Summary
In the era of targeted therapy and immunotherapy, the objective of dose finding is often to identify the optimal biological dose (OBD), rather than the maximum tolerated dose (MTD). We develop a utility-based Bayesian optimal interval (U-BOIN) phase I/II design to find the OBD. We jointly model toxicity and efficacy using a multinomial-Dirichlet model, and employ a utility function to measure dose risk-benefit trade-off. The U-BOIN design consists of two seamlessly connected stages. In stage I, the Bayesian optimal interval (BOIN) design is used to quickly explore the dose space and collect preliminary toxicity and efficacy data. In stage II, in light of accumulating efficacy and toxicity from both stages I and II, we continuously update the posterior estimate of the utility for each dose after each cohort, and use this information to direct the dose assignment and selection. Compared to existing phase I/II designs, one prominent advantage of the U-BOIN design is its simplicity for implementation. Once the trial is designed, it can be easily applied using predetermined decision tables, without complex model fitting and estimation. Our simulation study shows that, despite its simplicity, the U-BOIN design is robust and has high accuracy to identify the OBD. We extend the design to accommodate delayed efficacy by leveraging the short-term endpoint (e.g., immune activity or other biological activity of targeted agents), and using it to predict the delayed efficacy outcome to facilitate real-time decision making. A user-friendly software to implement the U-BOIN is freely available at www.trialdesign.org.
Keywords: Phase I/II, utility-based design, optimal biological dose, targeted therapy, immunotherapy
1 |. INTRODUCTION
Immunotherapy and targeted therapies have revolutionized cancer treatment. Unlike conventional chemotherapy, immunotherapy drugs do not target the tumor directly. Instead, they work by reactivating the immune system and, hence, reestablishing its capacity to combat tumors. A major class of immunotherapy drugs are monoclonal antibodies, known as immune checkpoint inhibitors (ICI) (e.g., nivolumab, pembrolizumab, and ipilimumab). Checkpoint proteins are receptors on immune cells that can be activated to block immune response, for example checkpoint proteins on T cells (e.g., PD-1 and CTLA-4). The ICI bind to the checkpoint receptors on T cells and release “brake” such that T cells can kill cancer cells, achieving treatment efficacy. Nivolumab and pembrolizumab are PD-1 inhibitors, and ipilimumab is CTLA-4 inhibitor.
Traditional dose-finding designs developed for chemotherapy aim to find the maximum tolerated dose (MTD). The underlying assumption is that both toxicity and efficacy monotonically increase with the dose and, thus, the MTD presents the most efficacious dose that is safe. This assumption, however, is often questionable for immunotherapy and targeted agents. Although it is reasonable to assume that toxicity increases with the dose, the same is not necessarily true for efficacy. For example, once the checkpoint binding is saturated, further incrementing the ICI dose does not increase treatment efficacy. For some monoclonal antibodies, it is observed that higher doses actually lead to lower efficacy.1 In addition, while increasing the dose of immunotherapy agents (or targeted biological agents) may improve efficacy, it still may cause substantial toxicity, due to over-activation of the immune system. When the overall benefit is limited, the value of increasing the dose becomes questionable.
To optimize the treatment benefit of immunotherapy and targeted therapy, therefore, it is important to consider toxicity and efficacy simultaneously and their risk-benefit trade-off during dose finding. The objective of dose finding for targeted therapy and immunotherapy is to identify the optimal biological dose (OBD), defined as the dose that has the highest desirability in terms of the risk-benefit trade-off. Our research is motivated by a phase I/II trial to identify the OBD of a novel humanized antiTROP2 monoclonal antibody in patients with an advanced solid tumor. Trophoblast cell-surface antigen 2 (TROP2) is associated with increased tumor growth. It is overexpressed in the majority of human epithelial cancers, including esophageal, breast, and lung cancers.2,3 Five doses will be studied in the trial. Toxicity will be graded according to the NCI-CTCAE version 4.03, with a 21-day assessment window using the scale of 5 grades. The dose limiting toxicity (DLT) is defined as toxicity with grade 3 or higher. Tumor response will be evaluated using RECIST version 1.1, scored as complete remission (CR), partial remission (PR), stable disease (SD) and progressive disease (PD). Response is defined as CR/PR. TROP2 expression will be measured on day 8, after dosing, to provide a biomarker for measuring the biological activity of the drug.
Here, we develop a utility-based seamless phase I/II design to find the OBD. We jointly model toxicity and efficacy using a multinomial-Dirichlet model and employ a utility function to measure dose risk-benefit trade-off. We show that the utility approach is flexible and more general, in that it contains the existing marginal toxicity-efficacy trade-off methods as special cases. The design consists of two seamlessly connected stages. In stage I, the Bayesian optimal interval (BOIN) design4 is used to quickly explore the dose space and collect preliminary toxicity and efficacy data. In stage II, in light of accumulating efficacy and toxicity from both stages I and II, we continuously update the posterior estimate of the utility for each dose after each cohort, and use this information to direct the dose assignment and selection. We refer to the resulting design as the utility-based BOIN (U-BOIN) design. To accommodate the delayed efficacy observed in some targeted and immunotherapy trials, we extend the U-BOIN design and use the short-term endpoint (e.g., immune activity or other biological activity of targeted agents) to predict the delayed efficacy outcome to facilitate real-time decision making.
Numerous phase I/II trial designs have been proposed. Thall and Russell5 developed a Bayesian phase I/II design that characterizes patient outcomes using a trinary variable that accounts for both toxicity and efficacy. Braun6 generalized the continual reassessment method (CRM)7 to accommodate toxicity and efficacy simultaneously. Thall and Cook8 presented the EffTox design, based on the trade-offs between toxicity and efficacy. Yin et al.9 proposed a phase I/II design that uses the odds ratio of the efficacy and toxicity as a measure of desirability. Yuan and Yin10 described a phase I/II design that jointly models toxicity and efficacy as time-to-event outcomes. Jin et al.11 proposed a phase I/II design that accommodates late-onset toxicity and efficacy. Liu and Johnson12 proposed a robust Bayesian phase I/II design, based on a flexible Bayesian dynamic model. Guo and Yuan13 proposed a personalized Bayesian phase I/II design that accounts for patient characteristics and biomarker information. Zang et al.14 proposed several practical phase I/II trial designs to find the OBD. Liu et al.15 proposed a Bayesian phase I/II trial design for immunotherapy that considers the immune response, toxicity, and efficacy. Yuan et al.16 provided a comprehensive coverage of phase I/II designs.
Compared to existing phase I/II designs, the proposed U-BOIN design has several strengths. First, the U-BOIN is simple and easy to implement. It is a model-assisted design in that it uses a model for efficient decision making, but its decision rule for dose escalation/de-escalation can be tabulated and included in the trial protocol before the trial starts.17,18,19 Once the trial is designed, it can be easily implemented using the predetermined decision table. No complicated computation or model estimation is needed. In contrast, most existing phase I/II designs are model-based designs, and require complicated model fitting and estimation after treating each cohort. Second, the U-BOIN is robust, because it models toxicity and efficacy at each dose independently, without imposing any parametric structure on dose-toxicity and dose-efficacy curves. In contrast, most existing designs assume that the dose-toxicity and dose-efficacy curves follow certain parametric forms (e.g., logistic model), and thus are susceptible to the influence of model misspecification.
Third, most existing phase I/II designs use the trade-off in the marginal toxicity and efficacy probabilities of a dose to measure the desirability of the dose, while the U-BOIN design uses the utility, which is not only easier to elicit from physicians, but also more flexible and general. We prove that the trade-off based on marginal toxicity and efficacy probabilities is a special case of the utility approach. Although utility has been used in previous designs,20,21,22 to the best of our knowledge, this paper is the first to formally show that the utility approach contains the trade-off based on marginal toxicity and efficacy probabilities as a special case. This provides a theoretical justification for using the utility approach. Lastly, the U-BOIN is capable of accommodating delayed efficacy, whereas most existing designs assume that the efficacy endpoint is quickly observed.
The remainder of this article proceeds as follows. Section 2 introduces the statistical model, utility function, dose-finding algorithm, and software to implement the U-BOIN design. Section 3 uses simulation to compare the U-BOIN design with an existing phase I/II design, and also assesses the robustness of the design using sensitivity analysis. Section 4 provides a brief summary.
2 |. METHODS
2.1 |. Efficacy-toxicity model
Consider a phase I/II trial with J doses under investigation. Let YE = 0,⋯,R − 1 denote the categorical efficacy endpoint with R levels, where a higher level represents a more desirable treatment response; and YT = 0,⋯,Q − 1 denote the categorical toxicity endpoint with Q levels, where a higher level represents a more severe toxicity. The bivariate outcomes (YE, YT) can be equivalently represented by a single variable Y with K = R×Q levels, where each level of Y maps to a distinct value of (YE, YT).
As an example, consider the conventional setting that both YE and YT are binary. Let YE = 1 denote response, 0 otherwise. Similarly, let YT = 1 denote DLT, 0 otherwise. Then, Y has K = 4 levels, with Y = 1, if (YE, YT) = (0, 1); Y = 2, if (YE, YT) = (0, 0); Y = 3, if (YE, YT) = (1, 1); and Y = 4, if (YE, YT) = (1, 0). The value of Y ascribed to each possible (YE, YT) is not critical, as long as we keep track of the mapping. Without loss of generality, we assume that Y = 1 denotes the least favorable clinical outcomes, and Y = K denotes the most favorable clinical outcomes.
Define and , with , where d denotes the dose level. We assume that Y follows a Dirichlet-multinomial model as follows:
| (1) |
| (2) |
where a1,...,aK > 0 are hyper-parameters. We set , such that the prior is vague and equivalent to a prior sample size of 1.
At an interim decision time, assume that nj patients have been treated at dose d = j, among which njk patients had outcome Y = k, where . Given the observed interim data Dj = (nj1,⋯,njk), the posterior distribution of is
| (3) |
2.2 |. Utility
We measure the desirability of the investigational doses using utility. Let ψk denote the utility ascribed to outcome Y = k, k = 1,⋯,K. The utility ψk should be elicited from physicians to reflect the risk-benefit trade-off underlying their medical decisions, which can be done using the following procedure:
Fix the value of the utility for the least desirable outcome Y = 1 as ψ1 = 0, and for the most desirable outcome Y = K as ψK = 100. For example, for binary YE and YT, the least desirable outcome is (YE = 0, YT = 1), i.e., (no response, DLT), and the most desirable outcome is (YE = 1, YT = 0), i.e., (response, no DLT).
Ask the clinician to use these two utilities as a reference to score the utility values ψ2,⋯,ψK−1 for the other K − 2 possible outcomes Y = 2,⋯,K − 1 to quantify the risk-benefit trade-off under each outcome.
Table 1 shows three examples of the utility function. The first two examples consider the scenario where both toxicity and efficacy are binary outcomes. Example 1 has utility values {ψ1 = 0, ψ2 = 30, ψ3 = 50, ψ4 = 100} for the outcomes {(YE = 0, YT = 1), (YE = 0, YT = 0), (YE = 1, YT = 1), (YE = 1, YT = 0)}. Compared to example 1, example 2 rewards the response (i.e., YE = 1) more, in the presence of DLT (i.e., YT = 1), by assigning a larger value to ψ3 (65 versus 50). This is appropriate for the trial where toxicity can be well managed and response is highly desirable (e.g., leading to long survival). Example 3 shows the case where YT has three levels (i.e., minor, moderate, and severe toxicity), and YE has also three levels (i.e., PD, SD, and CR/PR).
TABLE 1.
Examples of utility
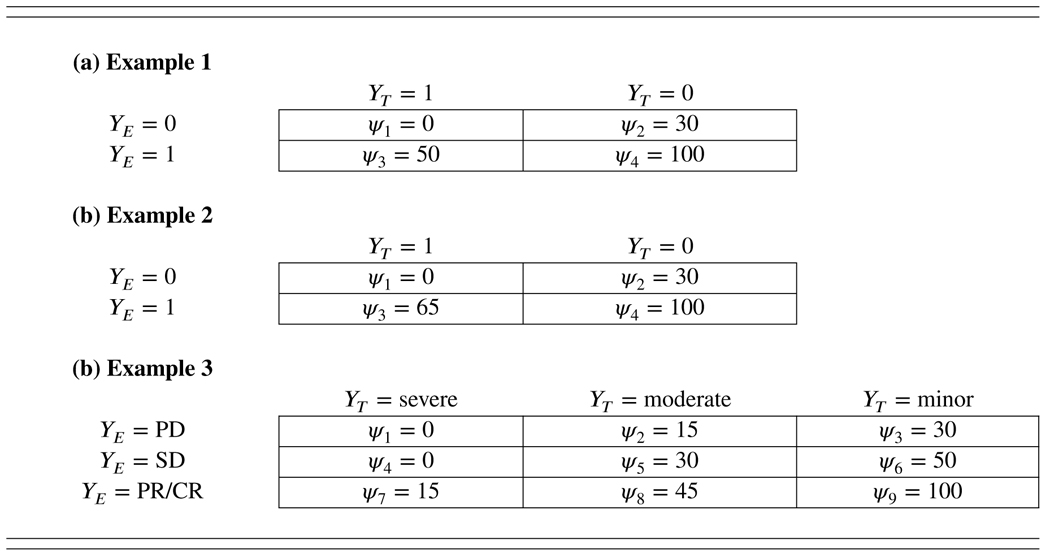 |
Note. PD: partial disease, SD: stable disease, PR: partial remission, CR: complete remission.
In our experience, clinicians quickly understand what the utilities mean and provide values for ψk’s, since the values reflect the actual clinical practice. After completing this process, simulation should be performed to verify the operating characteristics of the design. In some cases, the simulation results may motivate slight modification of some of the numerical utility values, although such modification typically has little or no effect on the design’s operating characteristics. One possible criticism for using the utility values is that they require subjective input. However, we are inclined to view this as a strength, rather than a weakness. The process of specifying the utility requires clinicians to carefully consider the potential risks and benefits of the treatment that underlie their clinical decision making in a more formal way and incorporate that into the trial. In addition, our simulation study and previous studies13,15,21 show that the design is generally not sensitive to the numerical values of the utility, as long as it reflects a similar trend.
Given the values of ψk, the true mean utility for dose j is given by
| (4) |
Since the true mean utility Uj depends on πjk, which is unknown, we need to estimate it, based on the observed data. Given the interim data D = {Dj}, the estimate of mean utility is given by
| (5) |
For the conventional setting with binary YT an YE, another common approach to defining the efficacy-toxicity trade-off is directly based on the marginal efficacy probability πE,j = Pr(YE = 1|d = j) and marginal toxicity probability πT,j = Pr(YT = 1|d = j),16 which can be expressed as
| (6) |
where ω is a prespecified weight. This trade-off function says that patients are willing to trade an increase of ω in the DLT rate for a unit increase in the efficacy rate. If ω = 0, we obtain the special case that the dose with the highest efficacy is the most desirable. The following theorem shows that this marginal-probability-based approach is a special case of the utility approach described above. The proof is provided in the Appendix.
Theorem 1.
Marginal-probability-based trade-off , defined in (6), is a special case of the utility method defined in (4), in the sense that for a pre-specified weight ω, we can find (ψ2, ψ3), such that , where ξ is a non-zero constant.
In the Supplementary Material, we provide an example to show how to map Uj with by choosing appropriate values of (ψ2, ψ3).
Liu and Johnson12 proposed another marginal-probability-based trade-off function
| (7) |
where ω1 and ω2 is a prespecified weight, I(·) is an indicator function, and ρ is a prespecified toxicity threshold deemed of substantial concern. Compared to in (6), this trade-off function is more flexible and allows to impose a higher penalty (i.e., ω1 + ω2) when the true DLT rate πT,j exceeds the threshold ρ. becomes when ω2 = 0. It is not clear if trade-off function can be expressed equivalently in the form of Uj as (4). Nevertheless, the proposed U-BOIN design can be used with different types of utility/trade-off, including . We plan to incorporate this functionality in our software provided later.
2.3 |. Optimal biological dose (OBD)
To define the OBD, we first define the admissible dose to safeguard patients from toxic or futile doses. As the objective here is to rule out toxic or futile doses, it is more natural to start with the definition of an inadmissible dose. Let denote the maximum tolerable DLT rate, and the lowest acceptable response rate. We define that dose j is inadmissible, if it meets either one or both of the following two criteria:
| (8) |
| (9) |
where CE and CT are probability cutoffs. In general, CT = 0.95 and CE = 0.9 work well, but should be calibrated using simulation to ensure desirable operating characteristics. This can be done easily using the software provided.
The admissible dose is then defined as the dose for which none of the criteria (8) and (9) is satisfied. When YT has more than 2 categories (e.g., grade 0–2, grade 3, and grade 4–5), for the purpose of defining the admissible dose, YT can be temporarily collapsed into DLT/no DLT (i.e., DLT = grade 3 and higher). This dichotomization simplifies the definition of the admissible dose and is often adequate to safeguard patients from overly toxic doses. While it is not necessary, more complicated safety criteria could be entertained to accommodate multiple categories. Similar dichotomization (i.e., response/no response) is also applied to YE, when it has more than two categories. Note that although we dichotomize YT and YE for defining the admissible dose, our model and utility are still based on their original scales. We define the OBD as the dose that is admissible and has the highest utility value, i.e.,
where denotes the set of admissible doses.
2.4 |. Phase I/II OBD finding algorithm
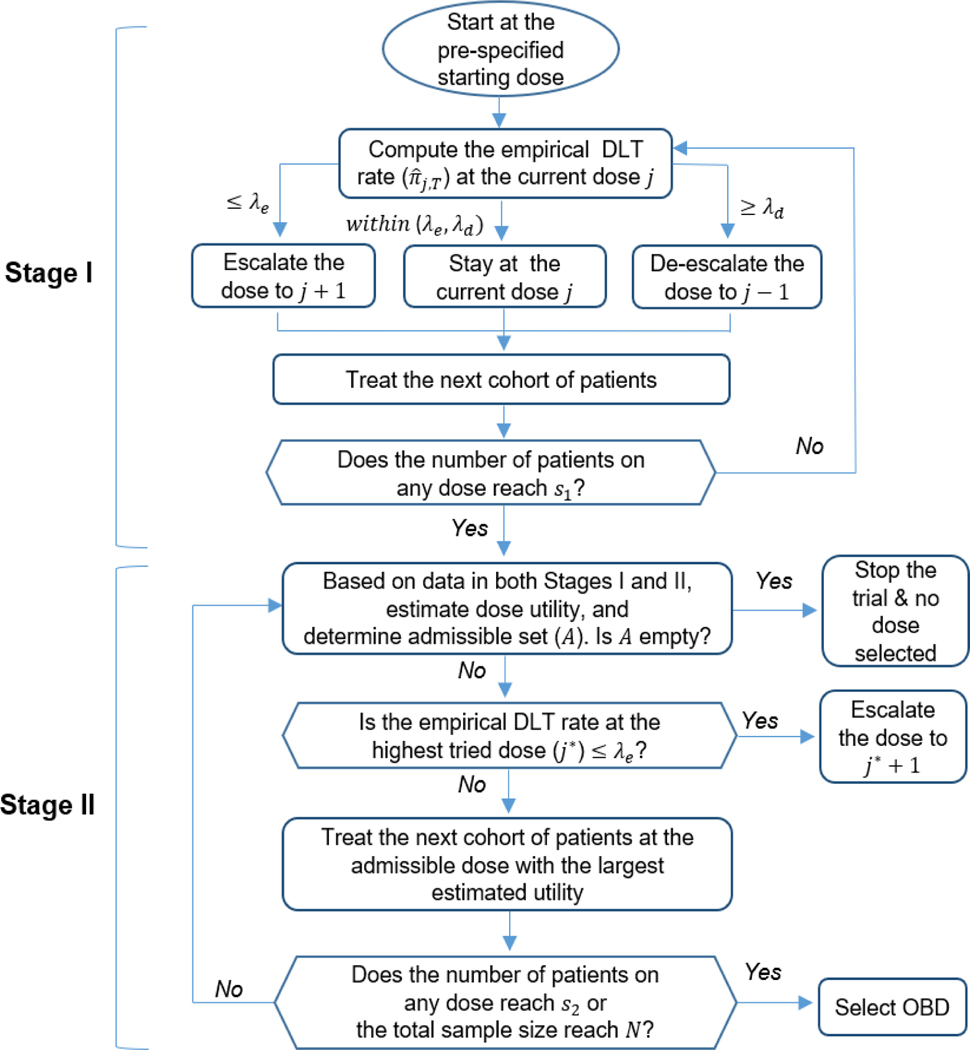
The U-BOIN design consists of two seamless, connected stages (Figure 1). The objective of stage I is to quickly explore the dose space to identify a set of admissible doses that are reasonably efficacious and safe for stage II. In stage I, we conduct dose escalation based on only the toxicity outcome, but efficacy data are also collected and will be used for decision making in stage II. Given the exploratory nature of stage I, if YT has more than two categories, we dichotomize it as DLT/no-DLT to facilitate the exploration of the dose space. This is in line with the clinical practice and serves well for the purpose of stage I. Note that for the estimation and finding the OBD (i.e., the primary objective of the trial), we retain the original scale of YT.
FIGURE 1.
Diagram of the U-BOIN design
Stage I dose escalation/de-escalation is guided by the BOIN design,4 which has been widely used in variety of oncology trials, including solid tumors,23,24 liquid tumors,25,26 and various treatment agents.27,28,29,30 Due to very limited data and large uncertainty, for patient safety, we set the target DLT rate , slightly lower than the maximum tolerable DLT rate , to ensure that stage I dose exploration concentrates around up to, but not exceeding, . Let denote the empirical (or maximum likelihood) estimate of πj,T, given by , where is the number of patients who experienced DLT at the dose level j; and λe and λd denote the predetermined optimal escalation boundary and de-escalation boundary. Table 2 provides the values of λe and λd for the commonly used target DLT rate ϕT, see Liu and Yuan4 for the derivation and formula to calculate λe and λd. The dose-finding algorithm in stage I proceeds as follows.
TABLE 2.
Dose escalation and de-escalation boundaries of the BOIN design
| Boundaries | Target DLT rate (ϕT) |
|||||
|---|---|---|---|---|---|---|
| 0.15 | 0.20 | 0.25 | 0.30 | 0.35 | 0.40 | |
| λe (escalation) | 0.118 | 0.157 | 0.197 | 0.236 | 0.276 | 0.316 |
| λd (de-escalation) | 0.179 | 0.238 | 0.298 | 0.358 | 0.419 | 0.480 |
| A1 | Patients in the first cohort are treated at dose level 1 or a prespecified starting dose. |
| A2 | Suppose j is the current dose, use the following rules to assign a dose to the next cohort of patients. |
Escalate the dose to j + 1 if .
De-escalate the dose to j − 1 if .
Otherwise, stay at the current dose j.
| A3 | Repeat step A2 until the number of patients treated on one of the doses reaches s1, and then move to stage II. We recommend s1 = 12 as the default value, while s1 = 9 to 15 generally yields good operating characteristics. |
Stage II proceeds as follows:
| B1 | Let j* denote the highest dose level that has been tried. If and j* is not the highest dose in the trial, escalate the dose to dj*+1 for treating the next cohort of patients; otherwise, proceed to step B2. |
| B2 | Given the observed interim data D collected in both stages I and II, determine the admissible dose set . If no dose is admissible, terminate the trial and no dose should be selected as the OBD. Otherwise, assign the next cohort of patients to the admissible dose (i.e., ∈ ) that has the largest posterior mean utility, which can be pre-tabulated. |
| B3 | Repeat steps B1 and B2 until reaching the prespecified maximum sample size N or the number of patients treated at one of the doses reaches s2 (> s1), and then select the OBD as the admissible dose (i.e., ∈ ) that has the largest posterior mean utility. For most trials, a value between 18 to 24 is a reasonable choice for s2. |
In stage I, following the BOIN design, we impose an overdose control rule as follows: if and nj ≥ 3, dose level j and higher are eliminated from the trial; the trial is terminated if the lowest dose level is eliminated, where is evaluated based on a beta-binomial model with the uniform prior. Once the trial move to stage II, this overdose control rule is seamlessly merged as the inadmissible rule (8). In the overdose control rule, we use , rather than ϕT, as the DLT rate threshold to ensure that the overdose control rule seamlessly connects with the inadmissible rule.
For stage II step B1, the reason that we perform dose escalation when is to allow the trial to continue exploring the dose space, given that the highest tried dose is safe, to reduce the risk of being stuck at a local suboptimal dose, due to a large variation caused by a small sample size. Besides the pick-the-winner (PW) approach (i.e., deterministically assigning the next cohort of patients to dose j ∈ that has the largest posterior mean utility), other strategies can also be used to assign patients. For example, we can adaptively randomize the next cohort of patients to dose j ∈ , with probability ωj proportional to its posterior mean utility, i.e.,
| (10) |
The adaptive randomization (AR) approach can reduce the risk of being stuck at a suboptimal dose, but as a tradeoff, it tends to treat fewer patients at the OBD. Another approach is equal randomization (ER), where the next cohort of patients are assigned to the admissible doses with equal probability, i.e.,
| (11) |
We compare the performance of PW, AR, and ER in our simulation study. None of the methods dominates the others. Thus, we generally recommend the PW approach because of its simplicity.
Unlike most existing phase I/II designs, which require complicated model fitting and estimation after each cohort to make the decision of dose assignment, one prominent advantage of the U-BOIN is that its dose assignment rules can be pre-tabulated in decision tables and included in the trial protocol before the trial starts. To conduct the trial, no complicated calculation is needed. The investigator can simply use the decision tables to determine dose assignment, e.g., determine whether escalation/de-escalation is needed (steps A2 and B1) or identify admissible doses (step B2). Because of this feature, the U-BOIN can be classified as a model-assisted design.17,18,19 Section 1 in the Supplementary Material provides an example of using the decision tables in a hypothetical phase I/II trial.
2.5 |. Delayed efficacy response
In some trials, efficacy endpoint YE requires a long time to be ascertained. In our motivating example, it takes three months to evaluate YE. The long assessment window causes a major logistics issue for decision making in stage II. For example, given that the accrual rate is three patients per month, and that patients are treated in cohorts of three, on average, six new patients will be accrued while waiting to evaluate the previous three patients’ outcomes. This begs the question: how can new patients receive timely treatment, when the previous patients’ outcomes are not yet observed?
Statistically, this means that D are not fully observed, as some YE’s are unavailable. As a result, the mean utility estimate (5) and the inadmissible criterion (9) cannot be evaluated for making interim decisions. This issue is known as the late-onset or delayed-outcome issue, which has been studied in literature. Cheung and Chappell31 proposed a weighting method to handle late-onset toxicity for phase I clinical trials. Yuan and Yin10 modeled toxicity and efficacy as time-to-event outcomes that accommodate the unobserved as censored events. Liu et al.32 and Jin et al.11 treated unobserved outcomes as missing data, and proposed a Bayesian data augmentation approach to predict unobserved toxicity and/or efficacy outcomes to facilitate decision making. Cai et al.33 took the multiple imputation approach to handle unobserved efficacy outcomes in phase II trials.
We follow the approach of Cai et al.33 and use multiple imputation to handle unobserved YE. The innovation here is that we use the measure of biological activity, which is routinely recorded in targeted and immunotherapy trials, as an ancillary variable to help predict (or impute) YE. Examples of the measure of biological activity include immune response (e.g., CD8+ T cell count) in immunotherapy trials and gene expression related to the pathway targeted by the treatment agent. The measure of biological activity is often quickly observable after drug administration and correlated with the clinical response. Daud et al.34 showed that the abundance of CD8+ T cells predict response to anti-PD1 therapy, and advocated using the immune activity to predict the likelihood of achieving a clinical response to the PD-1 pathway inhibitor. For ease of exposition, hereafter we use the immune response, denoted by YI, as the example to illustrate our approach, but YI can be any reasonable biological activity measure predictive of treatment efficacy. We assume that YI is quickly observable, thus its value is always available at the time of decision making.
Given the observed value of YI, we predict the unobserved YE, based on the following scaled logistic regression model:
| (12) |
where πE,j is the probability of efficacy for dose j, β0 and β1 are regression parameters, and 0 < λ ≤ 1 is a plateau (or scale) parameter used to reflect that the probability of clinical response often levels out after the immune activity reaches a certain level. Under this model, the probability of efficacy πE,j increases with YI, and then plateaus at the value of λ when YI is sufficiently large. When λ = 1, it becomes a standard logistic regression model. We do not include dose level j in the model, because the treatment effect of immunotherapy is mediated by the immune response, and thus it is often reasonable to assume that, conditional on YI, πE,j is independent of j. If this assumption is not plausible in some situations, one can simply add j as a covariate to the model. In addition, more biomarkers, when available, can be added to model (12) as predictors to improve the prediction accuracy. One attractive property of our imputation approach is that, when the imputation model (12) is misspecified, its impact on the design diminishes over time and eventually goes away when the trial is completed. This is because, as the trial proceeds, more and more patients’ YE become observed, and accordingly fewer and fewer percentage of YE needs prediction. As a result, our method is generally robust to model misspecification, as shown later in the sensitivity analysis.
In terms of prior specifications in model (12), following Gelman et al.,35 we assume that the model parameters (λ, β0, β1) are independent and have their own prior distributions. We specify a uniform distribution for the scale parameter . The magnitude of the coefficients (β0 and β1) could be very large or small, depending on different trials. This makes it difficult to specify standard prior distributions for the coefficients. To tackle this problem, we first standardize the variable YI. In our application, YI is continuous; we standardize it to have a mean of 0 and a standard deviation of 0.5. We regulate the prior distributions to make sure that a typical change in a covariate should not lead to a dramatic change in the efficacy rate.
After standardizing data, a change of 2.5 on the logit scale can move the probability of a favorable response to the therapy from 0.2 to 0.75. We assume that the effect of the immune response is unlikely to be more dramatic than that. This typically is true for immunotherapy trials, as the efficacy rate is rarely outside of that range. We assign a normal distribution with a mean of 0 and a standard deviation 1.25 for β0: β0 ~ N(0, 1.252). The parameter β1 is supposed to be positive, due to the positive relationship between immune response and tumor response, and thus we assign a gamma distribution with a shape parameter of 1 and a rate parameter of 1.2: . The priors ensure that a change in the covariate YI from one standard deviation below the mean to one standard deviation above the mean will lead to an absolute change that is mostly less than 2.5 on the logit scale.
Let f(β0, β1, λ) denote the joint prior distribution of (β0, β1, λ). The posterior distribution of (β0, β1, λ) is given by
| (13) |
where is the immune response for the ith patient treated with dose j and yE,ji is the tumor response for this patient.
At an interim decision time, let and denote the observed and missing parts of YE. We impute the value of YE,mis using multiple imputations as follows.
Conditional on observed data , sample L draws of (λ, β0, β1) from their posterior distribution (13) using the adaptive rejection Metropolis sampling.36 A certain number of burn-in iterations are typically needed before collecting the posterior draws. In our simulation, we set the number of burn-in iterations as L′ = L/2.
Thin the posterior draws by taking a sample after every L′/H draw, resulting in a total of H sets of posterior draws of (λ, β0, β1), denoted as .
Impute YE,mis for H times, where the hth imputed value is generated by drawing a random sample from Bernoulli(q), where h = 1,…,H and . We denoted the H sets of imputed values as .
After filling in YE,mis with each of the H imputed values, we obtained an H imputed complete dataset, D(1),⋯,D(H), where D(h) is obtained by filling in YE,mis with , h = 1,⋯, H. Then, the estimate of mean utility (5) is given by
| (14) |
and the left side of the inadmissible criterion (9) is calculated as
| (15) |
The dose finding follows the same algorithm described in section 2.4. Little and Rubin37 suggested that, for practical use, the number of multiple imputation (H) can be sufficient when H = 5 or greater. In our simulation, we perform H = 20 imputations.
To further improve the efficiency of the design, YI can also be used to refine the admissible rule. The rationale is that when YE takes a long time to be observed, the inadmissible criterion (9) is not effective for screening out ineffective doses, because a high percentage of YE may not be observed at the time of making interim decisions. Since YI is quickly observable, it can be used, supplementary to YE, to improve the power of identifying effective doses or safeguard patients from ineffective doses. Specifically, define , and let denote the lowest acceptable mean immune response. We define dose j inadmissible if it meets at least one of the toxicity and futility criteria in (8) and (9), and
| (16) |
where CI is a pre-specified probability threshold. In other words, if a dose has little activity to activate immune system, it is deemed unpromising and inadmissible. Again, the admissible dose is defined as the dose that is not inadmissible. The posterior probability can be evaluated based on the Bayesian normal model:
| (17) |
2.6 |. Software and trial implementation
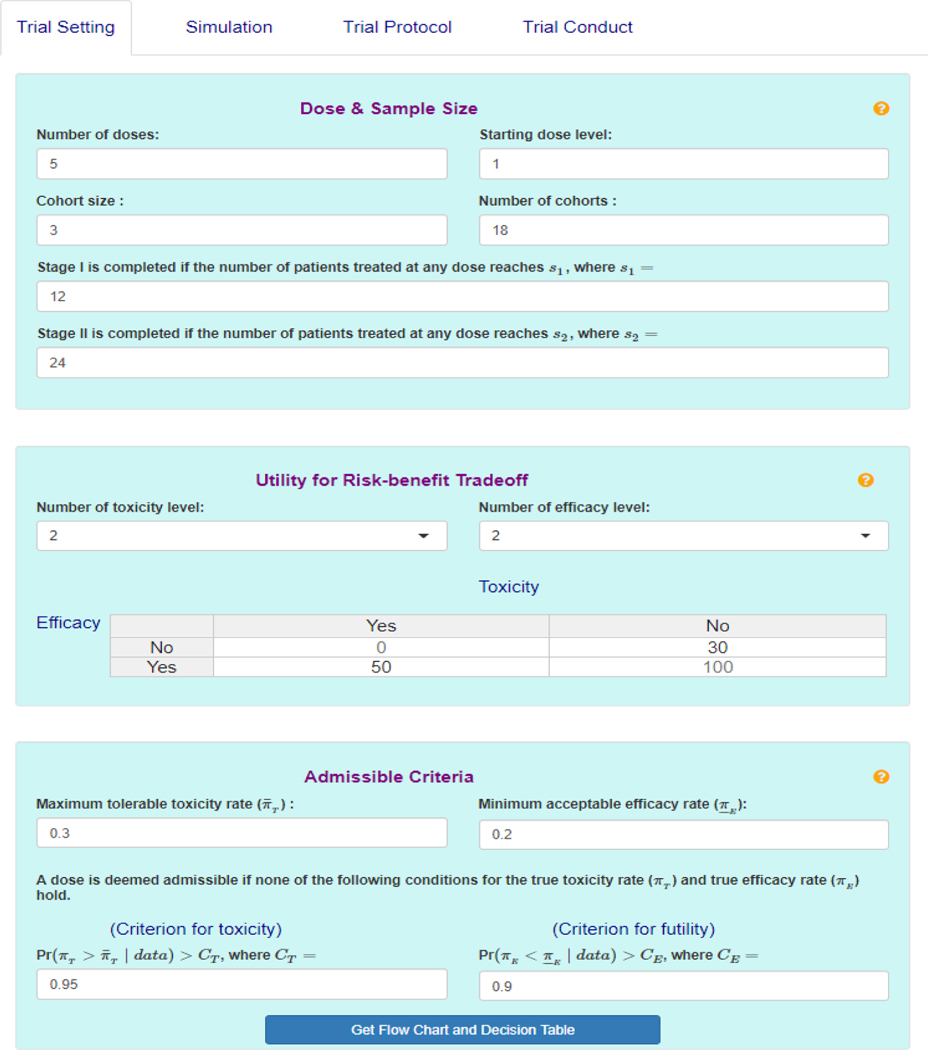
To facilitate the use of the U-BOIN design, we develop an easy-to-use web app, which is freely available at http://www.trialdesign.org. Figure 2 shows the graphical user interface of the app. Extensive help files are available by clicking on the yellow question mark at the upper right corner of each tab.
FIGURE 2.
The user interface of the U-BOIN software
To design a trial, follow the steps:
Specify the design parameters (e.g., dose information, sample size, cohort size, utility function, admissible criteria, etc.), under the Trial Setting tab. After parameters are entered, design diagram and decisions tables will be provided under the tab.
Generate the operating characteristics of the design by supplying different scenarios (under the Simulation tab).
Download trial protocol under Trial Protocol tab. The protocol includes (1) a brief description of the U-BOIN design, (2) decision tables based on input under Trial Setting, and (3) operating characteristics for the scenarios entered under Simulation.
To conduct a trial, use the decision tables generated under Trial Setting or use the Trial Conduct tab. The app will return the dose for treating the next cohort of patients if the data provided indicates that the trial is not completed, and it will select the OBD if the trial is completed.
3 |. NUMERICAL STUDY
3.1 |. Simulation A
Simulation A considers the case that YT and YE are quickly ascertainable. Following our motivating trial, we consider J = 5 doses, and the total sample size N = 54 patients with s1 = 12. The minimum acceptable efficacy rate is = 0.2, and the maximum acceptable DLT rate is = 0.30. We set by using δ = 0.05. For the inadmissible criteria (i.e., equations (8) and (9)), probability cutoffs are set as CT = 0.95 and CE = 0.9, based on simulation calibration. The elicited utility is presented in Table 1 as example 1.
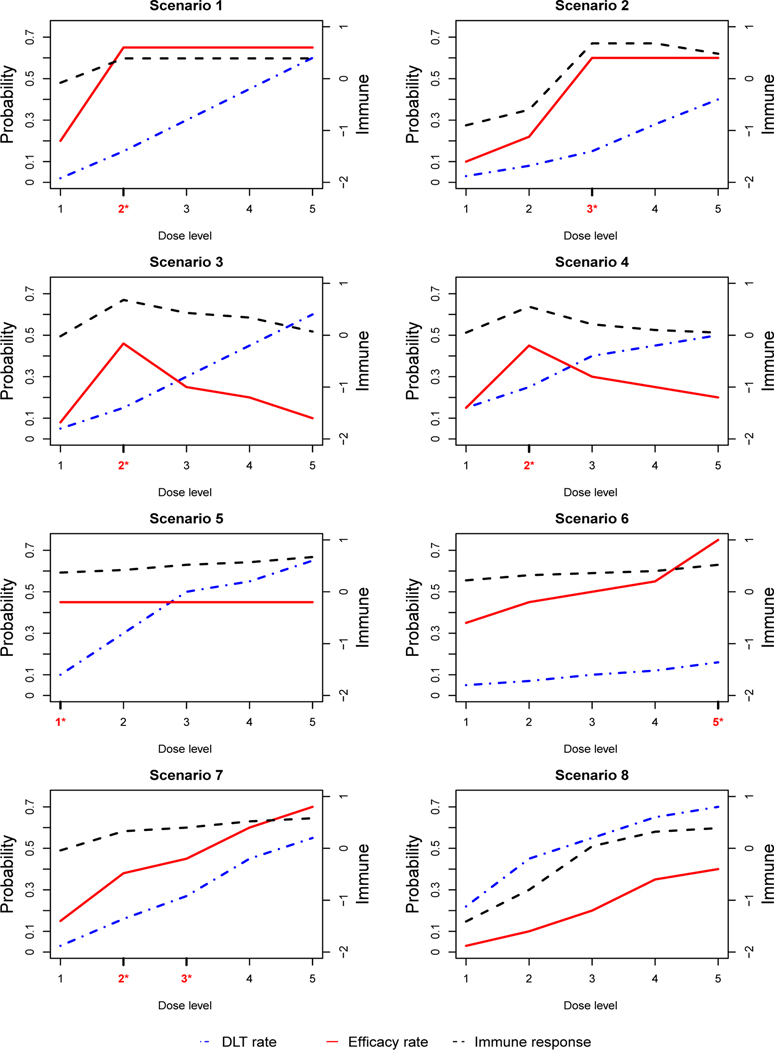
We consider 8 representative scenarios that differ in the shape of the dose-toxicity and dose-efficacy curves, and the location of the OBD. The scenarios are shown in Figure 3. In scenarios 1 and 2, the dose-response curve increases with the dose level, and then plateaus. In scenarios 3 and 4, the dose-response curve increases to an optimal point, and then decreases. In scenario 5, the dose-response curve levels off at the first dose level. In scenarios 6, 7, and 8, all dose-response curves monotonically increase. Scenario 6 has the last dose level being the OBD. Scenario 7 has two OBDs, located at dose level 2 and 3. Scenario 8 has no OBD since the doses are either futile or overly toxic. Under each scenario, we generate (YT, YE), based on a Gumbel model:
| (18) |
where yE, yT ∈ {0,1}, and the association parameter c was set as 0.2. The values of πE,j and πT,j (i.e., the marginal efficacy rate and DLT rate) for each dose level are provided in Table 3. We compare the proposed U-BOIN design with the EffTox design.8 We consider the two most important metrics for comparison: (1) percentage of correct selection (PCS), which is the probability of correctly identifying the OBD, and (2) patient allocation, which referred to the average number of patients assigned to each dose. In the EffTox design, the toxicity-efficacy trade-off is constructed based on three equally desirable trade-off target probabilities: (πE = 0.2, πT = 0), (πE = 1, πT = 0.7), and (πE = 0.45, πT = 0.3). Under each scenario, we performed 2,000 simulations.
FIGURE 3.
Simulation scenarios. The dash-dotted line (blue) is the dose-toxicity curve, the solid line (red) is the dose-efficacy curve, and the dashed line (black) is the dose-immune response curve. The OBD is highlighted by red asterisk in the x-axis. Simulation A considers only the efficacy and toxicity curves, while simulation B considers efficacy, toxicity, and immune response.
TABLE 3.
Results of Simulation A, including the selection percentage (selection %), the average number of patients treated at each dose (No. of patients), and the percentage of early stopping. The optimal biological dose (OBD) is bolded. In scenario 8, the OBD does not exist, and thus the percentage of early stopping is bolded.
| Design | Dose Level |
% of early stopping | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Scenario 1 | |||||||
| DLT rate | 0.02 | 0.15 | 0.30 | 0.45 | 0.60 | ||
| Efficacy rate | 0.20 | 0.65 | 0.65 | 0.65 | 0.65 | ||
| Utility | 43.0 | 69.0 | 63.0 | 56.0 | 50.0 | ||
| EffTox | Selection % | 2.0 | 50.0 | 45.0 | 2.0 | 0.0 | 0.0 |
| No. of patients | 4.3 | 22.7 | 23.8 | 2.8 | 0.4 | ||
| U-BOIN | Selection % | 1.7 | 72.9 | 22.4 | 2.8 | 0.0 | 0.2 |
| No. of patients | 6.2 | 29.9 | 13.8 | 3.5 | 0.5 | ||
| Scenario 2 | |||||||
| DLT rate | 0.03 | 0.08 | 0.15 | 0.28 | 0.40 | ||
| Efficacy rate | 0.10 | 0.22 | 0.60 | 0.60 | 0.60 | ||
| Utility | 36.0 | 43.0 | 66.0 | 60.0 | 55.0 | ||
| EffTox | Selection % | 0.0 | 4.0 | 60.0 | 29.0 | 7.0 | 0.0 |
| No. of patients | 3.4 | 4.9 | 26.4 | 14.2 | 5.1 | ||
| U-BOIN | Selection % | 1.1 | 3.2 | 65.7 | 24.9 | 4.3 | 0.8 |
| No. of patients | 4.9 | 7.5 | 24.4 | 12.7 | 4.3 | ||
| Scenario 3 | |||||||
| DLT rate | 0.05 | 0.15 | 0.30 | 0.45 | 0.60 | ||
| Efficacy rate | 0.08 | 0.46 | 0.25 | 0.20 | 0.10 | ||
| Utility | 34.0 | 56.0 | 37.0 | 29.0 | 18.0 | ||
| EffTox | Selection % | 12.0 | 70.0 | 10.0 | 1.0 | 0.0 | 7.0 |
| No. of patients | 10.8 | 25.5 | 11.5 | 2.9 | 1.0 | ||
| U-BOIN | Selection % | 1.2 | 92.2 | 4.0 | 0.4 | 0.0 | 2.1 |
| No. of patients | 6.6 | 34.7 | 8.9 | 2.6 | 0.4 | ||
| Scenario 4 | |||||||
| DLT rate | 0.15 | 0.25 | 0.40 | 0.45 | 0.50 | ||
| Efficacy rate | 0.15 | 0.45 | 0.30 | 0.25 | 0.20 | ||
| Utility | 36.0 | 52.0 | 36.0 | 32.0 | 27.0 | ||
| EffTox | Selection % | 36.0 | 47.0 | 5.0 | 2.0 | 2.0 | 9.0 |
| No. of patients | 19.4 | 19.0 | 7.5 | 2.6 | 2.3 | ||
| U-BOIN | Selection % | 11.9 | 74.1 | 3.7 | 0.4 | 0.0 | 9.9 |
| No. of patients | 15.0 | 29.5 | 5.1 | 1.1 | 0.2 | ||
| Scenario 5 | |||||||
| DLT rate | 0.10 | 0.30 | 0.50 | 0.55 | 0.65 | ||
| Efficacy rate | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | ||
| Utility | 58.0 | 50.0 | 42.0 | 40.0 | 36.0 | ||
| EffTox | Selection % | 69.0 | 27.0 | 2.0 | 0.0 | 0.0 | 2.0 |
| No. of patients | 29.7 | 17.3 | 4.9 | 1.0 | 0.3 | ||
| U-BOIN | Selection % | 75.4 | 22.8 | 1.5 | 0.2 | 0.0 | 0.2 |
| No. of patients | 33.1 | 16.5 | 3.7 | 0.5 | 0.1 | ||
| Scenario 6 | |||||||
| DLT rate | 0.05 | 0.07 | 0.10 | 0.12 | 0.16 | ||
| Efficacy rate | 0.35 | 0.45 | 0.50 | 0.55 | 0.75 | ||
| Utility | 53.0 | 59.0 | 61.0 | 64.0 | 75.0 | ||
| EffTox | Selection % | 10.0 | 12.0 | 26.0 | 24.0 | 29.0 | 0.0 |
| No. of patients | 8.6 | 7.9 | 14.2 | 11.1 | 12.1 | ||
| U-BOIN | Selection % | 5.9 | 11.7 | 13.1 | 13.6 | 55.7 | 0.0 |
| No. of patients | 7.0 | 8.8 | 9.0 | 9.1 | 20.1 | ||
| Scenario 7 | |||||||
| DLT rate | 0.03 | 0.16 | 0.27 | 0.45 | 0.55 | ||
| Efficacy rate | 0.15 | 0.38 | 0.45 | 0.60 | 0.70 | ||
| Utility | 40.0 | 51.0 | 51.0 | 53.0 | 55.0 | ||
| EffTox | Selection % | 1.0 | 33.0 | 54.0 | 9.0 | 1.0 | 2.0 |
| No. of patients | 4.2 | 13.8 | 25.9 | 7.0 | 2.3 | ||
| U-BOIN | Selection % | 2.0 | 45.0 | 41.0 | 10.0 | 1.0 | 1.0 |
| No. of patients | 5.1 | 20.4 | 20.0 | 6.7 | 1.2 | ||
| Scenario 8 | |||||||
| DLT rate | 0.22 | 0.45 | 0.55 | 0.65 | 0.70 | ||
| Efficacy rate | 0.03 | 0.10 | 0.20 | 0.35 | 0.40 | ||
| Utility | 25.0 | 23.0 | 25.0 | 30.0 | 31.0 | ||
| EffTox | Selection % | 1.0 | 6.0 | 6.0 | 0.0 | 0.0 | 87.0 |
| No. of patients | 4.6 | 6.2 | 6.3 | 2.3 | 1.2 | ||
| U-BOIN | Selection % | 0.8 | 5.5 | 1.7 | 0.0 | 0.0 | 92.0 |
| No. of patients | 14.3 | 9.7 | 1.6 | 0.1 | 0.0 | ||
Table 3 summarizes the operating characteristics for the designs. The U-BOIN design outperforms EffTox as it has a larger PCS and allocates more patients on the OBD. In scenarios 1 and 2, the dose-response curve increases first, and then plateaus, and the OBDs (dose level 2 and 3, respectively) are one dose level lower than the MTD (i.e., dose level with DLT rate closest to the target DLT rate). The U-BOIN has a 23% higher PCS and assigns seven more patients on the OBD than EffTox does in scenario 1. Similarly, the U-BOIN has a larger PCS in scenario 2, for which the two designs have comparable patient allocation. In scenarios 3 and 4 where the dose-response curve increases to an optimal point and then decreases, U-BOIN has 22% and 27% higher PCS than EffTox, respectively. Moreover, U-BOIN assigns nine and ten more patients on the OBD, respectively in the two scenarios. In scenario 5 where the OBD is located on the first dose, and the response rate does not change with dose levels, U-BOIN has a 6% higher PCS and assigns three more patients on the OBD. In scenario 6, where all doses are safe and the OBD is the last dose level, the U-BOIN has a 27% higher PCS and assigns eight more patients on the OBD. This is because EffTox cannot distinguish well the suboptimal doses from the OBD in this scenario, subsequently assigning more patients on dose level 3 and 4 and selecting one of the dose levels as the OBD 55% of the time. U-BOIN has comparable performance to EffTox when there are two OBDs (scenario 7), in terms of PCS and patient allocation. When there is no OBD due to toxicity or futility (scenario 8), U-BOIN has a larger chance to stop the trial early.
3.2 |. Simulation B
Simulation B considers the case that YT is quickly ascertainable, but YE takes a long time to be scored with the assessment window of 3 months. We assume that patient accrual follows a Poisson process, with the rate of 3 patients per month. We simulated YT and YE based on the same Gumbel model and 8 scenarios, as described in Simulation A. The reason we chose the same simulation scenarios is that, by doing so, the results from Simulation A (i.e., YE is always observed) can be used as a benchmark to evaluate the performance of the design in Simulation B (i.e., YE is partially observed, due to delayed response). To generate a delayed response, for patients who experience efficacy in the assessment window (i.e., YE = 1), we simulate their time to efficacy from a truncated Weibull distribution with a support of (0, 3) months. The shape and scale parameter for the Weibull distribution are chosen such that the efficacy rate at the end of assessment time matches those in Table 3, and that 90% of the responses occur in the latter half of the assessment window (i.e., (1.5, 3) months). The immune response YI is generated from N(μI,j, 1), where μI,j is plotted in Figure 3. We set CI = 0.9. Under each scenario, 2,000 simulations are performed.
Table 4 shows the simulation results. The PCS of the OBD and the number of patients allocated to the OBD are generally comparable to the results in Simulation A (i.e., the optimal benchmark with fully observed data) and U-BOIN still outperforms the EffTox design. The results indicate that U-BOIN efficiently handles the delayed efficacy response. Because the U-BOIN design does not need to suspend accrual to wait YE to be fully observed and allows real-time decision making, it has great potential to shorten the trial duration.
TABLE 4.
Results of Simulation B, including the selection percentage (selection %), the average number of patients treated at each dose (No. of patients), the percentage of early stopping, and the trial duration. The optimal biological dose (OBD) is bolded. In scenario 8, the OBD does not exist, and thus the percentage of early stopping is boldeded.
| Design | Dose Level |
% of early stopping | Duration (month) | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||
| Scenario 1 | ||||||||
| EffTox | Selection % | 2.0 | 50.0 | 45.0 | 2.0 | 0.0 | 0.0 | 45.9 |
| No. of patients | 4.3 | 22.7 | 23.8 | 2.8 | 0.4 | |||
| U-BOIN | Selection % | 0.9 | 68.9 | 24.1 | 2.9 | 0.3 | 2.8 | 20.8 |
| No. of patients | 6.4 | 29.2 | 13.7 | 3.6 | 0.5 | |||
| Scenario 2 | ||||||||
| EffTox | Selection % | 0.0 | 4.0 | 60.0 | 29.0 | 7.0 | 0.0 | 43.8 |
| No. of patients | 3.4 | 4.9 | 26.4 | 14.2 | 5.1 | |||
| U-BOIN | Selection % | 0.0 | 0.2 | 64.1 | 22.4 | 4.9 | 8.3 | 20.0 |
| No. of patients | 4.3 | 6.1 | 24.5 | 12.1 | 4.1 | |||
| Scenario 3 | ||||||||
| EffTox | Selection % | 12.0 | 70.0 | 10.0 | 1.0 | 0.0 | 7.0 | 45.6 |
| No. of patients | 10.8 | 25.5 | 11.5 | 2.9 | 1.0 | |||
| U-BOIN | Selection % | 0.8 | 89.6 | 6.0 | 0.8 | 0.0 | 2.8 | 20.7 |
| No. of patients | 6.7 | 33.6 | 9.5 | 2.9 | 0.4 | 2.8 | ||
| Scenario 4 | ||||||||
| EffTox | Selection % | 36.0 | 47.0 | 5.0 | 2.0 | 2.0 | 9.0 | 47.8 |
| No. of patients | 19.4 | 19.0 | 7.5 | 2.6 | 2.3 | |||
| U-BOIN | Selection % | 6.8 | 70.4 | 3.4 | 0.4 | 0.0 | 19.1 | 18.8 |
| No. of patients | 13.6 | 27.9 | 4.7 | 1.0 | 0.2 | 19.1 | ||
| Scenario 5 | ||||||||
| EffTox | Selection % | 69.0 | 27.0 | 2.0 | 0.0 | 0.0 | 2.0 | 50.0 |
| No. of patients | 29.7 | 17.3 | 4.9 | 1.0 | 0.3 | |||
| U-BOIN | Selection % | 72.9 | 22.7 | 2.2 | 0.2 | 0.0 | 2.0 | 20.7 |
| No. of patients | 32.9 | 16.4 | 3.7 | 0.5 | 0.0 | 2.0 | ||
| Scenario 6 | ||||||||
| EffTox | Selection % | 10.0 | 12.0 | 26.0 | 24.0 | 29.0 | 0.0 | 43.4 |
| No. of patients | 8.6 | 7.9 | 14.2 | 11.1 | 12.1 | |||
| U-BOIN | Selection % | 4.2 | 10.3 | 12.2 | 13.6 | 59.2 | 0.4 | 21.0 |
| No. of patients | 6.5 | 8.6 | 9.5 | 9.9 | 19.4 | 0.4 | ||
| Scenario 7 | ||||||||
| EffTox | Selection % | 33.0 | 45.0 | 45.0 | 43.0 | 40.0 | 46.5 | |
| No. of patients | 1.0 | 33.0 | 54.0 | 9.0 | 1.0 | 2.0 | ||
| U-BOIN | Selection % | 5.0 | 45.6 | 33.1 | 10.5 | 1.7 | 4.1 | 20.7 |
| No. of patients | 8.5 | 22.7 | 15.6 | 5.2 | 0.8 | 4.1 | ||
| Scenario 8 | ||||||||
| EffTox | Selection % | 1.0 | 6.0 | 6.0 | 0.0 | 0.0 | 87.00 | 16.6 |
| No. of patients | 4.7 | 6.1 | 6.3 | 2.4 | 1.3 | |||
| U-BOIN | Selection % | 0.0 | 0.4 | 0.4 | 0.0 | 0.0 | 99.10 | 8.8 |
To examine the performance of U-BOIN when there are delayed outcomes at a relatively smaller sample size, we conduct the simulation again using N = 39. Results show that U-BOIN still maintains its great operating characteristics, even when sample size is relatively small. The simulation results are provided in Table S8 of the Supplementary Material. We also provide operating characteristics for eight additional representative scenarios (Scenarios A1-A8) for both Simulation A (Table S9) and Simulation B (Table S10). The results, again, show that U-BOIN has robust performance in various scenarios in comparison to EffTox.
3.3 |. Sensitivity analysis
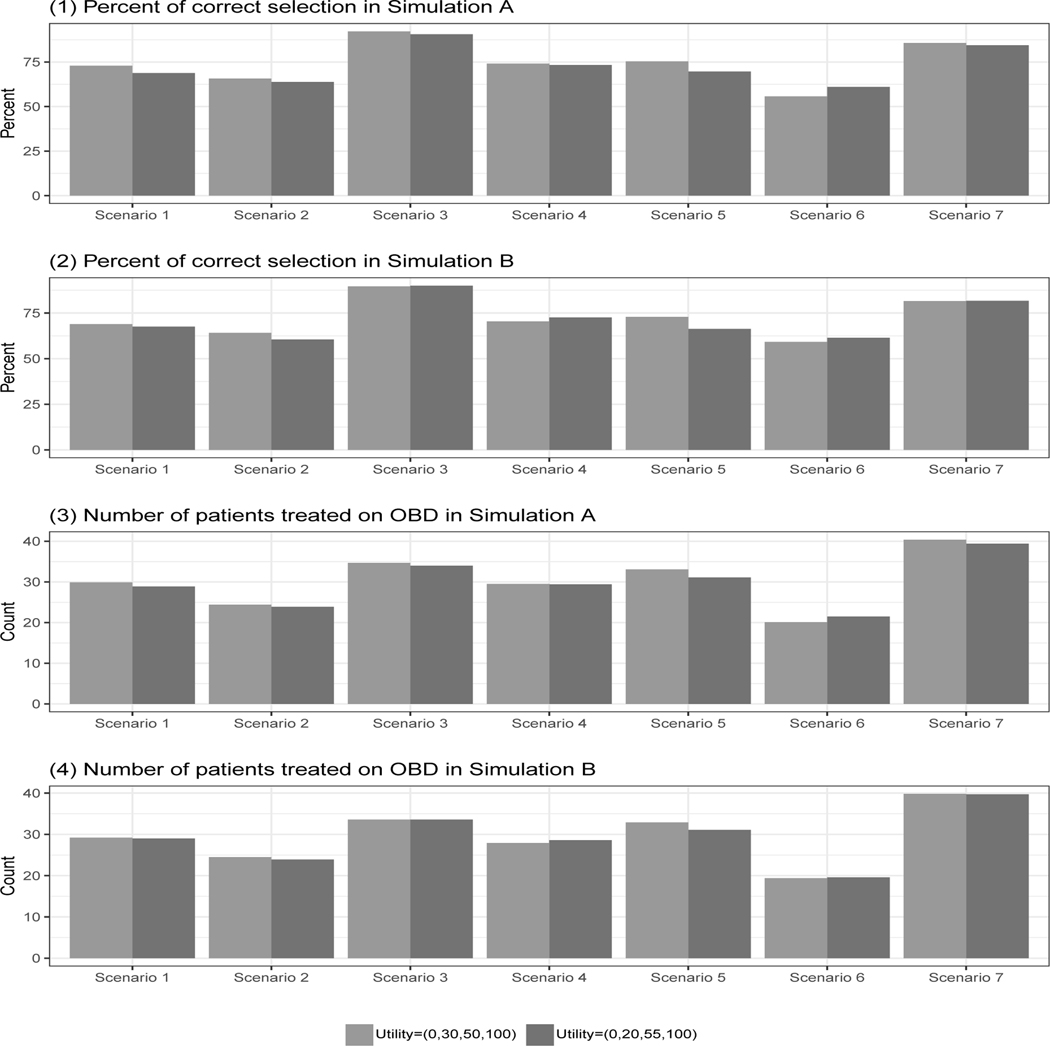
We conduct sensitivity analyses to assess the robustness of the U-BOIN design by using a different set of utility values Ψ′ = {ψ1 = 0, ψ2 = 20, ψ3 = 55, ψ4 = 100}, which assigns a lower score for (YE = 0, YT = 1) and a higher score for (YE = 1, YT = 1), indicating that patients are willing to tolerate a higher toxicity risk in order to attain a higher efficacy. We also evaluate the performance of U-BOIN using different patient allocation strategies (i.e., step B2 of Stage II of the dose finding algorithm): PW, AR, and ER.
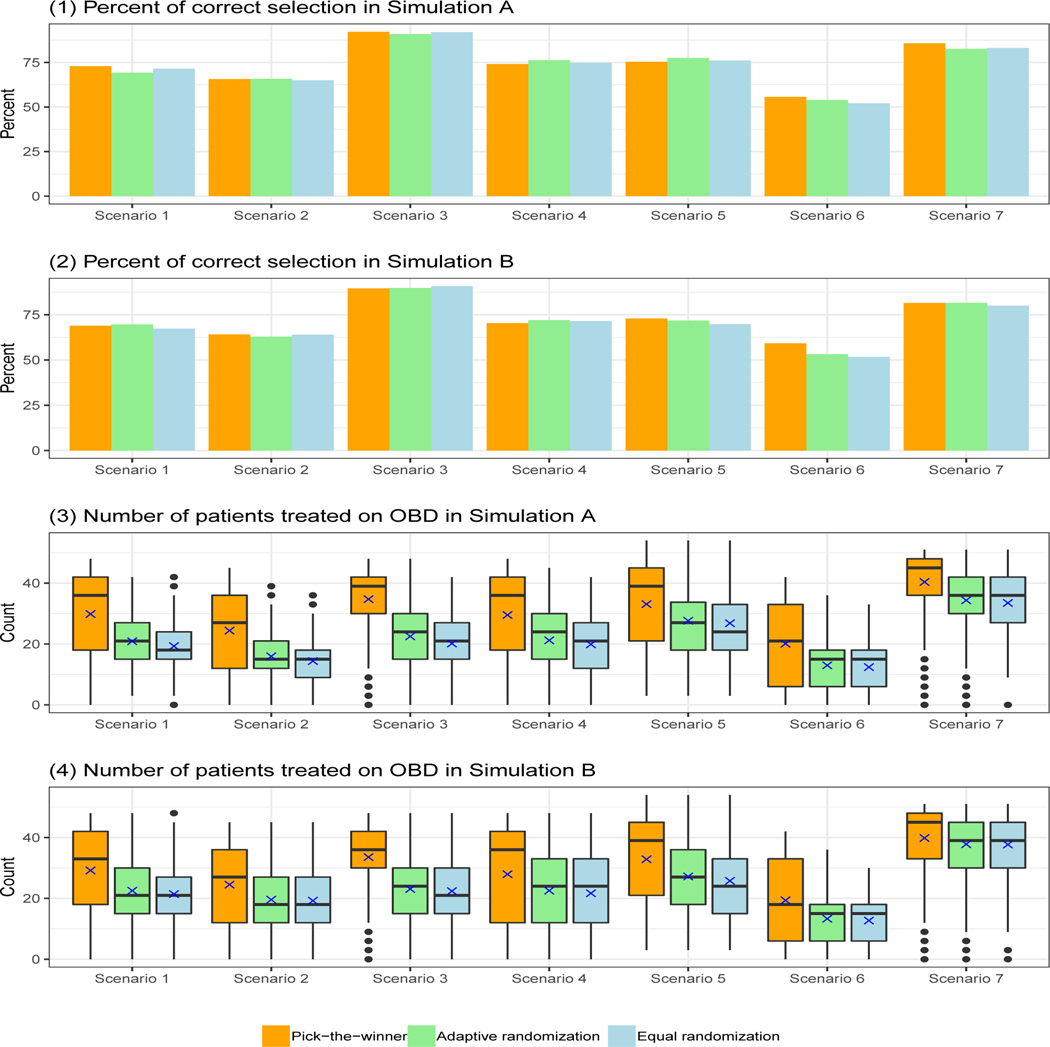
The simulation results (see Figure 4) show that the U-BOIN design performs well with comparable PCS and patient allocations for the two different utilities. This characteristic is important, since two clinicians might have slightly different opinions about the specific utility value assigned to an outcome combination. Our design shows robustness for this type of deviation. Figure 5 shows the results under different patient allocation strategies. The PCS is comparable among the PW, AR, and ER strategies. The difference among the three strategies mainly lies in the number of patients treated on OBD. The PW approach, on average, assigns significantly more patients on the OBD than both the ER and AR approaches, while it has larger variability. Since the variation is more towards the higher end (i.e., more patients are treated on OBD), the PW approach is desirable in this simulation study.
FIGURE 4.
Results of sensitivity analysis for different utilities. Scenario 8 is not included, as the OBD does not exist in that scenario.
FIGURE 5.
Results of sensitivity analysis for different patient allocation strategies: pick-the-winner (PW), adaptive randomization (AR), and equal randomization (ER). Scenario 8 is not included, as the OBD does not exist in that scenario.
Note that in this Simulation A, we specify s2 = N for fair comparison with the EffTox design, which does not stop the trial early on the basis of the number of patients treated on a dose. A sensitivity analysis using s2 = {18, 21} is provided in Section 5 of the Supplementary Material. The result in Figure S1 shows that using the recommended values, the change in the percentage of correct selection is negligible, but the saving in sample size is substantial.
For the case with delayed YE, we performed three additional sensitivity analyses by (1) assuming that the efficacy assessment window is two months or four months; (2) specifying more vague prior distributions for the coefficients β0 and β1 with β0 ~ N(0, 3.752) and β1 ~ Gamma(shape = 1, rate = 0.4), both of which have standard deviations that are three times the previous values in section 2.5; and (3) considering two additional scenarios (scenarios B1 and B2 in Table S11), where YI and YE are weakly associated with Pearson’s correlation coefficients −0.15 and 0.2, respectively. The results show that the U-BOIN design is also robust to the length of assessment window, prior distribution of the prediction model (Figure S2), and the association between YI and YE.
4 |. SUMMARY
We propose the U-BOIN, a seamless phase I/II model-assisted design, to identify the OBD for targeted and immunotherapy trials. The U-BOIN design accounts for the efficacy-toxicity trade-off using a utility function. Unlike most existing phase I/II designs, which require complicated real-time model fitting and estimation to make dose assignment decisions, the U-BOIN is simple and easy to implement. The dose assignment rules of the U-BOIN can be pre-tabulated in decision tables and included in the trial protocol before onset of the trial. To conduct the trial, no complicated calculation is needed. The investigator can simply use the decision tables to make the decision of dose escalation/de-escalation. Simulation studies show that compared to a more complicated model-based phase I/II design, the U-BOIN has higher accuracy to identify OBD and is more robust. To facilitate the use of the U-BOIN design in clinical trials, we develop a user-friendly software freely available at www.trialdesign.org.
While this article focuses on immunotherapy and targeted trials, U-BOIN also can be used for conventional cytotoxic agent trials. In such cases, both toxicity and efficacy typically increase with the dose, but may do so at different rates. It is likely that increasing the dose causes much higher toxicity, with limited efficacy gain. The idea of risk-benefit tradeoff, and thus finding the optimal dose, is still generally applicable here for use in most medical decisions in practice.
As most model-assisted designs, U-BOIN models efficacy and toxicity at each dose independently, whereas model-based phase I-II designs (e.g., EffTox design) model efficacy and toxicity across all doses, through imposing a parametric dose-efficacy and -toxicity curve model. As a result, one may worry about the potential efficiency loss for U-BOIN. Our numerical study and previous studies show that, for the purpose of dose finding, the efficiency loss caused by using only local data (in model-assisted designs, such as U-BOIN) is minimal or negligible. This can be explained as follows. First, although U-BOIN models only the local data at the current dose, its dose-finding algorithm (e.g., escalate the dose if the current dose is safe, and de-escalate the dose if the current dose is toxic) implicitly uses the dose-toxicity order information across the doses. In addition, in practice, the parametric dose-efficacy and -toxicity model assumed by model-based designs is more likely to be misspecified than correctly specified. Thus, on average, borrowing information across doses through the parametric model leads to rather limited efficiency gain. Furthermore, such limited efficiency gain does not necessarily translate into performance gain. This is because, to make correct decisions of dose assignment and selection, we only need to correctly estimate the rank of utility across the doses. A slightly more variability on the estimate of the utility has no or negligible impact on the performance of the design.
Supplementary Material
APPENDIX
A PROOF
Theorem 1.
Marginal-probability-based trade-off , defined in (6), is a special case of the utility method defined in (4), in the sense that for a pre-specified weight ω, we can find (ψ2, ψ3), such that , where ξ is a non-zero constant.
Proof
If there is a constant ξ, such that , then, is a special case of .
To fix scale for utility, we propose in this paper that ψ1 = 0,ψ4 = 100. Then is a function of only ψ2 and ψ3.
It is obvious that by definition. Denote . If , then , and . Since , we have , which is nonzero constant.
In summary, for a pre-specified ω and , we can find (ψ2, ψ3) and a non-zero constant ξ, such that , which says that is a special case of Uj.
References
- 1.Reynolds AR. Potential relevance of bell-shaped and u-shaped dose-responses for the therapeutic targeting of angiogenesis in cancer. Dose-response 2010; 8(3): dose–response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakashima K, Shimada H, Ochiai T, et al. Serological identification of TROP2 by recombinant cDNA expression cloning using sera of patients with esophageal squamous cell carcinoma. International journal of cancer 2004; 112(6): 1029–1035. [DOI] [PubMed] [Google Scholar]
- 3.Ni IBP, Zakaria Z, Muhammad R, et al. Gene expression patterns distinguish breast carcinomas from normal breast tissues: the Malaysian context. Pathology-Research and Practice 2010; 206(4): 223–228. [DOI] [PubMed] [Google Scholar]
- 4.Liu S, Yuan Y. Bayesian optimal interval designs for phase I clinical trials. Journal of the Royal Statistical Society: Series C (Applied Statistics) 2015; 64(3): 507–523. [Google Scholar]
- 5.Thall PF, Russell KE. A strategy for dose-finding and safety monitoring based on efficacy and adverse outcomes in phase I/II clinical trials. Biometrics 1998: 251–264. [PubMed] [Google Scholar]
- 6.Braun TM. The bivariate continual reassessment method: extending the CRM to phase I trials of two competing outcomes. Controlled clinical trials 2002; 23(3): 240–256. [DOI] [PubMed] [Google Scholar]
- 7.O’Quigley J, Pepe M, Fisher L. Continual reassessment method: a practical design for phase 1 clinical trials in cancer. Biometrics 1990: 33–48. [PubMed] [Google Scholar]
- 8.Thall PF, Cook JD. Dose-finding based on efficacy–toxicity trade-offs. Biometrics 2004; 60(3): 684–693. [DOI] [PubMed] [Google Scholar]
- 9.Yin G, Li Y, Ji Y. Bayesian dose-finding in phase I/II clinical trials using toxicity and efficacy odds ratios. Biometrics 2006; 62(3): 777–787. [DOI] [PubMed] [Google Scholar]
- 10.Yuan Y, Yin G. Bayesian dose finding by jointly modelling toxicity and efficacy as time-to-event outcomes. Journal of the Royal Statistical Society: Series C (Applied Statistics) 2009; 58(5): 719–736. [Google Scholar]
- 11.Jin IH, Liu S, Thall PF, Yuan Y. Using data augmentation to facilitate conduct of phase I–II clinical trials with delayed outcomes. Journal of the American Statistical Association 2014; 109(506): 525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, Johnson VE. A robust Bayesian dose-finding design for phase I/II clinical trials. Biostatistics 2016; 17(2): 249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo B, Yuan Y. Bayesian phase I/II biomarker-based dose finding for precision medicine with molecularly targeted agents. Journal of the American Statistical Association 2017; 112(518): 508–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zang Y, Lee JJ, Yuan Y. Adaptive designs for identifying optimal biological dose for molecularly targeted agents. Clinical Trials 2014; 11(3): 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu S, Guo B, Yuan Y. A Bayesian phase I/II trial design for immunotherapy. Journal of the American Statistical Association 2018: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan Y, Nguyen HQ, Thall PF. Bayesian designs for phase I–II clinical trials. Chapman and Hall/CRC. 2016. [Google Scholar]
- 17.Yan F, Mandrekar SJ, Yuan Y. Keyboard: a novel Bayesian toxicity probability interval design for phase I clinical trials. Clinical Cancer Research 2017; 23(15): 3994–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou H, Yuan Y, Nie L. Accuracy, safety, and reliability of novel phase I trial designs. Clinical Cancer Research 2018; 24(18): 4357–4364. [DOI] [PubMed] [Google Scholar]
- 19.Yuan Y, Lee JJ, Hilsenbeck SG. Model-Assisted Designs for Early Phase Clinical Trials: Simplicity Meets Superiority (under revision). Journal of Clinical Oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houede N, Thall PF, Nguyen H, Paoletti X, Kramar A. Utility-based optimization of combination therapy using ordinal toxicity and efficacy in phase I/II trials. Biometrics 2010; 66(2): 532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray TA, Yuan Y, Thall PF, Elizondo JH, Hofstetter WL. A utility-based design for randomized comparative trials with ordinal outcomes and prognostic subgroups. Biometrics 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray TA, Thall PF, Yuan Y, McAvoy S, Gomez DR. Robust treatment comparison based on utilities of semi-competing risks in non-small-cell lung cancer. Journal of the American Statistical Association 2017; 112(517): 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim B A Phase II Study of Triple Combination of Atezolizumab + Cobimetinib + Eribulin (ACE) in Patients With Recurrent/Metastatic Inflammatory Breast Cancer. https://clinicaltrials.gov/ct2/show/NCT03202316. Published February 8, 2019. Accessed March 18, 2019..
- 24.Wu J TG02 Plus Dose-Dense or Metronomic Temozolomide Followed by Randomized Phase II Trial of TG02 Plus Temozolomide Versus Temozolomide Alone in Adults With Recurrent Anaplastic Astrocytoma and Glioblastoma. https://clinicaltrials.gov/ct2/show/NCT02942264. Published January 4, 2019. Accessed March 18, 2019..
- 25.Al-Atrash G. Nivolumab and Ipilimumab After Donor Stem Cell Transplant in Treating Participants With High Risk Refractory or Relapsed Acute Myeloid Leukemia. https://clinicaltrials.gov/ct2/show/NCT03600155. Published January 30, 2019. Accessed March 18, 2019..
- 26.Leonard AJ, Rutherford S. tudy of Venetoclax Plus DA-EPOCH-R for the Treatment of Aggressive B-Cell Lymphomas (V+DA-EPOCH-R).https://clinicaltrials.gov/ct2/show/NCT03036904. Published May 21, 2018. Accessed March 18, 2019.
- 27.Li J The Safety,Efficacy of Anti-EGFR Humanized Monoclonal Antibody Combined With Chemotherapy in Advanced Solid Tumors(HLX07Ib/II). https://clinicaltrials.gov/ct2/show/NCT03577704. September 3, 2018. Accessed March 18, 2019.. [Google Scholar]
- 28.Phan J Trial of Stereotactic HYpofractionateD RadioAblative (HYDRA) Treatment of Laryngeal Cancer. https://clinicaltrials.gov/ct2/show/NCT03114462. Published November 29, 2018. Accessed March 18, 2019.. [Google Scholar]
- 29.Loskog A Phase I/IIa Trial Evaluating Safety of LOAd703, an Armed Oncolytic Adenovirus for Pancreatic Cancer. https://clinicaltrials.gov/ct2/show/NCT02705196. January 8, 2019. Accessed March 18, 2019.. [Google Scholar]
- 30.Jazaeri YC. T Cell Immunotherapy for Advanced Ovarian Cancer. https://clinicaltrials.gov/ct2/show/NCT03318900. January 29, 2019. Accessed March 18, 2019.. [Google Scholar]
- 31.Cheung YK, Chappell R. Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics 2000; 56(4): 1177–1182. [DOI] [PubMed] [Google Scholar]
- 32.Liu S, Yin G, Yuan Y. Bayesian data augmentation dose finding with continual reassessment method and delayed toxicity. The annals of applied statistics 2013; 7(4): 1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai C, Liu S, Yuan Y. A Bayesian design for phase II clinical trials with delayed responses based on multiple imputation. Statistics in medicine 2014; 33(23): 4017–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daud AI, Loo K, Pauli ML, et al. Tumor immune profiling predicts response to anti–PD-1 therapy in human melanoma. The Journal of clinical investigation 2016; 126(9): 3447–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gelman A, Jakulin A, Pittau MG, Su YS, others. A weakly informative default prior distribution for logistic and other regression models. The Annals of Applied Statistics 2008; 2(4): 1360–1383. [Google Scholar]
- 36.Gilks WR, Best N, Tan K. Adaptive rejection Metropolis sampling within Gibbs sampling. Journal of the Royal Statistical Society: Series C (Applied Statistics) 1995; 44(4): 455–472. [Google Scholar]
- 37.Little RJ, Rubin DB. Statistical analysis with missing data. 333. John Wiley & Sons. 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.