Abstract
Endometriosis is an incurable gynecological disease characterized by the abnormal growth of endometrium-like tissue, characteristic of the uterine lining, outside of the uterine cavity. Millions of people with endometriosis suffer from pelvic pain and infertility. This review aims to discuss whether nanomedicines that are promising therapeutic approaches for various diseases have the potential to create a paradigm shift in endometriosis management. For the first time, the available reports and achievements in the field of endometriosis nanomedicine are critically evaluated, and a summary of how nanoparticle-based systems can improve endometriosis treatment and diagnosis is provided. Parallels between cancer and endometriosis are also drawn to understand whether some fundamental principles of the well-established cancer nanomedicine field can be adopted for the development of novel nanoparticle-based strategies for endometriosis. This review provides the state of the art of endometriosis nanomedicine and perspective for researchers aiming to realize and exploit the full potential of nanoparticles for treatment and imaging of the disorder.
Keywords: endometriosis, nanoparticles, nanomedicine, imaging, therapy
Graphical Abstract
This review article outlines the potential of nanomedicine for diagnosis and treatment of endometriosis, a gynecological disease characterized by the abnormal growth of endometrial tissue outside the uterus. The up-to-date knowledge and developments in the field of endometriosis nanomedicine are critically overviewed. In addition, this work discusses whether some fundamental principles of cancer nanomedicine can be adapted for endometriosis.
1. Introduction
Endometriosis is the presence of endometrium-like glands and stroma outside of the uterus. This painful gynecological disorder affects as many as 10% of women in their reproductive years.[1] Although the cause of endometriosis is not completely clear, the predominant theory, the Sampson Hypothesis, suggests that endometriosis forms when endometrial cells pass [retrograde] through the fallopian tubes during menstruation and seed sites in the peritoneal cavity.[1] Compared to cancer, endometriosis is considered a non-malignant condition. However, the heterotopic tissue forms “endometriotic” lesions that, can be broadly distributed, and in some instances (e.g., deep infiltrating disease), perforate the underlying organs resulting in life-threatening conditions.[2] Most frequently, women with endometriosis suffer from pelvic pain and infertility.[1] There is a lack of accurate, non-invasive, “point of care” test for endometriosis, forcing clinicians to rely on palpation, ultrasound, and magnetic resonance imaging (MRI). Laparoscopic surgery remains the gold standard for definitive diagnosis of the disease.[3] Consequently, the average time from the onset of symptoms to definitive diagnosis is more than 7 years.[4, 5] Because endometriotic tissue, like the “eutopic” endometrium (referred to hereafter as the endometrium), grows, degrades, and bleeds in response to ovarian hormone secretion each menstrual cycle, medical therapies rely mainly on hormonal manipulation.[6] However, these treatments disrupt fertility and often cause side effects resulting in diminished quality of life and are not suitable for long-term use. Patients wishing to improve fertility often seek surgical removal of the lesions and the associated adhesions.[7-9] Surgical intervention can be effective at removing endometriotic lesions, but successful treatment relies mainly on the skill of surgeons in identifying and removing affected tissues. Recurrence rates are as high as 20%, and some patients require multiple surgeries. Recurrence is due, in part, to incomplete resection and subsequent implantation events. Moreover, complications associated with surgery also contribute to overall risks. Therefore, there is considerable interest in the development of efficient non-surgical strategies for diagnosis and treatment of endometriosis.
Over the last twenty years, nanomaterials have been widely explored for imaging and treatment of various diseases, including cancer.[10-14] Numerous reports suggest that nanoparticles have the potential to improve conventional therapeutic (e.g., chemotherapy) and imaging (e.g., MRI) modalities for disease detection and treatment.[12, 15] Nanomaterials allow further development of new experimental treatment and imaging strategies,[16-19] including photothermal therapy,[20, 21] magnetic hyperthermia,[22], and photoacoustic imaging.[23] Nanoparticles are promising vehicles for the delivery of drugs and imaging agents to disease sites. For instance, they can solubilize otherwise insoluble compounds and, protect cargos such as nucleic acids and unstable drugs from hydrolysis, oxidation, or other degradative processes in the systemic circulation. Moreover, they can extend the circulation time of drugs in the blood, allowing them to reach their therapeutic targets, and to deliver greater amounts of drugs to their target sites more precisely while reducing systemic toxicity via passive targeting or through the use of active targeting moieties.[16, 17, 21, 24-29] Although the application of nanoparticles for imaging and treatment of endometriosis is a relatively new field, a growing number of reports suggest that nanomedicine has the potential to provide novel therapeutic and diagnostic strategies for this devastating disease (Table 1).
Table 1.
Summary of reported nanomedicines for endometriosis imaging and treatment.
| Application | Nanomaterial | Cargo | References |
|---|---|---|---|
| MRI | Iron oxide nanoparticles | N/A | Lee et al., 2012[49] Zhang et al., 2014[60] |
| Fluorescence imaging | PEG-PCL polymeric nanoparticles | NIR dye (SiNc) | Moses et al., 2020[11] |
| Photothermal therapy | Hollow gold nanospheres | N/A | Guo et al., 2017[43] |
| PEG-PCL polymeric nanoparticles | NIR dye (SiNc) | Moses et al., 2020[11] | |
| Gene Therapy | Lipid-chitosan micelles-DNA polyplex | Plasmid DNA | Zhao et al., 2012[45] |
| PAMAM dendrimer-DNA polyplex | Plasmid DNA | Wang et al., 2014[104] | |
| PEI–PEG–RGD-miRNA polyplex | miRNA | Liang et al., 2017[98] | |
| PEI-chitosan oligosaccharide–siRNA polyplex | siRNA | Zhao et al., 2016[61] | |
| Combinatorial therapy | PLGA polymeric nanoparticles | Curcumin & letrozole | Jana et al., 2014[100] |
| Epigallocatechin gallate & doxycycline | Singh et al., 2015[101] | ||
| Pain treatment | Lipid grafted chitosan micelles-coated nanostructured lipid carriers | P2X3 receptor antagonist (A317491) | Yuan et al., 2017[44] |
| Antioxidant therapy | Cerium oxide nanoparticles | N/A | Chaudhury et al., 2012[99] |
| Immunotherapy | Mesoporous silica nanoparticles | Immunomodulatory drug | Antsiferova et al., 2013[186] |
| PLGA polymeric nanoparticles | Antibody | Liu et al., 2017[103] | |
| Natural product-based therapy | Silicate nanocomposite (VB8) | Copaiba oleoresin | de Almeida Borges et al., 2016[198] Henriques da Silva et al., 2015[200] |
| PLGA polymeric nanoparticles | de Almeida Borges et al., 2018[199] | ||
| Local therapy | PEG&PCL nanofibers | Curcumin | Boroumand et al., 2019[102] |
| Biodistribution in patients | Lipid core nanoparticles | N/A | Pogdaec et al., 2019[70] |
To our knowledge, this is the first systematic and critical overview of the reported nanomedicine-based strategies. First, we discuss the potential mechanisms of nanoparticle accumulation and retention in endometriotic lesions following systemic administration. Second, we provide a brief overview of animal models of endometriosis available for nanoparticle evaluation. Third, nanoparticle-based imaging strategies are described. Fourth, we overview previously reported nanoparticle-based therapeutic modalities and their efficacy for endometriosis treatment. Finally, through this review, we discuss whether some fundamental principles of cancer nanomedicine can be used for the development of novel nanoparticle-based strategies for treatment and imaging of endometriosis.
2. Accumulation of Nanoparticles in Endometriosis Lesions
2.1. Passive Targeting
In the cancer field, it is believed that passive targeting of nanoparticles to solid cancer tumors is made possible through the exploitation of the so-called enhanced permeability and retention (EPR) effect.[30-32] Several characteristics of solid tumors contribute to the EPR effect, including hypervascularization, exaggerated vascular permeability, and impaired lymphatic drainage.[30-33] Therefore, long-circulating nanoparticles leak preferentially into tumor tissue through the pores of permeable tumor vessels and are then retained in the tumor bed due to reduced lymphatic drainage.[30, 31, 33] Previous reports suggest that endometriosis, like cancer, is an angiogenesis-dependent disease, and increased vasculature is often observed surrounding endometriotic lesions during laparoscopy.[34, 35] [36] Endometriotic lesions display a several-fold more newly formed, immature blood vessels in comparison to the endometrium and other tissues.[37] Recent literature strongly supports that angiogenesis is required for the implantation, survival, and growth of endometriotic lesions. Moreover, angiostatic agents have been shown inhibit the growth of endometriosis.[38] Furthermore, various factors (e.g., vascular endothelial growth factor (VEGF), bradykinin, reactive oxygen species, nitric oxide, prostaglandins) involved in vascular permeability and the EPR effect in solid cancer tumors are also associated with endometriosis.[31] For example, VEGF is highly overexpressed in endometriotic tissue and peritoneal fluid of affected patients, and it plays a central role in the process of endometrial angiogenesis. VEGF concentrations are correlated with neovascularization and increased vascular permeability in both cancer and endometriosis.[31, 36] The bradykinin system, a key contributor to the EPR effect in cancer tumors, is also reported to be involved in endometriosis-related pain, and kinins are secreted by endometrial lesions.[39, 40] Furthermore, the high level of reactive oxygen species (ROS) and reactive nitrogen species (e.g., nitric oxide) that play an important role in vascular permeability of cancer tumors[31] are strongly associated with the development and progression of endometriosis.[41] Taking all these facts into consideration, it is reasonable to hypothesize that systemically injected nanoparticles can accumulate in endometriotic lesions as well, by extravasation through immature, hyperpermeable blood vessels (Figure 1). Previous reports also suggest that passive targeting (EPR effect) is not a phenomenon exclusive to cancer tumors and is evident in other diseases and disorders (e.g., inflammation) where vascular permeability is increased.[31, 42] While the use of nanoparticles in the treatment and/or imaging of endometriosis remains relatively rare; there are several reports wherein the accumulation and retention of nanoparticles in endometriotic tissues is qualitatively and/or quantitatively described.[11, 43-45]
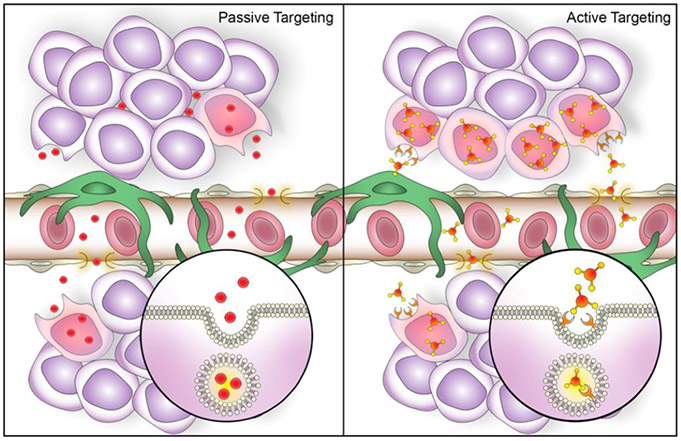
Figure 1.
Potential mechanisms of nanoparticle accumulation in endometriotic lesions following systemic administration. Passive targeting (Left panel): Nanoparticles extravasate from systemic circulation into lesion interstitium through pores in permeable blood vessels and undergo cellular internalization. Active targeting (Right panel): passively delivered nanoparticles equipped with surface ligands specific to receptors on endometriotic cells demonstrate increased cellular internalization and accumulation in endometriotic lesions. Created with Adobe Photoshop and Adobe Illustrator.
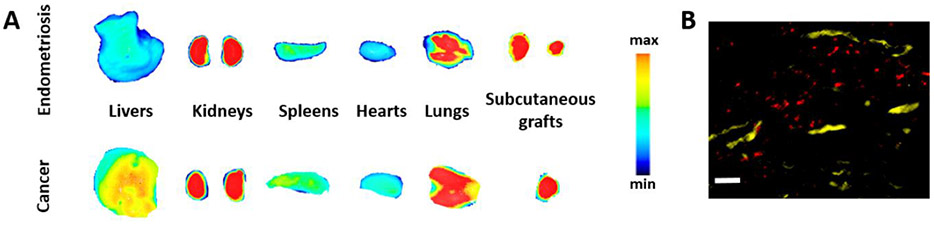
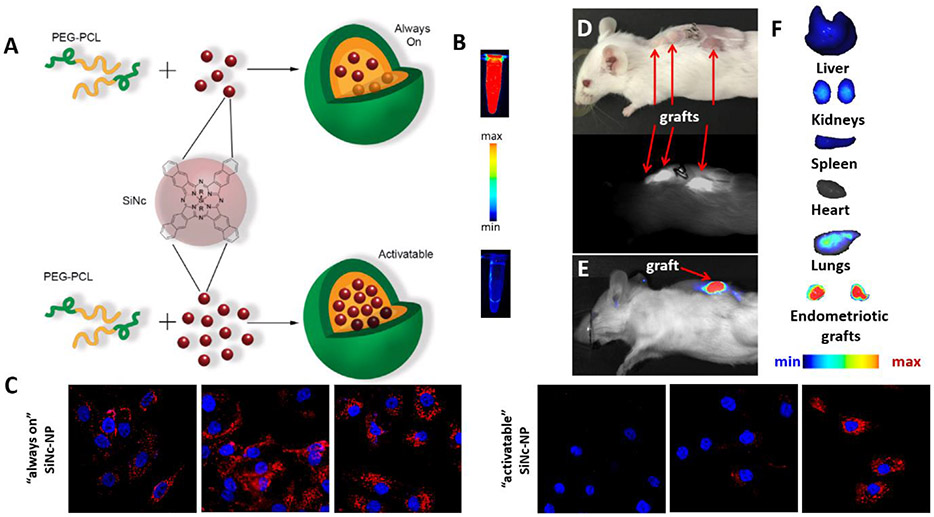
Taratula and colleagues reported that poly(ethylene glycol)-poly(ε-caprolactone) (PEG-PCL)-based polymeric nanoparticles (called SiNc-NP) with a hydrodynamic size of ~40 nm and slightly negative surface charge (−3 mV) efficiently accumulate in both endometriotic and cancer grafts and demonstrate similar biodistribution profiles within 24 h following their intravenous injection into mice bearing subcutaneous rhesus macaque endometriotic or human ovarian cancer xenografts (Figure 2A).[11, 21] Histological analysis further showed that these nanoparticles escape from the extensive blood vasculature and penetrate the endometriotic tissue (Figure 2B).[11]
Figure 2.
A) Fluorescence images of various organs, and endometriotic and ovarian cancer xenografts acquired at 24 h post-injection of near infrared (NIR) dye (SiNc) loaded PEG-PCL-based polymeric nanoparticles (SiNc-NP) into mice bearing subcutaneous endometriotic and ovarian cancer grafts. B) Fluorescence microscopy image of a section of endometriotic graft collected 24 h after intravenous injection of the tested nanoparticles. Red color indicates NIR fluorescence generated by NIR dye SiNc loaded into nanoparticles. Yellow color represents blood vessels stained with fluorescently labeled anti-CD31 antibody. Scale bar is 50 μm. A (top panel) and B adapted with permission.[11] Copyright 2020, Wiley-VCH. A (bottom panel) adopted under terms of the Creative Commons Attribution (CC BY-NC) license (https://creativecommons.org/licenses/by-nc/4.0/).[21] Copyright 2018, Ivyspring International Publisher.
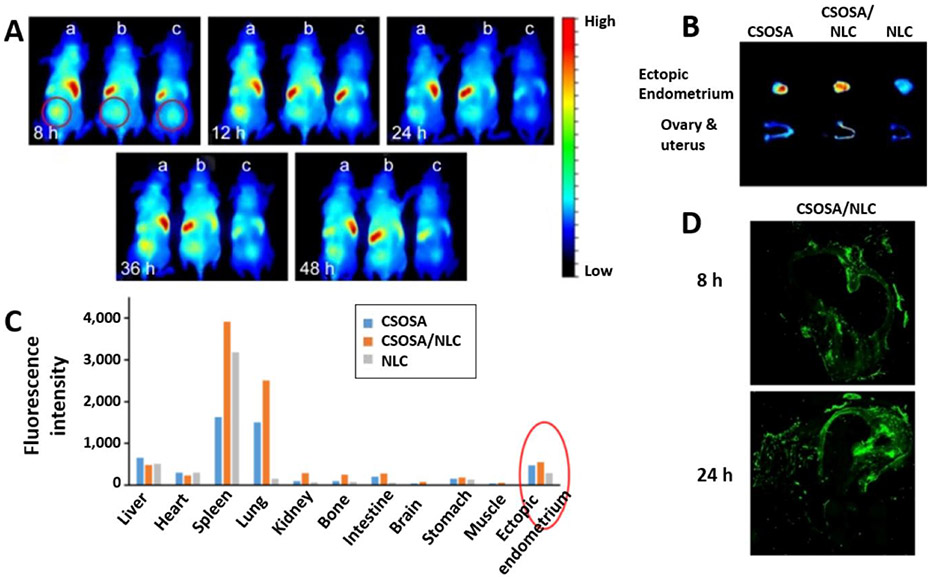
Recently, Yuan et al. demonstrated that three different types of near-infrared (NIR) dye-labeled nanoparticles, including chitosan oligosaccharide-g-stearic acid (CSOSA) polymer micelles (~68 nm), nanostructured lipid carriers (NLC, ~28 nm, and −18 mV), and CSOSA-coated NLC (~41 nm and +28 mV) could be detected in human endometriotic tissue (Figure 3A) subcutaneously implanted in mice at 8, 12, 24 and 48 h following tail-vein injection.[44] Ex-vivo fluorescence images of various tissues resected at 48 h post-injection further revealed that the accumulation of nanoparticles in endometriotic lesions was far higher than that observed in uterus, ovaries, kidneys, and some other organs, besides the liver, spleen, and lungs (Figure 3B and C). Finally, semi-quantitative analysis of fluorescence signal indicated that the accumulation of negatively charged NLC in endometriotic tissue was ~2 times lower when compared to positively charged NLC coated with CSOSA (Figure 3C), suggesting that surface charge may influence nanoparticle delivery and retention in endometriotic lesions. Previous reports suggest that the surface charge can have a dramatic effect on cellular internalization of nanoparticles, and positively charged nanoparticles are associated with higher cellular uptake. [46, 47] However, the role of surface charge in the internalization of nanoparticles by endometriotic cells remains to be investigated. The ability of the CSOSA-coated NLC to reach and penetrate endometriotic tissue at 8 and 24 h following systemic administration was also demonstrated histologically in rats bearing intraabdominal grafts of the uterine horn (Figure 3D). By using the same rat model, this research group demonstrated in a separate study that their CSOSA polymer micelles, electrostatically complexed with plasmid DNA (~135 nm and +6 mV), can be observed in abdominal endometriotic grafts as early as 1 h post-injection and remain there for at least 24 h.[45]
Figure 3.
A) NIR fluorescence images of female nude mice bearing subcutaneous human endometrial grafts (ectopic endometrium) recorded at 8, 12, 24, 36 and 48 h following tail vein injection of a) chitosan oligosaccharide-g-stearic acid (CSOSA) polymer micelles, b) nanostructured lipid carriers (NLC) and c) CSOSA-coated NLC (CSOSA/NLC) loaded with NIR dye DiR. The circles indicate the grafts. B) NIR fluorescent images of ectopic endometrium, ovary, and uterus resected at 48 h after tail vein injection of the tested nanoparticles. C) Accumulation of nanoparticles at 48 h post-injection in various tissues calculated as fluorescence per gram of tissue. The red circle indicates the distribution of CSOSA, CSOSA/NLC, and NLC in the ectopic endometrium. D) Fluorescence images of cryosections of the grafts observed following intravenous injection of the rat with fluorescein isothiocyanate (FITC)-labeled CSOSA/NLC at 8 and 24 h. Two pieces of rat uterine horn were sutured around mesenteric arteries, and two other pieces were sewn on each side of the peritoneum to induce endometriosis. Adapted with permission.[44] Copyright 2017, Dovepress.
Guo et al. also evaluated the biodistribution profiles of two types of nanoparticles with different sizes (10 nm vs. 40 nm) and chemical compositions following intravenous injection in mice bearing subcutaneous endometriotic grafts.[43] Although some differences were observed, the outcome revealed that both nanoparticles begin to accumulate in lesions within 12 h following systemic administration, extravasate from blood vessels into a deeper lesion matrix, and some are retained there for at least 144 h. Thus, following intravenous injection of mercaptopropionic acid-modified cadmium-telluride quantum dots (CdTe QD) with a hydrodynamic diameter of ~10 nm, the unambiguous fluorescence signal was detected in endometriotic lesions at 12 h, reaching peak accumulation at 24 h. In contrast to CdTe QD, the fluorescence signal from hollow gold nanospheres (HAuNS) with a hydrodynamic diameter of ~40 nm was observed in endometriotic lesions as early as 6 h post-injection, but the maximum intensity was not achieved until 72 h. It was hypothesized that the migration rate differs among nanoparticles of different sizes, inducing differential accumulation in the lesions. Quantitative analysis of the resected tissues at 72 h post-injection using inductively coupled plasma mass spectrometry (ICP-MS) further indicated that both QDs and HAuNS accumulated in the lesions at similar concentrations of ~5 μg Cd g−1 or Au g−1. Immunohistochemical evaluation of thin sections stained with CD31 antibody (an indicator of vascular endothelium) confirmed that both nanoparticles reach the vasculature and extravasate from there into tissues. The ICP-MS results also revealed that significant amounts of both nanoparticles reached the liver (~24 μg Cd g−1 and ~24 μg Au g−1) and spleen (~19 μg Cd g−1 and ~13 μg Au g−1) suggesting that the tested nanoparticles are cleared from the body by these organs. The tested elements were also detected in kidneys (~5 μg Cd g−1 and ~4 μg Au g−1), lungs (~2.5 μg Cd g−1 and ~5 μg Au g−1), and heart (~1.5 μg Cd g−1 and ~5 μg Au g−1). Finally, the ICP-MS measurements confirmed that Cd or Au was present in the lesions and tested organs at 144h post-injection. Of note, the previous report by the same research group revealed that the herein tested HAuNS demonstrated a similar biodistribution profile following systemic administration into mice bearing subcutaneous xenografts of lung (A549) or ovarian (SKOV-3) cancer cells.[48]
The studies described above, as well as additional reports (Table 1), reveal that nanoparticles with various core composition (e.g., Au, Cd, polymers, lipids, etc.), surface functionalization (e.g., PEG, peptides, mercaptopropionic acid, etc. ), size (10 – 150 nm) and surface charge (+30 – −20 mV) can accumulate in endometriotic tissue. Moreover, accumulation occurred within several hours (1 – 12 h) following systemic administration in mice and rats bearing subcutaneous and abdominal xenografts of human, monkey, and rodent endometrium.[11, 43, 44, 49] Histological analysis further demonstrates that these nanoparticles extravasate from blood vessels and penetrate endometriotic tissue.[11, 43] These results indicate that systemically injected nanoparticles can escape from the vasculature and accumulate in angiogenesis-dependent endometriotic lesions, possibly via the passive targeting mechanism, previously observed in cancer tumors and sites of infection/inflammation.[30, 31, 39, 42] This assumption is further supported by the fact that some nanoparticles demonstrate similar biodistribution profiles in rodent models of cancer and endometriosis.[11, 21, 43, 48] The available data also confirm that accumulated nanoparticles remain in endometriotic lesions for days, and neither of the reports demonstrated that nanoparticles were completely cleared from the diseased tissue.[11, 43] For example, the ICP-MS measurements provided by Guo et al. indicate that both tested QDs and HAuNS were present in the lesions at 144 h post-injection, although their concentration was ~3 - 5 times lower when compared to the 72 h time point.[43] Despite this experimental evidence, the mechanism of nanoparticle retention in endometriotic tissue is yet to be clarified and, therefore, additional studies are needed. The prolonged retention of nanoparticles in cancer tumors is explained by the lack of functional lymphatic drainage attributed to the poor development of the intratumoral lymphatic vessels.[32] Currently, there is no scientific evidence to hypothesize whether a similar mechanism inhibits the clearance of nanoparticles from endometriotic lesions. Furthermore, it is known that lymphangiogenesis is increased in ectopic endometriotic lesions compared to the endometrium and peripheral tissues.[50] The previous reports suggest that immune cells (e.g., macrophages) can be accountable for the retention of nanoparticles accumulated through permeable blood vessels in inflammatory tissues with functional lymphatic systems.[51, 52] Lee et al. also hypothesized that macrophages could internalize iron oxide nanoparticles after intravenous injection and facilitate their accumulation in endometriosis lesions, which are characterized by an increased presence of immune-related cells.[49] However, until now, it has not been demonstrated that this mechanism is responsible for nanoparticle retention in endometriosis.
2.2. Active Targeting
It is believed that active targeting is a complementary strategy to passive targeting aimed at increasing retention and accumulation of passively delivered nanoparticles in targeted tissues.[53, 54] This is achieved by modification of nanoparticles with targeting ligands (e.g., peptides, antibodies, small molecules) that can bind specific receptors overexpressed on target cells and facilitate internalization of the modified nanoparticles by these cells (Figure 1). Systemically injected nanoparticles, however, must first reach tissue expressing targeted receptors via passive targeting in order to take advantage of active targeting ligands.[54, 55] Active targeting has been extensively explored in cancer nanomedicine,[53, 54] and various reports ultimately concluded that in many cases, targeted nanoparticles outperformed non-targeted nanoparticles in vivo, affording at least a 2-fold increase in delivery efficiency to tumors.[56-58] Targeting ligands not only enhance the accumulation of various nanoparticles in tumors but, in some cases, also decrease their accumulation in healthy tissue, including organs of the reticuloendothelial system (e.g., liver).[15, 28, 58, 59] Several reports have already demonstrated that the accumulation of nanoparticles in endometriotic lesions can be enhanced by modifying their surface with targeting ligands that bind overexpressed receptors in endometriotic cells.[43, 60, 61] Collectively, these studies indicate that some receptors are overexpressed in both cancer and endometriosis and, therefore, active targeting strategies designed for cancer could be adapted for endometriosis.
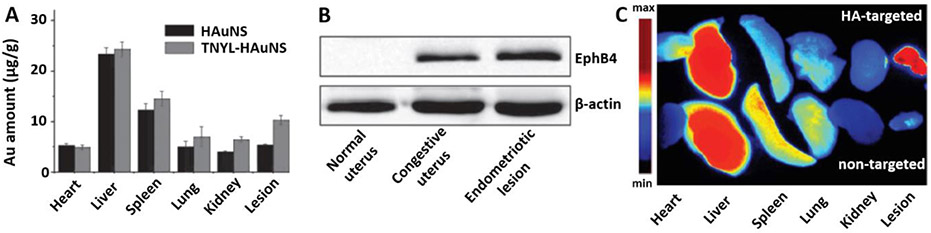
Guo et al. reported that the above mentioned hollow gold nanospheres (HAuNS) conjugated with TNYL peptide (TNYL-HAuNS), outperformed non-modified nanoparticles, affording ~2 times higher delivery efficiency to endometriotic lesions at 72 h post-injection (Figure 4A).[43] At the same time, the amount of both TNYL-modified and non-modified HAuNS was similar in other organs. The increase in nanoparticle concentration in lesions from mice administered TNYL-HAuNS was attributed to the targeting of the erythropoietin-producing hepatocellular receptor B4 (EphB4), overexpressed in endometriotic cells, with the TNYL peptide, which possesses a high binding affinity to this receptor. EphB4, a member of the family of receptor tyrosine kinases, is an indicator of high neovascularization in endometriotic lesions. The experimental data revealed that this receptor is highly expressed in endometriotic lesions, and expression was minimal in the uterus tissue (Figure 4B). However, EphB4 expression was significantly increased in the estrogenized uterus. Therefore, the authors suggested that Ephb4 receptor-targeted nanoparticles can be used for endometriosis treatment when the uterus is not in the cycling state (e.g., menses and estrus). The Ephb4 receptor has also been associated with tumor angiogenesis, growth, and metastasis, and is overexpressed in various cancer tissues.[62] By using mice simultaneously bearing both Ephb4 positive and Ephb4 negative cancer xenografts, this research group also revealed that the accumulation of TNYL-HAuNS was 3.2 times higher in Ephb4 positive tumor relative to Ephb4 negative tumor.[48] These results indicate that, in addition to passive targeting, exploitation of active targeting further enhances the accumulation of nanoparticles in cancer and endometriosis tissues overexpressing Ephb4 receptors.
Figure 4.
A) Gold (Au) content measured by ICP-MS in various tissues 72 h after intravenous injection of gold nanospheres (HAuNS) and HAuNS conjugated with TNYL peptide (TNYL-HAuNS) into nude mice bearing subcutaneous xenografts of rat uterus. B) Expression of EphB4 receptor of healthy mouse uterus, estrogen-treated uterus (e.g., congestive, a term used in the article), and endometriotic lesion by Western blot. Adapted with permission.[43] Copyright 2017, Wiley-VCH. C) Fluorescence images of various organs and endometriotic lesions resected at 48 h post-injection of non-targeted and hyaluronic acid (HA)-targeted nanoparticles into rats with endometriosis lesions. Endometriosis lesion animal model was created by transplanting autologous fragments of the uterus to the inner surface of the abdominal wall. Adapted with permission.[61] Copyright 2016, Dovepress.
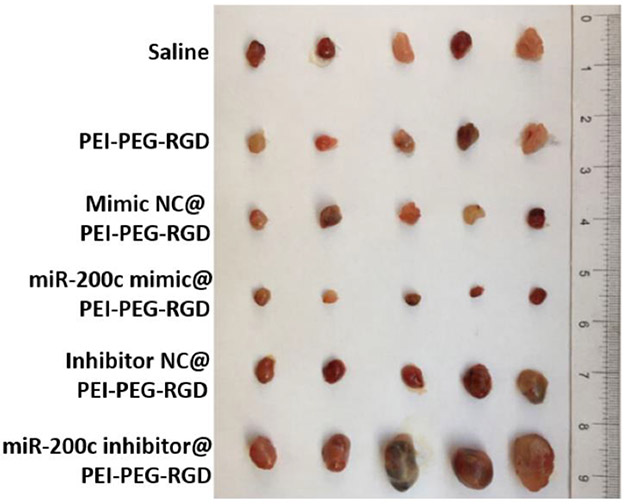
Several research groups evaluated hyaluronic acid (HA) as a potential targeting ligand to the CD44 receptor, which was previously suggested to be overexpressed in endometriosis cells and human endometriotic lesions.[63, 64] It was also revealed that CD44 expression in normal endometrium is detected only in the secretory but not in the proliferative phase, indicating hormonal regulation of this receptor.[65] Zhao et al. observed little to no fluorescence signal in intraabdominal endometriotic lesions of rats intravenously injected with polyethylenimine-grafted chitosan oligosaccharide nanoparticles (<150 nm) containing small interfering RNA (siRNA) and a fluorescent dye (DiR) (Figure 4C).[61] A very strong NIR fluorescence signal, however, was observed in the liver and less intense signal in the spleen and the lungs. When the same nanoparticles were decorated with hyaluronic acid, an intense fluorescence signal was observed in endometriotic lesions 48 h following intravenous injection, and a dramatically reduced signal was present in the spleen and lungs. Immunohistochemical analysis revealed that CD44 expression was significantly increased in endometriotic lesions when compared to endometrium collected from the same rats. In another study, Zhang et al. evaluated ~15 nm iron oxide nanoparticles modified with hyaluronic acid in a rat model of endometriosis. The ability of these nanoparticles to accumulate in CD44 expressing cancer tumors was previously reported.[66] The ICP-MS measurements and histological analysis revealed that these nanoparticles efficiently accumulated in the ectopic uterine tissues engrafted in the abdominal side of rats within 2 h following intravenous injection, and iron staining was observed in and around the blood vessels.[60] In contrast, little to no iron staining was detected in eutopic uterine tissues, demonstrating preferential accumulation of the targeted nanoparticles in endometriotic tissue. However, the expression levels of CD44 in the tested ectopic and eutopic uterine tissues were not provided, and the biodistribution profile of the non-targeted nanoparticles was not evaluated.
As a proliferative disease, endometriosis requires cholesterol in order to build new cellular membranes. In order to satisfy this increased demand, low-density lipoprotein (LDL) receptors in endometriotic foci are overexpressed.[67] This phenomenon has also been observed and exploited for drug delivery in various forms of cancer including ovarian and breast cancers.[68, 69] Therefore, the uptake of LDL and, by extension, lipid nanoparticles in endometriotic tissues could be increased. In a 2019 study, Bedin et al. quantified the uptake of lipid nanoparticles in endometriotic lesions of affected patients scheduled for surgical intervention.[70] One day before surgery, patients were intravenously administered 0.1 mL of solid cholesterol lipid core nanoparticles containing cholesterol [14C]-oleate. Following resection and biopsy, 14C in endometrial, peritoneal, and endometriotic tissues were evaluated using liquid scintillation spectrometry. The highest levels of uptake were observed in the peritoneum and endometrium of control subjects without endometriosis. In patients with intestinal endometriotic lesions, the highest uptake was observed in the endometrium. In endometriosis patients without intestinal lesions, the highest levels of uptake were observed in the peritoneum and endometrium. Although there was no significant difference in uptake between the intestinal and non-intestinal lesion groups, and the highest uptake was observed in healthy control patients, the uptake of lipid nanoparticles by endometriotic tissue, in general, is notable, and the first study of its kind thus there is no data to which to compare these results. Furthermore, although subjects received no hormone therapy for three months before surgery, it was not clear whether any were concurrently menstruating and, if so, during which menstrual stage nanoparticles were administered. Further investigation is needed to determine whether overexpressed LDL receptors can be exploited for targeted delivery of nanomedicines to endometriotic tissue, and the use of relevant animal models of the disease would allow researchers to optimize treatment strategies before translating any modality to a clinical setting.
2.3. Differential accumulation of nanoparticles in endometrium and endometriosis
While endometriosis is endometrium-like, the heterotopic tissues display striking differences from endometrium. These differences could reduce endometrial exposure to nanoparticles relative to the disease tissue. Endometrium within the uterus is histologically organized, a columnar/cuboidal luminal epithelium overlaying a thick lamina propria. In primates, the endometrium forms two distinct zones;[71] the upper (luminal) functionalis zone (e.g., stratum functionalis), and deeper basalis zone (e.g., stratum basalis), adjacent to the myometrium. Menstrual bleeding is restricted to the functionalis zone.[72, 73] Tubular glands extend from the basalis near the myometrium through the functionalis zone to the uterine lumen. The functionalis is vascularized by a unique arterial supply, the spiral arteries, which form columns arising from the myometrium. Blood drains from the functionalis zone via veins through the basalis.[74, 75] Lymphatic vessels are sparse and localized to the basalis.[76-78] The vascular basalis to functionalis gradients contribute to physiological hypoxia and regulation of VEGF, VEGF receptor (KDR), and matrix metalloproteinases (MMPs) in the functionalis zone. These gradients parallel tissue dissolution during menstruation.[79, 80] Thus, the administration of nanoparticles at times other than menstruation and ovulation could reduce endometrial effects.
Compared to uterine endometrium, the histological presentation of endometriosis appears to promote the passive accumulation of nanoparticles within lesions. For example, Yuan et al. demonstrated that nanoparticles preferentially accumulate in endometriotic lesions when compared to the uterus (Figure 3B).[44] Endometriosis is a heterogeneous growth of dysfunctional glands and stroma that invades tissues and organs outside the uterus.[81-83] The ectopic tissues appear histologically disorganized, and the type of lesion (ovarian, peritoneal, deep infiltrating) dramatically impacts the histological presentation.[11, 81-83] Endometriotic lesions lack spiral artery columns, and zonal gradients, as seen in the endometrium, are not present.[72] Abundant small vessels (veins and arteries) support the tissues, and lymphatic drainage is extensive.[50, 84, 85] Bleeding and tissue fragmentation associated with tissue breakdown accumulate in the formation of endometriotic cysts.[86, 87] Cyclic tissue dissolution and bleeding occur at foci throughout the endometriotic lesions with upregulation of matrix metalloproteinases and increased expression of HIF-1 alpha, VEGF, and VEGF receptors. As indicated earlier, this results in higher absolute levels of both VEGF and KDR in endometriotic lesions compared to the endometrium. VEGF is a robust vascular permeability factor and promotes an inflammatory process that could lead to increased passive accumulation of nanomedicines. Targeting KDR and VEGF-receptor C could increase specific targeting of endometriosis through the elevated levels of lymphatics.
During the primate menstrual cycle, estradiol (E2 ) secreted in the follicular phase stimulate cell proliferation in endometrial glands, stroma, and vasculature.[74] In the luteal phase, progesterone (P4 ) attenuates cell proliferation and induces secretory differentiation of the gland and decidualization of the stroma.[71] Regulaton of steroid hormone receptors in mice have been reviewed previously.[71, 88, 89] The decline in P4 and the end of the cycle stimulate endometrial breakdown, and menstruation ensues. It is noteworthy that compared to primates , mice do not menstruate and undergo a short estrous cycle (4–5 days), but the endometrial function is similarly regulated by E2 and P4 via these nuclear receptors. Therefore endometriotic tissues engrafted into immunodeficient mice require hormonal supplementation to maximize growth.
Endometriosis is an E2 dependent disorder, but the endometriotic response to cyclic changes in the steroid hormones is unpredictable relative to the endometrium.[89, 90] In contrast to uterine endometrium, endometriosis, displays a variable reduction in response to P4, a condition often referred to as P4 resistance where the effects of P on the differentiation of both the lesions and endometrium are attenuated.[89, 91, 92] E2 and P4 induce their effects via interactions with nuclear receptors. Two functionally distinct estrogen receptors (ESR-1; ERα and ESR-2; ERβ), and two structurally related P4 receptors (PGR-A and PGR-B) have been well-characterized.[94-96] Strong immuno staining for ESR-1 and PGR is present in the vascular pericytes and stroma; thus, it has been speculated that paracrine factors could mediate E2 regulation of vessel growth. ESR-2, however, is localized to many cell types in the endometrium, including vascular endothelial cells.[89] Therefore, the effect of E2 on endothelial cell proliferation could be mediated either directly via ESR-2 or indirectly via paracrine factors.
There is a growing number of reports that ESR-2 is overexpressed in endometriosis compared to the endometrium.[93, 98] Therefore, estrogen can act throughout the menstrual cycle to drive cell proliferation, including new vessel growth. Moreover, partial P4 action can increase the likelihood if dysfunctional bleeding in the lesions promoting inflammation and the influx of macrophages promoting the uptake of nanoparticles.
3. Endometriosis Animal Models
The assessment of novel nanoparticles for the treatment and imaging of endometriosis in women requires the use of animal models because these new nanomaterials have not been vetted for clinical use. Spontaneous endometriosis only occurs in women and menstruating Old World nonhuman primates (NHPs).[90] Therefore, NHPs, in theory, provide the most relevant model for human disease. As in women, there are no serological (non-invasive) tests for endometriosis in NHPs. Therefore, methods to create the disorder in macaques and baboons have been extensively studied.[89, 92] Artificial induction of endometriosis in primates was achieved in early studies through repositioning of the cervix in rhesus macaques and occlusion of the cervix in baboons, both methods increasing the exposure to retrograde menstruation and demonstrating the development of spontaneous endometriosis similar to that in humans.[91] Transplantation of endometrial fragments and endometriotic lesions are also efficient methods for induction of endometriosis and can be achieved either through surgical transplantation or injection of endometrial tissues, to the intraperitoneal cavities of primates.[93, 94] Overall, the primate models are more useful for studying the development and progression of endometriosis and allow comparison of physiological phenomena before and after disease induction. However, research with NHP models is limited by the high cost of animals and greater infrastructure for maintenance and handling of the animals. Therefore, the use of non-primate models has been explored extensively.[95]
Rodents display short estrous cycles and do not menstruate and, as such, do not develop spontaneous endometriosis. However, ectopic endometrium can be created in these animals by transplantation of uterine tissues. Transplantation models can be classified as homologous or heterologous, depending on the source of tissue and the animal into which the tissues will be transplanted. Homologous models are those in which endometrial fragments are transplanted to genetically similar, or syngeneic, immunocompetent animals. Heterologous models usually employ the collection of endometrial tissues from humans and their implantation in immunodeficient rodents, though this has also been performed using endometrium of primate origin. These rodent models are established similarly to primate models with regard to transplantation; tissues are surgically implanted or injected, generally, intraperitoneally or subcutaneously.[96, 97]
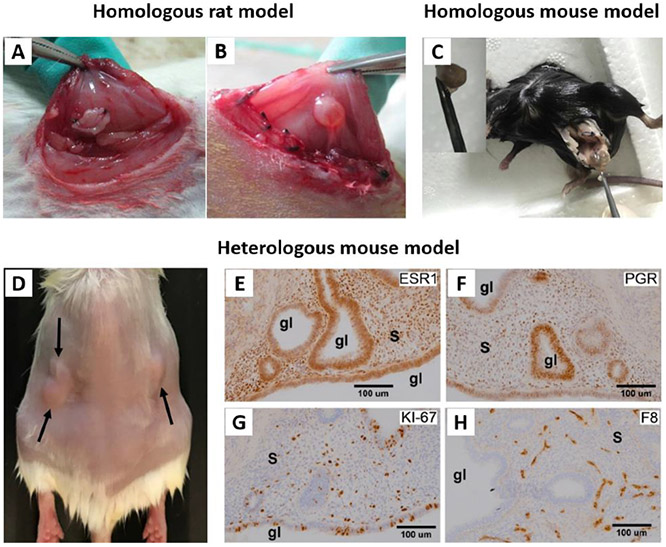
Despite the extensive use of non-primate models, these models have limitations relative to the development of new therapies for the disorder.[95] Nonhuman primates should be considered optimal animal models for nanoparticle development, but at the time of this literature review, there are no reports of primate endometriosis models being explicitly used in nanomedicine research. Several heterologous and homologous rodent models have been utilized for various nanomaterial-based imaging and treatment platforms (Table 2). Surgically-induced endometriosis in rats, through transplantation of autologous fragments of uterus to the inner surface of the abdominal wall (Figure 5A and B), is the most commonly used model in the reported studies (Table 2). The decision to use a rat model for these studies may be due, in part, to the larger size of the rat compared to the mouse, and the subsequent improved ability to locate the larger endometriotic lesions that form in, the larger rodents. This homologous rat model was employed to evaluate magnetic iron oxide nanoparticles as MRI contrast agents,[49, 60] the effect of various nanoparticle-mediated gene therapies on the progression of endometriotic lesions,[45, 61, 98] and the effectiveness of a small molecule, delivered via nanoparticles, in attenuation of endometriosis pain (Table 2).[44]
Table 2.
Summary of animal endometriosis models used to evaluate nanomedicines.
| Models | Description of animal model | Tested nanoparticle- based approach |
Reference |
|---|---|---|---|
| Homologous rat model | Abdominal grafts of autologous uterine tissue | Pain treatment | Yuan et al., 2017[44] |
| MRI | Lee et al., 2012[49] Zhang et al., 2014[60] |
||
| Gene (DNA) therapy | Zhao et al., 2012[45] | ||
| Gene (siRNA) therapy | Zhao et al., 2016[61] | ||
| Gene (miRNA) therapy | Liang et al., 2017[98] | ||
| Homologous mouse model | Abdominal grafts of mouse uterine tissue | Combinatorial therapy | Jana et al., 2014[100] Singh et al., 2015[101] |
| Antioxidant therapy | Chaudhury et al., 2012[99] | ||
| Local therapy | Boroumand et al., 2019[102] | ||
| Immunotherapy | Liu et al., 2017[103] | ||
| Heterologous mouse model | Subcutaneous grafts of NHP endometrium tissue | Photothermal therapy | Moses et al., 2020[11] |
| Subcutaneous grafts of human endometrium tissue | Drug delivery | Yuan et al., 2017[44] | |
| Subcutaneous grafts of rat uterine tissue | Photothermal therapy | Guo et al., 2017[43] | |
| Subcutaneous grafts of human endometrial cells | Gene (DNA) therapy | Wang et al., 2014[104] |
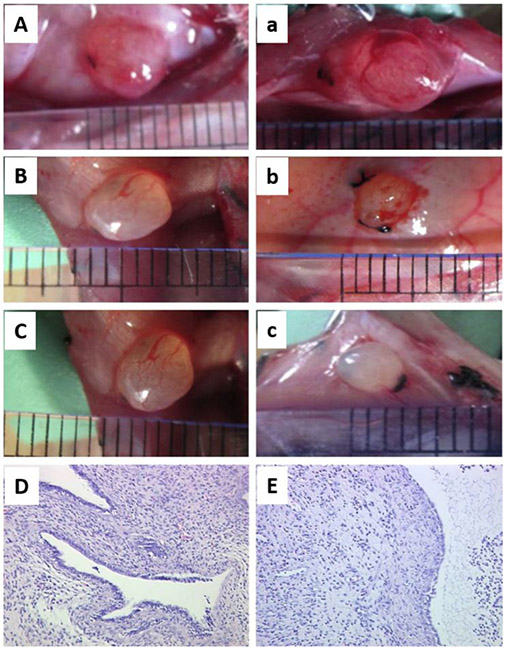
Figure 5.
A and B represent different stages of establishing homologous endometriosis model in rats. A) After transplantation of autologous uterus fragments to the inner surface of the abdominal wall. B) The transplanted fragment developed into ovoid, fluid-filled cystic lesion. Adapted with permission.[45] Copyright 2012, Elsevier. C) Homologous mouse model of endometriosis. To induce endometriotic cyst, a left uterine horn was removed from a C57 mouse, and separated endometrium tissue was sutured to the peritoneal wall. Adapted with permission.[103] Copyright 2017, Elsevier. D-H) Heterologous mouse model of endometriosis. D) SCID mouse bearing subcutaneous grafts of endometriotic tissue collected from rhesus macaques with advanced endometriosis. E-H) Images showing immunohistochemical staining (brown color) for E) ESR1, F) PGR, G) KI-67, and H) Factor 8 in endometriotic grafts collected from these mice. “s” indicates stroma and “gl” indicates glands. Adapted with permission.[11] Copyright 2020, Wiley-VCH.
Nanoparticles have also been tested in heterologous and homologous mouse models to image or treat endometriosis (Table 2). Homologous endometriosis models were established either by injecting suspension of minced uterine horns from donor mice into the peritoneal cavity of recipient animals[99-102] or surgical transplantation of autologous uterine fragments to the peritoneal wall (Figure 5C).[103] Cerium oxide nanoparticles were used to inhibit angiogenesis and decrease oxidative stress in these mouse models of endometriosis, as were polymeric nanoparticles loaded with a combination of epigallocatechin gallate and doxycycline.[99, 101] Nanomaterials have also been tested in these mice to investigate the use of immunotherapy to treat endometriosis,[103] as well as the therapeutic effects of molecules like letrozole and curcumin.[100, 102]
Finally, heterologous mouse models with subcutaneous grafts of human, macaque, and rat endometrial tissues, as well as grafts of human endometriotic cells, were employed for evaluation of several nanomedicine-based strategies (Table 2). The biodistribution of polymer- and lipid-based nanoparticles were assessed following tail vein injection in nude mice transplanted with human endometrial tissues and treated with 17-β estradiol.[44] Mice bearing subcutaneous grafts of adenovirus-GFP transfected primary cells isolated from human endometrium were used to evaluate therapeutic efficacy of nanoparticle-based antiangiogenesis gene therapy.[104] The effects of photothermal ablation on endometriotic lesions using a combination of systemically delivered gold nanoparticles and near-infrared (NIR) light were investigated in nude mice subcutaneously injected with fragments of rat uterus.[43] Finally, SCID mice subcutaneously engrafted with macaque endometrium were employed to evaluate nanoparticle-based photothermal therapy and NIR fluorescence imaging (Figure 5D).[11] It was reported that established lesions in rodents are similar to endometriosis those in macaques. To reproduce macaque length artificial “menstrual” cycles, animals were engrafted with implants releasing P4 and E2. Identical to macaque endometriosis, the established grafts are well-vascularized (Factor 8 staining, Figure 5H), and characterized by the presence of endometriotic glands and, stroma, estrogen receptor 1 (ESR1, Figure 5E), and P4 receptors (PGR, Figure 5F). Furthermore, the abundance of proliferating cells (KI-67 staining, Figure 5G) suggests that grafts grow in response to estrogen stimulation. The summarized articles in Table 2 represent, to date, the entirety of nanomaterial-based investigations using animal models of endometriosis.
4. Nanoparticle-Based Imaging Strategies
Because there are no serological diagnostic tests for endometriosis, there is a significant delay in the diagnosis of this disease, especially in young women.[4, 5] Laparoscopy with histological confirmation is currently the most reliable diagnostic procedure, but it is expensive, and carries surgical risks.[105] Moreover, several studies have shown that this approach is not always reliable, and only 54-67 % of suspected lesions are validated histologically. [105] Given this potential hazard, non-invasive medical imaging techniques such as ultrasound and MRI are also used to detect endometriosis.[105] Ultrasound is a readily available and inexpensive tool for the diagnosis of large endometriotic lesions and is currently the preferred method for initial assessments. However, the relatively small field of view, as well as dependency on the experience of the sonographer, pose significant limitations.[106] On the other hand, MRI provides better soft-tissue contrast and is being increasingly implemented in endometriosis evaluations and lesion detection, especially for those that are not visible under ultrasound.[107] Despite MRI‘s advantages, its low sensitivity hampers the visualization of small and deep infiltrating endometriotic lesions.[108] It is reported that hemorrhagic components associated with menstrual bleeding lead to hyperintense T1- and T2-weighted MR images (T1-WI/T2-WI) of endometriotic lesions.[109] Therefore, commonly available diagnostic T1 contrast agents such as paramagnetic gadolinium complexes provide limited useful information about endometriotic lesions. To address the current limitations in imaging and diagnosis of endometriosis, several research groups sought to improve both MRI techniques and laparoscopy using nanoparticles.
4.1. MRI
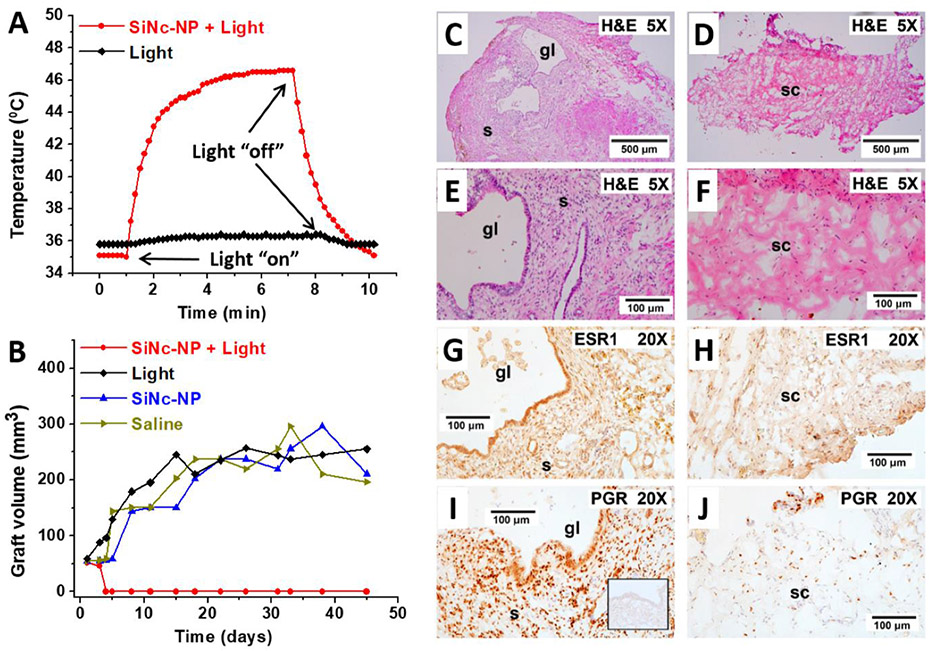
Several reports propose that the lesion-to-background signal ratio in MRI can be increased by implementing T2-negative contrast agents such as magnetic iron oxide nanoparticles.[49] Lee et al. investigated the use of ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles as an MRI contrast agent in an experimental rat intraperitoneal endometriosis model.[49] Images obtained 6 h following intravenous injection of either USPIO or gadolinium-labeled diethylenetriamine pentaacetic acid (Gd-DTPA) were interpreted independently by a radiologist and a gynecologic surgeon. Results indicated an increase in detection and border contrast of ectopic uterine tissues 3 mm or greater in size when using USPIO-enhanced T2-weighted images (T2-WI) in comparison to Gd-DTPA-enhanced T2-WI (Figure 6).
Figure 6.
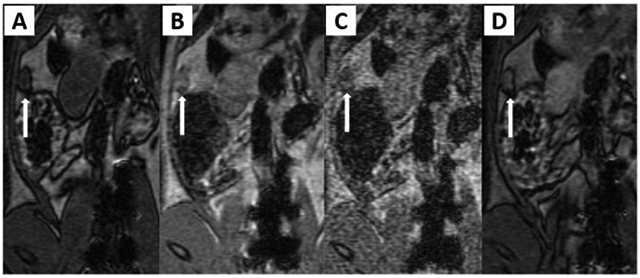
Four MR sequences for the detection of ectopic endometrial tissues. Endometriosis was surgically induced in the rats by transplanting an autologous fragment of ectopic uterine tissue onto the inner surface of the abdominal wall, the posterior surface of the uterine body and the arterial cascades (proximal and distal) of the small intestine adjacent to mesenteric blood vessels. A nodular lesion about 6.5 mm in size (arrow) on the abdominal wall reveals hypodense signal intensity on T1-WI (A), moderate enhancement on gadolinium-enhanced T1-WI (B), high signal intensity on T2-WI (C) and high signal intensity with peripheral dark signal intensity on USPIO-enhanced T2-W MR image (D). Reproduced with permission.[49] Copyright 2012, Elsevier.
In another report, Zhang et al. tested their previously synthesized hyaluronic acid (HA)-modified iron oxide nanoparticles (HAIONPs) for in vivo imaging of surgically induced endometriotic lesions in rats on a 3.0 Tesla MR scanner.[60] Following intravenous injection of nanoparticles, the MR signal for the wall of ectopic uterine tissue gradually declined, and the highest contrast enhancement was achieved after 2 h compared to earlier time points (Figure 7).
Figure 7.
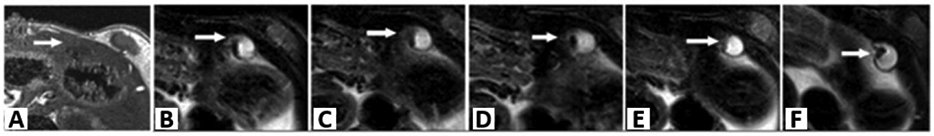
MR images of the ectopic endometriotic lesions at different time points. On T1WI, the ectopic uterine tissues (EUTs) (arrow) appeared as irregular cystic mass with low signal (A). On axial FS-T2WI before injection (B), the EUTs appeared to have slightly high signal intensity surrounding with intermediate signals of fibrous walls. At 15 min (C), 30 min (D), 60 min (E), and 120 min (F) post-injection, the wall of lesions was more clearly outlined, and the lesion to background contrast was improved compared with (A). Reproduced under terms of the Creative Commons Attribution License.[60] Copyright 2014, Zhang et al., published by PLOS ONE.
The available reports indicate that superparamagnetic nanoparticles can enhance the role of MRI in the diagnosis of endometriosis. However, additional research is required to demonstrate any potential that MRI may possess in diagnosing endometriosis
4.2. Fluorescence Imaging
The efficient detection and resection of endometriotic lesions depend on the surgeon’s ability to distinguish between healthy tissue and endometriosis during laparoscopy. Endometriotic lesions can have multiple forms and color manifestations.[110] Some lesions appear pigmented due to a higher level of hemosiderin content, giving them a dark blue or brown color, and are easier to identify intraoperatively. On the other hand, non-pigmented lesions are usually pale and more difficult to identify. Therefore, the utilization of real-time fluorescence surgical imaging techniques could enhance the detection of non-pigmented lesions. However, in order to visualize these lesions via fluorescence, there must be a cellular specific accumulation of fluorescent imaging agents. One such molecule for fluorescence image-guided surgery is protoporphyrin IX (PPIX), which has been shown to accumulate in cancer cells such as malignant gliomas after exogenous administration of aminolevulinic acid; the rate-limiting step in PPIX formation.[111] Owing to similarities between cancer and endometriotic lesions, the administration of exogenous ALA was also thought to induce the intracellular accumulation of fluorescent PPIX in lesions. Previous human and animal studies indicated that the administration of 5-ALA leads to preferential accumulation of PPIX in endometriotic lesions compared to adjacent normal peritoneum.[111, 112] Fluorescence was excited at 380 to 440 nm, spectra in the visible region were collected, and higher porphyrin fluorescence was observed in active peritoneal endometriosis relative to adjacent normal peritoneum.[113] Although the exact reason for the preferential accumulation of 5-ALA-induced PPIX in endometriotic lesions is still unclear, previous cancer studies have suggested the involvement of tumor-associated cellular alterations such as increased levels of the active transporter PEPT1 mRNA in renal cell carcinoma,[114] malfunctioning of the enzyme ferrochelatase that converts PPIX to heme in particular cancer types,[115] and mitochondrial energy metabolism disorder.[116] Although different cancer cells might exhibit different mechanisms for the accumulation of 5-ALA-induced PPIX, the observation that endometriotic tissues are also able to accumulate PPIX through exogenous ALA administration suggests the presence of potential similarity between cancer tissue and endometriosis.
The increased diagnostic accuracy of non-pigmented endometriotic lesions during laparoscopy after oral administration of ALA at different concentrations (20 mg kg−1 body weight, and 30 mg kg−1 body weight) to patients with endometriosis 10-14 h before surgery was documented in previous studies. Sensitivity and specificity were increased up to 100% and 75%, respectively, with fluorescence diagnosis as compared to 69% and 70% under white light.[117, 118] Although PPIX accumulation was shown to correlate with the availability of 5-ALA, none of the above studies took female sex hormones into consideration. Therefore, a different study evaluated ALA-induced PPIX fluorescence of normal endometrial epithelial cells in vitro in the presence of E2 or combination of E2 and P4.[119] The results showed higher fluorescence from PPIX accumulation in cells incubated with the hormones than cells without hormone treatment, suggesting their importance in PPIX accumulation.
Although fluorescence in endometriosis benefits from exogenous ALA administration, the effect was only limited to nonpigmented lesions and, thus, no fluorescence could be detected in pigmented and ovarian endometriosis using this approach.[113] However, previous sequential laparoscopic studies have shown that nonpigmented endometriotic implants usually evolve to pigmented lesions, in which exogenous ALA does not affect fluorescence.[120] In addition, there were also small false-positive fluorescent spots present and patients needed to avoid exposure to intense white light for a period of 24 - 48 h post ALA administration in order to prevent cutaneous phototoxic damage. Furthermore, ALA exhibits short half-life at physiological pH, and protoporphyrin IX has a short clearance time of 7.8 h.[112] These shortcomings have prompted researchers to look for a more precise fluorescence guided surgery technique such as the use of NIR dyes and light for endometriosis diagnosis.[121]
NIR light has relatively high tissue penetration and lower scattering with no tissue damage even after prolonged exposure.[122] Conversely, visible light is unable to penetrate deeper into blood and tissue because of energy depletion due to absorbance and scatter, allowing only surface features to be visible. In addition to higher penetration, major biomolecules such as deoxyhemoglobin, oxyhemoglobin, water, and lipids have local absorption minima in the NIR window, which spans from 650 to 900 nm.[122] Therefore, NIR fluorescence-guided surgery based on NIR dyes and activation light is a promising route to ensure real-time demarcation of endometriotic lesions, thereby decreasing the possibility of recurrence or healthy tissue damage.
One of the most widely used contrast agents for NIR imaging is the FDA approved tricarbocyanine dye indocyanine green (ICG).[123] Although it is extensively used in medical applications, most of the dye (~95%) remains intravascular due to its high affinity to plasma protein, which can limit its diffusion into target sites.[124] Furthermore, ICG in solution tends to aggregate and form oligomers even at low concentrations.[125] ICG readily succumbs to photobleaching and has also been shown to be relatively unstable in aqueous solution, though the albumin component in plasma aids in slowing the decay rate to some extent.[126] These changes, in addition to its high concentration dependence, cause a shift in the absorption spectra of the dye depending on the solvent in which it is dissolved, and can decrease its fluorescence.[127] In order to combat instability, researchers have used molecular additives like sodium polyaspartate (PASP) to prevent aggregate formation and enhance aqueous stability.[128] Previous studies have pointed to the benefits of using monoclonal antibody-dye conjugates to enhance delivery and accumulation of molecules of interest in endometriotic lesions. However, ICG by itself is unable to form these conjugates. Thus, only a modified version of ICG, such as ICG-sulfo-OSu, can be used for coupling.[129] Furthermore, even when this coupling is made possible using ICG-sulfo-OSu, a high fluorescence loss is observed upon protein binding. Therefore, to meet the need for tumor targeting contrast agents, researchers have focused their attention on other NIR fluorophores, such as IRDye 800CW, that can easily be conjugated to different biomolecules. According to clinicaltrial.gov, a clinical study (NCT02975219), currently in phase 1, is being conducted on the application of IRDye 800CW labeled bevacizumab (Bevacizumab-800CW) as a targeting fluorescent molecule for use in fluorescence-guided surgery in endometriosis. This is following an endometriotic tissue biomarker study, which reported the overexpression of vascular endothelial growth factor A (VEGF-A) in endometriosis, therefore suggesting the potential use of an anti-VEGF monoclonal antibody, bevacizumab, conjugated to isotopes or fluorescent agents for lesion visualization.[130] Although there does not seem to be endometriosis-specific photoimmunodetection and toxicity in animal studies using Bevacizumab-800CW, there have been other extensive studies to determine its safety and pharmaceutical quality, and to assess its potential for in-human use.[131] This could potentially improve outcomes in endometriosis treatment as previous studies have taken advantage of IRDye 800CW-antibody conjugates to enhance tumor-to-background contrast during fluorescence-guided surgery in cancer patients.[132] It is also important to mention that other clinical trials are currently underway, such as NCT03935165, that use ICG for fluorescence-guided surgery.
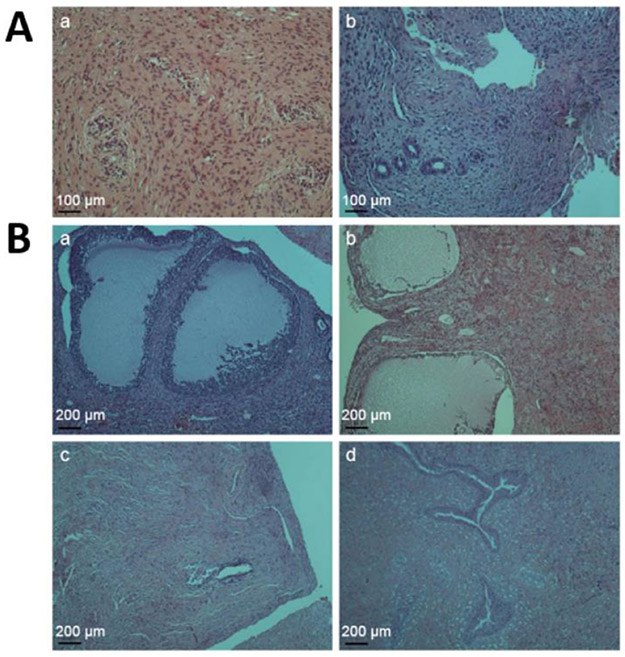
One study explored the potential use of nanoparticle-based platforms for the delivery of NIR fluorescent dyes after systemic administration to endometriotic lesions.[11] The research team implemented “activatable” silicon naphthalocyanine loaded PEG-PCL nanoparticles (SiNc-NP) that efficiently accumulate in endometriotic lesions following systemic administration. SiNc was chosen in this study because of its improved fluorescence efficiency and photostability as compared to other contrast agents such as ICG. Although SiNc is a promising contrast agent in fluorescence-guided surgery, its application is hindered by limited water solubility (<1 ng mL−1).[21] Therefore, the study used a biocompatible amphiphilic copolymer, PEG–PCL, consisting of hydrophilic poly(ethylene glycol) (PEG) and hydrophobic poly(ε-caprolactone) (PCL) blocks, to encapsulate SiNc and improve its aqueous solubility and delivery to endometriotic lesions (Figure 8A). It was demonstrated that the proximity of SiNc molecules inside the hydrophobic core of PEG–PCL polymer led to reversible intermolecular aggregate formation, which in turn led to the self-quenching of fluorescence (Figure 8A and B). This feature provides high contrast during fluorescence imaging of endometriosis because the developed “activatable” nanoparticles release SiNc molecules and reactivate their fluorescence following internalization by endometriotic cells (Figure 8C). SiNc-loaded PEG-PCL nanoparticles with “always on” fluorescence were used as a control (Figure 8A-C). In vivo studies confirmed that 24 h following systemic administration, the “activatable” SiNc-NPs accumulated in the subcutaneous endometriotic grafts resulting in the delineation of endometriotic lesions through NIR fluorescence (Figure 8D-F). These observations were made using an FDA-approved intraoperative imaging system, Fluobeam® 800, adding a layer of clinical relevancy and translational convenience (Figure 8D). These results are in line with a previous study that showed preferential accumulation and activation of SiNc-NP in ovarian cancer cells after systemic administration, which further strengthens the possible pathophysiological overlap between cancer tumors and endometriotic lesions.[21]
Figure 8.
A) Schematic illustration of “always on” and “activatable” SiNc-loaded PEG-PCL nanoparticles (SiNc-NP). Green and orange colors represent hydrophilic PEG outer shell and hydrophobic PCL core of nanoparticles, respectively. In contrast to ‘always on” SiNc-NP (top panel), “activatable” SiNc-NP (lower panel) contain a higher amount of SiNc molecules (dark red spheres) inside the hydrophobic cores, causing fluorescence self-quenching. B) Fluorescence images of “always-on” and “activatable” SiNc-NP solutions acquired using the Pearl Impulse Small Animal Imaging System. Scale bar: The blue and red colors reflect the lowest and highest fluorescence intensity, respectively. C) “Activatable” SiNc-NP generates fluorescence after internalization into endometriotic cells and subsequent relaxation of densely-packed, self-quenched SiNc molecules in the intracellular environment. Representative fluorescence microscopy images of macaque endometriotic stromal cells incubated with “always-on” SiNc-NP (left panel) and “activatable” SiNc-NP (right panel) for 1, 2, 4, and 24 h. The red and blue colors reflect the fluorescence signal generated by SiNc and NucBlue (nuclei staining), respectively. D) Photograph (top) and NIR fluorescence image (bottom), recorded with Fluobeam 800, of a mouse bearing endometriotic grafts 24 h after intravenous injection of “activatable” SiNc-NP. E - F) NIR fluorescence images of a mouse bearing endometriotic graft (E) and resected tissues (F) recorded with Pearl Impulse Small Animal Imaging System 24 h after intravenous injection of “activatable” SiNc-NP. Adapted with permission.[11] Copyright 2020, Wiley-VCH.
5. Nanoparticle-Based Treatment Modalities
The literature review suggests that various nanoparticle-based modalities were developed for endometriosis treatment, including gene therapy, phototherapy and immunotherapy (Table 1). Nanoparticles were employed as both therapeutic agents, and/or delivery vehicles for other drugs (e.g., siRNA, small molecules etc.). In addition to single-agent therapies,[44, 61, 99, 104] nanoparticle-based approaches allow concurrent delivery of combinations of therapeutic agents that target multiple pathways in endometriosis resulting in novel combinatorial therapies.[100, 101] The developed nanomedicines were tested for attenuation of endometriosis-related pain, reducing inflammation, addressing hormone balance, and eradication of lesions.[44, 100, 101] Importantly, the available reports illustrate the potential for some cancer nanomedicines to be adapted for endometriosis, because these two diseases share many pathophysiological features (e.g. angiogenesis, ROS etc.).[11, 43]
5.1. Therapeutic Targets
An optimal treatment strategy for any pathology with multiple therapeutic targets would entail the use of a single drug, or combination of drugs, that acts on more than one pathway.[13, 24, 133] Decades of cancer research using nanomaterial-based therapies has shown that encapsulation of different therapeutic molecules within the same delivery system can provide advantages over delivering the same agents in separate vehicles, such as nanoparticle uniformity, ratiometric drug loading, temporal drug release, synergistic effects, and reduced chemoresistance.[24, 134, 135] A number of different therapeutic pathways have been considered for development of novel single-agent and combinatorial nanomedicines for endometriosis.[11, 43, 44, 61, 98, 99, 104]
Oxidative stress (OS) has been implicated in various disease states, including cancer, endometriosis, and cardiovascular disease.[136, 137] It is believed that oxidative stress contributes to the pathogenesis of endometriosis by causing a general inflammatory response in the pelvic cavity.[41, 136, 138] This inflammatory reaction may increase local aromatase activity and estrogen levels. Similar to cancer tumors, ROS have been shown to increase the proliferation rate of endometriotic cells.[139] Oxidative stress has also been shown to induce overexpression of VEGF mRNA and increased angiogenesis.[140] Menstrual reflux is theorized to contribute to an imbalance in ROS and antioxidant defenses through deposition of erythrocytes, apoptotic endometrial tissue, and cellular debris in the peritoneal cavity.[141] The accumulation of this retrograde menstruation, and its subsequent hemolysis and degradation, is responsible for an abundance of hemoglobin (Hb), heme, and iron derivatives in the peritoneal environment. The effects of free, unchelated iron are numerous within the context of endometriosis in the peritoneal cavity; iron-induced damage to mesothelium creates adhesion sites for endometrial cells, angiogenesis is induced in endothelial cells due to activation of nuclear transcription factor kB (NF-kB), the proliferation of endometrial cells is increased by iron-containing proteins, and accumulation in peritoneal macrophages exceeds their capacity for sequestration of iron by ferritin.[141] As a result, oxidative stress phenomena have become popular targets for the development of therapeutic strategies, including those based on nanomaterials.
In addition to angiogenesis and oxidative stress, matrix metalloproteinase (MMP) activity has also been implicated in the implantation, proliferation, and persistence of endometriotic lesions.[142] MMP’s are a group of enzymes that contribute to the degradation of endometrial tissues during menstruation and are differentially expressed throughout the phases of the menstrual cycle.[143] MMP activity is believed to contribute to various stages of tumor development, including establishment, growth, neovascularization, and metastasis.[144] MMP’s may help in a similar way to establish endometriotic lesions through destruction of the extracellular matrix (ECM) of peritoneal mesothelium, allowing endometriotic cells to invade the surrounding tissues. Tissue inhibitors of metalloproteinases (TIMP) are endogenous proteins that bind and inhibit the activity of MMP’s, and imbalances in MMP/TIMP are implicated in the alteration of ECM degradation.
5.2. Combinatorial Therapies
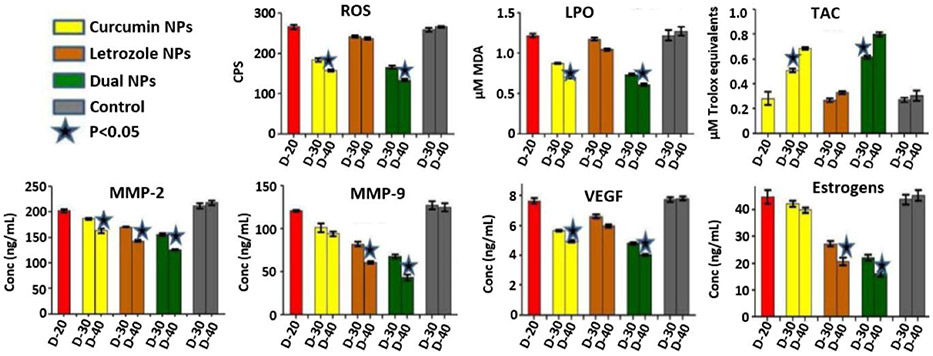
Given the fact that endometriosis is an estrogen (E2)-dependent disease, and oxidative stress, ECM degradation, and angiogenesis play an essential role in the pathogenesis of endometriosis, Jana et al. proposed a novel combinatorial therapy based on two therapeutic agents, curcumin and letrozole.[100] Curcumin (Cur) is known for its antioxidant, anti-angiogenic and anti-inflammatory activities,[145] while letrozole acts as an aromatase inhibitor, impairing the conversion of estrogen precursors to E2, and has been shown to reduce the size of endometriotic lesions and associated pain in patients.[146, 147] To improve the aqueous solubility of curcumin and enhance the delivery of both therapeutic agents, PLGA nanoparticles have been loaded with letrozole, curcumin, or a combination of both molecules, and their efficacy was evaluated in mice bearing intraperitoneal xenografts of fragmented endometrium of uteruses obtained from donor animals.[100] Following four intraperitoneal injections of either single or dual drug-loaded nanoparticles (Let-NP, Cur-NP, and Let-Cur-NP) within 20 days, visual inspection of the peritoneal cavities, and immunohistochemical analysis of the peritoneal tissue, suggested that all three formulations significantly reduced lesions and endometrial glands. In contrast to Let-Cur-NP and Let-NP, blood vessels, however, were still present around the endometrial tissue following Cur-NP-based treatment. Importantly, it was demonstrated that combinatorial therapy is more effective in changing oxidative stress parameters (ROS, lipid peroxidation (LPO) and total antioxidant capacity (TAC)), angiogenic marker (VEGF), matrix-degrading molecules (MMP-2 and MMP-9), and estrogen levels (E2, Figure 9). For example, ROS and LPO levels were significantly lower in mice treated with Cur-NP and Cur-Let-NP when compared to untreated endometriotic mice, with a slightly more pronounced reduction observed in mice treated with dual drug-loaded nanoparticles. Total antioxidant capacity was significantly higher in mice treated with Cur-NP and Cur-Let-NP, with slightly higher levels seen in dual drug-treated mice. MMP levels were most decreased in mice treated with Cur-Let-NP, but a significant reduction was also seen in mice treated with Let-NP when compared to untreated endometriotic mice. VEGF expression showed a similar trend to that seen in ROS and LPO levels; a reduction in both Cur-NP and Cur-Let-NP treated mice, with the most significant decrease in dual drug-treated subjects. Finally, estrogen levels in Let-NP and Cur-Let-NP groups were also significantly reduced, with the lowest levels observed in mice treated with dual drug-loaded NP. Based on the obtained results, researchers concluded that the evaluated combinatorial modality outperforms corresponding single treatments, and preclinical non-human primate studies are needed to further assess its safety and efficacy.
Figure 9.
Serum levels of oxidative stress markers (ROS, LPO, and TAC), MMP-2, MMP-9, VEGF, and E2 levels in mice with endometriosis before and after treatment with the following formulations: Control (0.9% NaCl), Let-Cur-NPs, Let-NPs and Cur-NPs. The red bar depicts mice with endometriosis on D20. D20, D30 and D40 indicate 20th (before treatment), 30th and 40th day (completion of treatment) after endometriosis induction. Adapted under terms of the Creative Commons Attribution License.[100] Copyright 2014, Jana SK, et al., published by Longdom Publishing SL.
Singh et al. also evaluated a multi-drug approach to treating endometriosis in a mouse model by targeting angiogenesis, oxidative stress, and MMP activity through the delivery of epigallocatechin gallate (EGCG) and the antibiotic doxycycline (Dox) in a single polymer-based nano-delivery system.[101] EGCG is known for its anti-angiogenic, antioxidant, and anti-inflammatory effects, as well as its inhibitory effect on MMP’s.[148] However, the application of EGCG is limited by its instability and unfavorable bioavailability due to poor absorption.[149] Doxycycline has also been shown to inhibit MMP activity in vitro and in vivo, but its therapeutic applications are similarly limited by its instability, rapid clearance, and inability to reach the site of action.[150] The authors evaluated the effects of single drug- (Doxycycline, DNP; EGCG, ENP) and dual drug-loaded (DENP) nanoparticles on angiogenesis, oxidative stress, and MMP activity following intraperitoneal injection in endometriosis-induced mice when compared to healthy mice. Oxidative stress markers, including ROS and LPO, were lower in mice treated with separate DNP and ENP, but were dramatically reduced in mice treated with combination DENP when compared to untreated endometriotic mice, and were strikingly similar to levels in healthy mice. A similar, but inverse, trend was observed with regard to total antioxidant capacity (TAC); untreated endometriotic mice and mice treated with DNP reflected lower TAC than mice treated with ENP. Mice treated with DENP had TAC levels almost identical to healthy mice. Serum MMP levels were significantly lower in mice with endometriosis when treated with single drug-loaded ENP and DNP when compared to untreated endometriotic mice. A more dramatic decrease in serum MMP levels was seen in mice treated with dual drug-loaded DENP, and levels were almost identical to that of healthy controls. An opposite effect was observed with regard to TIMP levels; untreated endometriotic mice displayed the lowest levels of TIMP-1, with a significant increase in mice treated with ENP and DNP. Healthy mice had the highest TIMP-1 levels, and endometriotic mice treated with DENP showed levels almost identical to healthy controls. Levels of TIMP-2 showed no significant difference across all groups. Angiogenic markers in mouse serum, including vascular endothelial growth factor (VEGF), adrenomedullin (ADM), tumor necrosis factor-alpha (TNF-α), and angiogenin, were quantified using ELISA. Across all four targets, levels were highest in untreated endometriotic mice, with a significant reduction in ENP- and DNP-treated subjects. Angiogenic markers in DENP-treated mice were even lower than in those treated with ENP or DNP and were comparable to levels in healthy mice. Histological analysis of endometriotic tissues appears to confirm the efficacy of single and dual drug-loaded nanoparticles; a remarkable reduction in the number of endometrial glands was observed in all three treatment groups, with the most dramatic decrease observed in those receiving combinatorial treatment. Immunohistochemical evaluation of endometrial tissues using anti-human platelet/endothelial cell adhesion molecule-1 (PECAM-1) revealed a significant reduction in newly formed microvessels across all treatment groups. The most dramatic reduction in the number and size of microvessels was observed in mice that received combinatorial treatment. Visual observation of peritoneal blood vessels further reinforced these findings, with numerous vessels visible in untreated endometriotic mice, a significant decrease in DNP- and ENP-treated mice, and the most pronounced reduction in DENP-treated mice.
In sum, the studies described above support the notion that co-delivery of multiple modalities in a single vehicle can have a more profound therapeutic effect that individual drugs delivered separately.
5.3. Single-Agent Therapies
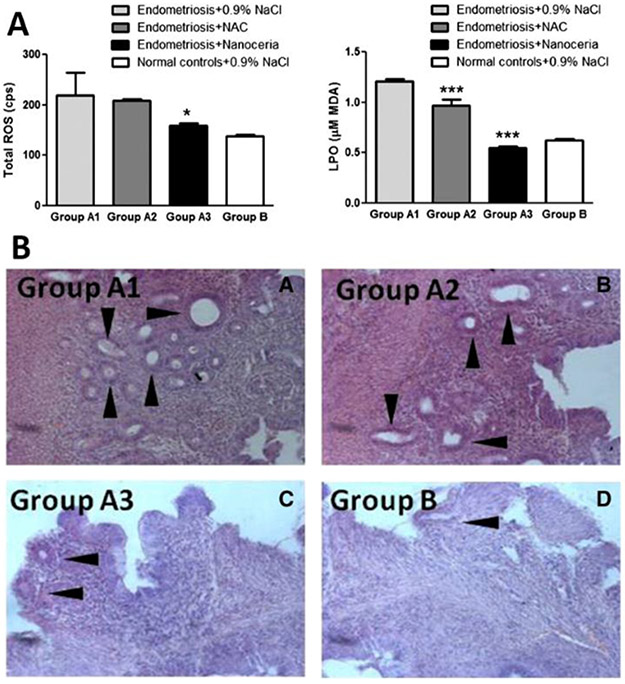
Therapeutic strategies based on a single agent that can target either one or multiple therapeutic pathways have also been developed for endometriosis treatment.[44, 99, 102] For example, Chaudhury et al. implemented cerium oxide nanoparticles (CNP or nanoceria) as a therapeutic agent in an attempt to reduce ROS levels and angiogenesis in the peritoneal cavity of mice with induced endometriosis.[99] The radical-scavenging capability of cerium-oxide nanoparticles is well documented in healthy subjects and various pathologies. At physiological pH, cerium exists in two different oxidation states, Ce4+ and Ce3+. Following scavenging of free radicals, Ce3+ is oxidized to Ce4+ and then converted back to Ce3+ through surface chemical reactions, allowing the molecule to be recycled.[151, 152] Several metal ions are known for their role in scavenging of free radicals, and some bacteria that contain truncated, dysfunctional genes for superoxide dismutase have even evolved to use high intracellular concentrations of metal ions as their sole defensive mechanism against ROS. The obtained results revealed that, following systemic administration of a single dose of cerium oxide nanoparticles (CNP) at 0.05 mg kg−1 into mice bearing abdominal endometriotic grafts, a significant reduction in ROS and lipid peroxidation (LPO) levels was observed (Figure 10A) in comparison to control mice and endometriotic mice treated with 0.9% NaCl or N-acetyl cysteine (NAC, an antiangiogenic agent). Immunostaining with CD34 antibodies (a marker for vasculature) revealed a notable decrease in CD34 expression in mice treated with CNP, suggesting a reduction in angiogenesis. Histological analysis of endometriotic tissues reinforced these findings by demonstrating a significant reduction in glands and stroma in mice treated with CNP (Figure 10B). Endometriosis is often associated with reduced fertility, and poor oocyte quality is considered to be one of the many causes of infertility. Examination of meiotic spindles of oocytes in mice treated with CNP revealed a remarkable increase in oocyte quality when compared to endometriotic mice administered 0.9% NaCl, and even control mice in which endometriosis was not induced.
Figure 10.
A) Serum levels of oxidative stress markers (ROS and LPO) in mice induced with endometriosis and injected with 0.9% NaCl (Group A1), NAC (Group A2), and Nanoceria (Group A3). Normal healthy mice injected with 0.9% NaCl were considered as controls (Group B). Data represented in mean±SD. B) Representative image of hematoxylin and eosin staining of the endometrial tissue from mice induced with endometriosis and injected with 0.9% NaCl (Group A1), NAC (Group A2), Nanoceria (Group A3), and normal healthy mice injected with 0.9% NaCl considered as controls (Group B). Arrowhead indicates the presence of endometrial glands in the tissue of each group. Adapted with permission.[99] Copyright 2013, Elsevier.
In contrast to the above-discussed nanomedicine-based platforms designed for systemic drug delivery, Boroumand et al. constructed biodegradable polymeric nanofibers for local and sustained release of curcumin in the peritoneum.[102] In vitro studies demonstrated that the developed platform could release ~50% of the drug over 30 days, and the implanted curcumin loaded nanofibers in the peritoneum of mice result in a considerable reduction of endometriotic lesions after 21 days. Histological analysis of the treated lesions further revealed a significant decrease in endometrial glands, stroma, and infiltration of inflammatory cells when compared to the control group.
A significant body of literature is dedicated not only to therapeutic strategies for the treatment of the underlying causes of endometriosis but also to the alleviation of endometriosis-related pain.[153] Pain experienced by patients with endometriosis has been characterized as a neuropathic and inflammatory pain and, as such, may be alleviated by therapies that target associated pathways.[154] One such pathway involves receptor P2X ligand-gated ion channel 3 (P2X3), which was previously investigated for its role in cancer-associated bone pain in rats.[155] A recent report also described the role of P2X3 in endometriosis pain via the ERK signaling pathway. A small molecule, A-317491, was previously identified as a selective antagonist of P2X3.[156] A 2017 study from Yuan, et al. attempted to determine the effects of this small molecule on endometriosis pain in a rat model of the disease, when delivered systemically by a chitosan oligosaccharide and stearic acid-coated lipid nanocarrier (CSOSA/NLC/A-317491).[44] Fluorescence microscopy revealed efficient in vitro internalization of CSOSA/NLC/A-317491 in PC12 rat adrenal medullary cells, selected for their high expression of P2X3, within 12 h of treatment. A-317491 reportedly affects pain signaling by blocking ATP-induced Ca2+ influx.[155-157] To confirm this, the authors used a fluorescence assay to measure intracellular calcium levels in PC12 cells following treatment with free A-317491 salt or CSOSA/NLC/A-317491. Results showed a significant decrease in intracellular Ca2+ following incubation with CSOSA/NLC/A-317491 for 12 h, or A-317491 salt for 1 h, when compared to untreated cells and cells treated with empty CSOSA/NLC nanoparticles, implying that the nanocarrier itself had no effect on Ca2+ influx and that these effects were, in fact, caused by the small molecule drug. In a rat model of endometriosis, CSOSA/NLC/A-317491 nanoparticles labelled with DiR showed efficient accumulation in subcutaneous endometrial grafts following system administration. To determine the effects of CSOSA/NLC/A-317491 on endometriosis hyperalgesia, the authors used paw withdrawal tests to assess nociceptive response to mechanical or thermal stimulus at 2, 4, 8, 12, 18, and 24 h following treatment with CSOSA/NLC/A-317491 or free A-317491 salt. Mechanical pain threshold (MPT) and heat source latency (HSL) were determined for each rat prior to surgery, and every week following surgery, to establish a baseline for nociceptive response in treatment and control animals. Baseline measurements for MPT and HSL following induction of endometriosis, but before treatment, revealed a significant increase in hyperalgesia of endometriotic rats in comparison to healthy rats from days 21 to 42 (Figure 11A and B). Measurements of MPT and HSL in healthy rats administered with CSOSA/NLC/A-317491 or A-317491 salt revealed no difference in nociceptive response when compared to healthy rats administered saline (Figure 11C and D). Following treatment, a notable increase in MPT and HSL was observed at 2 h, and 4 h in rats administered free A-317491 salt, but the effect had disappeared by 8 h (Figure 11E and F). In contrast, an equally significant increase in MPT and HSL was observed in rats administered CSOSA/NLC/A-317491 at 8 h, and this antinociceptive effect continued through 24 h. These results demonstrate the effectiveness of both free A-317491 salt and CSOSA/NLC/A-317491 in the alleviation of hyperalgesia, and suggest that a combinatorial treatment including both formulations of the small molecule may be even more effective than either treatment alone, theoretically, by providing a burst release of immediately available A-317491 salt to relieve hyperalgesia for the first 4 h following delivery, and an extended antinociceptive effect from CSOSA/NLC/A-317491. The authors would be well advised to repeat these experiments with different dosages of each formulation in an extended longitudinal study to determine how long these effects will last.
Figure 11.
Variations in pain behavior in rats. Notes: Mechanical (A) and heat (B) hyperalgesia behaviors measured every week after endometriotic or sham surgery. C and D) Hyperalgesia behavior observed at 2, 4, 8, 12, 18, and 24 h after drug administration in normal rats. E and F) Behavior tested after drug administration in endometriotic rats. Data are expressed as the mean (SEM; A and B, n=15; C–F, n=5). *P<0.05; **P<0.01; ***P<0.001. Abbreviations: CSOSA, chitosan oligosaccharide-g-stearic acid; Ctrl, control group; Em, endometriotic group; HSL, heat source latency; MPT, mechanical pain threshold; NLC, nanostructured lipid carrier; SEM, standard error of mean. Reproduced with permission.[44] Copyright 2017, Dovepress.
5.4. Gene Therapy
One of the more exciting and challenging developments in experimental medicine has been the emergence of gene therapy. This field focuses on the intracellular delivery of nucleic acids, either DNA or RNA, for the treatment of disease.[158] Depending on the desired molecular interaction, gene therapy can be used to produce functional proteins or to inhibit the expression of harmful genes following the transfection of target cells, and these effects can be achieved using various methods. Plasmid DNA encoding genes of interest can be delivered to the nucleus and can either be transiently expressed or stably integrated in the genome for long-term expression.[159] Messenger RNA (mRNA) can also be delivered to the cytosol, where free ribosomes translate the transcripts into functional proteins.[17] Small interfering RNA (siRNA), short hairpin RNA (shRNA), and micro RNA (miRNA) can be used to inhibit gene expression and/or protein activity by binding to target sites on genomic DNA, mRNA transcripts, or proteins.[160-162] Delivery of naked nucleic acid presents significant obstacles, including its inherent instability (especially RNA), immunological response following systemic administration, and difficulty in traversing negatively charged cellular membranes. Viral and non-viral vectors have been used successfully to protect nucleic acids from degradation, prevent their recognition by the immune system, and increase their transfection efficiency.[163, 164] Both categories of vectors can shield nucleic acids from enzymatic digestion. However, viral delivery vehicles demonstrate superior transfection efficiency, whereas non-viral vectors elicit little to no immunological response, and the decision to use one over the other is generally viewed as a trade-off between the two parameters. Gene therapy has been used with great success in the treatment of cancer and is a promising method for the treatment of endometriosis.[165] Experimental strategies for gene therapy of endometriosis have focused primarily on inhibition of angiogenesis, addressing hormonal imbalance, reducing inflammation, and attenuation of endometriosis-associated pain. For a review of gene therapy in endometriosis treatment, see Shubina, et al., 2013.[165] While almost all of the studies in the review as mentioned above employed viral vectors for nucleic acid delivery, the purpose of this section is to highlight gene therapy studies of endometriosis using non-viral vectors.
A gene therapy study from 2011 by Zhao, et al.[45] was inspired by an earlier report that pigment epithelium-derived factor (PEDF) was effective at inhibiting angiogenesis in an orthotopic model of metastatic osteosarcoma.[166] PEDF is a protein that has been shown to display potent antiangiogenic activity, negating the effects of several angiogenic factors including VEGF, and has previously been used to inhibit both cancer and endometriosis.[167-169] However, treatment using PEDF protein may require prolonged treatment with repeated administration due to its pharmacokinetic profile. The use of PEDF plasmid, delivered via stearic acid-grafted chitosan oligosaccharide micelles (CSO-SA/PEDF), showed a ~50% decrease in the volume of endometriotic lesions in rats two weeks after systemic administration (Figure 12A, B and C).[45] Histopathological analysis of endometriotic tissues showed a higher apoptotic index in the CSO-SA/PEDF treatment group relative to controls, in addition to a decrease in endometrial glands and stroma (Figure 12D and E). Furthermore, immunohistochemical analysis using anti-von Willebrand factor (vWF) revealed that microvessel density significantly decreased in CSO-SA/PEDF treated rats compared to control animals.
Figure 12.
A-C) The effects of drugs on endometriotic lesions in rats. The first group is the control group (A), the second group is CSO-SA/PEDF-treated group (B), and the third group is the danazol treated group (C). Danazol is a synthetic androgen, used in the treatment of endometriosis. Capital letters (A, B, C) stand for the mean of the lesion volumes of the endometriotic foci before drug treatment. There was no significant difference among three groups. Lowercase letters (a, b, c) stand for the mean of the lesion volumes after different treatment. In the control group the lesions grew to 69.11 ± 30.91 mm3, and those in the danazol and CSO-SA/PEDF groups were restrained. D and E) The light micrographs of endometriotic lesions. D) In the control group it shows abundant glands, and rich pseudostratified columnar epithelial cells can be seen clearly. E) Gland structure in CSO-SA/PEDF-treated group is disorganized in comparison to the control group and only simple squamous epithelial cells, stromal fibrosis can be observed. Reproduced with permission.[45] Copyright 2017, Elsevier.
The same group previously identified aquaporin 2 (AQP2) as a potential target for gene therapy of endometriosis due to its reported estrogen-response effect.[170] It was reported that an estrogen-response element was identified in the AQP2 promoter sequence, and estrogen exposure increased AQP2 expression in a dose-dependent manner in vitro. Furthermore, estrogen significantly increased migration, invasion, adhesion, and proliferation of immortalized human endometrial adenocarcinoma cells, and these features were significantly reduced following AQP2 expression knockdown using AQP2 siRNA. This led Zhao et al. to construct polymeric nanoparticles using chitosan oligosaccharide, polyethylenimine (PEI), AQP2 inhibitory siRNA, and hyaluronic acid (HA) as a targeting moiety to CD44 receptors, and evaluate their efficacy in a rat model of endometriosis through inhibition of AQP2.[61] Two weeks following intravenous injection of 5mg kg−1 (CSO-PEI/siRNA)HA nanoparticles, the inhibition rate of endometrial cyst growth increased by ~56% relative to controls, and disorganization and atrophy of endometrial glands and stroma was observed in treated animals (Figure 13A). The provided results also confirmed (CSO-PEI/siRNA)HA had no adverse effects on reproductive organs such as ovary and uterus (Figure 13B).
Figure 13.
A) Light micrographs of endometrial lesions. In the control group, it shows abundant glands, and pseudostratified columnar epithelial cells can be seen clearly (a). The amount of gland structure in (CSO-PEI/siRNA)HA-treated group is less than that of the control group, and only simple squamous epithelial cells and stromal fibrosis can be observed (b). B) The reproductive organs were examined by H&E staining under light microscopy. The structure of the ovary of the control group (a) and (CSO-PEI/siRNA)HA group (b); the structure of the uterus of the control group (c) and (CSO-PEI/siRNA)HA group (d). The obtained images do not show significant differences in the plane structure between the (CSO-PEI/siRNA)HA group and the control group. Reproduced with permission.[61] Copyright 2016, Dovepress.
Another antiangiogenic gene therapy strategy used polyamidoamine (PAMAM) dendrimer as a delivery vehicle for plasmid encoding endostatin (PAMAM-ES).[104] Endostatin acts as a potent inhibitor of angiogenesis by specifically inhibiting proliferation of endothelial cells, and was first described in mouse hemangioendothelioma.[171] The antiangiogenic properties of endostatin are well established in endometriosis, but earlier studies used recombinant endostatin protein whose short half-life requires long-term treatment with repeated administration, limiting its utility in a clinical setting. Following direct injection of PAMAM-ES in GFP-expressing subcutaneous endometriotic lesions in nude mice, a significant reduction in fluorescence as a measure of lesion size was noted in comparison to control mice over 30 days. After 30 days, excised lesions in PAMAM-ES treated mice were, indeed, significantly smaller than those in control animals, and expression of CD31, a marker of angiogenesis, was significantly reduced relative to controls. It should be noted that, while the findings of this study are promising, their results are dependent on direct injection of nanoparticles in subcutaneous endometriotic lesions. As a step further toward clinical translation, the authors would be well-advised to test this treatment modality following systemic administration.
Noncoding RNA (ncRNA) and long noncoding RNA (lncRNA) are RNA transcripts that are not translated into proteins, but which can often perform regulatory functions and can affect gene expression at the transcriptional or post-transcriptional level. Their involvement in regulatory functions such as translation, RNA splicing, DNA replication, and gene regulation has been well documented, as have their roles in various disease states including cancer and endometriosis.[172] One such lncRNA, metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), was found to influence epithelial-to-mesenchymal transition (EMT), a process by which epithelial cells acquire mesenchymal phenotypes, making them highly motile. EMT has, in turn, been implicated in the pathogenesis of endometriosis. Previous reports have suggested that EMT can be blocked by inhibition of zinc finger e-box binding homeobox 1 and 2 (ZEB1 and ZEB2) mRNA by members of the miR-200 family of miRNA.[173] A 2017 gene therapy study from Liang et al. found that MALAT1 acts as a decoy for miR-200c, and negates its suppressive effect on ZEB1 and ZEB2.[98] The authors constructed polyethylenimine-polyethylene glycol-arginine-glycine-aspartic acid (PEI-PEG-RGD) nanoparticles carrying miR-200c mimic RNA (miRNA@PEI-PEG-RGD) and evaluated their effect on ex vivo human endometriotic stromal cells (HESC’s) and in a subcutaneous rat model of endometriosis. Following transfection, proliferation and migration of HESC’s were significantly inhibited in comparison to controls. An miR-200c inhibitor was also shown to drastically upregulate HESC proliferation and migration. Downregulation of MALAT1 was shown to be affected by miR-200c in a dose-dependent manner, and several mesenchymal markers were significantly downregulated in HESC’s following treatment with miRNA@PEI-PEG-RGD. Western blot analysis confirmed decreased expression of ZEB1 and ZEB2 following treatment with miRNA@PEI-PEG-RGD, and upregulation of the same proteins when treated with miR-200c inhibitor. As evidenced by qRT-PCR analysis, transcript levels of MALAT1 in HESC’s treated with miR200c were significantly lower than in controls, whereas treatment with miR-200c inhibitor restored, and significantly increased, MALAT1 levels. Twenty days following direct intra-lesional injection of miRNA@PEI-PEG-RGD nanoparticles in a rat model, endometriotic cyst volume was significantly decreased in comparison to controls (saline, empty delivery vehicle, and scrambled miRNA). The administration of an miR200c inhibitor resulted in a profound increase in cyst volume, far beyond that of controls (Figure 14). Though these findings may reveal a novel therapeutic target for endometriosis, they are the result of direct injection of subcutaneous lesions and should be further explored following systemic administration of the non-viral gene therapy vector.
Figure 14.
Image of ectopic endometrial cysts resected 20 days after injection of various nanoparticles into rats. Mimic NC - negative control miRNA mimic, Inhibitor NC - negative control miRNA inhibitor. Reproduced under terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).[98] Copyright 2017, The Authors, published by BioMed Central, part of Springer Nature.
In summary, several gene therapy strategies developed for cancer research have been successfully applied in the experimental treatment of endometriosis, including viral and non-viral delivery vehicles. Both classes of transfection agents are quite effective at protecting nucleic acids and other cargo from degradation in systemic circulation and delivering them to disease sites via passive or active targeting. However, although gene therapy has been successful at affecting the expression of targeted or delivered genes and reducing lesion size in vivo, these strategies fall short of completely eradicating endometriotic lesions. Future studies may result in increased efficacy when nanomaterial-based strategies are applied as combinatorial treatments that target multiple genes or disease pathways, or that combine different strategies such as gene therapy and small drug molecules in a single treatment modality.
5.5. Phototherapy
Phototherapy is a therapeutic modality in which light energy is either focused and used to treat diseases directly, as in the treatment of jaundice, or used to activate a photosensitive agent (photosensitizer) to elicit a therapeutic response.[29] In the case of photosensitive agents’ application, phototherapy is generally classified into one of two categories, photothermal therapy (PTT) and photodynamic therapy (PDT). In PTT, light-absorbing molecules are used to convert light energy into heat energy and generate localized hyperthermia of the lesion. The generated heat leads to cell death mainly through induction of necrosis or apoptosis.[21, 29, 122, 174] On the other hand, photodynamic therapy is a method used to destroy diseased tissue mainly through the generation of toxic ROS from the interaction between excited photosensitizers and molecular oxygen.[21, 29, 122, 174] Both of these modalities have been explored widely in cancer studies,[21, 29, 122, 174] and would be preferred over radiation and chemotherapy in large part due to the tumor-specific accumulation and activation by biocompatible photosensitizers with safe, focused light, causing minimal damage to surrounding tissue.
Studies have utilized a variety of photosensitizers for PTT and PDT application in cancer and endometriosis. However, most commonly used organic photosensitizers are hydrophobic resulting in reduced water solubility, which makes them difficult to administer systemically, leading to inefficient delivery to target tissues.[175] In this regard, nanoparticles have proven to be a promising route to improve the efficiency of PTT and PDT, mainly due to their capability to efficiently deliver photosensitizing agents to diseased tissue, while simultaneously improving water solubility and stability in systemic circulation.[20, 21] Furthermore, these characteristics can be coupled with active targeting through surface modification of nanoparticles, via conjugation or other chemical alterations, in order to increase their selectivity and accumulation in disease sites.[176]
Nanoparticles are also versatile in their application. Some nanoparticles carry and deliver photosensitizers to lesions, while others act as photosensitizers themselves and generate heat upon exposure to light.[20, 21, 43] Considering the pathophysiological similarities between cancer and endometriosis (angiogenesis, EPR effect, etc.) nanoparticle based-PTT platforms can play a significant role in endometriosis treatment. However, the application of PTT in endometriosis is not as well explored as it is for cancer. To the best of our knowledge, only two studies used nanoparticle-based PTT for the eradication of endometriotic lesions.[11, 43] The first study, developed by Moses et al., employed silicon naphthalocyanine (SiNc) loaded PEG-PCL nanoparticles and NIR light for photothermal treatment of endometriosis.[11] SiNc was the photosensitizer of choice due to its high NIR absorption rates (ε = 2.5x105 M−1 cm−1) in the spectral range of 750 nm to 800 nm which corresponded to enhanced heating efficiency.[29] In vitro photothermal efficiency of the developed SiNc nanoparticles (SiNc-NP) was investigated by incubating monkey endometriotic stromal cells (30 μg mL−1) over a two day period. Subsequent exposure of incubated cells to NIR light (780 nm, 0.9 W cm−2) for 15 min increased the intracellular temperature to 53 °C leading to the death of 95% of the cells. SiNc-NP was then administered intravenously (3 mg kg−1) to endometriotic lesion-bearing mice via tail vein injection, and whole-body fluorescence imaging showed the nanoparticles efficiently accumulated in endometriotic lesions (Figure 2). Exposure of lesions to NIR light for 15 min, 24 hours post-injection, raised the intralesional temperature to 47 °C, leading to their eradication within 4 days (Figure 15A and B). Histological analysis confirmed the destruction of glands and stroma in endometriotic grafts, accompanied by a loss of ESR1 and PGR receptors, rendering them unresponsive to hormonal stimulation and eliminating their potential for growth under estrogen (Figure 15C-G). In addition, NIR light or SiNc-NP alone had no impact on the viability of endometriotic cells, confirming the photothermal phenomenon is mutually inclusive of both components (Figure 15B). This approach was originally developed by the same group to successfully eradicate tumors in murine cancer models with no sign of acute toxicity.[21]
Figure 15.
A) Representative temperature profile inside of endometriotic graft during photothermal therapy (PTT) mediated by intravenously injected SiNc-NP in combination with 780 nm light (0.9 W cm−2, red curve). Mice were injected with SiNc-NP 24 h prior to NIR light treatment. The black curve represents a temperature profile inside of endometriotic graft upon exposure to 780 nm light only (without SiNc-NP injection). B) Representative growth profiles of subcutaneous endometriotic grafts after the following treatments: 1) saline, mice injected with saline; 2) SiNc-NP, mice injected intravenously with SiNc-NP (3 mg SiNc per kg); 3) light, mice injected with saline and exposed to 780 nm light (0.9 W cm2) for 15 min at 24 h post-injection; 4) SiNc-NP + light, mice injected intravenously with SiNc-NP (3 mg SiNc per kg) and exposed to 780 nm light (0.9 W cm2) for 15 min at 24 h post-injection. C-G) Effect of PTT on graft histology and steroid receptor staining. In control mice not receiving PTT, the grafts displayed enlarged endometriotic glands and stroma (C and E). In these mice, the grafts stained strongly for both ESR1 and PGR (G and I). Endometriotic glands and stroma were lost after PTT and graft sites were replaced with murine connective Tissue (D and F). Minimal ESR1 and PGR staining nuclei were observed after PTT therapy (H and G. The inset in (I) shows irrelevant IgG control (anti-Br(d)U) showing staining specificity for both ESR1 and PGR. “gl” indicates glands; “s” indicates stroma; “sc” indicates stromal connective tissue with minimal ESR1 and PGR staining. 5X, 5× plan apochromatic objective; 20X, 20× plan apochromatic objective. Reproduced with permission.[11] Copyright, 2020, Wiley-VCH.
In the second study, Guo et al. investigated the efficiency of non-targeted hollow gold nanospheres (HAuNS) as well as TNYL tagged HAuNS (TNYL-HAuNS) in photothermal treatment of endometriotic lesions.[43] TNYL conjugation increased selective accumulation of nanoparticles in endometrial cells by binding to the overexpressed EphB4 receptor. Exposure to NIR light of passively and actively targeted gold photosensitizers, led to a temperature increase of an aqueous suspension. Biodistribution profiles revealed a significantly higher accumulation of targeted nanoparticles in lesions following systemic administration (Figure 4A). Exposure of endometriotic grafts to higher intensity NIR light (808 nm, 2 W cm−2) for 10 min on days 2 and 4 after injection of targeted and non-targeted nanoparticles inhibited the growth of lesions by 92.7% and 77.2%, respectively. However, NIR light at this intensity, as well as nanoparticles alone, caused minor inhibition to lesion growth. Lastly, as was the case in the first study, this system was also initially developed for photothermal ablation of cancer cells in mouse models.[48]
The excitation of some photosensitizers in lesions upon exposure to NIR light can also lead to cell death through PDT.[177] Large amounts of resources have been invested in exploring the cytotoxic effects of PDT in cancer studies. However, its application in endometriosis treatment is poorly investigated. A literature search showed the application of nanoparticles can enhance the efficiency of PDT in cancer but is yet to be applied for endometriosis. Earlier studies have used aminolaevulinic acid (ALA)-induced endogenous tissue photosensitizer Protoporphyrin IX (PPIX), previously used in cancer studies, to investigate its therapeutic potential in PDT-based endometriosis treatment.[178] PPIX was shown to have absorption wavelength around 405 nm and emission bands around 635 and 710 nm and was used for photodynamic cancer therapy, like superficial basal cell cancers and Bowen's disease.[179] Because of its therapeutic effect in cancer studies, others investigated its effect in endometriosis using exogenous 5-ALA as PPIX inducer in primary cultures of endometriotic epithelium.[180, 181] Although there were ALA resistant cells present, the majority of results indicated that light exposure of PPIX induced apoptosis of cells. However, in vivo studies revealed that nonspecific accumulation of a photosensitizer in various organs could potentially lead to healthy tissue damage upon exposure to light. In this regard, nanoparticle formulations can improve endometriotic lesion-specific delivery of 5-ALA and enhance the accumulation of PPIX. Furthermore, the formation of photoproducts through the interaction of PPIX with the resulting cytotoxic oxygen species led to rapid photobleaching, which reduced its treatment window.[182]
The above-discussed studies confirm that nanoparticle-based photothermal therapy can be useful in the elimination of endometriotic lesions and further suggest that other novel nanoparticles previously used in cancer have the potential to be repurposed for PTT treatment of endometriosis. However, due to limited tissue penetration, the intensity of light required for activation of currently available photosensitizers can only be achieved intra-operatively and, therefore, the developed phototherapies have the potential to be translated to clinical application, but likely limited as a component of surgical intervention. To our knowledge, the number of reports describing PDT application in endometriosis is sparse and, therefore, it is not clear whether PDT could be an efficient treatment for this disease.
5.6. Immunotherapy
Immunotherapy treats disease by either activation or suppression of the immune system, and its popularity has grown in recent years due to its use in the treatment of various cancers.[183] Activation immunotherapy initiates or amplifies an immune response by stimulating the immune system, either through targeting specific populations of immune cells such as macrophages, dendritic cells, natural killer cells, T cells, and other lymphocytes, or by non-specific stimulation of the immune system, in general, using cytokines and cell-signaling molecules.[184, 185] Suppressive immunotherapies inhibit immune responses in order to attenuate the effects of autoimmune disorders and allergic reactions or to prevent rejection of transplanted organs. While the use of nanoparticles in cancer immunotherapy, and the immunological aspects of endometriosis have both been explored, studies involving nanoparticle-mediated immunotherapy of endometriosis are scarce.
Macrophages play an essential role in many disease states including cancer, and a growing body of literature suggests that they are involved in regulating the implantation and progression of endometriosis. The ability of macrophages to recognize and clear endometrial cells from the intraperitoneal cavity is dependent on the functionality of membrane scavenger proteins SR-A1 and SR-B of the pattern recognition receptor (PRR) family. These PRR’s were shown to be underexpressed in peritoneal macrophages of women with endometriosis compared to those of healthy women, and their decreased expression was shown to result in impaired function of macrophages with regard to their interaction with endometrial cells. Antsiferova et al. attempted to increase the expression of these proteins and others in peritoneal macrophages through the delivery of glucosaminyl muramyldipeptide (GMDP) immobilized on the surface of mesoporous silica nanoparticles (UMNP) and aminopropyl functionalized silica nanoparticles (AMNP), and compare their activity to free GMDP.[186] GMDP is a derivative of muramyl dipeptide, which is the active fragment of bacterial cell wall peptidoglycan. It has been shown to stimulate adaptive and innate immune responses in mice, albeit with low bioavailability.[187] Phagocytic response to GMDP was shown to be regulated by another PRR, nucleotide-binding oligomerization domain 2 (NOD2).[188] Sol-gel silica materials are a popular class of carrier for drug molecules because of their biodegradability, high surface area, and absence of cytotoxicity or genotoxicity.[189] Peritoneal mononuclear cells were collected during laparoscopic examination of patients with endometriosis who had not received hormone therapy for at least three months prior to sample collection. Incubation of macrophages with GMDP-loaded UMNP or AMNP did not negatively affect the expression of functional membrane molecules, and flow cytometry revealed their strong interaction with empty, FITC-labelled UMNP and AMNP. Following 24 h incubation of macrophages with GMDP-AMNP, significantly higher expression of CD36 (SR-B) and CD204 (SR-A1) was observed using monoclonal antibody staining and flow cytometry, when compared to controls, whereas GMDP-UMNP had no significant effect. Also, qRT-PCR revealed a significant increase in expression of NOD2 in AMNP-treated cells, and no significant difference in expression was detected for UMNP, when compared to negative controls and cells treated with free GMDP. Receptor for advanced glycation end products (RAGE), another PRR associated with inflammatory response, was also significantly increased in GMDP-AMNP treated cells, compared to no significant difference when treated with GMDP-UMNP. Suppressor of cytokine signaling 1 (SOCS1), a negative-feedback regulator of PRR-induced signaling, was significantly inhibited by free GMDP and GMDP-UMNP but was unaffected by GMDP-AMNP in comparison to controls. The authors contend that the high level of suppression may be caused by cellular mechanisms that maintain the balance between the pro- and anti-inflammatory signals. A significant upregulation of matrix metalloproteinase 9 (MMP-9), and tissue inhibitor of metalloproteinase 1 (TIMP-1, the corresponding inhibitor of MMP-9), was also observed. As previously mentioned, MMP’s play a significant role in the ability of endometriotic cells to implant in peritoneal tissue. However, the literature also suggests that MMPs (e.g., MMP-9) are important for macrophages to degrade the extracellular matrix of cells designated for phagocytosis, and that suppression of this MMP is correlated with the severity of endometriosis.[190] The authors suggest that similar to the increase in SOCS1 mRNA, this upregulation of TIMP-1 may be a natural response to increased expression of MMP-9 in an attempt to prevent unbalanced macrophage activation. These are promising results in the sense that they help to identify possible therapeutic targets for the treatment of endometriosis, and the application of this treatment modality in an animal model is encouraged.
T cells are lymphocytes that originate in the thymus gland (hence their name), and they play many roles in regulating the immune response. There are several specialized subpopulations within the broader category of T cells, including regulatory T cells (Tregs). Tregs are responsible for suppressing excessive, deleterious immune responses by regulating other lymphocytes in order to maintain homeostasis and immunological self-tolerance, and their role in immunopathological conditions has been extensively investigated.[191] Cytotoxic T lymphocyte antigen 4 (CTLA-4) is an immune checkpoint receptor expressed by CD4 + CD25 + Tregs, and inhibition of this protein using anti-CTLA-4 antibody has been shown to disable the inhibitory mechanism of Tregs and boost the body’s immune response to cancer.[192] It was reported that high concentrations of CD4 + CD25 + Tregs had been found in the peritoneal fluid of women with endometriosis.[193] It has also been shown that endometrial stromal cell invasion and proliferation are stimulated by IL-10 and TGF-β secreted by CD4 + CD25 + Tregs.[194] A 2017 study attempted to determine whether PLGA nanoparticles loaded with anti-CTLA-4 antibody could more effectively inhibit the invasion and proliferation of endometriosis than free anti-CTLA-4 antibody in vitro, through suppression of CD4 + CD25 + Tregs isolated from endometriotic lesions of a mouse model and co-cultured with ectopic endometrial cells.[103] The authors created a mouse model of endometriosis by using the autologous endometrial transplantation method. Twenty-one days following implantation, peritoneal fluid containing Tregs was collected and treated with anti-CTLA-4 alone or PLGA-anti-CTLA-4 nanoparticles for 14 days. Prior to treatment, the concentration of CD4 + CD25 + Tregs in peritoneal fluid of endometriotic mice was determined to be more than twice that of healthy mice. At day 7, using flow cytometry, a significant decrease in CD4 + CD25 + Tregs was noted in nanoparticle-treated fluid of endometriotic mice following staining with FITC and APC. By day 14, this decrease was even more pronounced, whereas the level of CD4 + CD25 + Tregs in peritoneal fluid treated with free antibody alone remained largely unchanged. Endometrial grafts were dissociated into individual Tregs and ectopic endometrial cells (EEC) and co-cultured for 14 days. By days 7 and 14, a significant reduction in proliferation of EEC’s was noted in cultures treated with PLGA/anti-CTLA-4. In contrast, cells treated with free antibody showed almost no change in proliferation. A Matrigel assay showed similar results concerning the invasion of EEC’s; the invasion index of cells treated with PLGA/anti-CTLA-4 revealed significant inhibition when compared to untreated co-cultures, with levels matching that of negative controls by day 14. In contrast, cells treated with free antibody demonstrated little change in invasion index. Levels of IL-10 and TGF-β were similarly affected; treatment with nanoparticles significantly reduced the levels of cytokines, but treatment with free antibody showed almost no change when quantified using ELISA. To confirm that the decrease in proliferation and invasion was due to decreased expression of IL-10 and TGF-β, free antibodies for these proteins were used to treat EEC’s either alone, or in combination. Results showed that proliferation of EEC’s decreased significantly when treated with anti-IL-10 alone, and in combination with anti-TGF-β, but treatment with anti-TGF-β alone had no effect. Invasion of EEC’s was significantly inhibited following treatment with both individual and combined antibodies.
Taken together, the results of these studies indicate significant potential for immunotherapy to be an option for the treatment of endometriosis. As with other experimental treatments, better outcomes using immunotherapy may be achieved when combined with other treatment modalities to target multiple pathways for synergistic effects. Further application of immunotherapy in animal models is warranted, as are studies elucidating other shared immunological features of cancer and endometriosis.
5.7. Natural Product-Based Therapies
Many small molecules are known for their inhibitory effects on various diseases, including cancer and endometriosis.[195, 196] These molecules are sometimes derived or isolated from natural products and, as a result, may often only be available in the form of a crude extract containing multiple compounds. Copaiba oleoresin (CPO) is a natural product isolated from plants of the genus Copaifera, represented by 72 species, including Copaifera langsdorffii and contains several compounds, including β-caryophyllene, which binds directly to the CB2 endocannabinoid receptor. The endocannabinoid system plays a role in several physiological processes, including analgesia, neurogenesis, and immune cell function. A previous report already validated that an oral formulation of copaiba oil can inhibit the growth of endometriotic lesions in rats.[197] Therefore, several research groups proposed that encapsulating copaiba oil extracts within nanomaterials may provide advantages for their bioavailability, delivery, safety, and therapeutic effect.[198-200]
A 2016 study by de Almeida Borges et al. evaluated the effects of copaiba oleoresin, containing 5.05% w/w of β-caryophyllene, on endometrial cells when delivered using a PLGA nanoparticle formulation.[199] The authors collected endometrium from patients without endometriosis (CESCs), endometrium from patients with endometriosis (EuESCs), and ectopic endometrium from endometriotic lesions (EctESCs), and the established endometrial stromal cells from the obtained samples were incubated with empty and CPO-loaded nanoparticles for various periods. When treated with 300 μg mL−1 CPO-loaded nanoparticles, CESCs showed a slight inhibition compared to untreated cells and cells treated with empty PLGA nanoparticles only after 72 h incubation, indicating that PLGA itself had no effect on cells and that the observed minor inhibition of CESCs was the result of CPO. It was further revealed that the CPO-loaded nanoparticles preferentially reduce the viability of EuESCs and EctESCs by >50% after 72 h but their effect on both cell lines is similar.
In contrast to CPO-loaded PLGA nanoparticles, the same research group demonstrated that copaiba oleoresin encapsulated in a non-toxic lamellar silicate nanocomposite (Viscogel B8, VB8) significantly reduces the cell viability and proliferation of all three endometrial cell lines (CESCs, EuESCs, and EctESCs).[198] Moreover, the observed therapeutic effect of COP nanocomposites was the most pronounced in the endometrial stromal cells obtained from endometriotic lesions (EctESCs). For example, a 60% reduction in proliferation of EctESCs was observed when treated with 300 μg mL−1 VB8 COPA nanocomposite, while the proliferation of CESCs and EuSCs decreased by 20% and 35%, respectively, with the same formulation and concentration. At the same time, no reduction in proliferation was seen in cells treated with the same concentration of VB8 organoclay alone. These results are in agreement with a previous report by da Silva et al., indicating that copaiba oil-resin encapsulated in a similar VB8 nanocomposite (NanoCOR) has a more significant effect on the morphology, survival, and proliferation of human endometriotic cells (EctESCs) than on endometrial stromal cells (CESCs and EuESCs).[200] Moreover, the endometrial stromal cells obtained from patients with endometriosis (EuESCs) respond better to this formulation than the endometrial stromal cells obtained from patients without endometriosis (CESCs).
While these results suggest that some nanoparticle-based formulations of copaiba oleoresin are non-toxic and may inhibit the proliferation and viability of the human endometriotic stromal cells (EctESCs) in culture, it is still unclear whether this treatment modality would demonstrate the same effects in an animal model of endometriosis or human patients. Therefore, further experimentation is needed to determine whether similar results can be obtained in a relevant animal model of endometriosis. Because copaiba oleoresin nanocomposites made from VB8 organoclay affects the endometrial stromal cells obtained from patients without endometriosis (CESCs), although to a lesser extent, potential side effects of these formulations should be carefully evaluated.
6. Conclusion and Future Perspective
Although the application of nanomedicine for endometriosis is still in its infancy, the available reports suggest that nanoparticle-based strategies can shift the current paradigm for diagnosis and treatment of endometriosis. Importantly, the literature review reveals that nanoparticle-based platforms, designed for and validated in cancer studies, demonstrate similar biodistribution profiles and efficacy in rodent models of endometriosis and can potentially be employed for endometriosis detection and treatment. Moreover, these studies indicate that current knowledge and some fundamental principles of cancer nanomedicine can also be used to advance novel nanoparticle-based strategies for endometriosis.
Despite the available evidence that nanoparticles can accumulate in both cancer and endometriotic lesions, presumably via passive targeting (EPR effect), endometriosis, however, has different etiology and pathogenesis and, therefore, the exact mechanisms of nanoparticle accumulation in ectopic endometrium following systemic administration must be extensively investigated and clarified. Moreover, there is currently a debate regarding the role and significance of the EPR effect in nanoparticle delivery to cancer tissue. It has even been suggested, with convincing evidence, that inter-endothelial gaps and fenestrations in tumor vasculature may not be as ubiquitous as previously believed, and might not be responsible for the preferential “leakage” of nanoparticles into tumors. Instead, the majority of accumulated nanoparticles in cancer tissues from tumor vasculature may be attributable to active processes through endothelial cells.[201] Taking all factors into account, understanding the prevailing mechanisms responsible for nanoparticle accumulation and retention in endometriotic lesions is crucial for the successful future of endometriosis nanomedicine because it will guide the optimal design of efficient nanoparticles engineered for the specific pathophysiological features of this disease.
Because the potential of nanoparticle-based platforms for imaging and treatment of endometriosis has heretofore been demonstrated only in rodent models, there is also an urgent need to evaluate their efficacy in more clinically relevant animal models (e.g., monkeys) and human clinical trials. This is especially true for strategies involving hormonal manipulation, as endometriosis is estrogen-dependent, or progestin-resistant, and occurs naturally only in humans and some non-human primates. Over the years, cancer nanomedicines have shown significant therapeutic potential in various rodent models. However, the therapeutic outcomes of passively targeted nanoparticles in clinical practice are inconsistent mainly due to the inherent heterogeneity of the EPR effect among different patients, and even among different tumors in the same patient. Therefore, it is crucial to determine whether passive targeting is also heterogeneous in endometriosis patients and whether nanoparticles successfully tested in rodent models demonstrate similarly limited clinical outcomes. If this is the case, this knowledge will allow researchers to save precious time and resources by avoiding rodent studies and focusing instead on the development and evaluation of nanoplatforms in more relevant primate models that can be efficiently translated to the clinic.
Acknowledgements
Abraham S. Moses and Ananiya A. Demessie contributed equally to this work. Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R21HD098642, the National Cancer Institute of the National Institutes of Health under Award Numbers R01CA237569 and R37CA234006, College of Pharmacy at Oregon State University and Oregon National Primate Research Center (NIH/OD P51 OD011092). The funding sources had no involvement in the collection, analysis, and interpretation of the data or in the decision to submit the article for publication.
Biography

Oleh Taratula received his Ph.D. from Rutgers, The State University of New Jersey in 2008. He is currently an Associate Professor in the Department of Pharmaceutical Sciences, College of Pharmacy at Oregon State University. The primary focus of his research is the development of multifunctional nanomaterial-based platforms for imaging and specific eradication of cancer and endometriosis.

Ov D. Slayden is a Professor of Reproductive & Developmental Sciences at the Oregon National Primate Research Center. He received his Ph.D. at Oregon State University in 1991. His research interests include hormone action, contraception, and reproductive tract disorders, including endometriosis, endometrial bleeding, and polycystic ovarian syndrome. His laboratory conducts translational studies in nonhuman primates (NHPs) that bridge the gap between bench research and clinical trials. His goal is to leverage research in NHPs to improve reproductive health in women.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Abraham S. Moses, College of Pharmacy, Oregon State University, 2730 S Moody Avenue, Portland, Oregon 97201, USA
Ananiya A. Demessie, College of Pharmacy, Oregon State University, 2730 S Moody Avenue, Portland, Oregon 97201, USA
Olena Taratula, College of Pharmacy, Oregon State University, 2730 S Moody Avenue, Portland, Oregon 97201, USA.
Tetiana Korzun, College of Pharmacy, Oregon State University, 2730 S Moody Avenue, Portland, Oregon 97201, USA.
Ov D. Slayden, Division of Reproductive and Developmental Sciences, Oregon National Primate Research Center, Oregon Health & Science University, 505 NW 185th Avenue Beaverton, Oregon 97006, USA
Oleh Taratula, College of Pharmacy, Oregon State University, 2730 S Moody Avenue, Portland, Oregon 97201, USA.
References
- [1].Burney RO, Giudice LC, Fertil. Steril 2012, 98, 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Koninckx PR, Ussia A, Adamyan L, Wattiez A, Donnez J, Fertil. Steril 2012, 98, 564. [DOI] [PubMed] [Google Scholar]
- [3].Agarwal SK, Chapron C, Giudice LC, Laufer MR, Leyland N, Missmer SA, Singh SS, Taylor HS, Am. J. Obstet. Gynecol 2019, 220, 354.e1. [DOI] [PubMed] [Google Scholar]
- [4].Arruda MS, Petta CA, Abrao MS, Benetti-Pinto CL, Hum. Reprod 2003, 18, 756. [DOI] [PubMed] [Google Scholar]
- [5].Tapmeier TT, Nazri HM, Subramaniam KS, Manek S, Garbutt K, Flint EJ, Cheuk C, Hubbard C, Barrett K, Shepherd E, Zondervan KT, Becker CM, BMJ Open 2020, 10, e032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tosti C, Biscione A, Morgante G, Bifulco G, Luisi S, Petraglia F, Eur. J. Obstet. Gynecol. Reprod. Biol 2017, 209, 61. [DOI] [PubMed] [Google Scholar]
- [7].Parazzini F, Hum. Reprod 1999, 14, 1332. [DOI] [PubMed] [Google Scholar]
- [8].Marcoux S, Maheux R, Berube S, N. Engl. J. Med 1997, 337, 217. [DOI] [PubMed] [Google Scholar]
- [9].As-Sanie S, Black R, Giudice LC, Gray Valbrun T, Gupta J, Jones B, Laufer MR, Milspaw AT, Missmer SA, Norman A, Taylor RN, Wallace K, Williams Z, Yong PJ, Nebel RA, Am. J. Obstet. Gynecol 2019. [DOI] [PubMed] [Google Scholar]
- [10].Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S, Shin HS, J. Nanobiotechnol 2018, 16, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Moses AS, Taratula OR, Lee H, Luo F, Grenz T, Korzun T, Lorenz AS, Sabei FY, Bracha S, Alani AWG, Slayden OD, Taratula O, Small 2020, 16, e1906936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Majumder J, Taratula O, Minko T, Adv. Drug Deliv. Rev 2019, 144, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Garbuzenko OB, Kbah N, Kuzmov A, Pogrebnyak N, Pozharov V, Minko T, J. Control. Release 2019, 296, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kuzmov A, Minko T, J. Control. Release 2015, 219, 500. [DOI] [PubMed] [Google Scholar]
- [15].Taratula O, Kuzmov A, Shah M, Garbuzenko OB, Minko T, J. Control. Release 2013, 171, 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schumann C, Chan S, Millar JA, Bortnyak Y, Carey K, Fedchyk A, Wong L, Korzun T, Moses AS, Lorenz A, Shea D, Taratula O, Khalimonchuk O, Taratula O, Nanomedicine 2018, 14, 1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schumann C, Nguyen DX, Norgard M, Bortnyak Y, Korzun T, Chan S, Lorenz AS, Moses AS, Albarqi HA, Wong L, Michaelis K, Zhu X, Alani AWG, Taratula OR, Krasnow S, Marks DL, Taratula O, Theranostics 2018, 8, 5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schumann C, Taratula O, Khalimonchuk O, Palmer AL, Cronk LM, Jones CV, Escalante CA, Taratula O, Nanomedicine 2015, 11, 1961. [DOI] [PubMed] [Google Scholar]
- [19].Savla R, Garbuzenko OB, Chen S, Rodriguez-Rodriguez L, Minko T, Pharm. Res 2014, 31, 3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Duong T, Li X, Yang B, Schumann C, Albarqi HA, Taratula O, Taratula O, Nanomedicine 2017, 13, 955. [DOI] [PubMed] [Google Scholar]
- [21].Li X, Schumann C, Albarqi HA, Lee CJ, Alani AWG, Bracha S, Milovancev M, Taratula O, Taratula O, Theranostics 2018, 8, 767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Albarqi HA, Wong LH, Schumann C, Sabei FY, Korzun T, Li X, Hansen MN, Dhagat P, Moses AS, Taratula O, Taratula O, ACS Nano 2019, 13, 6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li W, Chen X, Nanomedicine (Lond) 2015, 10, 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Garbuzenko OB, Kuzmov A, Taratula O, Pine SR, Minko T, Theranostics 2019, 9, 8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li X, Taratula O, Taratula O, Schumann C, Minko T, Mini Rev. Med. Chem 2017, 17, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sapiezynski J, Taratula O, Rodriguez-Rodriguez L, Minko T, J. Control. Release 2016, 243, 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schumann C, Chan S, Khalimonchuk O, Khal S, Moskal V, Shah V, Alani AW, Taratula O, Taratula O, Mol. Pharmaceut 2016, 13, 2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Taratula O, Garbuzenko OB, Kirkpatrick P, Pandya I, Savla R, Pozharov VP, He HX, Minko T, J. Control. Release 2009, 140, 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Taratula O, Schumann C, Duong T, Taylor KL, Taratula O, Nanoscale 2015, 7, 3888. [DOI] [PubMed] [Google Scholar]
- [30].Maeda H, Adv. Enzym. Reg 2001, 41, 189. [DOI] [PubMed] [Google Scholar]
- [31].Maeda H, Proc. Japan Acad. Series B, Phys. Biol. Sci 2012, 88, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Matsumura Y, Maeda H, Cancer Res. 1986, 46, 6387. [PubMed] [Google Scholar]
- [33].Maeda H, Nakamura H, Fang J, Adv. Drug Deliv. Rev 2013, 65, 71. [DOI] [PubMed] [Google Scholar]
- [34].Ryan IP, Taylor RN, Obstet. Gynecol. Surv 1997, 52, 365. [DOI] [PubMed] [Google Scholar]
- [35].Groothuis PG, Nap AW, Winterhager E, Grummer R, Angiogenesis 2005, 8, 147. [DOI] [PubMed] [Google Scholar]
- [36].Rocha AL, Reis FM, Taylor RN, Obstet. Gynecol. Int 2013, 2013, 859619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schreinemacher MH, Backes WH, Slenter JM, Xanthoulea S, Delvoux B, van Winden L, Beets-Tan RG, Evers JL, Dunselman GA, Romano A, PLoS One 2012, 7, e33241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nap AW, Griffioen AW, Dunselman GA, Bouma-Ter Steege JC, Thijssen VL, Evers JL, Groothuis PG, J. Clin. Endocrinol. Metab 2004, 89, 1089. [DOI] [PubMed] [Google Scholar]
- [39].Maeda H, Cancer Sci. 2013, 104, 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yoshino O, Yamada-Nomoto K, Kobayashi M, Andoh T, Hongo M, Ono Y, Hasegawa-Idemitsu A, Sakai A, Osuga Y, Saito S, Eur. J. Pain 2017, 22, 501. [DOI] [PubMed] [Google Scholar]
- [41].Scutiero G, Iannone P, Bernardi G, Bonaccorsi G, Spadaro S, Volta CA, Greco P, Nappi L, Oxid. Med. Cell. Longev 2017, 2017, 7265238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Azzopardi EA, Ferguson EL, Thomas DW, J. Antimicrob. Chemother 2013, 68, 257. [DOI] [PubMed] [Google Scholar]
- [43].Guo X, Li W, Zhou J, Hou W, Wen X, Zhang H, Kong F, Luo L, Li Q, Du Y, You J, Small 2017, 13. [DOI] [PubMed] [Google Scholar]
- [44].Yuan M, Ding S, Meng T, Lu B, Shao S, Zhang X, Yuan H, Hu F, Int J Nanomedicine 2017, 12, 8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhao MD, Sun YM, Fu GF, Du YZ, Chen FY, Yuan H, Zheng CH, Zhang XM, Hu FQ, Biomaterials 2012, 33, 634. [DOI] [PubMed] [Google Scholar]
- [46].Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM, Proc. Natl. Acad. Sci. USA 2008, 105, 11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Frohlich E, Int. J. Nanomedicine 2012, 7, 5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang Z, Sun J, Qiu Y, Li W, Guo X, Li Q, Zhang H, Zhou J, Du Y, Yuan H, Hu F, You J, Biomaterials 2015, 68, 32. [DOI] [PubMed] [Google Scholar]
- [49].Lee HJ, Lee HJ, Lee JM, Chang Y, Woo ST, Magn. Reson. Imaging 2012, 30, 860. [DOI] [PubMed] [Google Scholar]
- [50].Jerman LF, Hey-Cunningham AJ, Biol. Reprod 2015, 92, 64. [DOI] [PubMed] [Google Scholar]
- [51].Ulbrich W, Lamprecht A, J. R. Soc. Interface 2010, 7 Suppl 1, S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nehoff H, Parayath NN, Domanovitch L, Taurin S, Greish K, Int. J. Nanomedicine 2014, 9, 2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bazak R, Houri M, El Achy S, Kamel S, Refaat T, J. Cancer Res. Clin. Oncol 2015, 141, 769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Morales-Cruz M, Delgado Y, Castillo B, Figueroa CM, Molina AM, Torres A, Milian M, Griebenow K, Drug Des. Devel. Ther 2019, 13, 3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rosenblum D, Joshi N, Tao W, Karp JM, Peer D, Nat. Commun 2018, 9, 1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sykes EA, Chen J, Zheng G, Chan WC, ACS Nano 2014, 8, 5696. [DOI] [PubMed] [Google Scholar]
- [57].Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW, Nature Rev. Mater 2016, 1, 16014. [Google Scholar]
- [58].Dai Q, Wilhelm S, Ding D, Syed AM, Sindhwani S, Zhang Y, Chen YY, MacMillan P, Chan WCW, ACS Nano 2018, 12, 8423. [DOI] [PubMed] [Google Scholar]
- [59].Shah V, Taratula O, Garbuzenko OB, Taratula OR, Rodriguez-Rodriguez L, M. T., Clin. Cancer Res 2013, 19, 6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhang H, Li J, Sun W, Hu Y, Zhang G, Shen M, Shi X, PLoS One 2014, 9, e94718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zhao MD, Cheng JL, Yan JJ, Chen FY, Sheng JZ, Sun DL, Chen J, Miao J, Zhang RJ, Zheng CH, Huang HF, Int. J. Nanomedicine 2016, 11, 1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chen Y, Zhang H, Zhang Y, Semin. Cancer Biol 2019, 56, 37. [DOI] [PubMed] [Google Scholar]
- [63].Iwase A, Kotani T, Goto M, Kobayashi H, Takikawa S, Nakahara T, Nakamura T, Kondo M, Bayasula, Nagatomo Y, Kikkawa F, Reprod. Sci 2014, 21, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kim HO, Yang KM, Kang IS, Koong MK, Kim HS, Zhang X, Kim I, J Reprod. Med 2007, 52, 207. [PubMed] [Google Scholar]
- [65].Poncelet C, Leblanc M, Walker-Combrouze F, Soriano D, Feldmann G, Madelenat P, Scoazec JY, Darai E, Acta Obstet. Gynecol. Scand 2002, 81, 195. [DOI] [PubMed] [Google Scholar]
- [66].Li J, He Y, Sun W, Luo Y, Cai H, Pan Y, Shen M, Xia J, Shi X, Biomaterials 2014, 35, 3666. [DOI] [PubMed] [Google Scholar]
- [67].Gibran L, Maranhao RC, Tavares ER, Carvalho PO, Abrao MS, Podgaec S, Hum. Reprod 2017, 32, 332. [DOI] [PubMed] [Google Scholar]
- [68].Graziani SR, Vital CG, Morikawa AT, Van Eyll BM, Fernandes HJ Junior, Kalil Filho R, Maranhao RC, Med. Oncol 2017, 34, 151. [DOI] [PubMed] [Google Scholar]
- [69].Pires LA, Hegg R, Valduga CJ, Graziani SR, Rodrigues DG, Maranhao RC, Cancer Chemother. Pharmacol 2009, 63, 281. [DOI] [PubMed] [Google Scholar]
- [70].Bedin A, Maranhao RC, Tavares ER, Carvalho PO, Baracat EC, Podgaec S, Clinics 2019, 74, e989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Slayden OD, Semin. Reprod. Med 2014, 32, 385. [DOI] [PubMed] [Google Scholar]
- [72].Rudolph-Owen LA, Slayden OD, Matrisian LM, Brenner RM, Biol. Reprod 1998, 59, 1349. [DOI] [PubMed] [Google Scholar]
- [73].Slayden OD, Brenner RM, Reprod. Biol. Endocrinol 2006, 4 Suppl 1, S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Girling JE, Rogers PA, Angiogenesis 2005, 8, 89. [DOI] [PubMed] [Google Scholar]
- [75].Rogers PA, Donoghue JF, Walter LM, Girling JE, Reprod. Sci 2009, 16, 147. [DOI] [PubMed] [Google Scholar]
- [76].Red-Horse K, Placenta 2008, 29 Suppl A, S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Donoghue JF, Lederman FL, Susil BJ, Rogers PA, Hum. Reprod 2007, 22, 1705. [DOI] [PubMed] [Google Scholar]
- [78].Rogers PA, Donoghue JF, Girling JE, Placenta 2008, 29 Suppl A, S48. [DOI] [PubMed] [Google Scholar]
- [79].Maybin JA, Hirani N, Jabbour HN, Critchley HO, Am. J. Pathol 2011, 178, 1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Nayak NR, Critchley HO, Slayden OD, Menrad A, Chwalisz K, Baird DT, Brenner RM, J. Clin. Endocrinol. Metab 2000, 85, 3442. [DOI] [PubMed] [Google Scholar]
- [81].Wang Y, Nicholes K, Shih IM, Ann. Rev. Pathol 2020, 15, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lagana AS, Garzon S, Gotte M, Vigano P, Franchi M, Ghezzi F, Martin DC, Int. J. Mol. Sci 2019, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Chapron C, Marcellin L, Borghese B, Santulli P, Nat. Rev. Endocrinol 2019, 15, 666. [DOI] [PubMed] [Google Scholar]
- [84].Clement PB, Adv. Anat. Pathol 2007, 14, 241. [DOI] [PubMed] [Google Scholar]
- [85].Jerman LF, Anderson L, Markham R, Hey-Cunningham AJ, Reprod. Sci 2020, 27, 977. [DOI] [PubMed] [Google Scholar]
- [86].Guo SW, Ding D, Shen M, Liu X, Reprod. Sci 2015, 22, 873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Brosens IA, Am. J. Obstet. Gynecol 1997, 176, 263. [DOI] [PubMed] [Google Scholar]
- [88].Slayden OD, Handb. Exp. Pharmacol 2016, 232, 191. [DOI] [PubMed] [Google Scholar]
- [89].Slayden OD, Martin LD, Curr. Women's Health Rev 2018, 14, 164. [Google Scholar]
- [90].Yamanaka A, Kimura F, Takebayashi A, Kita N, Takahashi K, Murakami T, Tohoku J. Exp. Med 2012, 226, 95. [DOI] [PubMed] [Google Scholar]
- [91].D'Hooghe TM, Bambra CS, Suleman MA, Dunselman GA, Evers HL, Koninckx PR, Fertil. Steril 1994, 62, 635. [PubMed] [Google Scholar]
- [92].Fazleabas AT, Methods Mol. Med 2006, 121, 95. [DOI] [PubMed] [Google Scholar]
- [93].Fazleabas AT, Brudney A, Gurates B, Chai D, Bulun S, Ann. NY Acad. Sci 2002, 955, 308. [DOI] [PubMed] [Google Scholar]
- [94].D'Hooghe TM, Bambra CS, Raeymaekers BM, De Jonge I, Lauweryns JM, Koninckx PR, Am. J. Obstet. Gynecol 1995, 173, 125. [DOI] [PubMed] [Google Scholar]
- [95].Pullen N, Birch CL, Douglas GJ, Hussain Q, Pruimboom-Brees I, Walley RJ, Hum. Reprod. Update 2011. [DOI] [PubMed] [Google Scholar]
- [96].Vernon MW, Wilson EA, Fertil. Steril 1985, 44, 684. [PubMed] [Google Scholar]
- [97].Somigliana E, Vigano P, Rossi G, Carinelli S, Vignali M, Panina-Bordignon P, Hum. Reprod 1999, 14, 2944. [DOI] [PubMed] [Google Scholar]
- [98].Liang Z, Chen Y, Zhao Y, Xu C, Zhang A, Zhang Q, Wang D, He J, Hua W, Duan P, Stem Cell Res. Ther 2017, 8, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Chaudhury K, Babu KN, Singh AK, Das S, Kumar A, Seal S, Nanomedicine 2013, 9, 439. [DOI] [PubMed] [Google Scholar]
- [100].Jana SK, Chakravarty B, Chaudhury K, J.Nanomed. Biother. Discov 2014, 4, 1 [Google Scholar]
- [101].Singh AK, Chakravarty B, Chaudhury K, J. Biomed. Nanotechnol 2015, 11, 789. [DOI] [PubMed] [Google Scholar]
- [102].Boroumand S, Hosseini S, Pashandi Z, Faridi-Majidi R, Salehi M, J. Mater. Sci. Mater. Med 2019, 31, 8. [DOI] [PubMed] [Google Scholar]
- [103].Liu Q, Ma P, Liu L, Ma G, Ma J, Liu X, Liu Y, Lin W, Zhu Y, Eur. J. Pharm. Sci 2017, 96, 542. [DOI] [PubMed] [Google Scholar]
- [104].Wang N, Liu B, Liang L, Wu Y, Xie H, Huang J, Guo X, Tan J, Zhan X, Liu Y, Wang L, Ke P, Biomed. Res. Int 2014, 2014, 546479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Hsu AL, Khachikyan I, Stratton P, Clin. Obstet. Gynecol 2010, 53, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Bazot M, Bharwani N, Huchon C, Kinkel K, Cunha TM, Guerra A, Manganaro L, Bunesch L, Kido A, Togashi K, Eur. Radiol 2017, 27, 2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Stratton P, Winkel C, Premkumar A, Chow C, Wilson J, Hearns-Stokes R, Heo S, Merino M, Nieman LK, Fertil. Steril 2003, 79, 1078. [DOI] [PubMed] [Google Scholar]
- [108].Lee N, Hyeon T, Chem. Soc. Rev 2012, 41, 2575. [DOI] [PubMed] [Google Scholar]
- [109].Togashi K, Nishimura K, Kimura I, Tsuda Y, Yamashita K, Shibata T, Nakano Y, Konishi J, Konishi I, Mori T, Radiology 1991, 180, 73. [DOI] [PubMed] [Google Scholar]
- [110].Murphy AA, Green WR, Bobbie D, dela Cruz ZC, Rock JA, Fertil. Steril 1986, 46, 522. [DOI] [PubMed] [Google Scholar]
- [111].Walter S, Susanne S, Simon W, Herbert S, Clemens F, Claudia G, Alwin EG, Rainer K, Hans JR, Neurosurgery 1998, 42, 518.9526986 [Google Scholar]
- [112].Yang JZ, Van Dijk-Smith JP, Van Vugt DA, Kennedy JC, Reid RL, Am. J. Obstet. Gynecol 1996, 174, 154. [DOI] [PubMed] [Google Scholar]
- [113].Hillemanns P, Weingandt H, Stepp H, Baumgartner R, Xiang W, Korell M, Am. J. Obstet. Gynecol 2000, 183, 52. [DOI] [PubMed] [Google Scholar]
- [114].da Rocha Filho HN, da Silva EC, Silva FR, Courrol LC, de Mesquita CH, Bellini MH, J. Fluores 2015, 25, 1363. [DOI] [PubMed] [Google Scholar]
- [115].Kemmner W, Wan K, Rüttinger S, Ebert B, Macdonald R, Klamm U, Moesta KT, FASEB J. 2008, 22, 500. [DOI] [PubMed] [Google Scholar]
- [116].Yang X, Palasuberniam P, Kraus D, Chen B, Int. J. Mol. Sci 2015, 16, 25865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Buchweitz O, Wülfing P, Staebler A, Kiesel L, J. Am. Assoc. Gynecol. Laparoscopists 2004, 11, 505. [DOI] [PubMed] [Google Scholar]
- [118].Malik E, Berg C, Meyhöfer-Malik A, Buchweitz O, Moubayed P, Diedrich K, Surg. Endosc 2000, 14, 452. [DOI] [PubMed] [Google Scholar]
- [119].Butowska W, Warchoł W, Nowak-Markwitz E, Wołuń-Cholewa M, Rocz. Akad. Med. Bialymst. (1995) 2004, 49, 123. [PubMed] [Google Scholar]
- [120].Jansen RP, Russell P, Am. J. Obstet. Gynecol 1986, 155, 1154. [DOI] [PubMed] [Google Scholar]
- [121].Owens EA, Lee S, Choi J, Henary M, Choi HS, Wiley Interdiscipl. Rev.: Nanomed. Nanobiotechnol 2015, 7, 828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Taratula O, Schumann C, Naleway MA, Pang AJ, Chon KJ, Mol. Pharmaceut 2013, 10, 3946. [DOI] [PubMed] [Google Scholar]
- [123].Starosolski Z, Bhavane R, Ghaghada KB, Vasudevan SA, Kaay A, Annapragada A, PLoS One 2017, 12, e0187563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Muckle TJ, Biochem. Med 1976, 15, 17. [DOI] [PubMed] [Google Scholar]
- [125].Zhou JF, Chin MP, Schafer SA, SPIE Proc. 1994, 2128, 495. [Google Scholar]
- [126].Gathje J, Steuer RR, Nicholes K, J. Appl. Physiol 1970, 29, 181. [DOI] [PubMed] [Google Scholar]
- [127].Landsman M, Kwant G, Mook G, Zijlstra W, J. Appl. Physiol 1976, 40, 575. [DOI] [PubMed] [Google Scholar]
- [128].Rajagopalan R, Uetrecht P, Bugaj JE, Achilefu SA, Dorshow RB, Photochem. Photobiol 2000, 71, 347. [DOI] [PubMed] [Google Scholar]
- [129].Ito S, Muguruma N, Hayashi S, Taoka S, Bando T, Inayama K, Sogabe M, Okahisa T, Okamura S, Shibata H, Bioorg. Med. Chem 1998, 6, 613. [DOI] [PubMed] [Google Scholar]
- [130].van den Berg LL, Crane LM, van Oosten M, van Dam GM, Simons AH, Hofker HS, Bart J, Int. J. Gynecol. Obstetr 2013, 121, 35. [DOI] [PubMed] [Google Scholar]
- [131].ter Weele EJ, van Scheltinga AGT, Linssen MD, Nagengast WB, Lindner I, Jorritsma-Smit A, de Vries EG, Kosterink JG, Lub-de Hooge MN, Eur. J. Pharmaceut. Biopharmaceut 2016, 104, 226. [DOI] [PubMed] [Google Scholar]
- [132].Marston JC, Kennedy GD, Lapi SE, Hartman YE, Richardson MT, Modi HM, Warram JM, J. Surg. Res 2019, 239, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Chen AM, Zhang M, Wei D, Stueber D, Taratula O, Minko T, He H, Small 2009, 5, 2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Hu CM, Aryal S, Zhang L, Ther. Deliv 2010, 1, 323. [DOI] [PubMed] [Google Scholar]
- [135].Garbuzenko OB, Saad M, Pozharov VP, Reuhl KR, Mainelis G, Minko T, Proc. Natl. Acad. Sci. USA 2010, 107, 10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S, Reprod. Biol. Endocrinol 2012, 10, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Siti HN, Kamisah Y, Kamsiah J, Vascul. Pharmacol 2015, 71, 40. [DOI] [PubMed] [Google Scholar]
- [138].Gupta S, Agarwal A, Krajcir N, Alvarez JG, Reprod. Biomed 2006, 13, 126. [DOI] [PubMed] [Google Scholar]
- [139].Ngo C, Chereau C, Nicco C, Weill B, Chapron C, Batteux F, Am. J. Pathol 2009, 175, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Platt DH, Bartoli M, El-Remessy AB, Al-Shabrawey M, Lemtalsi T, Fulton D, Caldwell RB, Free Radic. Biol. Med 2005, 39, 1353. [DOI] [PubMed] [Google Scholar]
- [141].Donnez J, Binda MM, Donnez O, Dolmans MM, Fertil. Steril 2016, 106, 1011. [DOI] [PubMed] [Google Scholar]
- [142].Sharpe-Timms KL, Cox KE, Ann. NY Acad. Sci 2002, 955, 147. [DOI] [PubMed] [Google Scholar]
- [143].Pitsos M, Kanakas N, Reprod. Sci 2009, 16, 717. [DOI] [PubMed] [Google Scholar]
- [144].McCawley LJ, Matrisian LM, Mol. Med. Today 2000, 6, 149. [DOI] [PubMed] [Google Scholar]
- [145].Arablou T, Kolahdouz-Mohammadi R, Biomed. Pharmacother 2018, 97, 91. [DOI] [PubMed] [Google Scholar]
- [146].Ailawadi RK, Jobanputra S, Kataria M, Gurates B, Bulun SE, Fertil. Steril 2004, 81, 290. [DOI] [PubMed] [Google Scholar]
- [147].Ferrero S, Venturini PL, Ragni N, Camerini G, Remorgida V, Drugs 2009, 69, 943. [DOI] [PubMed] [Google Scholar]
- [148].Singh BN, Shankar S, Srivastava RK, Biochem. Pharmacol 2011, 82, 1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Mochizuki M, Yamazaki S, Kano K, Ikeda T, Biochim. Biophys. Acta 2002, 1569, 35. [DOI] [PubMed] [Google Scholar]
- [150].Misra R, Acharya S, Dilnawaz F, Sahoo SK, Nanomedicine (Lond) 2009, 4, 519. [DOI] [PubMed] [Google Scholar]
- [151].Das M, Patil S, Bhargava N, Kang JF, Riedel LM, Seal S, Hickman JJ, Biomaterials 2007, 28, 1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Perez JM, Asati A, Nath S, Kaittanis C, Small 2008, 4, 552. [DOI] [PubMed] [Google Scholar]
- [153].Soares SR, Martinez-Varea A, Hidalgo-Mora JJ, Pellicer A, Fertil. Steril 2012, 98, 529. [DOI] [PubMed] [Google Scholar]
- [154].Howard FM, J. Minim. Invasive Gynecol 2009, 16, 540. [DOI] [PubMed] [Google Scholar]
- [155].Hansen RR, Nasser A, Falk S, Baldvinsson SB, Ohlsson PH, Bahl JM, Jarvis MF, Ding M, Heegaard AM, Eur. J. Pharmacol 2012, 688, 27. [DOI] [PubMed] [Google Scholar]
- [156].Cantin LD, Bayrakdarian M, Buon C, Grazzini E, Hu YJ, Labrecque J, Leung C, Luo X, Martino G, Pare M, Payza K, Popovic N, Projean D, Santhakumar V, Walpole C, Yu XH, Tomaszewski MJ, Bioorg. Med. Chem. Lett 2012, 22, 2565. [DOI] [PubMed] [Google Scholar]
- [157].Gever JR, Soto R, Henningsen RA, Martin RS, Hackos DH, Panicker S, Rubas W, Oglesby IB, Dillon MP, Milla ME, Burnstock G, Ford AP, Br. J. Pharmacol 2010, 160, 1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Que-Gewirth NS, Sullenger BA, Gene Ther. 2007, 14, 283. [DOI] [PubMed] [Google Scholar]
- [159].Chen S, Shimoda M, Chen J, Matsumoto S, Grayburn PA, Cell Cycle 2012, 11, 695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Hosseinahli N, Aghapour M, Duijf PHG, Baradaran B, J. Cell. Physiol 2018, 233, 5574. [DOI] [PubMed] [Google Scholar]
- [161].Izquierdo M, Cancer Gene Ther. 2005, 12, 217. [DOI] [PubMed] [Google Scholar]
- [162].Wang SL, Yao HH, Qin ZH, Expert Opin. Biol. Ther 2009, 9, 1357. [DOI] [PubMed] [Google Scholar]
- [163].Atkinson H, Chalmers R, Genetica 2010, 138, 485. [DOI] [PubMed] [Google Scholar]
- [164].Lundstrom K, Boulikas T, Technol. Cancer Res. Treat 2003, 2, 471. [DOI] [PubMed] [Google Scholar]
- [165].Shubina AN, Egorova AA, Baranov VS, Kiselev AV, Recent Pat. DNA Gene Seq 2013, 7, 169. [DOI] [PubMed] [Google Scholar]
- [166].Dass CR, Contreras KG, Dunstan DE, Choong PF, Biomaterials 2007, 28, 3026. [DOI] [PubMed] [Google Scholar]
- [167].Chuderland D, Hasky N, Ben-Ami I, Kaplan-Kraicer R, Grossman H, Shalgi R, Hum. Reprod 2013, 28, 1626. [DOI] [PubMed] [Google Scholar]
- [168].Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, Bouck NP, Science 1999, 285, 245. [DOI] [PubMed] [Google Scholar]
- [169].Manalo KB, Choong PF, Becerra SP, Dass CR, Expert Opin. Ther. Pat 2011, 21, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Zou LB, Zhang RJ, Tan YJ, Ding GL, Shi S, Zhang D, He RH, Liu AX, Wang TT, Leung PC, Sheng JZ, Huang HF, J. Clin. Endocrinol. Metab 2011, 96, E1399. [DOI] [PubMed] [Google Scholar]
- [171].O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J, Cell 1997, 88, 277. [DOI] [PubMed] [Google Scholar]
- [172].Ahn SH, Singh V, Tayade C, Fertil. Steril 2017, 107, 523. [DOI] [PubMed] [Google Scholar]
- [173].Kaller M, Hermeking H, Adv. Exp. Med. Biol 2016, 937, 71. [DOI] [PubMed] [Google Scholar]
- [174].Taratula O, Doddapaneni BS, Schumann C, Li X, Bracha S, Milovancev M, Alani A, Taratula O, Chem. Mater 2015, 27, 6155 [Google Scholar]
- [175].Hainfeld J, Slatkin D, Focella T, Smilowitz H, Brit. J. Radiol 2006, 79, 248. [DOI] [PubMed] [Google Scholar]
- [176].Konan YN, Gurny R, Allémann E, J. Photochem. Photobiol. B: Biol 2002, 66, 89. [DOI] [PubMed] [Google Scholar]
- [177].Veeranarayanan S, Mohamed MS, Poulose AC, Rinya M, Sakamoto Y, Maekawa T, Kumar DS, Theranostics 2018, 8, 5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].König K, Wyss-Desserich M-T, Tadir Y, Haller U, Tromberg B, Berns MW, Wyss P, Med. Laser Appl 2006, 21, 291. [Google Scholar]
- [179].Kennedy J, Pottier R, SPIE MILESTONE SERIES MS 1993, 82, 534. [Google Scholar]
- [180].Wołuń-Cholewa M, Szymanowski K, Nowak-Markwitz E, Warchoł W, Photodiagn. Photodyn. Ther 2011, 8, 58. [DOI] [PubMed] [Google Scholar]
- [181].Wolun-Cholewa M, Butowska W, Fischer N, Warcho W, Nowak-Markwitz E, Photomedi. Laser Surger 2009, 27, 295. [DOI] [PubMed] [Google Scholar]
- [182].Konig K, Photomedicine in Gynecology and Reproduction. Basel, Freiburg, Paris, London, New York, New Delhi, Bangkok, Singapore, Tokyo, Sydney: Karger; 2000, 86. [Google Scholar]
- [183].Mellman I, Coukos G, Dranoff G, Nature 2011, 480, 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].DeNardo DG, Ruffell B, Nat. Rev. Immunol 2019, 19, 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Rezvani K, Rouce RH, Front. Immunol 2015, 6, 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Antsiferova Y, Sotnikova N, Parfenyuk E, Biomed. Res. Int 2013, 2013, 924362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Shimizu T, Iwamoto Y, Yanagihara Y, Ikeda K, Achiwa K, Int. J. Immunopharmacol 1992, 14, 1415. [DOI] [PubMed] [Google Scholar]
- [188].Coulombe F, Fiola S, Akira S, Cormier Y, Gosselin J, PLoS One 2012, 7, e36734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Barnes CA, Elsaesser A, Arkusz J, Smok A, Palus J, Lesniak A, Salvati A, Hanrahan JP, Jong WH, Dziubaltowska E, Stepnik M, Rydzynski K, McKerr G, Lynch I, Dawson KA, Howard CV, Nano Lett. 2008, 8, 3069. [DOI] [PubMed] [Google Scholar]
- [190].Wu MH, Shoji Y, Wu MC, Chuang PC, Lin CC, Huang MF, Tsai SJ, Am. J. Pathol 2005, 167, 1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Kondelkova K, Vokurkova D, Krejsek J, Borska L, Fiala Z, Ctirad A, Acta Medica 2010, 53, 73. [DOI] [PubMed] [Google Scholar]
- [192].Korman AJ, Peggs KS, Allison JP, Adv. Immunol 2006, 90, 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Basta P, Majka M, Jozwicki W, Lukaszewska E, Knafel A, Grabiec M, Stasienko E, Wicherek L, Reprod. Biol. Endocrinol 2010, 8, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Li MQ, Wang Y, Chang KK, Meng YH, Liu LB, Mei J, Wang Y, Wang XQ, Jin LP, Li DJ, Cell Death Dis. 2014, 5, e1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Giudice LC, N. Engl. J. Med 2010, 362, 2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Peterson JR, Mitchison TJ, Chem. Biol 2002, 9, 1275. [DOI] [PubMed] [Google Scholar]
- [197].Nogueira Neto J, Lindoso MJ, Coelho LF, Carvalho RA, Rodrigues TG, Araujo AG, Girao MJ, Schor E, Acta Cir. Bras 2011, 26 Suppl 2, 20. [DOI] [PubMed] [Google Scholar]
- [198].de Almeida Borges VR, da Silva JH, Barbosa SS, Nasciutti LE, Cabral LM, de Sousa VP, Mater. Sci. Eng. C Mater. Biol. Appl 2016, 64, 310. [DOI] [PubMed] [Google Scholar]
- [199].de Almeida Borges VR, Tavares MR, da Silva JH, Tajber L, Boylan F, Ribeiro AF, Nasciutti LE, Cabral LM, de Sousa VP, Pharm. Dev. Technol 2018, 23, 343. [DOI] [PubMed] [Google Scholar]
- [200].Henriques da Silva J, Borges VR, Pereira Lda C, Ferrari R, de Mattos RM, Barros EG, Palmero CY, Fernandes PD, de Carvalho PR, Pereira de Sousa V, Cabral LM, Nasciutti LE, J. Pharm. Pharmacol 2015, 67, 1744. [DOI] [PubMed] [Google Scholar]
- [201].Sindhwani S, Syed AM, Ngai J, Kingston BR, Maiorino L, Rothschild J, MacMillan P, Zhang Y, Rajesh NU, Hoang T, Wu JLY, Wilhelm S, Zilman A, Gadde S, Sulaiman A, Ouyang B, Lin Z, Wang L, Egeblad M, Chan WCW, Nat. Mater 2020, 19, 566. [DOI] [PubMed] [Google Scholar]