Olorofim is a novel antifungal drug in phase 2 trials. It has shown promising in vitro activity against various molds, except for Mucorales. Initially, we observed a broad range of EUCAST MICs for Aspergillus fumigatus. Here, we explored the MIC variability in more detail and prospectively investigated the susceptibility of contemporary clinical mold isolates, as population data are needed for future epidemiological cutoff (ECOFF) settings. Fifteen A. fumigatus isolates previously found with low/medium/high MICs (≤0.
KEYWORDS: olorofim, F901318, Aspergillus, Scedosporium, EUCAST, antifungal susceptibility, DHODH, pyrE, Cyp51A, azole resistance
ABSTRACT
Olorofim is a novel antifungal drug in phase 2 trials. It has shown promising in vitro activity against various molds, except for Mucorales. Initially, we observed a broad range of EUCAST MICs for Aspergillus fumigatus. Here, we explored the MIC variability in more detail and prospectively investigated the susceptibility of contemporary clinical mold isolates, as population data are needed for future epidemiological cutoff (ECOFF) settings. Fifteen A. fumigatus isolates previously found with low/medium/high MICs (≤0.002 to 0.25 mg/liter) were tested repeatedly and EUCAST MICs read in a blinded fashion by three observers. pyrE, encoding the olorofim target enzyme dihydroorotate dehydrogenase (DHODH), was sequenced. A total of 1,423 mold isolates (10 Aspergillus species complexes [including 1,032 A. fumigatus isolates] and 105 other mold/dermatophyte isolates) were examined. Olorofim susceptibility (modal MIC, MIC50, MIC90, and wild-type upper limits [WT-ULs] [species complexes with ≥15 isolates]) was determined and compared to that of four comparators. MICs (mg/liter) were within two 2-fold dilutions (0.016 to 0.03) for 473/476 determinations. The MIC range spanned four dilutions (0.008 to 0.06). No significant pyrE mutations were found. Modal MIC/WT-UL97.5 (mg/liter) values were 0.03/0.06 (A. terreus and A. flavus), 0.06/0.125 (A. fumigatus and Trichophyton rubrum), and 0.06/0.25 (A. niger and A. nidulans). The MIC range for Scedosporium spp. was 0.008 to 0.25. Olorofim susceptibility was similar for azole-resistant and -susceptible isolates of A. fumigatus but reduced for A. montevidensis and A. chevalieri (MICs of >1). With experience, olorofim susceptibility testing is robust. The testing of isolates from our center showed uniform and broad-spectrum activity. Single-center WT-ULs are suggested.
INTRODUCTION
Olorofim (F901318) is a novel antifungal first-in-class orotomide compound that shows potent in vitro activity against a broad spectrum of pathogenic mold isolates by inhibiting dihydroorotate dehydrogenase (DHODH), which is involved in the de novo pyrimidine biosynthesis pathway. Although it lacks activity against Candida and Mucorales species, olorofim has shown promising in vitro and in vivo efficacy against endemic fungi and Aspergillus spp. (including cryptic species) and even activity against isolates of difficult-to-treat species, such as Scedosporium, Madurella mycetomatis, and some Fusarium species (1–13). Furthermore, olorofim retains efficacy against azole-resistant isolates of A. fumigatus in vitro and in vivo (1, 4, 13). This prompted the European Medicines Agency (EMA) Committee for Orphan Medicinal Products in 2016 to grant the drug orphan designation for scedosporiosis and invasive aspergillosis (IA). In 2019, the U.S. Food and Drug Administration (FDA) granted the drug Breakthrough Therapy Designation for the treatment of invasive mold infections (IFD) in patients with limited or no treatment options. The drug is presently being evaluated in an ongoing open-label, single-arm, phase 2b study (ClinicalTrials.gov registration no. NCT03583164) in patients with invasive mold infections due to olorofim-susceptible isolates of Lomentospora prolificans, Scedosporium spp., Aspergillus spp., and other resistant fungi in patients with limited treatment options.
To establish clinical breakpoints aiding in the identification of isolates that are likely to respond to treatment, it is of paramount importance to establish a reliable and robust susceptibility testing procedure and to acquire sufficient susceptibility data from clinical isolates. The CLSI and EUCAST have provided methods for the susceptibility testing of antifungal compounds (14, 15). Technical issues, which may influence intra- and interlaboratory variability in MIC determination, also should be investigated. We have previously examined the influence of various technical aspects, including the dilution procedure (ISO versus serial dilution), olorofim lot variation, different polystyrene plates, and reading method (visual versus spectrophotometric), and we found limited variation in EUCAST susceptibility test results (4). However, we did find a rather broad range of MIC values of a collection of clinical A. fumigatus isolates (unimodal range, <0.004 to 0.25 mg/liter) around a clear modal MIC of 0.06 mg/liter during our initial routine testing. We did not know whether this was due to inherent variation associated with the biological susceptibility method or if it presented true but subtle differences in susceptibility despite the fact that acquired olorofim resistance has not been reported in clinical isolates to date.
In this study, we investigated the reproducibility of MIC testing in more detail, including potential underlying resistance mechanisms. Furthermore, we investigated the olorofim performance against contemporary molds, including nation-wide Aspergillus surveillance.
RESULTS
MIC variability study.
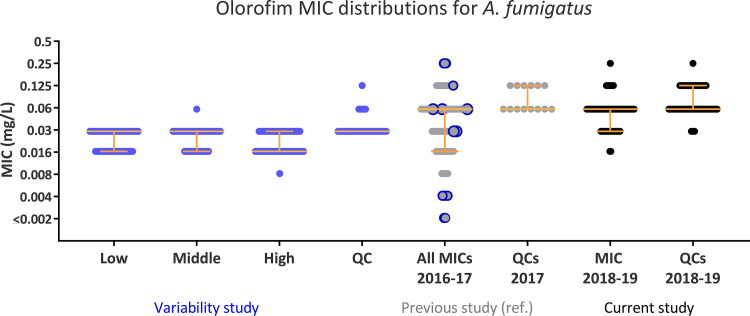
In total, 476 olorofim MICs were determined for the 15 isolates with low, medium, and high MICs; in addition, 76 MICs for the ATCC 204305 A. fumigatus quality control (QC) isolate were determined (see Table S1 in the supplemental material). The repeated MIC determination using one batch of plates showed excellent intra- and interreader reproducibility. MIC determinations were within one dilution of the modal MIC determined for three individual readers as well as overall for isolates (apart from isolate number 7793, where two of 28 MIC determinations were two 2-fold dilution steps below the modal MIC). Despite the original MICs spanning seven 2-fold dilution steps, all modal MICs and 473 of all MICs fell within two dilutions steps (0.016 to 0.03 mg/liter), and the entire range was within four dilution steps (0.008 to 0.06 mg/liter) (Fig. 1 and Table S1).
FIG 1.
Repetitive olorofim MICs against the 15 A. fumigatus isolates from the previous study (2016 to 2017) compared to the original A. fumigatus MICs of all isolates from that study (a), MICs against contemporary isolates in this study (2018 to 2019) (b), and QCs (A. fumigatus ATCC 204305) from the two study periods (c). The original MIC values for each isolate included in the variability study are marked in blue circles in the 2016–2017 data set. Yellow lines indicate the median and 25% interquartile range.
The target gene pyrE was sequenced to detect any potential resistance mutations. Thirteen of 15 isolates were identical to the pyrE sequence from the AF293 reference and, thus, considered wild type. One isolate had a single synonymous nucleotide polymorphism, and another isolate (SSI-7929, found with a near-modal MIC of 0.03 mg/liter) harbored a Q35L alteration (Table S1).
EUCAST MICs for clinical mold isolates.
In total, 1,423 mold isolates were referred for susceptibility testing and tested for olorofim susceptibility during the calendar years 2018 and 2019. The referred isolates included 1,318 (92.6%) Aspergillus species (of which 1,032 were A. fumigatus), 30 (2.1%) dermatophytes, 24 (1.9%) Fusarium spp., 20 (1.4%) Mucorales spp., 13 (0.9%) Scedosporium spp., and 18 (1.3%) other molds. The included number of isolates increased from 450 isolates in 2018 to 973 isolates in 2019.
Olorofim displayed potent in vitro activity across almost all examined Aspergillus species isolates, with a MIC range of 0.008 to 0.25 mg/liter (geometric mean [GM], 0.05 mg/liter; N = 1,312) (Table 1). The exceptions were (i) in the four isolates from Section Usti, where somewhat higher MICs were observed (range, 0.06 to 0.5 mg/liter; geometric mean, 0.21 mg/liter; N = 4) and (ii) in the two isolates from section Aspergillus, where olorofim MICs of >1 mg/liter were found. For A. fumigatus (1,032 isolates), the range (0.016 to 0.25 mg/liter), modal MIC (0.06 mg/liter), and statistical wild-type upper limits (WT-UL at 95% to 97.5% endpoints [WT-UL95–97.5]; the upper MIC value demarcates the end of the wild-type population) were similar for azole-susceptible and -resistant isolates. The WT-UL values for A. nidulans species complex (SC) and A. niger SC were one dilution higher than those for A. fumigatus (despite comparable modal MICs), whereas A. terreus SC and A. flavus SC were one dilution more susceptible than A. fumigatus (Table 1).
TABLE 1.
EUCAST MICs of olorofim against contemporary prospectively collected clinical mold isolates 2018 to 2019e
| Section, genus, and species | No. of isolates at MIC (mg/liter) of: |
MIC (mg/liter) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | 0.008 | 0.016 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | >1 | Range | Modal | GM | MIC50 | MIC90 | WT-UL95 | WT-UL97.5 | WT-UL99 | |
| Flavi | ||||||||||||||||||
| A. flavus SC | 48 | 13 | 27 | 7 | 1 | 0.016–0.125 | 0.03 | 0.029 | 0.03 | 0.06 | 0.06 | 0.06 | 0.06 | |||||
| A. terricola | 2 | 2 | 0.016 | NDd | ||||||||||||||
| Fumigati | ||||||||||||||||||
| A. fumigatus sensu lato | 1032 | 15 | 262 | 648 | 104 | 3 | 0.016–0.25 | 0.06 | 0.053 | 0.06 | 0.125 | 0.125 | 0.125 | 0.125 | ||||
| Azole susceptible | 920 | 13 | 235 | 588 | 83 | 1 | 0.016–0.25 | 0.06 | 0.053 | 0.06 | 0.06 | 0.125 | 0.125 | 0.125 | ||||
| Azole resistant | 112 | 2 | 27 | 60 | 21 | 2 | 0.016–0.25 | 0.06 | 0.058 | 0.06 | 0.125 | 0.125 | 0.125 | 0.25 | ||||
| A. thermomutatus | 11 | 1 | 2 | 5 | 3 | 0.016–0.125 | ND | (0.057) | (0.06) | (0.125) | ||||||||
| A. felis | 2 | 1 | 1 | 0.03–0.06 | ND | |||||||||||||
| A. hiratsukae | 1 | 1 | 0.016 | ND | ||||||||||||||
| Nidulantes | ||||||||||||||||||
| A. nidulans SC | 17 | 3 | 8 | 6 | 0.03–0.125 | 0.06 | 0.069 | 0.06 | 0.125 | 0.25 | 0.25 | 0.25 | ||||||
| A. quadrilineatus | 4 | 1 | 3 | 0.06–0.125 | ND | |||||||||||||
| A. spinulosporus | 4 | 1 | 3 | 0.03–0.06 | ND | |||||||||||||
| A. nidulans sensu stricto | 1 | 1 | 0.125 | ND | ||||||||||||||
| Nigri | ||||||||||||||||||
| A. niger SC | 129 | 1 | 9 | 58 | 48 | 9 | 0.016–0.25 | 0.06 | 0.080 | 0.06 | 0.125 | 0.25 | 0.25 | 0.25 | ||||
| A. tubingensis | 18 | 1 | 8 | 8 | 1 | 0.03–0.25 | 0.06–0.125 | 0.087 | 0.06 | 0.125 | 0.25 | 0.25 | 0.25 | |||||
| A. welwitschiae | 1 | 3 | 2 | 1 | 0.03–0.125 | ND | ||||||||||||
| Terrei | ||||||||||||||||||
| A. terreus SC | 64 | 7 | 21 | 27 | 9 | 0.008–0.06 | 0.03 | 0.023 | 0.03 | 0.06 | 0.06 | 0.06 | 0.125 | |||||
| Azole susceptible | 28 | 6 | 10 | 10 | 2 | 0.008–0.06 | 0.016–0.03 | 0.019 | 0.016 | 0.03 | 0.06 | 0.06 | 0.125 | |||||
| Azole resistant | 36 | 1 | 11 | 17 | 7 | 0.008–0.06 | 0.03 | 0.027 | 0.03 | 0.06 | 0.06 | 0.06 | 0.125 | |||||
| A. neoindicus | 1 | 1 | 0.016 | ND | ||||||||||||||
| Usti | ||||||||||||||||||
| A. ustus SC | 4 | 1 | 2 | 1 | 0.06–0.5 | ND | ||||||||||||
| A. calidoustus | 2 | 1 | 1 | 0.25–0.5 | ND | |||||||||||||
| A. pseudodeflectus | 1 | 1 | 0.25 | ND | ||||||||||||||
| Circumdati | ||||||||||||||||||
| A. ochraceus SC | 1 | 1 | 0.06 | ND | ||||||||||||||
| A. sclerotiorum | 1 | 1 | 0.008 | ND | ||||||||||||||
| A. westerdijkiae | 1 | 1 | 0.016 | ND | ||||||||||||||
| Clavati | ||||||||||||||||||
| A. giganteus | 1 | 1 | 0.016 | ND | ||||||||||||||
| Versicolores | ||||||||||||||||||
| A. sydowii | 4 | 2 | 2 | 0.08–0.016 | ND | |||||||||||||
| Aspergillus | ||||||||||||||||||
| A. montevidensis | 1 | 1 | 0.5 | ND | ||||||||||||||
| A. chevalieri | 1 | 1 | 0.5 | ND | ||||||||||||||
| Other molds | ||||||||||||||||||
| Scedosporium | ||||||||||||||||||
| Scedosporium species | 13 | 1 | 1 | 4 | 3 | 2 | 2 | 0.008–0.25 | ND | |||||||||
| S. apiospermum | 7 | 1 | 1 | 2 | 2 | 1 | 0.008–0.25 | ND | ||||||||||
| S. boydii | 5 | 1 | 1 | 2 | 1 | 0.03–0.25 | ND | |||||||||||
| Fusarium | ||||||||||||||||||
| Fusarium speciesa | 24 | 1 | 2 | 1 | 20 | 0.03 to >1 | >1 | |||||||||||
| F. proliferatum | 3 | 1 | 2 | 0.03–0.06 | ND | |||||||||||||
| Dermatophytes | ||||||||||||||||||
| T. rubrum | 24 | 1 | 2 | 4 | 15 | 1 | 1 | 0.008–0.25 | 0.06 | 0.048 | 0.06 | 0.06 | 0.125 | 0.125 | 0.125 | |||
| T. interdigitale | 4 | 2 | 1 | 1 | 0.03–0.125 | ND | ||||||||||||
| T. soudanense (T. rubrum complex) | 1 | 1 | 0.06 | ND | ||||||||||||||
| M. gypseum | 1 | 1 | 0.125 | ND | ||||||||||||||
| Mucorales | ||||||||||||||||||
| Mucorales speciesb | 20 | 1 | 19 | 1 to >1 | >1 | |||||||||||||
| Various rare molds | ||||||||||||||||||
| Other moldsc | 18 | 1 | 2 | 3 | 1 | 2 | 2 | 7 | 0.008 to >1 | 0.06 | ||||||||
| Total | 1,423 | 13 | 61 | 346 | 763 | 168 | 19 | 3 | 3 | 47 | 0.008 to >1 | 0.06 | ||||||
F. solani complex (n = 16), F. proliferatum (n = 3), F. oxysporum complex (n = 1), F. fujikuroi complex (n = 1; MIC 1 mg/liter), and F. dimerum complex (n = 1).
Rhizopus microsporus (n = 6), Rhizopus arrhizus (n = 5), Absidia corymbifera (n = 3), Mucor circinelloides (n = 3), Rhizopus pusillus (n = 2), and Mucorales species (n = 1).
Alternaria alternata, Paecilomyces variotii, Penicillium citrinum, Purpureocillium lilacinum, and Talaromyces amestolkiae (each n = 2); Alternaria infectoria, Exophiala phaeomuriformis, Acrophialophora levis, Penicillium crustosum, Penicillium spp, Rasamsonia aegroticola, Rasamsonia argillacea, and Stemphylium vesicarium (each n = 1).
ND, not determined.
For species with ≥15 isolates, the modal MIC, geometric mean (GM), MIC50, and MIC90 are stated. Modal MICs are highlighted in boldface. Various Aspergillus species complexes and non-aspergillus mold species are separated by dashed lines.
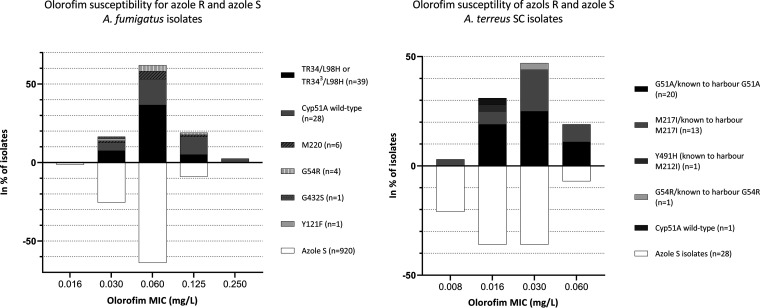
When comparing olorofim MICs between azole-susceptible and azole-resistant isolates, no definitive difference in susceptibility was found (geometric means, 0.053 versus 0.058 for A. fumigatus and 0.019 versus 0.027 for A. terreus, respectively). When comparing median MICs of azole-susceptible and -resistant isolates, this translated into slight differences (P values of 0.06 [approximate] and 0.02 [exact] for A. fumigatus and A. terreus). Similar distributions were found between azole-susceptible isolates and azole-resistant isolates with cyp51A mutations (Fig. 2).
FIG 2.
Comparison of olorofim MICs for isolates of A. fumigatus (left) and A. terreus SC (right) in relation to azole susceptibility (percentage of the total number of isolates with a given susceptibility classification). Azole-susceptible (S) isolates are shown below the x axis, whereas resistant (R) isolates are shown above the x axis. For A. fumigatus, only azole-resistant cyp51A sequenced isolates were included (79 of 112 azole-resistant isolates). For A. terreus, nonsequenced isolates with azole susceptibility profiles similar to those of other sequenced isolates from the same patient harboring Cyp51A alterations were included to show the MIC variability.
Olorofim’s in vitro activity against Scedosporium and dermatophyte species was similar to that against A. fumigatus (0.008 to 0.25 mg/liter) (Table 1). Finally, the in vitro activity against Fusarium and other mold species was diverse and species specific. The olorofim MICs against three isolates of F. proliferatum were low (0.03 to 0.06 mg/liter), whereas the MICs against the remaining Fusarium species were higher (MICs of ≥1 mg/liter). For the other mold species, no olorofim efficacy was observed for Alternaria, Exophiala, Purpureocillium, and Stemphylium isolates, whereas the MICs against the remaining species, including Rasamsonia aegroticola and Rasamsonia argillacea, were ≤0.5 mg/liter.
Comparing the in vitro activity against that of other mold-active agents (Table 2), olorofim was more potent on a milligram per milliliter basis against all species (apart from non-proliferatum Fusarium isolates). More than 20% of isolates from the following species had MICs that were above the ECOFF/A. fumigatus ECOFF: for the triazoles, A. terreus, other Aspergillus spp., and Fusarium spp.; for amphotericin B, A. terreus, other Aspergillus spp., other Fusarium spp., and Scedosporium spp.; and for olorofim, other Fusarium and Aspergillus spp. Only one isolate (2.1%) of A. flavus SC and three isolates (0.3%) of A. fumigatus had MICs above the olorofim WT-UL97.5.
TABLE 2.
In vitro susceptibility of contemporary mold isolates (from 2018 to 2019) to olorofim and four comparatorse
| Species (N) | Susceptibility value(s) for: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OLO |
AMB |
VRCb |
ITRb |
PRCb |
||||||
| Modea (range) (mg/liter) | % >WT-UL97.5/(>0.125 mg/liter) | Modea (range) (mg/liter) | % > ECOFF/(1 mg/liter) | Modea (range) (mg/liter) | % > ECOFF/(>1 mg/liter) | Modea (range) (mg/liter) | % > ECOFF/(>1 mg/liter) | Modea (range) (mg/liter) | % >ECOFF/(>0.25 mg/liter) | |
| A. flavus SC (48) | 0.03 (0.016–0.125) | 2.1 | 1 (0.05 to >4) | 2.1 | 1 (0.5–2) | 0 | 0.125 (0.06–1) | 0 | 0.125 (0.03–0.25) | 0 |
| A. fumigatus (1,032) | 0.06 (0.016–0.125) | 0.3 | 0.5 (0.06–2) | 0.5 | 0.5 (0.125 to >16) | 12.3 | 0.25 (0.06 to >16) | 12.1 | 0.125 (0.004 to >4) | 11.6 |
| A. nidulans SC (17) | 0.06 (0.03–0.125) | 0 | 0.5–1 (0.125–2) | 0 | 0.25 (0.125–0.5) | 0 | 0.125–0.25 (0.125–0.5) | 0 | 0.125 (0.06–0.5) | 0 |
| A. niger SC (129) | 0.06 (0.016–0.25) | 0 | 0.125 (0.03–1) | 0.8 | 1 (0.5–1) | 5.5 | 1 (0.06 to >16) | 18.6 | 0.25 (0.06–1) | 2.4 |
| A. terreus SC (64) | 0.03 (0.008–0.06) | 0 | 2 (0.5 to >4) | NDc (78.1) | 1 (0.25–16) | 28.1 | 0.125 (≤0.016 to >16) | 53.1 | 0.125/0.5d (0.03–1) | 48.4 |
| Other Aspergillus spp. (14) | ND (0.008 to >1) | (35.7) | ND (0.016 to >4) | (50) | ND (0.25–8) | (50.0) | ND (≤0.016 to >16) | (35.7) | ND (0.016 to >4) | (42.9) |
| F. proliferatum (3) | ND (0.03–0.06) | (0/3) | ND (0.5–1) | (0/3) | ND (2–4) | (3/3) | ND (>16) | (3/3) | ND (1 to >4)) | (3/3) |
| Other Fusarium spp. (21) | >1 (1 to >1) | (100) | 1 (0.5 to >4) | NDc (23.8) | 8 (2 to >16) | (100) | >16 (>16) | (100) | >4 (1 to >4) | (100) |
| Scedosporium spp. (13) | 0.03 (0.008–0.25) | (15.4) | ND (0.5 to >4) | (53.9) | ND 1 (0.25–2) | (7.7) | >16 (0.125 to >16) | (61.5) | ND (0.25–2) | (69.2) |
| T. rubrum (24) | 0.06 (0.008–0.25) | 4.2 | 1 (0.06–4) | (8.3) | 0.25–0.5 (0.06–0.5) | (0) | 0.125 (0.125–16) | (13.0) | 0.25 (0.125–0.5) | (37.5) |
The modal MIC was not determined for species with ≤15 isolates.
A. fumigatus isolates were classified as azole susceptible using E.Def 10.1 azole agar screening plates; hence, the MIC values for voriconazole/itraconazole/posaconazole were based on the MICs for the remaining 790/801/791 isolates.
The ECOFF (A. terreus)/tentative ECOFFs (F. fujikuroi SC and F. solani SC) are 8 mg/liter (www.eucast.org) and are ND (not determined) status due to the truncated range (percent above the A. fumigatus ECOFF [% > ECOFF], presented for comparison reasons).
Bimodal distribution.
Modal MIC, range, and percentage of isolates with MICs above those of the wild-type population/ECOFF are displayed. For olorofim, the WT-UL97.5 values for A. fumigatus (species with more or fewer than 15 isolates) are used. For the licensed comparators, EUCAST ECOFF (for various Aspergillus species, when available), T-ECOFF, or A. fumigatus ECOFF (no defined ECOFF) is used for comparison.
DISCUSSION
MIC variability study.
Broad MIC ranges can reflect either the presence of both wild-type and resistant isolates or technical issues causing low reproducibility. In our first study, isolates with low MICs were collected prior to July 2016, whereas all isolates with high MICs were collected during 2017, which suggests a change in susceptibility over time (4). Acquired resistance has been selected for in vitro and associated with alterations involving the Gly119 codon in DHODH (16). However, treatment-induced resistance seemed unlikely, as olorofim has not been licensed. Target sequencing revealed one alteration, Q35L, in a single isolate, but most likely, this is not of clinical significance, as it did not affect susceptibility. MIC values for repeated testing revealed excellent agreement across the isolates selected according to high, medium, and low MIC at initial testing, irrespective of the observer and with the observers blinded to the original MIC. Less variation was to be expected, as a single batch of plates was employed with eight olorofim determinations next to each other, in contrast to the multiple batches read on different days with different drugs in each row during the prospective routine setting. Moreover, we have previously observed slight trailing growth and an occasional occurrence of tiny dots in the center surface of the wells in supra-MIC wells and noted that this may challenge reproducible visual endpoint determination between observers and laboratories (4). This is particularly true as long as validated MIC targets and ranges for QC strains have not been established. Therefore, we hypothesize that the observed variation in MIC determination was an artifact due to the observers being new to olorofim susceptibility testing when it was introduced in 2016. In support of this, the evaluation of the MICs from our previous study revealed that a broader MIC range was found during May to November 2016 (modal MIC, 0.016; range, ≤0.002 to 0.06 mg/liter; seven dilution steps) than for the remaining time period (modal MIC, 0.06; modal range, 0.016 to 0.25; five dilution steps). This emphasizes the importance of using QC strains, especially when comparing MICs over time and between laboratories, and the need for proper training in the visual reading of olorofim MICs.
Contemporary olorofim susceptibility.
Olorofim displayed potent in vitro activity against Aspergillus species. This included both species intrinsically less susceptible to amphotericin B and/or the triazoles and isolates of A. fumigatus and A. flavus with acquired azole resistance (1, 4). This is corroborated by in vivo animal models of invasive aspergillosis, demonstrating in vivo activity against various Aspergillus isolates harboring both intrinsic and acquired resistance to polyene and the triazoles (10, 12, 13), and is consistent with the fact that olorofim has a unique target independent of the triazole target (5).
Epidemiological cutoff values (ECOFFs for EUCAST, ECVs for CLSI) on aggregated MIC distributions are obligatory ingredients for clinical breakpoint setting. The EUCAST SOP10.1 document stipulates that ECOFFs for antimicrobial agents should be based on at least five data sets, each with a minimum of 15 isolates from separate laboratories, totaling at least 100 isolates (www.eucast.org). There is a scarcity of population data using EUCAST (and, especially, CLSI) broth microdilution methods for the most common species of Aspergillus (Table 3) (1–5). Modal MIC, MIC50, and MIC90 values determined for A. fumigatus, A. flavus SC, and A. tubingensis in this study were within one 2-fold dilution of the EUCAST data set reported by other European experts and in close agreement with the values from our study for 2016 to 2017. This suggests that olorofim EUCAST testing is robust when performed in mycology laboratories (1, 2, 4) and is promising for future ECOFF settings. Olorofim in vitro susceptibility reports of cryptic species of Aspergillus as well as A. flavus have shown agreement between CLSI and EUCAST olorofim MIC determinations but with a tendency toward slightly lower (1 to 2 dilution steps) MICs for the CLSI than the EUCAST methodology (2, 10). For Scedosporium spp., we found MIC ranges similar to those previously reported using the CLSI methodology (6, 7). However, the direct comparison of olorofim MIC distributions between the two methodologies remains hampered by a paucity of data.
TABLE 3.
Summary of EUCAST and CLSI MICs for clinical Aspergillus isolates in this and previous studiese
| Species | Method | N | Value (mg/liter) for: |
Reference our source | ||||
|---|---|---|---|---|---|---|---|---|
| MIC50 | Mode | MIC90 | GM | Range | ||||
| A. calidoustus | EUCAST | 25 | 0.25 | 0.25 | 0.5 | 0.125–0.5 | 1 | |
| EUCASTa | 20 | 0.125 | 0.25 | 0.098 | 0.016–0.5 | 2 | ||
| CLSIa | 20 | 0.03 | 0.125 | 0.048 | 0.0016–0.25d | 2 | ||
| A. citrinoterreus | CLSIa | 27 | 0.016 | 0.016 | 0.03 | 0.008–0.06 | 3 | |
| CLSIa | 5 | 0.016 | 0.016 | 0.016 | 0.016 | 0.016–0.016 | 2 | |
| EUCASTa | 5 | 0.016 | 0.016 | 0.016 | 0.016 | 0.016–0.016 | 2 | |
| A. flavus (SC)b | EUCAST | 48 | 0.03 | 0.03 | 0.06 | 0.029 | 0.016–0.125 | This study |
| EUCAST | 12 | 0.03 | 0.03 | 0.06 | 0.05 | <0.004–0.06 | 4 | |
| EUCASTa | 10 | 0.03 | 0.03 | 0.06 | ND | 0.016–0.06 | 1 | |
| CLSIa | 19 | 0.021 | 0.016–0.06 | 5 | ||||
| A. fumigatus | EUCAST | 1,032 | 0.06 | 0.06 | 0.125 | 0.053 | 0.016–0.25 | This study |
| EUCAST | 235 | 0.06 | 0.06 | 0.125 | 0.037 | <0.004–0.25 | 4 | |
| EUCASTa,c | 143 | 0.03–0.125 | 0.03–0.125 | 0.06–0.125 | 0.016–0.025 | 1 | ||
| CLSI | 55 | 0.029 | 0.008–0.06 | 5 | ||||
| A. niger (SC)b | EUCAST | 129 | 0.06 | 0.06 | 0.125 | 0.080 | 0.016–0.25 | This study |
| EUCAST | 17 | 0.06 | 0.03/0.06 | 0.125 | 0.052 | 0.008–0.25 | 4 | |
| CLSIa | 19 | 0.031 | 0.016–0.06 | 5 | ||||
| A. nidulans (SC)b | EUCAST | 17 | 0.06 | 0.06 | 0.125 | 0.069 | 0.03–0.125 | This study |
| EUCASTa | 10 | 0.125 | 0.125 | 0.125 | 0.06–0.25 | 1 | ||
| A. thermomutatus | EUCAST | 11 | 0.06 | 0.06 | 0.125 | 0.057 | 0.016–0.125 | This study |
| EUCASTa | 10 | 0.016 | 0.016 | 0.016 | 0.016 | 0.016–0.016 | 2 | |
| CLSIa | 10 | 0.016 | 0.016 | 0.016 | 0.016 | 0.016–0.016 | 2 | |
| A. terreus (SC)b | EUCAST | 64 | 0.03 | 0.03 | 0.06 | 0.023 | 0.008–0.06 | This study |
| EUCAST | 5 | 0.022 | 0.008–0.03 | 4 | ||||
| CLSI | 21 | 0.014 | 0.004–0.03 | 5 | ||||
| CLSI | 42b | 0.004 | 0.004 | 0.008 | 0.002–0.008 | 3 | ||
| A. tubingensis | EUCAST | 18 | 0.06 | 0.06/0.125 | 0.125 | 0.03–0.25 | This study | |
| EUCASTa | 25 | 0.03 | 0.03 | 0.06 | 0.016–0.25 | 1 | ||
| EUCASTa | 20 | 0.06 | 0.06 | 0.051 | 0.03–0.125 | 2 | ||
| CLSIa | 20 | 0.06 | 0.125 | 0.053 | 0.03–0.125 | 2 | ||
For consistency, published MICs of 0.015, 0.031, 0.063, and 0.12 mg/liter were changed to 0.016, 0.03, 0.06, and 0.125 mg/liter, respectively.
Isolates are not all identified fully to species level; therefore, we use the term species complex (SC) for the present study and reference 4. For reference 5, the method of identification was not stated.
Several subgroups of A. fumigatus (with various azole susceptibility profiles) are pooled.
In Rivero-Menendez et al. (2), the range was indicated as 0.0015 to 0.25 mg/liter. However, 0.0015 is an off-scale concentration and outside the concentration range tested in the study (0.015 to 8 mg/liter); we assume this is a typing error and that the correct range should be 0.015 to 0.25 (or 0.016 to 0.25 mg/liter).
Only species with at least two studies each with a minimum of five isolates (≥20 isolates in total) are included. MIC50, mode, and MIC90 are presented in parentheses for sets with fewer than 10 isolates.
In this study, data sets for the olorofim in vitro susceptibility for the five most common species complexes of Aspergillus included population-based, nationwide data for 2019. All distributions were unimodal, and MICs were within five dilution steps. WT-UL97.5 values were from 0.06 mg/liter (A. terreus SC and A. flavus SC) over 0.125 mg/liter for A. fumigatus to 0.25 mg/liter (for A. niger SC and A. nidulans SC), with no sign of acquired resistance detected. With more than 10% of A. fumigatus isolates being azole resistant for at least one azole and an even higher proportion of A. terreus isolates being amphotericin B and azole resistant, olorofim is an interesting option for the future treatment of patients.
In contrast to Buil et al. (1), we found no difference between modal MICs or ranges for azole-susceptible and -resistant A. fumigatus isolates or altered modal MICs for resistant isolates harboring Cyp51A alteration TR34/L98H or TR343/L98H, or alterations involving M220 or G54, compared to azole-susceptible isolates. For A. terreus, M217I (which corresponds to M220 in A. fumigatus) was not associated with MICs lower than those observed with wild-type isolates, and the observed slight elevation in geometric mean (less than one dilution step) did not affect the WT-UL97.5 or range. In conclusion, we could not confirm a link between azole resistance and elevated olorofim MICs.
The low MICs for Scedosporium spp. and F. proliferatum show promise for the treatment of infections by these otherwise hard-to-treat molds, often exhibiting close to pan-antifungal resistance (17–20). Although Trichophyton infections are usually limited to the skin and rarely become invasive, recent reports of emerging terbinafine and even itraconazole resistance may call for new drugs for dermatophyte infections, and olorofim shows the promise of good in vitro efficacy as an oral formulation suitable for outpatient care (21–23).
In conclusion, our study suggests that olorofim MIC routine testing is reproducible, provided proper training, and compared to published data, interlaboratory variation is acceptable. We found unimodal MIC distributions and ranges of up to five two-fold dilution steps for the five most common Aspergillus species complexes and similar susceptibility of T. rubrum isolates, and we were able to suggest tentative cutoff values below or at 0.25 mg/liter. No acquired resistance or cross-resistance to other compounds was apparent.
MATERIALS AND METHODS
MIC variability study.
Fifteen A. fumigatus isolates from a previous olorofim study (4), spanning a broad range of MICs, were selected. The following isolates were selected: five with low olorofim MICs (≤0.002 mg/liter [n = 2] and 0.004 mg/liter [n = 3]), five with near-modal MICs (0.03 mg/liter [n = 2] and 0.06 mg/liter [n = 3]), and five with high MICs (0.125 mg/liter [n = 1] and 0.25 mg/liter [n = 4]). The olorofim MIC determinations were performed 10 times. The only exception was isolate SSI-8142 (MIC, 0.125 mg/liter), which was only tested 4 times on individual plates but with 6 subsequent repetitions on each of 6 plates to test for the potential influence of position in the plates. MICs were read by 2 to 3 observers blinded to the original MICs. Observers 1 and 2 determined the MICs of all isolates, whereas observer 3 determined only a random proportion. Olorofim susceptibility testing was performed as described below, and for all susceptibility testing, A. fumigatus ATCC 204305 was used as a QC strain on each plate as well as on a full plate (8 repetitions). Modal MICs and ranges were determined and compared to A. fumigatus MIC values obtained during routine prospective surveillance in 2016 to 2017 (4) as well as MICs obtained during 2018 to 2019.
Prospective evaluation of EUCAST olorofim in vitro susceptibility of contemporary mold isolates.
(i) Isolates and identification. The collection contained all isolates from clinical samples or pure cultures received at the mycology reference laboratory at Statens Serum Institut for identification and susceptibility testing during the calendar years 2018 and 2019. Statens Serum Institut perform susceptibility testing for all Danish mold isolates, apart from a few selected azole-susceptible mold isolates from 1 of 10 departments of clinical microbiology. From October 2018, data for A. fumigatus were collected nationwide due to the initiation of national surveillance. Duplicate isolates with the same species and overall resistance patterns were excluded if obtained within 21 days. Identification was done using macro- and micromorphology, supplemented by thermotolerance (incubation at 50°C) for Aspergillus fumigatus complex isolates, and the sequencing of β-tubulin (for Aspergillus), internal transcribed spacer regions ITS1 and ITS2 (ITS), and the translation elongation factor (TEF) (for Fusarium) was performed as previously described (24). The use of the term species complex (SC) is acknowledged for Aspergillus species other than A. fumigatus in the absence of detailed molecular identification.
(ii) Susceptibility testing. MICs were determined prospectively during routine susceptibility testing by following the E.Def.9.3.1 method (www.eucast.org). Pure antifungal substance was stored in aliquots at −80°C, and stock solutions were prepared in dimethyl sulfoxide (5,000 mg/liter; Sigma-Aldrich, Brøndby, Denmark) with amphotericin B and itraconazole (Sigma-Aldrich), voriconazole (Pfizer, Ballerup, Denmark), posaconazole (MSD, Ballerup, Denmark), and olorofim (F2G, Manchester, UK).
Final drug concentrations were the following: olorofim, 0.001 to 1 mg/liter (0.0005 to 0.5 mg/liter until early March 2018); amphotericin B and posaconazole, 0.004 to 4 mg/liter; itraconazole and voriconazole, 0.016 to 16 mg/liter. A portion of A. fumigatus isolates were not fully susceptibility tested but identified as susceptible to the azoles using only the EUCAST-validated 4-well azole screening agar methodology (VIPcheck, Nijmegen, The Netherlands) (25). Susceptibility classification was performed according to the interpretive breakpoint tables for MICs for antifungal agents, version 10.0, 2020 (26). Azole-resistant isolates of A. fumigatus and A. terreus were routinely cyp51A sequenced, as previously described (27, 28).
(iii) pyrE sequencing. PCR amplification of pyrE was accomplished using PCR primers AFDseq-F2 and AFDseq-R2 (see Table S2 in the supplemental material) and a touchdown-based PCR cycling method with decreasing annealing temperature (1°C/cycle), from 64°C to 57°C, followed by 30 cycles at 57°C. Full-length PCR amplicons were sequenced using the eight primers listed in Table S2.
Data management.
The olorofim range was determined for all species, whereas modal MICs, MIC50, and MIC90 were determined for species with ≥15 isolates. Statistical wild-type upper limits (WT-UL; the highest MIC for organisms without phenotypically detectable acquired resistance mechanisms) were determined using the ECOFFinder program, version 2.1, adopting 95%, 97.5%, and 99% subset endpoints (29). Geometric means were determined using GraphPad Prism, version 8.3.0, for Windows (GraphPad Software, San Diego, CA, USA;).
For A. fumigatus and A. terreus, olorofim activity was evaluated individually for azole-susceptible and -resistant isolates overall and by underlying resistance mechanism. In the analysis for A. terreus, we included azole-resistant, nonsequenced isolates if the patient was known to harbor resistant isolates and the resistance profile was similar to that of sequenced isolates (as fewer isolates were available). The median MICs of azole-susceptible and azole-resistant isolates of the two species were compared using the Mann-Whitney U test and GraphPad Prism.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by an unrestricted grant from F2G. The funder had no influence on the study design or on the analysis of results.
Outside the current study, the authors have the following conflicts of interest. K.M.T.A. has, over the past 5 years, received travel grants from Gilead, Pfizer, and the Nordic Society for Medical Mycology and a speaker honorarium (personal fee) from Pfizer. K.M.J. has, over the past 5 years, received travel grants from F2G and Amplyx and a meeting grant from MSD. R.K.J. has, over the past 5 years, received a travel grant and an unrestricted research grant from Gilead. M.C.A. has, over the past 5 years, received research grants/payment for contract work (paid to the SSI) from Amplyx, Basilea, Cidara, F2G, Gilead, Novabiotics, Scynexis, and T2Biosystems and speaker honoraria (personal fees) from Astellas, Gilead, Novartis, MSD, and SEGES. She is the current chairman of the EUCAST-AFST.
R.D. has no conflicts of interest to declare.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Buil JB, Rijs AJMM, Meis JF, Birch M, Law D, Melchers WJG, Verweij PE. 2017. In vitro activity of the novel antifungal compound F901318 against difficult-to-treat Aspergillus isolates. J Antimicrob Chemother 72:2548–2552. doi: 10.1093/jac/dkx177. [DOI] [PubMed] [Google Scholar]
- 2.Rivero-Menendez O, Cuenca-Estrella M, Alastruey-Izquierdo A. 2019. In vitro activity of olorofim (F901318) against clinical isolates of cryptic species of Aspergillus by EUCAST and CLSI methodologies. J Antimicrob Chemother 74:1586–1590. doi: 10.1093/jac/dkz078. [DOI] [PubMed] [Google Scholar]
- 3.Lackner M, Birch M, Naschberger V, Grässle D, Beckmann N, Warn P, Gould J, Law D, Lass-Flörl C, Binder U. 2018. Dihydroorotate dehydrogenase inhibitor olorofim exhibits promising activity against all clinically relevant species within Aspergillus section Terrei. J Antimicrob Chemother 73:3068–3073. doi: 10.1093/jac/dky329. [DOI] [PubMed] [Google Scholar]
- 4.Jørgensen KM, Astvad KMTT, Hare RK, Arendrup MC. 2018. EUCAST determination of olorofim (F901318) susceptibility of mold species, method validation, and MICs. Antimicrob Agents Chemother 62:e00487-18. doi: 10.1128/AAC.00487-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliver JD, Sibley GEMM, Beckmann N, Dobb KS, Slater MJ, McEntee L, Du Pré S, Livermore J, Bromley MJ, Wiederhold NP, Hope WW, Kennedy AJ, Law D, Birch M. 2016. F901318 represents a novel class of antifungal drug that inhibits dihydroorotate dehydrogenase. Proc Natl Acad Sci U S A 113:12809–12814. doi: 10.1073/pnas.1608304113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiederhold NP, Law D, Birch M. 2017. Dihydroorotate dehydrogenase inhibitor F901318 has potent in vitro activity against Scedosporium species and Lomentospora prolificans. J Antimicrob Chemother 72:1977–1980. doi: 10.1093/jac/dkx065. [DOI] [PubMed] [Google Scholar]
- 7.Biswas C, Law D, Birch M, Halliday C, Sorrell TC, Rex J, Slavin M, Chen SC-A. 2018. In vitro activity of the novel antifungal compound F901318 against Australian Scedosporium and Lomentospora fungi. Med Mycol 56:1050–1054. doi: 10.1093/mmy/myx161. [DOI] [PubMed] [Google Scholar]
- 8.Lim W, Eadie K, Konings M, Rijnders B, Fahal AH, Oliver JD, Birch M, Verbon A, van de Sande W. 2020. Madurella mycetomatis, the main causative agent of eumycetoma, is highly susceptible to olorofim. J Antimicrob Chemother 75:936–941. doi: 10.1093/jac/dkz529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talbot JJ, Frisvad JC, Meis JF, Hagen F, Verweij PE, Hibbs DE, Lai F, Groundwater PW, Samson RA, Kidd SE, Barrs VR, Houbraken J. 2019. cyp51A mutations, extrolite profiles, and antifungal susceptibility in clinical and environmental isolates of the Aspergillus viridinutans species complex. Antimicrob Agents Chemother 63:1–13. doi: 10.1128/AAC.00632-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Negri CE, Johnson A, McEntee L, Box H, Whalley S, Schwartz JA, Ramos-Martín V, Livermore J, Kolamunnage-Dona R, Colombo AL, Hope WW. 2018. Pharmacodynamics of the novel antifungal agent F901318 for acute sinopulmonary aspergillosis caused by Aspergillus flavus. J Infect Dis 217:1118–1127. doi: 10.1093/infdis/jix479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiederhold NP, Najvar LK, Jaramillo R, Olivo M, Birch M, Law D, Rex JH, Catano G, Patterson TF. 2018. The orotomide olorofim is efficacious in an experimental model of central nervous system coccidioidomycosis. Antimicrob Agents Chemother 62:e00999-18. doi: 10.1128/AAC.00999-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seyedmousavi S, Chang YC, Law D, Birch M, Rex JH, Kwon-Chung KJ. 2019. Efficacy of olorofim (F901318) against Aspergillus fumigatus, A. nidulans, and A. tanneri in murine models of profound neutropenia and chronic granulomatous disease. Antimicrob Agents Chemother 63:e00129-19. doi: 10.1128/AAC.00129-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hope WW, McEntee L, Livermore J, Whalley S, Johnson A, Farrington N, Kolamunnage-Dona R, Schwartz J, Kennedy A, Law D, Birch M, Rex JH. 2017. Pharmacodynamics of the orotomides against Aspergillus fumigatus: new opportunities for treatment of multidrug-resistant fungal disease. mBio 8:e01157-17. doi: 10.1128/mBio.01157-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arendrup MC, Meletiadis J, Mouton JW, Guinea J, Cuenca-Estrella M, Lagrou K, Howard SJ, Arendrup MC, Meletiadis J, Howard SJ, Mouton J, Guinea J, Lagrou K, Arikan-Akdagli S, Barchiesi F, Hamal P, Järv H, Lass-Flörl C, Mares M, Matos T, Muehlethaler K, Rogers TR, Torp Andersen C, Verweij P, Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). 2016. EUCAST technical note on isavuconazole breakpoints for Aspergillus, itraconazole breakpoints for Candida and updates for the antifungal susceptibility testing method documents. Clin Microbiol Infect 22:571.e1–571.e4. doi: 10.1016/j.cmi.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 15.CLSI. 2017. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, 3rd ed. CLSI standard M38. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 16.Buil J, Oliver J, Law D, Tehupeiory-Kooreman M, Rex J, Hokken M, Melchers W, Birch M, Verweij PE. 2019. Molecular mechanism and frequency of olorofim resistance in Aspergillus fumigatus, p P054. Abstr 9th Trends Med Mycol Conf 2019.
- 17.Tortorano AM, Richardson M, Roilides E, van Diepeningen A, Caira M, Munoz P, Johnson E, Meletiadis J, Pana ZD, Lackner M, Verweij P, Freiberger T, Cornely OA, Arikan-Akdagli S, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, Lanternier F, Pagano L, Skiada A, Akova M, Arendrup MC, Boekhout T, Chowdhary A, Cuenca-Estrella M, Guinea J, Guarro J, de Hoog S, Hope W, Kathuria S, Lortholary O, Meis JF, Ullmann AJ, Petrikkos G, Lass-Flörl C. 2014. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect 20:27–46. doi: 10.1111/1469-0691.12465. [DOI] [PubMed] [Google Scholar]
- 18.Lackner M, De Hoog GS, Verweij PE, Najafzadeh MJ, Curfs-Breuker I, Klaassen CH, Meis JF. 2012. Species-specific antifungal susceptibility patterns of Scedosporium and Pseudallescheria species. Antimicrob Agents Chemother 56:2635–2642. doi: 10.1128/AAC.05910-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alastruey-Izquierdo A, Cuenca-Estrella M, Monzon A, Mellado E, Rodriguez-Tudela JL. 2008. Antifungal susceptibility profile of clinical Fusarium spp. isolates identified by molecular methods. J Antimicrob Chemother 61:805–809. doi: 10.1093/jac/dkn022. [DOI] [PubMed] [Google Scholar]
- 20.Espinel-Ingroff A, Colombo AL, Cordoba S, Dufresne PJ, Fuller J, Ghannoum M, Gonzalez GM, Guarro J, Kidd SE, Meis JF, Melhem TMSC, Pelaez T, Pfaller MA, Szeszs MW, Takahaschi JP, Tortorano AM, Wiederhold NP, Turnidge J. 2016. International evaluation of MIC distributions and epidemiological cutoff value (ECV) definitions for Fusarium species identified by molecular methods for the CLSI broth microdilution method. Antimicrob Agents Chemother 60:1079–1084. doi: 10.1128/AAC.02456-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh A, Masih A, Monroy-Nieto J, Singh PK, Bowers J, Travis J, Khurana A, Engelthaler DM, Meis JF, Chowdhary A. 2019. A unique multidrug-resistant clonal Trichophyton population distinct from Trichophyton mentagrophytes/Trichophyton interdigitale complex causing an ongoing alarming dermatophytosis outbreak in India: genomic insights and resistance profile. Fungal Genet Biol 133:103266. doi: 10.1016/j.fgb.2019.103266. [DOI] [PubMed] [Google Scholar]
- 22.Saunte DML, Hare RK, Jørgensen KM, Jørgensen R, Deleuran M, Zachariae CO, Thomsen SF, Bjørnskov-Halkier L, Kofoed K, Arendrup MC. 2019. Emerging terbinafine resistance in Trichophyton: clinical characteristics, squalene epoxidase gene mutations, and a reliable EUCAST method for detection. Antimicrob Agents Chemother 63:e01126-19. doi: 10.1128/AAC.01126-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada T, Maeda M, Alshahni MM, Tanaka R, Yaguchi T, Bontems O, Salamin K, Fratti M, Monod M. 2017. Terbinafine resistance of Trichophyton clinical isolates caused by specific point mutations in the squalene epoxidase gene. Antimicrob Agents Chemother 61:e00115-17. doi: 10.1128/AAC.00115-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jørgensen KM, Astvad KMT, Hare RK, Arendrup MC. 2019. EUCAST susceptibility testing of isavuconazole: MIC data for contemporary clinical mold and yeast isolates. Antimicrob Agents Chemother 63:e00073-19. doi: 10.1128/AAC.00073-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guinea J, Verweij PE, Meletiadis J, Mouton JW, Barchiesi F, Arendrup MC, Arikan-Akdagli S, Castanheira M, Chryssanthou E, Friberg N, Järv H, Klimko N, Kurzai O, Lagrou K, Lass-Flörl C, Mares M, Matos T, Moore CB, Muehlethaler K, Rogers TR, Andersen CT, Velegraki A, Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). 2019. How to: EUCAST recommendations on the screening procedure E.Def 10.1 for the detection of azole resistance in Aspergillus fumigatus isolates using four-well azole-containing agar plates. Clin Microbiol Infect 25:681–687. doi: 10.1016/j.cmi.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Arendrup MC, Friberg N, Mares M, Kahlmeter G, Meletiadis J, Guinea J, Arendrup MC, Meletiadis J, Guinea J, Friberg N, Mares M, Kahlmeter G, Andersen CT, Barchiesi F, Chryssanthou E, Hamal P, Järv H, Klimko N, Kurzai O, Lagrou K, Lass-Flörl C, Matos T, Muehlethaler K, Rogers TR, Velegraki A. 17 June 2020. How to: interpret MICs of antifungal compounds according to the revised clinical breakpoints v. 10.0 European committee on antimicrobial susceptibility testing (EUCAST). Clin Microbiol Infect doi: 10.1016/j.cmi.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Mortensen KL, Mellado E, Lass-Flörl C, Rodriguez-Tudela JL, Johansen HK, Arendrup MC. 2010. Environmental study of azole-resistant Aspergillus fumigatus and other aspergilli in Austria, Denmark, and Spain. Antimicrob Agents Chemother 54:4545–4549. doi: 10.1128/AAC.00692-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arendrup MC, Jensen RH, Grif K, Skov M, Pressler T, Johansen HK, Lass-Flörl C. 2012. In vivo emergence of Aspergillus terreus with reduced azole susceptibility and a Cyp51a M217I alteration. J Infect Dis 206:981–985. doi: 10.1093/infdis/jis442. [DOI] [PubMed] [Google Scholar]
- 29.Turnidge J, Kahlmeter G, Kronvall G. 2006. Statistical characterization of bacterial wild-type MIC value distributions and the determination of epidemiological cutoff values. Clin Microbiol Infect 12:418–425. doi: 10.1111/j.1469-0691.2006.01377.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.