Congenital erythropoietic porphyria (CEP, MIM 263700) is a rare autosomal recessive disease caused by impaired activity of uroporphyrinogen III synthase (UROS), the fourth enzyme of the heme biosynthetic pathway.1 Accumulation of porphyrins in red blood cells, mainly uroporphyrinogen I (URO I) and coproporphyrinogen I (COPRO I), leads to ineffective erythropoiesis and chronic hemolysis. Porphyrin deposition in the skin is responsible for severe photosensitivity resulting in bullous lesions and progressive photomutilation. Treatment options are scarce, relying mainly on supportive measures and, for severe cases, on bone marrow transplantation (BMT). Increased activation of the heme biosynthetic pathway by gain-of-function mutations in ALAS2, the first and rate-limiting enzyme, results in a more severe phenotype.2 Iron deficiency promotes the binding of iron regulatory protein to the 5’ untranslated region iron-responsive elements of ALAS2 mRNA and therefore decreases its translation.3 Egan et al. observed a 32-year-old woman with CEP who exhibited spontaneous improvement in photosensitivity and hemolysis after chronic gastrointestinal bleeding that resulted in iron deficiency.4 They treated her with an iron chelator, resulting in correction of the hemolysis, decreased porphyrin levels and improved quality of life with reduced photosensitivity. We recently identified three CEP siblings with phenotypes ranging from moderate to asymptomatic which were modulated by iron availability, highlighting the importance of iron metabolism in the disease. Based on these data, we prospectively treated a CEP patient with phlebotomies to investigate the feasibility, safety and efficacy of this treatment. We observed discontinuation of hemolysis and a marked decrease in plasma and urine porphyrins. The patient reported a major improvement in photosensitivity. Finally, erythroid cultures were performed, demonstrating the role of iron in the rate of porphyrin production.
The study was conducted in accordance with the World Medical Association Declaration of Helsinki ethical principles for medical research involving human subjects.
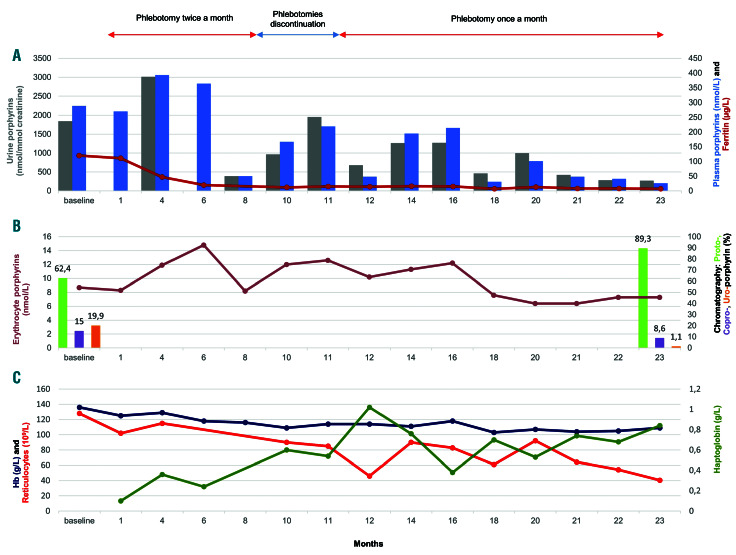
A 49-year-old Caucasian female was treated for CEP by repeated phlebotomies for almost 2 years. She was diagnosed with CEP at the age of 25 after experiencing symptoms since the age of 4, including photosensitivity with skin fragility, blistering and scarring, mouth lesions with erythrodontia, microstomia and gum recession, sclerodermiform retraction of the skin on the face and hands and loss of a distal phalange. She showed moderate chronic hemolysis. Genetic testing revealed a homozygous UROS variant, p.Gly58Arg, resulting in markedly decreased UROS activity in red blood cells (1.7 U/mg Hb/h, N>6). The patient was treated mainly with supportive measures, such as sun avoidance. At diagnosis, urine porphyrin levels were 2642 nmol/mmol of creatinine (URO 1602 nmol/mmol and COPRO 1040 nmol/mmol). Treatment started with the removal of 100 mL of blood twice a month for 1 month. The volume of blood was then increased by 50 mL each month or each 6 weeks until reaching 300 mL. This gradual increase of the volume of blood withdrawn was to avoid a strong induction of erythropoiesis. The iron store was efficiently depleted 8 months after the first phlebotomy, and the ferritin level decreased to 10.4 mg/L (Figure 1A). Concomitantly, we observed a significant decrease in urine and plasma porphyrin levels to 389 nmol/mmol and 50 nmol/L, respectively, corresponding to 79% and 85% decreases compared to the baseline levels. A progressive normalization of haptoglobin levels occurred which was associated with a decrease in reticulocyte count, showing discontinuation of hemolysis (Figure 1C). Photosensitivity markedly improved, and the urine became clearer. Phlebotomies were discontinued for 3 months, resulting in an increase in urine and plasma porphyrins (urine porphyrins 1956 nmol/mmol, plasma porphyrins 219 nmol/L) associated with worsening photosensitivity. Phlebotomies were reintroduced at month 11, on a monthly basis, progressively increasing the blood volume over 7 months until reaching 300 mL. Phlebotomies were then continued at 200 mL monthly. At month 18, low levels of urine and plasma porphyrins were obtained again (460 nmol/mmol and 31 nmol/L, respectively). Finally, at month 23, urine and plasma porphyrin levels were reduced to 271 nmol/mmol and 26 nmol/L (83% and 91% reductions compared to baseline), close to levels observed after BMT.5 During the course of treatment, total erythrocyte porphyrins levels were moderately reduced (8.7 mmol/L at baseline, 7.3 mmol/L at month 23; 16% decrease) (Figure 1B). Chromatography of erythrocyte porphyrins prior to phlebotomies revealed that these were composed of 62,4% protoporyphin IX (PPIX) (5.43 mmol/L), 19.9% URO (1.73 mmol/L) and 15% COPRO (1.30 mmol/L). At month 23 of treatment, erythrocyte porphyrin chromatography showed a large decrease in URO (1.1%, 0.08 mmol/L; 95% decrease compared to baseline) and COPRO (8.6%, 0.63 mmol/L, 51% decrease compared to baseline) and an increase in the amount of PPIX (89.3%, 6.52 mmol/L; 20% increase; 76% of zinc PPIX) due to iron-deficiency anemia. Hemoglobin levels remained greater than 10 g/dL. Clinical tolerance was good with moderate asthenia. The patient’s urine became clear. No adverse effects were detected. The biological and clinical improvement allowed the patient to have unrestricted exposure to sun during the summer without showing any signs of blistering.
Figure 1.
The patient’s clinical and biological parameters throughout treatment. (A) Urine porphyrins (gray), plasma porphyrins (blue) and ferritin (dark red). (B) Erythrocyte porphyrins (brown), protoporphyrins (light green), coproporphyrins (purple) and uroporphyrins (orange). (C) Hemoglobin (dark blue), reticulocytes (red) and haptoglobin (dark green). At baseline, the urine porphyrins/creatinine ratio ranged from 1845 to 2565 nmol/mmol (reference <30 nmol/mmol), plasma porphyrin concentrations ranged from 227 to 289 nmol/L (reference <20 nmol/L), reticulocyte counts ranged from 115 to 128 x 109/L (reference range, 20-80 x 109/L), the haptoglobin level was 0.16 g/L (reference range, 0.56-2 g/L) and ferritin ranged from 120 to 150 mg/L (reference range, 18-160 mg/L). Over the entire treatment course, ferritin levels were not greater than 17 mg/L.
Figure 2.
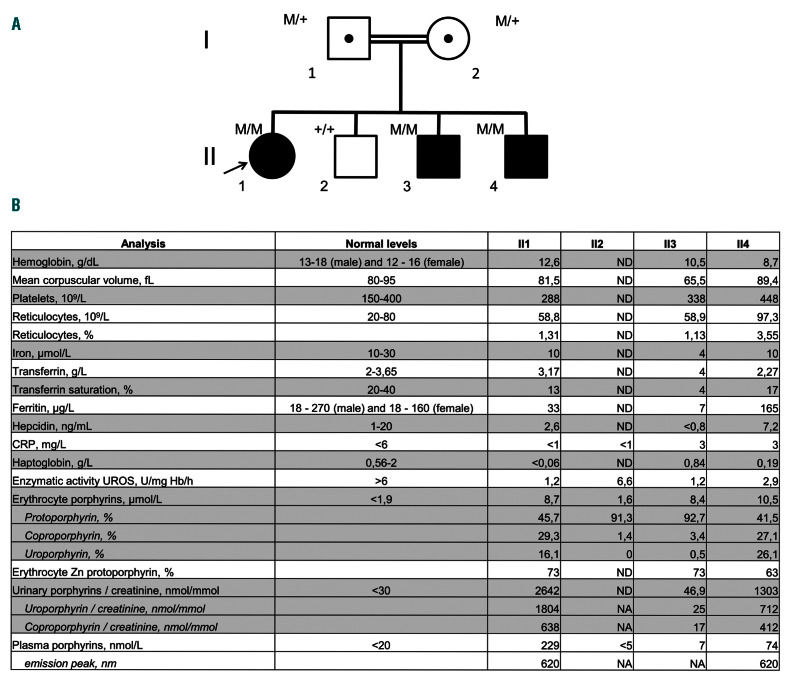
The pedigree of a family with congenital erythropoietic porphyria and biological parameters at diagnosis. (A) The family pedigree showing three of four siblings diagnosed with congenital erythropoietic porphyria (M: UROS c.660+4delA, +: wild-type allele). Genetic testing confirmed the diagnosis: patients II1, II3 and II4 are homozygous for the UROS mutation c.660+4delA, previously reported.6 (B) The siblings’ laboratory values at diagnosis. CRP: C-reactive protein; UROS: uroporphyrinogen III synthase; Zn: zinc; ND: not determined. NA: not applicable.
The impact of iron metabolism on the clinical expression of CEP is reinforced by the observation of a consanguineous Pakistani family with three of four children diagnosed with CEP (Figure 2A). Their UROS genotype was associated with a mild CEP phenotype in an Indian family.6 The proband of the Pakistani family (subject II1), a 9-year-old girl, exhibited photosensitivity, skin fragility and blistering, hirsutism and mild, compensated hemolysis without iron deficiency. She had markedly elevated urine and plasma porphyrin levels (Figure 2B). Her 5-year-old affected brother (subject II3) never experienced any symptoms of CEP despite unrestricted exposure to sun. His plasma porphyrin levels were in the normal range. Urine porphyrin levels, mostly consisting of isomer I, were almost normal and 56-fold lower than those of his sister. He had mild aregenerative microcytic anemia without hemolysis due to a profound iron deficiency. Glucose-6-phosphodehydrogenase and pyruvate kinase activity and hemoglobin electrophoresis were normal. There was no report of bleeding. Patients II1 and II3 had similar total erythrocyte porphyrin levels but their chromatographic profiles showed mainly URO and COPRO in subject II1, and 92% PPIX (zinc PPIX 73%) in subject II3, as observed in the patient treated with phlebotomies (Figure 2B). Patient II4, a 2-month-old baby, was free of skin symptoms but had normocytic anemia with moderate hemolysis. His ferritin levels were quite high at 165 μg/L. He had increased plasma and urine porphyrin levels.
Figure 3.
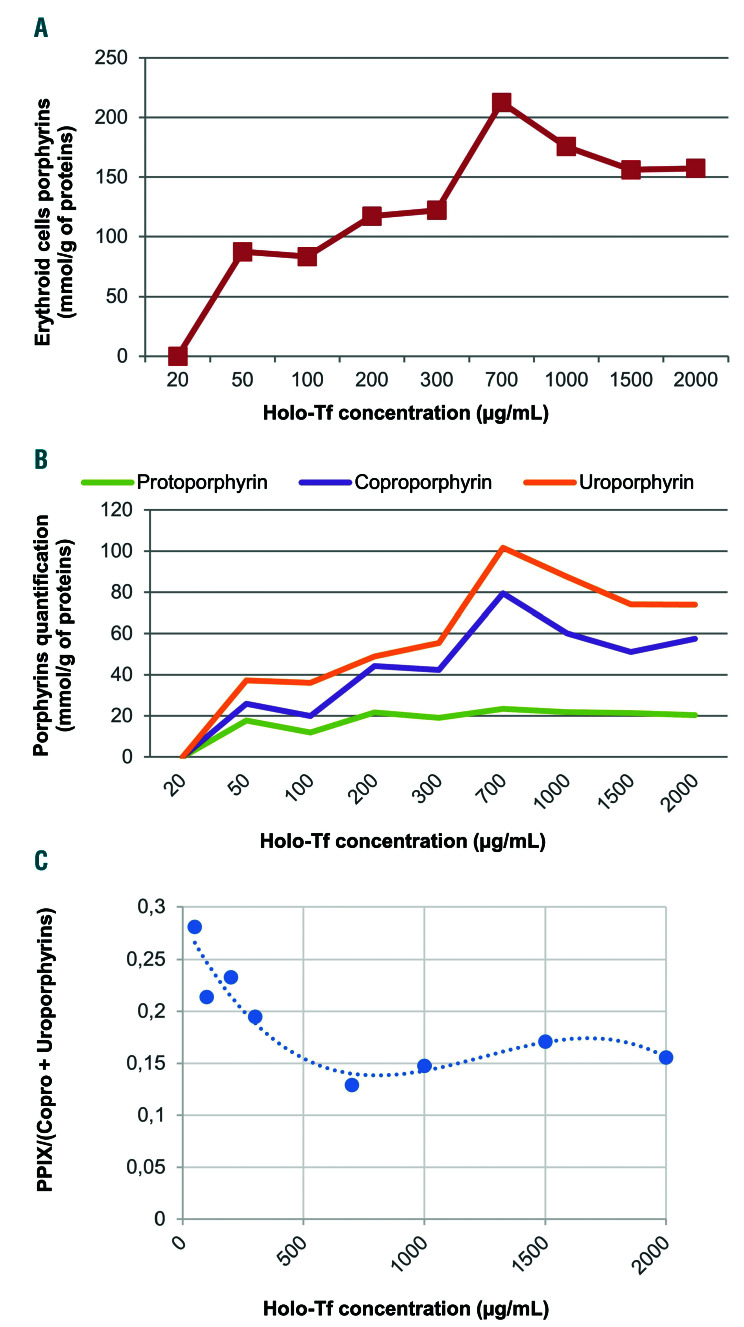
In vitro erythroid cell porphyrin analysis. Cells were harvested on day 18 for assessment of total erythroid cell porphyrins and porphyrin separation by high performance liquid chromatography with fluorescence detection. Erythrocyte, plasma and urine porphyrins were measured as previously described.15 (A) Porphyrin levels in erythroid cells derived from peripheral CD34+ cells, normalized by protein content after culture in increasing concentrations of holo-transferrin (Holo-Tf). Porphyrins were not detectable in the 20 mg/mL condition, presumably because of the technique’s insufficient sensitivity. (B) Quantification and separation of the different types of porphyrins in erythroid cells by chromatography. (C) The ratio of protoporphyrin IX (PPIX) to coproporphyrin (Copro) level plus uroproporphyrin level ranged from approximately 0.3 in iron-depleted conditions to 0.15 in iron-replete conditions.
To investigate the role of iron in the rate of porphyrin production we differentiated erythroid cells, derived from our patient’s peripheral CD34+ cells, by adapting the protocol of Mirmiran et al.7 by varying the holo-transferrin concentration from 20 to 2000 mg/mL (Online Supplementary Table S1). Blood was obtained from the CEP patient’s phlebotomy. Erythroid differentiation was monitored by flow cytometry using anti-CD36 and anti- CD235. Differentiation was similar in every condition of holo-transferrin concentration (Online Supplementary Figures S1 and S2). Total erythroid cell porphyrin measurements showed an increase in porphyrin levels, with approximately twice the level of porphyrin in the 700 mg/mL holo-transferrin condition, compared to the 200 mg/mL condition (Figure 3A). At higher concentrations (1000, 1500 and 2000 mg/mL), there were slight decreases in porphyrin levels, probably due to iron toxicity. Chromatography revealed that the higher levels of porphyrins were due to increases in URO and COPRO production, while PPIX levels remained stable (Figure 3B). These data show that CEP erythroid cells produce less URO and COPRO and that the proportion of PPIX increases when iron is less available. Thus, the in vitro findings confirm the in vivo observations that URO and COPRO levels decreased when the iron store was depleted
Treatment options for CEP patients are scarce. Photomutilation is prevented by sun avoidance. Hemolytic anemia is managed by hypertransfusions to decrease hematopoiesis and thereby porphyrin production. 8 This treatment has limited success and is responsible for severe secondary hemochromatosis.5 Patients often require BMT.9 An iron chelator (deferasirox) was successfully used to treat a patient with CEP who showed improved photosensitivity and erythropoiesis.4 The use of an iron chelator is a suitable treatment under iron overload, but its benefit and toxicity have not been evaluated in normal and iron-deficient states. Chronic deferasirox use is associated with toxicities such as gastrointestinal hemorrhage and renal and liver failure.10 Conversely, phlebotomies have been safely used as a long-term treatment in adults and children with hemoglobin SC disease to reduce blood viscosity.11,12 Nevertheless, an iron chelator could be used initially to deplete iron stores. Phlebotomy could then be used to maintain low ferritin levels. This sequential strategy would avoid initial induction of erythropoiesis and the long-term toxicity of iron chelators. After decreasing her iron stores, our patient’s urine porphyrin levels reached a level close to that observed in CEP patients after BMT.13 Iron-deficiency anemia was partially compensated by the correction of the hemolysis and the patient’s hemoglobin concentration remained above 10 g/dL. Finally, the observation of siblings with contrasting phenotypes modulated by iron availability highlights the importance of iron regulation in CEP. This study strengthens the hypothesis that the heme biosynthesis pathway can be slowed by inducing a mild iron-deficiency anemia. This is likely achieved by decreasing ALAS2 mRNA translation.4 More ALAS2 expression studies need to be performed to conclusively confirm this mechanism. 14 We can thus propose phlebotomies as a simple, universally available, well-tolerated and inexpensive strategy for treating CEP. Phlebotomies should be used in patients with hemolytic anemia without need of chronic transfusion or when BMT is not available, the latter remaining the first-line treatment in transfusiondependent patients.
Supplementary Material
Acknowledgments
The authors thank the affected individuals and their families who kindly contributed to this study and Dr N Talbi who helped greatly with monitoring the patient.
Funding Statement
Funding; this study was supported by a grant from Laboratory of Excellence GR-Ex, reference ANR-11-LABX-0051. AM was supported by a Labex GR-Ex scholarship. The Labex GR-Ex is funded by the program ‘‘Investissements d’avenir’’ of the French National Research Agency, reference ANR-11-IDEX-0005-02.
References
- 1.Erwin AL, Desnick RJ. Congenital erythropoietic porphyria: recent advances. Mol Genet Metab. 2019;128(3):288-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.To-Figueras J, Ducamp S, Clayton J, et al. ALAS2 acts as a modifier gene in patients with congenital erythropoietic porphyria. Blood. 2011;118(6):1443-1451. [DOI] [PubMed] [Google Scholar]
- 3.Bhasker CR, Burgiel G, Neupert B, Emery-Goodman A, Kühn LC, May BK. The putative iron-responsive element in the human erythroid 5-aminolevulinate synthase mRNA mediates translational control. J Biol Chem. 1993;268(17):12699-705. [PubMed] [Google Scholar]
- 4.Egan DN, Yang Z, Phillips J, Abkowitz JL. Inducing iron deficiency improves erythropoiesis and photosensitivity in congenital erythropoietic porphyria. Blood. 2015;126(2):257-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katugampola RP, Anstey AV, Finlay AY, et al. A management algorithm for congenital erythropoietic porphyria derived from a study of 29 cases. Br J Dermatol. 2012;167(4):888-900. [DOI] [PubMed] [Google Scholar]
- 6.Xu W, Warner CA, Desnick RJ. Congenital erythropoietic porphyria: identification and expression of 10 mutations in the uroporphyrinogen III synthase gene. J Clin Invest. 1995;95(2):905-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirmiran A, Schmitt C, Lefebvre T, et al. Erythroid-progenitor-targeted gene therapy using bifunctional TFR1 ligand-peptides in human erythropoietic protoporphyria. Am J Hum Genet. 2019; 104(2):341-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piomelli S, Poh-Fitzpatrick MB, Seaman C, Skolnick LM, Berdon WE. Complete suppression of the symptoms of congenital erythropoietic porphyria by long-term treatment with high-level transfusions. N Engl J Med. 1986;314(16):1029-1031. [DOI] [PubMed] [Google Scholar]
- 9.Peinado CM, Díaz De Heredia C, To-Figueras J, et al. Successful treatment of congenital erythropoietic porphyria using matched unrelated hematopoietic stem cell transplantation. Pediatr Dermatol. 2013;30(4):484-489. [DOI] [PubMed] [Google Scholar]
- 10.Kontoghiorghes GJ, Kontoghiorghe CN. Efficacy and safety of ironchelation therapy with deferoxamine, deferiprone, and deferasirox for the treatment of iron-loaded patients with non-transfusiondependent thalassemia syndromes. Drug Des Devel Ther. 2016; 10:465-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naessens V, Ward R, Kuo KHM. A proposed treatment algorithm for adults with haemoglobin SC disease. Br J Haematol. 2018;182(4):607-609. [DOI] [PubMed] [Google Scholar]
- 12.Lionnet F, Hammoudi N, Stojanovic KS, et al. Hemoglobin sickle cell disease complications: a clinical study of 179 cases. Haematologica. 2012;97(8):1136-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katugampola RP, Badminton MN, Finlay AY, et al. Congenital erythropoietic porphyria: a single-observer clinical study of 29 cases. Br J Dermatol. 2012;167(4):901-913. [DOI] [PubMed] [Google Scholar]
- 14.Barman-Aksözen J, Minder EI, Schubiger C, Biolcati G, Schneider-Yin X. In ferrochelatase-deficient protoporphyria patients, ALAS2 expression is enhanced and erythrocytic protoporphyrin concentration correlates with iron availability. Blood Cells Mol Dis. 2015;54(1):71-77. [DOI] [PubMed] [Google Scholar]
- 15.Deacon AC, Elder GH. Front line tests for the investigation of suspected porphyria. J Clin Pathol. 2001;54(7):500-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.