Abstract
BACKGROUND:
Diabetic retinopathy (DR) is a serious complication of diabetes that can cause visual impairment. The objective of this study was to estimate the prevalence and identify the determinants of DR in type 2 diabetes mellitus patients attending the diabetic center at Al-Noor Specialist Hospital in Makkah, Saudi Arabia.
MATERIALS AND METHODS:
A cross-sectional study was conducted in a sample of type 2 diabetic patients registered at the diabetic center at Al-Noor Specialist Hospital. Data was collected using a validated self-administered questionnaire. Grading of DR was done by slit-lamp examination and colored fundus photographs. Descriptive analysis included frequency and percentage for categorical variables, and mean, median, standard deviation (SD), and interquartile range, for continuous variables. Chi-square test used to test for association between two categorical variables; Student's t-test or Mann-Whitney U test as appropriate employed to compare continuous variable between two groups. Logistic regression analysis was utilized to identify correlates of DR after controlling for confounders.
RESULTS:
The study comprised 251 type 2 diabetic patients aged between 28 and 80 years, with an arithmetic mean of 56.8 and standard deviation of ±9.9 years. The prevalence of DR was 54.6%; mild nonproliferative (NP) type was present in 52.6% of the patients with DR, whereas severe NP type was present in 15.3% of them; the proliferative type was present in only 4.4% of those with DR. Multivariate logistic regression analysis revealed that patients who had had diabetes for a 11 to 16 years (adjusted odds ratio [AOR] = 3.52, P = 0.035), patients who did not take daily medications on time (AOR = 9.75, P = 0.008), patients who did not go for fundus examination annually (AOR = 3.62, P = 0.011), and patients with uncontrolled diabetes (AOR = 12.18, P < 0.001) were at higher significant risk for DR. Patients not treated with insulin were 70% less likely to develop DR (AOR = 0.30, P = 0.015). An increase of one unit in body mass index was significantly associated with increase in the probability of developing DR by 11% (AOR = 1.11, P = 0.024).
CONCLUSION:
DR is very prevalent in type 2 diabetic patients attending the diabetic center at Al-Noor Specialist Hospital, Makkah Al-Mukarramah; particularly the mild NP type.
Keywords: Diabetic retinopathy, risk factors, type 2 diabetes
Introduction
Diabetes mellitus (DM) is a global chronic disease affecting 366 million people, and this figure is expected to increase to 552 million by 2030.[1] According to the World Health Organization, Saudi Arabia has the second highest number of people with diabetes in the Middle East and 7th highest in the world with around 7 million diabetics and 3 million people who have prediabetes.[2]
Diabetes complications can be divided into three categories: metabolic complications (hyperglycemia/hypoglycemia), damage of the large blood vessels (macrovascular like cardiovascular and cerebrovascular disease), and damage of the small blood vessels (microvascular complications like nephropathy, neuropathy, and retinopathy); all these complications increase the rate of premature deaths.[3]
Diabetic retinopathy (DR) is one of the possible serious complications of diabetes. It can cause visual impairment or blindness, and it is confirmed that within 15 years of onset of diabetes, approximately 2% of patients may be blind, and about 10% may have visual impairments.[4]
The prevalence of DR in different regions of Saudi Arabia including Riyadh was 31%,[5] in Madinah was 36.8%, and in Abha was 36.4%.[6]
Screening for DR is essential for the early detection of the condition because the progression of the disease is rapid, therapy can be beneficial in the early stages, and most patients have no symptoms until very late stages. The screening should be done by an ophthalmologist or trained photographer and reader for retinal photography. According to the American Diabetes Association, patients with type 1 diabetes should have an annual examination for DR beginning 5 years after the onset of their disease, whereas those with type 2 diabetes should have an immediate examination at the time of diagnosis. Routine follow-up should be done every 1–2 years if there is no evidence of retinopathy, but if DR is noticed at any stage, retinal examinations should be repeated frequently depending on the stage of DR.[7]
DR is divided into nonproliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR). NPDR is classified into four stages. The first stage known as “mild NPDR” is characterized by the presence of at least one microaneurysms. The second stage is “moderate NPDR” and characterized by the presence of intraretinal hemorrhage or venous beading or intraretinal microvascular abnormalities (IRMA) but less than severe NPDR. The third stage is “severe NPDR” characterized by the presence of intraretinal hemorrhage in all four quadrants or venous beading in 2 or more quadrants or IRMA in one or more quadrants. PDR is characterized by neovascularization arising from the disc and retinal vessels and can result in vitreous hemorrhage and traction retinal detachment.[8]
The proportion of DR increases with the duration of diabetes, uncontrolled diabetes, and the presence of associated conditions such as hypertension (HTN), dyslipidemia, smoking, nephropathy, and pregnancy.[9]
The aim of this study was to estimate the prevalence and identify the determinants of DR in type 2 DM patients registered in December 2019, in the diabetic center at Al-Noor Specialist Hospital, Makkah Al-Mukarramah, Saudi Arabia.
Materials and Methods
A cross-sectional study was conducted in Makkah Al-Mukarramah, Saudi Arabia. There are two diabetic centers in the government sector in Al-Noor and Hera hospitals in Makkah Al-Mukarramah. Diabetic patients are referred to Al-Noor diabetic center from different hospitals and primary healthcare centers (PHCs) within and outside Makkah Al-Mukarramah.
A total of 860 type 2 diabetic patients attended the fundus examination clinic in the diabetic center in December 2018 according to Al-Noor Diabetic Center statistics. The prevalence of DR reported from a previous study done in Abha (Saudi Arabia) was 36%,[6] a confidence level of 95%, and a tolerable error of 5%. The sample size for this study using the Raosoft statistical program[10] was determined as 251 patients.
The sample size (n = 251) was divided by 20 working days of the month of December 2019; the research included nearly 13 patients in the study each day. There is one fundus examination clinic in Al-Noor Diabetic Center; the researcher followed a systematic random sampling technique to select seven patients from the patient appointment list for the morning clinic and six from the list of appointments for the afternoon clinic.
A self-administered questionnaire in Arabic was designed by the researchers according to guidelines of previous research and used for data collection after its face validation by two diabetes consultants. This included the following parts: sociodemographic and personal data including age, gender, nationality, and place of residence; clinical data including duration of diabetes, compliance with diet, meditation, and annual screening of the fundus, diabetes follow-up in the diabetic center or PHCs and number of visits/year, regular referral to annual screening from PHCs, use of insulin, oral hypoglycemic agents, other comorbidities HTN or dyslipidemia, complications of diabetes, hemoglobin A1c (HbA1c) controlled or not controlled according to ADA criteria:[11]
HbA1c <7% was considered as controlled in a nonpregnant adult and HbA1c <7.5% considered as controlled in healthy older adults, <8% in older adults with complex/intermediate (multiple coexisting chronic illnesses or +2 instrumental activities of daily living [ADL] impairments), and <8.5% considered as controlled in older people with very complex/poor health (long-term care or end-stage chronic illnesses or +2 ADL dependencies), body mass index (BMI)[12] and grading of DR classification[8] into NPDR and PDR, NPDR divided into mild, moderate, severe, and very severe; the diagnosis and grading of DR were done by slit-lamp examination with a Volk 90 D lens and colored fundus photographs using canon cr-2.
Ethical approval was obtained from the Institutional Review Board of the Ministry of Health in the Makkah region vide number H-02-K-076-0719-160 dated 03/11/2019. Informed written consent was taken from all the study participants.
Using SPSS Statistical Program for the Social Sciences version 25 (IBM Corp, Armonk, NY), descriptive statistics were performed using frequency and percentage for categorical variables, mean, median, standard deviation (SD), and interquartile range, depending on the distribution of a continuous variable. Bivariate analysis was performed to identify factors associated significantly with DR, using Chi-square to test for association between two categorical variables, Student's t-test to compare mean of normally distributed variable between two groups, and Mann–Whitney test to compare abnormally distributed variable between two groups. Multivariate logistic regression analysis was utilized to identify predictors of DR after controlling for confounders. P < 0.05 and 95% confidence interval (CI) were considered for statistical significance.
Results
The study comprised 251 type 2 diabetic patients whose ages ranged between 28 and 80 years, with a mean of 56.8 and SD of (±9.9) years. Females represented 61.8% of the participants, the majority (88%) were Saudi nationals living in Makkah (94%) and 76.9% were married. Those who had bachelor's degrees represented 29.5%, whereas 23.5% of the participants were illiterates [Table 1].
Table 1.
Distribution of diabetic retinopathy among patients with diabetes by various demographic and diabetesrelated characteristics, Al-Noor Specialist Hospital, Makkah, Saudi Arabia
| Characteristics | Total N (%) | Diabetic retinopathy | P-value | |
|---|---|---|---|---|
| No (n=114) N (%) | Yes (n=137) N (%) | |||
| Sociodemographic characteristics | ||||
| Gender | ||||
| Male | 96 (38.2) | 34 (35.4) | 62 (64.6) | 0.012 |
| Female | 155 (61.8) | 80 (51.6) | 75 (48.4) | |
| Age (years), mean±SD | 56.8±9.9 | 53.7±9.2 | 59.4±9.7 | <0.001 |
| Nationality | ||||
| Saudi | 221 (88.0) | 103 (46.6) | 118 (53.4) | 0.305 |
| Non-Saudi | 30 (12.0) | 11 (36.7) | 19 (63.3) | |
| Marital status | ||||
| Married | 193 (76.9) | 89 (46.1) | 104 (53.9) | 0.966 |
| Single | 7 (2.8) | 3 (42.9) | 4 (57.1) | |
| Divorced | 15 (6.0) | 7 (46.7) | 8 (53.3) | |
| Widowed | 36 (14.3) | 15 (41.7) | 21 (58.3) | |
| Place of residence | ||||
| Inside Makkah city | 236 (94.0) | 107 (45.3) | 129 (54.7) | 0.920 |
| Outside Makkah city | 15 (6.0) | 7 (46.7) | 8 (53.3) | |
| Level of education | ||||
| Bachelors’ | 74 (29.5) | 43 (58.1) | 31 (41.9) | 0.031 |
| Secondary school | 48 (19.1) | 21 (43.8) | 27 (56.3) | |
| Middle school | 42 (16.7) | 17 (40.5) | 25 (59.5) | |
| Elementary school | 28 (11.2) | 8 (28.6) | 20 (71.4) | |
| Illiterate | 59 (23.5) | 25 (42.4) | 34 (57.6) | |
| Diabetes-related characteristics | ||||
| Duration of diabetes (years) | ||||
| <5 | 56 (22.3) | 40 (71.4) | 16 (28.6) | <0.001 |
| 5-10 | 69 (27.5) | 43 (62.3) | 26 (37.7) | |
| 11-16 | 92 (36.6) | 28 (30.4) | 64 (69.6) | |
| >16 | 34 (13.6) | 3 (8.8) | 31 (91.2) | |
| Controlled HbA1c | 111 (44.2) | 83 (74.8) | 28 (25.2) | <0.001 |
| Uncontrolled HbA1c | 140 (55.8) | 31 (22.1) | 109 (77.9) | |
| Family history of diabetes | ||||
| Yes | 152 (60.6) | 67 (44.1) | 85 (55.9) | 0.597 |
| No | 99 (39.4) | 47 (47.5) | 52 (52.5) | |
| Adherence to diabetic healthy diet | ||||
| Yes | 106 (42.2) | 55 (51.9) | 51 (48.1) | 0.078 |
| No | 145 (57.8) | 59 (40.7) | 86 (59.3) | |
| Taking daily medications on time | ||||
| Yes | 231 (92.0) | 111 (48.1) | 120 (51.9) | 0.003 |
| No | 20 (8.0) | 3 (15.0) | 17 (85.0) | |
| Number of daily antihyperglycemic medications | ||||
| One | 53 (21.1) | 17 (32.1) | 36 (67.9) | 0.006 |
| Two | 109 (43.4) | 61 (56.0) | 48 (44.0) | |
| Three | 60 (23.9) | 28 (46.7) | 32 (53.3) | |
| >three | 29 (11.6) | 8 (27.6) | 21 (72.4) | |
| Using of insulin to treat diabetes | ||||
| Yes | 118 (47.0) | 28 (23.7) | 90 (76.3) | <0.001 |
| No | 133 (53.0) | 86 (64.7) | 47 (35.3) | |
| Frequency of diabetes checkup | ||||
| Every 6 months | 200 (79.7) | 99 (49.5) | 101 (50.5) | 0.004 |
| Every 3 months | 38 (15.1) | 8 (21.1) | 30 (78.9) | |
| <every 3 months | 13 (5.2) | 7 (53.8) | 6 (46.2) | |
| The main center for diabetes follow-up | ||||
| Primary health care | 95 (37.8) | 48 (50.5) | 47 (49.5) | 0.148 |
| Al-Noor diabetic center | 153 (61.0) | 66 (43.1) | 87 (56.9) | |
| Private center | 3 (1.2) | 0 | 3 (100) | |
| The doctor at the center arranges the referral for fundus examination annually | ||||
| Yes | 212 (84.5) | 101 (47.6) | 111 (52.4) | 0.099 |
| No | 39 (15.5) | 13 (33.3) | 26 (66.7) | |
| Annual fundus examination | ||||
| Yes | 178 (71.9) | 95 (53.4) | 83 (46.6) | <0.001 |
| No | 73 (28.1) | 19 (26.0) | 54 (74.0) | |
| Other chronic diseases | ||||
| Hypertension | ||||
| Yes | 160 (63.7) | 63 (39.4) | 97 (60.6) | 0.008 |
| No | 91 (36.3) | 51 (56.0) | 40 (44.0) | |
| Dyslipidemia | ||||
| Yes | 109 (43.4) | 39 (35.8) | 70 (64.2) | 0.007 |
| No | 142 (65.6) | 75 (52.8) | 67 (47.2) | |
| Chronic kidney disease | ||||
| Yes | 32 (12.7) | 5 (15.6) | 27 (84.4) | <0.001 |
| No | 219 (87.3) | 109 (49.8) | 110 (50.2) | |
| Hypothyroidism | ||||
| Yes | 34 (13.5) | 17 (50.0) | 17 (50.0) | 0.564 |
| No | 217 (86.5) | 97 (44.7) | 120 (55.3) | |
| Other diabetic complications | ||||
| Diabetic foot ulcer | ||||
| Yes | 79 (31.5) | 21 (26.6) | 58 (73.4) | <0.001 |
| No | 172 (68.5) | 93 (54.1) | 79 (45.9) | |
| Amputation | ||||
| Yes | 7 (2.8) | 2 (28.6) | 5 (71.4) | 0.306 |
| No | 244 (97.2) | 112 (45.9) | 132 (54.1) | |
| Renal insufficiency | ||||
| Yes | 24 (9.6) | 4 (16.7) | 20 (83.3) | 0.002 |
| No | 227 (90.4) | 110 (48.5) | 117 (51.5) | |
| Myocardial infarction | ||||
| Yes | 27 (10.8) | 7 (25.9) | 20 (74.1) | 0.031 |
| No | 224 (89.2) | 107 (47.8) | 117 (52.2) | |
| Heart failure | ||||
| Yes | 35 (13.9) | 7 (20.0) | 28 (80.0) | 0.001 |
| No | 216 (86.1) | 107 (49.5) | 109 (50.5) | |
| Peripheral neuropathy | ||||
| Yes | 157 (62.5) | 67 (42.7) | 90 (57.3) | 0.259 |
| No | 94 (37.5) | 47 (50.0) | 47 (50.0) | |
| Glaucoma | ||||
| Yes | 19 (7.6) | 2 (10.5) | 17 (89.5) | 0.001 |
| No | 232 (92.4) | 112 (48.3) | 120 (51.7) | |
| Cataract | ||||
| Yes | 80 (31.9) | 12 (15.0) | 68 (85.0) | <0.001 |
| No | 171 (68.1) | 102 (59.6) | 69 (40.4) | |
SD=Standard deviation, HbA1c=Glycated hemoglobin
The duration of diabetes ranged between 11 and 16 years in 36.6% of the patients, whereas for 13.6%, it exceeded 16 years. There was a family history of diabetes in 60.6% of the patients; mostly from parents (89.4%). Of the patients, 42.2% self-reported adherence to diabetic healthy diet; 43.3% indicated that they avoided unhealthy diet. Ninety-two percent claimed that they adhered to the daily medication. Two out of 43.3% of the participants took two daily antihyperglycemic medications. For 47%, insulin was the medication for treating the diabetes. More than three-quarters of the patients (79.7%) reported that they went for a checkup every 6 months. Regarding the main center for diabetes follow-up, most patients (61%) indicated that they attended Al-Noor diabetic center; the majority of patients (84.5%) reported that their doctor had arranged the referral for fundus examination for them every year. A history of an annual fundus examination was reported by 71.9% of patients. The most common reported reasons for the lack of annual eye check were that they forgot the appointment (8.4%) and the belief that having an annual eye test was unnecessary (7.5%). For 63%, there was a history of HTN and for 43.4%, it was dyslipidemia [Table 1]. The most common diabetic complication was DR, followed by cataracts (31.9%), diabetic foot ulcer (31.5%), and heart failure (13.9%). Glycemic control was observed in 111 patients (44.2%).
Table 2 summarizes BMI and chemistry tests in type 2 diabetic patients. BMI ranged between 20 and 61 kg/m2(28.51 ± 5.78). Low-density lipoprotein ranged between 31 and 232 mg/dL (111.98 ± 33.53). Triglycerides ranged between 24 and 450 mg/dL (156.77 ± 73.89), whereas glomerular filtration rate (GFR) ranged between 4 and 197 (86.15 ± 24.82).
Table 2.
Description of the body mass index and chemistry tests among diabetic patients attending diabetic clinic at Al-Noor Specialist Hospital, Makkah, Saudi Arabia
| Range | Mean±SD | Median (IQR) | |
|---|---|---|---|
| BMI (kg/m2) | 20-61 | 28.51±5.78 | 28 (24-31) |
| LDL (mg/dL) | 31-232 | 111.98±33.53 | 109 (90-133) |
| TG | 24-450 | 156.77±73.89 | 135 (115-177) |
| GFR | 4-197 | 86.15±24.82 | 89 (76-102) |
BMI=Body mass index, SD=Standard deviation, IQR=Interquartile range, LDL=Low-density lipoprotein, TG=Triglycerides, GFR=Glomerular filtration rate
In univariable analysis, DR was significantly associated with male patients, older age patients, patients with elementary school education only, long duration of disease, uncontrolled HbA1c, adherence to medications and healthy diet, the number of daily antihyperglycemic medications, the use of insulin, regular diabetes follow-up, regular fundus examination, patients with history of HTN, dyslipidemia, chronic kidney disease, patients with diabetic complications (cataract, glaucoma, diabetic foot ulcer, renal insufficiency, myocardial infarction [MI], and heart failure), and patients with higher BMI and lower GFR. Patients' nationality, marital status, place of residence, family history of diabetes, main center for diabetes follow-up, history of hypothyroidism, and presence of neuropathy or history of amputation were not significantly associated with the development of DR [Tables 1 and 3].
Table 3.
Comparison of the body mass index and chemistry tests between Diabetic patients with and without diabetic retinopathy
| Diabetic retinopathy | P-value | ||
|---|---|---|---|
| NO Median (IQR) | Yes Median (IQR) | ||
| BMI | 26 (23-28.25) | 29 (26.5-33) | <0.001 |
| LDL | 109 (90.75-129.25) | 112 (85-135) | 0.847 |
| TG | 135.5 (124-156) | 133 (97-209) | 0.932 |
| GFR | 92.5 (85-104.5) | 83 (63-100) | <0.001 |
BMI=Body mass index, SD=Standard deviation, IQR=Interquartile range, LDL=Low-density lipoprotein, TG=Triglycerides, GFR=Glomerular filtration rate
The prevalence of DR was 54.6% as demonstrated in Figure 1; mild nonproliferative (NP) type was present in 28.7% of patients (52.6% of those with DR), whereas severe NP type was present in 8.4% of patients (15.3% of those with DR), whereas proliferative type was present in only 2.4% of patients (4.4% of those with DR).
Figure 1.
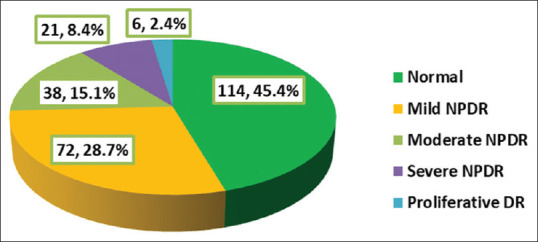
Prevalence and grade of diabetic retinopathy among the type 2 diabetic patients, Diabetic Center, Al-Noor Specialist Hospital, Makkah
Table 4 presents the results of multivariate logistic regression analysis. It revealed that patients with duration of diabetes ranging between 11 and 16 years were at almost three-folded risk of developing DR compared to those with duration <5 years (adjusted odds ratio [AOR] = 3.52, 95% CI: 1.10–11.33, P = 0.035). Patients who did not take daily medications on time were at higher significant risk for developing DR compared to others (AOR = 9.75, 95% CI: 1.82–52.29), P = 0.008. Compared to patients treated with insulin, those not treated with insulin were 70% less likely to develop DR (AOR = 0.30, 95% CI: 0.11–0.79), P = 0.015. Patients who did not have annual fundus examination were at about 4-fold risk of developing DR compared to others (AOR = 3.62, 95% CI: 1.35–9.71), P = 0.011. Patients with no history of MI, glaucoma, and cataract were at lower risk of having DR compared to those with these diseases (AOR = 0.15, 95% CI: 0.03–0.79, P = 0.026; AOR = 0.06, 95% CI: 0.01–0.41, P = 0.004; and AOR = 0.13, 95% CI: 0.05–0.38. P < 0.001), respectively. Patients with uncontrolled diabetes were at a significantly higher risk of developing DR compared to those whose disease was controlled (AOR = 12.18, 95% CI: 4.65–31.89), P < 0.001. An increase of one unit in BMI was significantly associated with an increase in the probability of developing DR by 11% (AOR = 1.11, 95% CI: 1.01–1.21), P = 0.024. Patients' gender, age, educational level, with hyperglycemia, frequent checkups, number of daily antihyperglycemic medications, HTN, dyslipidemia, chronic renal disease, diabetic foot ulcer, renal insufficiency, heart failure, and GFR were not significantly associated with the development of DR after controlling for confounders.
Table 4.
Correlates of diabetic retinopathy among type 2 diabetic patients attending diabetic clinic at Al-Noor Specialist Hospital, Makkah, Saudi Arabia
| β | SE | AOR | 95% CI | P-value | |
|---|---|---|---|---|---|
| Duration of diabetes (years) | |||||
| <5 (n=56)a | 1.0 | - | - | ||
| 5-10 (n=69) | 0.039 | 0.589 | 1.04 | 0.33-3.30 | 0.947 |
| 11-16 (n=92) | 1.259 | 0.596 | 3.52 | 1.10-11.33 | 0.035 |
| >16 (n=34) | 1.328 | 0.899 | 3.77 | 0.62-21.98 | 0.140 |
| Taking daily medications on time | |||||
| Yes (n=231)a | 1.0 | - | - | ||
| No (n=20) | 2.277 | 0.857 | 9.75 | 1.82-52.29 | 0.008 |
| Using of insulin to treat diabetes | |||||
| Yes (n=118)a | 1.0 | - | - | ||
| No (n=133) | -1.199 | 0.494 | 0.30 | 0.11-0.79 | 0.015 |
| Annual fundus examination | |||||
| Yes (n=178) | 1.0 | - | - | ||
| No (n=73) | 1.287 | 0.503 | 3.62 | 1.35-9.71 | 0.011 |
| Myocardial infarction | |||||
| Yes (n=27)a | 1.0 | - | - | ||
| No (n=224) | -1.930 | 0.867 | 0.15 | 0.03-0.79 | 0.026 |
| Glaucoma | |||||
| Yes (n=19)a | 1.0 | - | - | ||
| No (n=232) | -2.847 | 0.995 | 0.06 | 0.01-0.41 | 0.004 |
| Cataract | |||||
| Yes (n=80)a | 1.0 | - | - | ||
| No (n=171) | -2.036 | 0.540 | 0.13 | 0.05-0.38 | <0.001 |
| Glycemic control | |||||
| Controlled (n=111)a | 1.0 | - | -- | ||
| Uncontrolled (n=140) | 2.500 | 0.491 | 12.18 | 4.65-31.89 | <0.001 |
| BMI | 0.103 | 0.046 | 1.11 | 1.01-1.21 | 0.024 |
aReference category. B=Slop, SE=Standard error, AOR=Adjusted odds ratio, CI=Confidence interval
Discussion
DR is one of the main DM complications which can lead to blindness, if not discovered early and properly managed.[13,14] Since exploring the magnitude of DR problem and identifying its associated risk factors are essential for the achievement of better management of the status at earlier stages, the present study was carried out to estimate the prevalence and identify determinants of DR in type 2 DM patients registered in December 2019 in the diabetic center at Al-Noor Specialist Hospital, Makkah Al-Mukarramah, Saudi Arabia.
The prevalence of DR in the current study is very high (54.6%), though lower figures have been reported in other local and regional studies. The prevalence rate of DR in Saudi Arabian cities was 36.8%, 16% in Taif,[15,16] Al-Madinah Al-Munawarah: 36.1%,[15,17] Jazan: 27.8%,[4] Al-Hassa: 30%,[18] and Abha: 36.4%.[6] In the regional studies, the prevalence rate of DR was 19% in UAE,[19]12% in Kuwait,[20]34.1% in Jordan,[21]20.5% in Egypt,[14]42.4% in Oman,[22] and 26.6% in Iran.[23] However, another Jordanian study reported a higher rate of 64%[24] Furthermore, lower rates than those reported in the present study have been observed internationally. According to a study carried out by Yau et al., the global prevalence was 34.6%,[25] but other studies show the prevalence in India as 21.7%,[26]12.2% in Spain,[27]27.9% in China,[28] and 40.3% in the USA.[29]
The variation in the prevalence of DR between different studies and the relatively higher prevalence in the present study could be attributed to the use of different methods of diagnosis such as the use of slit-lamp examination and colored fundus photographs using canon cr-2 for diagnosis and grading of DR in the current study while most of the other studies depended on data from record files. Furthermore, variation in the sociodemographic and clinical characteristics of patients in various studies could partially explain this difference in the prevalence rate. Another important factor for this high prevalence rate is that the study was done in a highly specialized diabetic center which receives severe complicated diabetic patients referred from different other centers.
In the present study, mild NP type was present in 52.6% of type 2 diabetic patients with DR, whereas severe NP type was present in 15.3% of patients with DR, and the proliferative type was present in only 4.4%. In another Saudi study carried out in Abha, Saudi Arabia,[6]57.5% of the patients with DR had mild NPDR, 19.9% had moderate NPDR and 11.0% had severe NPDR, and 11.6% of diabetic patients had PDR. In Al-Madinah Al-Munawarah, Saudi Arabia,[17]13.6% of diabetic patients had mild NPDR, 8% had moderate NPDR, 8.1% had severe NPDR, and 6.4% had PDR. Some other studies also revealed that the mild type of NPDR was the most common, and the severe forms of NPDR and PDR were the least reported.[4,16,18]
In the current study, after controlling for confounders in multivariate logistic regression analysis, diabetic patients with moderate duration of diabetes (11 and 16 years) were at the highest risk for developing DR even more than those with longer duration of diabetes. The possibility here is that the longer duration of diabetes (>16 years) could be a reflection of better glycemic control and consequently a lower rate of complications such as DR. In other studies,[6,16,17,19,23] patients with longer duration of DM were at higher risk of having DR.
In the present study, an increase in BMI was associated with an increased probability of developing DR. The same has been documented in studies carried out in Saudi Arabia,[16] UAE,[19] and Iran.[23]
As expected, the present study revealed that patients who did not take daily medications on time as well as those who did not have their eyes checked annually by an ophthalmologist were at a higher significant risk of developing DR. Furthermore, in Al-Madinah Al-Munawarah,[17] regular fundus examination was a significant predictor for controlling and preventing retinal complications.
In addition, in the present study, patients treated with insulin were at higher risk of developing DR. The same has been documented in Abha, Saudi Arabia,[6] and a meta-analysis of seven cohort studies[30] in Thailand.[31] However, in a study carried out in Taif by Alharthi et al.,[16] DR was more reported in patients treated by oral hypoglycemic drugs only and least in those treated by both insulin and oral hypoglycemic drugs. The mechanism of the association between DR and insulin use is still unclear.[30]
Our study showed a significant association between other diabetic complications, particularly MI, glaucoma, and cataract, and DR. Other investigators reported an association between diabetic neuropathy and nephropathy from one side and DR from the other side,[6,32] and some others reported an association with cardiac problems.[33] A recently published systematic review revealed an association between DR and all other micro- and macrovascular complications of diabetes.[34]
In consonance with many other studies,[6,16,17,34] the current study revealed that patients with uncontrolled diabetes were at a higher risk of developing DR compared to those with controlled disease.
Some studies reported an association between the onset of the disease at an older age or at a younger age and the development of DR.[6] In the present study, older patient's age was a significant predictor for DR in bivariate analysis; however, after controlling for confounders in multivariate analysis, it was not significant; it was confounded by the duration of diabetes.
In the present survey and in accord with others,[16] HTN, dyslipidemia, and chronic renal diseases were significantly associated with DR in the bivariate analysis. However, their effects disappeared in multivariate analysis. Other studies have reported HTN as a significant predictor for developing DR.[6,24]
Among limitations of the current study is the fact that the study was done in one center only, which, therefore, affects the generalizability of results. However, this center is one of the biggest in Makkah and receives most referred cases of complicated DM could partly explain the high prevalence observed in our study.
Conclusion
DR particularly the mild NP type was very prevalent in type 2 diabetic patients attending the diabetic center at Al-Noor Hospital, Makkah Al-Mukarramah, Saudi Arabia; more than half of the patients had uncontrolled blood sugar. Predictors for the development of DR in type 2 diabetic patients were duration of diabetes ranging between 11 and 16 years, history of not taking daily medications on time, treatment with insulin, noncompliance with annual fundus examination, history of MI, glaucoma and cataract, uncontrolled diabetes, and increase in BMI. According to the study results, the following are recommended.
Screening for DR is necessary to reduce the burden caused by blindness, particularly for those with higher duration of diabetes, those treated with insulin, and those with cataract or glaucoma. Must raise awareness among physicians and diabetic patients of the importance of regular screening for diabetes complications, including annual retinal examination and the control of blood sugar. Physicians should encourage and help diabetic patients to obtain and maintain an ideal body weight and take their medications as prescribed on time. Further studies of patients from other health-care facilities are necessary.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Robert AA, Al Dawish MA, Braham R, Musallam MA, Al Hayek AA, Al Kahtany NH. Type 2 diabetes mellitus in Saudi Arabia: Major challenges and possible solutions. Curr Diabetes Rev. 2017;13:59–64. doi: 10.2174/1573399812666160126142605. [DOI] [PubMed] [Google Scholar]
- 3.Badedi M, Solan Y, Darraj H, Sabai A, Mahfouz M, Alamodi S, et al. Factors associated with long-term control of Type 2 diabetes mellitus. J Diabetes Res. 2016;2016:2109542. doi: 10.1155/2016/2109542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hajar S, Al Hazmi A, Wasli M, Mousa A, Rabiu M. Prevalence and causes of blindness and diabetic retinopathy in Southern Saudi Arabia. Saudi Med J. 2015;36:449–55. doi: 10.15537/smj.2015.4.10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elhadd TA, Al-Amoudi AA, Alzahrani AS. Epidemiology, clinical and complications profile of diabetes in Saudi Arabia: A review. Ann Saudi Med. 2007;27:241–50. doi: 10.5144/0256-4947.2007.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed RA, Khalil SN, Al-Qahtani MA. Diabetic retinopathy and the associated risk factors in diabetes Type 2 patients in Abha, Saudi Arabia. J Family Community Med. 2016;23:18–24. doi: 10.4103/2230-8229.172225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riddle MC, Bakris G, Blonde L, Boulton AJ, D'alessio D, de Groot M, et al. Standard medical care in diabetes 2018. Diabetes Care Clin Diabetes. 2018;36:14–37. [Google Scholar]
- 8.Diabetic retinopathy: Classification and clinical features - UpToDate. [[Last accessed on 2019 Oct 26]]. Available from: https://www.uptodate.com/contents/diabeticretinopathy-classification-and-clinical-features?search=diabeticretinopathy&source=search_result&selectedTitle=1~92&usage_type=default&display_rank=1#H7 .
- 9.Lima VC, Cavalieri GC, Lima MC, Nazario NO, Lima GC. Risk factors for diabetic retinopathy: A case-control study. Int J Retina Vitreous. 2018;2:21. doi: 10.1186/s40942-016-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sample size calculator by Raosoft [online] [[Last accessed on 2004 Oct 16]]. Available from: http://www.raosoft.com/samplesize.html .
- 11.American Diabetes association. [[Last accessed on 2019 Jan 01]]. Available from: https://professional.diabetes.org/content-page/practice-guidelinesresources .
- 12.American Heart association, Obesity and Overweight [online] [[Last accessed on 2019 Nov 20]]. Available from: https://www.heart.org/en/healthy-living/healthy-eating/losing-weight/bmi-in-adults?uid=1974 .
- 13.Nentwich MM, Ulbig MW. Diabetic retinopathy-ocular complications of diabetes mellitus. World J Diabetes. 2015;6:489–99. doi: 10.4239/wjd.v6.i3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macky TA, Khater N, Al-Zamil MA, El Fishawy H, Soliman MM. Epidemiology of diabetic retinopathy in Egypt: A hospital-based study. Ophthalmic Res. 2011;45:73–8. doi: 10.1159/000314876. [DOI] [PubMed] [Google Scholar]
- 15.Al Ghamdi AH, Rabiu M, Hajar S, Yorston D, Kuper H, Polack S. Rapid assessment of avoidable blindness and diabetic retinopathy in Taif, Saudi Arabia. Br J Ophthalmol. 2012;96:1168–72. doi: 10.1136/bjophthalmol-2012-301874. [DOI] [PubMed] [Google Scholar]
- 16.Alharthi AS, Almutairi MZ, Alswat AH, Al-Wagdani HA, Al Ghamdi A. Prevalence and potential risk factors of diabetic retinopathy among Type 2 diabetics patients in diabetic center, Taif city, KSA. Egypt J Hosp Med. 2018;70:1455–63. [Google Scholar]
- 17.El-Bab MF, Shawky N, Al-Sisi A, Akhtar M. Retinopathy and risk factors in diabetic patients from Al-Madinah Al-Munawarah in the Kingdom of Saudi Arabia. Clin Ophthalmol. 2012;6:269–76. doi: 10.2147/OPTH.S27363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan AR, Wiseberg JA, Lateef ZA, Khan SA. Prevalence and determinants of diabetic retinopathy in Al Hasa region of Saudi Arabia: Primary health care centre based cross-sectional survey, 2007-2009. Middle East Afr J Ophthalmol. 2010;17:257–63. doi: 10.4103/0974-9233.65502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Maskari F, El-Sadig M. Prevalence of diabetic retinopathy in the United Arab Emirates: A cross-sectional survey. BMC Ophthalmol. 2007;7:11. doi: 10.1186/1471-2415-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Shammari KH, Al-Meraghi O, Nasif A, Al-Otaibi S. The prevalence of diabetic retinopathy and associated risk factors in Type 2 diabetes mellitus in Al-Naeem area (Kuwait) Middle East J Family Med. 2005;3:1–8. [Google Scholar]
- 21.Al-Amer RM, Khader Y, Malas S, Abu-Yaghi N, Al-Bdour M, Ajlouni K. Prevalence and risk factors of diabetic retinopathy among Jordanian patients with Type 2 diabetes. Digit J Ophthalmol. 2008;14:42–9. doi: 10.5693/djo.01.2008.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haddad O, Saad MK. Prevalence and risk factors for diabetic retinopathy among Omani diabetics. Br J Ophthalmol. 1998;82:901–6. doi: 10.1136/bjo.82.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maghbooli Z, Pasalar P, Keshtkar A, Farzadfar F, Larijani B. Predictive factors of diabetic complications: A possible link between family history of diabetes and diabetic retinopathy. J Diabetes Metab Disord. 2014;13:55. doi: 10.1186/2251-6581-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Bdour MD, Al-Till MI, Abu Samra KM. Risk factors for diabetic retinopathy among Jordanian diabetics. Middle East Afr J Ophthalmol. 2008;15:77–80. doi: 10.4103/0974-9233.51997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gadkari SS, Maskati QB, Nayak BK. Prevalence of diabetic retinopathy in India: The all India ophthalmological society diabetic retinopathy eye screening Study 2014. Indian J Ophthalmol. 2016;64:38–44. doi: 10.4103/0301-4738.178144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Poncelas A, Miravet-Jiménez S, Casellas A, Barrot-de la Puente JF, Franch-Nadal J, López-Simarro F, et al. Prevalence of diabetic retinopathy in individuals with Type 2 diabetes who had recorded diabetic retinopathy from retinal photographs in Catalonia (Spain) Br J Ophthalmol. 2015;99:1628–33. doi: 10.1136/bjophthalmol-2015-306683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang G, Chen H, Chen W, Zhang M. Prevalence and risk factors for diabetic retinopathy in China: A multi-hospital-based cross-sectional study. Br J Ophthalmol. 2017;101:1591–5. doi: 10.1136/bjophthalmol-2017-310316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kempen JH, O'Colmain BJ, Leske CM, Haffner SM, Klein R, Moss SE, et al. The prevalence of diabetic retinopathy among adults in the United State. Arch Ophthalmol. 2004;122:552–63. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- 30.Zhao C, Wang W, Xu D, Li H, Li M, Wang F. Insulin and risk of diabetic retinopathy in patients with Type 2 diabetes mellitus: Data from a meta-analysis of seven cohort studies. Diagn Pathol. 2014;9:130. doi: 10.1186/1746-1596-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silpa-Archa S, Sukhawarn R. Prevalence and associated factors of diabetic retinopathy in chandrubeksa hospital, directorate of medical services, Royal Thai air force. J Med Assoc Thai. 2012;95(Suppl 4):S43–9. [PubMed] [Google Scholar]
- 32.Karlberg C, Falk C, Green A, Sjølie AK, Grauslund J. Proliferative retinopathy predicts nephropathy: A 25-year follow-up study of Type 1 diabetic patients. Acta Diabetol. 2012;49:263–8. doi: 10.1007/s00592-011-0304-y. [DOI] [PubMed] [Google Scholar]
- 33.Huang CC, Lee JJ, Lin TK, Tsai NW, Huang CR, Chen SF, et al. Diabetic retinopathy is strongly predictive of cardiovascular autonomic neuropathy in Type 2 diabetes. J Diabetes Res. 2016;2016:6090749. doi: 10.1155/2016/6090749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearce I, Simó R, Lövestam-Adrian M, Wong DT, Evans M. Association between diabetic eye disease and other complications of diabetes: Implications for care. A systematic review. Diabetes Obes Metab. 2019;21:467–78. doi: 10.1111/dom.13550. [DOI] [PMC free article] [PubMed] [Google Scholar]


