Abstract
Cancer stem cells (CSCs) are a heterogeneous and dynamic self-renewing population that stands at the top of tumor cellular hierarchy and contribute to tumor recurrence and therapeutic resistance. As methods of CSC isolation and functional interrogation advance, there is a need for a reliable and accessible quantitative approach to assess heterogeneity and state transition dynamics in CSCs. We developed a high-throughput automated single cell imaging analysis (HASCIA) approach for the quantitative assessment of protein expression with single-cell resolution and applied the method to investigate spatiotemporal factors that influence CSC state transition using glioblastoma (GBM) CSCs (GSCs) as a model system. We were able to validate the quantitative nature of this approach through comparison of the protein expression levels determined by HASCIA to those determined by immunoblotting. A virtue of HASCIA was exemplified by detection of a subpopulation of SOX2-low cells, which expanded in fraction size during state transition. HASCIA also revealed that GSCs were committed to loose stem cell state at an earlier time point than the average SOX2 level decreased. Functional assessment of stem cell frequency in combination with the quantification of SOX2 expression by HASCIA defined a stable cutoff of SOX2 expression level for stem cell state. We also developed an approach to assess local cell density and found that denser monolayer areas possess higher average levels of SOX2, higher cell diversity, and a presence of a sub-population of slowly proliferating SOX2-low GSCs. HASCIA is an open source software that facilitates understanding the dynamics of heterogeneous cell population such as that of GSCs and their progeny. It is a powerful and easy-to-use image analysis and statistical analysis tool available at https://hascia.lerner.ccf.org.
Keywords: glioblastoma, neoplastic stem cells, stem cell state transition, local cell density, fluorescent antibody technique, single cell imaging, quantitative immunofluorescence analysis, automation/statistical and numerical data
HETEROGENEITY is a predominant feature of most biological systems (1–3) and emerging evidence supports the functional importance of cellular heterogeneity and noise for stability and plasticity in cell populations (4–6). Heterogeneity of response ensures robustness and stability of a population; phenotypic and genomic heterogeneity provides a substrate for adaptation and populational evolution. Complex tissue microenvironments are characterized by cellular hierarchies and uneven distribution of ligands, metabolites, and energy sources, which in turn further increases the heterogeneity of such systems. In cancers, intratumoral heterogeneity results in multiple cell subpopulations (7). Implications of such diversity include development of therapeutic resistance, tumor evolution, and cellular population drift. Many cancer types have been shown to exhibit remarkable cellular heterogeneity including glioblastoma (GBM). This primary malignant brain tumor is notorious for the development of therapeutic resistance, genetic and phenotypic diversity, and drastic temporal cellular drift (8).
Cancer stem cells (CSCs) have been first proposed as a model for acquisition of intratumoral heterogeneity (9). To date, there is substantial evidence to support that GBM CSCs (GSCs) are involved in establishment of a diverse tumor microenvironment (10–15). Recent advances in isolation, manipulation, and interrogation of CSCs have made it possible to recapitulate CSC behavior and study these cells in vitro (16).
Emergence of single-cell assessment approaches enabled the evaluation of cellular diversity. Single-cell DNA and RNA sequencing as well as flow cytometry and mass spectrometry have become widely used methods in the field of cancer research and have revolutionized our understanding of CSCs (17–20). However, most of the research efforts focus on static assessment of CSCs and underestimate spatial distribution of cell population. GSCs are a dynamic system: these cells can transition of the stem cell state via differentiation, as well as differentiated cells can acquire stem cell properties under certain conditions (hypoxia, acidic stress, metabolic stress, and chemotherapy). GSCs can form intricate communication network within GSC population as well as with other cell types within the tumor (21–24). However, there is a lack of experimental approaches to assess spatiotemporal aspects of GSC biology.
Immunofluorescence staining allows direct visualization of cellular antigens in vivo and in vitro. The method has remained a staple of antigen detection and phenotype assessment since 1940 (25). However, despite widely accessible digital image acquisition, immunofluorescence implementation in modern research remains mostly qualitative. A survey of PubMed using “Journal Article” [PT] AND 2017 [DP] AND “immunofluorescence” [TW] request revealed that only 13 out of 50 papers studied (26%) used immunofluorescence quantification techniques to report study results. Most of the 13 papers we reviewed utilized manual data quantification. An average modern smartphone possesses 2.5 times more computing power than 1985 supercomputer (26); therefore, computation speed and digital memory no longer pose limitations for most of the applications in modern science. In most areas of research, the real limitation comes from lack of accessible, user-friendly software.
Large-scale programmatically driven data analysis is being used progressively more in biology in areas such as genomics, transcriptomics, and mass spectrometry (27–30). Programming languages and computing environments such as R, Python, and MATLAB offer powerful tools for data processing and analysis (31–33). Scientific image analysis has become accessible through development of ImageJ—open source software by NIH (34,35). ImageJ and its specific application for biology—Fiji (36)—allows users to quantify a wide variety of parameters based on pixel intensity and image coordinates. The software offers tools and plugins for particle analysis and an easy-to-learn macro language for batch image processing. RStudio’s Shiny is a platform for development of user-friendly interfaces for R-based statistical inference and data visualization.
We utilized ImageJ macro and R languages, as well as the Shiny platform to create an accessible open source high-throughput automated single-cell imaging analysis (HASCIA) approach to immunofluorescence quantification. We applied the method to investigate population dynamics during GSC state transition and examined spatiotemporal factors that play a role in this process.
MATERIALS AND METHODS
GSC Derivation and Xenograft Maintenance
Previously established patient-derived xenograft (PDX) model of GBM (sample MDA23) (37) was obtained via a material transfer agreement from The University of Texas MD Anderson Cancer Center, Houston, TX (38). GBM cells were maintained using subcutaneous xenografts as previously described (39,40). All experiments utilizing mice were approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic. PDX model was maintained using immune-deficient NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice (Jackson Laboratory, Bar Harbor, ME) for the maintenance of tumor heterogeneity. Six-week-old female mice were subcutaneously injected in the flank with 2 × 106 freshly dissociated PDX GBM cells. Animals were sacrificed when tumor exceeded 5% of the animal’s body weight. Xenografted tumors were dissected mechanically and tumor cells were dissociated using papain dissociation kit (Worthington Biochemical Corporation, Lakewood, NJ). Based on previous evidence (41), we used CD133 surface marker to subsequently enrich GSCs utilizing CD133 Magnetic Bead Kit for Hematopoietic Cells (CD133/2; Miltenyi Biotech, San Diego, CA).
GSCs were propagated as a nonadherent sphere culture using Neurobasal complete medium (NBC) (Neurobasal medium; Life Technologies, Carlsbad, CA; supplemented with B27; Life Technologies), 1% penicillin/streptomycin (Life Technologies), 1 mM sodium pyruvate (Life Technologies), 2 mM l-glutamine (Life Technologies), 20 ng/ml EGF (R&D Systems, Minneapolis, MN), and 20 ng/ml FGF-2 (R&D Systems) in humidified incubator with 5% CO2. Only low passage cells (<10) were used in all experiments to prevent cellular drift in vitro.
GSC Treatments
For immunofluorescence staining and immunoblotting experiments, cells were cultured as adherent monolayer on plates or glass cover slips coated Geltrex (Thermo Fisher Scientific, Waltham, MA) (42,43). To demonstrate change in epidermal growth factor receptor (EGFR) activation, cells were cultured in three different conditions for 3 days: (1) NBC: supplemented with 20 ng/ml EGF and 20 ng/ml FGF-2, B27, 1% penicillin/streptomycin, 1 mM sodium pyruvate, and 2 mM l-glutamine; (2) NBF—Neurobasal medium supplemented with 10% fetal bovine serum (FBS; Sigma Aldrich, St. Louis, MO), B27, 1% penicillin/streptomycin, 1 mM sodium pyruvate, and 2 mM l-glutamine; and (3) DMEM + FBS—Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS and 1% penicillin/streptomycin. For state transition experiments, NBC with 25 ng/ml of bone morphogenic protein 4 (BMP4; R&D Systems) was used for 1–5 days to induce GSC differentiation. For the experiment in suspension culture, the GSCs were propagated for 3 days in NBC medium as sphere culture. The spheres were dissociated into single cells using Accutase (Life Technologies) and then used for staining.
Immunoblotting
Whole cell lysates were collected from adherent monolayer cell culture using 10% NP40 (Sigma Aldrich), 1 mM EDTA, 150 mM NaCl, 10 mM TrisCl, pH 7.5, lysis buffer supplemented with protease and phosphatase inhibitor cocktails (Sigma Aldrich). GSCs were analyzed by immunoblotting for the expression of phospho-EGFR (Y1068, 1:1,000; Cell Signaling, Danvers, MA), SOX2 (1:500; R&D Systems), and phospho-STAT3 (1:1,000, Y705; Cell Signaling). Anti-β-actin (1:5,000; Santa Cruz Biotechnology, Dallas, TX) was used for loading control.
Limiting Dilution Assay
After exposure to 1–5 days of BMP4, cells were plated in a 96-well format with 24 wells of each dilution: 0.78, 1.56, 3.125, 6.25, 12.5, 25, 50, and 100 cells/well. Number of wells positive for spheres was calculated 10 days later and stem cell frequencies were calculated using online tool available through the Walter and Eliza Hall Institute of Medical Research (http://bioinf.wehi.edu.au/software/elda/index.html) (44).
Proliferation Assay
CellTrace Far Red dye (Life Technologies) was used for the tracing of cell proliferation activity within the 5-day period. Adherent monolayer cells were incubated with 1:1,000 dilution of the dye concentrate in Neurobasal medium for 20 min at 37°C. Following 3× wash with fresh Neurobasal medium cells were incubated in NBC medium for 5 days. Cells retaining the highest amount of dye represented the slowly dividing pool whereas cells that divided multiple times had diluted the dye and therefore presented lower fluorescence intensity in far red channel.
HASCIA Method Description
Phase I: Immunofluorescence staining.
To enable high-throughput parallel analysis of multiple conditions and up to four technical replicates, we cultured cells in a 24-well format with glass cover-slips (Electron Microscopy Sciences, Hatford, PA) on the bottom of the wells. To ensure that stem cell phenotype is preserved during propagation as an adherent monolayer, we coated the glass cover-slips with laminin-rich Geltrex solution (Life Technologies). Cells were fixed with a 4% paraformaldehyde (w/v) in phosphate-buffered saline (PBS). After blocking and permeabilization with 2% donkey serum (EMD Millipore, Burlington, MA) and 0.1% triton (Sigma Aldrich), we incubated the cells with 150 μl of primary and subsequently 500 μl of secondary antibody solutions directly in the 24-well plates, washing 3× with 1,000 μl of PBS in between. Primary antibodies used were phospho-EGFR (Y1068; Cell Signaling), SOX2 (R&D Systems), and phospho-STAT3 (Y705; Cell Signaling). Secondary antibodies (Jackson ImmunoResearch, West Grove, PA) used were DL-488-conjugated donkey antimouse IgG, Cy3-conjugated donkey antimouse IgG, and DL-649-conjugated donkey antirabbit IgG.
As the last step, the internal controls (DNA and in some cases—actin) were stained by incubating the cells with Hoechst 33342 (Polyscience, Inc., Warrington, PA) and phalloidin-Alexa-594 (Invitrogen, Carlsbad, CA) solutions in PBS. Cover slips were picked up from the wells and mounted on slides using gelvatol mounting medium (PVA; Sigma Aldrich; glycerol; Sigma Aldrich; sodium azide; Thermo Fisher Scientific; TrisCl pH 8.5, water). For the experiment with suspension cells, we performed all of the described above steps using the single cell suspension. For the last step, we resuspended the stained cells in gelvatol mounting medium and place the suspension under a clear glass cover slip.
Phase II: Digital imaging.
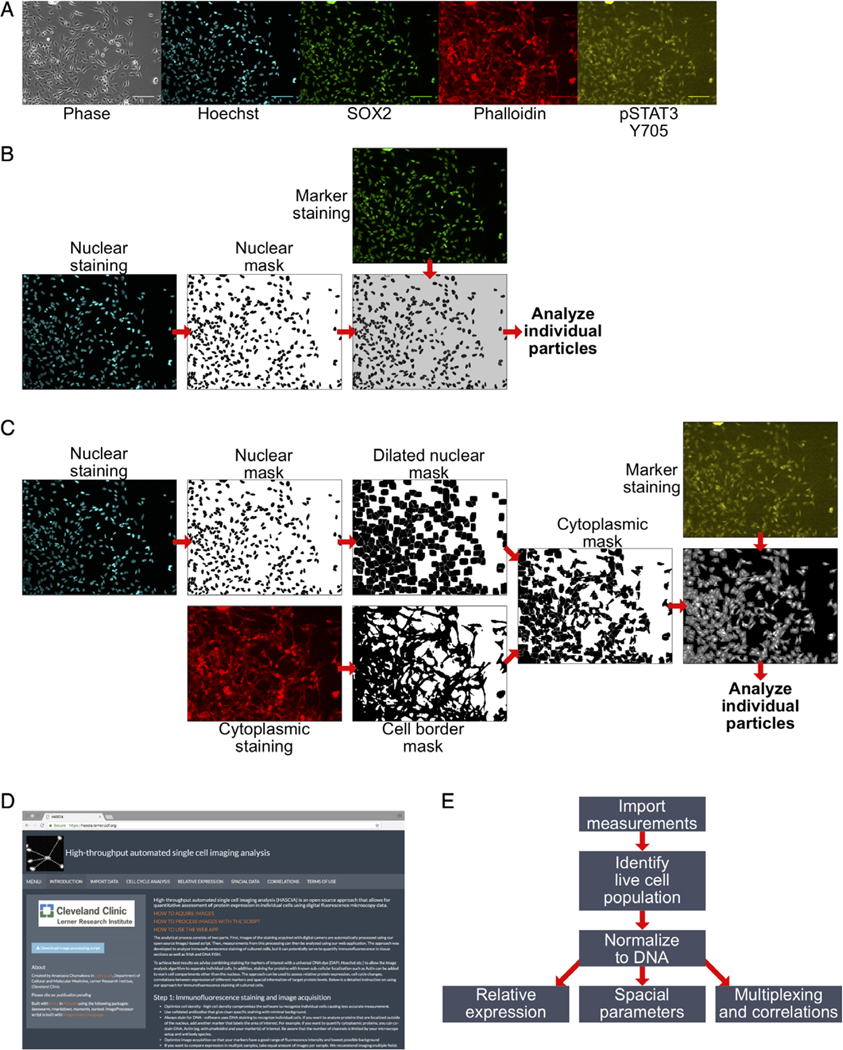
Imaging was carried out using Leica DM5000B microscope equipped with a Leica DFC310 FX Digital Color Camera (Fig. 1A). A total of 5–20 fields of view were captured from every cover slip in multiple channels.
Figure 1.
High-throughput automated single cell imaging analysis (HASCIA) method description. (A) immunofluorescence images of an adherent GSC monolayer; scale bar, 100 μm; phase, phase contrast; Hoechst, DNA staining; SOX2, stem cell marker; phalloidin, B-actin staining; pSTAT3 Y705, phosphorylated Y705 of STAT3 staining. (B) Algorithm for nuclear staining analysis. Nuclear staining images are used to create nuclear masks and separating particles. The mask is further used for the delineation of nuclear region in nuclear marker staining. Individual particles are subsequently analyzed using the particle analysis feature of ImageJ. (C) Algorithm for cytoplasmic staining analysis. Nuclear mask is created as in B with subsequent dilation to fill perinuclear area. Cytoplasmic standard staining (in this case, actin, stained by Phalloidin-Alexa594) is used to create cell border outlines. Combined dilated nuclear mask and cell border mask yield cytoplasmic mask that is then used to delineate individual cellular cytoplasm on images of marker staining. (D) Screen shot of https://hascia.lerner.ccf.org. (E) Algorithm for HASCIA web-application data processing. After image processing with ImageJ scrip, measurements are imported into the app, based on DNA intensity profiles, live cell population for every specimen is outlined, and expression in specimens is calculated by applying the normalization of marker intensity to DNA intensity in each individual particle. The normalized data can further be used to compare expression between specimens, analyze local cell density, and perform multiplexing to investigate correlations between parameters. [Color figure can be viewed at wileyonlinelibrary.com]
Phase III: Image processing.
An ImageJ-based image processing script was developed to enable analysis of digital images.
Script was created using ImageJ macro language (35,45) and under Fiji version 1.0 (36), additional plugins used: CLAHE (46). The flow of the script is shown below and in Figure 1B,C; code can be found by downloading the script file from https://hascia.lerner.ccf.org.
Images from each condition and staining are grouped into stacks to enable batch analysis;
Images are then normalized using shading images to cancel out any illumination, optics, camera, and/or shutter imperfections;
Based on the Hoechst DNA staining (nuclear internal control), a mask is created that delineates nuclear region and separates cells into individual particles using watershed functionality of ImageJ (Fig. 1B).
If the cytoplasmic staining needs to be analyzed, delineation of cell borders is performed using dilated nuclear mask and a mask of a cytoplasmic standard (phalloidin) (Fig. 1C).
The mask(s) is then applied to the staining of interest to measure fluorescence intensity in individual cells. The measurement is carried out using Analyze particles command optimized for automatic batch analysis of multiple image stacks.
The measurements obtained from image analysis include an image Label, Raw Integrated Density (RawIntDen), a measure of intensity of fluorescence in each particle, X and Y positions of each particle on the image, Feret diameter, a maximum particle caliper, and many additional parameters that can be used further for data analysis.
Phase IV: Data analysis.
For the analysis of single-cell measurements, we developed a web-application using R and Shiny platform and packages {beeswarm}, {rmarkdown}, {moments}, {nortest}, {shiny} (Fig. 1D,E). Public version of all the software is available at https://hascia.lerner.ccf.org.
Measurements from each marker as well as internal control created by image processing script are imported into the platform, organized, and then processed to analyze the following:
-
Cell cycle changes. DNA staining intensity is plotted as a histogram or as a Kernel density plot. The web-app attempts to automatically identify G1 population by identifying the highest peak. G2 peak is identified by multiplying G1 peak × value by 2 (G1x × 2). Boundaries of live cell population are then calculated with the following formula and applied to the plot:
0.5 × G1x > live population > G2x + 2 ×(G1x−0.5 × G1x)
Each graph can be manipulated to manually select G1 peak or manually outline viable cell population and exclude dead cells and cell aggregates from further analysis.
Relative expression of stained markers. Using RawIntDen as a measure of fluorescence intensity, level of expression in different tested conditions can be compared. There are also two possible approaches for the normalization of fluorescence intensity to internal control (i.e., DNA). The DNA intensity can be normalized to maximum of the selected live cell population and the marker intensity is divided by this normalized DNA value. Alternatively, marker intensity can be directly divided by DNA intensity. Both approaches enable more accurate per particle assessment of expression. Statistical analysis can be performed in this section: descriptive statistics include calculation of diversity coefficients, tests for normality, and % outliers. Relative expression between conditions can be compared using t-test, pairwise t-test, Mann–Whitney–Wilcoxon test, and Kruskal–Wallis test.
Spatial distribution of cells. Image Label is specified to identify cells located on the same image. Then, based on X and Y coordinates, position of the center of each particle is identified. Feret’s diameter is used as a measure of particle’s caliper and is used to obtain mean particle diameter. Two parameters can be calculated by our web-application: mean/median distance to top n number of closest neighbors (MDn) and number of particles within a certain radius of each cell (NWRn). Graphic representation of these parameters is outlined in Figure 4A. Similar to Relative expression section, comparative and descriptive statistical analyses can be performed on spatial parameters.
Correlations between multiple parameters including normalized and non-normalized marker expression, MDn and NWRn. Web application plots total population distribution and calculates Pearson’s correlation. A subset of the total population can be outlined and plotted independently.
Further Statistical Analysis
For the calculation and graphical presentation of diversity parameters, we processed data using R, RStudio interface and {base} R graphics. To characterize differences in cell populations, we used the following parameters:
Tsallis entropy (47,48) using Tsallis() function from {entro-part} R package;
Renyi entropy (49) using Renyi.z() function from {EntropyEstimation} R package;
-
Percent outliers (50) by determining Q1 (25%) and Q3 (75%) by quantile() function from {stats} and calculating interquartile range (IQR):
IQR =Q3–Q1
Lower outliers<Q1−1.5×IQR
Higher outliers>Q3+1.5×IQR
%OL=(N of lower outliers + N of higher outliers)/total population
Kolmogorov–Smirnov test (51) was performed by comparing sample distribution with a normal distribution with the same average and standard deviation using functions rnorm() and ks.test() from {stats} R package;
Anderson–Darling test (52) was performed using adtest() from {nortest} R package.
RESULTS
HASCIA Method Validation and Assessment of GSC Population Heterogeneity
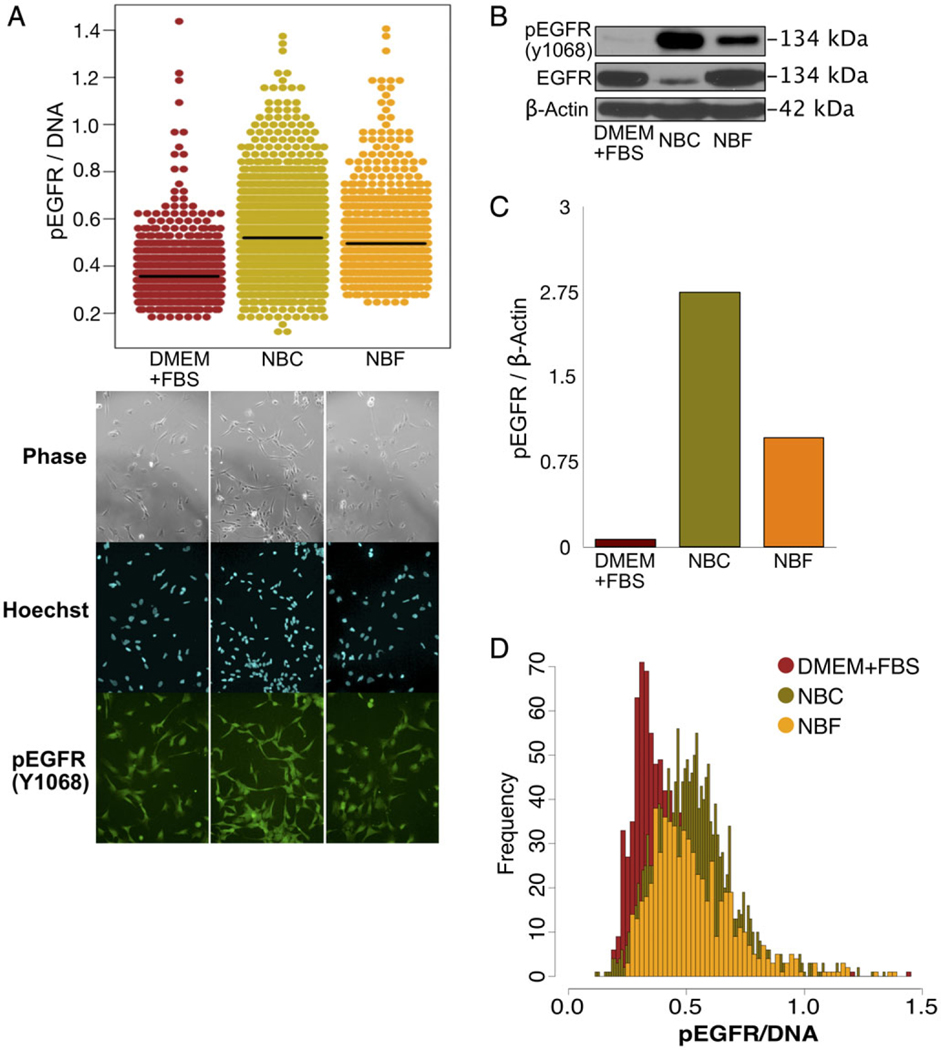
As a model of heterogeneous cell population, we used PDX GSCs. To validate the accuracy of assessing protein expression using HASCIA, we first compared cumulative protein expression levels determined by immunoblotting with mean immunofluorescence staining intensities measured by HASCIA. For this purpose, we chose EGFR activation, a signaling pathway that is well described and highly active in GSC population. To activate EGFR, we stimulated GSCs with NBC medium, which contains EGF. For the culture conditions without EGF, we used differentiation medium (DMEM + FBS) as well as Neurobasal medium without EGF and FGF but supplemented with FBS (NBF) as a control (Fig. 2A). Quantification of immunofluorescence staining for Tyr 1068 phosphorylated EGFR (pEGFR) using HASCIA revealed increased activation of the receptor when cells were cultured in GSC propagating NBC medium that contains EGF. Interestingly, insulin-containing (through B27 supplement) NBF medium was able to partially increase pEGFR levels compared to DMEM + FBS (without B27; Fig. 2A). The same EGFR activation pattern detected by immunoblotting in the parallel samples (Fig. 2B,C) validated that HASCIA can quantitatively determine the average protein expression of the population. Beyond the mean expression levels of pEGFP of the cultures, HASCIA revealed the heterogeneity of single-cell pEGFR/DNA level (Fig. 2D). We utilized previously described statistical approaches (50) to assess and compare the heterogeneity of pEGFR expression in GSC populations (Table 1). We compared diversity by calculating Tsallis and Renyi entropies and percent outliers in the three populations. Diversity was the highest in NBF differentiation inducing condition indicating that this medium was able to sustain wider spectrum of cell state. We were also able to assess normality of the expression distributions by Kolmogorov–Smirnov and Anderson-Darling tests. From these experiments it became apparent that when subjected to different media conditions response of the GSCs population is heterogeneous rather than binary. Population responds not only by shifting average expression but also by increasing or decreasing the diversity and number of outliers.
Figure 2.
HASCIA method validation and assessment of GSC population heterogeneity. (A) Immunofluorescence staining and quantification using HASCIA. Adherent CSC monolayer was treated for 3 days with DMEM + FBS, EGF-containing NBC or with NBF medium. The cells were then fixed and stained for phosphorylated Y1068 EGFR, and Hoechst was used to stain DNA as a nuclear standard. Quantification with HASCIA revealed significant differences in pEGFR expression between the three groups. (B) In parallel, after similar treatment an immunoblotting analysis was performed with whole cell lysates. (C) Densitometry analysis of the immunoblotting revealed similar trend of pEGFR changes with different media. (D) Histograms of expression distribution measured by HASCIA revealed differences in diversity and distribution shape in addition to changes in average pEGFR expression. [Color figure can be viewed at wileyonlinelibrary.com]
Table 1.
Diversity and normality of pEGFR expression distribution in GSCs
| DMEM + FBS | NBC | NBF | |
|---|---|---|---|
| Tsallis entropy | 1.4969 | 1.9069 | 1.9276 |
| Renyi entropy | 1.2773 | 1.7189 | 1.7413 |
| % outliers | 2.7701 | 2.4705 | 2.7331 |
| Kolmogorov–Smirnov index | 0.0886 | 0.0634 | 0.0932 |
| Kolmogorov–Smirnov P-value | 0.0069 | 0.0015 | 0.0090 |
| Anderson–Darling index | 19.0460 | 9.3235 | 11.7658 |
| Anderson–Darling P-value | 3.70E−24 | 1.37E−22 | 3.70E−24 |
Direct Transition Out of Stem Cell State
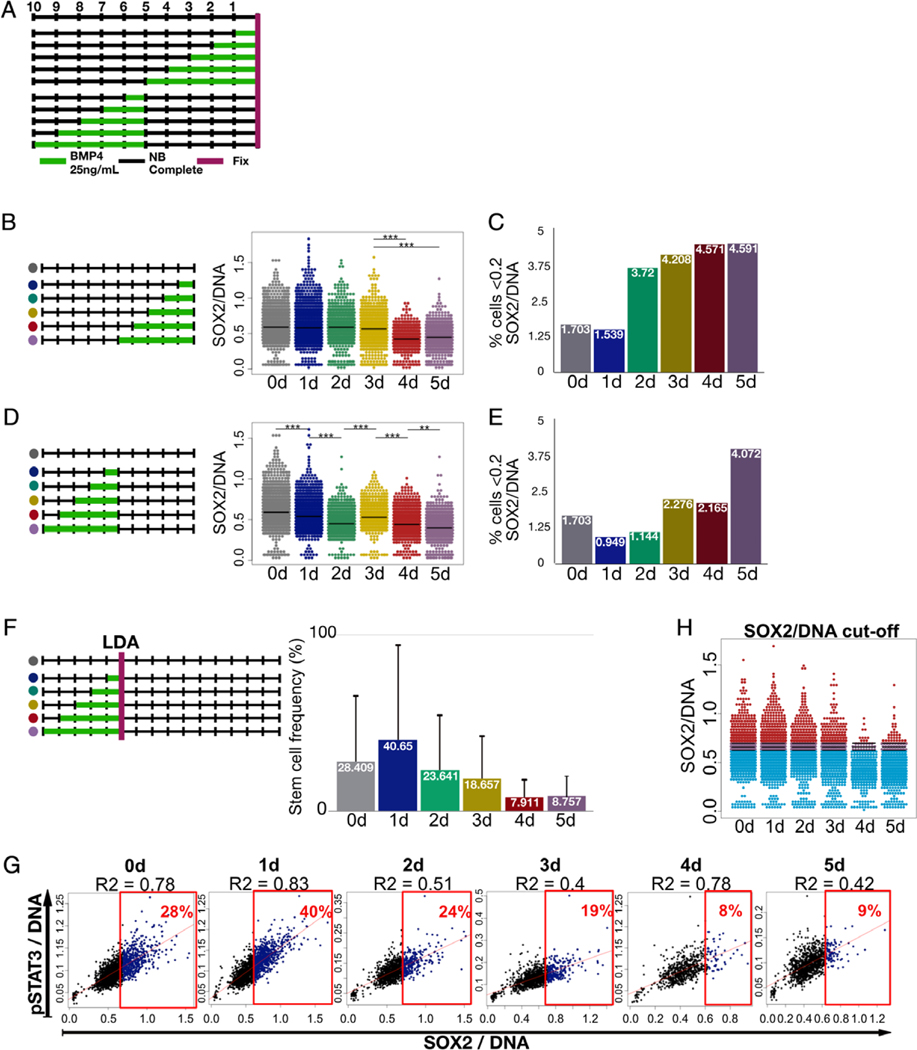
We next applied our method to investigate how GSC population responds to a differentiation stimulus over time (Fig. 3A). We first determined SOX2 and pSTAT3 expressions during a differentiation time courses as these markers have been described as predictors of stemness in GSCs (53,54). We used these markers as opposed to CD133 because they represent direct effector transcription factors for the stem cell program. To induce differentiation, GSCs were subjected to bone morphogenic protein 4 (BMP4) for 1–5 days and the cells were fixed and stained (Fig. 3B,C). Upon staining for SOX2, we observed a stable average SOX2 expression for the first 3 days and then a significant decline on days 4 and 5 (Fig. 3B). Interestingly, plotting SOX2 expression against pSTAT3 expression not only confirmed a strong correlation between the two markers but also revealed a sub-population of cells that expressed two markers at distinctively low levels separating this sub-population of cells from the majority (Fig. 3G). We measured the percentage of the SOX2-low/pSTAT3-low sub-population and plotted it against the time of treatment (Fig. 3C). An increase in SOX2-low population began to increase on day 2 of exposure to differentiation medium, suggesting a possible state transition in the population during this time. The expansion of the SOX2-low cell population preceded average SOX2 expression change by 2 days, which may indicate that state transition happens earlier than the cumulative marker expression change in the total population becomes apparent. Alternatively, these data may indicate a sub-population with different kinetics of state transition.
Figure 3.
GSC state transition and identification of SOX2 expression level in stem cell state. (A) Experimental design for state transition time courses. GSCs were cultured for total of 10 days in a 24-well plate. For differentiation induction experiments, cells were treated with NBC + BMP4 for the last 0–5 days and then fixed and stained. For reversal of differentiation experiment, cells were treated with NBC + BMP4 for 0–5 days and then returned to CSC-propagating NBC medium for 5 days before fixing and staining the cells. (B) Differentiation induction time course. Overall SOX2/DNA levels of the population showed significant decrease on days 4 and 5. (C) Quantification of SOX2-low population (<0.2 SOX2/DNA) revealed increase in this subpopulation percentage on day 2. (D) Pretreatment and reversal time course showed significant decrease in SOX2/DNA even after 1 day of pretreatment with BMP4. (E) Stable increase in the percentage of SOX2-low subpopulation (<0.2 SOX2/DNA) was observed only after 5 days of pretreatment. (F) Functional assessment of sphere forming capacity using LDA estimated stem cell frequency of cultures treated with BMP4 for varying durations. (G) Multiplexing of SOX2/DNA levels with pSTAT3/DNA from the direct time course (in A) helped outline estimated % of true stem cells (in D) and this way cutoff values of SOX2/DNA were determined. (H) For each culture with various duration of BMP4 treatment, top percentile, which correspond to sphere forming frequency (in F), of CSCs ranked according to SOX2 expression level were high lighted with red color, and the rest of the population was high lighted with blue color. Average cutoff between cutoffs of days 0–5 standard deviation (purple) was calculated. This cutoff value changed very little and independent of BMP4 exposure duration suggesting that the cells expressing SOX2 at higher than this level are capable of sphere formation. Thus, it divides each cell population into sphere-forming stem cells and nonsphere-forming cells. [Color figure can be viewed at wileyonlinelibrary.com]
State Transition and Recovery during Reversal of Differentiation
In attempt to identify a temporal point of no return for GSC state transition, we subjected cells to BMP4 pretreatment for 1–5 days, after which we returned stem cell propagating medium (NBC) for 5 days (Fig. 3D,E). Analysis of SOX2 expression using HASCIA image processing revealed that BMP4 treatment as short as 1 day suppressed average SOX2 expression level significantly lower than that in nontreated cells even after 5 days of recovery period in stem cell maintenance medium (Fig. 3D), indicating that commitment to exit stem cell state was made during this short exposure at least in some subpopulations. The longer the period of BMP4 pretreatment the lower the average SOX2 was observed. We also performed SOX2-low/pSTAT3-low sub-population quantification similar to the direct transition experiment (Fig. 3E). Surprisingly, only the cells pretreated for 5 days with BMP4 exhibited drastic increase in SOX2-low population. These data together with the results of direct transition out of stem cell state may indicate that marker expression changes are delayed in time from the actual state transition. To test that we performed a functional assessment of stem cell frequency using limiting dilution assay (LDA) (Fig. 3F). Estimated frequency of stem cells gradually decreased after day 2 of BMP4 exposure, which coincided with SOX2-low population expansion during direct time course. Together, these kinetic data from the time-courses and functional assay may indicate the beginning of state transition at day 2 with overall populational change in marker expression took place around day 4–5.
Identifying SOX2 Expression Level in a Stem Cell State
Since the time course and self-renewal (LDA) experiments were performed using parallel replicates from a common GSC population, we were able to directly compare the SOX2 expression levels with functional analysis. Using multiplex functionality of the HASCIA web-app we plotted the cell populations from the time course data of direct state transition experiment. We then outlined the top SOX2 expressing cells of the percentile that corresponded to the stem cell frequency determined by LDA for each time point (Fig. 3G). As a result, we identified a cutoff value of SOX2/DNA for each population. Interestingly, the cutoff did not change in populations exposed to BMP4 for different length of time (Fig. 3H). This finding strongly supports SOX2 as a consistent marker of stem cell state (55) and suggests that, during state transition, GSC population looses SOX2-high stem cells either through selection or through modulation of expression.
Role of Local Cell Density in Stem Cell Diversity and State Transition
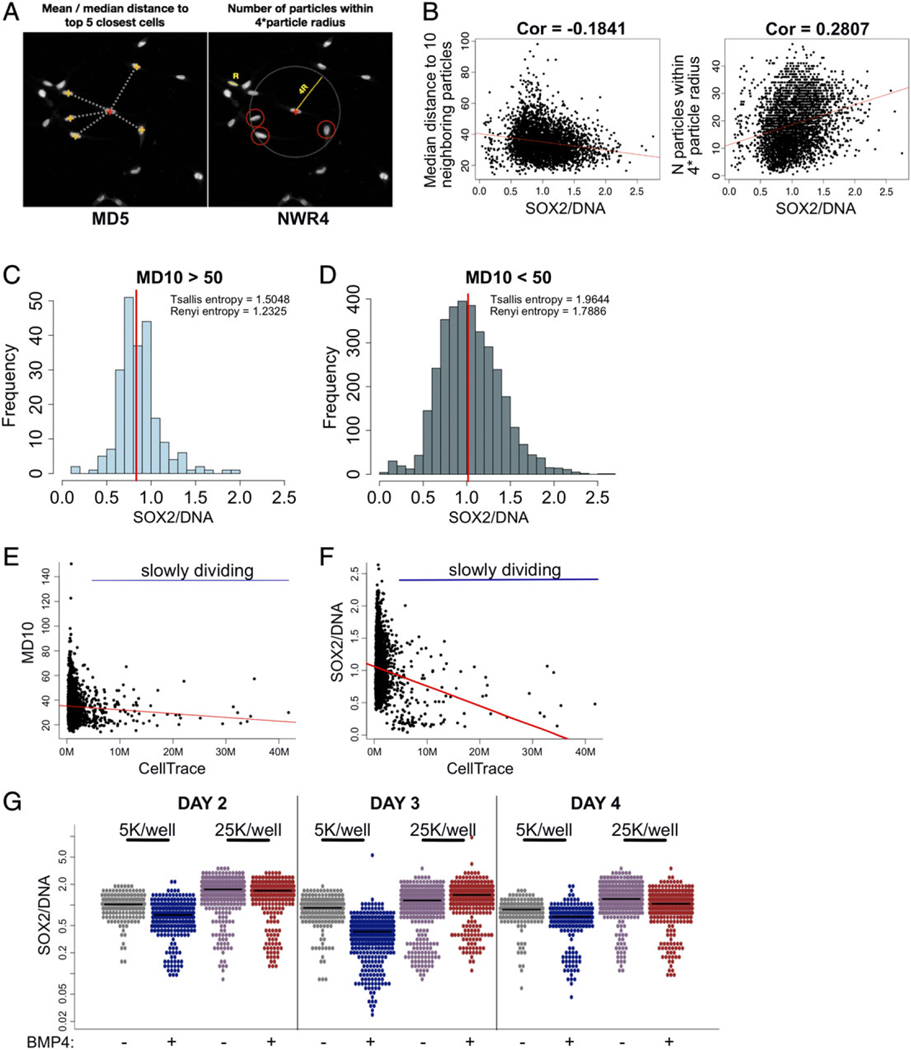
Importance of cell-to-cell contact and interaction with extracellular matrix for GSC propagation has been outlined in a several recent studies (56–58). HASCIA processing script measurements for each particle include coordinates of each nucleus centroid in the field of view as well as information about the diameter of the particle. We used these measurements to develop an automated quantification of two density parameters (Fig. 4A). We hypothesized that cell density influences stem cell state and state transition. First, we created an uneven culture of GSCs with areas of low and high cell density (Fig. 4B). We compared SOX2 expression with median distance to top 10 neighboring cells (MD10) and number of cells within four nuclear radii (NWR4). In these analyses, we found a weak correlation between the SOX2 expression and the two density parameters. The majority of SOX2-high cells were located in high-density areas (lower value for MD10 and higher value for NWR4). Sub-setting cells from low-density (MD10 > 50; Fig. 4C) and high-density (MD10 < 50; Fig. 4D) areas revealed a significant difference in diversity (Tsallis and Renyi entropies) of these two sub-populations. It should be noted that despite a small difference, average SOX2 expression was not significantly different in low-density vs. high-density areas. We then looked at proliferation of cells depending on density. Using CellTrace dye retention as a marker of slowly dividing cells, we cultured GSCs for 5 days. As expected, high-density areas of the monolayer contained slowly dividing cells (Fig. 4E). Interestingly, we observed SOX2-low population to be slower dividing (Fig. 4F). The results confirm that high-density areas are more diverse and contain both slowly dividing differentiated SOX2-low cells and rapidly dividing SOX2-high GSCs. Low-density areas on the contrary contain mostly rapidly proliferating cells with lower diversity of SOX2.
Figure 4.
Local cell density correlates with diversity of SOX2 expression and influences state transition. (A) Using particle coordinates HASCIA allows to quantify two spatial parameters: mean/median distance to top N neighboring particles (MDn) and number of particles within N× average particle radius (NWRn). (B) SOX2/DNA was plotted against MD10 and NWR4, which revealed significant correlation (Pearson’s test) between these parameters. Highest SOX2/DNA was observed in high-density areas. (C) Subset of CSC population in low local density (MD10 > 50) has a lower diversity (Tsallis and Renyi entropies) and slightly a lower average SOX2/DNA than (D) CSCs in high local density area (MD10 < 50). (E) 5-Day proliferation assay revealed slowly dividing cells (high retention of CellTrace dye) in high-density areas. (F) Slowly dividing population possesses lower SOX2/DNA level. (G) State transition time course of cells plated at 5,000/well and 25,000/well revealed faster response in low-density culture and higher, more stable SOX2/DNA level in high-density culture. [Color figure can be viewed at wileyonlinelibrary.com]
Finally, we looked at how local microenvironment affects state transition (Fig. 4G). Using BMP4 to facilitate differentiation, we plated cells into a 24-well plate at two different densities—5,000 and 25,000 cells/well—and measured SOX2 expression on days 2, 3, and 4. Consistent with findings in previous experiments, low-density monolayer had lower average SOX2 expression and response to BMP4 was apparent on day 2 in this population. We found that high-density culture responded to differentiating condition 2 days later than low-density culture.
Use of HASCIA in Suspension Culture
Traditionally, GSCs are cultured as suspension and form tumor spheres. While staining of these cells requires dissociation into single cells, all of the steps we utilized for adherent culture can be followed using single cell suspension. To demonstrate HASCIA’s functionality in this setting, we stained GSCs cultured in suspension for SOX2 and performed the analysis (Supporting Information Fig. 1). Heterogeneity of SOX2 expression was evident in the suspension population; however, we did not identify any sub-populations of cells in this setting. These findings suggest the importance of further investigation of differences between the adherently cultured cells and tumor spheres. We observed no correlation between local cell density and SOX2 expression in single-cell suspension, as local density on the cover slip was formed postfixation (Supporting Information Fig. 1).
DISCUSSION
For the accurate assessment and modeling of heterogeneous cell population dynamics, experimental approaches should enable the acquisition of quantifiable results. Biochemical approaches such as immunoblotting and classical qPCR assess cumulative or average genetic makeup or phenotype in a population based on the assessment of total cell lysates. Furthermore, biological systems present a significant level of noise; thus, high-throughput analysis techniques are warranted for multiple replicates to be included. Single-cell mass spectrometry and flow cytometry are methods that allow analyses with single-cell resolution, but their implementation is often limited due to high cost of equipment and dedicated software and technical complexity that requires users to undergo specific training. Immunofluorescence staining is a well established and relatively inexpensive approach, readily available in the majority of research laboratories. We present a solution for adapting this method to serve CSC research purposes with a single-cell resolution.
Our open source software is easy to follow and does not require lengthy training. Our results are consistent with previously published papers (59,60), suggesting that quantitative immunofluorescence staining is reliably comparable to cumulative expression analysis by immunoblotting. It allows for better data normalization, normalization to DNA content, which cancels out the general increase in the expression of many gene products due to cell cycle progression toward G2 cell cycle phase. Single-particle resolution allows assessing the heterogeneity of protein expression and its shift during response to biological stimuli in a cell population. Unlike commercially available options (Strataquest, MIPAR, etc.) (61,62) HASCIA is an open-source solution that can help guide early-stage experiments without requiring many resources. Several software solutions (such as Cell Profiler) (63) allow automated image quantification; however, they all require development statistical analysis pipelines by the user. Compared to these solutions, HASCIA has a ready-to-use statistical pipeline that is tailored toward analysis of heterogeneous populations, particularly CSCs. HASCIA yields statistically analyzed and graphically presented data, ready for publication. The software can potentially be optimized and used to analyze the images of tissue sections and RNA and DNA FISH (64).
Heterogeneity of a cell population is often a result of a distinct minority sub-population being included in the total cell pool. Our results revealed a minor sub-population of GSCs that is stable in a GSC propagating medium. Previous studies have suggested that multiple CSC subtypes may co-exist in the same tumor (23,65). Observed increase in this minor SOX2-low sub-population followed BMP4-induced state transition suggests that these cells may be further on a differentiation spectrum.
Temporal aspects of commitment for stem state transition in CSCs are understudied. Our results suggest that even though phenotypical shift in a population can be observed following up to 4 days of continuous differentiation stimulus, partial irreversible commitment to differentiation can happen following just 1 day of stimulation with a significant decrease in SOX2 appearing even after recovery. These results underline the long lasting effects of microenvironment on CSCs and the extent of population plasticity in PDX GSCs. SOX2 is an established progenitor cell program transcription factor in GSCs (66,67). Our results provide insight into a tight correlation between state transition and SOX2 expression. Narrow variation in identified SOX2 cutoff value defined by functional analysis supports the direct influence of this protein expression on sphere and tumor forming capacity.
Cell density is closely dependent on cell adhesion and cell-to-cell communication. Our group and many other researchers have previously outlined the importance of adhesion and cell communication molecules in CSC maintenance and expansion (20,68–71). However, in an experimental setting, cell density is often overlooked and poorly controlled for across studies in CSC field. By developing a coordinate analysis of immunofluorescence images, we were able to show that the local cell density impacted state transition dynamics, cellular diversity, and cell proliferation. Optimal cell density for every particular GSC specimen should be sought for the reliable representation of GBM biology in vitro.
In conclusion, our results obtained via HASCIA underlined the heterogeneity of GBM stem cells with a single-cell resolution. HASCIA can serve a useful and accessible tool to further interrogate heterogeneous and complex CSC behavior and is available through https://hascia.lerner.ccf.org.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Lathia Laboratory as well as Dr. Jacob Scott (Cleveland Clinic) and members of Scott Laboratory for constructive comments on the experimental design and manuscript. We would also like to thank Dr. Eldon Walker, Jeff Howell, and Dale Houston (Computing Services, Cleveland Clinic Lerner Research Institute) for help with web application testing and website assistance.
Grant sponsor: National Institutes of Health, Grant number: R03CA215939 to MH and NS089641, NS083629, and CA157948; Grant sponsor: Cleveland Clinic VeloSano Bike Ride; Grant sponsor: Cleveland Clinic Innovations; Grant sponsor: Lerner Research Institute; Grant sponsor: Case Comprehensive Cancer Center
Footnotes
Additional Supporting Information may be found in the online version of this article.
LITERATURE CITED
- 1.Elsasser WM. Outline of a theory of cellular heterogeneity. Proc Natl Acad Sci U S A 1984;81(16):5126–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heppner GH (1984). Tumor heterogeneity. Cancer Res, 44(6), 2259 LP-2265. Retrieved from http://cancerres.aacrjournals.org/content/44/6/2259.abstract [PubMed] [Google Scholar]
- 3.Fidler IJ, Hart IR. Biological diversity in metastatic neoplasms: Origins and implications. Science 1982;217(4564):998–1003. [DOI] [PubMed] [Google Scholar]
- 4.Suderman R, Bachman JA, Smith A, Sorger PK, Deeds EJ. Fundamental trade-offs between information flow in single cells and cellular populations. Proc Natl Acad Sci U S A 2017;114(22):5755–5760. 10.1073/pnas.1615660114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robustness Wollman R., accuracy, and cell state heterogeneity in biological systems. Curr Opin Syst Biol 2018;8:46–50. 10.1016/j.coisb.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altschuler SJ, Wu LF. Cellular heterogeneity: Do differences make a difference? Cell 2010;141(4):559–563. 10.1016/j.cell.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleppe M, Levine RL. Tumor heterogeneity confounds and illuminates: Assessing the implications. Nat Med 2014;20(4):342–344. 10.1038/nm.3522. [DOI] [PubMed] [Google Scholar]
- 8.Vartanian A, Singh SK, Agnihotri S, Jalali S, Burrell K, Aldape KD, Zadeh G. GBM’s multifaceted landscape: Highlighting regional and microenvironmental heterogeneity. Neuro Oncol 2014;16(9):1167–1175. 10.1093/neuonc/nou035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wicha MS, Liu S, Dontu G. Cancer stem cells: An old idea—A paradigm shift. Cancer Res 2006;66(4):1883–1886. 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 10.Infanger DW, Cho Y, Lopez BS, Mohanan S, Liu SC, Gursel D, Boockvar JA, Fischbach C. Glioblastoma stem cells are regulated by interleukin-8 signaling in a tumoral perivascular niche. Cancer Res 2013;73(23):7079–7089. 10.1158/0008-5472.CAN-13-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong BH, Shin H-D, Kim S-H, Mok H-S, Shim J-K, Lee J-H, Shin H-J, Huh Y-M, Kim E-H, Park E-K, et al. Increased in vivo angiogenic effect of glioma stromal mesenchymal stem-like cells on glioma cancer stem cells from patients with glioblastoma. International Journal of Oncology 2013;42(5):1754–1762. 10.3892/ijo.2013.1856. [DOI] [PubMed] [Google Scholar]
- 12.Lathia JD, Gallagher J, Myers JT, Li M, Vasanji A, McLendon RE, Hjelmeland AB, Huang AY, Rich JN. Direct in vivo evidence for tumor propagation by glioblastoma cancer stem cells. PLoS One 2011;6(9):e24807. 10.1371/journal.pone.0024807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borovski T, Beke P, van Tellingen O, Rodermond HM, Verhoeff JJ, Lascano V, Daalhuisen JB, Medema JP, Sprick MR. Therapy-resistant tumor microvascular endothelial cells contribute to treatment failure in glioblastoma multiforme. Oncogene 2013;32(12):1539–1548. 10.1038/onc.2012.172. [DOI] [PubMed] [Google Scholar]
- 14.Schonberg DL, Lubelski D, Miller TE, Rich JN. Brain tumor stem cells: Molecular characteristics and their impact on therapy. Mol Aspects Med 2014;39:82–101. 10.1016/j.mam.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CLL, Rich JN. Cancer stem cells in glioblastoma. Genes Dev 2015;29(12):1203–1217. 10.1101/gad.261982.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarrar A, Chumakova A, Hitomi M, Lathia JD. Chapter 3 Enrichment and Interrogation of Cancer Stem Cells. In: Liu H, Lathia J, editors. Elsevier, 2019. pp 59–98. 10.1016/b978-0-12-803892-5.00003-6. [DOI] [Google Scholar]
- 17.Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, Nainys J, Wu K, Kiseliovas V, Setty M, et al. Single-Cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment. Cell 2018. 10.1016/j.cell.2018.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho DWY, Yang ZF, Yi K, Lam CT, Ng MNP, Yu WC, Lau J, Wan T, Wang X, Yan Z, et al. Gene expression profiling of liver cancer stem cells by RNA-sequencing. PLoS One 2012;7(5):e37159. 10.1371/journal.pone.0037159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piccirillo SGM, Colman S, Potter NE, van Delft FW, Lillis S, Carnicer M-J, Kearney L, Watts C, Greaves M. Genetic and functional diversity of propagating cells in glioblastoma. Stem Cell Rep 2015;4(1):7–15. 10.1016/j.stemcr.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lathia JD, Li M, Sinyuk M, Alvarado AG, Flavahan WA, Stoltz K, Rosager AM, Hale J, Hitomi M, Gallagher J, et al. High-throughput flow cytometry screening reveals a role for junctional adhesion molecule a as a cancer stem cell maintenance factor. Cell Reports 2014;6(1):117–129. 10.1016/j.celrep.2013.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakano I, Garnier D, Minata M, Rak J. Extracellular vesicles in the biology of brain tumour stem cells—Implications for inter-cellular communication, therapy and biomarker development. Semin Cell Dev Biol 2015;40:17–26. 10.1016/j.semcdb.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Stockhausen M-T, Kristoffersen K, Stobbe L, Poulsen HS. Differentiation of glioblastoma multiforme stem-like cells leads to downregulation of EGFR and EGFRvIII and decreased tumorigenic and stem-like cell potential. Cancer Biol Ther 2014;15 (2):216–224. 10.4161/cbt.26736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suva ML, Rheinbay E, Gillespie SM, Patel AP, Wakimoto H, Rabkin SD, Riggi N, Chi AS, Cahill DP, Nahed BV, et al. Reconstructing and reprogramming the tumor-propagating potential of glioblastoma stem-like cells. Cell 2014;157(3):580–594. 10.1016/j.cell.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guichet P-O, Guelfi S, Teigell M, Hoppe L, Bakalara N, Bauchet L, Duffau H, Lamszus K, Rothhut B, Hugnot J-P. Notch1 stimulation induces a vascularization switch with pericyte-like cell differentiation of glioblastoma stem cells. Stem Cells 2015;33(1):21–34. [DOI] [PubMed] [Google Scholar]
- 25.Coons AH, Creech HJ, Jones RN. Immunological properties of an antibody containing a fluorescent group. Proc Soc Exp Biol Med 1941;47(2):200–202. 10.3181/00379727-47-13084P. [DOI] [Google Scholar]
- 26. https://pages.experts-exchange.com/processing-power-compared.
- 27.Oesper L, Satas G, Raphael BJ. Quantifying tumor heterogeneity in whole-genome and whole-exome sequencing data. Bioinformatics 2014;30(24):3532–3540. 10.1093/bioinformatics/btu651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen M, Carrasquillo MM, Funk C, Heavner BD, Zou F, Younkin CS, Burgess JD, Chai H-S, Crook J, Eddy JA, et al. Human whole genome genotype and transcriptome data for Alzheimer’s and other neurodegenerative diseases. Scientific Data 2016;3:160089. Retrieved from. 10.1038/sdata.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt A, Forne I, Imhof A. Bioinformatic analysis of proteomics data. BMC Syst Biol 2014;8(Suppl 2):S3–S3. 10.1186/1752-0509-8-S2-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng Z, Konopleva M, Andreeff M. Single-cell mass cytometry of acute myeloid leukemia and leukemia stem/progenitor cells. Methods Mol Biol 2017;1633:75–86. 10.1007/978-1-4939-7142-8_5. [DOI] [PubMed] [Google Scholar]
- 31. https://www.r-project.org/
- 32. https://www.python.org/
- 33. https://www.mathworks.com/products/matlab.html.
- 34. https://imagej.nih.gov/ij/index.html.
- 35.Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods 2012;9(7):671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods 2012;9:676. Retrieved from–682. 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong J, Park SY, Nguyen N, Ezhilarasan R, Martinez-Ledesma E, Wu S, Henry V, Piao Y, Tiao N, Brunell D, et al. The polo-like kinase 1 inhibitor volasertib synergistically increases radiation efficacy in glioma stem cells. Oncotarget 2018;9(12): 10497–10509. 10.18632/oncotarget.24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhat KPL, Balasubramaniyan V, Vaillant B, Ezhilarasan R, Hummelink K, Hollingsworth F, Wani K, Heathcock L, James JD, Goodman LD, et al. Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer Cell 2013;24:331–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, McLendon RE, Hjelmeland AB, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell 2010;6(5):421–432. 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006;444(7120):756–760. 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 41.Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res 2007;67:4010–4015. [DOI] [PubMed] [Google Scholar]
- 42.Fael Al-Mayhani TM, Ball SLR, Zhao J-W, Fawcett J, Ichimura K, Collins PV, Watts C. An efficient method for derivation and propagation of glioblastoma cell lines that conserves the molecular profile of their original tumours. Journal of Neuroscience Methods 2009;176(2):192–199. 10.1016/j.jneumeth.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 43.Lathia JD, Hitomi M, Gallagher J, Gadani SP, Adkins J, Vasanji A, Liu L, Eyler CE, Heddleston JM, Wu Q, et al. Distribution of CD133 reveals glioma stem cells self-renew through symmetric and asymmetric cell divisions. Cell Death Dis 2011;2:e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu Y, Smyth GK. ELDA: Extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods 2009;347 (1–2):70–78. 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 45. https://imagej.nih.gov/ij/developer/macro/macros.html.
- 46.Zuiderveld K. Graphics gems IV. In: Heckbert PS, editor. San Diego, CA, USA: Academic Press Professional, Inc. Retrieved from, 1994; p. 474–485. http://dl.acm.org/citation.cfm?id=180895.180940. [Google Scholar]
- 47.Ricotta C, Szeidl L. Towards a unifying approach to diversity measures: Bridging the gap between the Shannon entropy and Rao’s quadratic index. Theor Popul Biol 2006; 70(3):237–243. 10.1016/j.tpb.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Tsallis C. Possible generalization of Boltzmann–Gibbs statistics. J Stat Phys 1988;52 (1–2):479–487. 10.1007/BF01016429. [DOI] [Google Scholar]
- 49.Renyi A. On measures of entropy and information. Proceedings of the fourth Berkeley symposium on mathematical statistics and probability, Volume 1: Contributions to the theory of statistics. Berkeley, CA: University of California Press. Retrieved from, 1961; p. 547–561. https://projecteuclid.org/euclid.bsmsp/1200512181. [Google Scholar]
- 50.Gough AH, Chen N, Shun TY, Lezon TR, Boltz RC, Reese CE, Wagner J, Vernetti LA, Grandis JR, Lee AV, et al. Identifying and Quantifying Heterogeneity in High Content Analysis: Application of Heterogeneity Indices to Drug Discovery. In: Paulmurugan R, editor. PLoS ONE. San Francisco: USA, 2014. 10.1371/journal.pone.0102678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kolmogorov A. Sulla Determinazione Empirica Di Una Legge Di Distribuzione. Giornale Dell 1933;4:83–91. [Google Scholar]
- 52.Anderson TW, Darling DA. Asymptotic theory of certain “goodness of fit” criteria based on stochastic processes. Ann Math Stat 1952;23(2):193–212. 10.1214/aoms/1177729437. [DOI] [Google Scholar]
- 53.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A 2003;100:15178–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sherry MM, Reeves A, Wu JK, Cochran BH. STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells. Stem Cells 2009;27: 2383–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hitomi M, Deleyrolle LP, Mulkearns-Hubert EE, Jarrar A, Li M, Sinyuk M, Otvos B, Brunet S, Flavahan WA, Hubert CG, et al. Differential Connexin Function Enhances Self-Renewal in Glioblastoma. Cell Reports 2015. 10.1016/j.celrep.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fidoamore A, Cristiano L, Antonosante A, d’Angelo M, Di Giacomo E, Astarita C, Giordano A, Ippoliti R, Benedetti E, Cimini A. Glioblastoma Stem Cells Microenvironment: The Paracrine Roles of the Niche in Drug and Radioresistance. Stem Cells International 2016;2016:6809105. 10.1155/2016/6809105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turaga SM, Lathia JD. Adhering towards tumorigenicity: Altered adhesion mechanisms in glioblastoma cancer stem cells. CNS Oncol 2016;5(4):251–259. 10.2217/cns-2016-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo Y, Stacey DW, Hitomi M. Post-transcriptional regulation of cyclin D1 expression during G2 phase. Oncogene 2002;21(49):7545–7556. 10.1038/sj.onc.1205907. [DOI] [PubMed] [Google Scholar]
- 59.Hitomi M, Yang K, Guo Y, Fretthold J, Harwalkar J, Stacey DW. p27Kip1 and cyclin dependent kinase 2 regulate passage through the restriction point. Cell Cycle 2006;5 (19):2281–2289. 10.4161/cc.5.19.3318. [DOI] [PubMed] [Google Scholar]
- 60. http://www.tissuegnostics.com/en/
- 61. https://www.mipar.us/#.
- 62.Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, et al. Cell-Profiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol 2006;7(10):R100. 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stacey DW, Hitomi M, Chen G. Influence of cell cycle and oncogene activity upon topoisomerase IIalpha expression and drug toxicity. Mol Cell Biol 2000;20(24): 9127–9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cioffi M, D’Alterio C, Camerlingo R, Tirino V, Consales C, Riccio A, Ierano C, Cecere SC, Losito NS, Greggi S, et al. Identification of a distinct population of CD133 (+)CXCR4(+) cancer stem cells in ovarian cancer. Scientific Reports 2015;5(10357). 10.1038/srep10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar B, Prasad MS, Bhat-Nakshatri P, Anjanappa M, Kalra M, Marino N, Storniolo AMV, Rao X, Liu S, Wan J, et al. Normal breast-derived epithelial cells with luminal and intrinsic subtype-enriched gene expression document inter-individual differences in their differentiation cascade. Cancer Research. 2018. 10.1158/0008-5472.CAN-18-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gangemi RMR, Griffero F, Marubbi D, Perera M, Capra MC, Malatesta P, Ravetti GL, Zona GL, Daga A, Corte G. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells 2009;27(1):40–48. 10.1634/stemcells.2008-0493. [DOI] [PubMed] [Google Scholar]
- 67.Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr H-A, Delaloye J-F, Huelsken J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 2011;481(7379):85–89. 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 68.Hale JS, Li M, Lathia JD. The malignant social network: Cell–cell adhesion and communication in cancer stem cells. Cell Adh Migr 2012;6(4):346–355. 10.4161/cam.21294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu S, Li N, Yu X, Xiao X, Cheng K, Hu J, Wang J, Zhang D, Cheng S, Liu S. Expression of intercellular adhesion molecule 1 by hepatocellular carcinoma stem cells and circulating tumor cells. Gastroenterology 2013;144(5):1031–1041.e10. 10.1053/j.gastro.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 70.Rentala S, Chintala R, Guda M, Chintala M, Komarraju AL, Mangamoori LN. Atorvastatin inhibited rho-associated kinase 1 (ROCK1) and focal adhesion kinase (FAK) mediated adhesion and differentiation of CD133+CD44+ prostate cancer stem cells. Biochem Biophys Res Commun 2013;441(3):586–592. 10.1016/j.bbrc.2013.10.112. [DOI] [PubMed] [Google Scholar]
- 71.Duzagac F, Inan S, Ela Simsek F, Acikgoz E, Guven U, Khan SA, Rouhrazi H, Oltulu F, Aktug H, Erol A, et al. JAK/STAT pathway interacts with intercellular cell adhesion molecule (ICAM) and vascular cell adhesion molecule (VCAM) while prostate cancer stem cells form tumor spheroids. Journal of B.U.ON. : Official Journal of the Balkan Union of Oncology 2015;20(5):1250–1257. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.