Abstract
The mechanism of toxic action for organophosphates (OPs) is the persistent inhibition of acetylcholinesterase (AChE) resulting in accumulation of acetylcholine and subsequent hyperstimulation of the nervous system. Organophosphates display a wide range of acute toxicities. Differences in the OP’s chemistries results in differences in the compound’s metabolism and toxicity. Acute toxicities of OPs appear to be principally dependent on compound specific efficiencies of detoxication, and less dependent upon efficiencies of bioactivation and sensitivity of AChE. Serine esterases, such as carboxylesterase (CaE) and butyrylcholinesterase (BChE), play a prominent role in OP detoxication. Organophosphates can stoichiometrically inhibit these enzymes, removing OPs from circulation thus providing protection for the target enzyme, AChE. This in vitro study investigated age-related sensitivity of AChE, BChE and CaE to twelve structurally different OPs in rat tissues. Sensitivity of esterases to these OPs was assessed by inhibitory concentration 50s (IC50s). The OPs displayed a wide range of inhibitory potency towards AChE with IC50s in the low nM-¼M range with no differences among ages; however, the CaE IC50s generally increased with age reflecting greater protection in adults. These results suggest age-related differences in acute toxicities of OPs in mammals are primarily a result of their detoxication capacities.
Keywords: organophosphate, acetylcholinesterase, butyrylcholinesterase, carboxylesterase
1. Introduction
Organophosphate (OP) insecticides are widely used for a variety of agricultural applications globally. Their major mechanism of toxic action is the inhibition of the serine hydrolase acetylcholinesterase (AChE) in synapses and neuromuscular junctions resulting in an accumulation of the neurotransmitter acetylcholine and ultimately hyperstimulation of the nervous system (Hayes and Laws, 1991). The majority of OP insecticides undergo bioactivation via cytochrome P450 (CYP) to oxon metabolites, which are active anticholinesterases (Sultatos et al., 1985; Chambers and Chambers, 1989; Fukuto, 1990).
Several studies have reported that adult animals are less susceptible to acute exposures of OP insecticides than younger animals (Gagne and Brodeur, 1972; Benke and Murphy, 1975, Pope et al., 1991; Atterberry et al., 1997). Non-target serine esterases, such as carboxylesterases (CaEs) and butyrylcholinesterases (BChEs), can stoichiometrically bind oxon metabolites of OPs and limit the amount of OP available to target AChE, thus providing some protection to the organism (Aldridge, 1953; Fukuto, 1990; Maxwell, 1992; Chambers and Carr, 1993; Moser and Padilla, 2016). Because CaE activity in rats increases with age from birth to young adulthood, neonates and juveniles would not have the same level of protection (detoxication) from an OP exposure as adult animals (Clement, 1984; Maxwell, 1992; Atterberry et al., 1997). Hinds et al. (2016) reported an increase in hepatic CaE activity with age in human pediatric patients indicating a potential for greater OP toxicity in children.
In addition to serine esterases (CaEs and BChEs), some OPs can be hydrolyzed effectively by A-esterases (Aldridge, 1953; Furlong et al., 1989; Pond et al., 1995). A-esterase, also termed paraoxonase (PON), is a calcium dependent enzyme synthesized in the liver and is associated with high density lipoproteins (HDLs) in the serum (Mackness et al., 1985; Furlong et al., 1989; Pond et al., 1995). Paraoxonase is a multigene family, including PON1, PON2, and PON3 (Primo-Parmo et al., 1996), with PON1 capable of catalytically hydrolyzing some OP compounds thus providing protection for the target enzyme AChE (Le et al., 1995; Pond et al., 1995; Mortensen et al., 1996). Similar to CaE, PON1 has been demonstrated to increase with age in rats from birth to young adulthood (Atterberry et al., 1997; Moser et al., 1998).
OP insecticides, with their diverse chemistries, display a wide range of acute toxicity levels. Generally, the insecticidal OPs are either O,O-dimethyl or O,O-diethyl phosphates, phosphorothionates, and phosphorothionothiolates (Fukuto, 1990; Hayes and Laws, 1991). The differences in the chemistries of the insecticides result in differences in the compounds’ metabolism (activation and detoxication) (Aizawa, 1982; Chambers et al., 1990; Atterberry et al., 1997; Mileson et al., 1998). Studies from our laboratory and others have demonstrated that the metabolic bioactivation of OPs to their active oxon metabolites can increase their potency as AChE inhibitors by several orders of magnitude (Chambers and Chambers, 1989; Forsyth and Chambers, 1989; Fukuto, 1990; Mileson et al., 1998).
In adult mammals, the acute toxicity levels of individual OP insecticides appear to be principally dependent on the compound-specific efficiencies of detoxication (BChE, CbxE, and PON), and less dependent upon the differences in CYP activation and/or sensitivity of the target enzyme, AChE (Chambers et al., 1990; Atterberry et al., 1997; Pope et al., 2005). Previous studies suggested few age-related differences in in vitro rat brain AChE sensitivity to chlorpyrifos-oxon and malaoxon (Mortensen et al., 1996, 1998) or paraoxon and methyl paraoxon (Benke and Murphy, 1975, Atterberry et al.,1997). Mortensen et al. (1998) also compared the sensitivity of purified AChE from brain and plasma of neonate and adult rats to chlorpyrifos-oxon and found no age-related differences. Kasteel et al. (2020) reported only the oxon metabolites and not the parent OP for chlorpyrifos, phosmet and diazinon inhibited human blood AChE in vitro and no differences in AChE IC50s among ages was observed in 20 human blood donor samples. Although age-related differences in CYP-mediated bioactivation (desulfuration) and detoxication (dearylation) of OPs has been documented (Atterberry et al., 1997), the maturation of OP detoxication enzyme levels appear to be the primary factor influencing the toxicity level for most OPs in young animals.
The current in vitro investigation was designed to determine the age-related sensitivity of acetylcholinesterase, butyrylcholinesterase and carboxylesterase to 12 OP compounds that display a variety of chemistries. The OPs chosen for this study include 8 diethyl and 4 dimethyl phosphates (the anticholinesterase metabolite of the OP) of which some are the active metabolites of commercially available insecticides and some are model compounds. This work will support other studies that have investigated the age-related differences in acute toxicity of a select number of commercially available organophosphates and provide additional information about the structure/anticholinesterase activity relationship for OPs as well as the sensitivity of OP detoxication enzymes.
2. Materials and Methods
2.1. Chemicals
All organophosphates/oxons (Table 1) were synthesized by the late Dr. Howard Chambers at Mississippi State University using standard procedures from commercially available intermediates (Chambers and Chambers, 1989; Meek et al., 2011), except diazoxon which was purchased from ChemService (West Chester, PA) and azinphos-methyl-oxon which was a generous gift of Bayer Crop Protection (Stilwell, KS). All organophosphates were at least 95% pure. All other reagent grade chemicals were purchased from Sigma Chemical Co. (St. Louis, MO). Figure 1 displays the chemical structures for the 12 OP compounds tested in this study.
Table 1.
Organophosphate compounds, the abbreviations used in this paper and the corresponding toxicities of their parent insecticides
| Organophosphate (active metabolite) | Commercial Pesticide or Model Compound | Rat Oral LD50 (mg/kg) |
|---|---|---|
| Azinphos-methyl-oxon | Azinphos-methyl | 12 mg/kg |
| Methyl paraoxon | Methyl parathion | 6 mg/kg |
| Dicapthon-oxon | Dicapthon | 400 mg/kg |
| Methyl coumaphos-oxon | Model Compound | NA |
| Paraoxon | Parathion | 2 mg/kg |
| Chlorpyrifos-oxon | Chlorpyrifos | 96 mg/kg |
| Ethyl cyanophos-oxon | Model Compound | NA |
| Ethyl ronnel-oxon | Model Compound | NA |
| Diazoxon | Diazinon | 1250 mg/kg |
| Naftalophos | Naftalophos | 140 mg/kg |
| Nitropyrifos-oxon | Model Compound | NA |
| Phoxim-oxon | Phoxim | 2000 mg/kg |
LD50 values (rat oral) for the commercial organophosphates (parent compound) are presented as reported in Meister et al., 1992 or EPA, 2006. NA (not available) = LD50 values have not been determined for model compounds (experimental use only).
Figure 1.
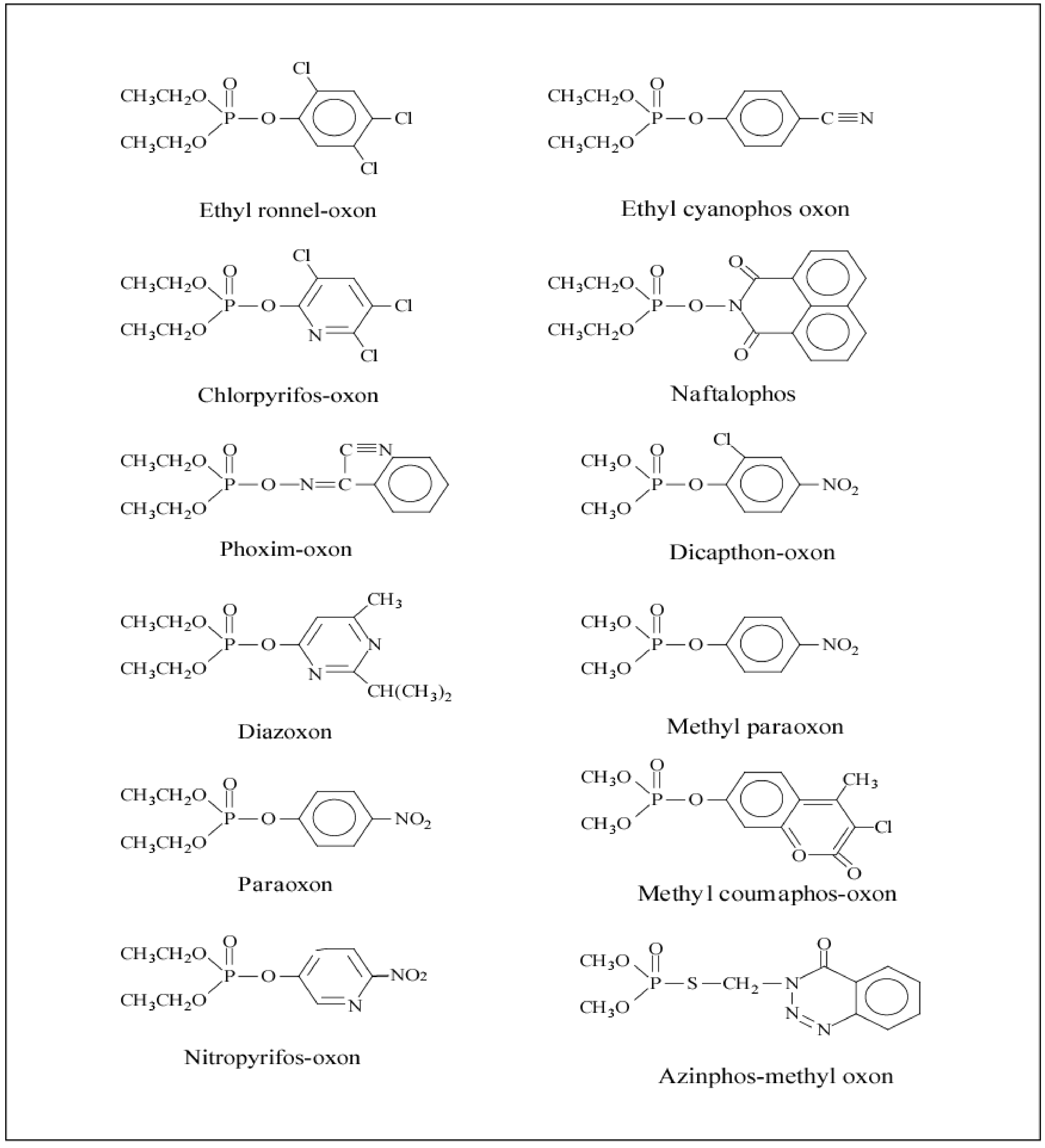
Chemical structures of 12 organophosphates/oxons.
2.2. Organophosphates
The 12 OPs tested in this study were selected for their differences in structure which were predicted to demonstrate unique inhibitory and detoxication potential. By selecting the active metabolites, i.e., oxons, for testing, this strategy eliminates the involvement of CYP-mediated bioactivation (desulfuration) and detoxication (dearylation) and allows for the determination of the sensitivity of the target enzyme, AChE, as well as the non-target protective esterases. Data from our laboratory and others suggests that esterase (carboxylesterase, butyrylcholinesterase and paraoxonase) mediated detoxication is more important in the overall toxicity level of OPs than the differential efficiencies of CYP-mediated bioactivation and detoxication. Of the 12 compounds selected for study, four (chlorpyrifos-oxon, paraoxon, methyl-paraoxon and diazoxon, and their parent insecticides) have an extensive literature database for comparing with data generated in this investigation. The additional eight compounds are either commercial insecticides, metabolites (oxons) of commercial insecticides, or model OPs with unique chemistries. These compounds were selected for their biochemical characteristics that were predicted to result in a wide range of potencies.
2.3. Animals
Rats were the source of tissues for the in vitro testing of OP potencies. Male Sprague Dawley-derived (Crl:CD(SD)BR) rats at postnatal days (PND) 1, 12, and 70 were obtained from breeding colonies derived from rats purchased from Charles River Laboratories and maintained at Mississippi State University (PND 1 and 12) or were purchased (PND 70) from Charles River Laboratories. Rats were housed in AAALAC accredited facilities within the College of Veterinary Medicine at Mississippi State University with temperature-controlled environments and 12 h dark-light cycle. Standard lab chow and tap water were provided ad libitum. All animal procedures received prior approval from the Mississippi State University Animal Care and Use Committee. Sex differences were not expected to be appreciable in neonates and juveniles; therefore, to reduce animal numbers and confounders such as time of estrus cycle in adult females, rats of only one sex (male) were chosen for study. The ages of rats were selected to represent a range of developmental stages and enzymatic activities. PND 1 was chosen as a neonatal age in rats, with rats of this age reported to have low levels of protective (detoxication) esterases. PND 12 was selected because studies have reported significant increases in protective esterases at this age in rats (Gagne and Brodeur, 1972). PND 70 was selected as young adults with a full complement of protective esterases (Atterberry et al., 1997). The choice of ages was not intended to equate to any particular age in humans.
2.4. Tissue Collection and Preparation
Brain, heart, skeletal muscle, lung, liver and blood were collected from naïve PND 1, 12, and 70 male Sprague Dawley rats. The tissues were rinsed in ice cold 0.9% saline, immediately snap-frozen in liquid nitrogen and stored at -80ºC. Serum was selected as a source of acetylcholinesterase instead of whole blood or erythrocytes because of less variability in acetylcholinesterase measurements, mostly due to the interference of the spectrophotometric (412nm) endpoint measurement from hemoglobin in whole blood and erythrocytes. The serum was prepared by centrifuging the blood at 10,000 g for 8 min, separated from sedimented erythrocytes, and was stored at -80ºC. Tissues were pooled to insure adequate amounts of tissue, especially for neonates, to compare all compounds. Three different pools of each rat tissue (brain, heart, skeletal muscle, and lung) were prepared to serve as experimental replication. Each pool consisted of tissues from six individual rats. Solid tissues were homogenized in 0.05 M Tris-HCl buffer (pH 7.4 at 37°C) with a motorized homogenizer. The more fibrous tissues (heart, lung and skeletal muscle) were filtered through fiberfill prior to assay. Serum was diluted with 0.05 M Tris-HCl buffer (pH 7.4 at 37°C). Immediately prior to testing, tissues were diluted to the following final concentrations (FC; mg tissue/ml 0.05 M Tris-HCl buffer (pH 7.4 at 37°C)): brain, 1 mg/ml; heart, 5 mg/ml; skeletal muscle, 5 mg/ml; lung, 2.5 mg/ml; serum, 10 ¼l/ml for cholinesterase assays and 0.5 mg/ml and 5 ¼l/ml for lung and serum carboxylesterase, respectively.
2.5. Esterase Activities
Enzymatic activity was determined for each tissue from PND 1, PND 12, and PND 70 rats. Acetylcholinesterase activity was determined for brain, heart, lung, skeletal muscle and serum using a discontinuous spectrophotometric assay (modification of Ellman et al., 1961) with acetylthiocholine as the substrate and 5,5′-dithio-bis(nitrobenzoic acid) (DTNB) as the chromogen (Chambers et al., 1988). Briefly, tissue homogenates were diluted (2 ml total assay volume) in buffer (0.05M Tris-HCl buffer (pH 7.4 at 37°C)), vortexed, and placed in a shaking water bath preheated to 37ºC. Additional tubes (blanks) containing eserine sulfate (FC, 10 ¼M) were included in the assay to inhibit AChE and correct for non-enzymatic hydrolysis. Each assay contained triplicate subsamples for each tissue as well as duplicate eserine sulfate blanks. Following the initial incubation (15 min), 20 ¼l of acetylthiocholine (FC, 1 mM) was added as a substrate for AChE and was incubated for an additional 15 min. The reaction was terminated and color was developed using 250 ¼l of a 5% SDS/0.024 M DTNB mixture (4:1). Absorbance was measured at 412 nm using a spectrophotometer. Serum butyrylcholinesterase activity was determined by the same method as serum AChE activity with the substitution of butyrylthiocholine (FC, 1mM) for acetylthiocholine as the substrate. Liver and serum carboxylesterase activities were determined according to the method of Carr and Chambers et al. (1991) using p-nitrophenyl valerate (pNPV) as the substrate. Dilute homogenates of liver or serum (total assay volume: 2 ml for liver, 1 ml for serum), were vortexed and placed in a shaking water bath preheated to 37ºC. Tubes (blanks) containing paraoxon (FC, 10 ¼M) were included in the assay to inhibit CaE and correct for non-enzymatic hydrolysis. Each assay contained triplicate subsamples for each tissue as well as duplicate paraoxon blanks. Following an initial incubation of 15 min, 20 ¼l of pNPV (FC, 500 ¼M) in ethanol vehicle was added as a substrate for the remaining uninhibited CaE and was incubated for an additional 15 min. The reaction was terminated with 500 ¼l of a 2% SDS/2% Tris-base mixture. Absorbance was measured at 405 nm using a spectrophotometer. The assays were run three independent times consisting of three subsamples for each replication using a unique set of reagents for each assay. Protein content for each tissue was quantified by the method of Lowry et al. (1951) using bovine serum albumin as the standard. Specific activities were calculated as nmoles product formed min-1mgP-1.
2.6. In Vitro Acetylcholinesterase IC50 Determination
The inhibitory concentration 50 (IC50), representing the concentration at which 50% of a given enzyme’s activity is inhibited, is often determined as an index of potency of an inhibitor. For this study, AChE IC50s were determined for each of 12 organophosphates in each of four rat tissues using a discontinuous spectrophotometric assay (as described above) with acetylthiocholine as the substrate and DTNB as the chromogen (Chambers et al., 1988). To diluted tissue homogenates, 20 ¼l of ethanol vehicle or one of five concentrations of each OP oxon in ethanol vehicle were added, vortexed, and placed in a shaking water bath preheated to 37ºC. Following the initial incubation (15 min), the remaining AChE activity was determined as described above. Percent inhibition of control (EtOH vehicle) absorbance was calculated for each concentration. IC50 values were determined by linear regression analysis of the plot of the logit of percent inhibition versus log10 oxon concentration. The best-fit line was drawn using points corresponding to the 20–80 percent AChE inhibition range and the equation of the best-fit line was solved for the x-intercept to determine the IC50. The procedure was used for brain, heart, lung, and skeletal muscle. The assays were run three independent times using a unique set of reagents for each assay.
2.7. In Vitro Serum AChE and BChE IC50 Determination
The determination of serum AChE and BChE IC50s requires the usage of specific inhibitors to separate AChE and BChE activities. For the determination of both serum AChE and BChE IC50s, 10 ¼l of serum was added to 990 ¼l of 0.05M Tris-HCl buffer (pH 7.4 at 37°C) for a total assay volume of 1 ml. Ten microliters of 0.1 M EDTA was added to the dilute serum to inhibit A-esterase (paraoxonase) preventing any catalytic hydrolysis of oxons by paraoxonase. Ethopropazine (FC, 1 ¼M), a selective BChE inhibitor, was added to inhibit all BChE activity leaving AChE functional. In parallel samples, a selective AChE inhibitor (BW284C51, FC 25) was added to inhibit all AChE activity leaving BChE functional. Ten microliters of ethanol vehicle or one of five concentrations of each OP in ethanol vehicle were added, vortexed, and placed in a shaking water bath preheated to 37ºC. Additional tubes (blanks) containing eserine sulfate (FC, 10 ¼M) were included in the AChE assay to inhibit AChE and correct for non-enzymatic hydrolysis. Blank tubes for the BChE assay contained iso-OMPA (tetraisopropyl pyrophosphoramide, FC, 10 ¼M) to inhibit BChE and correct for non-enzymatic hydrolysis. Each assay contained triplicate subsamples for vehicle controls and each inhibitor concentration as well as duplicate blanks. Following an initial incubation of 15 min, 10 ¼l of acetylthiocholine (FC, 1 mM) or butyrylthiocholine (FC, 1 mM) was added as a substrate for the remaining uninhibited AChE or BChE, respectively, and was incubated for an additional 15 min. The reaction was terminated and color was developed using 125 ¼l of a 5% SDS/0.24 M DTNB mixture (4:1). Absorbance was measured at 412 nm using a spectrophotometer. IC50s were determined as described above. The assays were run three independent times using a unique set of reagents for each assay.
2.8. In Vitro Liver and Serum Carboxylesterase IC50 Determination
Liver tissue was homogenized or serum diluted at a concentration of 0.5 mg/ml or ¼l/ml, respectively, in 0.05M Tris-HCl buffer (pH 7.4 at 37°C). The liver homogenate or diluted serum was assayed according to the method of Carr and Chambers et al. (1991) using p-nitrophenyl valerate (pNPV) as the substrate for CaE. To determine CaE IC50s for each of the 12 OPs, dilute homogenates (2 ml total assay volume for liver, 1 ml total assay volume for serum) were incubated with ethanol vehicle or one of five concentrations of each OP in ethanol vehicle (20 ¼l, for liver, or 10 ¼l, for serum) in a shaking water bath preheated to 37ºC. Tubes (blanks) containing paraoxon (FC, 10 ¼M) were included in the assay to inhibit CaE and correct for non-enzymatic hydrolysis. Each assay contained triplicate subsamples for vehicle controls and each inhibitor concentration as well as duplicate paraoxon blanks. Following an initial incubation of 15 min, pNPV (FC, 500 ¼M) in ethanol vehicle was added as a substrate for the remaining uninhibited CaE and was incubated for an additional 15 min. The reaction was terminated with SDS/2% Tris-base mixture (500 ¼l for liver, 250 ¼l for serum). Absorbance was measured at 405 nm using a spectrophotometer. IC50s were determined as described above. The assays were run three independent times using a unique set of reagents for each assay.
2.9. In Vitro Serum AChE IC50 Determination Using Specific Inhibitors
Subsequently, additional experiments were conducted with adult serum to selectively inhibit non-target (detoxication) esterases (paraoxonase, carboxylesterase and butyrylcholinesterase). For these experiments, 10 ¼l of EDTA (1 mM), 10 ¼l of saligenin cyclic phenylphosphonate, SCPP, (FC, 50 nM) and 10 ¼l ethopropazine (FC, 1 ¼M) were added to adult rat diluted serum to inhibit paraoxonase, carboxylesterase, and butyrylcholinesterase, respectively, leaving AChE functional. Ten microliters of ethanol vehicle or one of five concentrations of each OP in ethanol vehicle were added, vortexed, and placed in a shaking water bath preheated to 37ºC. Following the initial incubation (15 min), the remaining AChE activity was determined as described above for serum AChE. Absorbance was measured at 412 nm using a spectrophotometer. IC50 values were determined as described above. The assays were run three independent times using a unique set of reagents for each assay.
2.10. Statistics
Specific activities for each tissue were analyzed by an analysis of variance (ANOVA) using SAS software on a personal computer with mean separation by the Student-Newman-Keuls (SNK) post-hoc test. Significant difference among ages is reported for the p < 0.05 level. IC50s were calculated as the mean of three independent linear regressions using Excel software and mean IC50s were subsequently analyzed using SAS software on a PC with significant difference among ages determined by a lack of overlap of 95% Confidence Intervals for each compound.
3. Results
Acetylcholinesterase activities of brain significantly increased with age. Activity was 1.8-fold higher in juveniles than neonates and 2.2-fold higher in adults than neonates. The AChE activities of the peripheral tissues (heart, lung, and skeletal muscle) increased with age with PND 70 animals having significantly higher activities than PND 12s and PND 1s. Although activities were trending higher for PND 12s compared to PND 1s no significant differences were determined. Serum AChE activity was not significantly different among the three ages; however, serum BChE activities were significantly higher in PND 70 animals compared to PND 12 and PND 1 animals. Carboxylesterase activities for both liver and serum were significantly different among all three ages, increasing with age. The hepatic CaE activities of the PND 70 rats were about 2-fold higher than the PND 12 rats and about 8-fold higher than the PND 1 rats. Hepatic CaE activities increased 3.8-fold from PND 1 to PND 12. A similar increase was determined within serum among all three ages (Table 2). Adult rat tissues all had significantly higher protein levels than neonate or juvenile tissues (Table 3).
Table 2.
Specific activities for brain, heart, lung, skeletal muscle and serum acetylcholinesterase (AChE), serum butyrylcholinesterase (BChE), and liver and serum carboxylesterase (CaE) from rats of three ages (post-natal day, PND 1, 12 and 70).
| AChE Activities |
BChE Activities |
CaE Activities |
||||||
|---|---|---|---|---|---|---|---|---|
| Age | Brain | Heart | Lung | Skeletal Muscle | Serum | Serum | Liver | Serum |
| PND 1 | 43.4 ±7.1A | 19.9 ± 0.2 A | 17.9 ± 0.6A | 21.2 ± 0.3A | 10.4 ± 1.1A | 4.9 ± 0.2A | 147± 10A | 20 ± 6.2A |
| PND 12 | 79.4 ± 4.8B | 22.8 ± 0.7A | 20.6 ± 2.0A | 22.9 ± 0.7A | 11.4 ± 0.6A | 6.0 ± 0.2A | 558± 11B | 70 ± 6.3B |
| PND 70 | 98.8 ± 5.2C | 27.0 ± 0.9B | 25.4 ± 1.2B | 30.3 ± 1.2B | 12.9 ± 0.3A | 8.7 ± 0.4C | 1154±22C | 160 ± 7.1C |
Specific activities expressed as nmoles min−1 mgP−1, for AChE, BChE and CaE, means ± SEM of three independent replications. Means within a tissue not followed by the same letter are significantly different (p<0.05).
Table 3.
Protein levels for brain, heart, lung, skeletal muscle, serum and liver from rats of three ages (post-natal day, PND 1, 12 and 70).
| Protein μg |
||||||
|---|---|---|---|---|---|---|
| Age | Brain | Heart | Lung | Skeletal Muscle | Serum | Liver |
| PND 1 | 76 ± 0.9A | 137 ± 0.3 A | 107 ± 0.1A | 121 ± 0.1A | 74 ± 1.1A | 135 ± 1.2A |
| PND 12 | 96 ± 0.4A | 145 ± 0.1A | 126 ± 0.1A | 133 ± 0.9A | 96 ± 0.8B | 144 ± 0.7A |
| PND 70 | 138 ± 0.5B | 176 ± 0.1B | 149 ± 0.1B | 150 ± 1.2A | 109 ± 0.4C | 189 ± 0.8C |
Protein levels expressed as μg P, for AChE, BChE and CaE, means ± SEM of three independent replications. Means within a tissue not followed by the same letter are significantly different (p < 0.05).
IC50s were determined using equivalent amounts of tissue within a tissue for all three ages; therefore, the activities were different for some age groups. The diethyl insecticidal OPs were generally more potent AChE inhibitors than the dimethyl OPs as indicated by the IC50s for brain, heart, lung, skeletal muscle and serum (Table 4-5). The two model diethyl OPs, ethyl ronnel-oxon and ethyl cyanophos-oxon, were not potent inhibitors of AChE in any of the tissues. The more potent inhibitors of AChE, except for phoxim-oxon, contain a heterocyclic ring in their structure (Figure 1). The presence of the nitrogen in the ring and the difference in potency is evident when comparing chlorpyrifos-oxon and ethyl-ronnel-oxon, with chlorpyrifos-oxon (pyridine ring) about 70-fold more potent than ethyl-ronnel-oxon (aromatic ring) in brain and peripheral tissues (Table 4-5). Acetylcholinesterase IC50s within brain, heart, lung and skeletal muscle for both the diethyl and dimethyl organophosphates were not different among the three ages (PND 1, PND 12, and PND 70). The OP IC50s for AChE in the peripheral tissues typically exhibited greater variability than IC50s in brain tissue with the more potent OPs usually having less variability (Table 4-5).
Table 4.
Acetylcholinesterase inhibition by various organophosphates in rat cardiac, pulmonary, and muscle tissue.
| Oxon | Heart |
Lung |
Skeletal muscle |
||||||
|---|---|---|---|---|---|---|---|---|---|
| PND 1 |
PND 12 |
PND 70 |
PND 1 |
PND 12 |
PND 70 |
PND 1 |
PND 12 |
PND 70 |
|
| IC50 (nM) (95%CI) | IC50 (nM) (95%CI) | IC50 (nM) (95%CI) | IC50 (nM) (95%CI) | IC50 (nM) (95%CI) | IC50 (nM) (95%CI) | IC50 (nM) (95%CI) | IC50 (nM) (95%CI) | IC50 (nM) (95%CI) | |
| Azinphos-methyl-oxon | 430 (252,607) | 495 (317,673) | 212 (33,389) | 407 (256,558) | 281 (129,423) | 279 (172,430) | 154 (82,226) | 159 (101,218) | 83 (25,141) |
| Methyl paraoxon | 248 (36,461) | 583 (410,757) | 507 (334,681) | 455 (307,503) | 438 (290,586) | 158 (8307) | 266 (119,413) | 243 (123,363) | 184 (64,305) |
| Dicapthon-oxon | 67 (19,113) | 84 (46,122) | 34 (−4,73) | 109 (95,123) | 100 (86,115) | 116 (101,131) | 82 (64,101) | 64 (49,78) | 92 (78,107) |
| Methyl coumaphos-oxon | 955 (832,1077) | 987 (846,1109) | 894 (773,1017) | 474 (377,571) | 446 (350,543) | 295 (198,391) | 250 (162,348) | 230 (161,299) | 94 (25,163) |
| Paraoxon | 156 (115,197) | 167 (127,208) | 103 (62,143) | 69 (60,77) | 65 (57,74) | 54 (45,63) | 34 (9,59) | 47 (26,67) | 50 (29,70) |
| Chlorpyrifos-oxon | 5 (3,8) | 5 (3,7) | 3 (1,5) | 8 (6,10) | 7 (5,8) | 6 (5,8) | 6 (4,9) | 4 (2,6) | 9 (6,10) |
| Ethyl cyanophos-oxon | 1313 (1116,1510) | 1395 (1235,1556) | 1040 (880,1241) | 1163 (1038,1387) | 1217 (993,1443) | 812 (587,1036) | 791 (645,937) | 1022 (876,1169) | 733 (587,879) |
| Ethyl ronnel-oxon | 717 (704,729) | 721 (711,731) | 708 (706,717) | 652 (618,688) | 687 (652,722) | 699 (664,735) | 744 (726,762) | 752 (734,770) | 773 (755,791) |
| Diazoxon | 45 (32,57) | 43 (32,53) | 36 (26,47) | 134 (91,177) | 133 (90,177) | 221 (177,264) | 22 (7,37) | 38 (26,51) | 51 (37,62) |
| Naftalophos | 17 (6,27) | 11 (7,15) | 4 (1,7) | 43 (33,52) | 30 (21,39) | 44 (35,54) | 20 (3,37) | 17 (3,31) | 41 (27,55) |
| Nitropyrifos-oxon | 54 (44,64) | 61 (38,54) | 36 (28,44) | 46 (29,63) | 49 (32,66) | 24 (7,41) | 30 (17,43) | 29 (15,42) | 28 (15,42) |
| Phoxim-oxon | 15 (9,20) | 12 (7,16) | 8 (4,13) | 24 (19,29) | 21 (16,27) | 21 (15,26) | 14 (6,21) | 12 (5,18) | 17 (11,24) |
IC50s are expressed as means with 95% confidence intervals of three independent replications for each age group. No statistical differences were determined (p < 0.05) among ages within a tissue.
Table 5.
Acetylcholinesterase inhibition by various organophosphates in rat brain.
| Brain Acetylcholinesterase | |||
|---|---|---|---|
| PND 1 | PND 12 | PND 70 | |
| Oxon | IC50 nM (95%CI) | IC50 nM (95%CI) | IC50 nM (95%CI) |
| Azinphos-methyl-oxon | 115 (104,125) | 98 (68,109) | 96 (86,107) |
| Methyl paraoxon | 165 (132,198) | 118 (85,151) | 135 (102,168) |
| Dicapthon-oxon | 175 (144,205) | 131 (101,162) | 139 (108,170) |
| Methyl coumaphos-oxon | 124 (89,160) | 109 (73,144) | 74 (38,109) |
| Paraoxon | 32 (24,39) | 36 (28,44) | 25 (16,32) |
| Chlorpyrifos-oxon | 9 (6,12) | 8 (5,11) | 8 (5,11) |
| Ethyl cyanophos-oxon | 984 (864,1104) | 925 (805,1045) | 784 (664,904) |
| Ethyl ronnel-oxon | 622 (515,729) | 716 (609,822) | 691 (583,798) |
| Diazoxon | 224 (185,264) | 252 (212,291) | 273 (233,312) |
| Naftalophos | 62 (43,81) | 75 (57,94) | 64 (46,83) |
| Nitropyrifos-oxon | 40 (29,52) | 38 (26,49) | 34 (23,46) |
| Phoxim-oxon | 50 (38,63) | 52 (39,64) | 43 (30,55) |
IC50s are expressed as means with 95% confidence intervals of three independent replications for each age group. No statistical differences were determined (p < 0.05) among ages.
IC50s for serum AChE were generally equivalent in the neonates (PND 1) and juveniles (PND 12) but were significantly higher in adults (PND 70) for all OPs tested except azinphos-methyl-oxon, ethyl ronnel-oxon, and ethyl cyanophos (Table 6). The addition of specific inhibitors SCPP and ethopropazine for CaE and BChE, respectively, in adult (PND 70) serum reduced the AChE IC50s to values similar to those determined for the neonates and juveniles (Table 6). No significant differences were determined for azinphos-methyl-oxon, ethyl ronnel-oxon, and ethyl cyanophos among the three ages with or without CaE specific inhibitors. Three OPs, dicapthon-oxon, methyl coumaphos-oxon and nitropyrifos-oxon, exhibited IC50s that were significantly lower in adults than the corresponding neonatal and juvenile IC50s (Table 6).
Table 6.
Acetylcholinesterase inhibition by various organophosphates in rat serum.
| Serum Acetylcholinesterase | Serum + Selective Inhibitors | |||
|---|---|---|---|---|
| PND 1 | PND 12 | PND 70 | PND 70 | |
| Oxon | IC50 nM (95%CI) | IC50 nM (95%CI) | IC50 nM (95%CI) | IC50 nM (95%CI) |
| Azinphos-methyl-oxon | 117 (58,175) | 127 (68,185) | 178 (118,236) | 232 (160,304) |
| Methyl paraoxon | 137 (70,163) | 114 (68,161) | 219* (172,265) | 75 (18,131) |
| Dicapthon-oxon | 94 (86,101) | 84 (77,92) | 144* (137,152) | 46* (37,55) |
| Methyl coumaphos-oxon | 217 (195,238) | 212 (191,234) | 295* (273,317) | 138* (110,163) |
| Paraoxon | 56 (46,64) | 47 (38,56) | 106* (97,116) | 48 (36,60) |
| Chlorpyrifos-oxon | 10 (7,13) | 10 (6,13) | 54* (51,57) | 7 (3,11) |
| Ethyl cyanophos-oxon | 1140 (926,1354) | 1359 (1144,1573) | 1534 (1319,1748) | 1234 (972,1496) |
| Ethyl ronnel-oxon | 462 (409,515) | 477 (424,530) | 490 (437,543) | 399 (334,463) |
| Diazoxon | 157 (127,186) | 194 (164,223) | 309* (280,338) | 213 (177,248) |
| Naftalophos | 39 (29,49) | 34 (24,44) | 67* (57,77) | 32 (20,44) |
| Nitropyrifos-oxon | 46 (43,49) | 51 (47,54) | 83* (80,86) | 23* (19,27) |
| Phoxim-oxon | 30 (25,35) | 31 (26,36) | 109* (103,114) | 29 (23,35) |
IC50s are expressed as means with 95% confidence intervals of three independent replications for each age group. Means for each compound followed by an * are significantly different (p < 0.05) among ages within a tissue. Serum + selective inhibitors for CaE (SCPP) and BChE (ethopropazine).
Serum BChE IC50s generally increased with age (Table 7). BChE IC50s were significantly higher for PND 70 serum than PND 12 and PND 1 IC50s for all OPs except azinphos-methyl oxon and methyl paraoxon. Serum BChE was more sensitive to the diethyl OPs than the dimethyl OPs, as evidenced by the greater potency of the diethyl OPs. Azinphos-methyl-oxon and methyl coumaphos-oxon were not good inhibitors of serum BChE (Table 7).
Table 7.
Butyrylcholinesterase inhibition by various organophosphates in rat serum.
| Serum Butyrylcholinesterase | |||
|---|---|---|---|
| PND 1 | PND 12 | PND 70 | |
| Oxon | IC50 nM (95%CI) | IC50 nM (95%CI) | IC50 nM (95%CI) |
| Azinphos-methyl-oxon | 1051 (931,1382) | 1259 (1181,1337) | 1433 (1176,1691) |
| Methyl paraoxon | 389 (386,392) | 381 (357,406) | 394 (337,452) |
| Dicapthon-oxon | 58 (55,62) | 57 (53,61) | 185* (179,192) |
| Methyl coumaphos-oxon | 1223 (1137,1308) | 1212 (1191,1234) | 1469 (1116,1821) |
| Paraoxon | 47 (46,48) | 52 (50,54) | 76* (58,94) |
| Chlorpyrifos-oxon | 6 (5,7) | 7 (6,7) | 39* (30,49) |
| Ethyl cyanophos-oxon | 208 (167,249) | 224 (180,268) | 339* (282,396) |
| Ethyl ronnel-oxon | 2 (1,3) | 7 (3,8) | 35* (23,48) |
| Diazoxon | 8 (7,9) | 10 (9,11) | 31* (30,33) |
| Naftalophos | 5 (4,6) | 7 (6,7) | 30* (37,43) |
| Nitropyrifos-oxon | 15 (14,16) | 14 (13,15) | 64* (56,73) |
| Phoxim-oxon | 2 (1,3) | 31* (26,36) | 60* (57,64) |
IC50s are expressed as means with 95% confidence intervals of three independent replications for each age group. Means for each compound followed by an * are significantly different (p < 0.05) among ages within a tissue.
IC50s for hepatic CaE generally increased with age (Table 8). The hepatic CaE IC50s for methyl paraoxon, methyl coumaphos-oxon, chlorpyrifos-oxon and ethyl ronnel-oxon, were significantly higher for the PND 12 rats than the PND 1 rats. Hepatic IC50s were significantly higher for PND 70 rats compared to PND 1 rats for paraoxon, ethyl ronnel-oxon, diazoxon, and nitropyrifos-oxon. Additionally, CaE IC50s were significantly higher from livers of PND 70 rats compared to PND 12 rats for methyl paraoxon, methyl coumaphos-oxon, and chlorpyrifos-oxon. Because of low activity and limited sample volume, CaE IC50s were not measured in serum from neonatal rats (PND 1). IC50s for serum CaE generally increased with age from PND 12 to PND 70 with IC50s for methyl paraoxon, paraoxon and chlorpyrifos-oxon from PND 70 rats significantly higher than those from PND 12s. Hepatic and serum CaEs were more sensitive to the diethyl OPs than the dimethyl OP oxons, as evidenced by the greater potency from the diethyl OPs in PND 70 (highest CaE activity) liver and serum. The two most potent CaE inhibitors were chlorpyrifos-oxon and ethyl ronnel-oxon both with chlorinated rings in their structures.
Table 8.
Carboxylesterase inhibition by various organophosphates in rat hepatic tissue or serum.
| Carboxylesterase | |||||
|---|---|---|---|---|---|
| Liver IC50 (nM) (95%CI) | Serum IC50 (nM) (95%CI) | ||||
| Oxon | PND 1 | PND 12 | PND 70 | PND 12 | PND 70 |
| Azinphos-methyl-oxon | 182 (158,206) | 190 (98,282) | 337 (257,417) | 213 (70,359) | 282 (272,321) |
| Methyl paraoxon | 0.6 (0.4,0.9) | 3.2* (2.7,3.6) | 309* (260,357) | 49 (37,61) | 80* (73,87) |
| Dicapthon-oxon | 55 (27,83) | 61 (32,92) | 66 (28,105) | 61 (27,94) | 81 (74,87) |
| Methyl coumaphos- | 49 (36,62) | 89* (64,113) | 640* (550,730) | 31 (27,34) | 35 (25,44) |
| Paraoxon | 0.13 (0.1,0.2) | 0.14 (0.1,0.2) | 1.6* (0.5,2.8) | 2.5 (1.8,3.1) | 4.3* (35,5.1) |
| Chlorpyrifos-oxon | 0.02 (0.01,0.02) | 0.05* (0.04,0.05) | 0.16* (0.06,0.27) | 0.30 (0.2,0.3) | 0.70* (0.6,0.8) |
| Ethyl cyanophos- | 0.2 (0.1,0.3) | 0.2 (0.1,0.2) | 0.45 (0.2,0.7) | 0.17 (0.01,0.33) | 0.21 (0.08,0.35) |
| Ethyl ronnel-oxon | 0.04 (0.03,0.06) | 0.09* (0.08,0.1) | 0.20* (0.1,0.3) | 0.35 (0.24,0.46) | 0.49 (0.39,0.6) |
| Diazoxon | 0.2 (0.1,0.3) | 0.3 (0.2,0.3) | 1.1* (08,1.3) | 0.3 (0.2,0.5) | 0.5 (0.4,0.6) |
| Naftalophos | 5.0 (3.7,6.3) | 5.3 (4.9,5.6) | 5.7 (5.0,6.5) | 1.1 (0.7,1.5) | 1.3 (11,16) |
| Nitropyrifos-oxon | 10 (5,15) | 17 (11,23) | 30* (25,34) | 11 (6,16) | 16 (8,24) |
| Phoxim-oxon | 0.2 (0.1,0.3) | 0.2 (0.2,0.3) | 0.4 (0.3,0.5) | 0.9 (0.6,1.2) | 1.1 (0.7,1.5) |
IC50s are expressed as means with 95% confidence intervals of three independent replications for each age group. Means for each compound followed by an * are significantly different (p<0.05) among ages within a tissue.
4. Discussion
Acetylcholinesterase IC50s for the twelve organophosphates (OPs) in this study suggest that there is no age-related difference in the inhibitory potential toward AChE in the rat brain. Similar results were observed for the AChE IC50s in heart, lung and skeletal muscle. These results were not surprising with AChE having been determined to be from a single gene product (Massoulié et al., 2008). Kasteel et al. (2020) reported no age-related differences in the in vitro inhibitory potential of several OPs toward AChE in human blood donor samples. The decision to measure IC50s in equivalent tissue concentrations and not activity levels within a tissue could mean the number of enzyme active sites were not the same among the ages, which could result in slightly different IC50s. The diethyl OPs were generally more potent inhibitors of AChE than the dimethyl OPs, except for the two model compounds and diazoxon. Diazoxon was observed to be particularly unstable as well as it has a propensity to adhere to glass, which could account for the lower potency compared to the other diethyl insecticidal OPs. Acetylcholinesterase appears to be more sensitive to diethyl OPs containing heterocyclic rings in their structure compared to OPs containing aromatic rings, except for phoxim-oxon. For most of the OP compounds, slight increases in IC50s were observed with age but did not reach significance. The results are in agreement with previously reported data for chlorpyrifos-oxon (Mortensen et al., 1996; Atterberry et al., 1997) in rat brain. There were significant differences in serum AChE IC50s between the adult (PND 70) and young rats (PND 1 or PND12) for 9 of the 12 OPs tested; however, these differences were negated when a specific CaE inhibitor was included in the assays, indicating that the higher IC50s in the adult rat serum are most likely because of CaEs stoichiometrically destroying the OP and preventing its availability to inhibit AChE. This result correlates with the increase in CaE activity in the serum in adult rats compared to the juveniles and neonates.
Studies from our laboratory and others have demonstrated that young rats have lower CaE activity than adults (Moser et al., 1998; Atterberry et al., 1997). Atterberry et al. (1997) reported hepatic CaE activities in young rats (PND 3) were about 5-fold lower than adult rats (PND 70). Moser et al. (1998) also reported similar differences (6-fold increase) for serum CaE activity between the PND 3 and PND 70 rats. Atterberry et al. (1997) also reported that an age-related decrease in in vivo sensitivity to two OP insecticides, chlorpyrifos and parathion, paralleled the maturation of liver CaE with age in rats. This suggests that the age-related differences in acute toxicity levels that are associated with some organophosphate compounds are likely due to either a difference in the rate of CYP450-mediated metabolism to their bioactive metabolite or more likely to differences in the detoxication of the oxons by non-target esterases such as carboxylesterases and A-esterases (paraoxonases) (Gagne and Brodeur, 1972; Chambers et al., 1990; Forsyth and Chambers, 1989; Fukuto et al., 1990; Pope et al., 1991; Ma and Chambers, 1995; Pond et al., 1995; Atterberry et al., 1997; Moser et al., 1998). This hypothesis is supported by the data within this study.
The lower serum and hepatic CaE IC50s, reported here, suggest that younger rats may be more sensitive to some OPs. This greater sensitivity may be a result of lower concentrations of CaE and/or sensitivity of enzymes in younger ages. The age-related increase in serum AChE IC50s without a specific inhibitor for carboxylesterases (CaE activity present) further exhibits the protection CaE can provide the target enzyme (AChE) to OPs. As with AChE, the structure of the OP can affect the inhibitory potential for CaE. Data within this study suggest that the diethyl OPs are much better inhibitors of CaE than the dimethyl OPs. The in vitro data from this study correlate with data from our earlier studies comparing OP potencies for AChE in vitro and toxicities in vivo (Chambers et al., 1990; Chambers and Carr, 1993). The greater detoxication of many diethyl OPs helps explain the discrepancies between the AChE inhibitory potencies and acute toxicity. For example, chlorpyrifos-oxon (a diethyl OP) is a better inhibitor of AChE than methyl paraoxon or azinphos-methyl-oxon (dimethyl OPs); however, chlorpyrifos has a higher rat oral LD50 (96 mg/kg) than azinphos-methyl (12 mg/kg) or methyl parathion (6 mg/kg). Chlorpyrifos-oxon has a much lower CaE IC50 than azinphos-methyl-oxon or methyl paraoxon, indicating a higher affinity for the CaE and thus CaE potentially scavenges a greater amount from circulation resulting in greater protection. In addition, chlorpyrifos-oxon is hydrolyzed by PON much more effectively than most other OPs reducing the levels available to inhibit AChE. All the diethyl OPs exhibited IC50s in the low nanomolar range in both liver and serum indicating the high affinity for CaE and potential for greater detoxication. Pope et al. (1991) reported faster peak inhibition of brain cholinesterase activity in neonates compared to adults following chlorpyrifos exposure. This was most likely the result of lower detoxication (CaE and paraoxonase) enzymes in the neonates allowing more chlorpyrifos-oxon to reach the target brain AChE. Paraoxonase can hydrolyze some OPs very efficiently, such as chlorpyrifos-oxon, but typically is more important in chronic lower level OP exposures (Pond et al., 1995; Furlong et al., 1989; Atterberry et al., 1997). The lower levels of paraoxonase activity in juveniles can be important in the increased toxicity of some OPs in young animals (Atterberry et al., 1997; Li et al., 1997; Karanth and Pope, 2000). Of the 12 compounds tested (data not shown) only chlorpyrifos-oxon, diazoxon, paraoxon and nitropyrifos-oxon are hydrolyzed by paraoxonase and of those four only chlorpyrifos-oxon and diazoxon are efficiently hydrolyzed.
Exposures to high acute levels or chronic lower levels of some OPs can saturate the detoxication enzymes resulting in substantial brain AChE inhibition and toxicity (Chambers and Chambers, 1990). While detoxication of some of the OPs by CaEs and paraoxonases provides substantial protection, especially in mature animals, studies have reported that inhibition of the target enzyme, brain AChE, can occur prior to saturation of CaEs (Chambers and Chambers, 1990). CYP-mediated bioactivation for parathion can occur in the target site organ (brain) (Chambers et al., 1991). Although the CYP activity in the brain was reported to be low, the bioactivation to paraoxon was appreciable enough to produce brain AChE inhibition.
In addition to CaE and paraoxonase detoxication of OPs, the stoichiometric inhibition of BChE by OPs can reduce OP toxicity by scavenging OP molecules prior to reaching the target enzyme, AChE. OPs with higher affinities for BChE than AChE could be detoxified more efficiently than OPs that have a higher affinity for AChE. Adult rat serum BChE IC50s for the dimethyl OPs tested within this study were higher than serum AChE IC50s indicating that the dimethyl compounds have a greater affinity for AChE than BChE. This suggests that BChE would not scavenge dimethyl OPs well; therefore, BChE would not afford much protection from OPs with these structural characteristics. The reverse was observed for the diethyl OPs with adult rat serum BChE IC50s lower than adult rat serum AChE IC50s indicating greater diethyl phosphate affinity for BChE and subsequently the potential for increased detoxication. All of the diethyl OPs tested exhibited significantly higher BChE IC50s in adults than neonates and juveniles. This decrease in potency is most likely because of the maturation of BChE with age. Only one of the dimethyl OPs, dicapthon-oxon, tested within this study exhibited significantly higher IC50s in adult rat serum compared to neonates and juveniles, although the IC50s for the other three dimethyl OPs were trending higher with increasing age. Similar to AChE, serum BChE appears to be slightly more sensitive to diethyl phosphates containing heterocyclic rings in their structure compared to OPs containing aromatic rings. Li et al. (2000) reported BChE concentration was higher in adult heart tissue compared to neonates and subsequently Howard et al. (2007) reported that BChE activity in neonatal rat heart was more sensitive to inhibition by chlorpyrifos-oxon than activity in adult rat heart tissue. The stoichiometric inhibition of BChE by OPs can result in saturation of the enzyme which can lead to increased toxicity in high level exposures or chronic lower level exposures. Similarly, lower levels of BChE activity in the serum of younger animals may increase their susceptibility to OP toxicity. Kasteel et al. (2020) reported no differences in potency toward AChE (IC50s) for several OPs in human blood samples pre-treated with ethopropazine to inactivate BChE and EDTA to inactivate PON in vitro. In the same study, no intraspecies differences in AChE IC50s were observed among 20 human blood donor samples; however, differences in AChE IC50s were observed among the OPs (Kasteel et al. (2020) as was observed in this study with rat tissues.
5. Conclusion
The OP compounds investigated in this in vitro study displayed a wide range of inhibitory potential toward the target enzyme, AChE, as well as non-target detoxication enzymes, BChE and CaE, ranging over several orders of magnitude. Generally, the diethyl insecticidal oxons were more potent inhibitors than the dimethyl insecticidal oxons, which is in contrast to the acute toxicity of some of the parent insecticides. This discrepancy may be partially explained by the fact that many of the dimethyl compounds are poor inhibitors of the non-target esterases responsible for the stoichiometric detoxication of OPs. This study and others have reported age-related differences in the in vitro potency of some OP compounds in tissues from rats (Mortensen et al., 1996, 1998; Moser et al., 2016). In vitro studies can provide valuable insights into the metabolism and detoxication pathways of OPs to help explain the age-related differences in acute OP toxicity in vivo, that have been reported for many years (Benke and Murphy, 1975; Pope et al., 1991; Atterberry et al., 1997; Moser et al., 1998). Although the toxicological target for OPs is well described, the differences in bioactivation and detoxication with respect to age and sex must be considered when assessing risk. In vivo challenges of OPs are ultimately needed to address these differences; however, in vitro studies such as the one reported here can provide information (potency, kinetic rate constants, etc.) for the biochemical processes that can then be used in developing predictive models that, once validated, can reduce or replace the number of in vivo studies.
Highlights:
Organophosphates display a wide range of potency toward acetylcholinesterase
Organophosphate structure affects potency toward acetylcholinesterase
Age-related differences in detoxication of organophosphate affects acute toxicity
Organophosphate acute toxicity is principally dependent on efficiency of detoxication
Acknowledgement
Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number R01ES011287. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors wish to thank the late Dr. Howard Chambers for his help with technical procedures and synthesis of organophosphates used in this study.
Abbreviations:
- AChE
acetylcholinesterase
- BChE
butyrylcholinesterase
- CaE
carboxylesterase
- CYP 450
cytochrome P450
- DTNB
5,5′-dithio-bis(nitrobenzoic acid)
- OP
organophosphate
- PND
post natal day
- PON
paraoxonase
- pNPV
p-nitrophenyl valerate
- SCPP
saligenin cyclic phenylphosphonate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aizawa H (1982). Metabolic Maps of Pesticides. Academic Press, New York. [Google Scholar]
- Aldridge WN (1953). Serum esterases. I. Two types of esterase (A and B) hydrolysing p-nitrophenyl acetate, proprionate, and butyrate and a method for their determination. Biochem. J. 53:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atterberry TT, Burnett WT, and Chambers JE (1997). Age-related differences in parathion and chlorpyrifos toxicity in male rats: target and non-target sterase sensitivity and cytochrome P450-mediated metabolism. Toxicol. Appl. Pharmacol. 147:411–418. [DOI] [PubMed] [Google Scholar]
- Benke GM, and Murphy SD (1975). The influence of age on the toxicity and metabolism of methyl parathion and parathion in male and female rats. Toxicol. Appl. Pharmacol. 31:254–269. [DOI] [PubMed] [Google Scholar]
- Carr RL, and Chambers JE (1991). Acute effects of the organophosphate paraoxon on schedule-controlled behavior and esterase activity in rats: dose-response relationships. Pharmaco., Biochem., and Behavior, 40(4), 929–936. [DOI] [PubMed] [Google Scholar]
- Chambers HW, Brown B, and Chambers JE (1990). Noncatalytic detoxication of six organophosphorus compounds by rat liver homogenates. Pestic. Biochem. Physiol. 36:308–315. [Google Scholar]
- Chambers JE, and Carr RL (1993). Inhibition patterns of brain acetylcholinesterase and hepatic and plasma aliesterases following exposures to three phosphorothionate insecticides and their oxons in rats. Fund. Appl. Toxicol. 21:111–119. [DOI] [PubMed] [Google Scholar]
- Chambers HW and Chambers JE (1989). An investigation of acetylcholinesterase inhibition and aging and choline acetyltransferase activity following a high level acute exposure to paraoxon. Pestic. Biochem. Physiol. 33:125–131. [Google Scholar]
- Chambers JE and Chambers HW (1989). Oxidative desulfuration of chlorpyrifos, chlorpyrifos-methyl, and leptophos by rat brain and liver. J. Biochem. Toxicol. 4:201–203. [DOI] [PubMed] [Google Scholar]
- Chambers JE, and Chambers HW (1990). Time course of inhibition of acetylcholinesterase and aliesterases following parathion and paraoxon exposure in rats. Toxicol. Appl. Pharmacol. 103:420–429. [DOI] [PubMed] [Google Scholar]
- Chambers JE, Wiygul SM, Harkness JE, and Chambers HW (1988). Effects of acute paraoxon and atropine exposures on retention of shuttle avoidance behavior in rats. Neurosci. Res. Commun. 3:85–92 [Google Scholar]
- Clement JG (1984). Role of aliesterase in organophosphate poisoning. Fundam. Appl. Toxicol. 4:S96–S105. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V Jr., and Featherstone RM (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem.Pharmacol. 7:88–95. [DOI] [PubMed] [Google Scholar]
- Forsyth CS, and Chambers JE (1989). Activation and degradation of the phosphorothionate insecticides parathion and EPN by rat brain. Biochem. Pharmacol. 38:1597–1603. [DOI] [PubMed] [Google Scholar]
- Fukuto TR (1990). Mechanism of action of organophosphate and carbamate insecticides. Environ. Health Perspect. 87:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong CE, Richter R, Seidel S, Costa LG, and Motulsky A (1989). Spectrophotometric assays for the enzymatic hydrolysis of the active metabolites of chlorpyrifos and parathion by plasma paraoxonase/arylesterase. Anal. Biochem. 180:242–247. [DOI] [PubMed] [Google Scholar]
- Gagne J, and Brodeur J (1972). Metabolic studies on the mechanisms of increased susceptibility of weanling rats to parathion. Can. J. Physiol. Pharmacol. 50:202–215. [DOI] [PubMed] [Google Scholar]
- Hayes WJ and Laws ER (ed.). (1991). Handbook of Pesticide Toxicology, Vol. 3, Classes of Pesticides. Academic Press, Inc., NY. [Google Scholar]
- Hinds R, Simpson P, and McCarver D (2016). Age-dependent human hepatic carboxylesterase 1 (CES1) and carboxylesterase 2 (CES2) postnatal ontogeny. Drug Metab Disp. 44:959–966. [DOI] [PubMed] [Google Scholar]
- Karanth S, and Pope C (2000). Carboxylesterase and A-esterase activities during maturation and aging: relationship to the toxicity of chlorpyrifos and parathion in rats. Toxicol. Sci. 58:282–289. [DOI] [PubMed] [Google Scholar]
- Kasteel E, Nijmeijer SM, Darney K, Lautz LS, Dorne J, Kramer NI, and Westerink R (2020). Acetylcholinesterase inhibition in electric eel and human donor blood: an in vitro approach to investigate interspecies differences and human variability in toxicodynamics Arch Toxicol. 2020 October 10. doi: 10.1007/s00204-020-02927-8. Epub ahead of print. PMID: 33037899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WF, Furlong CE, and Costa LG (1995). Paraoxonase protects against chlorpyrifos toxicity in mice. Toxicol. Lett. 76:219–226. [DOI] [PubMed] [Google Scholar]
- Li W-F, Matthews C, Disteche CM, Costa LG, and Furlong CE (1997). Paraoxonase (PON1) gene in mice: sequencing, chromosomal localization, and developmental expression. Pharmacogenetics 7(2):137–144. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Rarr AL, and Randall RJ (1951). Protein determination with the Folin phenol reagent. J. Biol. Chem. 193:266–275. [PubMed] [Google Scholar]
- Ma T, and Chambers JE. 1995. A kinetic analysis of hepatic microsomal activation of parathion and chlorpyrifos in control and Phenobarbital-treated rats. J Biochem Toxicol. 10:63–8. [DOI] [PubMed] [Google Scholar]
- Mackness MI, Hallum SD, Peard T, Warner S, and Walker CH (1985). The separation of sheep and human serum A-esterase activity into the lipoprotein fraction by ultracentrifugation. Comp. Biochem. Physiol. 82B(4):675–677. [DOI] [PubMed] [Google Scholar]
- Massoulié J, Perrier N, Noureddine H, Liang D, and Bon S (2008). Old and new questions about cholinesterases. Chem. Biol. Interact. 175:30–44. [DOI] [PubMed] [Google Scholar]
- Maxwell DM (1992). The specificity of carboxylesterase protection against the toxicity of organophosphorus compounds. Toxicol. Appl. Pharmacol. 114:306–312. [DOI] [PubMed] [Google Scholar]
- Meek EC, Chambers HW, Coban A, Funck KE, Pringle RB, Ross MK and Chambers JE. (2012). Synthesis and in vitro and in vivo inhibition potencies of highly relevant nerve agent surrogates. Toxicol. Sci. 126(2):525–533. [DOI] [PubMed] [Google Scholar]
- Meister RT (ed.). (1992). Farm Chemicals Handbook ’92. Meister Publishing Company, Willoughby, OH. [Google Scholar]
- Mileson BE, Chambers JE, Chen WL, Dettbarn W, Ehrich M, Eldefrawi AT, Gaylor DW, Hamernik K, Hodgson E, Karczmar AG, Padilla S, Pope CN, Richardson RJ, Sauders DR, Sheets LP, Sultatos LG, and Wallace KB (1998). Common mechanism of toxicity: a case study of organophosphorus pesticides. Toxicol. Sci. 41:8–20. [DOI] [PubMed] [Google Scholar]
- Mortensen SR, Chanda SM, Hooper MJ, and Padilla S (1996). Maturational differences in chlorpyrifos-oxonase activity may contribute to age-related sensitivity to chlorpyrifos. J. Biochem. Toxicol. 11:279–287. [DOI] [PubMed] [Google Scholar]
- Mortensen SR, Hooper MJ, and Padilla S (1998). Rat brain acetylcholinesterase activity: developmental profile and maturational sensitvity to carbamate and organophosphorus inhibitors. Toxicology 125:13–19. [DOI] [PubMed] [Google Scholar]
- Moser VC, Chanda SM, Mortensen SR, and Padilla S (1998). Age and gender-related differences in sensitivity to chlorpyrifos in the rat reflect developmental profiles of esterase activities. Toxicol. Sci. 46:211–222. [DOI] [PubMed] [Google Scholar]
- Moser VC, and Padilla S (2016). Esterase detoxication of acetylcholinesterase inhibitors using human liver samples in vitro. Toxicology. 353–354:11–20. [DOI] [PubMed] [Google Scholar]
- Pond AL, Chambers HW, and Chambers JE (1995). Organophosphate detoxication potential of various rat tissues via A-esterase and aliesterase activities. Toxicol. Lett. 78:245–252. [DOI] [PubMed] [Google Scholar]
- Pope CN, Chakraborti TK, Chapman ML, Farrar JD, and Arthun D (1991). Comparison of in vivo cholinesterase inhibition in neonatal and adult rats by three organophosphorothionate insecticides. Toxicology 68:51–61. [DOI] [PubMed] [Google Scholar]
- Pope CN, Karanth S, and Liu J (2001). Phamacology and toxicology of cholinesterase inhbitiors: uses and misues of a common mechanism of action. Environ. Toxicol. Pharmacol. 19:433–446. [DOI] [PubMed] [Google Scholar]
- Primo-Parmo SL, Sorenson RC, Teiber J, and La Du BN (1996). The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics 33:498–507. [DOI] [PubMed] [Google Scholar]
- Sultatos LG, Minor LD, and Murphy SD (1985). Metabolic activation of phosphorothioate pesticides: Role of the liver. J. Pharmacol. Exp. Ther. 232, 624–628. [PubMed] [Google Scholar]
- US EPA (2006). Revised OP (Organophosphate) Cumulative Risk Assessment: Volume I. https://nepis.epa.gov. [Google Scholar]


