Abstract
There is a link between excessive alcohol drinking and an increased risk to develop cardiovascular disease, including alcoholic cardiomyopathy (ACM). This association warrants further research on the potential utility for the electrocardiogram (ECG) in the participatory management of the chronic consequences of alcohol use disorder (AUD). Our goal is to enhance understanding about the pernicious role alcohol plays on cardiac health using the ECG, an accessible, cost-effective, validated tool to inform novel targeted treatments for AUD. In this systematic review of human studies, we examine the relationship between abnormal clinically significant changes to ECG variables and excessive alcohol drinking with the goal of identifying key patterns specific to quantity of alcohol consumed. Three independent reviewers and one consensus reviewer, adhering to the PRISMA guidelines, conducted an initial review on studies published from database inception to April 19, 2019 using PubMed, Embase, CINAHL and COCHRANE databases. The initial search generated 2225 articles. The final selected number included 153 original papers. This systematic review provides evidence of patterns of clinically significant changes to ECG variables as a consequence of excessive alcohol consumption. Future directions include investigating whether a real-time assessment, such as the ECG, in conjunction with other key behavioral and cardiac measures can help clinicians and patients realize the progressive and insidious cardiac damage due to excessive alcohol consumption. This theory guided nurse science review supports the development of personalized symptom monitoring to deliver tailored feedback that illuminate risk factors as a potentially transformative approach in the management of AUD.
Keywords: ECG, alcohol use disorder, at-risk drinking, alcoholic cardiomyopathy
Introduction
Alcohol use disorder (AUD) is a significant public health issue and current research is focusing on individualized clinical course characteristics to develop novel, tailored, effective treatment strategies (Forouzanfar et al., 2016; Kazdin, 2008; Maisto, Kirouac, & Witkiewitz, 2014). Excessive alcohol use remains the third leading preventable cause of death in the United States (U.S.), with the Centers for Disease Control (CDC) reporting that there were approximately 88,000 deaths, annually, attributable to excessive alcohol use in the U.S. from 2006–2010 (U.S. Centers for Disease Control and Prevention, 2018). There is mounting evidence that screening for excessive alcohol drinking should be a routine component of an effective health assessment before physical and psychological harm develop and burdens to the health care system compound (Jones, Johnston, Biola, Gomez, & Crowder, 2018). Current statistics report only 10% of patients afflicted with AUD receive any type of treatment and <4% receive pharmacological treatment for AUD (Barata et al., 2017; Joudrey, Kladney, Cunningham, & Bachhuber, 2019). Patient’s lack of problem awareness is cited as a primary reason that prevents individuals from seeking treatment for AUD (Probst, Manthey, Martinez, & Rehm, 2015). The Middle Range Theory of Learned Response to Chronic Illness: The Self-Help Model introduces this concept of perception of severity of illness as a necessary antecedent to patient learning and provides a framework for this current theory guided nurse research (Braden, 1990).
Discrepancies exist in the literature between benefits versus harms of alcohol consumption on the cardiovascular system, especially as it relates to the quantity of alcohol someone consumes on a regular basis. That said, evidence supports a correlation between moderate-to-heavy alcohol consumption and one’s risk to develop cardiovascular diseases, including hypertension and alcoholic cardiomyopathy (ACM) (George & Figueredo, 2011; Gordon & Kännel, 1983; Guzzo-Merello et al., 2015; Husain, Ansari, & Ferder, 2014; Klatsky, 2015; Wood et al., 2018). ACM is defined as alcohol toxicity to the myocardial cells by ethanol and its metabolite (Maisch, 2016). The comorbidity of ACM with other cardiovascular risk factors associated with AUD contribute to a complex clinical presentation, delaying treatment, often resulting in cardiac damage that is significant and irreversible, for review see (Maisch, 2016; Mirijello et al., 2017). Given these shortcomings, more research is needed to better inform targeted evidenced-based, nurse symptom science, tailored to a client’s goals, perception of a need for treatment, and current disease symptomology in AUD.
The goal of this systematic review is to incorporate published findings on excessive alcohol consumption in humans and its harmful effects on cardiovascular functioning, specifically as reflected by clinically significant abnormalities in the routinely performed electrocardiogram (ECG). While non-specific changes in ECG tracing may be observed in association with excessive consumption of alcohol, this clinical scenario has not been investigated in a rigorous way. Information gleaned from this systematic review may inform larger projects aimed at developing targeted novel strategies managing excessive alcohol drinking behavior based on highly specific, meaningful, near-real time clinical assessments, augmented with the use of a very inexpensive, readily accessible, and well-validated clinical tool, the ECG. We have included a summary table illustrating basic cardiac physiology using ECG variables for quick reference highlighting clinically significant cardiac rhythm interpretation (Supplemental Table S1).
Methods
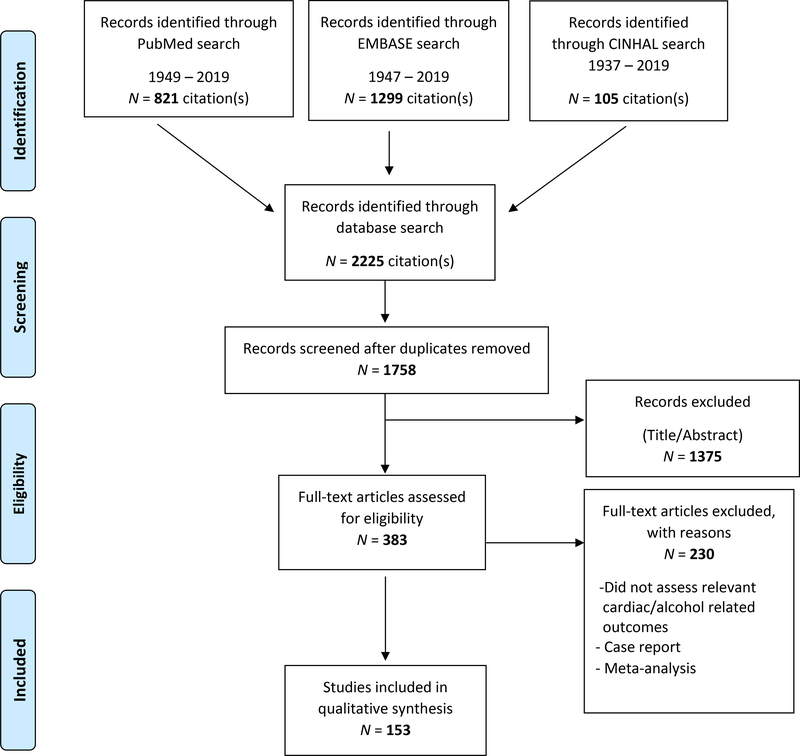
Research studies of varying designs, methodologies and length were included to investigate ECG abnormalities reported in exposure to excessive oral alcohol consumption in humans. The selection of papers for this systematic review followed the Preferred Reporting Items for Systematic Reviews, and Meta-Analysis (PRISMA) (Schulz, Altman, & Moher, 2010) (Figure 1). Three electronic databases (PubMed, Embase, and CINAHL) for studies published from database inception to April 19, 2019 were included for this systematic review. We also searched the Cochrane database which produced no results, a finding consistent with the fact that we excluded reviews and meta-analysis, as detailed below. Language was restricted to English.
Figure 1.
PRISMA Flow Diagram of Systematic Review
Due to the expansive time frame covered in this review, a comprehensive terminology describing excessive alcohol use and associated symptomology was necessary to properly translate the evolving medical terminology used in the literature, as further outlined in the Supplemental Table S2. In order to be included in this systematic review, studies had to report information on ECG tracing and interpretation on one or a combination of alcohol use disorder, alcohol dependence, alcohol abuse, alcohol misuse, excessive alcohol use, binge alcohol, heavy alcohol, harmful alcohol, unhealthy alcohol, chronic alcohol drinking, acute on chronic alcohol drinking and alcohol drinking in withdrawal. Search terms included free text and MESH terms from published articles for alcohol drinking and ECG concepts and are detailed in the Supplemental Table S2.
The goal of this work was to systematically review patterns of ECG abnormalities reported in exposure to excessive oral alcohol consumption in humans by conducting a systematic review of original empirical research data. For this reason, reviews, meta-analysis, anecdotical case reports as well as all animal studies were excluded. Additional studies were found by screening the reference lists of the eligible studies and forward search for subsequent citations of these papers.
Relevant studies were first screened by title and abstract. Studies were excluded unless the title or abstract focused on cardiovascular findings utilizing ECG interpretation in relation to excessive alcohol drinking symptomology. Where this determination was not clear, the full text of the articles was screened. Screening, selection, data extraction, and narrative synthesis were performed independently by three reviewers (LAF, DP and BDB). Differences in article selection, quality, and relevance were resolved by a consensus with a fourth independent reviewer (LL) for final determination. All reviewers have current research ethics and compliance training in Good Clinical Practice in Human Clinical Research and experience in AUD research and treatment, with three out of the four reviewers possessing clinical expertise in AUD research and treatment with training as either a registered nurse or a physician.
Results
As detailed in Figure 1, out of the 2225 articles initially reviewed, 153 satisfied the criteria of this systematic review. Studies were divided up in four categories based on ‘chronic’, ‘acute’, ‘acute on chronic’, and ‘withdrawal from excessive alcohol consumption’, as detailed in Table 1 and Supplementary Table S3.
Table 1.
Summary of Abnormal Clinically Significant ECG Main Findings
| Sample Group Studied | Key Clinically Significant Abnormal ECG Reports | |||||
|---|---|---|---|---|---|---|
| PR Interval Abnormality | P-wave Abnormality | QRS Complex Abnormality | ST Segment Abnormality | T-wave Abnormality | QTc Abnormality | |
| Chronic Alcohol Use n=93 | 12 | 17 | 53 | 20 | 34 | 23 |
| Acute Alcohol Use n=29 | 2 | 9 | 6 | 1 | 1 | 6 |
| Acute on Chronic Alcohol Use n=8 | 0 | 0 | 2 | 1 | 1 | 1 |
| Withdrawal from Alcohol Use n=23 | 1 | 3 | 15 | 6 | 14 | 7 |
n= number of publications in systematic review
QTc = Corrected QT Interval
PR Interval Abnormalities
Clinically significant PR Interval abnormalities were reported in all sample groups reviewed except for the Acute on Chronic Use group.
Chronic Alcohol Use Group (n=93):
From the studies reviewed in this group, 12 clinically significant abnormal PR Interval findings were reported as 3 increased prolongation PR Interval, 8 atrioventricular block (AV), and 1 decreased prolongation PR Interval.
Acute Alcohol Use Group (n=29):
From the studies reviewed in this group, 2 clinically significant abnormal PR Interval findings were reported as increased prolongation PR Interval.
Withdrawal from Alcohol Use Group (n=23):
From the studies reviewed in this group, 1 clinically significant abnormal PR Interval finding was reported as atrioventricular block (AV).
P-wave Abnormalities
Clinically significant P-wave abnormalities were reported in all sample groups reviewed except for the Acute on Chronic Use group.
Chronic Alcohol Use Group (n=93):
From the studies reviewed in this group, 17 clinically significant abnormal P-wave findings were reported as 6 premature atrial contractions (PAC), 2 nonspecific P-wave, 1 broad P-wave, 1 high P-wave, 1 notched P-wave, 3 increased P-wave voltage, and 3 increased P-wave duration.
Acute Alcohol Use Group (n=29):
From the studies reviewed in this group, 9 clinically significant abnormal P-wave findings were reported as 1 increased P-wave dispersion and 1 increased P-wave maximum, 1 decreased respiratory sinus arrythmia, 2 increased P-wave voltage, and 4 increased P-wave duration.
Withdrawal from Alcohol Use Group (n=23):
From the studies reviewed in this group, 3 clinically significant abnormal P-wave findings were reported as premature atrial contractions (PAC).
QRS Complex Abnormalities
Clinically significant QRS abnormalities were reported in all sample groups reviewed.
Chronic Alcohol Use Group (n=93):
From the studies reviewed in this group, 53 clinically significant abnormal QRS Complex findings were reported as 9 left ventricular hypertrophy (LVH), 8 right bundle branch block (RBBB), 5 premature ventricular contractions (PVC), 3 prolonged QRS Complex, 2 increased voltage QRS complex, 4 decreased voltage QRS complex, 1 increase duration in QRS complex, 2 left anterior hemiblock (LAH), 1 left posterior hemiblock (LPH), 12 left bundle branch block (LBBB), 1 intraventricular conduction defect, 2 left axis deviation, and 3 right axis deviation.
Acute Alcohol Use Group (n=29):
From the studies reviewed in this group, 6 clinically significant abnormal QRS Complex findings were reported as 1 PVC, 1 LBBB, 1 RBBB, 1 prolonged QRS complex, 1 increased voltage QRS complex, and 1 increased duration QRS complex.
Acute on Chronic Alcohol Use Group (n=8):
From the studies reviewed in this group, 2 clinically significant abnormal QRS Complex findings were reported as prolonged QRS complex.
Withdrawal from Alcohol Use Group (n=23):
From the studies reviewed in this group, 15 clinically significant abnormal QRS Complex findings were reported as 4 RBBB, 6 PVC, 1 left axis deviation, 2 LVH, 1 intraventricular conduction defect, and 1 decreased prolongation of QRS Complex.
ST Segment Abnormalities
Clinically significant ST Segment abnormalities were reported in all sample groups reviewed.
Chronic Alcohol Use Group (n=93):
From the studies reviewed in this group, 20 clinically significant abnormal ST segment findings were reported as 1 prolonged ST segment, 1 horizontal ST segment, 1 elevated ST segment, 6 depressed ST segments, and 11 nonspecific ST segments.
Acute Alcohol Use Group (n=29):
From the studies reviewed in this group, 1 clinically significant abnormal ST segment finding was reported as a depressed ST segment.
Acute on Chronic Alcohol Use Group (n=8):
From the studies reviewed in this group, 1 clinically significant abnormal ST segment findings was reported as a nonspecific ST segment.
Withdrawal from Alcohol Use Group (n=23):
From the studies reviewed in this group, 6 clinically significant abnormal ST segment findings were reported as 3 depressed ST segment, 1 prolonged ST segment, and 2 nonspecific ST segments.
T-wave Abnormalities
Clinically significant T-wave abnormalities were reported in all sample groups reviewed.
Chronic Alcohol Use Group (n=93):
From the studies reviewed in this group, 34 clinically significant abnormal T-wave findings were reported as 5 inverted T-waves, 2 spinous T- waves, 2 dimpled T-waves, 2 depressed T-waves, 2 flattened T-waves, 1 notched T-wave, 2 cloven T-waves, 2 increased voltage in T-waves, and 16 reports of nonspecific T-waves.
Acute Alcohol Use Group (n=29):
From the studies reviewed in this group, one clinically significant abnormal T-wave finding was reported as an increase in T-wave voltage.
Acute on Chronic Alcohol Use Group (n=8):
From the studies reviewed in this group, one clinically significant abnormal T-wave finding was reported as a nonspecific T-wave.
Withdrawal from Alcohol Use Group (n=23):
From the studies reviewed in this group, 14 clinically significant abnormal T-wave findings were reported as 5 inverted T-waves, 1 flattened T-wave, 1 tall T-wave, 1 peaked T-wave, 1 notched T-wave, 1 biphasic T-wave, and 4 nonspecific T-waves.
Corrected QT (QTc) Interval Abnormalities
Clinically significant QTc Interval abnormalities were reported in all sample groups reviewed.
Chronic Alcohol Use Group (n=93):
From the studies reviewed in this group, 23 clinically significant abnormal QTc Interval findings were reported as 23 prolonged QTc Intervals.
Acute Alcohol Use Group (n=29):
From the studies reviewed in this group, 6 clinically significant abnormal QTc Interval findings were reported as 6 prolonged QTc Intervals.
Acute on Chronic Alcohol Use Group (n=8):
From the studies reviewed in this group, 1 clinically significant abnormal QTc Interval finding was reported as a prolonged QTc Interval.
Withdrawal from Alcohol Use Group (n=23):
From the studies reviewed in this group, 7 clinically significant abnormal QTc findings were reported as 7 prolonged QTc Intervals.
Discussion
Critical View of the Studies Reported
The data collected from the 153 articles included in this systematic review reveal valuable insight into patterns of clinically significant ECG changes associated with excessive alcohol drinking behavior. The diversity and complexity of findings may reflect both differences in methodologies employed, e.g., study design, data collection and analyses, and heterogeneous effects of alcohol on the myocardial electrical conductivity. The latter aspect is supported by this work and reflects the significance in assessing an individual’s current clinical presentation, irrespective of disease progression. This work also supports future research on synchronization of possible interventions to an individual’s characteristics in an effort to overcome late-stage AUD treatment and potentially halt the progressive onset of ACM (Miller, 2005). In summary, albeit the heterogeneity of these findings does not rule out the possibility that many other factors beyond alcohol may influence cardiac function (for example smoking, hypertension, and poor nutritional status which are common comorbidities with AUD), this work supports a clinically significant effect of alcohol on cardiac physiology, as reflected by the ECG findings. Among them, dysrhythmias and alternations in QRS and/or QT appear from this work as the most relevant findings and are discussed next in greater detail.
Rhythm and Rate Determination.
Evidence of dysrhythmias reported in this systematic review reflect how excessive alcohol consumption plays a destructive role on the myocardial cells, consistent with dilation of the heart chambers, a known consequence of alcohol toxicity in both acute and chronic excessive alcohol consumption (Voskoboinik, Prabhu, Ling, Kalman, & Kistler, 2016). For example, alterations in electrolyte balance, a key clinical laboratory finding among AUD patients is associated with a variety of arrhythmias, e.g., sinus tachycardia, atrial and ventricular fibrillation, paroxysmal tachycardia, atrial flutter, sinus, and junctional tachycardia, as well as sudden sinus arrest. Clinical Implications: Excessive alcohol use may lead to dysrhythmias, which in turn adversely affect the cardiac output measure and increase one’s risk to develop seizures and eventual death, thus making dysrhythmias a medical emergency (Attilia et al., 2018; Mulholland & Brewer, 1990).
Axis Determination.
Evidence showing significant changes in ventricular muscular depolarization is reflected with reported alterations in the QRS complex in both amplitude and duration, in relation to excessive alcohol exposure. Changes to the QRS complex may be attributed to both intrinsic Intraventricular Conduction Delays (IVCD) (e.g., LBBB and RBBB) and extrinsic metabolic disturbances (e.g., hyperkalemia) (Mulholland & Brewer, 1990). Clinical Implications: Abnormal axis determination is a useful early alert prompting clinicians to further test for possible ventricular hypertrophy, acute myocardial infarction, hemiblocks, and positional change of the heart muscle due to mechanical shifts (Mulholland & Brewer, 1990). Additionally, QRS prolongation is associated with ventricular arrhythmias and sudden cardiac death (Patel et al., 2019). Important to note, in the differential diagnosis of axis deviation, comorbidities including hypertension should be considered in the general population in addition to those specifically presented among AUD patients.
Ventricular Contraction/Repolarization.
Evidence showing clinically significant QTc interval abnormalities is reflected with reported QTc prolongation in all sample groups reviewed. QT interval represents the onset of ventricular depolarization to the end of repolarization (Mulholland & Brewer, 1990). The QT interval is inversely proportional to the heart rate and highly sensitive to variations in electrolyte and environmental changes and is therefore corrected (QTc) for accurate interpretation. Although no specific method of QT measurement has been deemed most useful and/or accurate, The American Heart Association does provide a helpful recommendation for clinicians to quickly assess the QT interval by simply assessing if it is greater than half of the RR interval as an early indicator of clinical significance (Surawicz, Childers, Deal, & Gettes, 2009). Electrocardiographic evidence of a prolonged QT interval is an established risk factor for life threatening ventricular arrhythmias, specifically Torsades de Pointes (Tdp) (Malik & Camm, 2001). Clinical Implications: This is relevant to the field of addiction due to the strong association reported for an increased risk of adverse clinical outcomes and the combined use of over-the-counter and prescription medications with alcohol (Johnson & Seneviratne, 2014; White, Hingson, Pan, & Yi, 2011). As evidenced in this systematic review, medication management treating both excessive alcohol consumption as well as the myriad of comorbidities associated with this chronic disease reflects the standard practice. With an estimated 3% of all prescription medications identified as having potential QTc prolonging properties current ECG monitoring including QTc interpretation presents as a valid measure to strengthen immediate and potentially life-saving clinical treatment decisions per individual (Pourmand et al., 2017; Viskin, Justo, Halkin, & Zeltser, 2003).
Both pharmacokinetic and pharmacodynamic drug-alcohol interactions can significantly increase the risk of QTc prolongation. As for pharmacokinetics, the majority of drugs that potentially prolong the QTc interval are hepatically metabolized by the cytochrome isoenzymes CYP3A4, 1A2, and 2D6, with CYP3A4 responsible for the metabolism of approximately 50% of all drugs (Bauman, 2001). Most drugs that prolong the QTc interval work in a concentration-dependent manner. Furthermore, it is intuitive that inhibiting the metabolism of these commonly associated medications can significantly increase the risk for QTc prolongation. Pharmacodynamic interactions may also lead to QTc prolongation. These interactions occur as a result of synergistic or antagonistic pharmacologic properties and have been reported in Class III antiarrhythmics, antimicrobial agents, antifungals, antipsychotic agents, atypical antipsychotic agents, tricyclic antidepressants, selective serotonin reuptake inhibitors and non-sedating antihistamines (Al-Khatib, LaPointe, Kramer, & Califf, 2003; Chiang, 2006; Darpö, 2001; Haddad & Anderson, 2002; Kupec, 1998; Taylor, 2003; Vieweg & Wood, 2004; Yap & Camm, 2003).
Theory Driven-Precision Nurse Science
Nursing is a healing process informed by knowledge, expertise, intuition, creativity, and theory (Mariano, 2007; Pamela G Reed & Rawnsley, 2000). Patterns serve as agents for the discovery of meaningful and distinct mechanisms involved in human healing that provide insight into the person/environment interaction (P. G. Reed, 2019). Studies have demonstrated this powerful interrelationship, with patients reporting a significant increase in one’s perception of severity of illness with AUD, when provided with information about how excessive alcohol drinking is specifically harming their own bodies (Borok et al., 2013; Edlund, Booth, & Feldman, 2009). The detection and interpretation of symptoms remains an essential component in theoretical development of the self-care construct (Riegel, Jaarsma, Lee, & Strömberg, 2019). The Middle Range Theory of Learned Response to Chronic Illness: The Self-Help Model demonstrates a range of variables that mediate one’s response to the experience of chronicity, including perception of severity of illness as an antecedent to learning (LeFort, 2000).
The evidence gleaned from this systematic review builds on basic research, expanding nursing science and applied research, facilitating future conceptual development and theory testing specific to understanding this phenomenon in addiction. This work supports the inextricable link between theory, inquiry and evidence that contributes to the discipline of nursing’s distinct body of knowledge that translates the meaning, hope, and personal significance of the individual health experience (Barrett, 2002; Pamela G Reed, 1997).
Future Directions
Future large, cross-sectional, and prospective studies are needed to test the effectiveness of integrating one’s electrophysiological patterns as a meaningful learning assessment to improve early diagnosis, and early treatment engagement for the management of AUD. This aggregative synthesis assimilates key patterns related to the physiological response in the healing process of AUD and provides fertile ground for theoretical development ergo actionable knowledge in a participatory nurse practice. We encourage nurse scientists to use this data-inspired synthesis to develop creative new ways to approach AUD that have yet to be configured.
Conclusion
Variations in terminology, measurement, and analysis have evolved over the past eight decades, which is the timeframe covered by this systematic review. On the other hand, the ECG instrument itself has maintained its original importance and, in fact, continues to offer clinicians remarkable validity and reliability that has stood the test of time. This systematic review demonstrates clinically significant ECG interpretations to help illustrate changes in the normal physiological cardiac rhythm and activity in relation to excessive alcohol use. We believe this systematic review has the potential to set the basis for future work that may prove to be valuable, translatable evidence in the development of personalized nurse screening and educational strategies. The best method to evaluate the effectiveness of treatment strategies is to incorporate current symptomology data from the patients’ perspective (National Institutes of Health, 2019). It is therefore incumbent for both researchers and clinicians to seek novel and relevant information to translate clinical measures of health outcomes in the management of chronic disease. The past eight decades of research on cardiovascular disease and excessive alcohol exposure suggest there is potential value in integrating an individual’s electrophysiological patterning, along with other key behavioral, cognitive, and clinical findings, to help clinicians and patients realize the progressive and insidious myocardial damage in both the acute and chronic stages of AUD.
Supplementary Material
Acknowledgements
The authors would like to thank Diane Cooper, from the NIH Library for bibliographic assistance.
Financial support
The authors of this work are supported by and grateful to NIH intramural funding ZIA-AA000218 (Section on Clinical Psychoneuroendocrinology and Neuropsychopharmacology; PI: Dr. Lorenzo Leggio), jointly supported by NIAAA Division of Intramural Clinical and Biological Research and NIDA Intramural Research Program. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the funders, which had no role in the development of this article.
List of Abbreviations:
- ACM
Alcoholic cardiomyopathy
- AUD
Alcohol Use Disorder
- CDC
Centers for Disease Control
- ECG
Electrocardiogram
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- Al-Khatib SM, LaPointe NMA, Kramer JM, & Califf RM (2003). What clinicians should know about the QT interval. JAMA, 289(16), 2120–2127. [DOI] [PubMed] [Google Scholar]
- Attilia F, Perciballi R, Rotondo C, Capriglione I, Iannuzzi S, Attilia ML, … Fiore M (2018). Alcohol withdrawal syndrome: diagnostic and therapeutic methods. Rivista di Psichiatria, 53(3), 118–122. [DOI] [PubMed] [Google Scholar]
- Barata IA, Shandro JR, Montgomery M, Polansky R, Sachs CJ, Duber HC, … Josephson EB (2017). Effectiveness of SBIRT for alcohol use disorders in the emergency department: A systematic review. Western Journal of Emergency Medicine, 18(6), 1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett EA (2002). What is nursing science? Nursing Science Quarterly, 15(1), 51–60. doi: 10.1177/089431840201500109 [DOI] [PubMed] [Google Scholar]
- Bauman J (2001). The role of pharmacokinetics, drug interactions and pharmacogenetics in the acquired long QT syndrome. European Heart Journal Supplements, 3(suppl_K), K93–K100. [Google Scholar]
- Borok J, Galier P, Dinolfo M, Welgreen S, Hoffing M, Davis JW, … Karno M (2013). Why do older unhealthy drinkers decide to make changes or not in their alcohol consumption? Data from the Healthy Living as You Age Study. Journal of the American Geriatrics Society, 61(8), 1296–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden CJ (1990). A test of the self-help model: Learned response to chronic illness experience. Nursing Research, 39(1), 42–47. [PubMed] [Google Scholar]
- Chiang C (2006). Drug-induced long QT syndrome. Journal of Medical and Biological Engineering, 26(3), 107. [Google Scholar]
- Darpö B (2001). Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. European Heart Journal Supplements, 3(suppl_K), K70–K80. [Google Scholar]
- Edlund MJ, Booth BM, & Feldman ZL (2009). Perceived need for treatment for alcohol use disorders: results from two national surveys. Psychiatric Services, 60(12), 1618–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forouzanfar MH, Afshin A, Alexander LT, Anderson HR, Bhutta ZA, Biryukov S, … Charlson FJ (2016). Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015:A systematic analysis for the Global Burden of Disease Study 2015. The Lancet, 388(10053), 1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A, & Figueredo VM (2011). Alcoholic cardiomyopathy: A review. Journal of Cardiac Failure, 17(10), 844–849. [DOI] [PubMed] [Google Scholar]
- Gordon T, & Kännel W (1983). Drinking habits and cardiovascular disease: The Framingham Study. American Heart Journal, 105(4), 667–673. [DOI] [PubMed] [Google Scholar]
- Guzzo-Merello G, Segovia J, Dominguez F, Cobo-Marcos M, Gomez-Bueno M, Avellana P, … Garcia-Pavia P (2015). Natural history and prognostic factors in alcoholic cardiomyopathy. JACC Heart Failure, 3(1), 78–86. doi: 10.1016/j.jchf.2014.07.014 [DOI] [PubMed] [Google Scholar]
- Haddad PM, & Anderson IM (2002). Antipsychotic-related QTc prolongation, torsade de pointes and sudden death. Drugs, 62(11), 1649–1671. [DOI] [PubMed] [Google Scholar]
- Husain K, Ansari RA, & Ferder L (2014). Alcohol-induced hypertension: Mechanism and prevention. World Journal of Cardiology, 6(5), 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, & Seneviratne C (2014). Alcohol–medical drug interactions. Handbook of Clinical Neurology, 125, 543–559. [DOI] [PubMed] [Google Scholar]
- Jones Q, Johnston B, Biola H, Gomez S, & Crowder C (2018). Implementing standardized substance use disorder screening in primary care. Journal of the American Academy of PAs, 31(10), 42–45. [DOI] [PubMed] [Google Scholar]
- Joudrey PJ, Kladney M, Cunningham CO, & Bachhuber MA (2019). Primary care engagement is associated with increased pharmacotherapy prescribing for alcohol use disorder (AUD). Addiction Science & Clinical Practice, 14(1), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdin AE (2008). Evidence-based treatment and practice: New opportunities to bridge clinical research and practice, enhance the knowledge base, and improve patient care. American Psychologist, 63(3), 146. [DOI] [PubMed] [Google Scholar]
- Klatsky AL (2015). Alcohol and cardiovascular diseases: Where do we stand today? Journal of Internal Medicine, 278(3), 238–250. doi: 10.1111/joim.12390 [DOI] [PubMed] [Google Scholar]
- Kupec I (1998). Seldane and generic terfenidine withdrawn from market. Feb 27, 1998. Retrieved from Science Blog: http://www.scienceblog.com/community
- LeFort SM (2000). A test of Braden’s self-help model in adults with chronic pain. Journal of Nursing Scholarship, 32(2), 153–160. [DOI] [PubMed] [Google Scholar]
- Maisch B (2016). Alcoholic cardiomyopathy: The result of dosage and individual predisposition. Herz, 41(6), 484–493. doi: 10.1007/s00059-016-4469-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto SA, Kirouac M, & Witkiewitz K (2014). Alcohol use disorder clinical course research: Informing clinicians’ treatment planning now and in the future. J Stud Alcohol Drugs, 75(5), 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik M, & Camm AJ (2001). Evaluation of drug-induced QT interval prolongation. Drug Safety, 24(5), 323–351. [DOI] [PubMed] [Google Scholar]
- Mariano C (2007). Holistic nursing as a specialty: Holistic nursing—Scope and standards of practice. Nursing Clinics of North America, 42(2), 165–188. [DOI] [PubMed] [Google Scholar]
- Miller WR (2005). Are alcoholism treatments effective? The Project MATCH data: Response. BMC Public Health, 5(1), 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirijello A, Tarli C, Vassallo GA, Sestito L, Antonelli M, d’Angelo C, … Addolorato G (2017). Alcoholic cardiomyopathy: What is known and what is not known. European Journal of Internal Medicine. doi: 10.1016/j.ejim.2017.06.014 [DOI] [PubMed] [Google Scholar]
- Mulholland GC, & Brewer BB (1990). Improving your skills in 12 lead ECG interpretation: Philadelphia, PA: Williams & Wilkins. [Google Scholar]
- National Institutes of Health. (2019). Patient-Reported Outcomes Measurement Information System (PROMIS). Retrieved from https://www.nia.nih.gov/research/resource/patient-reported-outcomes-measurement-information-system-promis [DOI] [PMC free article] [PubMed]
- Patel SI, Ackerman MJ, Shamoun FE, Geske JB, Ommen SR, Love WT, … Lester SJ (2019). QT prolongation and sudden cardiac death risk in hypertrophic cardiomyopathy. Acta Cardiologica. 74(1), 53–58. [DOI] [PubMed] [Google Scholar]
- Pourmand A, Mazer-Amirshahi M, Chistov S, Sabha Y, Vukomanovic D, & Almulhim M (2017). Emergency department approach to QTc prolongation. The American journal of emergency medicine, 35(12), 1928–1933. [DOI] [PubMed] [Google Scholar]
- Probst C, Manthey J, Martinez A, & Rehm J (2015). Alcohol use disorder severity and reported reasons not to seek treatment: A cross-sectional study in European primary care practices. Substance Abuse Treatment, Prevention, and Policy, 10(1), 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed PG (1997). Nursing: The ontology of the discipline. Nursing Science Quarterly, 10(2), 76–79. [DOI] [PubMed] [Google Scholar]
- Reed PG (2019). Intermodernism: A Philosophical Perspective for Development of Scientific Nursing Theory. Advances in Nursing Science, 42(1), 17–27. [DOI] [PubMed] [Google Scholar]
- Reed PG, & Rawnsley M (2000). Nursing reformation: Historical reflections and philosophic foundations. Nursing Science Quarterly, 13(2), 129–136. [DOI] [PubMed] [Google Scholar]
- Riegel B, Jaarsma T, Lee CS, & Strömberg A (2019). Integrating symptoms into the middle-range theory of self-care of chronic illness. Advances in Nursing Science, 42(3), 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, & Moher D (2010). CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Annals of Internal Medicine, 152(11), 726–732. [DOI] [PubMed] [Google Scholar]
- Surawicz B, Childers R, Deal BJ, & Gettes LS (2009). AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part III:Intraventricular conduction disturbances a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society endorsed by the International Society for Computerized Electrocardiology. Journal of the American College of Cardiology, 53(11), 976–981. [DOI] [PubMed] [Google Scholar]
- Taylor D (2003). Antipsychotics and QT prolongation. Acta Psychiatrica Scandinavica, 107(2), 85–95. [DOI] [PubMed] [Google Scholar]
- U.S. Centers for Disease Control and Prevention. (2018). Alcohol use and health. Retrieved from http://www.cdc.gov/alcohol/fact-sheets/alcohol-use.htm
- Vieweg WVR, & Wood MA (2004). Tricyclic antidepressants, QT interval prolongation, and torsade de pointes. Psychosomatics, 45(5), 371–377. [DOI] [PubMed] [Google Scholar]
- Viskin S, Justo D, Halkin A, & Zeltser D (2003). Long QT syndrome caused by noncardiac drugs. Progress in Cardiovascular Diseases, 45(5), 415–427. [DOI] [PubMed] [Google Scholar]
- Voskoboinik A, Prabhu S, Ling L. h., Kalman JM, & Kistler PM (2016). Alcohol and atrial fibrillation: A sobering review. Journal of the American College of Cardiology, 68(23), 2567–2576. [DOI] [PubMed] [Google Scholar]
- White AM, Hingson RW, Pan I. j., & Yi H-Y (2011). Hospitalizations for alcohol and drug overdoses in young adults ages 18–24 in the United States, 1999–2008: Results from the Nationwide Inpatient Sample. J Stud Alcohol Drugs, 72(5), 774–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, … Burgess S (2018). Risk thresholds for alcohol consumption: Combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. The Lancet, 391(10129), 1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap YG, & Camm AJ (2003). Drug induced QT prolongation and torsades de pointes. Heart, 89(11), 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.