Periodontitis is a chronic inflammatory disease triggered by dysbiosis of the oral microbiome. Porphyromonas gingivalis is strongly implicated in periodontal inflammation, gingival tissue destruction, and alveolar bone loss through sustained exacerbation of the host response. Recently, the use of other bacterial species, such as Akkermansia muciniphila, has been suggested to counteract inflammation elicited by P. gingivalis. In this study, the effects of A. muciniphila and its pili-like protein Amuc_1100 on macrophage polarization during P. gingivalis infection were evaluated in a murine model of experimental periodontitis.
KEYWORDS: Porphyromonas gingivalis, Akkermansia muciniphila, host-microbe interactions, periodontitis, probiotics
ABSTRACT
Periodontitis is a chronic inflammatory disease triggered by dysbiosis of the oral microbiome. Porphyromonas gingivalis is strongly implicated in periodontal inflammation, gingival tissue destruction, and alveolar bone loss through sustained exacerbation of the host response. Recently, the use of other bacterial species, such as Akkermansia muciniphila, has been suggested to counteract inflammation elicited by P. gingivalis. In this study, the effects of A. muciniphila and its pili-like protein Amuc_1100 on macrophage polarization during P. gingivalis infection were evaluated in a murine model of experimental periodontitis. Mice were gavaged with P. gingivalis alone or in combination with A. muciniphila or Amuc_1100 for 6 weeks. Morphometric analysis demonstrated that the addition of A. muciniphila or Amuc_1100 significantly reduced P. gingivalis-induced alveolar bone loss. This decreased bone loss was associated with a proresolutive phenotype (M2) of macrophages isolated from submandibular lymph nodes as observed by flow cytometry. Furthermore, the expression of interleukin 10 (IL-10) at the RNA and protein levels was significantly increased in the gingival tissues of the mice and in macrophages exposed to A. muciniphila or Amuc_1100, confirming their anti-inflammatory properties. This study demonstrates the putative therapeutic interest of the administration of A. muciniphila or Amuc_1100 in the management of periodontitis through their anti-inflammatory properties.
INTRODUCTION
Periodontitis is a highly prevalent inflammatory disease, with more than 700 million cases reported globally (1). This disease is characterized by inflammation and destruction of tooth-supporting tissues including alveolar bone and cement, ultimately leading to tooth loss (2). Clinical symptoms depend on the severity of the disease and include periodontal pocketing, gingival swelling and bleeding, and tooth mobility and migration, reducing masticatory function, which consequently negatively influence the oral-health-related quality of life (3).
The inflammatory nature of periodontitis is induced via microbiome dysbiosis characterized by alterations in the abundance of keystone pathogens within a polymicrobial community that disrupt host-microbe homeostasis (4). Porphyromonas gingivalis is a keystone pathogen involved in periodontitis with the ability to modulate the composition and function of the oral microbiome and is strongly associated with severe periodontal lesions (5). Through interaction with gingival epithelial cells, P. gingivalis activates the innate immune response (6–8), inducing the recruitment of neutrophils and macrophages into the gingival tissue (9–12). This immune response drives the production of cytokines, proteases, and chemokines that drive P. gingivalis-associated tissue destruction (13, 14), while inhibiting the production of T-cell chemoattractants (15). The recruitment of macrophages is pivotal to the pathogenesis of periodontitis, due to their versatile functions. They participate in the initiation and resolution of inflammation, as well as in the activation of lymphocyte-mediated adaptive immunity and alveolar bone resorption (16–18). Levels of several proinflammatory cytokines appear elevated during chronic periodontitis; tumor necrosis factor alpha (TNF-α) is among the most prominent produced by M1 (CD80/86+) macrophages. In contrast, M2 (CD206/163+) macrophages are associated with the resolution of inflammation and express high levels of the metabolic marker Arg-1 and the anti-inflammatory cytokine interleukin 10 (IL-10). Both in vitro and in vivo findings have consistently suggested an enhanced phenotype and an increase in the number of M1-like macrophages recruited in periodontitis (19–22).
Current management of periodontitis aims to restore microbiome homeostasis and balance between bacterial insult and the host response. Conventional nonsurgical treatment for periodontitis consists of mechanical debridement of the periodontal lesion, scaling, and root planing, and for severe forms of the disease, the adjunctive administration of antibiotics/antiseptics can be applied with moderate success (23–25). However, the limitations of these therapeutic procedures and the risk of side effects, such as antibiotic resistance, emphasize the need for new pharmacological approaches targeting both microbiome dysbiosis and inflammation (26). Recently, probiotic supplementation has become a topic of interest for alternative therapeutic approaches (27). Several Lactobacillus strains, including Lactobacillus reuteri, Lactobacillus salivarius, and Lactobacillus brevis, have been evaluated in clinical trials as adjunctive therapeutics to mechanical treatment, with promising results (27–29). Mechanisms by which probiotic bacteria compete with the existing microbiome include influencing the host immune response to allow for colonization and manipulation of bacterium-bacterium interactions to create a niche to inhabit. Oral probiotics can take advantage of these mechanisms in the context of periodontitis to prevent formation of the pathogenic biofilm and to halt disease progression (30). However, none of the probiotics tested have moved into mainstream treatment plans for periodontitis (23).
Akkermansia muciniphila, a mucophilic Gram-negative symbiont, is found throughout the length of the gastrointestinal tract, with an enriched population in the colon (31). Current research has demonstrated the beneficial effect of the administration of A. muciniphila in the management of obesity and diabetes (32–34). It was recently reported that the administration of A. muciniphila to obese individuals contributed to weight loss and regulation of insulin, without any observed concerns related to its safety of use (35, 36). Furthermore, high doses of pasteurized A. muciniphila have shown negative in vitro genotoxicity results, combined with the absence of adverse effects in a 90-day toxicity study (36).
In the context of periodontitis, increased presence of A. muciniphila has been demonstrated in the oral cavities of healthy individuals, but it is totally absent from the microbiomes of those with severe periodontitis (37). In a recent murine model of experimental periodontitis, administration of A. muciniphila significantly reduced bone loss and periodontal tissue inflammation associated with P. gingivalis inoculation (38). The beneficial effects observed with the administration of A. muciniphila may be mediated by one of its surface molecules, the pili-like protein Amuc_1100 (39). The use of this protein in both in vitro and in vivo models has shown its ability to regulate the immune response and to increase transepithelial resistance and the expression of tight-junction proteins, highlighting its potential as a therapeutic compound (39–41). However, the mechanism through which A. muciniphila reduces gingival inflammation and the role of Amuc_1100 are still unclear.
In the present study, the impact of oral administration of A. muciniphila and its pili-like protein Amuc_1100 on the innate immune response in P. gingivalis-induced experimental periodontitis has been evaluated.
RESULTS
A. muciniphila and Amuc_1100 decrease alveolar bone loss associated with P. gingivalis infection.
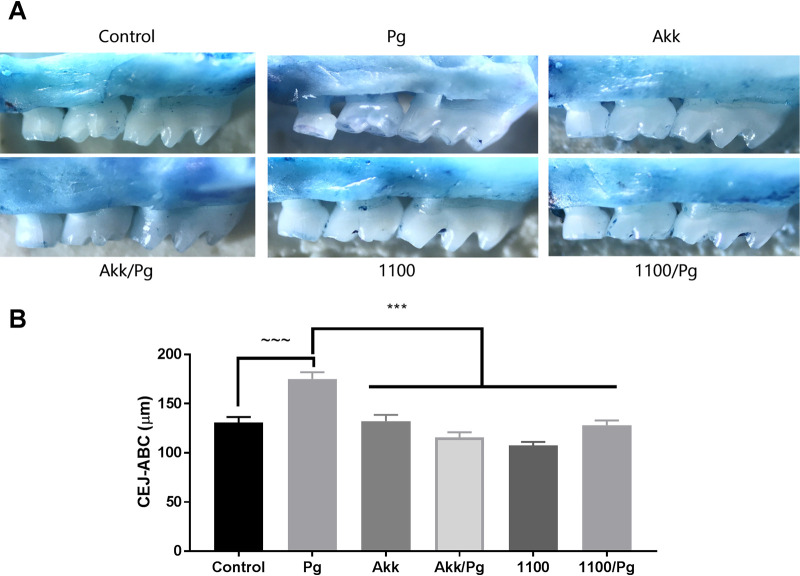
Alveolar bone resorption is a hallmark of periodontitis progression, and its prevention is a key clinical challenge in periodontal treatment (42). To evaluate the effects of A. muciniphila and Amuc_1100 on P. gingivalis-induced alveolar bone loss, mice were gavaged intraorally with P. gingivalis either alone or in combination with A. muciniphila or Amuc_1100. Alveolar bone resorption, characterized by the distance from the alveolar bone crest (ABC) to the cementoenamel junction (CEJ), was measured after 6 weeks (Fig. 1). After 6 weeks, P. gingivalis gavage induced significant alveolar bone loss (34% increase) in comparison with the bones of control mice (P > 0.05) (Fig. 1A and B). Additionally, the administration of A. muciniphila or Amuc_1100 to mice orally challenged with P. gingivalis exhibited decreased alveolar bone loss (a 34% decrease from bone loss in P. gingivalis-treated mice [P < 0.001]). Interestingly, the administration of A. muciniphila or Amuc_1100 alone did not induce significant alveolar bone loss, confirming their innocuity for periodontal tissues.
FIG 1.
A. muciniphila and Amuc_1100 reduce P. gingivalis-induced alveolar bone loss. Mice were divided at random into the following groups for gavage: PBS (control), P. gingivalis (Pg), A. muciniphila (Akk), P. gingivalis and A. muciniphila (Akk/Pg), Amuc_1100 (1100), and Amuc_1100 and P. gingivalis (1100/Pg). Mice were then monitored for 6 weeks. (A) Alveolar bone loss was observed after staining. (B) Alveolar bone loss was determined by the distance between the CEJ and ABC (in micrometers). All values are represented as means ± SEM (n, 6/group). Results were analyzed by one-way ANOVA, with differences considered significant at a P value of ≤0.05. ***, P ≤ 0.001. (Asterisks indicate comparisons with P. gingivalis; tildes indicate comparisons with the control.)
A. muciniphila and Amuc_1100 increase the presence of M2 macrophages during P. gingivalis infection.
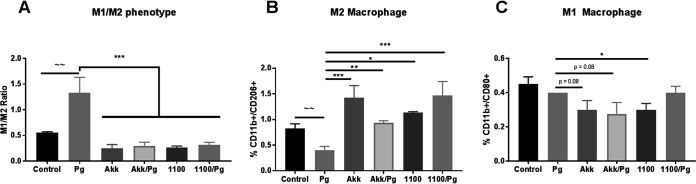
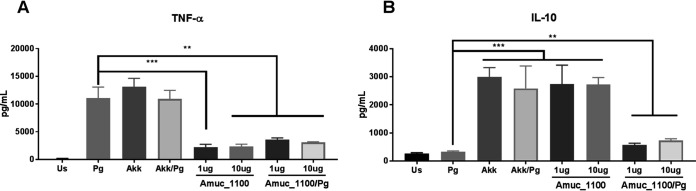
It has been demonstrated that intraoral inoculation with P. gingivalis increases M1 macrophages in both the gingival tissue and the submandibular lymph nodes (SMLNs), which drain the oral cavity. The influx of M1 macrophages in these mice was further associated with the extent of alveolar bone destruction (22). Therefore, to determine the mechanism by which the administration of A. muciniphila and Amuc_1100 modulates the P. gingivalis-elicited immune response, the macrophage population in the SMLNs was analyzed. Mice gavaged with P. gingivalis alone were subject to an M1/M2 ratio higher (150%) (P < 0.05) than those for control mice and the A. muciniphila- or Amuc_1100-treated group (Fig. 2A). The administration of A. muciniphila or Amuc_1100 significantly increased the population of anti-inflammatory M2 macrophages (Fig. 2B) while decreasing the population of inflammatory M1 macrophages (Fig. 2C) during P. gingivalis infection. These results were further corroborated in vitro, with bone marrow-derived macrophages (BMM) infected with P. gingivalis and exposed to A. muciniphila or Amuc_1100. The addition of A. muciniphila to P. gingivalis-infected BMM increased the secretion of IL-10 (7-fold) (Fig. 3B). Further, Amuc_1100 demonstrated the ability to increase the expression of IL-10 while simultaneously decreasing the secretion of tumor necrosis factor alpha (TNF-α) induced by P. gingivalis infection (Fig. 3A and B), supporting the ability of both A. muciniphila and Amuc_1100 to promote an M2 phenotype switch.
FIG 2.
A. muciniphila and Amuc_1100 increase the presence of M2 macrophages during P. gingivalis-induced experimental periodontitis. (A to C) SMLNs were isolated at the endpoint of the gavage period and analyzed for the presence of M1 macrophages (CD11b+ CD80+) (C) and M2 macrophages (CD11b+ CD206+) (B). Treatment groups were as follows: PBS (control), P. gingivalis (Pg), A. muciniphila (Akk), P. gingivalis and A. muciniphila (Akk/Pg), Amuc_1100 (1100), and Amuc_1100 and P. gingivalis (1100/Pg). All values are represented as means ± SEM (n, 6/group). Results were analyzed by one-way ANOVA, with differences considered significant at a P value of ≤0.05. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001. (Asterisks indicate comparisons with P. gingivalis; tildes indicate comparisons with the control.)
FIG 3.
A. muciniphila and Amuc_1100 increase the secretion of the anti-inflammatory mediator IL-10 by BMM infected with P. gingivalis. BMM were exposed to the control (Us), P. gingivalis alone (Pg) (MOI, 20:1), A. muciniphila alone (Akk) (MOI, 40:1), A. muciniphila and P. gingivalis (Akk/Pg), Amuc_1100 alone (1 μg/ml or 10 μg/ml), or Amuc_1100 and P. gingivalis (Amuc_1100/Pg) (1 μg/ml or 10 μg/ml). TNF-α (A) and IL-10 (B) concentrations were determined after 8 h of stimulation. All values are represented as means ± SEM (n, 5/group). Results were analyzed by one-way ANOVA, with differences considered significant at a P value of ≤0.05. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001. (Asterisks indicate comparisons with P. gingivalis.)
A. muciniphila and Amuc_1100 modulate the gingival cytokine environment during P. gingivalis infection.
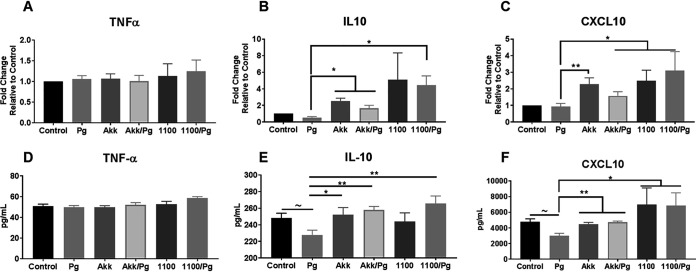
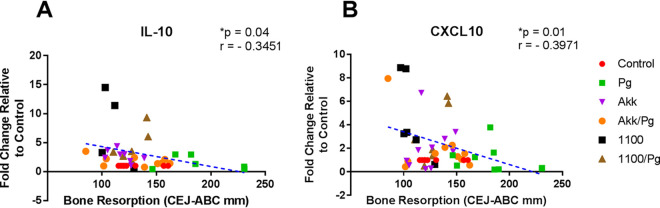
The cytokine environment of the gingival tissue plays an important role in the progression of periodontitis and determines the phenotype of the immune cells recruited at the lesion site. To evaluate how the administration of A. muciniphila and Amuc_1100 affects the cytokine environment, gingival tissue was isolated from mice after 6 weeks of gavage. The expression levels of the TNF-α, IL-10, and CXCL10 genes were evaluated in these tissues. Interestingly, analysis of the gingival tissues failed to show any significant changes in the expression of TNF-α at the mRNA or protein level in any group (Fig. 4A and D). As expected, the addition of either A. muciniphila or Amuc_1100 increased the expression of IL-10 at both the mRNA (3-fold) (P < 0.05) and protein (10%) (P < 0.05) levels over that in tissues harvested from P. gingivalis-gavaged mice (Fig. 4B and E). This increase in IL-10 expression was correlated with decreased alveolar bone loss (r = 0.3451; P < 0.05) (Fig. 5A). Additionally, increases in the expression of the T-cell chemoattractant CXCL10 were observed in the A. muciniphila- and Amuc_1100-treated groups at both the RNA (2-fold) (P < 0.05) and protein (40% and 100%, respectively) (P < 0.05) levels (Fig. 4C and F). Interestingly, the increase in CXCL10 expression was also correlated with the decreased alveolar bone loss observed in these groups (Fig. 5B).
FIG 4.
A. muciniphila and Amuc_1100 increase the levels of anti-inflammatory mediators in the gingival tissue during P. gingivalis infection. (A to C) Gingival tissues isolated at the endpoint of the gavage period were analyzed for mRNA expression. Mice were divided into the following groups: PBS (control), P. gingivalis (Pg), A. muciniphila (Akk), P. gingivalis and A. muciniphila (Akk/Pg), Amuc_1100 (1100), and Amuc_1100 and P. gingivalis (1100/Pg). The relative expression of TNF-α (A), IL-10 (B), and CXCL10 (C) mRNAs was determined by quantitative real-time PCR using TaqMan assays. The fold change was calculated using control tissue as a baseline and β-actin as a normalizing control. (D to F) Gingival tissue lysates were also analyzed for the protein concentrations of TNF-α (D), IL-10 (E), and CXCL10 (F) via ELISA. All values are represented as means ± SEM (n, 6/group). Results were analyzed by one-way ANOVA, with differences considered significant at a P value of ≤0.05. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001. (Asterisks indicate comparisons with P. gingivalis; tildes indicate comparisons with the control.)
FIG 5.
Increased expression of IL-10 and CXCL10 in the gingival tissues of mice exposed to A. muciniphila or Amuc_1100 correlated with reduced alveolar bone loss. Gingival tissues isolated at the endpoint of the gavage period were analyzed for mRNA expression. Mice were divided into the following groups: PBS (control), P. gingivalis (Pg), A. muciniphila (Akk), P. gingivalis and A. muciniphila (Akk/Pg), Amuc_1100 (1100), and Amuc_1100 and P. gingivalis (1100/Pg). The relative expression of mRNA was determined by quantitative real-time PCR using TaqMan assays. The fold change was calculated using control tissue as a baseline and β-actin as a normalizing control. All values are represented as means ± SEM (n, 6/group). Results were analyzed by Pearson’s correlation analysis, with differences considered significant at a P value of ≤0.05.
DISCUSSION
In this study, the protective effects of A. muciniphila administration on P. gingivalis-induced periodontitis were demonstrated. Furthermore, for the first time, the pili-like protein of A. muciniphila, Amuc_1100, has also shown its effectiveness at relieving periodontal inflammation. The reduced periodontal inflammation observed was associated with the recruitment of proresolution M2 macrophages, confirming the anti-inflammatory role of A. muciniphila and its derivatives.
During the progression of periodontitis, macrophages are among the most abundant cells in the gingival tissue, alongside neutrophils (43). Macrophages display functional versatility in response to local microenvironment factors such as cytokines, bacterial components, and Toll-like receptor (TLR) ligands. In response to infection, activated M1 macrophages constitute an important source of proinflammatory and tissue-destructive molecules (44). In patients with severe periodontitis, the presence of CD68-positive macrophages was correlated with increased collagen breakdown and increased severity of the disease (45). Oral administration of P. gingivalis in murine models has been shown to induce an influx of macrophages into the gingival tissue as well as in the SMLNs (21, 22). Furthermore, it has been demonstrated that this increase is dominated by M1 macrophages, disrupting the M1/M2 macrophage ratio in the oral cavity and leading to a hyperinflammatory environment (22). In our model, the introduction of either A. muciniphila or Amuc_1100 increased the proportion of M2 macrophages (CD206+), significantly reducing the M1/M2 ratio. This shift from an M1 to an M2 phenotype is crucial for wound healing and the resolution of the host inflammatory response (46–48) as lesions progress through the inflammation, proliferation, and remodeling phases. Without the switch from M1 to M2, wounds can stall in the first stage of wound healing, the inflammatory phase, promoting scarring and chronic inflammation (46).
The onset of periodontitis is due to an unresolved inflammatory host response driven by the dysbiotic oral microbiome. P. gingivalis fuels the inflammatory environment by preventing a return to homeostasis through an array of virulence factors that paralyze the innate and adaptive immune responses and suppress bacterial clearance (49, 50). The ability of A. muciniphila and Amuc_1100 to upregulate the expression of the T-cell chemoattractant CXCL10 during P. gingivalis infection potentially allows for increased bacterial clearance through the recruitment of Th1 T cells to the gingival tissue. CXCL10, a 10-kDa protein, regulates immune responses by activating T cells, eosinophils, monocytes, and natural killer cells through binding with CXCR3. Interestingly, CXCL10 has been suggested to have different roles in periodontal disease according to disease severity. It has been demonstrated that CXCL10 levels were increased in the saliva and sera of periodontitis patients (51, 52). However, it has also been demonstrated that the presence of CXCL10 is inversely correlated with periodontal disease inflammation (53). Additionally, the role of CXCL10 and it interactions with IL-10 should be discussed. In the context of HIV infection, it was observed that repeated lipopolysaccharide (LPS) exposure suppressed CXCL10 production in the brain through mediation by IL-10 (54). It has been demonstrated previously that A. muciniphila causes the expansion of specific CD4 T-cell populations in the gastrointestinal tract, helping to shape the immune environment of the intestine during homeostasis (55). In our model, the administration of A. muciniphila and Amuc_1100 may also be modulating the T-cell environment during P. gingivalis infection. In the context of periodontitis, the specific roles of CXCL10/IL-10 should therefore be investigated, as well as the impact of A. muciniphila on T-cell recruitment. Here, we demonstrate that the effects of A. muciniphila are not limited to its direct effect on P. gingivalis (38) but that it also acts indirectly through the modulation of the immune response. Additionally, the ability of A. muciniphila and Amuc_1100 to induce an anti-inflammatory environment has been demonstrated several times in the context of metabolic diseases (33, 41, 56–59); this work demonstrates the potential for A. muciniphila to promote a T-cell response in the oral cavity, which should be further investigated.
Amuc_1100, a 32-kDa transmembrane protein, is one of the most abundant proteins in the outer membrane of A. muciniphila. It has been demonstrated to be thermostable during the pasteurization process and can recapitulate the effects of both live and pasteurized A. muciniphila in vitro and in vivo through the activation of TLR2 (41). The effects of A. muciniphila and its protein components in decreasing systemic inflammation are believed to be driven by their effects on the intestinal barrier. A. muciniphila is often thought of as a “sentinel of the gut,” and research on this organism has focused on how its administration to the gut, or its presence, can affect both metabolic and infectious diseases (33, 35, 40, 56). Although these studies are focused on the presence of A. muciniphila in the intestinal tract and its correlation with systemic health, it has been demonstrated recently that A. muciniphila is also present in the oral cavities of healthy individuals but is completely absent from the microbiomes of those with severe periodontitis (37). Here, we confirm that in murine models of experimental periodontitis, the oral administration of A. muciniphila alleviates P. gingivalis-induced inflammation as well as the associated osteoclastic activity (38). These results demonstrate that the beneficial effects of A. muciniphila are not limited to supporting intestinal barrier function but that it also acts through host response modulation.
P. gingivalis-induced dysbiosis and inflammation have been linked to numerous systemic inflammatory diseases (34, 60–65). The altered innate immune response caused by periodontitis has been suggested to mediate this cross talk between oral and extraoral sites of inflammation. In fact, it has been reported previously that there is a specific correlation between periodontitis-activated macrophages and aortic inflammation, as observed in vivo in a ligature-induced experimental model of periodontitis (66). In such a periodontitis model, the presence of M1 macrophages was associated with increased adhesion to aortic endothelial cells through the NF-κB/VCAM-1 axis (66). Additionally, periodontitis-activated monocytes can prime Th17 cells for enhanced production of IL-17, a cytokine which is critical in the development of numerous diseases (67). Therefore, since the local administration of A. muciniphila or Amuc_1100 during P. gingivalis infection was able to shift the macrophage population in gingival tissues and in SMLNs, future research should investigate the effects of local probiotic administration on systemic inflammatory diseases associated with periodontitis.
To conclude, the results of this study support the conclusion that A. muciniphila and Amuc_1100 have a polarizing effect on the macrophage population in the oral cavity. The preference for an M2 phenotype and manipulation of the immune response prevent excessive bone loss associated with P. gingivalis infection. Therefore, A. muciniphila and Amuc_100 should be considered as promising tools for treating or preventing periodontitis. Additional research will need to determine the specific cellular mechanisms involved.
MATERIALS AND METHODS
Bacterial cultures.
A. muciniphila MucT (ATCC BAA-835) was grown anaerobically (N2–CO2, 80:20 [vol/vol]) in brain heart infusion broth supplemented with 3% commercial hog gastric mucin (Type III; Sigma-Aldrich) in 10-ml Hungate anaerobic tubes as described previously (68). The concentration of bacteria was determined by measuring optical density at 600 nm. Liquid cultures were washed and resuspended in sterile anaerobic phosphate-buffered saline (PBS) to the required concentration prior to each experiment.
P. gingivalis strain 381 was cultured and maintained in brain heart infusion medium supplemented with hemin (5 μg/ml) and menadione (1 μg/ml) in an anaerobic environment at 37°C as described previously (38). On the day of each experiment, bacteria were centrifuged and washed with PBS, and the concentration of bacteria was determined by measuring the optical density at 600 nm.
Gavage model.
Twelve-week-old male C57/BL6 mice were separated into six treatment groups: PBS (control), P. gingivalis alone, A. muciniphila alone, A. muciniphila and P. gingivalis administered at the same time, Amuc_1100 alone, and Amuc_1100 and P. gingivalis administered at the same time. Mice were given an oral gavage of P. gingivalis (5 × 108 CFU), A. muciniphila (109 CFU), Amuc_1100 (6 μg/mouse), or a combination of P. gingivalis and A. muciniphila or P. gingivalis and Amuc_1100, 3 days a week for 6 weeks. After the 6-week gavage period, mice were euthanized after isoflurane exposure. All animal experiments were approved by the New York Medical College Institutional Animal Care and Use Committee (protocol 55-2-0919H).
Morphometric analysis.
Palatal bone samples were processed for morphometric analysis as described previously (38). After the samples were boiled for 15 min to remove gingival tissue, they were washed in PBS and then exposed to 3% hydrogen peroxide overnight. The palatal bones were treated with 10% bleach for 1 min, washed with PBS three times for 5 min, and then dried at 37°C. Bone staining was performed at room temperature with 1% methylene blue (Sigma-Aldrich) for 1 min, and specimens were washed and dried for 30 min at 37°C. Bone loss was defined as the distance between the cementoenamel junction (CEJ) and the most coronal alveolar bone (69). Measurements were taken at six sites from the mesial aspect of the first molar to the distal aspect of the second molar. Bone loss was expressed in micrometers as means ± standard errors of the means (SEM).
SMLN macrophage isolation and flow cytometry.
The submandibular lymph nodes (SMLNs) were isolated, and a single-cell suspension was prepared via mechanical dissociation and resuspended in Hanks balanced salt solution (HBSS) (70). Cells were stained and analyzed on a FACSCelesta flow cytometer (Becton, Dickinson, San Diego, CA, USA) with dead cells excluded by a Zombie Violet Fixable Viability kit (BioLegend, San Diego, CA, USA). The following fluorescence-conjugated antibodies were used: CD11b (allophycocyanin [APC]-Cy; Thermo Fisher), CD206 (phycoerythrin [PE]; Thermo Fisher), and CD80 (APC; Thermo Fisher) (21, 22, 43). To determine the numbers of M1 versus M2 macrophages in the SMLNs, populations of cells double positive for CD11b and CD80 or CD11b and CD206 were gated off the total population of live cells. M1 versus M2 macrophage populations were expressed as a ratio and as percentages of live cells.
Gingival tissue homogenate.
Gingival tissues were isolated at the endpoint of the experiment and were directly frozen at −80°C. To generate tissue homogenates, samples were placed in 300 μl of radioimmunoprecipitation assay (RIPA) buffer supplemented with Halt protease inhibitor cocktail and a phosphatase inhibitor cocktail (Sigma-Aldrich) and were homogenized with a Qiagen TissueRuptor (71). Protein concentrations were determined by a DC (detergent-compatible) protein assay (Bio-Rad), and aliquots of homogenates were stored at −80°C until use.
Macrophage cultures.
Bone marrow-derived macrophages (BMM) were harvested as described previously (38). Briefly, bone marrow from the femurs and tibias of donor mice was isolated and cultured in 30% L-929-conditioned RPMI medium. After 1 week in culture, the isolated monocytes had differentiated into macrophages (BMM). One day prior to the experiments, the cell culture medium was replaced with fresh 30% L-929-conditioned RPMI medium. P. gingivalis or A. muciniphila was added to BMM cultures at a multiplicity of infection (MOI) of 20:1 or 40:1, respectively (38). Additionally, BMM were exposed to P. gingivalis (MOI, 20:1) and the pili-like protein Amuc_1100 (1 μg/ml or 10 μg/ml). BMM were incubated with bacteria for 8 h, and at the endpoint of each experiment, cells and supernatants were collected.
RNA extraction and qRT-PCR.
RNA from mouse gingival tissue was isolated and purified with the QIAshredder system and the RNeasy minikit (both from Qiagen) according to the manufacturer’s instructions. cDNA from total RNA was synthesized (500 ng RNA/reaction) using the QuantiTect reverse transcription kit (Qiagen) according to the manufacturer’s instructions. Quantitative real-time PCR (qRT-PCR) was performed using TaqMan Fast Advanced master mix and was run for the genes encoding the following proteins by using the probes (assay IDs given in parentheses) provided by Thermo Fisher: CXCL10 (Mm00445235), TNF-α (Mm00443258), and IL-10 (Mm99999062). Data were analyzed using QuantStudio 5 software, v1.4. The gene expression levels were normalized to β-actin levels (MM02619580_G1) and expressed relative to levels in control animals by following the 2–ΔΔCT method.
ELISA.
Concentrations of cytokines in culture supernatants as well as in the gingival tissue homogenate were measured by enzyme-linked immunosorbent assays (ELISA) using Invitrogen mouse IL-10, mouse TNF-α, and mouse CXCL10 kits according to the manufacturer’s instructions (Thermo Fisher).
Statistical analysis.
Results were analyzed by one-way analysis of variance (ANOVA), with differences considered significant at P values of <0.05, using GraphPad Prism 7 software.
ACKNOWLEDGMENTS
This research was funded by NIH/NIDCR, grant R01DE014079, and NIH/NHLBI, grant R01HL076801.
Pili-like protein Amuc_1100 was provided by W. M. de Vos and P. Cani of A-Mansia BioTech.
Author contributions to this paper were as follows. Conceptualization, H.M., R.I., and S.A.; Methodology, H.M., R.I., O.H., and S.A.; Data Curation, H.M., J.M.D., and S.A.; Writing — Original Draft, H.M., J.M.D., O.H., and S.A. All authors have read and agreed to the published version of the manuscript.
We declare no conflict of interest.
REFERENCES
- 1.Bernabe E, Marcenes W, Hernandez CR, Bailey J, Abreu LG, Alipour V, Amini S, Arabloo J, Arefi Z, Arora A, Ayanore MA, Bärnighausen TW, Bijani A, Cho DY, Chu DT, Crowe CS, Demoz GT, Demsie DG, Dibaji Forooshani ZS, Du M, El Tantawi M, Fischer F, Folayan MO, Futran ND, Geramo YCD, Haj-Mirzaian A, Hariyani N, Hasanzadeh A, Hassanipour S, Hay SI, Hole MK, Hostiuc S, Ilic MD, James SL, Kalhor R, Kemmer L, Keramati M, Khader YS, Kisa S, Kisa A, Koyanagi A, Lalloo R, Le Nguyen Q, London SD, Manohar ND, Massenburg BB, Mathur MR, Meles HG, Mestrovic T, Mohammadian-Hafshejani A, GBD 2017 Oral Disorders Collaborators, et al. 2020. Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: a systematic analysis for the Global Burden of Disease 2017 study. J Dent Res 99:362–373. doi: 10.1177/0022034520908533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinane DF, Stathopoulou PG, Papapanou PN. 2017. Periodontal diseases. Nat Rev Dis Primers 3:17038. doi: 10.1038/nrdp.2017.38. [DOI] [PubMed] [Google Scholar]
- 3.Oliveira LM, de David SC, Ardenghi TM, Moreira CHC, Zanatta FB. 2020. Gingival inflammation influences oral health-related quality of life in individuals living in a rural area of southern Brazil. J Clin Periodontol 47:1028–1039. doi: 10.1111/jcpe.13333. [DOI] [PubMed] [Google Scholar]
- 4.Hajishengallis G. 2020. New developments in neutrophil biology and periodontitis. Periodontol 2000 82:78–92. doi: 10.1111/prd.12313. [DOI] [PubMed] [Google Scholar]
- 5.Hajishengallis G, Darveau RP, Curtis MA. 2012. The keystone-pathogen hypothesis. Nat Rev Microbiol 10:717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cloitre A, Halgand B, Sourice S, Caillon J, Huck O, Bugueno IM, Batool F, Guicheux J, Geoffroy V, Lesclous P. 2019. IL-36γ is a pivotal inflammatory player in periodontitis-associated bone loss. Sci Rep 9:19257. doi: 10.1038/s41598-019-55595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bugueno IM, Batool F, Keller L, Kuchler-Bopp S, Benkirane-Jessel N, Huck O. 2018. Porphyromonas gingivalis bypasses epithelial barrier and modulates fibroblastic inflammatory response in an in vitro 3D spheroid model. Sci Rep 8:14914. doi: 10.1038/s41598-018-33267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bugueno IM, Batool F, Korah L, Benkirane-Jessel N, Huck O. 2018. Porphyromonas gingivalis differentially modulates apoptosome apoptotic peptidase activating factor 1 in epithelial cells and fibroblasts. Am J Pathol 188:404–416. doi: 10.1016/j.ajpath.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Bryzek D, Ciaston I, Dobosz E, Gasiorek A, Makarska A, Sarna M, Eick S, Puklo M, Lech M, Potempa B, Potempa J, Koziel J. 2019. Triggering NETosis via protease-activated receptor (PAR)-2 signaling as a mechanism of hijacking neutrophils function for pathogen benefits. PLoS Pathog 15:e1007773. doi: 10.1371/journal.ppat.1007773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huck O, Al-Hashemi J, Poidevin L, Poch O, Davideau J-L, Tenenbaum H, Amar S. 2017. Identification and characterization of microRNA differentially expressed in macrophages exposed to Porphyromonas gingivalis infection. Infect Immun 85:e00771-16. doi: 10.1128/IAI.00771-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huck O, You J, Han X, Cai B, Panek J, Amar S. 2018. Reduction of articular and systemic inflammation by Kava-241 in a Porphyromonas gingivalis-induced arthritis murine model. Infect Immun 86:e00356-18. doi: 10.1128/IAI.00356-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sochalska M, Potempa J. 2017. Manipulation of neutrophils by Porphyromonas gingivalis in the development of periodontitis. Front Cell Infect Microbiol 7:197. doi: 10.3389/fcimb.2017.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bates AM, Fischer CL, Abhyankar VP, Johnson GK, Guthmiller JM, Progulske-Fox A, Brogden KA. 2018. Matrix metalloproteinase response of dendritic cell, gingival epithelial keratinocyte, and T-cell transwell co-cultures treated with Porphyromonas gingivalis hemagglutinin-B. Int J Mol Sci 19:3923. doi: 10.3390/ijms19123923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen I, Hajishengallis G. 2016. Major neutrophil functions subverted by Porphyromonas gingivalis. J Oral Microbiol 8:30936. doi: 10.3402/jom.v8.30936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jauregui CE, Wang Q, Wright CJ, Takeuchi H, Uriarte SM, Lamont RJ. 2013. Suppression of T-cell chemokines by Porphyromonas gingivalis. Infect Immun 81:2288–2295. doi: 10.1128/IAI.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasturk H, Kantarci A, Van Dyke T. 2012. Oral inflammatory diseases and systemic inflammation: role of the macrophage. Front Immunol 3:118. doi: 10.3389/fimmu.2012.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alvarez C, Monasterio G, Cavalla F, Córdova LA, Hernández M, Heymann D, Garlet GP, Sorsa T, Pärnänen P, Lee H-M, Golub LM, Vernal R, Kantarci A. 2019. Osteoimmunology of oral and maxillofacial diseases: translational applications based on biological mechanisms. Front Immunol 10:1664. doi: 10.3389/fimmu.2019.01664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayala A, Chung CS, Grutkoski PS, Song GY. 2003. Mechanisms of immune resolution. Crit Care Med 31(8 Suppl):S558–S571. doi: 10.1097/01.CCM.0000081438.04801.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez OA, Novak MJ, Kirakodu S, Stromberg A, Nagarajan R, Huang CB, Chen KC, Orraca L, Martinez-Gonzalez J, Ebersole JL. 2015. Differential gene expression profiles reflecting macrophage polarization in aging and periodontitis gingival tissues. Immunol Invest 44:643–664. doi: 10.3109/08820139.2015.1070269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu T, Zhao L, Huang X, Ma C, Wang Y, Zhang J, Xuan D. 2016. Enhanced activity of the macrophage M1/M2 phenotypes and phenotypic switch to M1 in periodontal infection. J Periodontol 87:1092–1102. doi: 10.1902/jop.2016.160081. [DOI] [PubMed] [Google Scholar]
- 21.Lam RS, O’Brien-Simpson NM, Holden JA, Lenzo JC, Fong SB, Reynolds EC. 2016. Unprimed, M1 and M2 macrophages differentially interact with Porphyromonas gingivalis. PLoS One 11:e0158629. doi: 10.1371/journal.pone.0158629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam RS, O’Brien-Simpson NM, Lenzo JC, Holden JA, Brammar GC, Walsh KA, McNaughtan JE, Rowler DK, Van Rooijen N, Reynolds EC. 2014. Macrophage depletion abates Porphyromonas gingivalis-induced alveolar bone resorption in mice. J Immunol 193:2349–2362. doi: 10.4049/jimmunol.1400853. [DOI] [PubMed] [Google Scholar]
- 23.Sanz M, Herrera D, Kebschull M, Chapple I, Jepsen S, Beglundh T, Sculean A, Tonetti MS, on behalf of the EFP Workshop Participants and Methodological Consultants. 2020. Treatment of stage I–III periodontitis—the EFP S3 level clinical practice guideline. J Clin Periodontol 47(Suppl 22):4–60. doi: 10.1111/jcpe.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eickholz P, Koch R, Kocher T, Hoffmann T, Kim T-S, Meyle J, Kaner D, Schlagenhauf U, Harmsen D, Harks I, Ehmke B. 2019. Clinical benefits of systemic amoxicillin/metronidazole may depend on periodontitis severity and patients’ age: an exploratory sub-analysis of the ABPARO trial. J Clin Periodontol 46:491–501. doi: 10.1111/jcpe.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van der Weijden GA, Dekkers GJ, Slot DE. 2019. Success of non-surgical periodontal therapy in adult periodontitis patients: a retrospective analysis. Int J Dent Hyg 17:309–317. doi: 10.1111/idh.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Dyke TE. 2020. Shifting the paradigm from inhibitors of inflammation to resolvers of inflammation in periodontitis. J Periodontol doi: 10.1002/JPER.20-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin-Cabezas R, Davideau J-L, Tenenbaum H, Huck O. 2016. Clinical efficacy of probiotics as an adjunctive therapy to non-surgical periodontal treatment of chronic periodontitis: a systematic review and meta-analysis. J Clin Periodontol 43:520–530. doi: 10.1111/jcpe.12545. [DOI] [PubMed] [Google Scholar]
- 28.Higuchi T, Suzuki N, Nakaya S, Omagari S, Yoneda M, Hanioka T, Hirofuji T. 2019. Effects of Lactobacillus salivarius WB21 combined with green tea catechins on dental caries, periodontitis, and oral malodor. Arch Oral Biol 98:243–247. doi: 10.1016/j.archoralbio.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 29.Riccia DD, Bizzini F, Perilli MG, Polimeni A, Trinchieri V, Amicosante G, Cifone MG. 2007. Anti-inflammatory effects of Lactobacillus brevis (CD2) on periodontal disease. Oral Dis 13:376–385. doi: 10.1111/j.1601-0825.2006.01291.x. [DOI] [PubMed] [Google Scholar]
- 30.Schlagenhauf U, Rehder J, Gelbrich G, Jockel-Schneider Y. 2020. Consumption of Lactobacillus reuteri-containing lozenges improves periodontal health in navy sailors at sea: a randomized controlled trial. J Periodontol doi: 10.1002/JPER.19-0393. [DOI] [PubMed] [Google Scholar]
- 31.Geerlings S, Kostopoulos I, de Vos W, Belzer C. 2018. Akkermansia muciniphila in the human gastrointestinal tract: when, where, and how? Microorganisms 6:75. doi: 10.3390/microorganisms6030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, Dumas ME, Rizkalla SW, Doré J, Cani PD, Clément K, Le Mouhaër S, Cotillard A, Kennedy SP, Pons N, Le Chatelier E, Almeida M, Quinquis B, Galleron N, Batto JM, Renault P, Zucker JD, Ehrlich SD, Blottière H, Leclerc M, Juste C, De Wouters T, Lepage P, MICRO-Obes Consortium. 2016. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65:426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 33.Zhao S, Liu W, Wang J, Shi J, Sun Y, Wang W, Ning G, Liu R, Hong J. 2017. Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J Mol Endocrinol 58:1–14. doi: 10.1530/JME-16-0054. [DOI] [PubMed] [Google Scholar]
- 34.Mulhall H, Huck O, Amar S. 2020. Porphyromonas gingivalis, a long-range pathogen: systemic impact and therapeutic implications. Microorganisms 8:869–815. doi: 10.3390/microorganisms8060869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, de Barsy M, Loumaye A, Hermans MP, Thissen JP, de Vos WM, Cani PD. 2019. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med 25:1096–1103. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Druart C, Plovier H, Van Hul M, Brient A, Phipps KR, de Vos WM, Cani PD. 2020. Toxicological safety evaluation of pasteurized Akkermansia muciniphila. J Appl Toxicol doi: 10.1002/jat.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coretti L, Cuomo M, Florio E, Palumbo D, Keller S, Pero R, Chiariotti L, Lembo F, Cafiero C. 2017. Subgingival dysbiosis in smoker and non-smoker patients with chronic periodontitis. Mol Med Rep 15:2007–2014. doi: 10.3892/mmr.2017.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huck O, Mulhall H, Rubin G, Kizelnik Z, Iyer R, Perpich JD, Haque N, Cani PD, de Vos WM, Amar S. 2020. Akkermansia muciniphila reduces Porphyromonas gingivalis-induced inflammation and periodontal bone destruction. J Clin Periodontol 47:202–212. doi: 10.1111/jcpe.13214. [DOI] [PubMed] [Google Scholar]
- 39.Ottman N, Reunanen J, Meijerink M, Pietila TE, Kainulainen V, Klievink J, Huuskonen L, Aalvink S, Skurnik M, Boeren S, Satokari R, Mercenier A, Palva A, Smidt H, De Vos WM, Belzer C. 2017. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS One 12:e0173004. doi: 10.1371/journal.pone.0173004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anhê FF, Marette A. 2017. A microbial protein that alleviates metabolic syndrome. Nat Med 23:11–12. doi: 10.1038/nm.4261. [DOI] [PubMed] [Google Scholar]
- 41.Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, Myridakis A, Delzenne NM, Klievink J, Bhattacharjee A, van der Ark KCH, Aalvink S, Martinez LO, Dumas M-E, Maiter D, Loumaye A, Hermans MP, Thissen J-P, Belzer C, de Vos WM, Cani PD. 2017. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med 23:107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 42.Hienz SA, Paliwal S, Ivanovski S. 2015. Mechanisms of bone resorption in periodontitis. J Immunol Res 2015:615486–615410. doi: 10.1155/2015/615486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parisi L, Gini E, Baci D, Tremolati M, Fanuli M, Bassani B, Farronato G, Bruno A, Mortara L. 2018. Macrophage polarization in chronic inflammatory diseases: killers or builders? J Immunol Res 2018:8917804. doi: 10.1155/2018/8917804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mosser DM, Edwards JP. 2008. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou L-N, Bi C-S, Gao L-N, An Y, Chen F, Chen F-M. 2019. Macrophage polarization in human gingival tissue in response to periodontal disease. Oral Dis 25:265–273. doi: 10.1111/odi.12983. [DOI] [PubMed] [Google Scholar]
- 46.Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. 2018. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol 9:419. doi: 10.3389/fphys.2018.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kotwal GJ, Chien S. 2017. Macrophage differentiation in normal and accelerated wound healing. Results Probl Cell Differ 62:353–364. doi: 10.1007/978-3-319-54090-0_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ley K. 2017. M1 means kill; M2 means heal. J Immunol 199:2191–2193. doi: 10.4049/jimmunol.1701135. [DOI] [PubMed] [Google Scholar]
- 49.Hajishengallis G. 2014. The inflammophilic character of the periodontitis-associated microbiota. Mol Oral Microbiol 29:248–257. doi: 10.1111/omi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hajishengallis G, Lamont RJ. 2014. Breaking bad: manipulation of the host response by Porphyromonas gingivalis. Eur J Immunol 44:328–338. doi: 10.1002/eji.201344202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aldahlawi S, Youssef A-R, Shahabuddin S. 2018. Evaluation of chemokine CXCL10 in human gingival crevicular fluid, saliva, and serum as periodontitis biomarker. J Inflamm Res 11:389–396. doi: 10.2147/JIR.S177188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimada Y, Tabeta K, Sugita N, Yoshie H. 2013. Profiling biomarkers in gingival crevicular fluid using multiplex bead immunoassay. Arch Oral Biol 58:724–730. doi: 10.1016/j.archoralbio.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 53.Gemmell E, Carter CL, Seymour GJ. 2001. Chemokines in human periodontal disease tissues. Clin Exp Immunol 125:134–141. doi: 10.1046/j.1365-2249.2001.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maingat F, Viappiani S, Zhu Y, Vivithanaporn P, Ellestad KK, Holden J, Silva C, Power C. 2010. Regulation of lentivirus neurovirulence by lipopolysaccharide conditioning: suppression of CXCL10 in the brain by IL-10. J Immunol 184:1566–1574. doi: 10.4049/jimmunol.0902575. [DOI] [PubMed] [Google Scholar]
- 55.Ansaldo E, Slayden LC, Ching KL, Koch MA, Wolf NK, Plichta DR, Brown EM, Graham DB, Xavier RJ, Moon JJ, Barton GM. 2019. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science 364:1179–1184. doi: 10.1126/science.aaw7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. 2016. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe–/– mice. Circulation 133:2434–2446. doi: 10.1161/CIRCULATIONAHA.115.019645. [DOI] [PubMed] [Google Scholar]
- 57.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, De Vos WM, Cani PD. 2013. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhai R, Xue X, Zhang L, Yang X, Zhao L, Zhang C. 2019. Strain-specific anti-inflammatory properties of two Akkermansia muciniphila strains on chronic colitis in mice. Front Cell Infect Microbiol 9:239. doi: 10.3389/fcimb.2019.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Earley H, Lennon G, Balfe Á, Coffey JC, Winter DC, O'Connell PR. 2019. The abundance of Akkermansia muciniphila and its relationship with sulphated colonic mucins in health and ulcerative colitis. Sci Rep 9:15683. doi: 10.1038/s41598-019-51878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huck O, Saadi-Thiers K, Tenenbaum H, Davideau J-L, Romagna C, Laurent Y, Cottin Y, Roul JG. 2011. Evaluating periodontal risk for patients at risk of or suffering from atherosclerosis: recent biological hypotheses and therapeutic consequences. Arch Cardiovasc Dis 104:352–358. doi: 10.1016/j.acvd.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 61.Linden GJ, Lyons A, Scannapieco FA. 2013. Periodontal systemic associations: review of the evidence. J Clin Periodontol 40:S8–S19. doi: 10.1111/jcpe.12064. [DOI] [PubMed] [Google Scholar]
- 62.Nibali L, Tatarakis N, Needleman I, Tu Y-K, D'Aiuto F, Rizzo M, Donos N. 2013. Association between metabolic syndrome and periodontitis: a systematic review and meta-analysis. J Clin Endocrinol Metab 98:913–920. doi: 10.1210/jc.2012-3552. [DOI] [PubMed] [Google Scholar]
- 63.Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, Taylor R. 2012. Periodontitis and diabetes: a two-way relationship. Diabetologia 55:21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chou Y-Y, Lai K-L, Chen D-Y, Lin C-H, Chen H-H. 2015. Rheumatoid arthritis risk associated with periodontitis exposure: a nationwide, population-based cohort study. PLoS One 10:e0139693. doi: 10.1371/journal.pone.0139693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nascimento GG, Peres MA, Mittinty MN, Peres KG, Do LG, Horta BL, Gigante DP, Corrêa MB, Demarco FF. 2017. Diet-induced overweight and obesity and periodontitis risk: an application of the parametric G-formula in the 1982 Pelotas birth cohort. Am J Epidemiol 185:442–451. doi: 10.1093/aje/kww187. [DOI] [PubMed] [Google Scholar]
- 66.Miyajima S, Naruse K, Kobayashi Y, Nakamura N, Nishikawa T, Adachi K, Suzuki Y, Kikuchi T, Mitani A, Mizutani M, Ohno N, Noguchi T, Matsubara T. 2014. Periodontitis-activated monocytes/macrophages cause aortic inflammation. Sci Rep 4:5171. doi: 10.1038/srep05171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng W-C, van Asten SD, Burns LA, Evans HG, Walter GJ, Hashim A, Hughes FJ, Taams LS. 2016. Periodontitis-associated pathogens P gingivalis and A. actinomycetemcomitans activate human CD14+ monocytes leading to enhanced Th17/IL-17 responses. Eur J Immunol 46:2211–2221. doi: 10.1002/eji.201545871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Derrien M, Vaughan EE, Plugge CM, de Vos WM. 2004. Akkermansia municiphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol 54:1469–1476. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 69.Li CH, Amar S. 2007. Morphometric, histomorphometric, and microcomputed tomographic analysis of periodontal inflammatory lesions in a murine model. J Periodontol 78:1120–1128. doi: 10.1902/jop.2007.060320. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Zhang W, Xu L, Jin J-O. 2016. Porphyromonas gingivalis lipopolysaccharide induced proliferation and activation of natural killer cells in vivo. Molecules 21:1086. doi: 10.3390/molecules21081086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoshinaka K, Shoji N, Nishioka T, Sugawara Y, Hoshino T, Sugawara S, Sasano T. 2014. Increased interleukin-18 in the gingival tissues evokes chronic periodontitis after bacterial infection. Tohoku J Exp Med 232:215–222. doi: 10.1620/tjem.232.215. [DOI] [PubMed] [Google Scholar]