Mantle cell lymphoma (MCL) accounts for ~6% of all non-Hodgkin lymphomas (NHL) with an aggressive clinical course in patients, especially after early relapse.1 Lack of cure for relapsed/refractory (R/R) MCL with conventional therapy1 has resulted in a search for targeted therapies. CDK4 and CDK6 inhibitors have emerged as therapeutic options for R/R MCL because MCL cell lines and patient-derived samples that express high levels of cyclin D1 are highly sensitive to CDK4 and CDK6 inhibitors.2 Oral abemaciclib is a potent and selective CDK4 and CDK6 inhibitor that reduced tumor growth in human xenograft models with MCL.3 In a phase I study of patients with MCL, palbociclib, another CDK4 and CDK6 inhibitor, was shown to overcome resistance to ibrutinib, a first-in-class bruton tyrosine kinase (BTK) inhibitor.4 Here, we evaluated the efficacy, safety, and pharmacokinetic profile of abemaciclib in patients with R/R MCL in a phase II trial.
In this multi-center, open-label, single arm trial, patients ≥18 years of age with R/R MCL received 200 mg oral abemaciclib Q12H (every 12 hours) each day of a 28- day cycle (Online Supplementary Appendix). The study enrolled 28 patients in eight centers in France and Germany from March 2013 to September 2015 (Online Supplementary Figure S1). Most patients were male (60.7%) and white (96.4%) with a median age of 70 years (range, 53-83) (Online Supplementary Table S1). The median number of prior therapies was three (range, 1-6) and the majority of the patients (67.8%) had received ≤3 prior lines of therapies. Seven patients had received prior stem cell transplant and median time to treatment from stem cell transplant was 46 months (range, 18-87 months). During the study, patients completed a median of six cycles (range, 1-32).
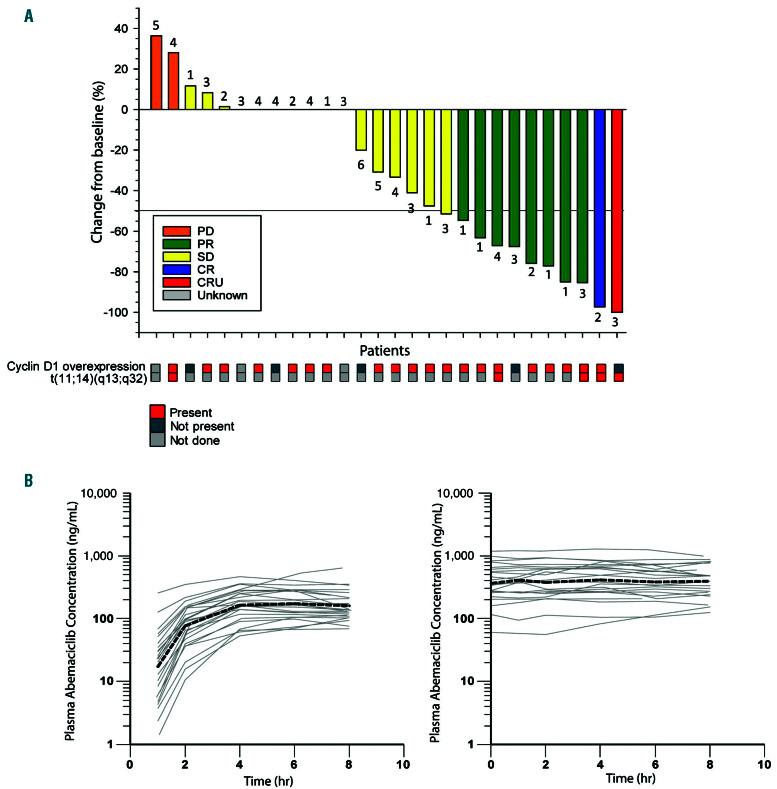
Fluorescence in-situ hybridization (FISH)/cytogenetics showed that all evaluable samples (n=5) from patients had the t(11;14)(q13;q32) translocation, which is a genetic hallmark of MCL and four (14.3%) among them overexpressed cyclin D1 (Figure 1A). In addition, cyclin D1 was overexpressed in 16 more patients (57.1%) as evidenced by immunohistochemistry although the t(11;14)(q13;q32) translocation could not be verified in these patients due to lack of evaluable samples.
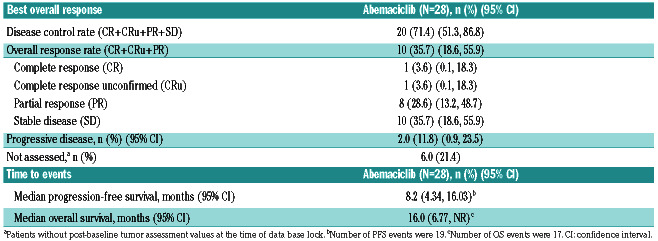
Primary objective was disease control rate (DCR) based on the Response Criteria for NHL (including bone marrow evaluation).5 Key secondary objectives included the objective response rate (ORR), duration of response (DoR), progression-free survival (PFS) and overall survival (OS). Single-agent abemaciclib demonstrated a DCR of 71.4% (95% Confidence interval [CI]: 51.3-86.8). ORR was 35.7% (95% CI: 18.6, 55.9) including two complete response (CR) (7.1%) (CR, n=1; CR unconfirmed [CRu], n=1) and eight partial response (PR) (28.6%) (Table 1; Figure 1A). Median time to best response was 110.5 days. At the end of cycle 2, 22 patients were evaluated; one patient had CRu, four had PR, 15 had stable disease (SD) and two had progressive disease (PD). At a median follow- up time of 13.8 months, median DoR was 12.39 months (95% CI: 3.19, not reached [NR]), median PFS was 8.18 months (95% CI: 4.34-16.03) and median OS was 16.03 months (95% CI: 6.77, NR; Online Supplementary Figure S2). A correlation could not be made between efficacy, and gene translocation and cyclin D1 expression due to the small number of samples and lack of sufficient information on the biomarkers.
In the subgroup of patients who had received ≤3 prior therapies DCR was higher (84.2%; n=16; 95%CI: 60.4-96.6) than those who received >3 prior therapies (44.4%; n=4; 95% CI: 13.7-78.8). A similar trend was observed for ORR (47.4%; 95% CI: 24.5-71.1 vs. 11.1%; 95% CI: 0.3-48.3), DoR (12.39 months vs. 6.67 months), PFS (12.85 months vs. 5.09 months) and OS (NR vs. 8.18 months; Online Supplementary Table S2). Thus, abemaciclib was more clinically active in patients who had received ≤3 prior therapies than those who received higher numbers of prior therapies. In patients who received temsirolimus, an mTOR inhibitor, as prior therapy (n=14), ORR was 14.3% (95% CI: 1.8-42.8) vs. 57.1% (95% CI: 28.9-82.3) in those who did not receive temsirolimus.
Dose reductions and dose omissions were reported for 78.6% and 75% of the patients, respectively. The median relative dose intensity was 71.5%. The median time to dose reduction was 28 days (range, 15–117) for those who had received ≤3 prior therapies and 15 days (range, 15–43) for patients who had received >3 prior therapies.
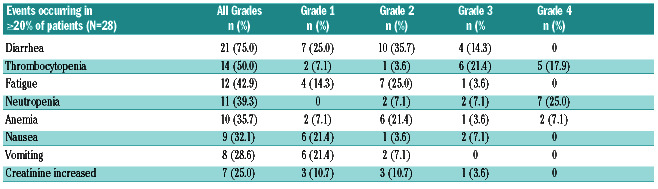
Safety was assessed per Common Terminology Criteria for Adverse Events (CTCAE) v4.0. The most frequent treatment-emergent adverse event (TEAE) of any grade were diarrhea, thrombocytopenia, fatigue and neutropenia (Table 2). Diarrhea was the most common TEAE and was reported by 75% of the patients with most (60.7%) experiencing low grade diarrhea (grade 1 or 2) in cycle 1. Grade 3 diarrhea occurred less frequently (14.3%). Per protocol, diarrhea was managed with over-the-counter medications, such as loperamide, or dose reduction. 12.5% of patients required dose reduction due to diarrhea, and no patient discontinued due to this adverse event. 50% of the patients who experienced diarrhea received anti-diarrheal medication (loperamide). Fatigue was also predominantly of low grade; grade 3 and 4 events were reported for thrombocytopenia (n=11) and neutropenia (n=9) and were likely related to study drug. A total of 42.9% of the patients reported at least 1 serious adverse event (SAE). Five patients experienced grade ≥3 events, likely related to the study drug (n=1 each of lobar pneumonia and lung infection, dehydration and pyrexia, nausea, grade 5 sepsis, and somnolence). There were five fatal events reported that were considered by investigators as due to AE (one patient due to grade 5 meningitis that was unrelated to the study drug, one patient due to grade 5 sepsis possibly related to the study drug, one patient due to grade 4 sepsis unrelated to the study drug, one patient due to grade 5 reversible posterior leukoencephalopathy unrelated to the study drug, and one patient due to grade 3 lung infection possibly related to the study drug; Online Supplementary Figure S3).
Table 1.
Summary of overall best response.
Figure 1.
Anti-tumor activity and pharmacokinetics of abemaciclib. (A) Change in tumor size at best response. Best overall response was based on investigator assessment. Number above or below each bar is the number of treatment regimens prior to study entry. Cyclin D1 expression and t(11;14)(q13;q32) translocation in each patient is shown below the response. (B) Abemaciclib plasma concentration-time profiles following oral administration of single (left panel) and multiple (right panel) doses of 200 mg abemaciclib every 12 hours, depicted as individual (gray continuous lines) and geometric mean (black broken line).
Table 2.
Treatment-emergent adverse events.
Pharmacokinetic (PK) evaluations included assessing plasma concentrations of abemaciclib and its metabolites by liquid chromatography–mass spectrometry (LC-MS) method. The median abemaciclib tmax after a single dose was 5.7 hours (range, 3.9-8.0 hours) (Figure 1B). The mean (coefficient of variation) steady-state abemaciclib trough concentration was 364 ng/mL (85%), indicating a high degree of interindividual variability in exposure. After single and multiple doses of abemaciclib, the mean accumulation ratio based on Cmax was 2.14 for abemaciclib and 3.91 to 5.17 for its metabolites, LSN2839567, LSN3106726, and LSN3106729 (Online Supplementary Table S3).
In this single arm phase II trial, abemaciclib monotherapy demonstrated clinical activity and a manageable safety profile in patients with R/R MCL. The ORR of 35.7% achieved with abemaciclib was similar to the 33% ORR with bortezomib,6 28% with lenalidomide,7 and 47% with temsirolimus,8 which are approved agents for MCL treatment. This study did not investigate the effect of abemaciclib in patients who previously received BTK inhibitors or lenalidomide as these compounds were not approved at the time of enrollment; the results post temsirolimus, however, suggest a potential influence of prior pathway specific treatment. Compared to this, ORR was higher with BTK inhibitors; 81% with alacabrutinib9 and 68% with ibrutinib.10 However, de novo or acquired resistance to BTK inhibition11 followed by uncontrolled growth of resistant MCL cells have led to poor prognosis. Therefore, the current challenge in the treatment of R/R MCL is to overcome the resistance to BTK inhibitors by choosing combination therapies targeting non-overlapping pathways.
Simultaneous inhibition of BTK and BCL2 with ibrutinib and venetoclax in a phase II trial, improved patient outcomes at 16 weeks (CR: 42%) compared to historical controls at the same time point (9%).12 A CR of 37% was demonstrated in a phase I trial of R/R MCL patients who were treated with a combination of ibrutinib and palbociclib. 4 Prolonging cell cycle arrest using a CDK4 and CDK6 inhibitor was reported to have reverted ibrutinib resistance.13 These data are promising and indicate that abemaciclib may have a potential role in the treatment of R/R MCL. It is important to explore the CDK4 and CDK6 inhibitors in combination with BTK inhibitors with potential synergistic effects.
Previous PK assessments performed in Colo-205 xenograft tumors showed that continuous inhibition of CDK4 and CDK6 and the resulting cell-cycle arrest were associated with an abemaciclib plasma concentration of approximately 200 ng/mL.14 In this study, although the mean steady state trough abemaciclib plasma concentrations in patients were higher than the levels associated with durable cell cycle arrest in preclinical models, the range of the observed concentrations was consistent with patients with solid tumors.14 Similar to abemaciclib, its major metabolites, LSN2839567 and LSN3106726, also inhibit CDK4 and CDK6 with similar potencies in in vitro biochemical and cell-based assays and the metabolite exposure achieved in patients with MCL at a dosage of 200 mg twice daily exceeds the 50% inhibition concentration (IC50) for CDK4/cyclin D1 and CDK6/cyclin D1.15 Thus, the exposure of abemaciclib and its active metabolites is consistent with what is expected to yield biological activity. However, the optimal abemaciclib dose in MCL based on the relationship between exposure, efficacy, and safety requires further elucidation.
In conclusion, this study demonstrated that singleagent abemaciclib dosed on a continuous schedule has clinical activity in patients with R/R MCL who received multiple prior systemic therapies. The safety profile of abemaciclib in this patient group is generally consistent with other abemaciclib studies on advanced breast cancer except for higher thrombocytopenia. Additional clinical trials of abemaciclib in combination with current preferred therapies such as a BTK inhibitors are needed to determine the synergistic effects and positioning of CDK4 and CDK6 inhibitors in MCL.
Supplementary Material
Acknowledgments
The authors thank the investigators and staff who conducted this study, and the patients and their families for their participation. Nirmala Xavier, employee of Eli Lilly and Company, provided medical writing support for this manuscript.
Funding Statement
Funding: this study was funded by Eli Lilly and company.
References
- 1.Arora PC, Portell CA. Novel therapies for relapsed/refractory mantle cell lymphoma. Best Pract Res Clin Haematol. 2018;31(1):105-113. [DOI] [PubMed] [Google Scholar]
- 2.Gong X, Litchfield LM, Webster Y, et al. Genomic aberrations that activate D-type cyclins are associated with enhanced sensitivity to the CDK4 and CDK6 inhibitor abemaciclib. Cancer Cell. 2017;32(6):761-776. [DOI] [PubMed] [Google Scholar]
- 3.Dempsey JA, Chan EM, Burke TF, Beckmann RP. Abstract LB-122: LY2835219, a selective inhibitor of CDK4 and CDK6, inhibits growth in preclinical models of human cancer. Cancer Res. 2013;73(8 Suppl):LB-122. [Google Scholar]
- 4.Martin P, Bartlett NL, Blum KA, et al. A phase I trial of ibrutinib plus palbociclib in previously treated mantle cell lymphoma. Blood. 2019;133(11):1201-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244-1253. [DOI] [PubMed] [Google Scholar]
- 6.Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24(30):4867-4874. [DOI] [PubMed] [Google Scholar]
- 7.Goy A, Sinha R, Williams ME, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol. 2013;31(29):3688-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rule S, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus: 3-year follow-up of patients with previously treated mantle cell lymphoma from the phase 3, international, randomized, open-label RAY study. Leukemia. 2018;32(8):1799-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M, Rule S, Zinzani PL, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;391(10121):659-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao X, Lwin T, Silva A, et al. Unification of de novo and acquired ibrutinib resistance in mantle cell lymphoma. Nat Commun. 2017;8:14920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med. 2018;378(13):1211-1223. [DOI] [PubMed] [Google Scholar]
- 13.Chiron D, Di Liberto M, Martin P, et al. Cell-cycle reprogramming for PI3K inhibition overrides a relapse-specific C481S BTK mutation revealed by longitudinal functional genomics in mantle cell lymphoma. Cancer Discov. 2014;4(9):1022-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tate SC, Cai S, Ajamie RT, et al. Semi-mechanistic pharmacokinetic/ pharmacodynamic modeling of the antitumor activity of LY2835219, a new cyclin-dependent kinase 4/6 inhibitor, in mice bearing human tumor xenografts. Clin Cancer Res. 2014;20(14): 3763-3774. [DOI] [PubMed] [Google Scholar]
- 15.Burke T, Torres R, McNulty A, et al. Abstract 2830: The major human metabolites of abemaciclib are inhibitors of CDK4 and CDK6. Cancer Res. 2016;76(14 Suppl):2830. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.