Abstract
Acute invasive fungal rhinosinusitis is a rare, although highly morbid, infection primarily affecting immunosuppressed individuals. The same population is at particularly high risk of complications and mortality in the setting of SARS-CoV-2 infection and coronavirus disease 2019 (COVID-19)-related acute respiratory distress syndrome. The authors present a case of acute invasive fungal rhino-orbital mucormycosis in a patient with COVID-19 and discuss the prevalence, diagnosis, and treatment of fungal coinfections in COVID-19. Prompt recognition, initiation of therapy, and consideration of the challenges of rapidly evolving COVID-19 therapy guidelines are important for improving patient survival.
The authors report a case of acute invasive rhino-orbital mucormycosis in the setting of concurrent COVID-19-associated acute respiratory distress syndrome and the dilemma posed of treating both entities concurrently.
Acute invasive fungal rhinosinusitis (AIFRS) is a rare, life-threatening infection with a high risk of mortality.1 It commonly affects immunocompromised individuals, including those with hematological malignancy, diabetes mellitus, and organ transplantation.2 The global pandemic of coronavirus disease 2019 (COVID-19) has infected >18 million people internationally and over 4 million in the United States alone.3 Caused by coronavirus-2 (SARS-CoV-2), the disease may progress to acute respiratory distress syndrome (ARDS), a condition that increases the susceptibility of pulmonary fungal coinfections.4
The authors present a case of invasive fungal rhinosinusitis with orbital involvement in a patient with COVID-19 and discuss the prevalence of fungal coinfection with COVID-19, as well as the diagnostic and management challenges faced in critically ill patients. This case report, including the collection and evaluation of protected patient health information, was performed in compliance with the provisions of the United States of America Health Insurance Portability and Accountability Act of 1996 and adhered to the World Medical Association’s ethical principles for medical research involving human subjects outlined in the Declaration of Helsinki as amended in 2013.
CASE PRESENTATION
A 60-year-old man with a history of poorly controlled insulin-dependent diabetes, asthma, hypertension, hyperlipidemia, and recent travel to Mexico presented to an outside emergency department with dyspnea and hypoxia. Testing negative for SARS-CoV-2, he was discharged home with a diagnosis of bronchitis and treated with antibiotics and home oxygen. One week later, he represented with worsening symptoms. Although serum glucose was mildly elevated (105–143 mg/dl), he had marked hyperglycemia (600 mg/dl) the night before. After testing positive for SARS-CoV2, he was transferred to the University of California, San Francisco for a higher level of care for COVID-19 and the associated ARDS. On arrival, because of increasing oxygen demand, he was intubated in the intensive care unit and started on remdesivir. The following day, the patient was found to have prominence of the right eye. Computed tomography angiography of the brain, orbits, and neck revealed right globe proptosis with asymmetric retrobulbar fat stranding and extensive opacification of right maxillary, ethmoid, and frontal sinuses. There was also partial opacification of the right sphenoid sinus and erosions of the lamina papyracea (Fig. 1). The clinical and radiographic findings were highly suspicious for acute invasive fungal rhinosinusitis with orbital involvement. Hemoglobin A1c was found to be 14.0%. Intranasal tissue culture and biopsy was performed via bedside nasal endoscopy on hospital day 2. Frozen sections were not performed due to prolonged decontamination requirements for the cryotome in the setting of known COVID-19 infection; thus, the tissues were formalin fixed before processing. The patient was started on intravenous vancomycin and cefepime, antifungal coverage with liposomal amphotericin B, and strict glucose management. On hospital day 4, histopathology demonstrated mucormycosis with angioinvasion, and therefore, caspofungin was added to the medical regimen (Fig. 2). Fungal cultures ultimately grew Rhizopus species. The patient’s respiratory status was tenuous, requiring prone positioning 16+ hours daily to maintain oxygenation on 100% FiO2 and thus precluded MRI evaluation or surgical management.
FIG. 1.
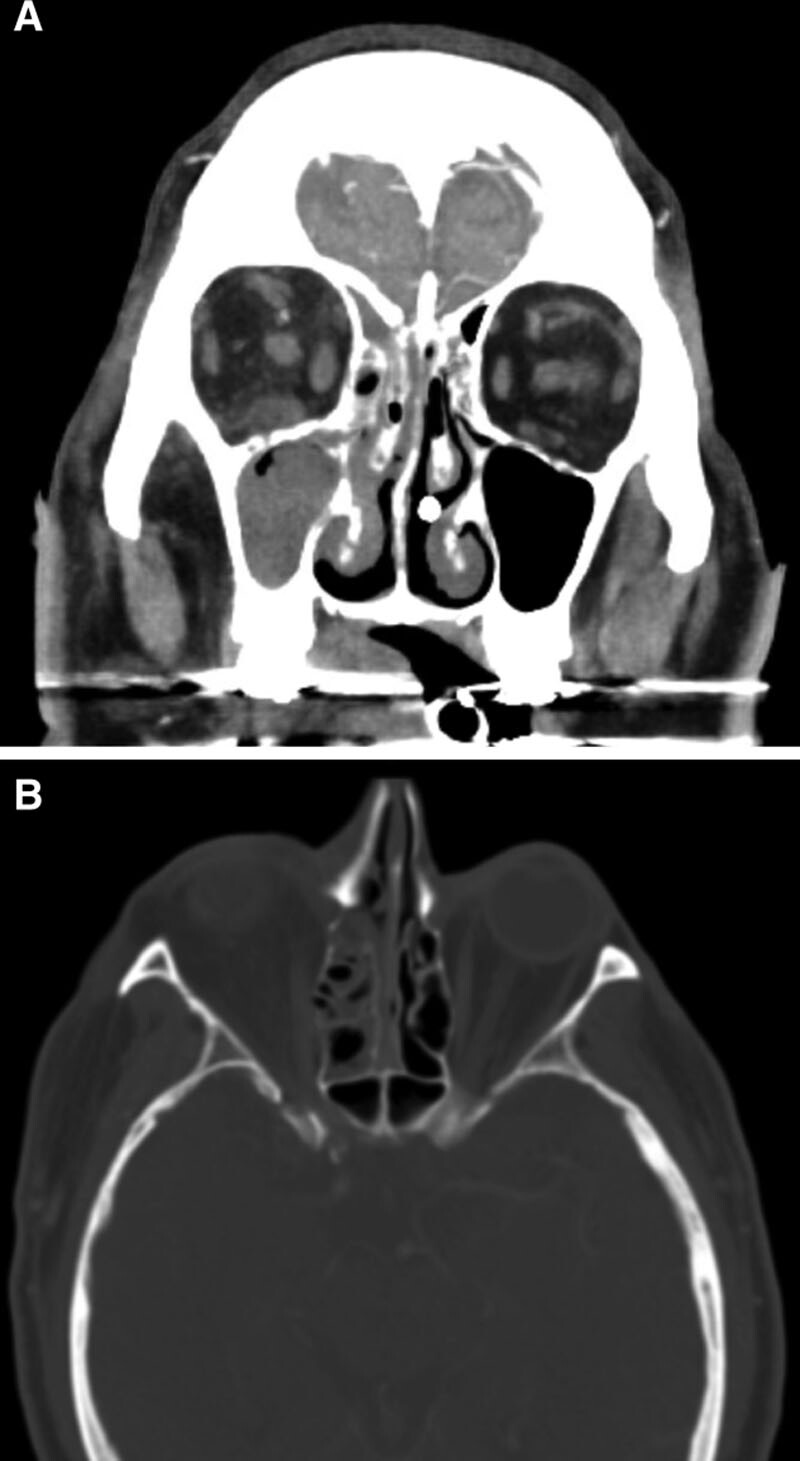
Postcontrast CT of the head. A, Coronal sections with a soft tissue window demonstrating maxillary and ethmoid sinusitis, asymmetric contrast enhancement and stranding of the intraconal and extraconal orbital fat on the right compared to the left side, and subtle enlargement and enhancement of the right inferior and medial rectus muscles. B, Axial sections with a bone window demonstrating bony dehiscence of the lamina papyracea along the right medial orbital wall.
FIG. 2.
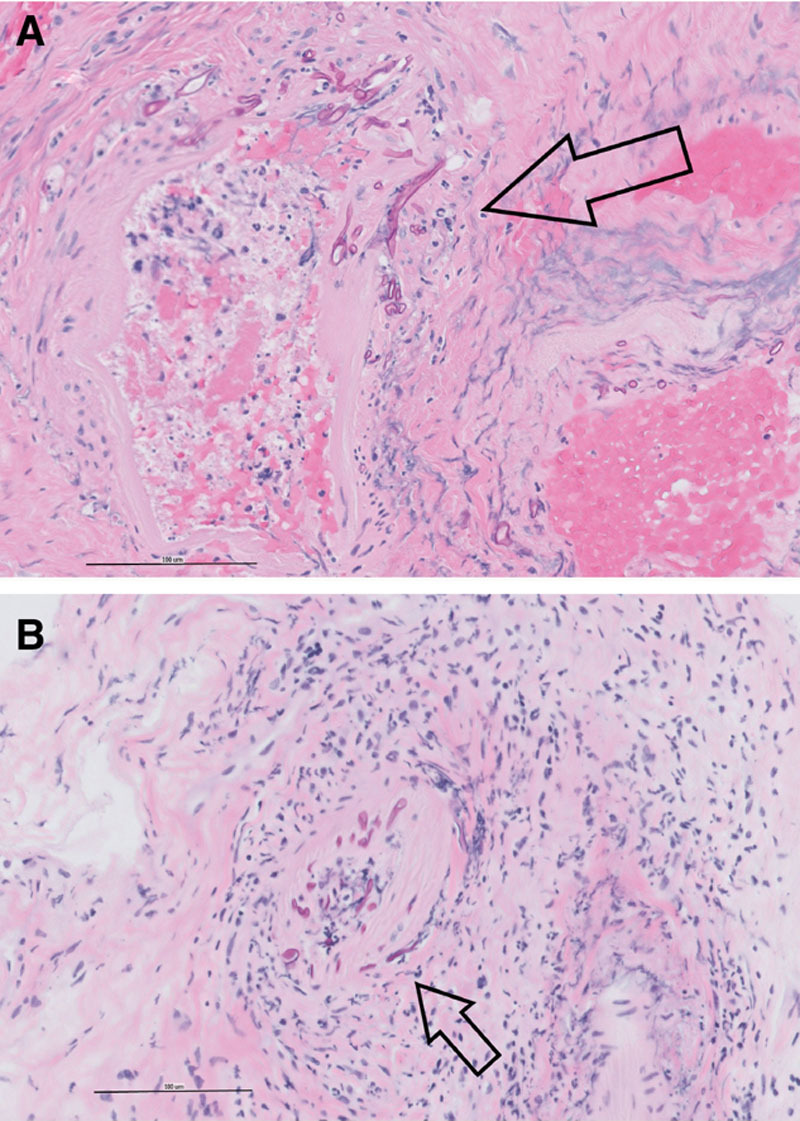
Sinus histopathology. A, Bone and sinus tissue (400×, H&E): Fungal forms infiltrating arteries (arrow). B, Sinus tissue (400×, H&E): Area of acute inflammation and fungus infiltrating arterial wall (arrow).
The ophthalmology service was consulted on hospital day 5, once histopathology confirmed invasive fungal infection. On bedside ophthalmologic examination, the right eye had mild proptosis with erythema and edema of the eyelids and conjunctival chemosis. The right pupil was fixed and mid-dilated due to an old third nerve palsy, while the left was pharmacologically constricted by his sedative medications, limiting assessment for a relative afferent pupillary defect. The patient was unable to participate with vision or motility testing due to intubation before ophthalmic examination. The remainder of the examination including dilated fundus examination was unremarkable. The patient was treated with a series of 3 daily retrobulbar injections of liposomal amphotericin B (3.5 mg/dl; off-label usage) to the right orbit. Concurrently, the patient was also started on a 10-day course of dexamethasone 6 mg daily and a single dose of convalescent plasma as a treatment for COVID-19. Unfortunately, the dexamethasone led to hyperglycemia that was difficult to manage despite aggressive insulin usage. On hospital day 10, the patient had improved respiratory status, allowing endoscopic surgical debridement by otolaryngology. COVID-19 infection control precautions included the use of powered air purifying respirators and the exclusion of trainees from operative management to minimize the risk of exposure to medical staff. He was subsequently transitioned from liposomal amphotericin B to posaconazole due to acute kidney injury. His health status continued to decline despite ongoing treatment, and he unfortunately expired on hospital day 31 from ARDS due to COVID-19.
DISCUSSION
The reported prevalence of AIFRS with orbital involvement in the United States is estimated to be 1.7 per 1 million.5 It is characterized by direct invasion and necrosis of local structures followed by rapid progression and angioinvasion from the nasal and sinus mucosa into the orbit and brain. While many fungal species can cause AIFRS, it most commonly involves Aspergillus, Rhizopus, Mucor, and Rhizomucor.6 Mortality is high, ranging between 50% and 80%, with factors including intracranial or orbital involvement, irreversible immune suppression, and mucormycosis leading to poorer outcomes.1,2
It is well established that hematological malignancy, diabetes mellitus, pharmacological immunosuppression, and HIV are the most frequent risk factors for AIFRS. While orbital cellulitis has been reported in the setting of COVID-19, little is known about the impact of COVID-19 on AIFRS and other fungal infections.7 Globally, fungal co-infections with Aspergillus and Candida in patients with COVID-19 have been reported in a limited number of studies.4 van Arkel et al.8 showed that 6 of 31 critically ill COVID-19 patients (19.4%) were found to have secondary Aspergillus infections. This suggests a potential increased risk for developing invasive pulmonary aspergillosis in COVID-19 as has been previously described in up to one-third of critically ill patients with severe influenza.8 Given the robust inflammation and concurrent immunosuppression evident in COVID-19, it is believed that the dysregulated immune response provides an hospitable environment for the development of fungal co-infections.9 It is unknown whether sinus tissues are similarly at increased risk for fungal infection in the setting of COVID-19 infection; however, there are shared risk factors for COVID-19 and invasive fungal sinusitis, including pre-existing immunosuppression from comorbidities such as diabetes mellitus.10
A diagnosis of AIFRS requires both microbiological and histologic evidence of fungi.11 Clinical presentation may be variable, making early diagnosis and prompt initiation of antifungal therapy challenging. MRI has been shown to be more sensitive than CT for detecting AIFRS, with perisinus invasion being the most diagnostic finding.12 Confirmation of fungal elements by the frozen section is traditionally preferred over paraffin due to rapid processing and overall higher sensitivity and specificity.1 However, the concern for SARS-CoV-2 exposure and infection control has shifted practices regarding sample processing and diagnostic testing. Recent literature has shown that the high temperature required for formalin-fixed paraffin-embedded processing may inactivate the virus and be preferable for histopathological diagnosis of samples in patients coinfected with COVID-19 over frozen sections.13–15 Real-time PCR has also been shown to accurately detect zygomycetes.16
Treatment of AIFRS requires a multimodal approach involving antifungal therapy, surgical debridement, and reversal of immunosuppression to the degree possible. Hyperglycemia, diabetic ketoacidosis, and metabolic disturbances provide a favorable environment for fungal growth and should be aggressively addressed with glycemic control and electrolyte repletion.1 Concurrently, surgical debridement of necrotic tissue and antifungal therapy with liposomal amphotericin B or combination therapy with amphotericin B and posaconazole or caspofungin have been shown to improve survival.2 For AIFRS patients with orbital involvement, a complication seen in 50%–60% of cases, a recent retrospective study at the authors’ institution concluded that there was no added survival benefit from orbital exenteration as other studies have also demonstrated.2,17,18 The authors suggest a conservative step-wise approach based on clinical examination and imaging findings, beginning with minimally invasive retrobulbar injections.2,19–21
To the best of the authors’ knowledge, this case represents the first report of acute invasive fungal rhinosinusitis with orbital involvement in a patient with COVID-19. The patient faced numerous challenges to early diagnosis and prompt therapy. Poorly controlled diabetes and COVID-19 status may have worked synergistically to increase the patient’s susceptibility for fungal co-infection.22 An unstable hemodynamic and respiratory status with an inability to lie supine without oxygen desaturation made MRI imaging unfeasible. While bedside nasal endoscopy was performed in a timely manner, histologic processing protocols in the setting of active COVID infection potentially delayed confirmation of mucormycosis and subsequent retrobulbar amphotericin injections by 2–3 days. Surgical debridement was delayed due to a tenuous respiratory status. Moreover, he was administered corticosteroids given the latest evidence from the UK RECOVERY trial that demonstrated improved survival among mechanically ventilated patients with severe COVID-19-associated ARDS treated with dexamethasone.23 In the present case, it was hoped that improvement of the patient’s respiratory status would facilitate more effective surgical management of the fungal disease, outweighing the potential deleterious effect on the fungal infection from steroid-induced hyperglycemia and immune suppression. In the future, alternative therapeutic approaches for COVID-19 patients with fungal coinfection may include the use of biologics such as tocilizumab, an IL-6 inhibitor that has shown promising results to reduce mortality in COVID-associated ARDS.24,25
In summary, this case highlights the difficult management decisions associated with co-infection of SARS-CoV2 and Rhizopus. These diseases share risk factors, have independently high mortality rates, but currently have conflicting management principles.
Footnotes
This research was supported, in part, by the UCSF Vision Shared Resource Core Grant (NIH/NEI P30 EY002162) and an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology at UCSF.
The authors have no financial or conflicts of interest to disclose.
REFERENCES
- 1.Deutsch PG, Whittaker J, Prasad S.Invasive and non-invasive fungal rhinosinusitis—a review and update of the evidence. Medicina. 2019; 55:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirabayashi KE, Idowu OO, Kalin-Hajdu E, et al.Invasive fungal sinusitis: risk factors for visual acuity outcomes and mortality. Ophthalmic Plast Reconstr Surg. 2019; 35:535–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong E, Du H, Gardner L.An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020; 20:533–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song G, Liang G, Liu W.Fungal co-infections associated with global COVID-19 pandemic : a clinical and diagnostic perspective from China. Mycopathologia. 2020; 185:599–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, Kim JD, Beaver HA, et al.Rhino-orbital mucormycosis treated successfully with posaconazole without exenteration. Neuroophthalmology. 2013; 37:198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez IJ, Crocetta FM, Demattè M, et al.Acute invasive fungal rhinosinusitis in immunocompromised patients: role of an early diagnosis. Otolaryngol Head Neck Surg. 2018; 159:386–393 [DOI] [PubMed] [Google Scholar]
- 7.Turbin RE, Wawrzusin PJ, Sakla NM, et al.Orbital cellulitis, sinusitis and intracranial abnormalities in two adolescents with COVID-19. Orbit. 2020; 39:305–310 [DOI] [PubMed] [Google Scholar]
- 8.van Arkel ALE, Rijpstra TA, Belderbos HNA, et al.COVID-19-associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020; 202:132–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gangneux JP, Bougnoux ME, Dannaoui E, et al.Invasive fungal diseases during COVID-19: we should be prepared. J Mycol Med. 2020; 30:100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erener S.Diabetes, infection risk and COVID-19. Mol Metab. 2020; 39:101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skiada A, Lass-Floerl C, Klimko N, et al.Challenges in the diagnosis and treatment of mucormycosis. Med Mycol. 2018; 56suppl_193–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groppo ER, El-Sayed IH, Aiken AH, et al.Computed tomography and magnetic resonance imaging characteristics of acute invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg. 2011; 137:1005–1010 [DOI] [PubMed] [Google Scholar]
- 13.Henwood AF.Coronavirus disinfection in histopathology. J Histotechnol. 2020; 43:102–104 [DOI] [PubMed] [Google Scholar]
- 14.Guerini-Rocco E, Taormina SV, Vacirca D, et al. SARS-CoV-2 detection in formalin-fixed paraffin-embedded tissue specimens from surgical resection of tongue squamous cell carcinoma. J Clin Pathol. 2020; 73:754–757 [DOI] [PubMed] [Google Scholar]
- 15.Iwen PC, Stiles KL, Pentella MA.Safety considerations in the laboratory testing of specimens suspected or known to contain the severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2). Am J Clin Pathol. 2020; 153:567–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hata DJ, Buckwalter SP, Pritt BS, et al.Real-time PCR method for detection of zygomycetes. J Clin Microbiol. 2008; 46:2353–235818480229 [Google Scholar]
- 17.Turner JH, Soudry E, Nayak JV, Hwang PH.Survival outcomes in acute invasive fungal sinusitis: a systematic review and quantitative synthesis of published evidence: survival outcomes in invasive fungal sinusitis. Laryngoscope. 2013; 123:1112–1118 [DOI] [PubMed] [Google Scholar]
- 18.Kashkouli MB, Abdolalizadeh P, Oghazian M, et al.Outcomes and factors affecting them in patients with rhino-orbito-cerebral mucormycosis. Br J Ophthalmol. 2019; 103:1460–1465 [DOI] [PubMed] [Google Scholar]
- 19.Wakabayashi T, Oda H, Kinoshita N, et al.Retrobulbar amphotericin B injections for treatment of invasive sino-orbital aspergillosis. Jpn J Ophthalmol. 2007; 51:309–311 [DOI] [PubMed] [Google Scholar]
- 20.Mainville N, Jordan DR.Orbital apergillosis treated with retrobulbar amphotericin B. Orbit. 2012; 31:15–17 [DOI] [PubMed] [Google Scholar]
- 21.Colon-Acevedo B, Kumar J, Richard MJ, et al.The role of adjunctive therapies in the management of invasive sino-orbital infection. Ophthalmic Plast Reconstr Surg. 2015; 31:401–405 [DOI] [PubMed] [Google Scholar]
- 22.Arastehfar A, Carvalho A, van de Veerdonk FL, et al. COVID-19 associated pulmonary aspergillosis (CAPA)—from immunology to treatment. J Fungi (Basel). 2020; 6:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horby P, Lim WS, Emberson JR, et al. RECOVERY Collaborative Group Dexamethasone in hospitalized patients with COVID-19 - preliminary report. N Engl J Med. 2020. Jul 17:NEJMoa2021436 [Google Scholar]
- 24.Xu X, Han M, Li T, et al.Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020; 117:10970–10975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winn BJ.Is there a role for insulin-like growth factor inhibition in the treatment of COVID-19-related adult respiratory distress syndrome? Med Hypotheses. 2020; 144:110167. [DOI] [PMC free article] [PubMed] [Google Scholar]


