheres report maximal phagocytosis by mouse/rat macrophages for an intermediate particle size (2 to 3 μm) (48). Individual conidia are usually within such a size range. On this basis, it is unlikely that shCD5-induced agglutination could improve phagocytosis. The direct effects of shCD5 on fungal viability we report for C. albicans (but not C. neoformans) (Fig. 4C and Fig. S3) also may play a role. Whether the binding of shCD5 to fungal cells would also facilitate interactions with phagocytic/cytotoxic cells and/or production of proinflammatory cytokines (TNF-α and IFN-γ) by immune cells in a CD5 ligand-driven manner is also unknown. The elusive identification of a bona fide endogenous cell-bound CD5 ligand(s) (14, 16) may be indicative of the low affinity of such CD5-ligand interaction(s), contradicting a significant contribution. On the other hand, it is known that membrane-bound CD5 acts as a negative modulator of T and B1a cell differentiation and activation responses (17, 18). Thus, shCD5 binding to fungal MAMPs (namely, β-1,3-glucans) could prevent putative inhibitory effects of T and/or B1a cell-mediated antifungal responses resulting from fungal MAMP ligation of membrane-bound CD5 (decoy effect). Moreover, previous studies from our group demonstrate that shCD5 (either transgenically expressed or exogenously infused) potentiates antitumor and autoimmune responses by reducing the frequency of regulatory T and B cells, and this could also be helpful in early phases of fungal infection (26, 27).
Finally, we explored the use of shCD5 as an adjunctive antifungal immunotherapy to currently available antimycotic drugs, such as FLC, at suboptimal doses (10-fold lower than the optimal 10 mg/kg/day for a period of 7 days) (33). FLC, a first-line treatment for several fungal infections (including invasive candidiasis), does not interfere with β-glucan biosynthesis (32). Under these conditions, survival rates of C. albicans-infected CD1 mice treated with FLC increased from 40% to 80% when combined with shCD5. These results support the use of shCD5 as adjunctive immunotherapy to reduce the adverse effects associated with current antimycotic drugs without compromising their efficacy and, at the same time, expand their antifungal spectrum.
MATERIALS AND METHODS
Recombinant proteins.
The production and purification of recombinant shCD5 was carried out as previously described (49). Briefly, protein-free culture supernatants from SURECHO-M cell transfectants stably expressing shCD5 were generated by Selexis (Geneva, Switzerland) and further subjected to size exclusion chromatography protocols, available from PX’Therapeutics (Grenoble, France). Purified shCD5 protein (>90% purity) was stored at −80°C in phosphate-buffered saline (PBS) plus 10% glycerol, pH 7.4, until used. Human serum albumin (HSA) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Mouse models of fungal sepsis.
Male, 8- to 10-week-old CD1 (ICR) and NOD-scid IL-2Rgnull (NSG) mice were purchased from Charles River Laboratories (France). All of the procedures were approved by the Animal Experimentation Ethical Committee of the University of Barcelona (CEEA 82/16 and 315/16). C. albicans (SC5314; ATCC MYA‐2876) infection was performed by intravenous (i.v.) injection of 3 × 102 to 3 × 104 CFU/g. Fungal infection induced by C. neoformans H99 was performed by intranasal (i.n.) instillation of 3 × 104 CFU/g into mice anesthetized with ketamine (100 mg/kg; Ketamidor) plus xylazine (10 mg/kg; Rompun) (50). When indicated, single-dose HSA (1.25 mg/kg, i.v.) or shCD5 (0.625 to 2.5 mg/kg, i.v.) and daily fluconazole (0.1 to 10 mg/kg, i.p.; B/Braun) were administered at different time points. Mouse survival and body weight loss were monitored daily.
Fungal load assessment.
At the desired time point postinfection, organs were aseptically removed, weighed, and homogenized in PBS using 40-μm cell strainers (Biologix). Serial dilutions of the homogenates were plated on Sabouraud’s dextrose agar (SDA; Conda), and the number of CFU per gram of organ was counted after 48 h of incubation at 30°C.
Serum and tissue cytokine measurements.
Blood samples obtained by cardiac puncture were kept on ice until centrifugation (2,000 rpm) for 10 min at 4°C for serum recovery and storage at −80°C until used. Kidney samples were homogenized with a tissue disrupter (Ultra-Turrax T20; IKA) in PBS with cOmplete protease inhibitor cocktail (Roche). After centrifugation at 12,000 × g for 10 min at 4°C, the supernatant was recovered and stored at –80°C until used. Mouse IL-6, IFN-γ, TNF-α, IL-1β, and IL-10 cytokine levels were determined by using commercially available enzyme-linked immunosorbent assay (ELISA) kits (BD OptEIA; BD Biosciences Pharmingen) by following the manufacturer's instructions.
qRT-PCR analyses.
Total kidney RNA samples (0.5 μg) isolated by using the PureLink RNA minikit (Ambion, Life Technologies) were retrotranscribed into cDNA by using the high-capacity cDNA kit (Life Technologies). Cytokine mRNA levels were assessed by quantitative real-time PCR (qRT-PCR) with TaqMan fast universal PCR master mix (Life Technologies) using a 7900HT fast real-time PCR system (Applied Biosystems) and the following TaqMan probes (Thermo Fisher): IFN-γ (Mm00801778_m1), TNF-α (Mm00443260_m1), IL-1β (Mm00434228_m1), IL-10 (Mm00439614_m1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Mm99999915_g1). Cytokine mRNA expression was adjusted by GAPDH expression as 2ΔΔCT, where ΔΔCT = (CTgene of interest sample − CTGAPDH sample) − (CTgene of interest basal – CTGAPDH basal).
Analysis of tissue leukocyte infiltrates.
Whole spleen and kidneys were digested for 20 min at 37°C in PBS containing collagenase D (0.5 mg/ml for kidneys and 1 mg/ml for spleens; Roche) and DNase I (0.1 mg/ml; Roche). Following disaggregation through 40-μm cell strainers, cell suspensions were washed twice with PBS plus 2% fetal bovine serum (FBS; BioWest), and erythrocytes were lysed with 4 ml of red blood cell lysis solution buffer (RBC; eBioscience) for 4 min at room temperature. After a second wash, cells were counted and adjusted to 107 cells/ml in PBS plus 2% FBS. Samples (106 cells) then were incubated for 15 min at room temperature in blocking solution (PBS plus 2% FBS and anti-mouse CD16/CD32) prior to staining with the following monoclonal antibodies (MAbs): phycoerythrin (PE)-labeled anti-NK1.1 (PK136; BD Biosciences) and anti-CD11b (M1/70; TONBO), allophycocyanin (APC)-labeled anti-Gr-1 (RB6-8C5; TONBO) and anti-CD3 (145-2C11; TONBO), fluorescein isothiocyanate (FITC)-labeled anti-CD45 (30-F11; BioLegend), peridinin chlorophyll protein-Cy5.5-labeled anti-CD11c (N418; TONBO), and violetFluor 450-labeled anti-B220 (RA3-6B2; TONBO). A LIVE/DEAD fixable near-IR dead cell stain kit (Invitrogen) was used to exclude dead cells. Stained cells were analyzed in a FACS Canto II flow cytometer (Becton, Dickinson, USA) and the data processed with Flow Jo software (Tree Star, USA).
Ex vivo C. albicans and spleen cell cocultures.
CD1 splenocytes (5 × 106 cells/ml) were suspended in RPMI 1640 medium with l-glutamine plus 10% FBS and 50 μM 2-mercaptoethanol (2-ME) and then cocultured for 24 h at 37°C and 5% CO2 with heat-killed C. albicans (0.5 × 106 CFU/ml) in 96-well plates in the presence of shCD5 (1 or 10 μg/ml) or vehicle (PBS plus 10% glycerol). Heat-killed C. albicans was prepared by incubation for 30 min at 100°C. Mouse IFN-γ, TNF-α, IL-1β, and IL-10 cytokine levels in culture supernatants were measured using commercially available ELISA kits (BD OptEIA; BD Biosciences Pharmingen).
For fungal cell killing assays, CD1 splenocytes (106 cells/ml) were suspended in RPMI 1640 medium with l-glutamine plus 10% FBS and 50 μM 2-ME and cocultured for 2 h at 37°C and 5% CO2 in 96-well plates with live C. albicans (0.5 × 106 cells/ml) in the presence of shCD5 (1 or 10 μg/ml) or vehicle. The cells then were lysed with water, and the number of viable CFU was assessed by seeding and subsequent incubation for 48 h at 30°C on SDA plates. Killing activity was calculated as the percentage of nonviable CFU in the presence of splenocytes with regard to nonviable CFU in the absence of cells.
Fungal viability assays.
C. albicans (0.5 × 106/ml) cells were suspended in RPMI 1640 medium with l-glutamine plus 10% FBS and 50 μM 2-ME and cultured for 2 h at 37°C in 96-well plates in the presence of shCD5 (1 or 10 μg/ml) or vehicle. Fungal serial dilutions then were seeded on SDA plates and incubated for 48 h at 30°C for CFU assessment. Viability was calculated as the percentage of CFU in the presence of shCD5 with regard to viable CFU in the absence of the protein.
Statistical analyses.
GraphPad Prism 6 software was used for statistical comparisons (GraphPad Software Inc., San Diego, CA). Statistical parameters, including the exact value of n, precision measures (means ± standard errors of the means [SEM]), and statistical test and significance are reported in the figure legends. Data are judged to be statistically significant when P values are ≤0.05.
Supplementary Material
ACKNOWLEDGMENTS
We thank Marcos Isamat for critically reviewing and editing the manuscript.
M.V.-D.A., C.C., S.C.-L., M.M.-F., I.S., J.G.-L., G.M.-E., and E.C. performed the experiments. M.V.-D.A. and F.L. wrote the paper. M.V.-D.A., E.C., O.Z., and F.L. designed and supervised the project. All authors discussed the results and participated in revising the paper.
This work was supported by the Spanish Ministerio de Economía y Competitividad (MINECO; SAF-2016-80535-R, PID2019-106658RB-I00, and PCIN-2015-070 to F.L.; SAF2017-86912-R to O.Z.), cofinanced by the European Development Regional Fund “A way to achieve Europe” and Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR; 2017/SGR/1582) from Generalitat de Catalunya. M.V.-D.A., C.C., S.C.-L., I.S., J.G.-L., and E.C. are recipients of fellowships from the Spanish MINECO (BES-2014-069237 and BES-2017-082107), Spanish Ministerio de Educación, Cultura y Deporte (FPU15/02897), Portuguese Fundação para a Ciência e a Tecnologia (SFRH/BD/75738/2011), Uruguayan Agencia Nacional de Investigación e Innovación (POS-FCE-2018-1-1007796), and European Community Seventh Framework Program (BIOTRACK, FP7/2007/2013; 229673), respectively.
F.L. is the inventor of patent WO2009153336A1. The rest of the authors have no competing financial interests to declare.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Hope W, Drusano GL, Rex JH. 2016. Pharmacodynamics for antifungal drug development: an approach for acceleration, risk minimization and demonstration of causality. J Antimicrob Chemother 71:3008–3019. doi: 10.1093/jac/dkw298. [DOI] [PubMed] [Google Scholar]
- 2.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis 43:25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 3.Salazar F, Brown GD. 2018. Antifungal innate immunity: a perspective from the last 10 years. J Innate Immun 10:373–325. doi: 10.1159/000488539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodridge HS, Wolf AJ, Underhill DM. 2009. β-Glucan recognition by the innate immune system. Immunol Rev 230:38–50. doi: 10.1111/j.1600-065X.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD. 2007. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao X, Guo Y, Jiang C, Chang Q, Zhang S, Luo T, Zhang B, Jia X, Hung M-C, Dong C, Lin X. 2017. JNK1 negatively controls antifungal innate immunity by suppressing CD23 expression. Nat Med 23:337–346. doi: 10.1038/nm.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thornton BP, Vĕtvicka V, Pitman M, Goldman RC, Ross GD. 1996. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J Immunol 156:1235–1246. [PubMed] [Google Scholar]
- 8.Swidergall M, Solis NV, Lionakis MS, Filler SG. 2018. EphA2 is an epithelial cell pattern recognition receptor for fungal β-glucans. Nat Microbiol 3:53–61. doi: 10.1038/s41564-017-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li SS, Ogbomo H, Mansour MK, Xiang RF, Szabo L, Munro F, Mukherjee P, Mariuzza RA, Amrein M, Vyas JM, Robbins SM, Mody CH. 2018. Identification of the fungal ligand triggering cytotoxic PRR-mediated NK cell killing of Cryptococcus and Candida. Nat Commun 9:751. doi: 10.1038/s41467-018-03014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Means TK, Mylonakis E, Tampakakis E, Colvin RA, Seung E, Puckett L, Tai MF, Stewart CR, Pukkila-Worley R, Hickman SE, Moore KJ, Calderwood SB, Hacohen N, Luster AD, El Khoury J. 2009. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J Exp Med 206:637–653. doi: 10.1084/jem.20082109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vera J, Fenutria R, Canadas O, Figueras M, Mota R, Sarrias M-R, Williams DL, Casals C, Yelamos J, Lozano F. 2009. The CD5 ectodomain interacts with conserved fungal cell wall components and protects from zymosan-induced septic shock-like syndrome. Proc Natl Acad Sci U S A 106:1506–1511. doi: 10.1073/pnas.0805846106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velasco-de-Andrés M, Català C, Casadó-Llombart S, Simões I, Zaragoza O, Carreras E, Lozano F. 24 April 2020. The lymphocyte scavenger receptor CD5 plays a nonredundant role in fungal infection. Cell Mol Immunol. doi: 10.1038/s41423-020-0434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.PrabhuDas MR, Baldwin CL, Bollyky PL, Bowdish DME, Drickamer K, Febbraio M, Herz J, Kobzik L, Krieger M, Loike J, McVicker B, Means TK, Moestrup SK, Post SR, Sawamura T, Silverstein S, Speth RC, Telfer JC, Thiele GM, Wang X-Y, Wright SD, El Khoury J. 2017. A consensus definitive classification of scavenger receptors and their roles in health and disease. J Immunol 198:3775–3789. doi: 10.4049/jimmunol.1700373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgueño‐Bucio E, Mier‐Aguilar CA, Soldevila G. 2019. The multiple faces of CD5. J Leukoc Biol 105:891–904. doi: 10.1002/JLB.MR0618-226R. [DOI] [PubMed] [Google Scholar]
- 15.Martinez VG, Moestrup SK, Holmskov U, Mollenhauer J, Lozano F. 2011. The conserved scavenger receptor cysteine-rich superfamily in therapy and diagnosis. Pharmacol Rev 63:967–1000. doi: 10.1124/pr.111.004523. [DOI] [PubMed] [Google Scholar]
- 16.Consuegra-Fernández M, Aranda F, Simões I, Orta M, Sarukhan A, Lozano F. 2015. CD5 as a target for immune-based therapies. Crit Rev Immunol 35:85–115. doi: 10.1615/critrevimmunol.2015013532. [DOI] [PubMed] [Google Scholar]
- 17.Tarakhovsky A, Kanner SB, Hombach J, Ledbetter JA, Müller W, Killeen N, Rajewsky K. 1995. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science 269:535–537. doi: 10.1126/science.7542801. [DOI] [PubMed] [Google Scholar]
- 18.Bikah G, Carey J, Ciallella JR, Tarakhovsky A, Bondada S. 1996. CD5-mediated negative regulation of antigen receptor-induced growth signals in B-1 B cells. Science 274:1906–1909. doi: 10.1126/science.274.5294.1906. [DOI] [PubMed] [Google Scholar]
- 19.Sarhan MA, Pham TNQ, Chen AY, Michalak TI. 2012. Hepatitis C virus infection of human T lymphocytes is mediated by CD5. J Virol 86:3723–3735. doi: 10.1128/JVI.06956-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mourglia-Ettlin G, Miles S, Velasco-De-Andrés M, Armiger-Borràs N, Cucher M, Dematteis S, Lozano F. 2018. The ectodomains of the lymphocyte scavenger receptors CD5 and CD6 interact with tegumental antigens from Echinococcus granulosus sensu lato and protect mice against secondary cystic echinococcosis. PLoS Negl Trop Dis 12:e0006891. doi: 10.1371/journal.pntd.0006891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenz LL. 2009. CD5 sweetens lymphocyte responses. Proc Natl Acad Sci U S A 106:1303–1304. doi: 10.1073/pnas.0812579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramos-Casals M, Font J, García-Carrasco M, Calvo J, Places L, Padilla O, Cervera R, Bowen MA, Lozano F, Ingelmo M. 2001. High circulating levels of soluble scavenger receptors (sCD5 and sCD6) in patients with primary Sjögren’s syndrome. Rheumatology 40:1056–1059. doi: 10.1093/rheumatology/40.9.1056. [DOI] [PubMed] [Google Scholar]
- 23.Aibar J, Martínez-Florensa M, Castro P, Carrasco E, Escoda-Ferran C, Fernández S, Butjosa M, Hernández C, Rinaudo M, Lozano F, Nicolás JM. 2015. Pattern of soluble CD5 and CD6 lymphocyte receptors in critically ill patients with septic syndromes. J Crit Care 30:914–919. doi: 10.1016/j.jcrc.2015.04.120. [DOI] [PubMed] [Google Scholar]
- 24.Sundberg I, Rasmusson AJ, Ramklint M, Just D, Ekselius L, Cunningham JL. 2020. Daytime melatonin levels in saliva are associated with inflammatory markers and anxiety disorders. Psychoneuroendocrinology 112:104514. doi: 10.1016/j.psyneuen.2019.104514. [DOI] [PubMed] [Google Scholar]
- 25.Calvo J, Places L, Espinosa G, Padilla O, Vilà JM, Villamor N, Ingelmo M, Gallart T, Vives J, Font J, Lozano F. 1999. Identification of a natural soluble form of human CD5. Tissue Antigens 54:128–137. doi: 10.1034/j.1399-0039.1999.540203.x. [DOI] [PubMed] [Google Scholar]
- 26.Fenutría R, Martinez VG, Simões I, Postigo J, Gil V, Martínez-Florensa M, Sintes J, Naves R, Cashman KS, Alberola-Ila J, Ramos-Casals M, Soldevila G, Raman C, Merino J, Merino R, Engel P, Lozano F. 2014. Transgenic expression of soluble human CD5 enhances experimentally-induced autoimmune and anti-tumoral immune responses. PLoS One 9:e84895. doi: 10.1371/journal.pone.0084895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simões IT, Aranda F, Carreras E, Andrés MV, Casadó-Llombart S, Martinez VG, Lozano F. 2017. Immunomodulatory effects of soluble CD5 on experimental tumor models. Oncotarget 8:108156–108169. doi: 10.18632/oncotarget.22564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gow NAR, Odds FC, Van Nuffel L. 2000. Survival in experimental Candida albicans infections depends on inoculum growth conditions as well as animal host. Microbiology 146:1881–1889. doi: 10.1099/00221287-146-8-1881. [DOI] [PubMed] [Google Scholar]
- 29.Zaragoza O, Alvarez M, Telzak A, Rivera J, Casadevall A. 2007. The relative susceptibility of mouse strains to pulmonary Cryptococcus neoformans infection is associated with pleiotropic differences in the immune response. Infect Immun 75:2729–2739. doi: 10.1128/IAI.00094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.García-Barbazán I, Trevijano-Contador N, Rueda C, de Andrés B, Pérez-Tavárez R, Herrero-Fernández I, Gaspar ML, Zaragoza O. 2016. The formation of titan cells in Cryptococcus neoformans depends on the mouse strain and correlates with induction of Th2-type responses. Cell Microbiol 18:111–124. doi: 10.1111/cmi.12488. [DOI] [PubMed] [Google Scholar]
- 31.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. 2002. NOD/SCID/γcnull mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 32.Nami S, Aghebati-Maleki A, Morovati H, Aghebati-Maleki L. 2019. Current antifungal drugs and immunotherapeutic approaches as promising strategies to treatment of fungal diseases. Biomed Pharmacother 110:857–868. doi: 10.1016/j.biopha.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 33.MacCallum DM, Odds FC. 2004. Need for early antifungal treatment confirmed in experimental disseminated Candida albicans infection. Antimicrob Agents Chemother 48:4911–4914. doi: 10.1128/AAC.48.12.4911-4914.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armstrong-James D, Brown GD, Netea MG, Zelante T, Gresnigt MS, van de Veerdonk FL, Levitz SM. 2017. Immunotherapeutic approaches to treatment of fungal diseases. Lancet Infect Dis 17:e393–e402. doi: 10.1016/S1473-3099(17)30442-5. [DOI] [PubMed] [Google Scholar]
- 35.Posch W, Steger M, Wilflingseder D, Lass-Flörl C. 2017. Promising immunotherapy against fungal diseases. Expert Opin Biol Ther 17:861–870. doi: 10.1080/14712598.2017.1322576. [DOI] [PubMed] [Google Scholar]
- 36.Volman TJH, Hendriks T, Goris RJA. 2005. Zymosan-induced generalized inflammation: experimental studies into mechanisms leading to multiple organ dysfunction syndrome. Shock 23:291–297. doi: 10.1097/01.shk.0000155350.95435.28. [DOI] [PubMed] [Google Scholar]
- 37.Erwig LP, Gow NAR. 2016. Interactions of fungal pathogens with phagocytes. Nat Rev Microbiol 14:163–176. doi: 10.1038/nrmicro.2015.21. [DOI] [PubMed] [Google Scholar]
- 38.van de Veerdonk FL, Kullberg B-J, Netea MG. 2012. Adjunctive immunotherapy with recombinant cytokines for the treatment of disseminated candidiasis. Clin Microbiol Infect 18:112–119. doi: 10.1111/j.1469-0691.2011.03676.x. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Rubio R, de Oliveira HC, Rivera J, Trevijano-Contador N. 2019. The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front Microbiol 10:2993. doi: 10.3389/fmicb.2019.02993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Becker KL, Ifrim DC, Quintin J, Netea MG, van de Veerdonk FL. 2015. Antifungal innate immunity: recognition and inflammatory networks. Semin Immunopathol 37:107–116. doi: 10.1007/s00281-014-0467-z. [DOI] [PubMed] [Google Scholar]
- 41.Almeida F, Wolf JM, da Silva TA, DeLeon-Rodriguez CM, Rezende CP, Pessoni AM, Fernandes FF, Silva-Rocha R, Martinez R, Rodrigues ML, Roque-Barreira MC, Casadevall A. 2017. Galectin-3 impacts Cryptococcus neoformans infection through direct antifungal effects. Nat Commun 8:1968. doi: 10.1038/s41467-017-02126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linden JR, Kunkel D, Laforce-Nesbitt SS, Bliss JM. 2013. The role of galectin-3 in phagocytosis of Candida albicans and Candida parapsilosis by human neutrophils. Cell Microbiol 15:1127–1142. doi: 10.1111/cmi.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jouault T, Behi M-E, Martínez-Esparza M, Breuilh L, Trinel P-A, Chamaillard M, Trottein F, Poulain D. 2006. Specific recognition of Candida albicans by macrophages requires galectin-3 to discriminate saccharomyces cerevisiae and needs association with TLR2 for signaling. J Immunol 177:4679–4687. doi: 10.4049/jimmunol.177.7.4679. [DOI] [PubMed] [Google Scholar]
- 44.Porte R, Davoudian S, Asgari F, Parente R, Mantovani A, Garlanda C, Bottazzi B. 2019. The long pentraxin PTX3 as a humoral innate immunity functional player and biomarker of infections and sepsis. Front Immunol 10:794. doi: 10.3389/fimmu.2019.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lo Giudice P, Campo S, Verdoliva A, Rivieccio V, Borsini F, De Santis R, Salvatori G. 2010. Efficacy of PTX3 in a rat model of invasive aspergillosis. Antimicrob Agents Chemother 54:4513–4515. doi: 10.1128/AAC.00674-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaziano R, Bozza S, Bellocchio S, Perruccio K, Montagnoli C, Pitzurra L, Salvatori G, De Santis R, Carminati P, Mantovani A, Romani L. 2004. Anti-Aspergillus fumigatus efficacy of pentraxin 3 alone and in combination with antifungals. Antimicrob Agents Chemother 48:4414–4421. doi: 10.1128/AAC.48.11.4414-4421.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diniz SN, Nomizo R, Cisalpino PS, Teixeira MM, Brown GD, Mantovani A, Gordon S, Reis LFL, Dias AAM. 2004. PTX3 function as an opsonin for the dectin-1-dependent internalization of zymosan by macrophages. J Leukoc Biol 75:649–656. doi: 10.1189/jlb.0803371. [DOI] [PubMed] [Google Scholar]
- 48.Champion JA, Walker A, Mitragotri S. 2008. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res 25:1815–1821. doi: 10.1007/s11095-008-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarrias MR, Padilla O, Monreal Y, Carrascal M, Abian J, Vives J, Yélamos J, Lozano F. 2004. Biochemical characterization of recombinant and circulating human Spalpha. Tissue Antigens 63:335–344. doi: 10.1111/j.0001-2815.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- 50.Perfect JR, Lang SD, Durack DT. 1980. Chronic cryptococcal meningitis: a new experimental model in rabbits. Am J Pathol 101:177–194. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.