Abstract
Preclinical models that faithfully recapitulate tumor heterogeneity and therapeutic response are critical for translational breast cancer research. Immortalized cell lines are easy to grow and genetically modify to study molecular mechanisms, yet the selective pressure from cell culture often leads to genetic and epigenetic alterations over time. Patient-derived xenograft (PDX) models faithfully recapitulate the heterogeneity and drug response of human breast tumors. PDX models exhibit a relatively short latency after orthotopic transplantation that facilitates the investigation of breast tumor biology and drug response. The transplantable genetically engineered mouse models allow the study of breast tumor immunity. The current protocol describes the method to orthotopically transplant breast tumor fragments into the mammary fat pad followed by drug treatments. These preclinical models provide valuable approaches to investigate breast tumor biology, drug response, biomarker discovery and mechanisms of drug resistance.
Keywords: Cancer Research, Issue 159, breast cancer, preclinical model, mammary fat pad transplantation, tumor biology, drug response, biomarker
Introduction
Most breast cancer deaths can be ascribed to recurrent disease that is resistant to conventional therapies1,2. The inter- and intra-tumor heterogeneity of breast cancers contribute to therapy resistance. Moreover, tumor heterogeneity can impinge on accurate prognosis and challenge disease management3,4. Identification of predictive biomarkers of response will significantly improve clinical outcomes of patients with breast cancer. Even though most breast cancer types are immunologically ‘cold’ tumors that are likely unresponsive to immunotherapy, immune checkpoint inhibitors have shown promise in clinical trials2,5. For example, a phase III trial showed improved disease-free survival (DFS) and preliminary evidence that atezolizumab (monoclonal antibody against PD-L1) combined with nab-paclitaxel may provide an overall survival benefit as compared with nab-paclitaxel alone in tumors with ≥1% PD-L1 staining6. Development of therapies that sensitize breast tumors to immunotherapy will revolutionize treatment regimens.
Preclinical models that faithfully recapitulate human breast cancer heterogeneity and drug response are critical to study tumor biology and identify potential biomarkers for targeted therapy. Immortalized cell lines are widely used for breast cancer research since these cell lines are easy to grow and genetically modify to study molecular mechanisms. However, due to the selective pressure from long term cell culture in vitro, genetic drift may occur over time and breast cancer cell lines may carry cell line-specific genomic alterations that are distinct from aberrations in primary breast tumors7,8,9.
Patient-derived xenograft (PDX) tumor chunks are able to recapitulate the heterogeneity of human disease, and are histologically and immunohistochemically similar to the tumor of origin10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29. Importantly, PDX models are phenotypically stable across multiple transplantations as evidenced by histology, transcriptome, proteome and genomic analysis10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29. PDX models show treatment responses comparable to those observed clinically10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29. PDX models for estrogen receptor positive (ER+), progesterone receptor positive (PR+), epidermal growth factor 2 positive (ERBB2+, HER2+) and triple negative breast cancer (TNBC) PDX models have been established, and provide an excellent platform to test endocrine-, chemo- and targeted therapies. However, one main caveat of PDX models at present is the lack of a functional immune system in the mouse.
The genetically engineered mouse models (GEMM), such as Trp53 homozygous null, cMyc, Wnt1, PyMT, or Her2 overexpression models, allow the study of spontaneous tumor initiation, progression and metastasis in the context of an intact immune system. However, the tumor latency is long, which makes it difficult to conduct preclinical trials with multiple arms30,31. However, GEMM can be transplanted to syngeneic hosts to generate sufficient numbers of tumors that closely recapitulate human tumors32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55. For example, the mammary epithelium from a p53-null BALB/c mouse was transplanted into the cleared fat pads of syngeneic wild-type recipient mice to form primary tumors, which can be further transplanted into syngeneic hosts56,57. The p53-null tumors recapitulated different subtypes of human tumors.
The combination of PDX models and transplantable GEMM provides valuable preclinical tools to investigate breast tumor biology, drug response and anti-tumor immunity. In the current protocol, a method of orthotopic transplantation of PDX and GEMM tumor fragments into the mouse mammary fat pad is described. These models are amenable for serial passages and usually retain a stable phenotype. To mitigate the risk of genetic drift or loss of heterogeneity across passages over time, multiple tissue fragments are cryopreserved at each passage for subsequent transplantation in the event that biological or morphological changes are observed over time29,58.
Protocol
All protocols using animals have been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC). The tumor fragments, around 1–2 mm3 in size, are from viably frozen stock obtained from the Patient-Derived Xenograft and Advanced In Vivo Models Core at Baylor College of Medicine.
1. Preparation of cryopreserved mammary tumor fragments for transplantation
Transfer the cryovial with tumor fragments from liquid nitrogen to a 37 °C water bath.
Agitate the cryovial with an occasional gentle flick during thawing.
After the tissue is thawed, take the cryovial out of water bath and mix by gentle inversion.
Dry off the outside of the cryovial and spray with 70% ethanol. Transfer it to a biosafety hood.
Transfer the thawed tumor tissues into a 15 mL conical tube filled with 10 mL of cold Dulbecco’s modified Eagle medium (DMEM). Mix well by inverting the tube. Allow the tissue fragments to settle to the bottom of the tube.
Aspirate the supernatant and resuspend in 10 mL of cold DMEM. Mix well by inverting the tube. Allow the tissue fragments to settle to the bottom of the tube.
-
Aspirate the supernatant and resuspend in 10 mL of cold DMEM. Place the tube on ice.
NOTE: The tissue is ready for transplantation.
2. Collection and preparation of fresh mammary tumor for transplantation
-
Euthanize the breast tumor-bearing mouse.
NOTE: PDX host may be SCID/Beige, NSG or NRG female mice while in the current study female Balb/c mice aged 3–5 weeks are used.
-
Spray the tumor region of the euthanized mouse with 70% ethanol.
NOTE: Try to avoid hair with the tumor sample which might cause contamination of tumor fragments for cryostorage or transplantation.
With serrated forceps to pinch and lift up the skin surrounding the tumor, use scissors to make a short incision.
-
Separate the tumor from the skin with the scissors to dissect the whole tumor from the host mouse. Trim off any remaining mouse fat pad tissue from the outer surface of the tumor. Place the tumor in a 15 mL conical tube filled with 5 mL of cold DMEM.
NOTE: Use tumors at a maximum size of 1 cm diameter since larger tumors are likely to contain necrotic cores.
In a biosafety hood, transfer the dissected tumor to a sterile 10 cm Petri dish containing enough DMEM to prevent drying.
-
Cut the tumor into 1 mm3 fragments with scalpel or blade under aseptic conditions.
NOTE: The scalpel or blade should be exposed under UV in the biosafety hood for at least 20 min prior to use.
-
Transfer the tumor fragments to a 15 mL conical tube filled with cold DMEM. Place the tube on ice.
NOTE: The tissue is ready for transplantation. The dissected tumor fragments from step 2.4 can be, 1) snap frozen in liquid nitrogen for protein and RNA/DNA extraction, 2) fixed with 4% paraformaldehyde (PFA) or 10% neutral buffered formalin (NBF) for hematoxylin and eosin (H&E) and immunohistochemistry (IHC) analysis, or 3) cryopreserved in 1.25 mL freezing medium (10% dimethyl sulfoxide [DMSO], 40% DMEM and 50% fetal bovine serum [FBS]) by slow freezing in a −80 °C freezer overnight and then transferring to liquid nitrogen for long-term storage.
3. Prepare animal for surgery
For animal pain management, subcutaneously inject buprenorphine sustained release 60 min prior to the surgery at a dose of 1 mg/kg or follow institution’s guide for 72 h of analgesic coverage.
Set up the surgical suite according to institutional guidelines for aseptic surgeries.
-
Anesthetize a 4-week old female in an induction chamber of the isoflurane anesthesia machine at the rate of ~11.25 mL/h. Transfer the mouse to procedure area, onto the sterile (silicone rubber) surgery board, where it will receive anesthesia through a small facemask. Put the mouse on its back and tape the legs down in their natural positions.
NOTE: SCID/Beige, NSG or NRG are used for PDX and Balb/c is used for GEMM transplant models.
Apply ophthalmic ointment to the eyes to prevent dryness.
-
Confirm the appropriate level of sedation by toe pinch.
NOTE: No response/movement of the animal indicates that the animal is sufficiently anesthetized and ready for surgery.
Shave the mouse on the lower abdomen, especially the region around the fourth nipple where the surgery will take place.
Using a circular motion and starting in the center of the surgery site, work toward the outer edges with povidone-iodine surgical scrub, followed by removal of povidone-iodine with a 70% isopropyl ethanol pad. Repeat two additional times.
4. Transplantation of tumor fragments into the fourth (inguinal) mammary fat pad
-
Use a sterile surgical drape to cover the body of the animal except the incision site.
NOTE: Confirm the appropriate level of sedation by toe pinch before making the incision.
Using serrated forceps pinch and lift up the skin at the #4 nipple.
With the blunt side down, use scissors to make a short (about 1 cm), parasagittal incision, from the #4 nipple towards the head.
Using a cotton tipped applicator, separate the skin from the peritoneum on the medial side of the incision.
While holding the lateral side of the incision, gently peel the #4 mammary fat pad from the skin using the same swab.
Once the fat pad is separated, pin down the skin with a 27 G needle close to the animal’s body.
-
If it is experimentally necessary to clear the fat pad of endogenous mouse mammary epithelium perform the following steps.
Using the serrated forceps, gently extend the fat pad away from the body and locate the inguinal lymph node, which is beneath the intersection points of the major vessels in the gland (close to where the forceps will be holding the gland).
-
Carefully cauterize the vessels medial to the lymph node and the fat joining the fourth and fifth fat pads which forms a triangular area.
NOTE: Temporarily turn off the oxygen source for this step.
Using micro dissecting scissors, cut each cauterized vessel one at a time (to ensure there is no bleeding after each cut) until the section of the fat pad that contains the lymph node is removed.
Discard the “cleared” fat pad tissue.
Hold the fourth mammary fat pad using blunt separation forceps. With the other hand, insert the closed tip of the angled fine forceps into the middle of the fat pad above the remaining vessel and close to the wall of the peritoneum. Slowly open the forceps to make a small pocket. Remove the angled fine forceps from the fat pad.
Using the angled fine forceps, take a piece of tumor fragment and insert it into the pocket.
Slowly open the forceps tips to release the tumor fragment into the fat pad pocket.
-
Withdraw the angled fine forceps carefully.
NOTE: Look at the pocket to confirm that the tumor fragment remains in the pocket of the mammary fat pad when withdrawing the forceps. Tumors may be implanted into both contralateral fat pads when excess tumor tissue is needed for experiments or banking. Animals with double-sided transplants are not recommended for treatment studies because of known interactions between contralateral tumors. Alternatively, a trocar device might be used for the implantation process.
Starting from the base of the incision, collect the skin on each side using the claw and serrated forceps. Bring the two sides together and lift slightly to prepare the skin for wound clip application. Uncurl the edges of the incision with the claw forceps and pinch the edges together to form a continuous surface at the top.
Holding the two sides together with serrated forceps, use the wound clip applicator to place a wound clip in the center of the incision. If necessary, apply tissue adhesive to the ends of the incision in order to keep them closed and secured.
Place the animal into a clean cage that is on a warming surface. Monitor for bleeding, signs of dehiscence, and pain during the postsurgical period. The animal should be up and moving within minutes postsurgery.
Pay close attention to the incision site and overall health of the animals for at least 3 days following surgery. Follow institutional guidelines for pain management.
Clean the surgical tools for 10 s in a glass beads sterilizer before continuing with the next transplant. Wait for the tools to cool down prior to use.
-
Repeat steps 4.1–4.15 until all mice are transplanted.
NOTE: Estrogen supplementation is required for ER+ tumors, which can be supplied through water or slow release pellets59.
5. Monitoring tumor growth in response to drug treatment
NOTE: Palpable tumors of established transplantable PDXs and p53-null tumors takes between 2 weeks and 8 weeks to develop after surgery, depending on intrinsic tumor growth rates.
- Measure tumors in two dimensions using calipers twice a week. Calculate the volume using the formula:
where W is width and L is length of the tumor. -
Start drug treatment when the tumor volume reaches 150–300 mm3.
NOTE: Depending on the property of the drugs, oral gavage or intraperitoneal injection may be used to deliver the drugs.
Collect tumor samples and perform IHC staining60, protein and RNA/DNA isolation and immune phenotyping61 for various purposes. Collect blood and perform immune phenotyping or use it for pharmacokinetic/pharmacodynamic studies.
Representative Results
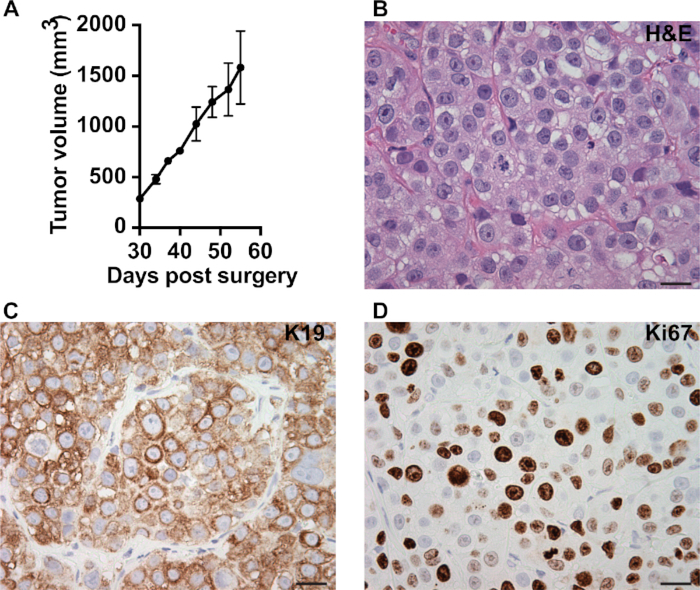
Figure 1 shows the equipment (Figure 1A) and key procedures (Figure 1B) of orthotopic transplantation. Figure 2 shows characterization of a transplanted PDX tumor (MC1). Tumor fragments (1 mm3) of MC1 model were transplanted into the #4 fat pad of SCID/Beige mice. One month later, the average tumor size reached around 350 mm3. Tumor volume was monitored twice a week for one month. Normally we obtain palpable tumors for various PDX or GEMM in around 2 weeks to 8 weeks with 1 mm3 tumor fragments transplanted (Figure 2A). Tumor samples can be collected for morphology and signaling analysis (Figure 2B–D). H&E staining was performed to analyze the pathology (Figure 2B). IHC was used to monitor markers for specific cell lineage (keratin 19 (K19), epithelial cell, Figure 2C), cell status (Ki67, proliferation, Figure 2D) or signaling molecule of interest.
Figure 1: Schematic showing the surgery technique.
(A) Surgical equipment required for the orthotopic transplantation. (B) Representative image showing the exposure of mammary fat pad for tumor trunk transplantation.
Figure 2: Characterization of the transplanted tumors.
(A) Representative kinetics of tumor growth measured by a caliper. Tumor volume (mm3) = W2 × L/2 (W = width and L = length). (B) H&E staining showing pathology of MC1 PDX. IHC showing epithelial marker keratin 19 (C) and proliferation maker Ki67 (D) in MC1 PDX. Scale bar = 20 μm, magnification = 40×.
Discussion
To reduce variations in tumor growth across animals, it is critical to cut the tumor tissue into 1 mm3 fragments for transplantation. Models that grow soft tissue are harder to work with and the tumor fragments need to be cut slightly larger (1–2 mm3). When placing the tissue into the mammary fat pad pocket take care not to split the tissue into multiple pieces as this will result in multiple small tumors or oddly shaped tumors.
In addition, use fresh tumor for transplanting animals that will be used for drug treatment studies. Implanting tissue from cryopreservation will yield a more variable take rate and slightly slower growth kinetics. Once tumors grow from the cryopreserved material, the second transplant generation will yield the typical take rate and growth kinetics for that model. Moreover, try to use tumors with no or mild necrosis for transplantation. For most models this will be a size range of 400–600 mm3. If an obvious necrotic core is observed, use tissue from the outer layer of the tumor for transplantation and do not use the necrotic areas. It is important to keep the tumor tissue on ice and to protect from drying.
To reduce variability among tumor chunks from GEMM that may have been derived from the periphery or tumor core with different microenvironments, an alternative method is to prepare a primary cell suspension and transplant approximately 5,000–30,000 cells depending upon the tumor model into the mammary fat pad. The limited collagenase digestion is carried out with 1 mg/mL type I collagenase in DMEM/F12 without any additives for 2 h at 37 °C rotating at 125 rpm. Mammary tumor cells can be enriched by 3 short centrifugation steps. Briefly, transfer the cell suspension to a 15 mL conical tube and centrifuge at 450 × g for 7 s. Aspirate the supernatant and resuspend the pellet in 10 mL of 1× Dulbecco’s phosphate buffered saline (DPBS). Repeat the pulse centrifugation for two more times. This will help randomize differences between chunks.
Transplantation of normal mammary epithelium will not regenerate a morphologically normal and functional ductal tree in the presence of endogenous mouse epithelium. It is necessary to remove the endogenous mouse epithelium (clearing) for the normal epithelial transplant to grow62. However, neoplastic tissue is able to grow even in the presence of intact endogenous mouse epithelium. Yet this does not necessarily mean no such inhibitory signals exist. Mammary fat pad clearing is necessary for certain experimental protocols to prohibit the interaction of endogenous mouse mammary epithelium with the engrafted material. In addition, the endogenous epithelium may complicate some downstream analysis such as genome, transcriptome and proteome analysis.
The PDX model and transplantable GEMM can faithfully recapitulate the heterogeneity of clinical subtypes and the response to drug therapy of human breast cancer. Importantly, these models are easy to transplant and maintain a stable phenotype during a limited number of serial passages. Tumor growth can be easily measured with calipers. One caveat of the PDX model and transplantable GEMM is that these models do not recapitulate early steps of tumor initiation. Also, PDX models lack the interaction of the tumor with a functional immune system. These preclinical models provide a valuable system to study breast cancer biology and evaluate drug response. Combining drug response with the genomic and proteomic information for each tumor model will facilitate the identification of biomarkers for response prediction and treatment resistance mechanisms. These types of data may lead to novel targeted therapies that could be used alone and in combination with chemotherapy or immunotherapy to improve patient outcomes.
Acknowledgments
This work was supported by the National Institutes of Health (R37CA228304 and R01HL146642 to Xi Chen, CA148761 to Jeffrey M. Rosen), US Department of Defense (W81XWH-19-1-0524 to Xi Chen, W81XWH-19-1-0035 to Xiangdong Lv), American Cancer Society (RSG-18-181-01-TBE to Xi Chen) and Cancer Prevention and Research Institute of Texas (RR150009 CPRIT Scholar in Cancer Research Award to Xi Chen), the Patient-Derived Xenograft and Advanced In Vivo Models Core at Baylor College of Medicine (funding from RP170691 CPRIT Core Facility Award and NCI-CA125123 P30 Cancer Center Support Grant).
Footnotes
Disclosures
MTL is the founder of a limited partner in StemMed, Ltd. and founder and manager in StemMed Holdings, its general partner. MTL is a founder of and an equity holder in Tvardi Therapeutics, Inc. LED is a compensated employee of StemMed, Ltd.
References
- 1.Waks AG, Winer EP Breast Cancer Treatment: A Review. JAMA. 321 (3), 288–300 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Harbeck N et al. Breast cancer. Nature Reviews Disease Primers. 5 (1), 66 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Harbeck N, Salem M, Nitz U, Gluz O, Liedtke C Personalized treatment of early-stage breast cancer: present concepts and future directions. Cancer Treatment Reviews. 36 (8), 584–594 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Zardavas D, Irrthum A, Swanton C, Piccart M Clinical management of breast cancer heterogeneity. Nature Reviews Clinical Oncology. 12 (7), 381 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Esteva FJ, Hubbard-Lucey VM, Tang J, Pusztai L Immunotherapy and targeted therapy combinations in metastatic breast cancer. The Lancet Oncology. 20 (3), e175–e186 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Schmid P et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. New England Journal of Medicine. 379 (22), 2108–2121 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Tsuji K et al. Breast cancer cell lines carry cell line-specific genomic alterations that are distinct from aberrations in breast cancer tissues: comparison of the CGH profiles between cancer cell lines and primary cancer tissues. BMC Cancer. 10 (1), 15 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neve RM et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 10 (6), 515–527 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke R Human breast cancer cell line xenografts as models of breast cancer-the immunobiologies of recipient mice and the characteristics of several tumorigenic cell lines. Breast Cancer Research and Treatment. 39 (1), 69–86 (1996). [DOI] [PubMed] [Google Scholar]
- 10.DeRose YS et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nature Medicine. 17 (11), 1514 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuperwasser C et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proceedings of the National Academy of Sciences of the United States of America of the United States of America. 101 (14), 4966–4971 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaillant F et al. Targeting BCL-2 with the BH3 mimetic ABT-199 in estrogen receptor-positive breast cancer. Cancer Cell. 24 (1), 120–129 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Li S et al. Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell Reports. 4 (6), 1116–1130 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeRose YS et al. Patient-derived models of human breast cancer: protocols for in vitro and in vivo applications in tumor biology and translational medicine. Current Protocols in Pharmacology. 60 (1), 14.23.11–14.23.43 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 100 (7), 3983–3988 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marangoni E et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clinical Cancer Research. 13 (13), 3989–3998 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Zhang H et al. Patient-derived xenografts of triple-negative breast cancer reproduce molecular features of patient tumors and respond to mTOR inhibition. Breast Cancer Research. 16 (2), R36 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shultz LD, Ishikawa F, Greiner DL Humanized mice in translational biomedical research. Nature Reviews Immunology. 7 (2), 118 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Sheffield LG, Welsch CW Transplantation of human breast epithelia to mammary-gland-free fat-pads of athymic nude mice: Influence of mammotrophic hormones on growth of breast epithelia. International Journal of Cancer. 41 (5), 713–719 (1988). [DOI] [PubMed] [Google Scholar]
- 20.Sebesteny A et al. Primary human breast carcinomas transplantable in the nude mouse. Journal of the National Cancer Institute. 63 (6), 1331–1337 (1979). [PubMed] [Google Scholar]
- 21.Sakakibara T et al. Growth and metastasis of surgical specimens of human breast carcinomas in SCID mice. The Cancer Journal from Scientific American. 2 (5), 291–300 (1996). [PubMed] [Google Scholar]
- 22.Rae-Venter B, Reid LM Growth of human breast carcinomas in nude mice and subsequent establishment in tissue culture. Cancer Research. 40 (1), 95–100 (1980). [PubMed] [Google Scholar]
- 23.Outzen H, Custer R Brief communication: Growth of human normal and neoplastic mammary tissues in the cleared mammary fat pad of the nude mouse. Journal of the National Cancer Institute. 55 (6), 1461–1466 (1975). [DOI] [PubMed] [Google Scholar]
- 24.Noël A et al. Heterotransplantation of primary and established human tumour cells in nude mice. Anticancer Research. 15 (1), 1–7 (1995). [PubMed] [Google Scholar]
- 25.Naundorf H, Fichtner I, Büttner B, Frege J Establishment and characterization of a new human oestradiol-and progesterone-receptor-positive mammary carcinoma serially transplantable in nude mice. Journal of Cancer Research and Clinical Oncology. 119 (1), 35–40 (1992). [DOI] [PubMed] [Google Scholar]
- 26.Murthy MS, Scanlon EF, Jelachich ML, Klipstein S, Goldschmidt RA Growth and metastasis of human breast cancers in athymic nude mice. Clinical and Experimental Metastasis. 13 (1), 3–15 (1995). [DOI] [PubMed] [Google Scholar]
- 27.Fichtner I, Becker M, Zeisig R, Sommer A In vivo models for endocrine-dependent breast carcinomas: special considerations of clinical relevance. European Journal of Cancer. 40 (6), 845–851 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Ding L et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 464 (7291), 999 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X et al. A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Cancer Research. 73 (15), 4885–4897 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borowsky AD Choosing a mouse model: experimental biology in context-the utility and limitations of mouse models of breast cancer. Cold Spring Harbor Perspectives in Biology. 3 (9), a009670 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caligiuri I, Rizzolio F, Boffo S, Giordano A, Toffoli G Critical choices for modeling breast cancer in transgenic mouse models. Journal of Cellular Physiology. 227 (8), 2988–2991 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Backlund MG et al. Impact of ionizing radiation and genetic background on mammary tumorigenesis in p53-deficient mice. Cancer Research. 61 (17), 6577–6582 (2001). [PubMed] [Google Scholar]
- 33.Jerry D et al. A mammary-specific model demonstrates the role of the p53 tumor suppressor gene in tumor development. Oncogene. 19 (8), 1052–1058 (2000). [DOI] [PubMed] [Google Scholar]
- 34.Hüsler MR et al. Lactation-induced WAP-SV40 Tag transgene expression in C57BL/6J mice leads to mammary carcinoma. Transgenic Research. 7 (4), 253–263 (1998). [DOI] [PubMed] [Google Scholar]
- 35.Simin K et al. pRb inactivation in mammary cells reveals common mechanisms for tumor initiation and progression in divergent epithelia. PLoS Biology. 2 (2), e22 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandgren EP et al. Inhibition of mammary gland involution is associated with transforming growth factor α but not c-myc-induced tumorigenesis in transgenic mice. Cancer Research. 55 (17), 3915–3927 (1995). [PubMed] [Google Scholar]
- 37.Gallahan D et al. Expression of a truncated Int3 gene in developing secretory mammary epithelium specifically retards lobular differentiation resulting in tumorigenesis. Cancer Research. 56 (8), 1775–1785 (1996). [PubMed] [Google Scholar]
- 38.Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 55 (4), 619–625 (1988). [DOI] [PubMed] [Google Scholar]
- 39.Guy CT, Cardiff R, Muller WJ Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Molecular and Cellular Biology. 12 (3), 954–961 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guy CT et al. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proceedings of the National Academy of Sciences of the United States of America. 89 (22), 10578–10582 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu X et al. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nature Genetics. 22 (1), 37 (1999). [DOI] [PubMed] [Google Scholar]
- 42.Maroulakou IG, Anver M, Garrett L, Green JE Prostate and mammary adenocarcinoma in transgenic mice carrying a rat C3 (1) simian virus 40 large tumor antigen fusion gene. Proceedings of the National Academy of Sciences of the United States of America. 91 (23), 11236–11240 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin Y et al. Characterization of medroxyprogesterone and DMBA-induced multilineage mammary tumors by gene expression profiling. Molecular Carcinogenesis. 44 (1), 42–50 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Cressman VL et al. Mammary tumor formation in p53-and BRCA1-deficient mice. Cell Growth and Differentiation-Publication American Association for Cancer Research. 10 (1), 1–10 (1999). [PubMed] [Google Scholar]
- 45.Li Z et al. ETV6-NTRK3 fusion oncogene initiates breast cancer from committed mammary progenitors via activation of AP1 complex. Cancer Cell. 12 (6), 542–558 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pond AC et al. Fibroblast growth factor receptor signaling dramatically accelerates tumorigenesis and enhances oncoprotein translation in the mouse mammary tumor virus-Wnt-1 mouse model of breast cancer. Cancer Research. 70 (12), 4868–4879 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sinn E et al. Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo. Cell. 49 (4), 465–475 (1987). [DOI] [PubMed] [Google Scholar]
- 48.Muller WJ et al. The int-2 gene product acts as an epithelial growth factor in transgenic mice. The EMBO Journal. 9 (3), 907–913 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu S et al. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell. 15 (6), 539–550 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torres-Arzayus MI et al. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell. 6 (3), 263–274 (2004). [DOI] [PubMed] [Google Scholar]
- 51.Chan SR et al. STAT1-deficient mice spontaneously develop estrogen receptor α-positive luminal mammary carcinomas. Breast Cancer Research. 14 (1), R16 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang Z et al. Rb deletion in mouse mammary progenitors induces luminal-B or basal-like/EMT tumor subtypes depending on p53 status. The Journal of Clinical Investigation. 120 (9), 3296–3309 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adams JR et al. Cooperation between Pik3ca and p53 mutations in mouse mammary tumor formation. Cancer Research. 71 (7), 2706–2717 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Pei X-H et al. CDK inhibitor p18INK4c is a downstream target of GATA3 and restrains mammary luminal progenitor cell proliferation and tumorigenesis. Cancer Cell. 15 (5), 389–401 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bultman S et al. Characterization of mammary tumors from Brg1 heterozygous mice. Oncogene. 27 (4), 460 (2008). [DOI] [PubMed] [Google Scholar]
- 56.Jerry D et al. A mammary-specific model demonstrates the role of the p53 tumor suppressor gene in tumor development. Oncogene. 19 (8), 1052 (2000). [DOI] [PubMed] [Google Scholar]
- 57.Zhang M et al. Identification of tumor-initiating cells in a p53-null mouse model of breast cancer. Cancer Research. 68 (12), 4674–4682 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Landis MD, Lehmann BD, Pietenpol JA, Chang JC Patient-derived breast tumor xenografts facilitating personalized cancer therapy. Breast Cancer Research. 15 (1), 201 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang X, Lewis MT Establishment of Patient-Derived Xenograft (PDX) Models of Human Breast Cancer. Current Protocols in Mouse Biology. 3 (1), 21–29 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Chi V, Chandy KG Immunohistochemistry: paraffin sections using the Vectastain ABC kit from vector labs. Journal of Visualized Experiments. (8), e308 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao N et al. Pharmacological targeting of MYC-regulated IRE1/XBP1 pathway suppresses MYC-driven breast cancer. Journal of Clinical Investigation. 128 (4), 1283–1299 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DeOme K, Faulkin L, Bern HA, Blair PB Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Research. 19 (5), 515 (1959). [PubMed] [Google Scholar]