Abstract
Aim:
To develop a conjugate of vitamin B12 bound to the glucagon-like peptide-1 receptor (GLP-1R) agonist exendin-4 (Ex4) that shows reduced penetrance into the central nervous system while maintaining peripheral glucoregulatory function.
Methods:
We evaluated whether a vitamin B12 conjugate of Ex4 (B12-Ex4) improves glucose tolerance without inducing anorexia in Goto-Kakizaki (GK) rats, a lean type 2 diabetes model of an understudied but medically compromised population of patients requiring the glucoregulatory effects of GLP-1R agonists without anorexia. We also utilized the musk shrew (Suncus murinus), a mammalian model capable of emesis, to test B12-Ex4 on glycaemic profile, feeding and emesis.
Results:
In both models, native Ex4 and B12-Ex4 equivalently blunted the rise in blood glucose levels during a glucose tolerance test. In both GK rats and shrews, acute Ex4 administration decreased food intake, leading to weight loss; by contrast, equimolar administration of B12-Ex4 had no effect on feeding and body weight. There was a near absence of emesis in shrews given systemic B12-Ex4, in contrast to reliable emesis produced by Ex4. When administered centrally, both B12-Ex4 and Ex4 induced similar potency of emesis, suggesting that brain penetrance of B12-Ex4 is required for induction of emesis.
Conclusions:
These findings highlight the potential therapeutic value of B12-Ex4 as a novel treatment for type 2 diabetes devoid of weight loss and with reduced adverse effects and better tolerance, but similar glucoregulation to current GLP-1R agonists.
Keywords: animal pharmacology, antidiabetic drug
1 |. INTRODUCTION
Nausea and vomiting are omnipresent side effects of human diseases and pharmacotherapies that have spawned significant drug discovery efforts to combat their many causes.1 Glucagon-like peptide-1 receptor (GLP-1R)-based therapies for the treatment of type 2 diabetes (T2D), such as Exendin-4 (Ex4) and liraglutide, are no exception,2 causing nausea and vomiting in ~25% to 50% of patients prescribed these drugs.3–7
We have recently reported that a vitamin B12 conjugate of Ex4 (hereafter called ‘B12-Ex4’)8,9 shows retention of glucoregulation comparable with native Ex4 without producing Ex4-associated anorexia and conditioned taste avoidance in lean, healthy rats. Furthermore, B12-Ex4 co-localized with insulin-containing β-cells within the pancreas, while brain penetrance of fluorescently tagged B12-Ex4 was not detected within the hypothalamus, nor the area postrema (AP), nor the nucleus tractus solitarius (NTS) of the hindbrain. These results corroborated that the incretin action on the pancreas10–12 and hypoglycaemic effects of Ex4 remained intact when bound to B12, while central sites of Ex4 action controlling energy homeostasis and nausea/malaise (e.g. the brainstem AP and NTS, as well as hypothalamic nuclei)13–15 were putatively not agonized following B12-Ex4 treatment. The B12-Ex4 construct may be of considerable clinical utility given the potential for retained glycaemic control in combination with improved tolerability and associated patient compliance through a mechanism of action that simultaneously limits central nervous system (CNS) penetrance and retains peripheral (i.e. pancreatic) pharmacodynamics.
Weight loss is considered desirable for the majority of patients with T2D considered overweight or obese, as they may benefit from improvements in insulin signalling and overall health with concomitant adipose tissue loss.16,17 However, patients with T2D who also suffer from co-morbidities associated with cachexia (e.g. cancer,18 cystic fibrosis,19 HIV,20 chronic obstructive pulmonary disease21 and sarcopenia22), would also benefit from use of the front-line treatments for T2D in the form of GLP1-R agonists, without affecting their body weight. In addition, ~10% of patients with T2D, such as those potentially with polygenic/inherited T2D, are not overweight/obese,23 and weight loss below normal weight status is undesirable. To this end, given our previous work in healthy, lean rats, where we showed comparable glycaemic control without anorexia/weight loss following acute B12-Ex4 treatment compared with native Ex4, we employ the use of the Goto-Kakizaki (GK) rat, a model of spontaneous insulin resistance and T2D.24 These animals develop T2D early in life and maintain a lean phenotype (see Ostenson and Efendic25 for a discussion/review), making this strain ideal to model T2D without concomitant obesity.
The musk shrew (Suncus murinus) is a well-established model of emesis26 and feeding behaviour27 and is suitable for examination of the GLP-1 system as this species exhibits glucoregulatory, hypophagic and emetic responsivity following administration of current Food and Drug Administration-approved GLP-1R agonists.14,15 Here, we employ behavioural, immunohistochemical and pharmacological analyses to examine the effects of B12-Ex4 versus Ex4 administration on glycaemic control, emesis and feeding behaviour in shrews.
We report both the preservation of hypoglycaemic responses and superior tolerance of B12-Ex4 over native Ex4 in musk shrews and the GK rat. B12-Ex4 shows substantially decreased effects on emesis compared with the native drug, while maintaining an incretin profile of glucoregulatory effects with no change in energy balance in shrews. These data support the potential clinical utility of B12-Ex4 in lean patients, where anorexia and weight loss are undesirable outcomes of T2D management and/or where dose scheduling of GLP-1R agonists is of major concern for patient compliance because of considerations of extensive episodes of nausea and vomiting.
2 |. METHODS
2.1 |. Drugs
B12-Ex4 was synthesized, characterized and screened as previously described.8 For all systemic administrations, B12-Ex4 and Ex4 (Bachem, USA) were dissolved in 0.9% saline and administered intraperitoneally (IP). B12-Ex4 and Ex4 were dissolved in artificial cerebro-spinal fluid (aCSF) for intracerebroventricular (ICV) delivery. Liraglutide (Sigma-Aldrich) was dissolved in PBS and injected IP. Fluorophore-labelled B12-Ex4 (Cy5-B12-Ex4) was synthetized as previously described.9
2.2 |. Animals
Experimentally naive adult male shrews (n = 81) weighing ~70–80 g used in this study were offspring from a colony maintained at the University of Pittsburgh Cancer Institute (a Taiwanese strain derived from stock supplied by the Chinese University of Hong Kong). At ~2 months of age, the animals were moved to the University of Pennsylvania, where all studies presented here were conducted.
Shrews were single-housed in plastic cages (37.3 × 23.4 × 14 cm, Innovive), fed ad libitum with a mixture of feline (75%, Laboratory Feline Diet 5003, Lab Diet) and ferret food (25%, High Density Ferret Diet 5LI4, Lab Diet) and had ad libitum access to tap water except where noted. Experimentally naive adult male GK rats (n = 21, Taconic) weighing ~350 g upon arrival were individually housed in hanging wire mesh cages. Rats were fed ad libitum with a chow diet (NIH-31 M Diet, Taconic) and had ad libitum access to tap water, except where noted. All animals were housed under a 12-hour/12-hour light/dark cycle in a temperature- and humidity-controlled environment. All procedures were approved by the Institutional Care and Use Committee of the University of Pennsylvania.
2.3 |. In vivo study design
Shrews and GK rats were habituated to single housing in their home cages and IP injections at least 1 week prior to experimentation. All experimental injections in shrews were separated by at least 72 hours. Experimental testing in GK rats was conducted every 7 days. In vivo experiments used a within-subjects, Latin-square design, except for immunohistochemical studies, which were conducted between subjects.
2.4 |. Stereotaxic surgery in shrews
The surgical procedure was similar to that previously described.14 Shrews (n = 8) were deeply anaesthetized with a triple cocktail of KAX (ketamine [180 mg/kg; Butler Animal Health Supply], acepromazine [1.28 mg/kg; Butler Animal Health Supply] and xylazine [5.4 mg/kg; Anased]) and placed into a stereotaxic frame equipped with custom-made ear bars (Kopf Instruments). An incision was made, the temporalis muscles on either side of the sagittal crest were displaced, and the area was cleaned of connective tissue. A hole was made according to the following coordinates: 8.2 mm rostral to lambda and 0.9 mm lateral to the midline. A 26-gauge stainless steel guide cannula (C315G/SPC, PlasticOne) was lowered 1.2 mm below the surface of the dura (2 mm above the lateral ventricle) and cemented to the skull using two screws. After surgery, animals received 2 mL of warm saline and analgesia (Meloxicam, 5 mg/kg, IP, Boehringer Ingelheim). Shrews were allowed 7 days of recovery before the beginning of the experiment. During drug administration, the guide cannula was fitted with a 33-gauge injection cannula (C315I/SPC, PlasticOne) that extended 2 mm beyond the tip of the guide cannula. At the end of the experiments, 1 μL of Evans Blue dye was injected ICV following termination of the animals with IP KAX. Brains were immediately removed and frozen in cold hexane on dry ice. Coronal sections were performed to confirm the site of injection. Only those animals with blue staining in the ventricles were included in the analysis.
2.5 |. Effects of Ex4 and B12-Ex4 on glycaemic control in shrews
The protocol for performing an intraperitoneal glucose tolerance test (IPGTT) in shrews was similar to that previously used in mice.9 One hour before dark onset, shrews (n = 13) were food- and water-deprived. Four hours later (t = −30 minutes), baseline blood glucose (BG) levels were determined from a small drop of tail blood and measured using a standard glucometer (AccuCheck). Immediately following this blood sample, each shrew received an IP injection of 5 nmol/kg Ex4 (i.e. ~20 μg/kg), 5 nmol/kg B12-Ex4 or vehicle (sterile saline, 1 mL/100 g body weight). BG was measured 30 minutes later (t = 0 minutes), then each shrew received an IP bolus of glucose (2 g/kg). Subsequent BG readings were taken at 20, 40, 60 and 120 minutes after glucose injection. After the final BG reading, food and water were returned. The dose selection of Ex4 was based on the literature and pilot experiments showing reliable BG suppression following glucose challenge in shrews.15 Intraperitoneal delivery was chosen as the route of glucose administration because of the emetogenic properties of Ex4, which could produce expulsion of glucose delivered to the gastrointestinal tract and confound interpretation of results because of the potential variability of intestinal glucose absorption.
To evaluate the effects of Ex4 and B12-Ex4 on baseline BG without subsequent glucose administration, Ex4, B12-Ex4 or vehicle was administered in a separate cohort of animals (n = 5). Shrews were food- and water-deprived as described above. Four hours later (t = −30 minutes), baseline BG levels were determined, and each shrew received an IP injection of 5 nmol/kg Ex4, 5 nmol/kg B12-Ex4 or vehicle (1 mL/100 g BW sterile saline). BG was measured 30 minutes later (t = 0 minutes), followed by an IP saline injection (1 mL/100 g BW). Subsequent BG readings were taken at 20, 40, 60 and 120 minutes after glucose injection. After the final BG reading, food and water were returned.
2.6 |. Effects of Ex4 and B12-Ex4 on energy balance in shrews
Shrews (n = 14) were adapted to powdered food for 5 days and habituated to IP injections for 2 days prior to experimentation. In one cohort (n = 8), shortly before dark onset, shrews received an IP injection of 5 nmol/kg Ex4, 5 nmol/kg B12-Ex4 or vehicle. In another sub-group of animals (n = 6), 50 nmol/kg Ex4 (i.e. ~200 μg/kg), 50 nmol/kg B12-Ex4 or vehicle was injected. Food intake was evaluated using our custom-made automated feedometers,28 which consisted of a standard plexiglass rodent housing cage (29 × 19 × 12.7 cm) with mounted food hoppers resting on a plexiglass cup (to account for spill-age) attached to a load-cell. Shrews had ad libitum access to powdered food through a circular (3 cm diameter) hole in the cage. Food weights were measured every 10 seconds to the nearest 0.1 g (Arduino 1.8.8). Data obtained from the feedometer were processed and converted by computer software (Processing 3.4 and Microsoft Excel) to determine food ingestion each 10 seconds. Total food intake curves were cumulated for 3, 6 and 24 hours.
2.7 |. Emetogenic properties of systemic Ex4 and B12-Ex4
Shrews (n = 24) were habituated to IP injections and to clear plastic observation chambers (23.5 × 15.25 × 17.8 cm) for 2 consecutive days prior to experimentation. In one group (n = 16), the animals were injected IP with 5 nmol/kg Ex4, 5 nmol/kg B12-Ex4 or vehicle, then video-recorded (Vixia HF-R62, Canon) for 90 minutes; the camera was positioned at a slight angle above the chamber. After 90 minutes, the animals were returned to their cages. In a second cohort (n = 8), the animals received supra-pharmacological doses of 50 nmol/kg Ex4, B12-Ex4 or vehicle, and were then video-recorded as described. Analysis of emetic episodes was carried out by an observer blinded to treatment groups. Emetic episodes were characterized by strong rhythmic abdominal contractions associated with either oral expulsion from the gastrointestinal tract (i.e. vomiting) or without the passage of materials (i.e. retching movements). An emetic bout was defined as a series of one or more emetic episodes that occurred within 1 minute of each other. Latency to the first emetic episode, total number of emetic episodes and the number of emetic episodes per minute, as well as emetic bouts, were calculated.
2.8 |. Emetogenic properties of centrally administered Ex4 and B12-Ex4
Lateral ventricle cannulated shrews (n = 8) were habituated to observation chambers for 2 days prior to experimentation, as in peripheral administration experiments. Ex4 (0.24 nmol, i.e. 1 μg), 0.24 nmol B12-Ex4 or vehicle (aCSF) was infused ICV (total volume 1 μL). Shrews were then video-recorded for 120 minutes. After 120 minutes, the animals were returned to their cages. Each treatment was 72 hours apart. Analysis of emetic episodes was performed as described above.
2.9 |. Assessment of neuronal activation in the dorsal vagal complex following Ex4, B12-Ex4 or saline treatments in shrews
Body weight-matched shrews (n = 13) received an IP injection of 5 nmol/kg Ex4, 5 nmol/kg B12-Ex4 or vehicle, just prior to dark onset. Food was removed to avoid feeding-related changes in c-Fos expression between groups. Three hours later, shrews were deeply anaesthetized with an IP triple cocktail of KAX and transcardially perfused with phosphate buffered saline (PBS, 0.1 M, pH 7.4; Boston Bioproducts), followed by 4% paraformaldehyde (PFA) in PBS. Brains were removed and postfixed in 4% PFA for 48 hours and then stored in 30% sucrose for 2 days. Brains were subsequently frozen in cold hexane and stored at −20°C until further processing. Thirty micrometre-thick frozen coronal sections containing the dorsal vagal complex (DVC) were cut in a cryomicrotome (CM3050S, Leica Microsystem), then collected and stored in cryoprotectant (30% sucrose, 30% ethylene glycol, 1% polyvinyl-pyrrolidone-40, in PBS) at −20°C until further processing. Immunohistochemistry was conducted according to a previously described procedure.29 Briefly, free-floating sections were washed with PBS (3 × 8 minutes), incubated in PBS containing 0.3% Triton X-100 (PBST) and 5% normal donkey serum (NDS) for 1 hour, followed by an overnight incubation with rabbit anti-Fos antibody (1:1000 in PBST; s2250; Cell Signaling). After washing (3 × 8 minutes) with PBS, sections were incubated with the secondary antibody donkey anti-rabbit Alexa Fluor 555 (1:500 in 5% NDS PBST; Jackson Immuno Research Lab-oratories) for 2 hours at room temperature. After final washing (3 × 8 minutes in 0.1 M PBS) the sections were mounted onto glass slides (Superfrost Plus, VWR) and coverslipped with Fluorogel (Electron Microscopy Sciences). A total of three DVC sections per animal were used to quantify the number of c-Fos–labelled cells in the AP and NTS of the DVC (~200–250 μm rostral to the obex). c-Fos-positive neurons were visualized and quantified manually in a blind fashion using fluorescence microscopy (20x; Nikon 80i, NIS Elements AR 3.0).
2.10 |. Evaluation of B12-Ex4 presence/penetrance in the pancreas
Shrews (n = 4) received an IP injection of 5 nmol/kg Cy5-B12-Ex4 and were transcardially perfused 3 hours later as described above. Pancreases were immediately dissected. Thirty micrometre-thick frozen sagittal sections were processed via immunohistochemistry for insulin and coverslipped with 4′,6-diamidino-2-phenylindole mounting medium as previously described.9 Sections were visualized with confocal microscopy (Leica SP5 X) using the 488 and 594 laser lines with 10x and 20x objectives. All images were collected sequentially to avoid contamination of signals from other fluorophores.
2.11 |. Effects of B12-Ex4, Ex4 and liraglutide on glycaemic control, insulin profile and energy balance in GK rats
GK rats (n = 21) were adapted to insertion of a gavage for 1 week before the beginning of the experiment. On the test day, animals were food deprived 2 hours before dark onset and, just after onset of the dark phase, water was also removed from the cage. Three hours later, a small drop of blood was collected from the tail tip and analysed for BG level using a standard glucometer (AccuCheck). Immediately after this baseline BG reading (t = −60 minutes), each rat received an IP injection of 5 nmol/kg Ex4, 5 nmol/kg B12-Ex4, liraglutide (100 μg/kg) or vehicle (1 mL/kg sterile saline). Doses of drugs were selected based on previous reports9,30,31 and pilot studies conducted in GK rats. BG was measured 60 minutes later (t = 0 minutes) and each rat received an oral gavage of glucose (1 g/kg). Subsequent BG readings were taken at 20, 40, 60, 120 and 180 minutes after glucose gavage. After the final BG reading, food and water were returned.
2.12 |. Statistical analyses
All data were expressed as mean ± SEM. For behavioural studies, data were analysed by a repeated measures one- or two-way ANOVA, followed by Tukey’s post hoc tests. For all statistical tests, a P-value of less than 0.05 was considered significant. Data were analysed using Prism GraphPad 8.0. BG levels measured during the oral glucose tolerance tests (OGTTs) and IPGTTs were analysed using a repeated measures two-way ANOVA followed by Tukey’s post hoc test. Each area under the curve (AUC) was calculated from 0 to 120 minutes using the trapezoidal method. The resulting AUCs were analysed using a repeated measures one-way ANOVA followed by Tukey’s post hoc test. The total numbers of emetic episodes and emetic bouts were analysed using a repeated measures one-way ANOVA followed by Tukey’s post hoc test. Data obtained from the feedometer were analysed with repeated measures one-way ANOVA followed by Tukey’s post hoc test. For the immunohistological study, statistical comparisons were performed using a one-way ANOVA followed by Tukey’s post hoc test.
3 |. RESULTS
3.1 |. Study 1: systemically delivered B12-Ex4 enhances glucose clearance relative to Ex4 and co-localizes with insulin in pancreatic β-cells in the shrew
We first tested whether B12-Ex4 reduces BG following an IPGTT in shrews. We observed that shrews treated with equimolar B12-Ex4 and native Ex4 display similar improvements in glucose clearance following glucose load compared with saline controls (Treatment × Time interaction F[10, 140] = 5.522; P < .0001, Figure 1A). Post hoc analyses showed that both compounds significantly suppressed BG at 20, 40 and 60 minutes after glucose administration compared with saline treatment (all P < .001). Furthermore, the AUC for B12-Ex4 did not differ from native Ex4 following glucose administration (Treatment effect F (2, 28) = 7.218; P < .003, Cbi-Ex4 vs. Ex4; P > .05, Figure 1B). Additionally, we administered Ex4, B12-Ex4 or saline without subsequent glucose injection and observed that both Ex4 and B12-Ex4 did not produce basal BG change (Treatment × Time interaction F[10, 60] = 0.1682; P > .05) or impact the glucose AUC (Treatment effect F (2, 8) = 1.652; P > .05, Figure 1C,D). To assess whether B12-Ex4 is taken up by insulin-producing cells in the pancreas, shrews were injected IP with a fluorescently labelled version of B12-Ex4 (Cy5-B12-Ex4, 5 nmol/kg) and sacrificed 3 hours after delivery. Results show that upon systemic administration, Cy5-B12-Ex4 co-localized with insulin in pancreatic β-cells (Figure 1E).
FIGURE 1.
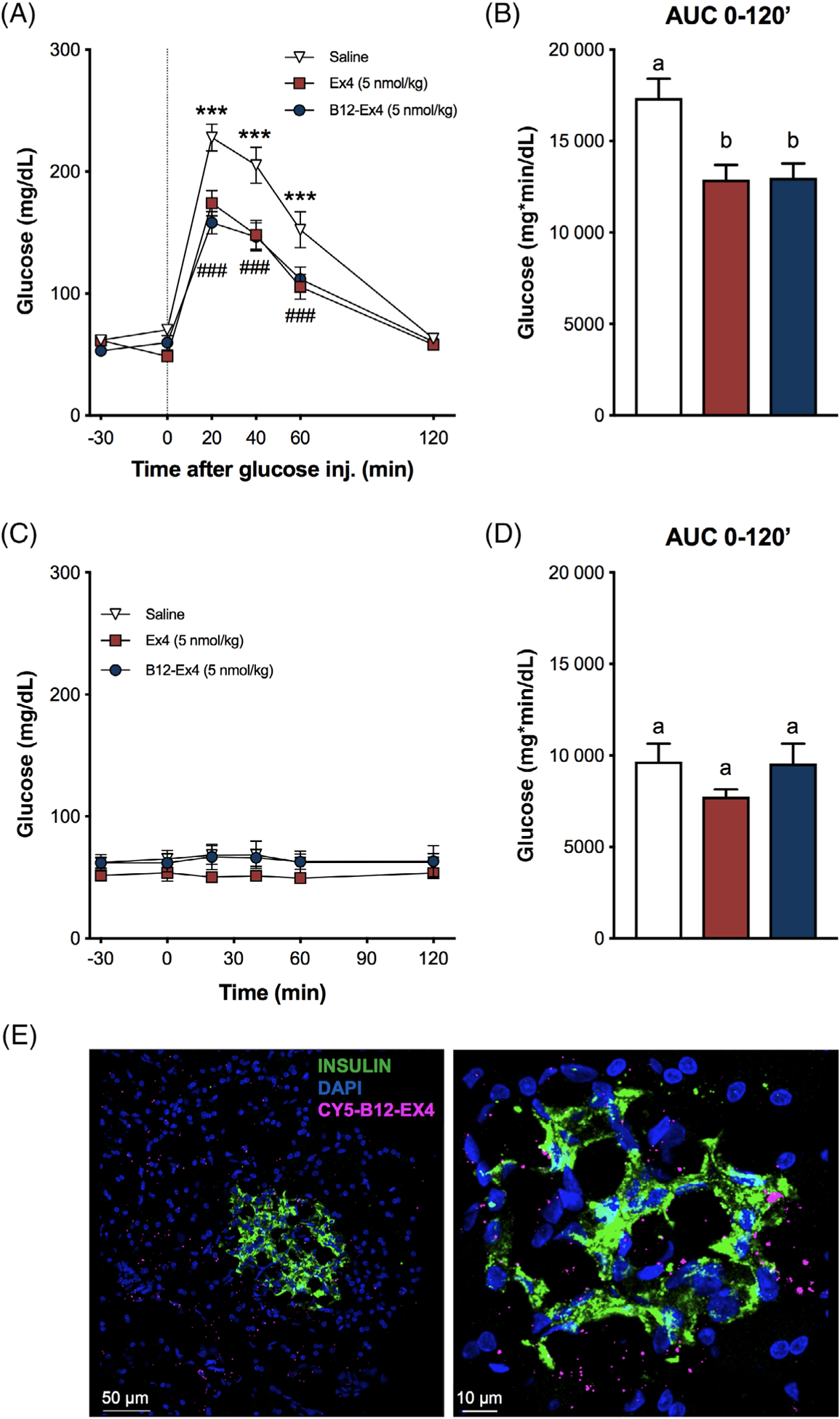
Systemically delivered B12-Ex4 enhances glucose clearance during an intraperitoneal glucose tolerance test (IPGTT) and co-localizes with insulin in pancreatic β-cells in shrews. (A) In an IPGTT, Ex4 (5 nmol/kg, i.e. ~20 μg/kg) and B12-Ex4 (5 nmol/kg) showed a similar potency in suppressing blood glucose (BG) levels after intraperitoneal (IP) glucose administration (2 g/kg, IP) compared with saline; saline versus B12-Ex4: *** P < .001; saline versus Ex4: ### P < .001. (B) Area under the curve (AUC) analysis from 0 (i.e. postglucose bolus) to 120 minutes; B12-Ex4 and Ex4 similarly reduced AUCs compared with saline. (C, D) To investigate the effects of B12-Ex4 and Ex4 on baseline glucose homeostasis, the same doses of Ex4, B12-Ex4 or saline were administered without subsequent glucose administration. Ex4 and B12-Ex4 treatments were no longer effective in reducing BG levels and had no effect on AUC. (E) Systemically injected fluorophore-labelled B12-Ex4 (Cy5-B12-Ex4, 5 nmol/kg) co-localized with insulin in shrew pancreatic tissue supporting the hypothesis that B12-Ex4 acts at the pancreas to improve glycaemic control. All data are expressed as mean ± SEM. Data in (A) and (C) were analysed with repeated measurements two-way ANOVA followed by Tukey’s post hoc test. Data in (B) and (D) were analysed with repeated measurements one-way ANOVA followed by Tukey’s post hoc test. Means with different letters are significantly different (P < .05). In (A, B), n = 13, within subject; in (C-D), n = 5, within subject
3.2 |. Study 2: B12-Ex4 does not alter food intake or body weight, contrary to administration of Ex4, which does reduce food intake and leads to body weight loss in the shrew
Shrews received an IP injection of Ex4 (5 nmol/kg), B12-Ex4 (5 nmol/kg) or saline, to test whether B12-Ex4 administration alters food intake or body weight in this species. In line with previous reports,14 systemic administration of Ex4 produced hypophagia in shrews at all measured time points (all P < .05, Treatment × Time interaction F (6, 42) = 4.097; P < .003, Figure 2A,B) and body weight loss 24 hours after injection (all P < .05, Treatment effect F (2, 14) = 6.9; P < .01, Figure 2C). By contrast, no significant effects on food intake (all P > .05, Figure 2A,B) or body weight (P > .05, Figure 2C) were observed following B12-Ex4 treatment. In a second experiment, shrews were injected IP with a supra-pharmacological dose of Ex4 (50 nmol/kg, i.e. ~200 μg/kg), B12-Ex4 (50 nmol/kg) or saline. Ex4 induced anorexia at all measured time points (all P < .05, Treatment × Time interaction F (6, 30) = 6.894; P < .001, Figure 2D,E), and 24 hours body weight loss (P < .05, Treatment effect F (2, 10) = 12.94; P < .002, Figure 2F). By contrast, administration of equimolar doses of B12-Ex4 did not induce hypophagia (Figure 2D,E) or body weight loss compared with saline-treated animals (Figure 2F).
FIGURE 2.
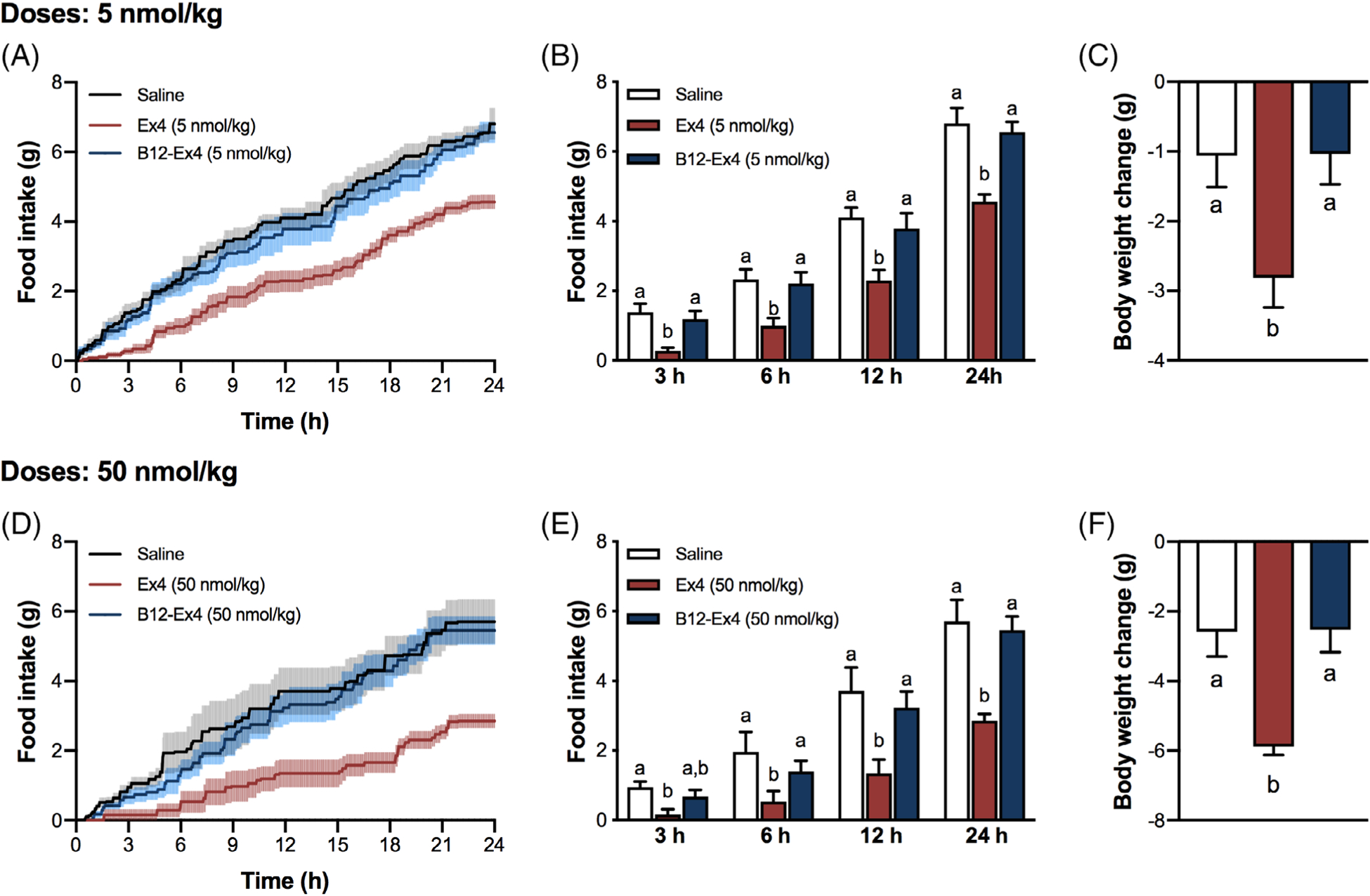
Pharmacological and supra-pharmacological doses of B12-Ex4 do not affect feeding and body weight in shrews. (A, B) Ex4 (5 nmol/kg) suppressed feeding at 3, 6, 12 and 24 hours, whereas equimolar doses of B12-Ex4 had no effect on food intake. (C) Ex4-induced anorexia was accompanied by body weight loss. No significant changes in body weight were observed after B12-Ex4 administration compared with controls. (D, E) Ex4 (50 nmol/kg, i.e. ~200 μg/kg) suppressed eating at all measured time points. By strict contrast, supra-pharmacological equimolar doses of B12-Ex4 did not show any effects. (F) While severe body weight loss occurred following supra-pharmacological doses of Ex4, no significant changes occurred in the B12-Ex4–treated animals. All data are expressed as mean ± SEM. Data were analysed with repeated measurements one-way ANOVA followed by Tukey’s post hoc test. Means with different letters are significantly different (P < .05). In (A-C), n = 8, within subject; in (D-F), n = 6, within subject
3.3 |. Study 3: B12-Ex4 treatment produces only slight emesis compared with the potent emetic effects of Ex4
Ex4 (5 nmol/kg) induced emesis in the majority of the shrews tested (Figure 3A). By contrast, only one shrew experienced emesis after equimolar B12-Ex4 administration (P < .001). The number of single emetic episodes (Treatment effect F (2, 30) = 9.368; P < .002, Figure 3B) and emetic bouts (Treatment effect F (2, 30) = 20.5; P < .0001, Figure 3C) in the 90 minutes window after drug administration were also significantly reduced after B12-Ex4 compared with Ex4 and did not differ from the control condition. Ex4 triggered emesis with an average latency of 19 ± 5 minutes (Figure 3D). A heatmap representation of the Ex4 and B12-Ex4 effects on emetic intensity and latency for each individual animal across time can be seen in Figure 3E.
FIGURE 3.
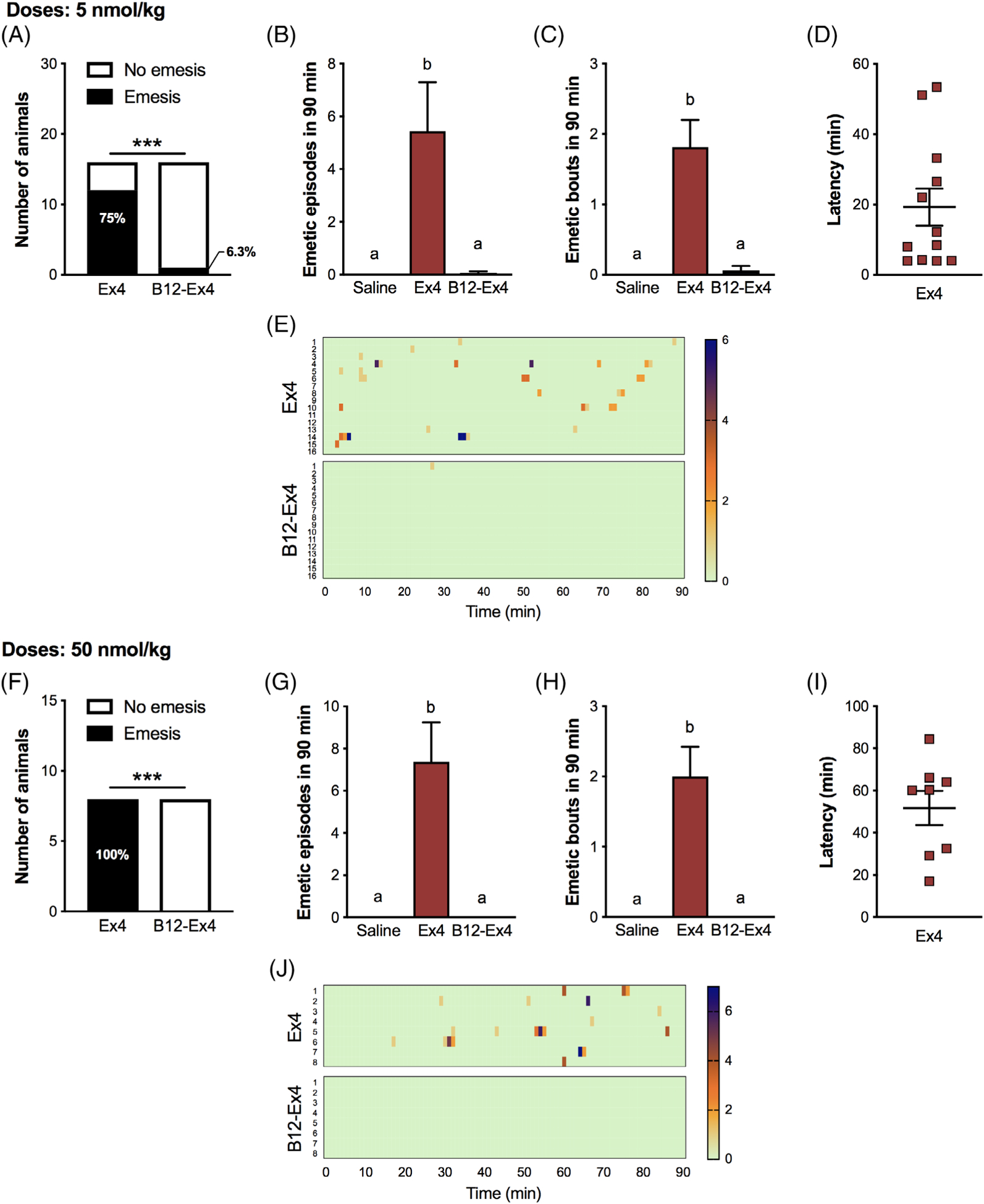
Emesis in shrews is significantly reduced following B12-Ex4 treatment compared with native Ex4, denoting improved tolerance across different doses. (A) The percentage of shrews experiencing emesis was significantly different between Ex4 (5 nmol/kg) and equimolar B12-Ex4 (*** P < .001). None of the animals experienced emesis following saline administration (data not shown). (B) The number of single emetic episodes following Ex4 (5 nmol/kg), equimolar B12-Ex4 or saline systemic administration was recorded for 90 minutes. Ex4 induced robust emetic responses that were not observed after B12-Ex4 or saline injections. (C) The number of emetic bouts was also lower after B12-Ex4 treatment compared with Ex4 and it did not differ from saline controls. (D) Latency to the first emetic episode in Ex4 animals that exhibited emesis. (E) Heatmap showing the emetic latency and intensity, as well as the number of emetic episodes induced by Ex4 or B12-Ex4 for each animal across time. A similar experiment was conducted with supra-pharmacological doses of Ex4 and B12-Ex4. Astonishingly, no emesis occurred after B12-Ex4 administration. (F) The percentage of shrews experiencing emesis was completely reversed between Ex4 (50 nmol/kg, i.e. ~200 μg/kg) and equimolar B12-Ex4 (*** P < .001). No animal experienced emesis following saline injection (data not shown). (G, H) The number of emetic episodes and bouts following Ex4 (50 nmol/kg), equimolar B12-Ex4 or saline administration was analysed over 90 minutes. Also, at this dosage Ex4 induced robust emetic responses. (I) Latency to the first emetic episode in Ex4 shrews that exhibited emesis. (J) Heatmap showing the emetic latency and intensity, as well as the number of emetic episodes for each animal across time. All data are expressed as mean ± SEM. Data in (A, F) were analysed with Fisher’s exact test. Data in (B, C, G, H) were analysed with repeated measurements one-way ANOVA followed by Tukey’s post hoc test. In (A-C), n = 16, within-subject; in (F-H), n = 8, within subject. Means with different letters are significantly different (P < .05)
Next, we tested supra-pharmacological doses of Ex4 and B12-Ex4 and observed that Ex4 induced profound emesis, reflected by both the number and severity of emetic episodes, in 100% (i.e. 8/8, P < .001) of the shrews tested, while an equimolar dose of B12-Ex4 did not induce emesis in shrews (Treatment effect on single emetic episodes F (2, 14) = 15.39; P < .0003, and Treatment effect on emetic bouts F (2, 14) = 20.4; P < .0001, Figure 3G,H). The average latency to first emetic episode induced by Ex4 was 52 ± 8 minutes (Figure 3I). The emetic profiles of each animal following B12-Ex4 or Ex4 administration are represented in Figure 3J.
3.4 |. Study 4: systemically administered B12-Ex4 does not induce c-Fos in the AP or the NTS, while central administration of B12-Ex4 and Ex4 similarly produce emesis
Ex4, but not B12-Ex4, induced c-Fos immunofluorescence compared with saline-treated controls within the NTS (Treatment effect F (2, 10) = 28.82; P < .0001) and AP (Treatment effect F (2, 10) = 6.62; P < .02, Figure 4A,B). ICV administration of Ex4 (0.24 nmol) and B12-Ex4 (0.24 nmol) induced emesis with similar emetogenic profiles. We observed no difference in the percentage of shrews experiencing emesis following central B12-Ex4 or Ex4 administration (Figure 4C). Furthermore, the number of single emetic episodes (Figure 4D), the number of emetic bouts (Figure 4E), as well as the latency to the first emetic response (Figure 4F), were not statistically different between B12-Ex4 and Ex4. ICV administration of aCSF did not induce any emesis in shrews (data not shown).
FIGURE 4.
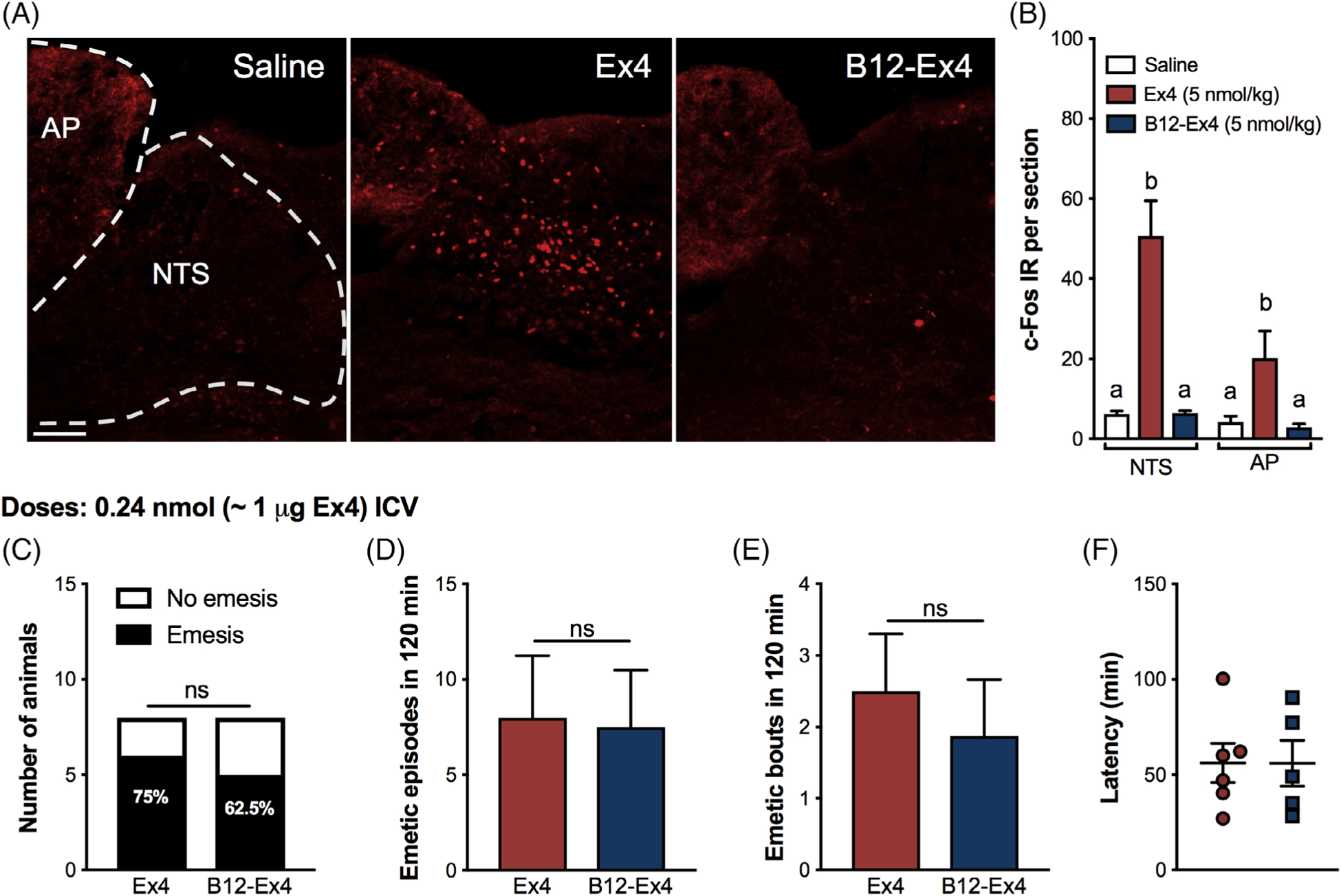
Systemic administered B12-Ex4 does not activate the area postrema (AP) or the nucleus tractus solitarius (NTS) in shrews but it causes emesis when administered centrally. (A) Representative immunostainings of the AP/NTS region showing the c-Fos response following saline (n = 4), Ex4 (5 nmol/kg, n = 4) and equimolar B12-Ex4 (n = 5) systemic treatment. (B) Peripheral Ex4 administration significantly increased the number of c-Fos immunoreactive (IR) cells in the AP/NTS of shrews 3 hours after injection. The number of c-Fos–positive cells in the AP/NTS was significantly lower in B12-Ex4–treated animals and it did not differ from saline-treated shrews. (C) Ex4 (0.24 nmol, i.e. 1 μg), equimolar B12-Ex4 or vehicle was infused into the lateral ventricle. The percentage of shrews showing emesis was similar between Ex4 and B12-Ex.4. (D, E) The number of single emetic episodes or bouts following Ex4, B12-Ex4 or saline was recorded for 120 minutes. Both Ex4 and B12-Ex4 induced comparable emetic responses, while none of the animals experienced emesis following vehicle delivery (data not shown). (F) Latency to the first emetic episode in Ex4- and B12-Ex4–treated animals that exhibited emesis did not differ. Data in (B) were analysed with one-way ANOVA followed by Tukey post hoc test. Means with different letters are significantly different from each other (P < .05). In (C) the analysis was performed with Fisher’s exact test. Data in (D-F) were analysed with Student t-test (n = 8, within subject). Values are expressed as mean ± SEM. Scale bar 100 μm
3.5 |. Study 5: B12-Ex4 enhances glucose clearance during an OGTT without inducing anorexia and body weight loss in the GK rat
We tested the effects of Ex4, B12-Ex4 and liraglutide on oral glucose tolerance, food intake and body weight in GK rats. Similar to the effects of liraglutide (100 μg/kg), B12-Ex4 reduced the glucose level following an OGTT compared with saline at 20 and 40 minutes after glucose load, and to native Ex4 (5 nmol/kg) at 120 and 180 minutes after glucose load (all P < .01, Treatment × Time interaction F[18, 360] = 11.92; P < .0001, Figure 5A). However, while both liraglutide and native Ex4 suppressed 24 hours food intake leading to 24 hours body weight loss (all P < .05), no hypoghagic or body weight suppressive effects were observed after B12-Ex4 administration in this lean diabetic model (Treatment effect on food F(3, 60) = 95.41; P < .0001, and Treatment effect on body weight F(3, 60) = 28.21; P < .0001, Figure 5B,C).
FIGURE 5.
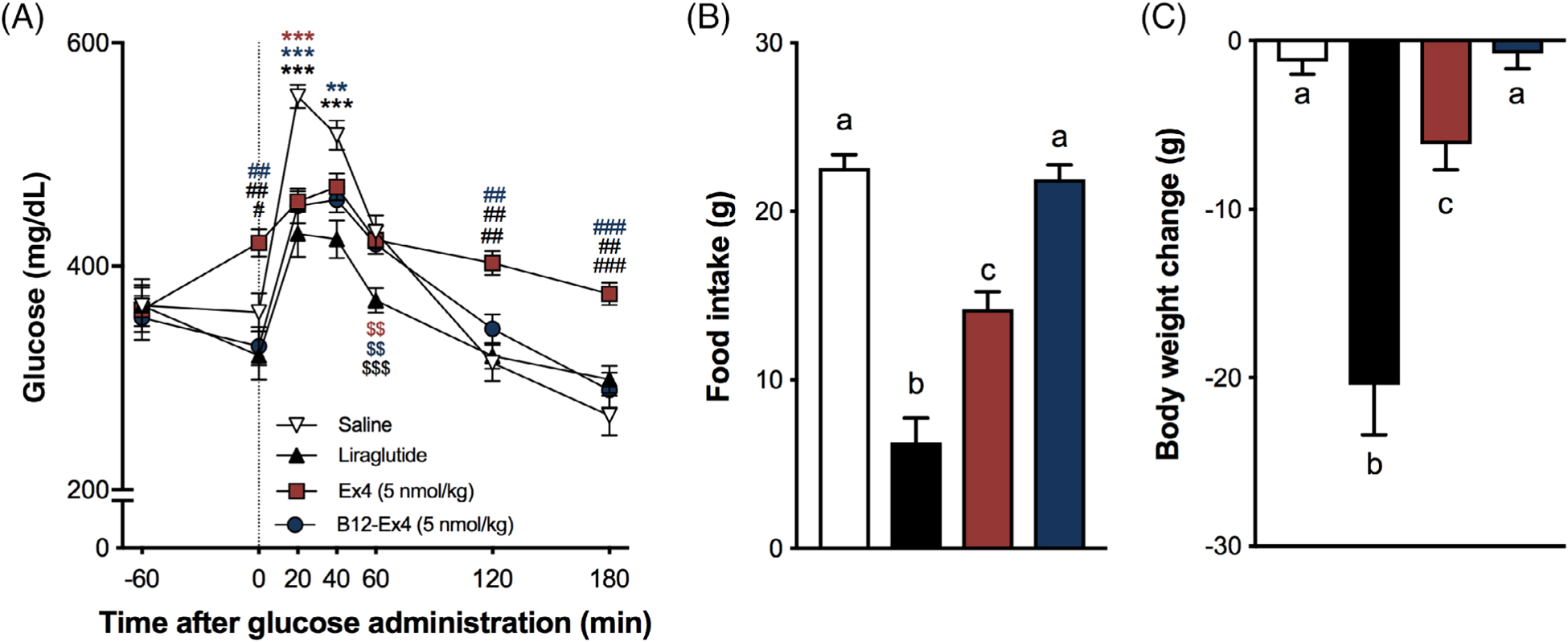
B12-Ex4 enhances glucose clearance during an oral glucose tolerance test (OGTT) without inducing anorexia and body weight loss in the Goto-Kakizaki lean diabetic rat model. (A) In an OGTT, liraglutide (100 μg/kg, i.e. 26.6 nmol/kg) and 5 nmol/kg B12-Ex4 suppressed blood glucose levels after intraperitoneal (IP) glucose administration (1 g/kg, PO) compared with 5 nmol/kg Ex4 (i.e. ~20 μg/kg) and saline. * saline versus others; #: Ex4 versus others; $: liraglutide versus others. *, #, $: P < .05; **, ##, $$: P < .01; ***, ###, $$$: P < .001. (B) Liraglutide and Ex4 induced a strong anorectic effect, which was not observed after B12-Ex4 treatment. (C) Hypophagia was accompanied by body weight loss in liraglutide- and Ex4-treated animals, while no significant body weight change was observed following B12-Ex4. Data were analysed with repeated measure two-way ANOVA (A) or one-way ANOVA (B, C) followed by Tukey post hoc test; n = 21, within subject. All data are expressed as mean ± SEM. Means with different letters are significantly different from each other (P < .05)
4 |. DISCUSSION
Existing GLP-1-based pharmacotherapies are excellent glucoregulatory drugs for treating hyperglycaemia in the majority of patients with T2D despite reported nausea and vomiting. These phenomena, however, are gaining notoriety as overlooked major side effects in many patients, which reduce patient compliance and make current GLP-1R agonists ‘suboptimal’.3–7,32,33 Designing and testing second-generation GLP-1-based drugs aimed at improving patient tolerance while preserving or enhancing glucoregulatory control therefore provides clear clinical value. Here, we report that a B12-Ex4 conjugate previously shown to exert comparable glycaemic control with limited anorexia and nausea-like effects compared with native Ex4 in rats9 produced comparable glycaemic control, and better tolerance, in an emetic mammalian species and in a lean polygenetic rat model of T2D.
In line with previous reports in lean, non-diabetic rats and shrews,9,14,30,31 both native Ex4 doses (5 and 50 nmol/kg) tested here induced anorexia and body weight loss over 24 hours in shrews. By contrast, no such effects occurred after B12-Ex4 treatments, even at supra-pharmacological doses (i.e. 50 nmol/kg). The lack of short-term effects on feeding was unexpected given reports of a role of vagal afferent signalling in the mediation of the early-phase anorexia induced by both GLP-1 and native Ex4 in rats.34,35 Our previous report also observed this effect in lean, healthy rats treated with the highest dose of B12-Ex4.9 This incongruency, however, could be explained by broad species differences in metabolism between rats and shrews, rather than by the direct action of the drug.
Furthermore, B12-Ex4 administration resulted in a near complete absence of emesis compared with potent emetic effects of Ex4 in the shrew. In fact, we observed that ~10-fold the threshold concentration necessary for glucoregulation did not cause emesis with B12-Ex4, while all animals tested with this dose of Ex4 experienced emesis. Together, these findings show that it is possible to both physiologi-cally and pharmacologically separate the emetic potential of GLP-1R agonists like Ex4 from their glucoregulatory role, and that a wide range of B12-Ex4 doses produce limited anorectic or emetic side effects. These data suggest that a B12-Ex4 conjugate could improve T2D medication adherence compared with existing GLP-1 mimetic drugs. This is particularly relevant given the pleiotropic nature of GLP-1, with a myriad of effects, mainly beneficial, which render GLP-1 analogues as one of the best current treatments for T2D. Indeed, alternative therapies approved for the treatment of T2D (e.g. dipeptidyl peptidase-4 inhibitors, metformin and sodium-glucose co-transporter-2 inhibitors) do not achieve the same overall positive outcome on glucose handling compared with GLP-1R agonists. Importantly, these alternative strategies also lead to modest but significant body weight reductions.36,37
Preventing access to CNS GLP-1Rs is the probable explanation for the lack of B12-Ex4–induced emesis in the shrew. Our immunohistological evidence supports this notion as we observed sparse c-Fos activation following systemic B12-Ex4 administration in the AP and NTS, two hindbrain nuclei known to mediate feeding behaviour and emesis,13,38–41 while native Ex4 produced robust activation of these nuclei. To provide functional evidence that B12 conjugation itself does not impact the emetic nature of Ex4, we centrally administered B12-Ex4 directly into the lateral ventricle of the shrew, which produced a comparable emetic profile with that of native Ex4 (e.g. the number of emetic episodes, latency to vomit, and the proportion of treated animals experiencing emesis). These data suggest that when centrally delivered, and thus bypassing the blood–brain barrier, B12-Ex4 is a stimulus that can produce an emetic profile consistent with Ex4 alone.
To speculate how conjugation to B12 reduces CNS penetrance of Ex4, increased polarity may be an important variable rather than the increased molecular size alone, given that GLP-1R agonists with greater molecular masses, such as liraglutide, have been shown to penetrate into the hindbrain and to stimulate direct GLP-1R activation.30,42,43 It is also possible that the binding of B12-Ex4 to the B12 carrier proteins transcobalamin (TC) or haptocorrin (HC) in serum, previously confirmed for this construct,9 further reduces brain penetrance because its size (now >60 KDa) either actively transports the conjugate to systemic targets (via TC), maintains the conjugate in serum (via HC), or protects the conjugate from proteolytic degradation (via TC or HC),8 as is typical for dietary B12 alone.44 As noted, however, the shrew has both TC and HC, unlike rodents, which have only TC. In comparison with studies performed for B12-Ex4 in rodents,9 we see comparable pharmacodynamics (in terms of illness behaviours and glucoregulation), suggesting that the presence of both TC and HC is not specifically necessary for the conjugate. It may be that the benefits of conjugation lie solely with the B12 molecule itself, or simply through the effects of binding to any B12 binding protein in terms of protection and/or mitigation of hindbrain access. Our data suggest that it is the unique structure of B12-Ex4, rather than simply mass, which drives its unique pharmacodynamic profile and underlying properties.
Pancreatic β-cells expressing the GLP-1R represent the probable cellular substrate mediating the hypoglycaemic effects of B12-Ex4 in shrews. Our proof of concept (via microscopy) shows that a fluorescently tagged B12-Ex4 conjugate (Cy5-B12-Ex4) co-localizes with insulin in the shrew pancreatic β-cells. By employing fluorescently tagged Ex4 in combination with Cy5-B12-Ex4, further investigations are warranted to examine the binding specificity of B12-Ex4 in the pancreas. Nevertheless, the current data support our previous work in rodents9 and strengthen the notion that the incretin effect occurring after GLP-1 analogue administration is mediated by direct activation of GLP-1Rs expressed on pancreatic β-cells, mimicking the paracrine actions of pancreatic-derived GLP-1.10–12
It is also possible that GLP-1Rs expressed in other peripheral tissues may be engaged by B12-Ex4 and thus contribute to its beneficial effects on glucose homeostasis. For instance, it has been shown that low doses of Ex4 that mimic endogenous GLP-1 release can modulate glycaemia and gastric emptying via vagal afferent signalling.35 Further, GLP-1Rs are also abundantly expressed on enteric neurons, and emerging evidence suggests a role of the enteric nervous system in the mediation of effects induced by GLP-1 analogues.45–48 Whether B12-Ex4 acts on GLP-1Rs expressed in the periphery beyond the pancreas, possibly within the peripheral and enteric nervous system, and to what degree these hypothetical actions contribute to the observed augmented glucoregulation, requires further investigation.
We also report that B12-Ex4 improves glucose clearance relative to Ex4 in a lean, polygenetic model of T2D, the GK rat. Similar to liraglutide, B12-Ex4 does not produce the transient stress-induced hyperglycaemic response in the GK rat commonly observed from Ex4 across rat models (lean, obese or GK),49,50 a phenomenon tied to the high CNS penetrance of Ex4. Consistent with the lack of a CNS stress-mediated hyperglycaemia reported for Ex4,49,50 B12-Ex4 did not produce the documented CNS-dependent outcomes of anorexia and body weight loss in this lean model of T2D.
In summary, the data presented here in shrews and GK rats support our hypothesis that limiting or blocking Ex4 entry into the brain dramatically reduces the side effects of the Ex4 drug (i.e. prevents anorexia and emesis), while maintaining the glucoregulatory efficacy of the compound. The overall outcomes of this work highlight the translational potential of B12-Ex4–based pharmacotherapies for use in lean T2D patients where weight loss is an undesirable outcome, although chronic studies are needed to elucidate the efficacy, tolerability and pharmacodynamics of B12-Ex4 in long-term treatment. Albeit small, there is the possibility that B12-Ex4 chronic exposure would interfere with normal B12 physiology, potentially reducing the amount of dietary B12 available in the body.51 Further, while we do not predict tachyphylaxis, the extent to which the observed positive effects will be maintained requires future empirical testing. Nonetheless, the current data underlie the potential use of B12-Ex4 for patients with T2D who either do not tolerate current GLP-1 agonists or suffer from co-morbidities associated with an anorectic/cachectic state, for which the body weight-suppressing effects of a standard GLP-1R agonist are an unwanted side effect, with the loss of nausea/emesis an additional benefit.
ACKNOWLEDGMENTS
The authors would like to thank Sam Fortin and Rinzin Lhamo for technical assistance. This work was supported by NIH-DK-112812 (B.C.D.), NIH-DK-097675 (R.P.D.), NIH-DK-115762 (M.R.H.), SNF P2ZHP3_178114, P400PB_186728 (T.B.) and NIH-CA-201962 (C.C.H.).
Funding information
This work was supported by National Institutes of Health-DK-112812 (B.C.D.), NIH-DK-097675 (R.P.D.), NIH-DK-115762 (M.R.H.), SNF P2ZHP3_178114, P400PB_186728 (T.B.) and NIH-CA-201962 (C.C.H.)
Footnotes
CONFLICTS OF INTEREST
BCD receives research funding from Eli Lilly & Co. and Pfizer, Inc. and provided remunerated consultancy services for Pfizer Inc. not supporting these studies. RPD is a scientific advisory board member and received funds from Xeragenx LLc (St. Louis, NY) and Balchem, New Hampton, New York, which were not used in support of these studies. MRH receives research funding from Zealand Pharma, Novo Nordisk, Eli Lilly & Co. and Boehringer Ingelheim that was not used in support of these studies. RPD is an inventor of the patents associated with this work. The other authors have no competing interests to declare.
REFERENCES
- 1.Sanger GJ, Andrews PLR. A history of drug discovery for treatment of nausea and vomiting and the implications for future research. Front Pharmacol. 2018;9:913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab. 2017;19(4): 524–536. [DOI] [PubMed] [Google Scholar]
- 3.Bergenstal RM, Wysham C, Macconell L, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376(9739):431–439. [DOI] [PubMed] [Google Scholar]
- 4.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27(11):2628–2635. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28(5):1092–1100. [DOI] [PubMed] [Google Scholar]
- 6.John LE, Kane MP, Busch RS, Hamilton RA. Expanded use of exenatide in the management of type 2 diabetes. Diabetes Spectr. 2007;20:59–63. [Google Scholar]
- 7.Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28(5):1083–1091. [DOI] [PubMed] [Google Scholar]
- 8.Bonaccorso RL, Chepurny OG, Becker-Pauly C, Holz GG, Doyle RP. Enhanced peptide stability against protease digestion induced by intrinsic factor binding of a vitamin B12 conjugate of exendin-4. Mol Pharm. 2015;12(9):3502–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mietlicki-Baase EG, Liberini CG, Workinger JL, et al. A vitamin B12 conjugate of exendin-4 improves glucose tolerance without associated nausea or hypophagia in rodents. Diabetes Obes Metab. 2018;20 (5):1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamont BJ, Li YZ, Kwan E, Brown TJ, Gaisano H, Drucker DJ. Pancreatic GLP-1 receptor activation is sufficient for incretin control of glucose metabolism in mice. J Clin Investig. 2012;122(1):388–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith EP, An Z, Wagner C, et al. The role of beta cell glucagon-like peptide-1 signaling in glucose regulation and response to diabetes drugs. Cell Metab. 2014;19(6):1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chambers AP, Sorrell JE, Haller A, et al. The role of pancreatic preproglucagon in glucose homeostasis in mice. Cell Metab. 2017;25 (4):927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology. 2012;62(5–6):1916–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan SW, Lin G, Yew DT, Yeung CK, Rudd JA. Separation of emetic and anorexic responses of exendin-4, a GLP-1 receptor agonist in Suncus murinus (house musk shrew). Neuropharmacology. 2013;70:141–147. [DOI] [PubMed] [Google Scholar]
- 15.Chan SW, Lin G, Yew DT, Rudd JA. A physiological role of glucagon-like peptide-1 receptors in the central nervous system of Suncus murinus (house musk shrew). Eur J Pharmacol. 2011;668(1–2): 340–346. [DOI] [PubMed] [Google Scholar]
- 16.Andersen A, Lund A, Knop FK, Vilsboll T. Glucagon-like peptide 1 in health and disease. Nat Rev Endocrinol. 2018;14(7):390–403. [DOI] [PubMed] [Google Scholar]
- 17.Bray GA, Fruhbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet. 2016;387(10031):1947–1956. [DOI] [PubMed] [Google Scholar]
- 18.Gallo M, Muscogiuri G, Felicetti F, et al. Adverse glycaemic effects of cancer therapy: indications for a rational approach to cancer patients with diabetes. Metabolism. 2018;78:141–154. [DOI] [PubMed] [Google Scholar]
- 19.Moheet A, Moran A. CF-related diabetes: containing the metabolic miscreant of cystic fibrosis. Pediatr Pulmonol. 2017;52:S37–S43. [DOI] [PubMed] [Google Scholar]
- 20.Noubissi EC, Katte JC, Sobngwi E. Diabetes and HIV. Curr Diab Rep. 2018;18(11):125. [DOI] [PubMed] [Google Scholar]
- 21.Glaser S, Kruger S, Merkel M, Bramlage P, Herth FJ. Chronic obstructive pulmonary disease and diabetes mellitus: a systematic review of the literature. Respiration. 2015;89(3):253–264. [DOI] [PubMed] [Google Scholar]
- 22.Mesinovic J, Zengin A, De Courten B, Ebeling PR, Scott D. Sarcopenia and type 2 diabetes mellitus: a bidirectional relationship. Diabetes Metab Syndr Obes. 2019;12:1057–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caspard H, Jabbour S, Hammar N, Fenici P, Sheehan JJ, Kosiborod M. Recent trends in the prevalence of type 2 diabetes and the association with abdominal obesity lead to growing health disparities in the USA: an analysis of the NHANES surveys from 1999 to 2014. Diabetes Obes Metab. 2018;20(3):667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goto Y, Kakizaki M, Masaki N. Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J Exp Med. 1976;119 (1):85–90. [DOI] [PubMed] [Google Scholar]
- 25.Ostenson CG, Efendic S. Islet gene expression and function in type 2 diabetes; studies in the Goto-Kakizaki rat and humans. Diabetes Obes Metab. 2007;9((Suppl 2)):180–186. [DOI] [PubMed] [Google Scholar]
- 26.Ueno S, Matsuki N, Saito H. Suncus murinus: a new experimental model in emesis research. Life Sci. 1987;41(4):513–518. [DOI] [PubMed] [Google Scholar]
- 27.Andrews PL, Friedman MI, Liu YL, Smith JE, Sims DW. Potential energetic implications of emesis in the house musk shrew (Suncus murinus). Physiol Behav. 2005;84(4):519–524. [DOI] [PubMed] [Google Scholar]
- 28.Nasir S, Aguilar D. Congestive heart failure and diabetes mellitus: balancing glycemic control with heart failure improvement. Am J Cardiol. 2012;110(9 Suppl):50B–57B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borner T, Shaulson ED, Ghidewon MY, et al. GDF15 induces anorexia through nausea and emesis. Cell Metab. 2020.31(2):351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology. 2011;152(8):3103–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayes MR, Kanoski SE, Alhadeff AL, Grill HJ. Comparative effects of the long-acting GLP-1 receptor ligands, liraglutide and exendin-4, on food intake and body weight suppression in rats. Obesity. 2011;19(7): 1342–1349. [DOI] [PubMed] [Google Scholar]
- 32.Zhao P, Liang YL, Belousoff MJ, et al. Activation of the GLP-1 receptor by a non-peptidic agonist. Nature. 2020;577(7790):432–436. [DOI] [PubMed] [Google Scholar]
- 33.Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724–1732. [DOI] [PubMed] [Google Scholar]
- 34.Labouesse MA, Stadlbauer U, Weber E, Arnold M, Langhans W, Pacheco-Lopez G. Vagal afferents mediate early satiation and prevent flavour avoidance learning in response to intraperitoneally infused exendin-4. J Neuroendocrinol. 2012;24(12):1505–1516. [DOI] [PubMed] [Google Scholar]
- 35.Krieger JP, Arnold M, Pettersen KG, Lossel P, Langhans W, Lee SJ. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes. 2016;65(1):34–43. [DOI] [PubMed] [Google Scholar]
- 36.Brunton S GLP-1 receptor agonists vs. DPP-4 inhibitors for type 2 diabetes: is one approach more successful or preferable than the other? Int J Clin Pract. 2014;68(5):557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Hu Y, Xu Y, Jia Y, Miao L, Wang G. Comparison of exenatide and metformin monotherapy in overweight/obese patients with newly diagnosed type 2 diabetes. Int J Endocrinol. 2017;2017: 9401606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hornby PJ. Central neurocircuitry associated with emesis. Am J Med. 2001;111((Suppl 8A)):106S–112S. [DOI] [PubMed] [Google Scholar]
- 39.Miller AD, Leslie RA. The area postrema and vomiting. Front Neuroendocrinol. 1994;15(4):301–320. [DOI] [PubMed] [Google Scholar]
- 40.Grill HJ, Hayes MR. The nucleus tractus solitarius: a portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. Int J Obes. 2009;33((Suppl 1)):S11–S15. [DOI] [PubMed] [Google Scholar]
- 41.Price CJ, Hoyda TD, Ferguson AV. The area postrema: a brain monitor and integrator of systemic autonomic state. Neuroscientist. 2008;14 (2):182–194. [DOI] [PubMed] [Google Scholar]
- 42.Sisley S, Gutierrez-Aguilar R, Scott M, D’Alessio DA, Sandova DA, Seeley RJ. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J Clin Investig. 2014;124(6):2456–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124(10):4473–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green R, Allen LH, Bjorke-Monsen AL, et al. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017;3:17040. [DOI] [PubMed] [Google Scholar]
- 45.Richards P, Parker HE, Adriaenssens AE, et al. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes. 2014;63(4):1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varin EM, Mulvihill EE, Baggio LL, et al. Distinct neural sites of GLP-1R expression mediate physiological versus pharmacological control of incretin action. Cell Rep. 2019;27(11):3371–3384. [DOI] [PubMed] [Google Scholar]
- 47.Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740–756. [DOI] [PubMed] [Google Scholar]
- 48.Washington MC, Raboin SJ, Thompson W, Larsen CJ, Sayegh AI. Exenatide reduces food intake and activates the enteric nervous system of the gastrointestinal tract and the dorsal vagal complex of the hindbrain in the rat by a GLP-1 receptor. Brain Res. 2010;1344:124–133. [DOI] [PubMed] [Google Scholar]
- 49.Gao W, Jusko WJ. Pharmacokinetic and pharmacodynamic modeling of exendin-4 in type 2 diabetic Goto-Kakizaki rats. J Pharmacol Exp Ther. 2011;336(3):881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perez-Tilve D, Gonzalez-Matias L, Aulinger BA, et al. Exendin-4 increases blood glucose levels acutely in rats by activation of the sym-pathetic nervous system. Am J Physiol Endocrinol Metab. 2010;298(5): E1088–E1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lildballe DL, Mutti E, Birn H, Nexo E. Maximal load of the vitamin B12 transport system: a study on mice treated for four weeks with high-dose vitamin B12 or cobinamide. PLoS One. 2012;7(10): e46657. [DOI] [PMC free article] [PubMed] [Google Scholar]


