Abstract
Protein handling, modification and folding in the endoplasmic reticulum (ER) are tightly regulated processes that determine cell function, fate and survival. In several tumour types, diverse oncogenic, transcriptional and metabolic abnormalities cooperate to generate hostile microenvironments that disrupt ER homeostasis in malignant and stromal cells, as well as infiltrating leukocytes. These changes provoke a state of persistent ER stress that has been demonstrated to govern multiple pro-tumoural attributes in the cancer cell while dynamically reprogramming the function of innate and adaptive immune cells. Aberrant activation of ER stress sensors and their downstream signalling pathways have therefore emerged as key regulators of tumour growth and metastasis as well as response to chemotherapy, targeted therapies and immunotherapy. In this Review, we discuss the physiological inducers of ER stress in the tumour milieu, the interplay between oncogenic signalling and ER stress response pathways in the cancer cell and the profound immunomodulatory effects of sustained ER stress responses in tumours.
The endoplasmic reticulum (ER) is a central organelle where secreted and transmembrane proteins are synthesized, folded and modified. Although this process is exquisitely regulated, multiple external factors and cell-intrinsic events can disrupt the protein-folding capacity of this organelle and provoke a state of ER stress that is characterized by the build-up of misfolded or unfolded proteins. Diverse genetic, transcriptional and metabolic abnormalities enriched in tumours create adverse microenvironments that cause persistent ER stress in tumour cells, which ultimately impact their function, fate and survival (FIG. 1). In mammalian cells, three ER transmembrane proteins operate as sensors of ER stress1: activating transcription factor 6 (ATF6), inositol-requiring enzyme 1α (IRE1α) and PRKR-like ER kinase (PERK) (BOX 1). Under conditions of proteostasis, the molecular chaperone binding-immunoglobulin protein (BiP; also known as GRP78) binds to these sensors and maintains them in an inactive state2,3. During periods of ER stress, BiP shows higher affinity binding for misfolded or unfolded proteins and therefore dissociates from the sensors. This event enables their activation and the subsequent induction of the unfolded protein response (UPR; also known as the ER stress response), which is an adaptive mechanism capable of reinstating ER homeostasis through multiple mechanisms encompassing transcriptional reprogramming and mRNA decay, global translational attenuation, removal of misfolded proteins via the ER-associated protein degradation (ERAD) system and recycling of misfolded proteins and cellular materials through the induction of autophagy (BOX 1). Productive, non-lethal, ER stress responses restore ER homeostasis, and thus promote cell adaptation to stress and survival. By contrast, unresolved or extreme ER stress can lead to cell death3. For instance, proteasome inhibitors used as anticancer agents induce unresolved lethal ER stress4. In addition, professional secretory cells with a constitutively active UPR are highly susceptible to additional ER stress, thus triggering cell death1. Importantly, it is becoming clear that signalling through ER stress sensors can further modulate UPR-independent transcriptional and metabolic pathways in a cell-specific and context-dependent manner, consequently governing cellular phenotypes that are implicated in cancer initiation, progression and response or resistance to therapy. As the ER is in close and dynamic contact with other organelles such as the nucleus, mitochondria and Golgi apparatus, ER-intrinsic alterations can drastically impact the regulation of global cellular processes1. This Review focuses on the common drivers of ER stress in the tumour microenvironment (TME), the interplay between oncogenic events and ER stress responses, and the mechanisms by which ER stress response pathways influence diverse tumorigenic and immune-regulatory programmes to dictate malignant progression, antitumour immunity and response to treatment.
Fig. 1 |. Inducers of endoplasmic reticulum stress in the tumour microenvironment.
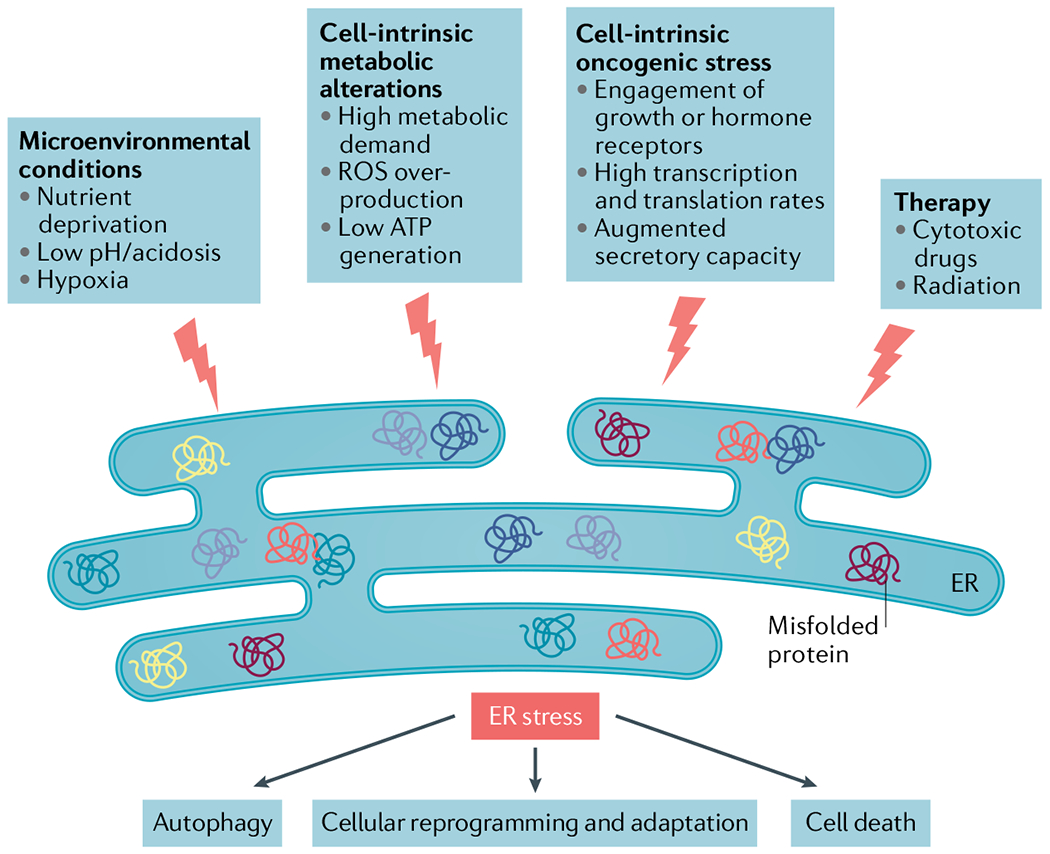
The uncontrolled proliferative capacity of malignant cells in growing tumours engenders hostile microenvironments characterized by high metabolic demand, hypoxia, nutrient limitations and acidosis, which in turn provoke disruption of calcium and lipid homeostasis in multiple cell types inhabiting this milieu. Collectively, these harsh conditions alter the protein-folding capacity of the endoplasmic reticulum (ER) in both cancer cells and infiltrating immune cells, thereby promoting accumulation of misfolded or unfolded proteins within this organelle and, consequently, ER stress. Oncogenic events in cancer cells further contribute to this state by elevating their global transcription and translation rates. The unfolded protein response (UPR) is subsequently activated as an attempt to restore ER homeostasis and promote adaptation to diverse insults in the tumour. Certain therapeutic modalities can also trigger ER stress in the cancer cell to alter their normal behaviour in the tumour microenvironment (TME). Depending on the magnitude of ER stress, the cell type and the specific pathological context, ER stress responses can have multiple effects ranging from cellular reprogramming and adaptation to autophagy and apoptosis. Owing to the additive effects of various ER stressors concurrently enriched in the TME during cancer initiation, progression and therapy, robust and persistent UPR activation is mostly evidenced in cancer cells and tumour-infiltrating immune cells in vivo, which has been challenging to recapitulate under in vitro conditions. ROS, reactive oxygen species.
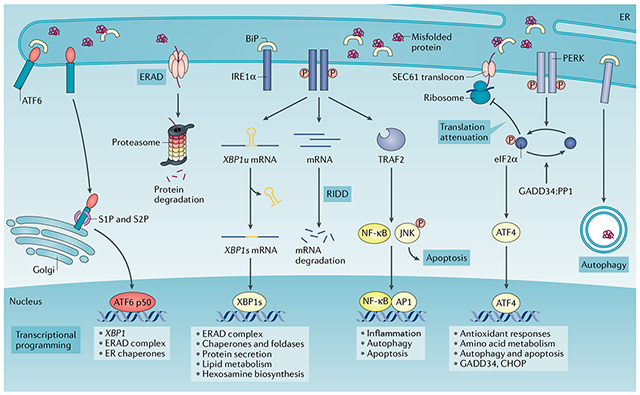
Box 1 |. Sensing and responding to endoplasmic reticulum stress: canonical roles of the unfolded protein response.
Protein folding and modification in the endoplasmic reticulum (ER) is a highly regulated process. Yet various intrinsic and extrinsic stressors can disrupt proteostasis in this organelle, leading to ER stress. The unfolded protein response (UPR) is a highly conserved, adaptive mechanism with three branches that coordinates responses to the detrimental accumulation of unfolded or misfolded proteins. Indeed, the UPR is instigated by perturbation of ER homeostasis owing to alterations in protein synthesis rates, mutations that lead to protein misfolding or fluctuations in the ER folding environment, which include, but are not limited to, alterations in the redox state, nutrient status and calcium levels19,169. In metazoans, the UPR is initiated by three ER-resident transmembrane proteins that function as sensors of protein-folding stress: inositol-requiring protein 1α (IRE1α), PRKR-like ER kinase (PERK) and activating transcription factor 6 (ATF6). Under normal conditions, the molecular chaperone binding-immunoglobulin protein (BiP) binds to these sensors to restrain their activation3. However, during periods of ER stress, BiP is titrated away from the sensors owing to its capacity to bind misfolded proteins with higher affinity, thus leading to activation of the ER stress sensors and their downstream signalling. IRE1α–X-box binding protein 1 (XBP1) is the most evolutionarily conserved arm of the UPR170,171. Upon activation, IRE1α oligomerizes and undergoes autophosphorylation, causing a conformational change that activates its RNase domain to excise a 26-nucleotide fragment from the XBP1 mRNA in the cytosol172. This unconventional splicing event generates a modified mRNA isoform (XBP1s) that codes for the functionally active protein XBP1 (also known as XBP1s), which operates as a potent and multifunctional transcription factor172. In the absence of IRE1α activation, unspliced XBP1 (XBP1u) is translated into a highly unstable protein with poor transcriptional activation properties. This unique activation mechanism allows for rapid XBP1 isoform switching in response to changes in ER proteostasis, enabling the induction of genes encoding chaperones, foldases and ER-associated protein degradation (ERAD) components, as well as transcriptional networks controlling lipid and hexosamine biosynthetic programmes172,173. Peptides and unfolded proteins can also directly bind to the major histocompatibility complex (MHC)-like groove in the ER-luminal domain of the IRE1α core, causing allosteric changes that lead to its activation174,175. Under particular conditions, the RNase domain of IRE1α can also degrade certain mRNAs in a process termed regulated IRE1-dependent decay (RIDD), which reduces the burden of proteins entering the ER176,177. By interacting with different adaptor and modulator proteins, IRE1α can further activate the JUN N-terminal kinase (JNK), p38 and nuclear factor-κB (NF-κB) pathways, thus modulating inflammation, autophagy and apoptosis57,61,178. Additional ER stress or UPR-independent functions of IRE1α include the regulation of cytoskeleton remodelling by binding to filamin A and the control of mitochondrial function by facilitating calcium transfer between the ER and the mitochondrion179,180.
PERK is a serine–threonine kinase, the best-characterized substrate of which is the eukaryotic translation initiation factor 2α (eIF2α)181. PERK-dependent phosphorylation of eIF2α reduces protein synthesis by globally inhibiting 5′ cap-dependent translation while selectively increasing the cap-independent translation of ATF4. ATF4 subsequently induces the transcription factor C/EBP homologous protein (CHOP, also known as DDIT3) and multiple other genes involved in the regulation of autophagy, amino acid metabolism, antioxidant responses and cell death182–184. Under ER stress, ATF6 is translocated to the Golgi apparatus where it is cleaved by S1P (also known as MBTPS1) and S2P (also known as MBTPS2) proteases, releasing its N-terminal fraction (ATF6 p50) that functions as a transcription factor185. Cleaved ATF6 (ATF6 p50) forms a heterodimer with XBP1 and mediates the induction of the primary (unspliced) XBP1 transcript and other genes that are involved in protein folding and degradation in the ER, such as molecular chaperones, foldases and ERAD system components186,187. AP1, activator protein 1; GADD34:PP1, growth arrest and DNA-damaged protein 34 bound to serine/threonine protein phosphatase 1; P, phosphorylation; TRAF2, tumour necrosis factor receptor-associated factor 2.
Common drivers of ER stress in the TME
Multiple stressors enriched in the TME dynamically perturb the protein-folding capacity of the ER in malignant and stromal cells (FIG. 1). As it is difficult to recapitulate the physiological array of stressors in vitro, it is crucial to study ER stress biology in vivo using preclinical cancer models and freshly isolated patient-derived specimens. Various experimental tools have been developed to detect and monitor ER stress responses. The majority of these involve the use of elegant reporter cell lines or transgenic mice that express fluorescent proteins upon activation of specific arms of the UPR5–7. However, these tools have not been extensively utilized in cancer research, and future preclinical studies would benefit from using these reporters to monitor ER stress in vivo. As these systems cannot be exploited to analyse human clinical specimens, phosphorylation of ER stress sensors, induction of UPR canonical target genes, changes in ER morphology and specific modifications in ER-resident proteins have all been used as putative indicators of ER stress. The future development of novel imaging and biochemical approaches that enable accurate and direct assessment of ER stress in samples from patients with cancer will be instrumental to advance the field and direct potential clinical interventions using UPR modulators.
Hypoxia.
Lack of oxygen is a common feature in the TME that perturbs ER homeostasis and induces stress in this compartment8–11 (FIG. 1). While formation of disulfide bonds during protein synthesis can occur without oxygen, post-translational folding or isomerization are oxygen-dependent processes. The perturbation of post-translational oxygen-dependent disulfide bond formation contributes to hypoxia-induced ER stress12. Furthermore, hypoxia restricts the function of the oxygen-dependent ER localized oxidoreductase ERO1α, which is required for disulfide bond formation and protein folding13. Oxygen is also necessary for lipid desaturation. Hypoxic cells have decreased content of desaturated lipids, which limits ER expansion and thus triggers ER stress14. However, it is noteworthy that only extreme hypoxia induces robust ER stress, with modest hypoxia (1–5% O2) having minimal effects on UPR activation15,16. This observation has raised the possibility that the magnitude of ER stress and UPR activation inside the tumour are spatially controlled.
Nutrient availability.
Metabolic stress, characterized by insufficient or excessive nutrient supply in comparison with normal cellular energetic needs, can readily disrupt ER homeostasis (FIG. 1). Glucose and glutamine availability are intimately connected with ER stress in multiple ways. The lack of glucose or glutamine interrupts the hexosamine biosynthetic pathway (HBP), which uses these two nutrients to generate uridine diphosphate-N-acetylglucosamine (UDP-GlcNAc) that is necessary for N-linked glycosylation and folding of proteins in the ER17,18. Glucose restriction also affects ATP production, which operates as an energy source and a phosphate donor required for protein folding in the ER19. Furthermore, limited glucose results in dysregulated calcium flux in the ER mediated by sarcoplasmic/ER calcium ATPase (SERCA) activity20. Insufficient amino acid availability is another key stressor in the TME. Amino acid starvation activates the kinase GCN2 that induces eukaryotic translation initiation factor 2α (eIF2α) phosphorylation and activates the integrated stress response (ISR)21, which has emerged as an essential stress adaptation mechanism in cancer cells. Lastly, obesity is often associated with higher cancer risk22, and saturated long-chain fatty acids such as palmitate and stearate that are enriched in high-fat diets cause alterations in ER membrane size, composition and fluidity, which can, in turn, affect calcium stores and impair protein glycosylation, eventually leading to ER stress23.
Intracellular accumulation of reactive oxygen species.
Protein folding in the ER is heavily dependent on the redox status of this organelle. Intracellular accumulation of reactive oxygen species (ROS) in response to external conditions or evoked by diverse signalling events can drastically perturb ER proteostasis24 (FIG. 1). For instance, limiting levels of glutamine fuels ER stress by disrupting glutathione production and altering the redox milieu of the ER lumen25. High amounts of ROS can also be generated at the inner membrane of mitochondria as a by-product of the electron transport chain (ETC), especially during fatty acid β-oxidation (FAO)26. Moreover, signalling events initiated by the sensing of pro-inflammatory cytokines and growth factors, or upon engagement of pattern recognition receptors (PRRs), can result in sustained activation of downstream NADPH oxidases (NOXs) that generate copious amounts of ROS26. In these settings, excessive intracellular ROS accumulation can provoke protein-folding stress by altering ER-resident calcium channels27 and by promoting the generation of lipid peroxidation by-products that form stable adducts with ER-resident protein chaperones28,29. ROS overproduction drives PERK-mediated stabilization of nuclear factor erythroid 2-related factor 2 (NRF2) to limit the oxidative damage induced by these radicals30.
Low pH.
Cancer cells use aerobic glycolysis as a central metabolic pathway, thereby producing lactic acid that lowers the pH of the surrounding microenvironment (FIG. 1). Activation of proton-sensing receptors upon detection of low pH can trigger the three arms of the UPR in various cell types31,32, likely by disrupting intracellular calcium homeostasis and/or inducing ROS overproduction32–35.
ER stress responses in the cancer cell
The UPR in oncogenic transformation and tumour growth.
Beyond the adverse environmental conditions generated by tumours, genetic alterations in the cancer cell can fuel ER stress and promote persistent activation of UPR pathways (FIG. 1). For instance, loss of tumour suppressors and hyperactivation of oncogenes readily increase protein synthesis to meet the increased metabolic demand during tumorigenesis. In addition, proliferating cancer cells require rapid ER expansion for division and allocation to daughter cells36. Orchestrating ER stress responses is a highly dynamic process that could result in both pro-survival and pro-apoptotic outputs. Indeed, cell fate determination appears to depend on the intensity and duration of the UPR (FIG. 2; BOX 2). In the past 15 years, multiple studies have uncovered relevant roles for ER stress response pathways in cancer initiation and progression.
Fig. 2 |. The magnitude of endoplasmic reticulum stress and its differential outcomes in malignant cells.
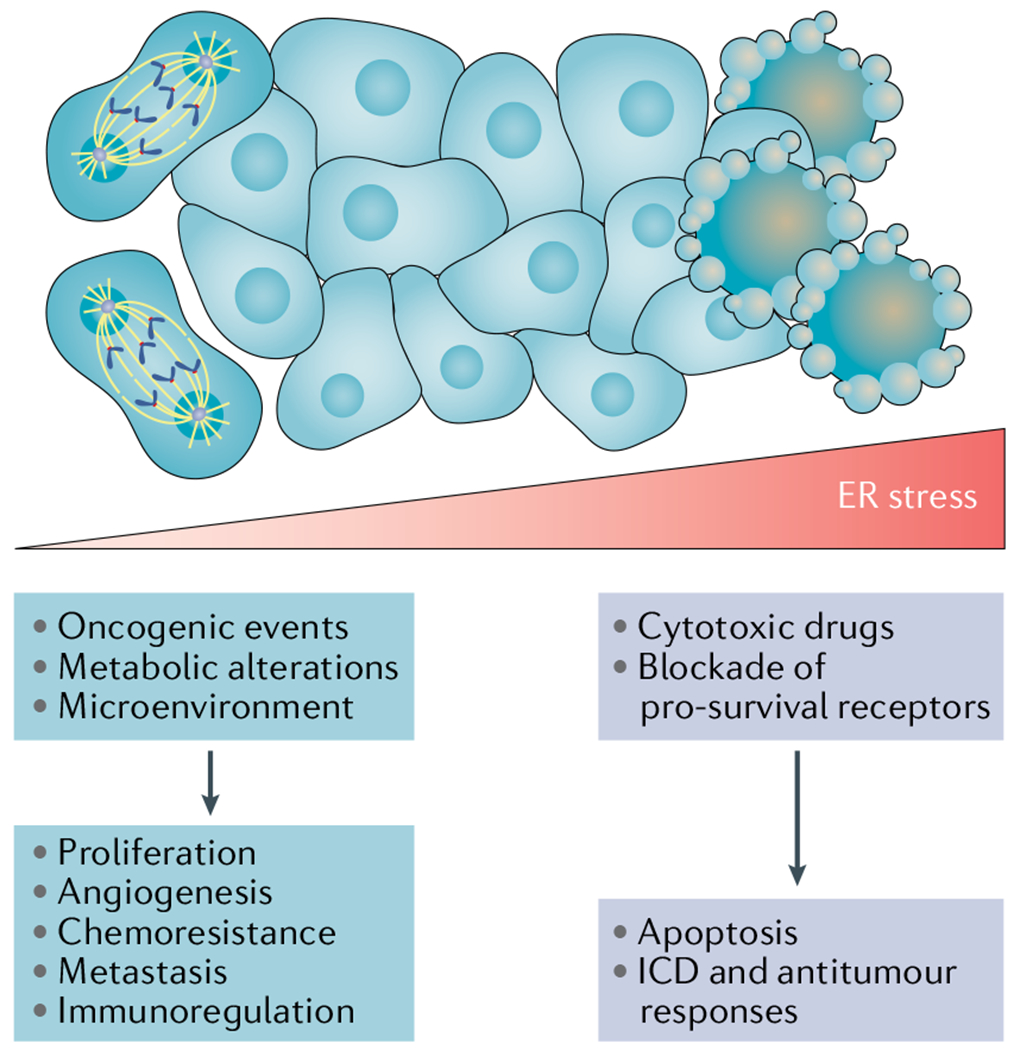
Persistent, yet moderate, endoplasmic reticulum (ER) stress responses fuelled by oncogenic pathways, metabolic changes and conditions of the tumour microenvironment stimulate several mechanisms that promote cancer cell proliferation, metastasis, chemoresistance, angiogenesis and immune evasion. By contrast, extreme ER stress caused by the uncontrolled accumulation of misfolded proteins in this organelle can lead to a terminal unfolded protein response (UPR) that induces cell death. For instance, proteasome inhibitors have been shown to trigger proapoptotic ER stress responses in multiple myeloma cells by hyperactivating the PRKR-like ER kinase (PERK)–eukaryotic translation initiation factor 2α (eIF2α)–activating transcription factor 4 (ATF4)–C/EBP homologous protein (CHOP) arm of the UPR. Of note, exposure to some cytotoxic agents, such as anthracyclines, can trigger ER stress responses that promote immunogenic cell death (ICD) capable of eliciting antitumour immunity (BOX 2). Hence, the consequences of UPR activation, either pro-survival or pro-apoptotic, are determined by the duration and intensity of the stress.
Box 2 |. Endoplasmic reticulum stress responses and immunogenic cell death.
Lethal endoplasmic reticulum (ER) stress responses induced by some forms of chemotherapy have been shown to trigger immunogenic cell death (ICD) that evokes protective antitumour immune responses (FIG. 2). Although this topic was previously reviewed elsewhere188, recent studies have expanded our understanding of the role of ER stress response factors in the induction of ICD. For instance, treatment with cytotoxic drugs of the anthracycline family has been demonstrated to trigger overproduction of reactive oxygen species (ROS) in the cancer cell. This process causes severe ER stress and induces the localization of the ER-associated chaperone calreticulin to the cancer cell surface while promoting the release of damage-associated molecular patterns (DAMPs), such as double-stranded DNA (dsDNA), ATP and high mobility group protein B1 (HMGB1) prior to causing cell death189,190. Calreticulin binds to scavenger receptors and CD91, whereas HMGB1 concurrently triggers Toll-like receptor 2 (TLR2) and/or TLR4 signalling on neighbouring phagocytic immune cells. These events initiate various signalling pathways that, ultimately, facilitate the attraction, licensing and maturation of dendritic cells capable of priming or reactivating tumour-specific T cells189,190. Recent studies by Kroemer and colleagues revealed that ICD-inducing anthracyclines and pharmacological agents that provoke tetraploidization in the cancer cell preferentially enhance the phosphorylation of eukaryotic translation initiation factor 2α (eIF2α) without activating other ER stress response factors, such as activating transcription factor 4 (ATF4), ATF6 or X-box binding protein 1s (XBP1s)191. Furthermore, it has been shown that inhibition of the pro-survival receptor tyrosine kinase ephrin type-B receptor 4 (EPHB4) also induces eIF2α phosphorylation and ICD in prostate cancer cells by suppressing glucose uptake and reducing the intracellular ATP levels192. Interestingly, specific polyphenol fractions isolated from the plant Caesalpinia spinosa can induce canonical ICD and T cell-driven antitumour immunity in preclinical models of melanoma and breast cancer193,194, but recent in vitro mechanistic experiments suggest that these effects are predominantly mediated by activation of the ER stress sensor PRKR-like ER kinase (PERK), independently of eIF2α phosphorylation195.
In contrast to the ICD-promoting effects of PERK or eIF2α signalling described above, the inositol-requiring protein 1α (IRE1α)–XBP1 arm of the unfolded protein response was shown to mediate resistance to ICD in colorectal cancer cells treated with the combination of chemotherapy and monoclonal antibodies blocking the epidermal growth factor receptor (EGFR)196. However, the mechanisms by which IRE1α–XBP1 signalling antagonizes ICD in this setting remain elusive. Furthermore, it was reported that reducing dietary protein content by ~25% could induce ER stress and IRE1α overactivation in malignant cells of the tumour microenvironment in various mouse models of cancer197. In this particular setting, IRE1α activation was found to promote antitumour immune responses by triggering regulated IRE1-dependent decay of RNA (RIDD) and the consequential generation of small RNA fragments that stimulated the antiviral innate immune response receptor RIG-I in the cancer cell197. However, the potential involvement of ICD as a mediator of this process was not determined. Lastly, it would be worth testing whether ICD-inducing binding-immunoglobulin protein (BiP) inhibitors, such as HA15 and KP1339, can enhance the therapeutic effects of immune checkpoint blockers.
Oncogenic transformation is a multistep process that exploits the UPR to overcome diverse barriers. Hyperactivation of MYC in normal epithelial cells induces massive proteotoxic stress and leads to decreased cell survival37. Yet, cells enduring MYC-induced stress exhibit enhanced activation of the UPR in multiple human cancers, including lymphoma, neuroblastoma, prostate cancer and breast cancer37–42. Whereas normal mammary epithelial cells or cancer cells expressing low levels of MYC are resistant to X-box binding protein 1 (XBP1) ablation, MYC-hyperactivated cells are highly sensitive to the genetic or pharmacological inactivation of IRE1α–XBP1 (REFS37,40). Therefore, an intact UPR is critical for adaptation to stress caused by MYC-driven oncogenic transformation (FIG. 3).
Fig. 3 |. Integration of oncogenic programmes and endoplasmic reticulum stress responses in the cancer cell.
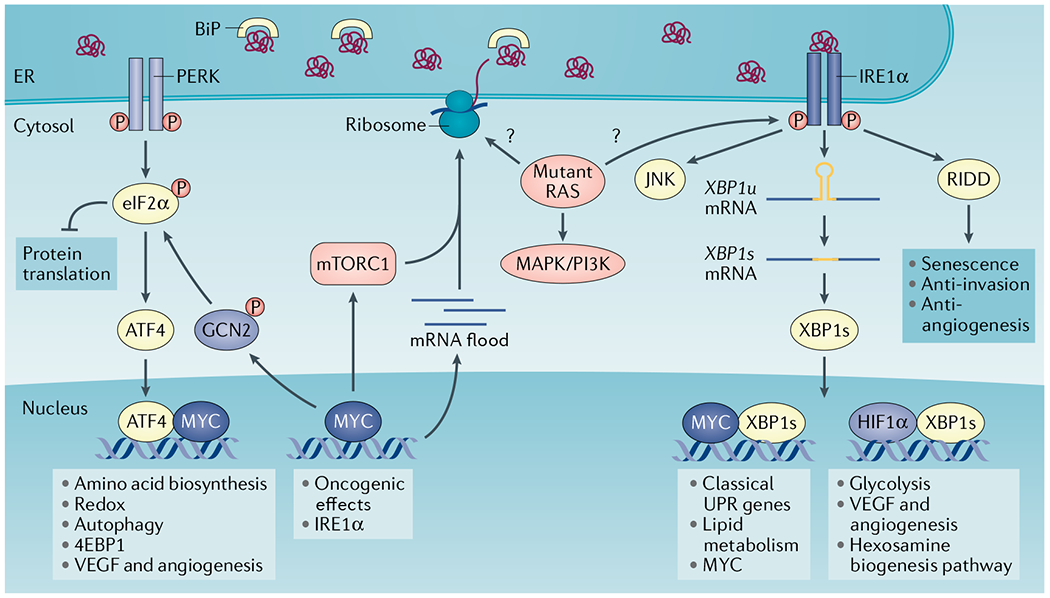
Oncogenic MYC activates the unfolded protein response (UPR) through multiple mechanisms. MYC-induced upregulation of global transcription (mRNA flood) and translation increases ribosome biogenesis and protein load in the endoplasmic reticulum (ER), thus activating all branches of the UPR. MYC further binds to promoter and enhancer regions in the gene encoding inositol-requiring protein 1α (IRE1α), positively regulating its transcription and augmenting IRE1α protein levels. MYC can also form a heterodimer with X-box binding protein 1s (XBP1s) in the nucleus to regulate classical UPR genes and lipid metabolism genes. Of note, XBP1s has been shown to promote MYC transcription in prostate cancer cells and natural killer cells. MYC engages PRKR-like ER kinase (PERK) and general control non-derepressible 2 (GCN2) kinase to induce eukaryotic translation initiation factor 2α (eIF2α) phosphorylation and the integrated stress response. MYC can also interact with activating transcription factor 4 (ATF4) to regulate amino acid transporters and biosynthesis, antioxidant pathways and autophagy. The MYC–ATF4 complex regulates eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1) to reduce translation and proteotoxic stress. mTOR complex 1 (mTORC1) activation induces protein synthesis and ER overload that activates the UPR. In turn, the IRE1α–tumour necrosis factor receptor-associated factor 2 (TRAF2)–JUN N-terminal kinase (JNK)–insulin receptor substrate 1 (IRS1) axis has been shown to restrict mTORC1 activity. Mutant RAS is integrated within the UPR in a context-specific manner. Mutant HRAS preferentially induces IRE1α activity in keratinocytes through an unknown mechanism. In primary human melanocytes, HRAS-G12V–PI3K, but not BRAF-V600E, increases ER content and induces activation of all UPR branches. It is unclear whether mutant RAS enhances global protein translation and protein load in the ER, which promotes ER stress in all cancer types. BiP, binding-immunoglobulin protein; HIF1α, hypoxia-inducible factor 1α; P, phosphorylation; RIDD, regulated IRE1-dependent decay of RNA; VEGF, vascular endothelial growth factor.
Mutant RAS is another oncogenic driver that interplays with the UPR (FIG. 3). HRAS-G12V triggers IRE1α and ATF6 activation to promote premature senescence and suppress neoplastic growth in melanocytes43. The regulated IRE1-dependent decay of RNA (RIDD) activity of IRE1α has been shown to mediate premature senescence in keratinocytes through degradation of inhibitor of DNA binding 1 (ID1) mRNA. However, this process was independent of XBP1 splicing or mitigation of ER stress using compounds that promote protein folding44. By contrast, IRE1α–XBP1 signalling enhances HRAS-induced proliferation of keratinocytes44. Genetic screens in yeast have also identified IRE1α as a synthetic lethal partner of mutant KRAS45. Nevertheless, the mechanisms behind the cell-specific function of IRE1α upon mutant RAS activation remain to be determined. Furthermore, the functional importance of XBP1 splicing versus RIDD outputs of the IRE1α RNase activity in mutant RAS-driven tumour initiation and progression warrants further studies using preclinical animal models.
After oncogenic transformation, cancer cells undergo persistent, non-lethal, ER stress responses coordinated by IRE1α and PERK activation that facilitate adaptation and tumour growth (FIG. 3). Constitutive activation of IRE1α promotes XBP1-dependent expression of classical UPR genes, such as chaperones, foldases and the ERAD machinery. These effectors support the degradation of misfolded proteins while enhancing protein folding and secretion, thus sustaining the cytoprotective function of the UPR. IRE1α–XBP1 signalling is also involved in reprogramming of cancer metabolism. XBP1 regulates hypoxia responses and glycolysis by forming a heterodimer with hypoxia-inducible factor 1α (HIF1α) and controlling the expression of key regulators including glucose transporter 1 (GLUT1) and lactate dehydrogenase A (LDHA) in triple-negative breast cancer (TNBC)16,46,47 (FIG. 3). As mentioned above, the HBP is the branch of glycolysis responsible for the generation of UDP-GlcNAc, a key substrate for protein glycosylation, which has been implicated in cancer progression48. XBP1 directly binds to the promoter of the rate-limiting enzyme glutamine:fructose-6-phosphate amidotransferase 1 (GFAT1) to enhance the HBP49 (FIG. 3). XBP1 also controls lipid metabolism by transcriptionally inducing key enzymes such as stearoyl-CoA-desaturase 1 (SCD1), fatty acid synthase (FASN), ATP-citrate lyase (ACLY), acetyl-CoA carboxylase 1 (ACC1), 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) and 3-hydroxy-3-methylglutaryl-coenzyme A synthase 1 (HMGCS1)40,50. MYC-overexpressing tumours rely on XBP1-regulated SCD1 to generate unsaturated lipids that maintain ER homeostasis and cell growth40. In mouse models of pancreatic cancer, depletion of SWI/SNF chromatin remodeller SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 (Smarcb1) activates mutant KRAS-independent MYC and IRE1α–MKK4-mediated mesenchymal reprogramming, which provides a potential therapeutic target for the aggressive mesenchymal subtype of pancreatic cancer51. These mechanisms ensure an efficient adaptation to various oncogenic and metabolic stresses that cancer cells encounter during progression.
Recent studies have also begun to reveal the function of IRE1α-driven RIDD in cancer. In glioblastoma cell lines in vitro, the RNase activity of IRE1α plays two contrasting functions: XBP1 promotes infiltration by myeloid cells (described below) while also increasing angiogenesis and enhancing expression of migration and invasion markers52. By contrast, RIDD attenuates angiogenesis and cancer cell migration52. Therefore, the balance between XBP1 activation and RIDD has been proposed to be associated with the clinical outcome of patients with glioblastoma52. RIDD is also a key determinant of normal cell fate instructed by PERK and IRE1α crosstalk via death receptor 5 (DR5). ER stress can initiate apoptosis through PERK–C/EBP homologous protein (CHOP)-activated intracellular DR5 independent of its canonical extracellular ligand tumour necrosis factor-related apoptosis-inducing ligand (TRAIL)53. Misfolded proteins also act as direct ligands that activate DR5 intracellularly, promoting apoptosis53. The RIDD activity of IRE1α counteracts this process by degrading the DR5 mRNA54, but PERK can attenuate IRE1α phosphorylation and RNase activity through the phosphatase RNA polymerase II-associated protein 2 (RPAP2)55. Although the balance between PERK activation and IRE1α RIDD activity governs cell fate, the function of PERK-regulated and IRE1α-regulated DR5 in cancer cells remains to be determined. Under excessive ER stress, IRE1α RNase hyperactivation has been shown to promote apoptosis by cleaving select microRNAs (miR-17, miR-34a, miR-96 and miR-125b), allowing translation of proapoptotic caspase 2 and induction of the mitochondrial apoptosis pathway56. However, the implications of IRE1α-regulated microRNA function in cancer also remain largely unexplored.
The cytoplasmic domain of IRE1α interacts with tumour necrosis factor receptor-associated factor 2 (TRAF2) to stimulate the JUN N-terminal kinase (JNK) pathway and promote apoptosis by suppressing BCL-2 activity and inducing BIM (also known as BCL2L11) function57–59. Hence, long-term uncontrolled IRE1α activation is harmful to cancer cells. IRE1α undergoes autophosphorylation, but additional direct substrates of the IRE1α kinase domain remain largely unexplored. IRE1α has been shown to promote XBP1-independent signal transducer and activator of transcription 3 (STAT3) phosphorylation and growth of hepatocellular carcinoma (HCC)60. In addition, the kinase activity of IRE1α is required for ER stress-induced nuclear factor-κB (NF-κB) activation by forming a complex with IκB kinase (IKK) through TRAF2 (REF.61). The IRE1α–TRAF2–apoptosis signal-regulating kinase 1 (ASK1)–JNK axis also promotes NF-κB and activator protein 1 (AP1) activation to enhance inflammatory responses in cancer cells62,63 (BOX 1).
Beyond its common pro-tumorigenic function, IRE1α–XBP1 signalling was recently reported to suppress tumour growth in germinal centre B cell-like diffuse large B-cell lymphoma (GCB-DLBCL). IRE1α expression is silenced by enhancer of zeste homologue 2 (EZH2), blocking XBP1 activation in GCB-DLBCL. However, this phenotype can be rescued by ectopic overexpression of XBP1s leading to suppression of GCB-DLBCL progression in xenograft mouse models64. Therefore, IRE1α–XBP1 downregulation appears to distinguish GCB-DLBCL from other DLBCL subtypes, but the underlying mechanism accounting for the distinct tumour suppressor function of IRE1α–XBP1 in this specific cancer subtype remains elusive.
BiP is overexpressed in multiple human cancers and promotes tumour growth via diverse mechanisms, such as enhancing growth factor maturation and secretion, suppressing apoptosis and promoting angiogenesis2,65,66. A fraction of BiP localizes on the cancer cell surface2,67, where it mediates signal transduction by forming complexes with other surface proteins. For instance, cell surface-localized BiP increases AKT signalling to enhance prostate cancer cell survival68, and it interacts with Cripto, a multifunctional cell surface protein, to facilitate prostate cancer growth by suppressing transforming growth factor-β (TGFβ) signalling69. Therefore, BiP has been considered an attractive therapeutic target in human cancers.
PERK employs various mechanisms to regulate tumour progression. First, a key function of this sensor is to control oxidative stress by increasing biosynthesis of the antioxidant glutathione70. PERK phosphorylates NRF2, causing its dissociation from the Kelch-like ECH-associated protein 1 (KEAP1) complex and enabling its function as a transcriptional inducer of protective antioxidant responses71. PERK activation also upregulates ERO1α to facilitate protein folding in the ER72,73. The resultant increase in oxidative protein folding capacity is beneficial for tumour growth. Second, the PERK–eIF2α axis attenuates global translation and enhances autophagy to promote cytoprotective UPR functions in MYC-driven lymphoma41. Third, both PERK and GCN2 are activated by MYC to phosphorylate eIF2α and induce ATF4 (REF.39). Although eIF2α transiently slows down translation, this is not sufficient to alleviate MYC-induced proteotoxic stress39. Hence, ATF4 forms a complex with MYC to directly upregulate eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1) and suppress mTOR complex 1 (mTORC1)-dependent signalling and prevent proteotoxicity upon MYC activation39 (FIG. 3). ATF4 also regulates amino acid transporters to sustain tumour growth39,74. PERK and GCN2 need to be simultaneously suppressed to reduce ATF4, therefore impeding MYC-driven tumour progression39. Fourth, PERK regulates lipid metabolism and phospholipid biology via multiple mechanisms. PERK controls the expression of key lipogenic enzymes, such as FASN, ACLY and SCD1, by promoting the maturation and activation of sterol regulatory element-binding protein (SREBP) in mammary epithelial cells75. PERK-dependent eIF2α phosphorylation and inhibition of protein synthesis triggers the depletion of insulin-induced gene 1 protein (INSIG1), an ER-localized protein that retains the SREBP–SREBP cleavage-activating protein (SCAP) complex in the ER membrane, and results in the translocation of SREBP to the Golgi for cleavage and activation75,76. PERK also has lipid kinase activity, which phosphorylates diacylglycerol (DAG) and generates phosphatidic acid as a major product. The lipid kinase activity of PERK is regulated by the PI3K p85 subunit and mediates mTOR, AKT and ERK1 and ERK2 activation during ER stress77. However, the PERK-dependent regulation of lipid metabolism in tumour progression warrants further investigation. Lastly, PERK-driven regulation of non-coding RNA is critical to promote cancer cell survival. miR-211 is a PERK-dependent pro-survival microRNA in mouse mammary tumours and human B cell lymphoma that induces histone H3 lysine 27 (H3K27) methylation at the CHOP promoter to repress its expression and sustain cell survival78. MYC-dependent activation of the PERK–miR-211 axis also suppresses the circadian regulators brain and muscle ARNT-like 1 (BMAL1) and circadian locomotor output cycles kaput (CLOCK) to reduce circadian oscillation and protein synthesis, thus promoting the progression of Burkitt’s lymphoma79.
Although both IRE1α and PERK function to alleviate ER stress and maintain cancer cell survival, multiple studies suggest that they are not functionally redundant in most cases. For instance, in MYC-driven cancers, ablation of either IRE1α or PERK reduced tumour progression37,40,41. How cancer cells maintain the pro-tumoural functions of IRE1α and PERK without triggering their pro-apoptotic activity is not well understood. In addition to the increased protein synthesis and folding demand, it is also crucial to understand whether oncogenic signalling directly regulates the key UPR factors through ER stress-independent mechanisms, and if so, what is the advantage of such specific regulation?
The UPR in metastasis and dormancy.
Whereas the function of the UPR in primary tumour progression has been studied extensively, its role in the multistep metastatic programme remains incompletely defined. Cancer cells can reprogramme distant organ niches to facilitate the colonization and survival of metastatic cells, a process termed pre-metastatic niche (PMN) formation80,81. Hypoxia upregulates lysyl oxidase (LOX) in oestrogen receptor-negative breast tumours to promote PMN formation in the bone80. Interestingly, XBP1 binds to the LOX promoter and regulates its expression82, raising the possibility that IRE1α–XBP1 could contribute to PMN via this factor. During metastasis, cancer cells undergo anoikis, a form of programmed cell death, after losing contact with the extracellular matrix (ECM) and neighbouring cells83,84. Several studies suggest that the PERK–eIF2α axis suppresses anoikis and is required for tumour invasion and metastasis85–87. PERK is also selectively activated in cells undergoing epithelial-to-mesenchymal transition (EMT) with enhanced secretory capacity85. Inactivation of PERK compromised the in vitro invasion of breast cancer cells undergoing EMT85. Metastatic cells experience higher levels of oxidative stress in circulation and distant tissues than cancer cells in primary tumours88. Metabolic adaptations, such as the synthesis of antioxidants, are indispensable for the survival and eventual outgrowth of cancer cells at distant sites. The PERK branch promotes antioxidant responses through ATF4 and NRF2, and, thus, possibly benefits metastatic cells by alleviating oxidative stress71.
Recent studies also show an increased UPR in dormant malignant cells from patients and mouse models of cancer, including breast cancer, squamous carcinoma, colorectal carcinoma and pancreatic ductal adenocarcinoma (PDAC)89–93. The UPR may induce dormancy as an adaptation to survive the hostile microenvironments of distal organs90–92. For example, both BiP and the ER molecular chaperone GRP94 (also known as endoplasmin) are highly expressed in disseminated tumour cells (DTCs) present in the bone marrow of patients with breast cancer89. IRE1α activation might promote cancer cell entry into quiescence by inducing p38, a critical regulator of tumour dormancy in human cancers90–92,94. MKK6 and p38 induce the nuclear translocation and activation of ATF6 in dormant squamous carcinoma cells. ATF6 is essential for quiescent, but not proliferative, squamous carcinoma cells to adapt to chemotherapy, nutritional stress and the hostile microenvironment in vivo through the induction of GTP binding protein RHEB and mTOR signalling independent of AKT90. PERK inhibits the translation of cyclin D1 and cyclin D3 and cyclin-dependent kinase 4 (CDK4), thus restricting cancer cells in the G0–G1 phase91,95. Overactivation of the PERK pathway is evident in dormant PDAC DTCs present in the liver of patients and mouse models, where the unresolved ER stress appears to determine the fate of the PDAC DTCs93. Although these studies suggest the importance of the UPR in the survival of dormant cancer cells, some questions remain unanswered. First, as DTCs have very limited protein synthesis, how is the UPR activated in these quiescent cells and maintained at a high level? Second, unresolved ER stress usually leads to cell lethality, so how do DTCs tolerate and exploit this persistent ER stress? Collectively, activation of the UPR might be instrumental in promoting stressed cancer cells to enter into dormancy and sustain their initial survival.
Modulation of the tumour immune microenvironment by ER stress in the cancer cell.
A body of work has indicated that cancer cell-intrinsic ER stress responses can influence malignant progression by altering the function of immune cells that coexist in the TME (FIG. 4). Early studies suggested that induction of ER stress and activation of the UPR could inhibit surface expression of major histocompatibility complex class I (MHC-I) molecules, likely via XBP1s and ATF6 overexpression96. Eliciting ER stress in mouse EL4 lymphoma cells by exposure to palmitate or glucose deprivation was found to cause eIF2α-mediated inhibition of protein synthesis and subsequent impairment of optimal peptide loading onto MHC-I proteins, which compromised their stability and normal surface localization97. Furthermore, ER-stressed epithelial cells demonstrated XBP1-dependent induction of miR-346, which post-transcriptionally repressed expression of the ER transporter involved in antigen processing 1 (TAP1) that is implicated in optimal ER peptide influx and MHC-I antigen loading98. Despite these interesting observations, it remains unclear whether persistent induction of UPR-associated pathways in cancer cells facilitates tumour immune evasion by blunting MHC-I-mediated antigen presentation to CD8+ T cells.
Fig. 4 |. Immunomodulatory effects of endoplasmic reticulum stress signals in the tumour microenvironment.
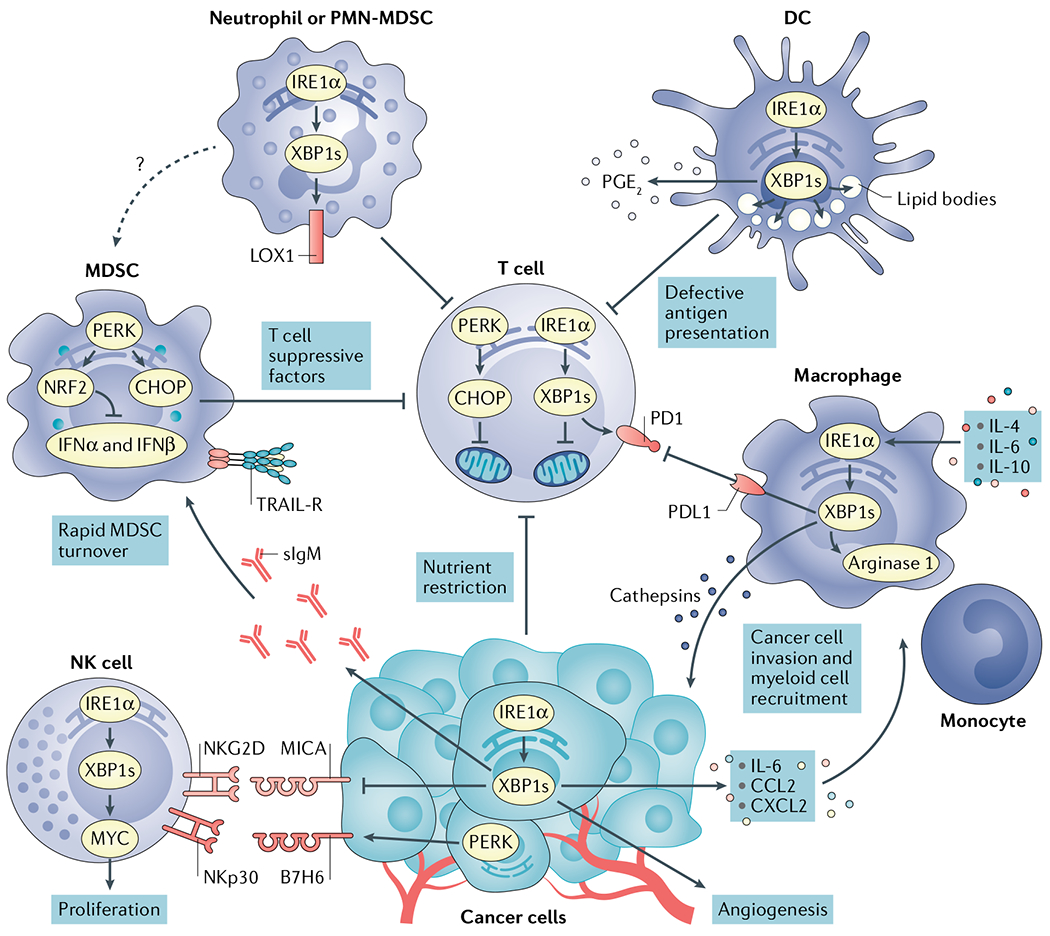
Cancer cells undergoing activation of inositol-requiring protein 1α (IRE1α) or PRKR-like ER kinase (PERK) modulate tumour recognition by natural killer (NK) cells while secreting mediators that promote angiogenesis and recruitment of myeloid cell types to tumour sites. Both IRE1α and PERK are well established to regulate angiogenesis168. X-box binding protein 1s (XBP1s) and activating transcription factor 4 (ATF4) directly bind to the vascular endothelial growth factor (VEGF) promoter to regulate its expression. Nutrient restriction, reactive oxygen species (ROS) accumulation or the presence of soluble factors that blunt glucose uptake cause endoplasmic reticulum (ER) stress and chronic activation of the IRE1α–XBP1 and PERK–C/EBP homologous protein (CHOP) arms of the unfolded protein response (UPR) in intratumoural T cells, provoking mitochondrial dysfunction and inhibition of their optimal anticancer effector function. High levels of cholesterol in the tumour microenvironment (TME) can also activate IRE1α–XBP1 signalling in intratumoural T cells to induce programmed cell death protein 1 (PD1) expression and limit their protective activity. Myeloid-derived suppressor cells (MDSCs) exploit PERK to control antitumour immunity via CHOP-mediated expression of T cell suppressive factors and by inducing nuclear factor erythroid 2-related factor 2 (NRF2)-driven responses that inhibit production of protective type I interferon. ER stress in MDSCs has also been associated with their elevated expression of tumour necrosis factor-related apoptosis-inducing ligand-receptors (TRAIL-Rs) and rapid turnover in the TME. ER stress-related gene signatures and expression of lectin-type oxidized LDL receptor 1 (LOX1) distinguish normal neutrophils from polymorphonuclear (PMN)-MDSCs in patients with cancer. In addition, ER-stressed neutrophils acquire immunosuppressive attributes and overexpress LOX1 via IRE1α activation. ROS accumulation fuels ER stress and persistent IRE1α–XBP1 activation in tumour-associated dendritic cells (DCs), driving uncontrolled lipid droplet formation that inhibits their capacity to present local antigens to intratumoural T cells. ER-stressed DCs have also been shown to overproduce the immunosuppressive lipid mediator prostaglandin E2 (PGE2) via IRE1α–XBP1 activation, presumably contributing to immune escape in cancer. In macrophages, the IRE1α–XBP1 branch has been shown to promote expression of cathepsins, PD1 ligand 1 (PDL1) and Arginase 1, further promoting cancer cell invasion and immunosuppression in the TME. CCL2, CC-chemokine ligand 2; CXCL2, CXC-chemokine ligand 2; IFNα, interferon-α; IL-6, interleukin-6; MICA, MHC class I polypeptide-related sequence A; NKGD2, natural killer group 2D; sIgM, secretory immunoglobulin M.
ER stress responses in the cancer cell have been proposed to alter natural killer (NK) cell-mediated recognition of tumours. The IRE1α–XBP1 arm repressed expression of the NK group 2D (NKG2D) ligand MHC class I polypeptide-related sequence A (MICA) in human melanoma cell lines undergoing in vitro-induced ER stress99 (FIG. 4), and reduced MICA expression in human melanoma samples was negatively associated with the intrinsic levels of XBP1s in the same specimen99. Whereas it is unknown whether disabling IRE1α–XBP1 in malignant cells can promote NK cell-driven antitumour responses, another study indicated that activation of the PERK–eIF2α arm of the UPR in melanoma cells undergoing pharmacological ER stress could induce expression of B7H6 (FIG. 4), which is a ligand for the NK cell receptor NKp30 (REF.100). Accordingly, ER-stressed melanoma cells overexpressing B7H6 were sensitized to killing by chimeric antigen receptor (CAR) T cells specifically redirected against this ligand100. Therefore, additional research is necessary to discern how activation of specific ER stress sensors in malignant cells either prevents or promotes tumour recognition by NK cells. Nevertheless, studies have indicated that ER-stressed cancer cells can drastically alter the recruitment and function of immune cells at tumour locations (FIG. 4). IRE1α overactivation in TNBC cells facilitates the production of pro-inflammatory and immunomodulatory cytokines, such as interleukin-6 (IL-6), IL-8, CXC-chemokine ligand 1 (CXCL1) and granulocyte–macrophage colony-stimulating factor (GM-CSF)101. Ablating IRE1α remodelled the TME in TNBC by increasing pericyte levels and promoting vascular normalization while decreasing accumulation of cancer-associated fibroblasts (CAFs) and myeloid-derived suppressor cells (MDSCs)102. Work by Chevet and colleagues further demonstrated that IRE1α–XBP1 signalling in glioblastoma cells promoted their capacity to express IL-6, CC-chemokine ligand 2 (CCL2) and CXCL2, which can participate in the chemo-attraction of macrophages and monocytes to the TME52 (FIG. 4). Furthermore, the extent of XBP1s expression in patient-derived glioblastoma samples correlated with increased macrophage infiltration in the same specimen52. Nevertheless, additional research is necessary to functionally define whether IRE1α-driven overexpression of pro-inflammatory factors in cancer cells promotes progression in these two malignancies by modulating the tumour immune contexture.
ER-stressed cancer cells can release additional factors that recruit or alter the function of myeloid cells in the tumour. Administration of the ER stressor thapsigargin to mice bearing CT26-derived colon tumours promoted the recruitment and immunosuppressive activity of MDSCs, which could be attenuated upon treatment with compounds that relieve protein-folding stress103. In genetically engineered mouse models of chronic lymphocytic leukaemia (CLL), malignant cells exploited the IRE1α–XBP1 arm to facilitate the overproduction of secretory immunoglobulin M (sIgM), which in turn promoted the accumulation and immunoregulatory function of MDSCs, presumably via signalling through sialic acid-binding immunoglobulin-like lectin G (Siglec-G) and/or complement receptors expressed on these innate immune cells104 (FIG. 4). Disabling IRE1α–XBP1 in CLL cells reduced MDSC accumulation and controlled their immunosuppressive activity, thus delaying malignant progression in this model104. HCC cells treated with the ER stressor tunicamycin released exosomes containing high amounts of miR-23a-3p, which upregulated programmed cell death protein 1 ligand 1 (PDL1) expression in macrophages via modulation of the PTEN–AKT pathway105. Macrophages exposed to exosomes derived from ER-stressed HCC cells demonstrated suppressive activity towards CD8+ T cells105, and histological expression of ER stress response markers BiP, ATF6, PERK and IRE1α in human HCC specimens correlated with increased infiltration by CD68+PDL1+ macrophages and poor patient prognosis105. Of note, a recent study demonstrated that ER stress sensor IRE1α facilitates production of hepatocyte-derived extracellular vesicles (EVs) that recruit macrophages to the liver to promote inflammation in the setting of diet-induced steatohepatitis106. In addition, mouse myeloid cells exposed to factors secreted by prostate cancer, lung cancer or melanoma cells experiencing ER stress demonstrated UPR activation that was accompanied by the induction of pro-tumorigenic and immunosuppressive functions. This process, termed ‘transmissible ER stress’107, was shown to upregulate immunosuppressive Arginase 1 and prostaglandin E2 (PGE2) in dendritic cells (DCs) while simultaneously inhibiting their capacity to cross-present antigens to CD8+ T cells108. Therefore, DCs conditioned in vitro with supernatants from ER-stressed cancer cells acquired an immunosuppressive phenotype that stimulated tumour growth after adoptive transfer into mice bearing B16.F10 melanomas108. As the mechanisms mediating this type of transmissible ER stress are unknown, it would be pertinent to test whether exosomes or EVs released from ER-stressed cancer cells are implicated in the process. Indeed, EVs have been shown to contain diverse types of RNAs that could trigger ISR-dependent p38 activation in normal cells located in distal organs, which would subsequently phosphorylate interferon-α/β receptor 1 (IFNAR1) to enable its degradation by β-transducin repeat-containing protein (β-TRCP)109,110. Hence, this form of transmissible stress may have major implications in the regulation of antitumour immunity and PMN formation.
Other studies have indicated that ER stress in the cancer cell can modulate T cell-mediated control of tumour growth, metastasis and response to immunotherapy. In mouse models of pancreatic cancer, artificial overexpression of XBP1s in malignant cells, together with systemic T cell depletion using antibody-based approaches, facilitated the outgrowth of macrometastatic lesions93. This phenotype was attributed to reduced ER stress in cancer cells overexpressing XBP1s (REF.93), but additional studies are necessary to address whether enforced expression of this transcription factor in pancreatic cancer cells induces UPR-independent pro-tumorigenic or immunosuppressive programmes that stimulate metastatic outgrowth. In a different setting, analysis of mice lacking the ubiquitin ligase ring finger protein 5 (RNF5) revealed diminished IRE1α–XBP1 activation and decreased expression of UPR gene markers in their intestinal epithelial cells, which was associated with CCL5-mediated DC recruitment and activation, and reduced production of antimicrobial peptides111. These effects altered the gut microbiota composition and promoted the development of T cell-driven responses capable of restraining melanoma growth in mouse models111. Knocking down XBP1 directly in melanoma cells enhanced the immunotherapeutic effects of treatment with antibodies blocking programmed cell death protein 1 (PD1) and cytotoxic T lymphocyte-associated antigen 4 (CTLA4). Importantly, reduced expression of XBP1s, ATF4 and BiP in pretreatment tumour biopsy samples correlated with improved responses and extended survival in various cohorts of patients with melanoma receiving anti-CTLA4 therapy111. Interestingly, expression of the active form of ATF6 in intestinal epithelial cells was shown to promote microbial dysbiosis and innate immune changes that facilitated microbiota-dependent colorectal tumorigenesis. Hence, elevated expression of ATF6 was associated with reduced disease-free survival of patients with colorectal cancer112. Collectively, these studies indicate that ER-stressed malignant cells can orchestrate various immune-evasive mechanisms in the TME to facilitate malignant progression.
The UPR in intratumoural immune cells
The recognition and elimination of neoplasms by the immune system requires the efficient and timely expression of several molecules that act in concert to dictate the activation, trafficking, proliferation, differentiation and fate of cancer-reactive immune cells. Mounting durable immune responses against tumours is a complex systemic process that involves the coordination of metabolic, transcriptional and translational programmes in innate and adaptive immune cells. As the ER functions as a cellular hub that senses and integrates various intracellular alterations, as well as extracellular conditions and factors, intrinsic disruption of ER homeostasis in tumour-infiltrating leukocytes has emerged as a key mechanism promoting malignant progression and immune escape by cancer cells.
Various conditions in the TME contribute to sustaining detrimental ER stress responses in infiltrating immune cells (FIG. 1). The high metabolic demand and unrestrained proliferative capacity of malignant cells drastically alter the nutrient composition of the tumour milieu. For instance, cancer cells rapidly intake and consume glucose and glutamine to support various metabolic processes associated with uncontrolled cell division113. Therefore, tumour-infiltrating immune cells have limited access to key nutrients implicated in major metabolic processes required for protein folding and the generation of effective anticancer responses114. As described above, intracellular ROS accumulation and acidosis in the TME can also readily dampen the protein-folding capacity of the ER, triggering persistent ER stress responses in tumour-infiltrating leukocytes. Whether hypoxia promotes ER stress in intratumoural immune cells has not been established and deserves further investigation.
The following subsections summarize recent studies revealing that tumour-infiltrating leukocytes undergo persistent activation of ER stress signalling pathways that, beyond triggering the canonical UPR, modulate major transcriptional and metabolic programmes in an immune cell-specific manner (FIG. 4). Therefore, targeting ER stress sensors, or their associated UPR pathways, might be useful to enhance the effects of immune checkpoint blockade and adoptive T cell immunotherapies in solid tumours currently refractory to these approaches.
Myeloid cells.
In mouse models of metastatic ovarian cancer, dysfunctional tumour-associated DCs demonstrated accumulation of intracellular ROS that provoked ER stress and persistent activation of the UPR by generating lipid peroxidation by-products that modified ER-resident proteins28. Sustained IRE1α–XBP1 activation in these tumour-associated DCs not only upregulated multiple UPR factors but also induced transcriptional activation of pathways driving triglyceride biosynthesis and lipid droplet formation, which ultimately hindered their antigen-presenting capacity28 (FIG. 4). Of note, previous studies had established that aberrant lipid accumulation and uncontrolled lipid droplet formation are a central feature of tolerogenic or immunosuppressive DCs in patients with cancer and mouse models of disease115,116. Treatment with antioxidants or hydrazine derivatives that sequester lipid peroxidation by-products mitigated IRE1α–XBP1 induction in DCs exposed to tumour-derived soluble factors28. Moreover, selective ablation of IRE1α–XBP1 in DCs using conditional knockout mice or small interfering RNA (siRNA)-loaded nanoparticles controlled abnormal lipogenesis in tumour-associated DCs while simultaneously enhancing their antigen-presenting capacity in the TME28. Disabling IRE1α–XBP1 in DCs using these approaches delayed ovarian cancer progression in different mouse models and extended their survival by eliciting adaptive antitumour immunity28. Furthermore, elevated expression of ER stress gene markers in DCs isolated from human ovarian cancer samples correlated with decreased intratumoural T cell infiltration in the same specimens28. Subsequent studies uncovered that IRE1α–XBP1 signalling not only controlled antigen presentation by DCs but also expression of key immunosuppressive factors. This arm of the UPR was demonstrated to drive the synthesis of multiple prostaglandins, including the potent lipid mediator PGE2, by mouse bone marrow-derived DCs and human monocyte-derived DCs undergoing ER stress or stimulated via PRRs117. Upon activation by IRE1α, XBP1s transcriptionally induced two genes encoding enzymes necessary for inducible PGE2 biosynthesis, namely prostaglandin-endoperoxide synthase 2 (Ptgs2; encoding COX2) and prostaglandin E synthase (Ptges; encoding mPGES1)117. Furthermore, DCs, macrophages and neutrophils lacking either IRE1α or XBP1 demonstrated impaired production of PGE2 under ER stress or inflammatory settings, both in vitro and in vivo117. Ablation of IRE1α or XBP1 was subsequently found to decrease expression of PTGS2 by human TNBC cells102. As PGE2 coordinates crucial immune-evasive mechanisms in cancer118, these findings raise the possibility that persistent IRE1α–XBP1 activation in intratumoural myeloid subsets, or cancer cells, could further promote malignant progression by enhancing biosynthesis of this immunosuppressive lipid mediator.
TME-enriched cytokines such as IL-4, IL-6 and IL-10 were shown to trigger IRE1α–XBP1 signalling in macrophages via activation of STAT3 and STAT6 (REF.119). This process promoted expression and secretion of ECM-degrading cathepsins that facilitated macrophage-mediated cancer cell invasion in in vitro models119. Uptake of oxidized low-density lipoprotein (ox-LDL) via the scavenger receptor CD36 caused ER stress in macrophages, which in turn enhanced expression of the receptor and promoted intracellular lipid accumulation through IRE1α and ATF6 (REF.120). Although lipid-laden macrophages have been shown to exhibit robust immunosuppressive capacity that promotes tumour growth121, whether this process is mediated by ER stress responses induced by the TME has not been determined. Nonetheless, recent findings indicate that tumour-associated macrophages (TAMs) in B16F10 melanoma mouse models demonstrate robust activation of IRE1α–XBP1, which promoted their capacity to express immunoregulatory PDL1 and Arginase 1 (REF.122) (FIG. 4). Of note, classical IRE1α-dependent gene signatures were postulated to be associated with higher expression of CD274 (encoding PDL1) in human melanoma samples122, and B16F10 tumour-bearing mice selectively lacking IRE1α in macrophages showed a significant increase in survival compared with their wild-type counterparts122. Nonetheless, whether these protective effects were mediated by the induction of antitumour T cell responses was not determined.
ER stress responses have also been shown to play a major role in coordinating the immunoregulatory activity of MDSCs in cancer. Early work by Gabrilovich and colleagues showed that MDSCs in various mouse models of cancer demonstrated signs of ER stress associated with the regulation of their fate and turnover123. MDSCs exhibited lower viability and a shorter half-life than neutrophils and monocytes due to increased apoptosis mediated by TRAIL receptors (TRAIL-Rs) and caspase 8 activation (FIG. 4). TRAIL-R expression in MDSCs was linked to the intrinsic induction of ER stress gene markers, and the short lifespan of peripheral MDSCs promoted their expansion in the bone marrow123. Supporting these initial observations, a recent study reported that TRAIL-R expression in various cell types, including macrophages, can operate as stress-associated molecular patterns that mediate inflammatory or apoptotic responses induced by ER stress124. Furthermore, upregulation of ER stress-related gene signatures and surface expression of the lectin-type oxidized LDL receptor 1 (LOX1) specifically distinguished low-density immunosuppressive polymorphonuclear MDSCs (PMN-MDSCs) from high-density neutrophils in patients with cancer125 (FIG. 4). Of note, human primary neutrophils undergoing pharmacologically induced ER stress upregulated LOX1 and became highly suppressive towards T cells, a process that could be mitigated by disabling the IRE1α RNAse domain125.
Beyond the role of IRE1α–XBP1 in tumour-infiltrating myeloid cells, seminal work by the group of Paulo Rodriguez has further demonstrated a major immunoregulatory and tumorigenic function for the PERK–CHOP arm of the UPR in MDSCs (FIG. 4). ROS and peroxynitrites (PNTs) potently induced CHOP in tumour-associated MDSCs, which promoted their accumulation and T cell suppressive function in various mouse models of cancer126. CHOP deficiency reprogrammed MDSCs towards an immunostimulatory cell type capable of activating cancer-specific T cells that restrained tumour growth126. Although CHOP induction during ER stress is predominantly driven by ATF4, it remains unknown whether ATF4 can modulate MDSC function independently of CHOP. Nonetheless, a more recent study by the same group demonstrated that deleting or targeting PERK in MDSCs could be used to elicit type I interferon-mediated antitumour immune responses in various mouse models of cancer127. Tumour-associated MDSCs demonstrated robust PERK activation that promoted resistance to oxidative stress by phosphorylating and activating the transcription factor NRF2 (FIG. 4), which induces cellular redox transcripts that alleviate the effects of ROS accummulation71. Genetic or pharmacological targeting of PERK compromised NRF2 signalling in MDSCs and disrupted their mitochondrial homeostasis, provoking cytosolic accumulation of mitochondrial DNA127. This process triggered stimulator of interferon genes (STING)-dependent production of type I interferon and subsequent induction of antitumour immune responses capable of enhancing the effects of immune checkpoint blockade and adoptive T cell immunotherapy127.
Collectively, these findings indicate that sustained activation of ER stress sensors IRE1α and PERK in tumour-associated myeloid cells promotes cancer progression by shaping a tolerogenic and immunosuppressive TME. Additional research is necessary to evaluate potential XBP1-independent roles of IRE1α activation in intratumoural myeloid cells. Likewise, it remains to be determined whether the ATF6 arm further modulates the activity of myeloid cells to impact adaptive antitumour immunity and cancer progression.
T cells.
Beyond altering the function of myeloid cells, the TME can directly control adaptive immune responses by causing metabolic perturbations and mitochondrial dysfunction in infiltrating T cells114,128,129. How metabolic stress signals are sensed and integrated by intratumoural T cells is an area of active research, and increasing experimental evidence now indicates that adverse conditions in the TME can provoke maladaptive ER stress responses that control the metabolic fitness and effector profile of intratumoural T cells (FIG. 4).
T cells isolated from human ovarian cancer specimens, including solid tumours and ascites fluid, demonstrated robust XBP1 splicing and upregulation of ER stress response gene markers, which was associated with diminished intratumoural T cell infiltration and reduced interferon-γ (IFNG) mRNA expression130. Soluble factors in the ovarian TME suppressed expression of GLUT1 on T cells and, hence, impaired their capacity to import this nutrient. Defective N-linked glycosylation, decreased mitochondrial respiration and reduced production of IFNγ were observed in ER-stressed T cells under glucose deprivation or exposed to ascites supernatants derived from patients with ovarian cancer130. Mechanistically, aberrant IRE1α–XBP1 activation during glucose restriction decreased the abundance of glutamine transporters in T cells, limiting the influx and usage of this amino acid as an alternative carbon source to sustain mitochondrial respiration in the absence of glucose130 (FIG. 4). Abrogating IRE1α–XBP1 signalling in glucose-deprived or ascites-exposed T cells augmented their mitochondrial respiration and IFNγ production130. Furthermore, ovarian cancer-bearing mice that lacked IRE1α or XBP1 selectively in T cells exhibited delayed malignant progression and increased survival, which was accompanied by intratumoural T cell reprogramming characterized by the induction of immune-activating gene networks and enhanced effector capacity against cancer cells130. Subsequent studies reported that elevated levels of cholesterol in B16 melanoma tumours promoted ER stress in infiltrating CD8+ T cells, a process that contributed to their exhausted phenotype via XBP1s-mediated upregulation of PD1 (REF.131) (FIG. 4). Silencing XBP1 in cancer-specific CD8+ T cells enhanced their antitumour activity and enabled superior control of B16 melanoma cells growing in the lung. By contrast, enforcing XBP1s expression in cancer-reactive CD8+ T cells promoted malignant progression and metastatic disease131. It remains to be determined how tumour-released cholesterol evokes or amplifies ER stress in intratumoural T cells. Identifying the transcriptional programmes that XBP1s controls in melanoma-infiltrating CD8+ T cells to promote their exhaustion also deserves further investigation. Taken together, these findings indicate that although IRE1α–XBP1 supports ER expansion and the resolution of protein-folding stress in this organelle under nutrient-rich conditions132, persistent activation of this UPR branch is detrimental to T cells residing in the TME where nutrient availability is restricted or altered.
Hyperactivation of the PERK–CHOP pathway in intratumoural T cells has also emerged as a critical mediator of immune evasion in cancer. In various mouse models of cancer, CHOP was found to inhibit IFNγ production in intratumoural CD8+ T cells by directly repressing the type 1 T helper (TH1) transcription factor T-box expressed in T cells (T-BET)133. CHOP also negatively regulated glycolysis and mitochondrial respiration in activated CD8+ T cells133 (FIG. 4), but the underlying mechanisms remain to be determined. Genetic or pharmacological targeting of this branch of the UPR enhanced the cytotoxic activity of cancer-reactive T cells in the TME and other aspects of their effector capacity, which was found to improve the effects of both immune checkpoint blockade and adoptive T cell immunotherapy in multiple different mouse models of cancer133,134. Importantly, CHOP overexpression in tumour-infiltrating T cells correlated with poor clinical outcome in patients with ovarian cancer133.
NK cells.
Recent studies indicate that IRE1α–XBP1 signalling is necessary for the optimal proliferative capacity of NK cells under homeostatic conditions and in the setting of mouse viral infections and melanoma models135. Mechanistically, XBP1s was found to induce MYC and promote mitochondrial respiration to support NK cell proliferation135 (FIG. 4). Intravenous injection of B16F10 melanoma cells into conditional knockout mice lacking IRE1α or XBP1 in NK cells resulted in decreased intratumoural NK cell infiltration, increased lung nodules and reduced host survival, compared with their wild-type counterparts135. Although NK cells promote intratumoural recruitment of DC populations that orchestrate protective T cell-mediated responses against melanoma136, it was not established whether DC-driven adaptive immunity was compromised under these conditions. Furthermore, whether pharmacological targeting of IRE1α alters the expansion and/or function of intratumoural NK cells in vivo has not been determined and also deserves investigation. Likewise, the role of ATF6 or PERK activation in intratumoural NK cell function remains elusive.
Pharmacological modulation of the UPR
Induction of unresolved or lethal ER stress, or suppression of UPR-driven cytoprotective functions, could be exploited to restrain tumour growth. Furthermore, multiple standard of care therapies perturb ER homeostasis and trigger adaptive ER stress responses in the cancer cell that promote tumour growth and mediate resistance to treatment (BOX 3). Approaches combining standard therapies with UPR modulators have shown remarkable efficacy in preclinical cancer models and hence warrant future consideration in patients with cancer. Drugs modulating ER stress and/or the UPR have been extensively reviewed elsewhere137. Therefore, this section will briefly focus on some pharmacological UPR modulators that have been shown to induce antitumour effects in preclinical models of cancer.
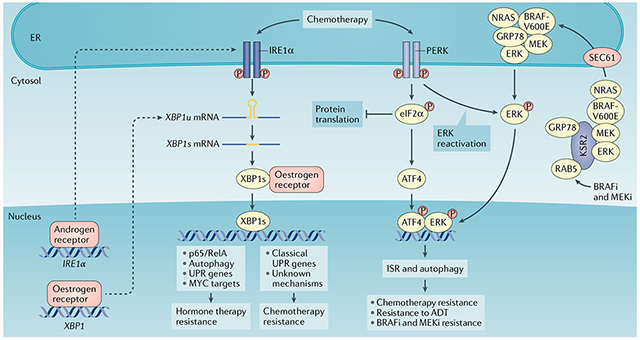
Box 3 |. The unfolded protein response and resistance to cancer therapy.
Various standard anticancer treatments can induce endoplasmic reticulum (ER) stress and activate the unfolded protein response (UPR) in malignant cells as an adaptive pro-survival mechanism.
Chemotherapy resistance
The UPR is associated with chemotherapy responses in human breast cancers198. High binding-immunoglobulin protein (BiP) expression correlates with shorter relapse-free survival in patients with breast cancer who received doxorubicin adjuvant therapy199. BiP inhibits doxorubicin-induced apoptosis by suppressing BAX and caspase 7 activation200. Paradoxically, BiP positivity in tumours from patients with breast cancer treated with doxorubicin and cyclophosphamide followed by taxane predicts better clinical outcome199. Similarly, expression of BiP in MCF7 human breast cancer cells confers greater sensitivity to sequential doxorubicin and taxane treatment199. It is unclear why taxane is able to reverse the correlation between BiP levels and chemosensitivity. However, taxanes can induce activation of inositol-requiring protein 1α (IRE1α), activating transcription factor 6 (ATF6) and PRKR-like ER kinase (PERK) in breast cancers198,201. Inhibition of the IRE1α RNase activity with MKC8866 substantially enhanced the response of a triple-negative breast cancer (TNBC) patient-derived xenograft (PDX) model and MDA-MB-231 breast cancer xenografts to taxane37,101. Another IRE1α RNase inhibitor, B-I09, similarly improved the in vitro cytotoxicity of doxorubicin or vincristine in Burkitt’s lymphoma cells40. In colon cancer cells, PERK–nuclear factor erythroid 2-related factor 2 (NRF2) regulates multidrug resistance-associated protein 1 (MRP1) to mediate cancer cell resistance to ER stress and chemotherapy202. Silencing PERK reduced tumour growth and restored chemosensitivity in resistant HT29 colon cancer xenografts. In addition, ATF4 activation induces autophagy, which mediates breast cancer resistance to taxane198.
Hormone therapy resistance
IRE1α and PERK are important for resistance to endocrine therapy in hormone-dependent breast cancers146. X-box binding protein 1 (XBP1) is a direct target of the oestrogen receptor203, and its expression is upregulated in breast cancer cells resistant to anti-oestrogen therapy204,205. Enforced expression of XBP1 confers oestrogen-independent growth of breast cancers and resistance to tamoxifen and fulvestrant treatment through multiple mechanisms, including autophagy, nuclear factor-κB (NF-κB) activation and regulation of the ER206,207. BHPI is an effective antagonist of oestrogen receptor-α that selectively inhibits growth of oestrogen receptor-expressing breast cancer xenografts and ovarian cancer cell lines208. Of note, BHPI activates the UPR by inhibiting protein synthesis through activating phospholipase Cγ (PLCγ) and depleting ER calcium stores208. Therefore, BHPI enhances the cytotoxic effects of endocrine therapy by inducing lethal ER stress responses in these cancer cells208.
Targeted therapy resistance
The combination of a BRAF inhibitor and a MEK inhibitor (BRAFi and MEKi) is the standard of care for BRAF-mutant melanoma and lung cancer209. Interestingly, treatment with these inhibitors results in SEC61-dependent translocation of MAPK into the ER, leading to PERK-mediated ERK re-phosphorylation and reactivation209. In turn, ERK phosphorylates ATF4 and upregulates autophagy to induce resistance to therapy with BRAF and MEK inhibitors209. Upregulation of BiP levels and ATF4 phosphorylation are observed in tumours from patients resistant to these inhibitors209, and targeting ATF4 re-sensitized the tumours to BRAF and MEK combined inhibitor treatment. It is unclear why MAPK needs to translocate to the ER to escape the drug treatment. Whether the eukaryotic translation initiation factor 2α (eIF2α) inhibitor ISRIB could be used to reverse tumour resistance to BRAF and MEK combined inhibition in BRAF-mutant melanoma and lung cancer deserves further attention. ADT, androgen deprivation therapy; GRP78, 78-kDa glucose-regulated protein; ISR, integrated stress response; KSR2, kinase suppressor of Ras 2; P, phosphorylation.
IRE1α inhibitors.
IRE1α has two druggable enzymatic domains: the kinase domain and the endoribonuclease domain (BOX 1). IRE1α kinase inhibitors have shown remarkable in vivo efficacy in xenograft models of multiple myeloma. The IRE1α kinase inhibitor Compound 18, also known as KIRA8 or AMG-18, restrains multiple myeloma growth and augments the response of these tumours to established front-line drugs, the proteasome inhibitor bortezomib and the immunomodulatory drug lenalidomide138. Importantly, kinase-dead IRE1α mutants are refractory to the inhibitor treatment, validating the on-target effects of this compound. Similarly, the IRE1α RNase inhibitor MKC3946 significantly enhances cytotoxicity induced by bortezomib or the heat shock protein 90 (HSP90) inhibitor 17-AAG in xenograft models of multiple myeloma139. However, it is noteworthy that genetic ablation of IRE1α, inactivating mutations in XBP1 or deletion of XBP1 in multiple myeloma was reported to induce bortezomib resistance via de-differentiation of plasma cells into progenitors140. It is unclear whether the de-differentiation of plasma cells is specific to complete genetic deletion of IRE1α or XBP1, or could also be induced by inhibition of IRE1α kinase or RNase activity. Further studies are necessary to understand the discrepancy between genetic and pharmacological effects, which will provide valuable information for potential clinical application of these inhibitors in patients with multiple myeloma. In xenograft mouse models of TNBC, suppression of IRE1α kinase activity with Compound 18 hindered tumour growth and sensitized tumours to anti-VEGFA therapy102. Treatment of pancreatic neuroendocrine tumours (PanNETs) with Compound 18 also reduced tumour growth and prolonged host survival in a RIP-Tag2 genetically engineered mouse model of this malignancy141.
IRE1α RNase inhibitors, including B-I09, STF083010, MKC3946 and MKC8866, have been extensively tested in mouse models of breast cancer, prostate cancer, melanoma, lymphoma, multiple myeloma and CLL37,40,101,142–146. B-I09 has been proven a safe and selective IRE1α RNase inhibitor suitable for in vivo use. Treatment with B-I09 suppressed leukaemic growth in mouse models of CLL without causing systemic toxicity and synergized with ibrutinib, a US Food and Drug Administration (FDA)-approved Bruton tyrosine kinase (BTK) inhibitor, to induce apoptosis in human cell lines of B cell leukaemia, lymphoma and multiple myeloma142. B-I09 administration also sensitized MYC-driven Burkitt’s lymphoma and neuroblastoma to the chemotherapy doxorubicin in mouse models40. Treatment with another IRE1α RNase inhibitor, MKC8866, enhanced the effects of the chemotherapy docetaxel to induce complete and durable responses in a MYC-driven patient-derived xenograft (PDX) model of TNBC37. Long-term administration of MKC8866 caused minimal toxicity to normal mouse tissues and is currently being tested in a phase I clinical trial for patients with relapsed or refractory metastatic breast cancer146. In mouse models of glioblastoma, intracerebral administration of MKC8866 improved the antitumour effects of surgical resection combined with radiotherapy and chemotherapy147. Lastly, treatment with STF083010 enhanced the efficacy of adoptive immunotherapy using tumour-specific CD8+ T cells in mouse models of melanoma131. Additional mechanistic studies of how RIDD influences tumour progression are needed to better direct the design of therapeutic strategies using IRE1α RNase inhibitors. Overall, these preclinical studies suggest that using IRE1α pharmacological inhibitors may be beneficial to improve the clinical outcome of patients with cancer with poor response to chemotherapy, drug resistance and/or disease recurrence.
PERK inhibitors.
PERK inhibitors GSK2606414 and GSK2656157 suppress tumour growth in human xenograft models of different cancers137. Altered amino acid metabolism, decreased blood vessel density and decreased vascular perfusion are potential mechanisms for the observed antitumour activity of these inhibitors. GSK2656157 also sensitizes colon cancer cells to 5-fluorouracil (5-FU) chemotherapy148. Interestingly, GSK2606414 administration reactivates T cell function and enhances responses to PD1 blockade in mouse models of immunogenic sarcoma134. Despite the marked therapeutic efficacy, the on-target toxicity is a concern for PERK abrogation149. PERK inhibition causes serious toxic effects in the pancreas and markedly suppresses insulin production78,150,151. Recent reports also indicate that both GSK2606414 and GSK2656157 exhibit PERK-independent off-target effects including targeting RIPK1 to completely repress tumour necrosis factor (TNF)-mediated receptor-interacting serine/threonine-protein kinase 1 (RIPK1) kinase-dependent cell death152. GSK2606414 and the IRE1α kinase inhibitor KIRA6 are also potent KIT inhibitors that suppress its tyrosine kinase activity. By contrast, a series of 1H-pyrazol-3(2H)-one molecules have recently been identified as new PERK inhibitors153. Structure-based design and optimization led to identification of the compounds AMG44 and AMG52 as potent and highly selective PERK inhibitors153. These two compounds demonstrate good pharmacokinetic properties for in vivo use. Notably, prolonged treatment with AMG44 did not induce pancreatic toxicity and was well tolerated when used as an experimental approach to control the regulatory activity of MDSCs and induce antitumour immunity in various preclinical models of cancer127. LY-4 is a potent third-generation PERK inhibitor with good selectivity154. This compound has shown impressive in vivo efficacy against BRAFV600E mutant melanoma and MYC-driven lymphoma (specifically in transgenic mice lacking GCN2) without pancreatic or overt toxicities39,154. Therefore, AMG44 and LY-4 are emerging as selective and well-tolerated PERK inhibitors that warrant further preclinical and clinical investigation to evaluate their antitumour efficacy and potential side effects in combination with cytotoxic drugs or targeted therapies.
eIF2α inhibitors.
eIF2α is the key node of the ISR21. ER stress-driven PERK activation, amino acid starvation-induced GCN2, double-stranded RNA (dsRNA)-induced protein kinase RNA-activated (PKR; also known as EIF2AK2) or haem deficiency-induced haem-regulated inhibitor (HRI; also known as EIF2AK1) phosphorylates eIF2α, which converts eIF2 from a substrate to a competitive inhibitor of its guanine nucleotide exchange factor, eIF2B, and inhibits translation21. ISRIB is a potent eIF2α inhibitor that suppresses the effects of eIF2α phosphorylation by activating eIF2B155,156. When used as a single agent, ISRIB significantly suppressed PTEN-deficient and MYC-overexpressing prostate cancer progression, extending the survival of tumour-bearing mice42. Importantly, administration of ISRIB alone could cause regression of advanced prostate tumours after 3 weeks of treatment without overt side effects42. Studies of the role of IRE1α, PERK and the ISR in tumour progression and metastasis suggest that these pathways are not functionally redundant16,37,39–41,85. Hence, it would be pertinent to test whether there is a therapeutic window to simultaneously target IRE1α and the ISR in cancer cells, and whether this combination therapy may induce synergistic effects in suppressing tumour progression and metastasis without toxicity to normal tissues.
BiP inhibitors.
BiP is upregulated in multiple different human cancers, plays pro-tumoural roles in mouse models of malignancy and promotes resistance to anticancer therapies157. Silencing of BiP in glioblastoma, bladder or breast cancer cell lines enhances their responses to chemotherapy158,159. The overexpression of BiP and the existence of cancer type-specific variants of BiP in human cancers provide a therapeutic window making BiP an attractive therapeutic target2. The ruthenium-based BiP inhibitor KP1339 induces extensive ER stress and immunogenic cell death (ICD) (BOX 2) in various mouse models of cancer160–163. Importantly, a phase I study of KP1339 showed acceptable tolerability and disease stabilization in 10 out of 38 patients with metastatic neuroendocrine tumours, non-small cell lung carcinoma (NSCLC) or colon cancer161,164. HA15 is another compound that targets BiP and triggers unresolved ER stress that leads to cancer cell death both in vitro and in vivo. As such, HA15 elicited marked antitumour effects in xenograft models of melanoma and was effective in slowing down BRAF inhibitor-resistant melanoma without major side effects165. Furthermore, cell-surface BiP appears to be a viable therapeutic target. Synthetic chimeric peptides composed of BiP binding motifs fused to a cell death-inducing sequence can suppress tumour growth in xenograft models of prostate cancer and breast cancer166. A cancer cell-specific O-linked carbohydrate moiety in cell surface-localized BiP also provides a potential target for immunotherapy and antibody-based therapy in gastric cancer167.
Concluding remarks
Persistent ER stress is an emerging hallmark of cancer, which is driven by multiple metabolic and oncogenic abnormalities in the TME that perturb protein-folding homeostasis in both malignant cells and infiltrating immune cells. Constitutively active ER stress responses enable malignant cell adaptation to oncogenic and environmental challenges while orchestrating diverse immunomodulatory mechanisms that promote malignant progression. Systematic deconvolution of the precise impact of the UPR on individual cell types in the TME, and especially on cancer cell metabolic reprogramming in vivo, should be carried out in the future. Although multiple studies have elucidated the function and mechanisms of the UPR in each step of tumorigenesis, additional studies are also necessary to understand the role of ER stress in cancer metastasis and therapy resistance. In particular, a deeper mechanistic understanding of the UPR in the rate-limiting steps of the metastatic cascade, particularly in the context of TME reprogramming during metastasis, is also imperative for the rational design of effective therapeutic interventions that address the current clinical challenges and improve patient outcome (BOX 3).
Besides immune cells, endothelial cells and CAFs are important components of the TME that significantly contribute to tumour progression. Although limited knowledge is available on the role of ER stress in these cells in the TME, there is evidence indicating the functional impact of ER stress responses in endothelial cells under normal physiological conditions. For instance, BiP relocalizes from the ER to the cell surface and interacts with T-cadherin to promote endothelial cell survival via the PI3K–AKT pathway168. Further studies to dissect the role of the UPR in endothelial cells, CAFs and other stromal cells in the TME during cancer progression and therapy are necessary.
Targeting ER stress responses alone can destabilize some aggressive features of cancer cells while enhancing antitumour immunity, but this may not lead to therapeutic efficacy that is superior to standard interventions. However, increasing evidence demonstrates that modulation of ER stress sensors or UPR-associated factors creates vulnerabilities that markedly sensitize aggressive tumours to cytotoxic drugs, targeted therapies and immunotherapy. Larger preclinical studies using PDXs and immunocompetent mouse models that recapitulate human tumour heterogeneity, together with retrospective analyses of clinical trial specimens, could help uncover potent UPR-targeted combination treatments to elicit durable responses that prevent cancer progression and/or recurrence in patients.
Acknowledgements
X.C. has been supported by the National Institutes of Health (NIH) (R37CA228304, P50CA186784 and R01HL146642), the US Department of Defense Congressionally Directed Medical Research Programs (W81XWH1910524), the American Cancer Society (RSG-18-181-01-TBE), the Cancer Prevention and Research Institute of Texas (RR150009 CPRIT Scholar in Cancer Research award) and Susan G. Komen for the Cure (CCR16380871). J.R.C.-R. has been supported by the NIH (R01NS114653 and R21CA248106), the US Department of Defense Ovarian Cancer Research Program (W81XWH-16-1-0438 and W81XWH-20-1-0191), Stand Up to Cancer (SU2C-AACR-IRG-03-16 and SU2C-AACR-PS24), the Pershing Square Sohn Cancer Research Alliance, the Mary Kay Foundation, the Daedalus Fund for Innovation, the Mark Foundation for Cancer Research ASPIRE Award and the Wade F. B. Thompson/Cancer Research Institute CLIP grant. The authors apologize to colleagues whose work is not cited in this Review due to space limitations.
Glossary
- Proteostasis
The regulation of a balanced and functional proteome.
- Isomerization
The transformation of a molecule into a different isomer.
- Hypoxia
A low level of oxygen tension.
- Integrated stress response (ISR)
An adaptive signalling pathway activated by various forms of cellular stress, including endoplasmic reticulum stress, which helps maintain cellular integrity under unfavourable conditions such as nutrient restriction and oxidative stress.
- Reactive oxygen species (ROS)
A natural by-product of the metabolism of oxygen that is more reactive than molecular oxygen.
- ER-associated protein degradation (ERAD)
A pathway that targets misfolded proteins in the endoplasmic reticulum (ER) for ubiquitylation and degradation by the proteasome in the cytoplasm.
- Autophagy
A regulated cellular process that delivers dysfunctional cellular constituents to the lysosomes for degradation and recycling.
- Electron transport chain (ETC)
A series of enzymatic reactions occurring in the inner mitochondrial membrane that shuttles electrons from NADH and FADH2 to oxygen. During this process, protons pumped from the mitochondrial matrix to the intermembrane space are used to produce ATP.
- Fatty acid β-oxidation (FAO; also known as β-oxidation)
The breakdown of fatty acids to generate acetyl-CoA, which is incorporated into the tricarboxylic acid (TCA) cycle.
- Pattern recognition receptors (PRRs)
Germline-encoded proteins that sense invading pathogens or endogenous damage signals to initiate and regulate immune responses.
- Glycolysis
The oxygen-independent metabolic pathway that converts glucose into pyruvate.
- Regulated IRE1-dependent decay of RNA (RIDD)
The mechanism of microRNA or mRNA degradation caused by hyperactivation of the ribonuclease domain of inositol-requiring enzyme 1α (IRE1α).
- Damage-associated molecular patterns (DAMPS)
Host-derived molecules that can initiate and drive a non-infectious inflammatory response. By contrast, pathogen-associated molecular patterns are molecules derived from an infectious non-self-entity, which also stimulate an inflammatory response.
- Epithelial-to-mesenchymal transition (EMT)
A cellular developmental process by which epithelial cells acquire a mesenchymal-like phenotype. This process is accompanied by the loss of cell adhesion properties and augmented motility, and is therefore exploited by cancer cells to metastasize.
- Myeloid-derived suppressor cells (MDSCs)
A heterogeneous group of immune cells from the myeloid lineage with relatively immature but potent immunosuppressive phenotypes. MDSCs are classified into two major subtypes: monocytic MDSCs (M-MDSCs) and granulocytic MDSCs (polymorphonuclear (PMN)-MDSCs).
- Acidosis
A process that increases acidity in the blood and other tissues.
- Peroxynitrites (PNTs)
Powerful oxidants produced by the reaction between nitric oxide and superoxide radicals that cause lipid peroxidation, as well as protein and DNA damage.
Footnotes
Competing interests
X.C. and J.R.C.-R. hold patents on the use of ER stress and UPR modulators for cancer therapy. X.C. reports research funding from Fosun Pharma.
Peer review information
Nature Reviews Cancer thanks E. Chevet, C. Koumenis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
References
- 1.Hetz C, Zhang K & Kaufman RJ Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol 21, 421–438 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang M, Wey S, Zhang Y, Ye R & Lee AS Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid. Redox Signal 11, 2307–2316 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walter P & Ron D The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Lee AH, Iwakoshi NN, Anderson KC & Glimcher LH Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc. Natl Acad. Sci. USA 100, 9946–9951 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwawaki T, Akai R, Kohno K & Miura M A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat. Med 10, 98–102 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Merksamer PI, Trusina A & Papa FR Real-time redox measurements during endoplasmic reticulum stress reveal interlinked protein folding functions. Cell 135, 933–947 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiotto MT et al. Imaging the unfolded protein response in primary tumors reveals microenvironments with metabolic variations that predict tumor growth. Cancer Res. 70, 78–88 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keith B & Simon MC Hypoxia-inducible factors, stem cells, and cancer. Cell 129, 465–472 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koumenis C ER stress, hypoxia tolerance and tumor progression. Curr. Mol. Med 6, 55–69 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Kaelin WG Jr. & Ratcliffe PJ Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Jiang D, Niwa M & Koong AC Targeting the IRE1 α-XBP1 branch of the unfolded protein response in human diseases. Semin. Cancer Biol 33, 48–56 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koritzinsky M et al. Two phases of disulfide bond formation have differing requirements for oxygen. J. Cell Biol 203, 615–627 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.May D et al. Ero1-Lα plays a key role in a HIF-1-mediated pathway to improve disulfide bond formation and VEGF secretion under hypoxia: implication for cancer. Oncogene 24, 1011–1020 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Young RM et al. Dysregulated mTORC1 renders cells critically dependent on desaturated lipids for survival under tumor-like stress. Genes Dev. 27, 1115–1131 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals that constitutive mTORC1 activity renders hypoxic cells dependent upon exogenous desaturated lipids to expand the ER and prevent the cancer cells from lethal ER stress.
- 15.Koumenis C & Wouters BG “Translating” tumor hypoxia: unfolded protein response (UPR)-dependent and UPR-independent pathways. Mol. Cancer Res 4, 423–436 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Chen X et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1α pathway. Nature 508, 103–107 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denzel MS & Antebi A Hexosamine pathway and (ER) protein quality control. Curr. Opin. Cell Biol 33, 14–18 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Domblides C, Lartigue L & Faustin B Metabolic stress in the immune function of T cells, macrophages and dendritic cells. Cells 7, 68 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braakman I & Bulleid NJ Protein folding and modification in the mammalian endoplasmic reticulum. Annu. Rev. Biochem 80, 71–99 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Moore CE, Omikorede O, Gomez E, Willars GB & Herbert TP PERK activation at low glucose concentration is mediated by SERCA pump inhibition and confers preemptive cytoprotection to pancreatic β-cells. Mol. Endocrinol 25, 315–326 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pakos-Zebrucka K et al. The integrated stress response. EMBO Rep. 17, 1374–1395 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park J, Morley TS, Kim M, Clegg DJ & Scherer PE Obesity and cancer — mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol 10, 455–465 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei Y, Wang D, Topczewski F & Pagliassotti MJ Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol. Endocrinol. Metab 291, E275–E281 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Shimizu Y & Hendershot LM Oxidative folding: cellular strategies for dealing with the resultant equimolar production of reactive oxygen species. Antioxid. Redox Signal 11, 2317–2331 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Pavlova NN & Thompson CB Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine. EMBO J. 36, 1302–1315 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liou GY & Storz P Reactive oxygen species in cancer. Free Radic. Res 44, 479–496 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorlach A, Bertram K, Hudecova S & Krizanova O Calcium and ROS: a mutual interplay. Redox Biol. 6, 260–271 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cubillos-Ruiz JR et al. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell 161, 1527–1538 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study unveils that IRE1α–XBP1 overactivation causes intratumoural DC dysfunction.
- 29.Vladykovskaya E et al. Lipid peroxidation product 4-hydroxy-trans-2-nonenal causes endothelial activation by inducing endoplasmic reticulum stress. J. Biol. Chem 287, 11398–11409 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bobrovnikova-Marjon E et al. PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene 29, 3881–3895 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang X et al. Functional interaction between responses to lactic acidosis and hypoxia regulates genomic transcriptional outputs. Cancer Res. 72, 491–502 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong L, Krewson EA & Yang LV Acidosis activates endoplasmic reticulum stress pathways through GPR4 in human vascular endothelial cells. Int. J. Mol. Sci 18, 278 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maeyashiki C et al. Activation of pH-sensing receptor OGR1 (GPR68) induces ER stress via the IRE1α/JNK pathway in an intestinal epithelial cell model. Sci. Rep 10, 1438 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun X et al. Deletion of the pH sensor GPR4 decreases renal acid excretion. J. Am. Soc. Nephrol 21, 1745–1755 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teixeira J et al. Extracellular acidification induces ROS- and mPTP-mediated death in HEK293 cells. Redox Biol. 15, 394–404 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Babour A, Bicknell AA, Tourtellotte J & Niwa M A surveillance pathway monitors the fitness of the endoplasmic reticulum to control its inheritance. Cell 142, 256–269 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao N et al. Pharmacological targeting of MYC-regulated IRE1/XBP1 pathway suppresses MYC-driven breast cancer. J. Clin. Invest 128, 1283–1299 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tameire F, Verginadis II & Koumenis C Cell intrinsic and extrinsic activators of the unfolded protein response in cancer: mechanisms and targets for therapy. Semin. Cancer Biol 33, 3–15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tameire F et al. ATF4 couples MYC-dependent translational activity to bioenergetic demands during tumour progression. Nat. Cell Biol 21, 889–899 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie H et al. IRE1α RNase-dependent lipid homeostasis promotes survival in Myc-transformed cancers. J. Clin. Invest 128, 1300–1316 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with Zhao et al. (2018), this paper demonstrates that MYC hyperactivation is synthetic lethal with IRE1α or XBP1 inhibition in multiple MYC-driven human cancers.
- 41.Hart LS et al. ER stress-mediated autophagy promotes Myc-dependent transformation and tumor growth. J. Clin. Invest 122, 4621–4634 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals that MYC induces ER stress responses and that MYC-driven tumours depend on PERK-regulated autophagy to survive.
- 42.Nguyen HG et al. Development of a stress response therapy targeting aggressive prostate cancer. Sci. Transl Med 10, eaar2036 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that advanced prostate cancer with MYC amplification and PTEN loss activates eIF2α to protect them from excessive protein synthesis.
- 43.Denoyelle C et al. Anti-oncogenic role of the endoplasmic reticulum differentially activated by mutations in the MAPK pathway. Nat. Cell Biol 8, 1053–1063 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Blazanin N et al. ER stress and distinct outputs of the IRE1α RNase control proliferation and senescence in response to oncogenic Ras. Proc. Natl Acad. Sci. USA 114, 9900–9905 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sustic T et al. A role for the unfolded protein response stress sensor ERN1 in regulating the response to MEK inhibitors in KRAS mutant colon cancers. Genome Med. 10, 90 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang D et al. Comprehensive analysis of the unfolded protein response in breast cancer subtypes. JCO Precis. Oncol 10.1200/PO.16.00073 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romero-Ramirez L et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 64, 5943–5947 (2004). [DOI] [PubMed] [Google Scholar]; This study is the first to demonstrate that hypoxia regulates IRE1α–XBP1 and that XBP1 is necessary for cancer cells to survive under hypoxia.
- 48.Akella NM, Ciraku L & Reginato MJ Fueling the fire: emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol. 17, 52 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang ZV et al. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell 156, 1179–1192 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study is the first to demonstrate that XBP1 regulates the hexosamine biosynthetic pathway.
- 50.Lee AH, Scapa EF, Cohen DE & Glimcher LH Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320, 1492–1496 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study uncovers that XBP1 controls multiple enzymes involved in lipid metabolism and de novo lipogenesis.
- 51.Genovese G et al. Synthetic vulnerabilities of mesenchymal subpopulations in pancreatic cancer. Nature 542, 362–366 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lhomond S et al. Dual IRE1 RNase functions dictate glioblastoma development. EMBO Mol. Med 10, e7929 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lam M, Marsters SA, Ashkenazi A & Walter P Misfolded proteins bind and activate death receptor 5 to trigger apoptosis during unresolved endoplasmic reticulum stress. eLife 9, e52291 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu M et al. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science 345, 98–101 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang TK et al. Coordination between two branches of the unfolded protein response determines apoptotic cell fate. Mol. Cell 71, 629–636.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Upton JP et al. IRE1α cleaves select microRNAs during ER stress to derepress translation of proapoptotic caspase-2. Science 338, 818–822 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Urano F et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287, 664–666 (2000). [DOI] [PubMed] [Google Scholar]
- 58.Szegezdi E, Logue SE, Gorman AM & Samali A Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 7, 880–885 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pihan P, Carreras-Sureda A & Hetz C BCL-2 family: integrating stress responses at the ER to control cell demise. Cell Death Differ. 24, 1478–1487 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu Y et al. Dual role for inositol-requiring enzyme 1α in promoting the development of hepatocellular carcinoma during diet-induced obesity in mice. Hepatology 68, 533–546 (2018). [DOI] [PubMed] [Google Scholar]
- 61.Hu P, Han Z, Couvillon AD, Kaufman RJ & Exton JH Autocrine tumor necrosis factor α links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1α-mediated NF-κB activation and down-regulation of TRAF2 expression. Mol. Cell Biol 26, 3071–3084 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nishitoh H et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 16, 1345–1355 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pathinayake PS et al. Understanding the unfolded protein response in the pathogenesis of asthma. Front. Immunol 9, 175 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bujisic B et al. Impairment of both IRE1 expression and XBP1 activation is a hallmark of GCB DLBCL and contributes to tumor growth. Blood 129, 2420–2428 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Dong D et al. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res. 68, 498–505 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Fu Y et al. Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of ER chaperone GRP78/BiP in prostate epithelium. Proc. Natl Acad. Sci. USA 105, 19444–19449 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shin BK et al. Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J. Biol. Chem 278, 7607–7616 (2003). [DOI] [PubMed] [Google Scholar]
- 68.Misra UK, Deedwania R & Pizzo SV Activation and cross-talk between Akt, NF-κB, and unfolded protein response signaling in 1-LN prostate cancer cells consequent to ligation of cell surface-associated GRP78. J. Biol. Chem 281, 13694–13707 (2006). [DOI] [PubMed] [Google Scholar]
- 69.Shani G et al. GRP78 and Cripto form a complex at the cell surface and collaborate to inhibit transforming growth factor β signaling and enhance cell growth. Mol. Cell Biol 28, 666–677 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rouschop KM et al. PERK/eIF2α signaling protects therapy resistant hypoxic cells through induction of glutathione synthesis and protection against ROS. Proc. Natl Acad. Sci. USA 110, 4622–4627 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cullinan SB et al. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell Biol 23, 7198–7209 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li G et al. Role of ERO1-α-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J. Cell Biol 186, 783–792 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marciniak SJ et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18, 3066–3077 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harding HP et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 (2003). [DOI] [PubMed] [Google Scholar]
- 75.Bobrovnikova-Marjon E et al. PERK-dependent regulation of lipogenesis during mouse mammary gland development and adipocyte differentiation. Proc. Natl Acad. Sci. USA 105, 16314–16319 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee JN & Ye J Proteolytic activation of sterol regulatory element-binding protein induced by cellular stress through depletion of Insig-1. J. Biol. Chem 279, 45257–45265 (2004). [DOI] [PubMed] [Google Scholar]
- 77.Bobrovnikova-Marjon E et al. PERK utilizes intrinsic lipid kinase activity to generate phosphatidic acid, mediate Akt activation, and promote adipocyte differentiation. Mol. Cell Biol 32, 2268–2278 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chitnis NS et al. miR-211 is a prosurvival microRNA that regulates chop expression in a PERK-dependent manner. Mol. Cell 48, 353–364 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bu Y et al. A PERK–miR-211 axis suppresses circadian regulators and protein synthesis to promote cancer cell survival. Nat. Cell Biol 20, 104–115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study establishes the connection between the UPR and circadian rhythms in regulating cancer cell survival.
- 80.Cox TR et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature 522, 106–110 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Hoshino A et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang Y et al. XBP1–LOX axis is critical in ER stress-induced growth of lung adenocarcinoma in 3D culture. Am. J. Transl Res 9, 700–707 (2017). [PMC free article] [PubMed] [Google Scholar]
- 83.Paoli P, Giannoni E & Chiarugi P Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta Mol. Cell Res 1833, 3481–3498 (2013). [DOI] [PubMed] [Google Scholar]
- 84.Simpson CD, Anyiwe K & Schimmer AD Anoikis resistance and tumor metastasis. Cancer Lett. 272, 177–185 (2008). [DOI] [PubMed] [Google Scholar]
- 85.Feng Y.-x. et al. Epithelial-to-mesenchymal transition activates PERK–eIF2α and sensitizes cells to endoplasmic reticulum stress. Cancer Discov. 4, 702 (2014). [DOI] [PubMed] [Google Scholar]
- 86.Avivar-Valderas A et al. PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol. Cell. Biol 31, 3616 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dey S et al. ATF4-dependent induction of heme oxygenase 1 prevents anoikis and promotes metastasis. J. Clin. Invest 125, 2592–2608 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Piskounova E et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527, 186 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bartkowiak K et al. Discovery of a novel unfolded protein response phenotype of cancer stem/progenitor cells from the bone marrow of breast cancer patients. J. Proteome Res 9, 3158–3168 (2010). [DOI] [PubMed] [Google Scholar]
- 90.Schewe DM & Aguirre-Ghiso JA ATF6α–Rheb–mTOR signaling promotes survival of dormant tumor cells in vivo. Proc. Natl Acad. Sci. USA 105, 10519 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ranganathan AC, Ojha S, Kourtidis A, Conklin DS & Aguirre-Ghiso JA Dual function of pancreatic endoplasmic reticulum kinase in tumor cell growth arrest and survival. Cancer Res. 68, 3260 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with Schewe and Aguirre-Ghiso (2008), this paper reveals the function and mechanism of action of ATF6 and PERK in dormant cancer cells.
- 92.Ranganathan AC, Zhang L, Adam AP & Aguirre-Ghiso JA Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer Res. 66, 1702 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pommier A et al. Unresolved endoplasmic reticulum stress engenders immune-resistant, latent pancreatic cancer metastases. Science 360, eaao4908 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ranganathan AC, Adam AP, Zhang L & Aguirre-Ghiso JA Tumor cell dormancy induced by p38SAPK and ER-stress signaling: an adaptive advantage for metastatic cells? Cancer Biol. Ther 5, 729–735 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brewer JW & Diehl JA PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc. Natl Acad. Sci. USA 97, 12625–12630 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Almeida SF, Fleming JV, Azevedo JE, Carmo-Fonseca M & de Sousa M Stimulation of an unfolded protein response impairs MHC class I expression. J. Immunol 178, 3612–3619 (2007). [DOI] [PubMed] [Google Scholar]
- 97.Granados DP et al. ER stress affects processing of MHC class I-associated peptides. BMC Immunol. 10, 10 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bartoszewski R et al. The unfolded protein response (UPR)-activated transcription factor X-box-binding protein 1 (XBP1) induces microRNA-346 expression that targets the human antigen peptide transporter 1 (TAP1) mRNA and governs immune regulatory genes. J. Biol. Chem 286, 41862–41870 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Obiedat A et al. Transcription of the NKG2D ligand MICA is suppressed by the IRE1/XBP1 pathway of the unfolded protein response through the regulation of E2F1. FASEB J. 33, 3481–3495 (2019). [DOI] [PubMed] [Google Scholar]
- 100.Obiedat A et al. The integrated stress response promotes B7H6 expression. J. Mol. Med 98, 135–148 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Logue SE et al. Inhibition of IRE1 RNase activity modulates the tumor cell secretome and enhances response to chemotherapy. Nat. Commun 9, 3267 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Harnoss JM et al. IRE1α disruption in triple-negative breast cancer cooperates with anti-angiogenic therapy by reversing ER stress adaptation and remodeling the tumor microenvironment. Cancer Res. 80, 2368–2379 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee BR et al. Elevated endoplasmic reticulum stress reinforced immunosuppression in the tumor microenvironment via myeloid-derived suppressor cells. Oncotarget 5, 12331–12345 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tang CH et al. Secretory IgM exacerbates tumor progression by inducing accumulations of MDSCs in mice. Cancer Immunol. Res 6, 696–710 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu J et al. Endoplasmic reticulum stress causes liver cancer cells to release exosomal miR-23a-3p and up-regulate programmed death ligand 1 expression in macrophages. Hepatology 70, 241–258 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article reports that ER-stressed cancer cells release immunomodulatory exosomes.
- 106.Dasgupta D et al. IRE1A stimulates hepatocyte-derived extracellular vesicles that promote inflammation in mice with steatohepatitis. Gastroenterology 159, 1487–1503 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mahadevan NR et al. Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc. Natl Acad. Sci. USA 108, 6561–6566 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mahadevan NR et al. Cell-extrinsic effects of tumor ER stress imprint myeloid dendritic cells and impair CD8+ T cell priming. PLoS ONE 7, e51845 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bhattacharya S et al. Anti-tumorigenic effects of type 1 interferon are subdued by integrated stress responses. Oncogene 32, 4214–4221 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ortiz A et al. An interferon-driven oxysterol-based defense against tumor-derived extracellular vesicles. Cancer Cell 35, 33–45.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li Y et al. Gut microbiota dependent anti-tumor immunity restricts melanoma growth in Rnf5−/− mice. Nat. Commun 10, 1492 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study discovers that XBP1 expression in cancer cells limits the efficacy of immune checkpoint blockade.
- 112.Coleman OI et al. Activated ATF6 induces intestinal dysbiosis and innate immune response to promote colorectal tumorigenesis. Gastroenterology 155, 1539–1552.e12 (2018). [DOI] [PubMed] [Google Scholar]
- 113.Pavlova NN & Thompson CB The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ho PC et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 162, 1217–1228 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Herber DL et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat. Med 16, 880–886 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gao F et al. Radiation-driven lipid accumulation and dendritic cell dysfunction in cancer. Sci. Rep 5, 9613 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chopra S et al. IRE1α– XBP1 signaling in leukocytes controls prostaglandin biosynthesis and pain. Science 365, eaau6499 (2019). [DOI] [PubMed] [Google Scholar]; This study demonstrates that the IRE1α–XBP1 arm mediates prostanoid production by ER-stressed immune cells.
- 118.Wang D & DuBois RN The role of prostaglandin E2 in tumor-associated immunosuppression. Trends Mol. Med 22, 1–3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yan D, Wang HW, Bowman RL & Joyce JA STAT3 and STAT6 signaling pathways synergize to promote cathepsin secretion from macrophages via IRE1α activation. Cell Rep. 16, 2914–2927 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yao S et al. Endoplasmic reticulum stress promotes macrophage-derived foam cell formation by up-regulating cluster of differentiation 36 (CD36) expression. J. Biol. Chem 289, 4032–4042 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu H et al. Lipid droplet-dependent fatty acid metabolism controls the immune suppressive phenotype of tumor-associated macrophages. EMBO Mol. Med 11, e10698 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Batista A et al. IRE1α regulates macrophage polarization, PD-L1 expression and tumor survival. PLoS Biol. 18, e3000687 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Condamine T et al. ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis. J. Clin. Invest 124, 2626–2639 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sullivan GP et al. TRAIL receptors serve as stress-associated molecular patterns to promote ER-stress-induced inflammation. Dev. Cell 52, 714–730 (2020). [DOI] [PubMed] [Google Scholar]
- 125.Condamine T et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci. Immunol 1, aaf8943 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article reports that overexpression of ER stress-related gene signatures distinguishes PMN-MDSCs from regular neutrophils.
- 126.Thevenot PT et al. The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity 41, 389–401 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study uncovers that the UPR factor CHOP drives immunosuppression in tumours.
- 127.Mohamed E et al. The unfolded protein response mediator PERK governs myeloid cell-driven immunosuppression in tumors through inhibition of STING signaling. Immunity 52, 668–682.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; This report reveals that PERK activation in tumour-associated MDSCs inhibits protective type I interferon responses.
- 128.Chang CH et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Scharping NE et al. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity 45, 701–703 (2016). [DOI] [PubMed] [Google Scholar]
- 130.Song M et al. IRE1α– XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature 562, 423–428 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that aberrant IRE1α–XBP1 activation causes T cell metabolic dysfunction in ovarian cancer.
- 131.Ma X et al. Cholesterol induces CD8+ T cell exhaustion in the tumor microenvironment. Cell Metab. 30, 143–156.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bettigole SE & Glimcher LH Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol 33, 107–138 (2015). [DOI] [PubMed] [Google Scholar]
- 133.Cao Y et al. ER stress-induced mediator C/EBP homologous protein thwarts effector T cell activity in tumors through T-bet repression. Nat. Commun 10, 1280 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article uncovers that the UPR factor CHOP inhibits antitumour T cell function.
- 134.Hurst KE et al. Endoplasmic reticulum stress contributes to mitochondrial exhaustion of CD8+ T cells. Cancer Immunol. Res 7, 476–486 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dong H et al. The IRE1 endoplasmic reticulum stress sensor activates natural killer cell immunity in part by regulating c-Myc. Nat. Immunol 20, 865–878 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bottcher JP & Reis ESC The role of type 1 conventional dendritic cells in cancer immunity. Trends Cancer 4, 784–792 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hetz C, Axten JM & Patterson JB Pharmacological targeting of the unfolded protein response for disease intervention. Nat. Chem. Biol 15, 764–775 (2019). [DOI] [PubMed] [Google Scholar]
- 138.Harnoss JM et al. Disruption of IRE1α through its kinase domain attenuates multiple myeloma. Proc. Natl Acad. Sci. USA 116, 16420–16429 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals that targeting the IRE1α kinase domain is an effective and safe strategy against multiple myeloma.
- 139.Vincenz L, Jager R, O’Dwyer M & Samali A Endoplasmic reticulum stress and the unfolded protein response: targeting the Achilles heel of multiple myeloma. Mol. Cancer Ther 12, 831–843 (2013). [DOI] [PubMed] [Google Scholar]
- 140.Leung-Hagesteijn C et al. Xbp1s-negative tumor B cells and pre-plasmablasts mediate therapeutic proteasome inhibitor resistance in multiple myeloma. Cancer Cell 24, 289–304 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moore PC et al. Parallel signaling through IRE1α and PERK regulates pancreatic neuroendocrine tumor growth and survival. Cancer Res. 79, 6190–6203 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tang CH et al. Inhibition of ER stress-associated IRE-1/XBP-1 pathway reduces leukemic cell survival. J. Clin. Invest 124, 2585–2598 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sheng X et al. IRE1α–XBP1s pathway promotes prostate cancer by activating c-MYC signaling. Nat. Commun 10, 323 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Papandreou I et al. Identification of an Ire1α endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood 117, 1311–1314 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mimura N et al. Blockade of XBP1 splicing by inhibition of IRE1α is a promising therapeutic option in multiple myeloma. Blood 119, 5772–5781 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jin Y & Saatcioglu F Targeting the unfolded protein response in hormone-regulated cancers. Trends Cancer 6, 160–171 (2020). [DOI] [PubMed] [Google Scholar]
- 147.Le Reste PJ et al. Local intracerebral inhibition of IRE1 by MKC8866 sensitizes glioblastoma to irradiation/chemotherapy in vivo. Cancer Lett. 494, 73–83 (2020). [DOI] [PubMed] [Google Scholar]
- 148.Shi Z et al. Activation of the PERK–ATF4 pathway promotes chemo-resistance in colon cancer cells. Sci. Rep 9, 3210 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Atkins C et al. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 73, 1993–2002 (2013). [DOI] [PubMed] [Google Scholar]
- 150.Harding HP, Zyryanova AF & Ron D Uncoupling proteostasis and development in vitro with a small molecule inhibitor of the pancreatic endoplasmic reticulum kinase, PERK. J. Biol. Chem 287, 44338–44344 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yu Q et al. Type I interferons mediate pancreatic toxicities of PERK inhibition. Proc. Natl Acad. Sci. USA 112, 15420–15425 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rojas-Rivera D et al. When PERK inhibitors turn out to be new potent RIPK1 inhibitors: critical issues on the specificity and use of GSK2606414 and GSK2656157. Cell Death Differ. 24, 1100–1110 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Smith AL et al. Discovery of 1H-pyrazol-3(2H)-ones as potent and selective inhibitors of protein kinase R-like endoplasmic reticulum kinase (PERK). J. Med. Chem 58, 1426–1441 (2015). [DOI] [PubMed] [Google Scholar]
- 154.Pytel D et al. PERK is a haploinsufficient tumor suppressor: gene dose determines tumor-suppressive versus tumor promoting properties of PERK in melanoma. PLoS Genet. 12, e1006518 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rabouw HH et al. Small molecule ISRIB suppresses the integrated stress response within a defined window of activation. Proc. Natl Acad. Sci. USA 116, 2097–2102 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tsai JC et al. Structure of the nucleotide exchange factor eIF2B reveals mechanism of memory-enhancing molecule. Science 359, eaaq0939 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee AS GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 67, 3496–3499 (2007). [DOI] [PubMed] [Google Scholar]
- 158.Pyrko P, Schonthal AH, Hofman FM, Chen TC & Lee AS The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 67, 9809–9816 (2007). [DOI] [PubMed] [Google Scholar]
- 159.Reddy RK et al. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J. Biol. Chem 278, 20915–20924 (2003). [DOI] [PubMed] [Google Scholar]
- 160.Heffeter P et al. The ruthenium compound KP1339 potentiates the anticancer activity of sorafenib in vitro and in vivo. Eur. J. Cancer 49, 3366–3375 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schoenhacker-Alte B et al. Sensitivity towards the GRP78 inhibitor KP1339/IT-139 is characterized by apoptosis induction via caspase 8 upon disruption of ER homeostasis. Cancer Lett. 404, 79–88 (2017). [DOI] [PubMed] [Google Scholar]
- 162.Flocke LS, Trondl R, Jakupec MA & Keppler BK Molecular mode of action of NKP-1339 — a clinically investigated ruthenium-based drug — involves ER- and ROS-related effects in colon carcinoma cell lines. Invest. New Drugs 34, 261–268 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wernitznig D et al. First-in-class ruthenium anticancer drug (KP1339/IT-139) induces an immunogenic cell death signature in colorectal spheroids in vitro. Metallomics 11, 1044–1048 (2019). [DOI] [PubMed] [Google Scholar]
- 164.Burris HA et al. Safety and activity of IT-139, a ruthenium-based compound, in patients with advanced solid tumours: a first-in-human, open-label, dose-escalation phase I study with expansion cohort. ESMO Open 1, e000154 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cerezo M et al. Compounds triggering ER stress exert anti-melanoma effects and overcome BRAF inhibitor resistance. Cancer Cell 29, 805–819 (2016). [DOI] [PubMed] [Google Scholar]; This study discovers HA15 as a small molecule that targets BiP and overcomes BRAF inhibitor resistance in melanoma cells.
- 166.Arap MA et al. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell 6, 275–284 (2004). [DOI] [PubMed] [Google Scholar]
- 167.Rauschert N et al. A new tumor-specific variant of GRP78 as target for antibody-based therapy. Lab. Invest 88, 375–386 (2008). [DOI] [PubMed] [Google Scholar]
- 168.Binet F & Sapieha P ER stress and angiogenesis. Cell Metab. 22, 560–575 (2015). [DOI] [PubMed] [Google Scholar]
- 169.Braakman I & Hebert DN Protein folding in the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol 5, a013201 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cox JS, Shamu CE & Walter P Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73, 1197–1206 (1993). [DOI] [PubMed] [Google Scholar]
- 171.Morl K, Ma W, Gething M-J & Sambrook J A transmembrane protein with a cdc2+CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell 74, 743–756 (1993). [DOI] [PubMed] [Google Scholar]
- 172.Yoshida H, Matsui T, Yamamoto A, Okada T & Mori K XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 (2001). [DOI] [PubMed] [Google Scholar]
- 173.Calfon M et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92–96 (2002). [DOI] [PubMed] [Google Scholar]
- 174.Karagoz GE et al. An unfolded protein-induced conformational switch activates mammalian IRE1. eLife 6, e30700 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gardner BM & Walter P Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science 333, 1891–1894 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hollien J et al. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J. Cell Biol 186, 323–331 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hollien J & Weissman JS Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313, 104 (2006). [DOI] [PubMed] [Google Scholar]
- 178.Nguyên DT et al. Nck-dependent activation of extracellular signal-regulated kinase-1 and regulation of cell survival during endoplasmic reticulum stress. Mol. Biol. Cell 15, 4248–4260 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Carreras-Sureda A et al. Non-canonical function of IRE1α determines mitochondria-associated endoplasmic reticulum composition to control calcium transfer and bioenergetics. Nat. Cell Biol 21, 755–767 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals that IRE1α controls ER to mitochondria crosstalk to regulate cellular metabolism.
- 180.Urra H et al. IRE1α governs cytoskeleton remodelling and cell migration through a direct interaction with filamin A. Nat. Cell Biol 20, 942–953 (2018). [DOI] [PubMed] [Google Scholar]
- 181.Harding HP, Zhang Y & Ron D Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271–274 (1999). [DOI] [PubMed] [Google Scholar]
- 182.Lu PD, Harding HP & Ron D Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol 167, 27 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vattem KM & Wek RC Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl Acad. Sci. USA 101, 11269 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu Z, Lv Y, Zhao N, Guan G & Wang J Protein kinase R-like ER kinase and its role in endoplasmic reticulum stress-decided cell fate. Cell Death Dis. 6, e1822–e1822 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Haze K, Yoshida H, Yanagi H, Yura T & Mori K Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 10, 3787–3799 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lee K et al. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 16, 452–466 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lee AH, Iwakoshi NN & Glimcher LH XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell Biol 23, 7448–7459 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rufo N, Garg AD & Agostinis P The unfolded protein response in immunogenic cell death and cancer immunotherapy. Trends Cancer 3, 643–658 (2017). [DOI] [PubMed] [Google Scholar]
- 189.Kepp O et al. Crosstalk between ER stress and immunogenic cell death. Cytokine Growth Factor Rev. 24, 311–318 (2013). [DOI] [PubMed] [Google Scholar]
- 190.Serrano-Del Valle A, Anel A, Naval J & Marzo I Immunogenic cell death and immunotherapy of multiple myeloma. Front. Cell Dev. Biol 7, 50 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bezu L et al. eIF2α phosphorylation is pathognomonic for immunogenic cell death. Cell Death Differ. 25, 1375–1393 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sagar V et al. EPHB4 inhibition activates ER stress to promote immunogenic cell death of prostate cancer cells. Cell Death Dis. 10, 801 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gomez-Cadena A et al. Immune-system-dependent anti-tumor activity of a plant-derived polyphenol rich fraction in a melanoma mouse model. Cell Death Dis. 7, e2243 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Uruena C et al. Multifunctional T lymphocytes generated after therapy with an antitumor gallotanin-rich normalized fraction are related to primary tumor size reduction in a breast cancer model. Integr. Cancer Ther 14, 468–483 (2015). [DOI] [PubMed] [Google Scholar]
- 195.Prieto K et al. Polyphenol-rich extract induces apoptosis with immunogenic markers in melanoma cells through the ER stress-associated kinase PERK. Cell Death Discov. 5, 134 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pozzi C et al. The EGFR-specific antibody cetuximab combined with chemotherapy triggers immunogenic cell death. Nat. Med 22, 624–631 (2016). [DOI] [PubMed] [Google Scholar]
- 197.Rubio-Patino C et al. Low-protein diet induces IRE1α-dependent anticancer immunosurveillance. Cell Metab. 27, 828–842.e7 (2018). [DOI] [PubMed] [Google Scholar]
- 198.Avril T, Vauleon E & Chevet E Endoplasmic reticulum stress signaling and chemotherapy resistance in solid cancers. Oncogenesis 6, e373 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lee E, Nichols P, Groshen S, Spicer D & Lee AS GRP78 as potential predictor for breast cancer response to adjuvant taxane therapy. Int. J. Cancer 128, 726–731 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lee E et al. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Res. 66, 7849–7853 (2006). [DOI] [PubMed] [Google Scholar]
- 201.Notte A et al. Taxol-induced unfolded protein response activation in breast cancer cells exposed to hypoxia: ATF4 activation regulates autophagy and inhibits apoptosis. Int. J. Biochem. Cell Biol 62, 1–14 (2015). [DOI] [PubMed] [Google Scholar]
- 202.Salaroglio IC et al. PERK induces resistance to cell death elicited by endoplasmic reticulum stress and chemotherapy. Mol. Cancer 16, 91 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Carroll JS et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122, 33–43 (2005). [DOI] [PubMed] [Google Scholar]
- 204.Mao C, Livezey M, Kim JE & Shapiro DJ Antiestrogen resistant cell lines expressing estrogen receptor α mutations upregulate the unfolded protein response and are killed by BHPI. Sci. Rep 6, 34753 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cook KL et al. Knockdown of estrogen receptor-α induces autophagy and inhibits antiestrogen-mediated unfolded protein response activation, promoting ROS-induced breast cancer cell death. FASEB J. 28, 3891–3905 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gomez BP et al. Human X-box binding protein-1 confers both estrogen independence and antiestrogen resistance in breast cancer cell lines. Faseb J. 21, 4013–4027 (2007). [DOI] [PubMed] [Google Scholar]
- 207.Hu R et al. NF-κB signaling is required for XBP1 (unspliced and spliced)-mediated effects on antiestrogen responsiveness and cell fate decisions in breast cancer. Mol. Cell Biol 35, 379–390 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Andruska ND et al. Estrogen receptor α inhibitor activates the unfolded protein response, blocks protein synthesis, and induces tumor regression. Proc. Natl Acad. Sci. USA 112, 4737–4742 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ojha R et al. ER translocation of the MAPK pathway drives therapy resistance in BRAF-mutant melanoma. Cancer Discov. 9, 396–415 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


