Abstract
Introduction
Recent research suggests that health disparities among low-SES and ethnic minority populations may originate from prenatal and early life exposures. Postpartum maternal depressive symptoms have been linked to poorer infant physical health, yet prenatal depressive symptoms not been thoroughly examined in relation to infant health.
Methods
In a prospective study of low-income Mexican American mothers and their infants, women (N = 322, median age 27.23, IQR = 22.01–32.54) completed surveys during pregnancy (median gestation 39.50, IQR = 38.71–40.14 weeks) and 12 weeks after birth. We investigated (1) if prenatal depressive symptoms predicted infant physical health concerns at 12 weeks of age, (2) whether these associations occurred above and beyond concurrent depressive symptoms, and (3) if birth weight, gestational age, and breastfeeding were mediators of prenatal depression predicting subsequent infant health.
Results
Higher prenatal depressive symptoms were associated with more infant physical health concerns at 12 weeks (p < .001), after accounting for 12-week maternal depressive symptoms, breastfeeding, gestational age, and birth weight. Twelve-week maternal depressive symptoms were concurrently associated with more infant health concerns (p < .01). Birth weight, gestational age, and breastfeeding were not associated with maternal depression or infant health concerns.
Discussion
Results establish a link between prenatal depressive symptoms and an elevated risk of poor health evident shortly after birth. These findings underscore the importance of the prenatal period as a possible sensitive period for infants’ health, and the need for effective interventions for depression during pregnancy to mitigate potentially teratogenic effects on the developing fetus and reduce risks for later health concerns.
Keywords: Prenatal depression, Mother, Infant, Health
Introduction
In the U.S., low-income and Hispanic individuals experience higher rates of chronic diseases, including diabetes, liver disease, and hypertension (Dominguez et al. 2015). There is growing awareness of prenatal origins of adult disease (Calkins and Devaskar 2011), suggesting that events affecting the fetus during pregnancy could influence long-term health. The fetal origins of adult disease (FOAD) hypothesis argues that insults to development during sensitive periods result in permanent changes that affect lifetime health (Calkins and Devaskar 2011). Recent research demonstrates that the presence of prenatal maternal depressive symptoms is capable of directly affecting the developing fetus, with potential consequences for infant and child health (Seckl 2001). Approximately 10% of pregnant women experience significant depressive symptoms (Bennett et al. 2004), with higher prevalence (34%) among Mexican-Americans (Lara et al. 2009). Consistent with FOAD, prenatal depressive symptoms have been linked to poor birth outcomes (Grote et al. 2010), reduced breastfeeding (Figueiredo et al. 2014), and more infant illnesses and antibiotic use in the first year of life (Beijers et al. 2010).
In populations with fewer resources and less access to care, the consequences of maternal prenatal depressive symptoms for infant health could contribute to already problematic health disparities. In a study consisting of primarily low-income minority women, persistent depressive symptoms spanning from prenatal to 9–12 months postpartum were associated with increased risks of infant hospitalization (Chung et al. 2004). In addition, depression during pregnancy has been associated with poorer infant growth and a higher incidence of diarrheal infection in rural Pakistan (Rahman et al. 2004). Despite compelling arguments for further study, there is a dearth of research examining the effects of prenatal depressive symptoms on infant physical health.
The majority of research on maternal depression and its association with infant health outcomes has been limited to the postpartum period. For example, maternal depression at four months postpartum predicts slower weight gain, poorer sleep, and more physical health concerns in infants of low-socioeconomic status (SES) Mexican-American women (Gress-Smith et al. 2012). Although maternal depression at any point throughout childhood is a risk factor for poor child outcomes, the impact of maternal depression during pregnancy may be particularly deleterious (Field et al. 2011). Specifically, some have hypothesized that exposure to environmental insults such as maternal prenatal depression during critical periods of fetal development may “program”, or exert enduring structural and functional changes to, the development of critical physiological systems, with implications for lifespan health (Seckl 2001). Indeed, some evidence suggests that prenatal exposure to maternal depression may be implicated in adult disease (see Kajantie 2006 for a review). Nonetheless, there is little data available regarding the extent to which associations between maternal depression during pregnancy are prospectively associated with children’s health.
There are several indirect pathways by which maternal prenatal depressive symptoms may negatively affect infant physical health. Prenatal depression has been linked to low birth weight and early gestational age (Field et al. 2004; Grote et al. 2010). Low birth weight children have higher rates of health problems, including asthma, respiratory infections, ear infections, and more emergency room visits and school absence (Hack et al. 1995). Compared to children born full-term, preterm and late preterm children experience higher parent-reported disease burden at ages three and five, including more hospital admissions, asthma and wheezing, long-term illnesses, and mortality that extend across the lifespan (Boyle et al. 2012). Prenatal depression has also been linked to decreased intent to breastfeed (Fairlie et al. 2009), quicker cessation of breastfeeding (Ystrom 2012), and shorter durations of exclusive breastfeeding (Figueiredo et al. 2014). In turn, children who are not breastfed are more likely to experience long-term poorer health, including gas-troenteritis, asthma, diabetes, obesity, and cancer (Chung et al. 2007; Rossiter et al. 2015; Midodzi et al. 2010). Consequently, the mechanisms underlying infant health development and its link to maternal depressive symptoms should be clarified by examining birth outcomes and breastfeeding behavior as mediators.
The Present Study
This longitudinal study addresses the need for research on prenatal depression as a predictor of infant health, with exploration of potential mediating factors. Our first aim was to examine the relation of prenatal depressive symptoms to 12-week infant health concerns. We expected that women reporting higher depressive symptoms during pregnancy would have infants with more mother-reported health concerns. Our second aim was to evaluate whether prenatal depressive symptoms predict infant health above and beyond prediction from concurrent maternal depressive symptoms. We hypothesized that prenatal depressive symptoms would remain associated with infant health at 12 weeks after accounting for 12-week maternal depressive symptoms. Our third aim was to evaluate objectively measured birth outcomes (birth weight, gestational age) and breastfeeding as mediators of the relation of prenatal depressive symptoms to infant health concerns. We expected that higher depressive symptoms would be associated with more health concerns due to lower birth weight, earlier gestational age, and breastfeeding as pathways of influence.
Methods
Participants
Participants were 322 low income Mexican American mother-infant dyads. Women were eligible if they were age 18 or older, fluent in either Spanish or English, self-identified as Mexican or Mexican American, expected and delivered a singleton baby, had no prenatal indication of a significant health or developmental problem with the baby, and reported low income status (eligible for Medicaid or self-reported income below $25,000).
Procedures
Participants were recruited at county-operated prenatal care clinics in the Phoenix, Arizona metropolitan area. Women were recruited at any point during pregnancy before 36 weeks gestation by a bilingual female interviewer. Fifty-six percent of eligible women who were approached agreed to schedule a prenatal interview at their homes. All women provided informed consent and contact information at the prenatal visit. The parent longitudinal study included home visits prenatally as well as 6, 12, 18, and 24 weeks postpartum; we analyzed only prenatal and 12-week interview data. To reduce the burden of ongoing data collection with short time intervals, the study used a “planned missingness” design (Enders 2010); all participants completed the prenatal visit, but two-thirds of participants were randomly assigned to complete the 12-week visit based on a computer algorithm conducted prior to study recruitment. Women who did not complete the 12-week home visit completed a brief phone survey to assess depressive symptoms.
Visits were conducted orally by interviewers in the participants’ language of choice: English (18%) or Spanish (82%). To account for variability in literacy, interviewers read survey items aloud and women were given written and graphic response descriptions. Prenatal visits occurred any time prior to birth (M = 35.40, SD = 2.49 weeks gestation). Postpartum interviews were scheduled to occur within one week of the infant’s chronological age for normal gestation; for infants born earlier than 37 weeks gestation, interview dates were scheduled based on corrected age. Visits lasted approximately 1.5–2 h. Women were compensated $75 for their participation in the prenatal visit and $50 for the 12-week visit. Of the 322 women who completed the prenatal visit, 205 (95% of those assigned) completed the 12-week visit. Approval to conduct this study was obtained from all relevant Institutional Review Boards and data collection procedures complied with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Measures
Depressive Symptoms
Prenatal and 12-week postpartum depressive symptoms were assessed using the ten-item Edinburgh Postpartum Depression Scale (EPDS) (Cox et al. 1987). The EPDS has been validated in English (Cox et al. 1987) and Spanish (Garcia-Esteve et al. 2003). Possible responses for each item ranged from zero (“Not at all/Never”) to three (“As much as you always do/most of the time”). Item scores were summed (maximum possible score = 30; prenatal α = 0.86; 12-week α = 0.835). Higher scores reflect higher levels of depressive symptoms.
Infant Health Concerns
Mothers reported health concerns on the 8-item Baby Health Questionnaire (Gress-Smith et al. 2012) at 12 weeks postpartum. The Baby Health Questionnaire asks about minor health problems commonly encountered in infancy to assess general health. Women were asked to endorse medical issues that had occurred for their infant, including: rash, colic, cold, fever, cough, ear infections, vomiting, and diarrhea. Endorsed items were summed to create an “infant health concerns score”. Higher scores reflect more health concerns (maximum possible score = 8).
Birth Outcomes
Infant gestational age and birth weight were collected from medical record abstraction at the delivery hospital.
Breastfeeding
At 12 weeks postpartum, women indicated whether they were currently breastfeeding. Breastfeeding was coded “0” if women endorsed bottle-feeding exclusively and “1” if they endorsed either breastfeeding plus bottle-feeding or exclusive breastfeeding.
Control Variables
Women provided demographic information on country of birth, age at intake, years of education completed, marital/partner status, number of other children, and estimated yearly household income.
Analytic Plan
Descriptive statistics and zero-order correlations were computed using SPSS v24. Multiple linear regressions were computed using MPlus 6.12 with the full sample of 322 women and their infants. To account for data missing-at-random, Full Information Maximum Likelihood estimation was used, a less biased procedure than other methods of estimation (Enders 2010). For maternal depression variables, an error in response sets led to one item on the prenatal measure being treated as missing data for 99 participants, and two items on the 12-week measure treated as missing for 64 participants. Multiple imputation using Mplus was used to address missing item-level values for these participants (Reiter and Raghunathan 2007).
For each regression, we tested appropriateness of the model using fit statistics (Chi square, RMSEA, CFI, and SRMR) as well as effect sizes. Continuous variables were centered at the sample mean. To address the first aim, a multiple regression model was computed with infant health concerns as the dependent variable and prenatal depressive symptoms as the independent variable. To address our second aim, a second regression model was computed with infant health concerns as the dependent variable, prenatal depressive symptoms and concurrent depressive symptoms as independent variables, with romantic partner and mother age as covariates. To address the third aim to examine mediators of the association between prenatal depressive symptoms and infant health concerns, three models were computed using gestational age, infant birth weight, and breastfeeding as putative mediators of prenatal depressive symptoms in predicting infant health concerns.
Results
Descriptive statistics and correlations are shown in Table 1. Median age was 27.79 (IQR = 22–33), 11 years education (IQR = 8–12), median number of other children was 2 (IQR = 1–3), 86% were born in Mexico, and 77% reported a romantic partner. Zero-order correlations are shown in Table 2. Prenatal depressive symptoms were positively correlated with 12-week infant health concerns (p < .01) and 12-week depressive symptoms (p < .01). Having a romantic partner was associated with fewer prenatal depressive symptoms (p < .05).
Table 1.
Descriptive statistics for variables of interest
| % | Median | Inter-quartile range | Minimum | Maximum | |
|---|---|---|---|---|---|
| Mother education (years) | 11 | 8–12 | 0 | 18 | |
| Mother age | 27.23 | 22.01–32.54 | 18 | 42 | |
| Born in Mexico | 86 | ||||
| Current romantic partner | 77 | ||||
| Number of other children | 2 | 1–3 | 0 | 9 | |
| Prenatal depressive symptoms | 5.00 | 1.00–9.25 | 0 | 24 | |
| 12-week depressive symptoms | 2 | 0–6 | 0 | 18 | |
| Birth variables | |||||
| Gestational age (weeks) | 39.50 | 38.71–40.14 | 26 | 42 | |
| Birth weight (g) | 3360 | 3115–3670 | 1190 | 4935 | |
| 12-week breastfeeding | 31 | ||||
| 12-week health concerns | 3 | 1–4 | 0 | 8 |
Breastfeeding was coded 0 if no breastfeeding, 1 if breastfeeding (some of the time or exclusively) was endorsed
Table 2.
Zero-order correlations
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mother education | - | |||||||||||
| 2 | Mother age | −0.283** | - | ||||||||||
| 3 | Romantic partner status | 0.008 | 0.143* | - | |||||||||
| 4 | Number other children | −0.426** | 0.612** | 0.106 | - | ||||||||
| 5 | Spanish language | −0.245** | 0.394** | 0.168** | 0.311** | - | |||||||
| 6 | Household income | 0.244** | 0.062 | 0.177** | −0.088 | −0.088 | - | ||||||
| 7 | Prenatal depressive symptoms | 0.044 | 0.001 | −0.113* | 0.001 | −0.029 | −0.017 | - | |||||
| 8 | 12-week depressive symptoms | 0.010 | 0.047 | −0.047 | −0.035 | −0.105 | 0.090 | 0.408** | - | ||||
| 9 | Gestational age (weeks) | −0.011 | −0.085 | 0.049 | −0.021 | 0.009 | −0.034 | −0.032 | 0.018 | - | |||
| 10 | Birth weight (g) | −0.110 | 0.142* | 0.101 | 0.178** | 0.064 | 0.039 | −0.019 | 0.037 | 0.521** | - | ||
| 11 | 12-week breastfeeding | −0.073 | 0.148* | 0.154* | 0.064 | 0.216** | 0.060 | 0.026 | −0.013 | 0.093 | 0.048 | - | |
| 12 | 12-week health concerns | 0.191** | −0.282** | 0.083 | −0.194** | −0.146* | 0.085 | 0.313** | 0.290** | −0.006 | −0.024 | 0.072 | - |
p < .05.
p < .01.
Romantic partner status was coded 0 if no partner, 1 if a partner was endorsed. Spanish language was coded 0 if English and 1 if Spanish. Breastfeeding was coded 0 if no breastfeeding, 1 if breastfeeding (some of the time or exclusively) was endorsed
For our first aim, romantic partner and mother age were included as covariates because they were correlated with infant health concerns. Education, household income, immigrant status, and number of other children were correlated with health concerns and were examined in the model; however, they were not included in the final model because they were unrelated with infant health concerns and negatively affected fit within the model. For the final model (Table 3, Aim 1), fit was very good (χ2 = 6.85, p = .23; RMSEA = 0.03 (90% CI 0–0.09), CFI = 0.99, SRMR = .04). Prenatal depressive symptoms significantly predicted more infant health concerns at twelve weeks (β = 0.06, SE = 0.01, p < .001; R2 = 0.22, SE = 0.05, p < .001). Older women (β= .29, SE = 0.06, p < .001) and those with romantic partners (β = 0.42, SE = 0.14, p < .01) reported fewer infant health concerns.
Table 3.
Results from multiple regressions predicting 12-week infant health concerns
| Dependent variable: 12-week infant health concerns | Aim 1 | Aim 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| B | β | S.E. | p | B | β | S.E. | p | |
| Prenatal depressive symptoms | 0.113 | 0.344 | 0.060 | < 0.001 | 0.087 | 0.266 | 0.067 | < 0.001 |
| Romantic partner | 0.738 | 0.175 | 0.059 | 0.003 | 0.716 | 0.171 | 0.058 | 0.003 |
| Mother age | − 0.079 | − 0.288 | 0.057 | < 0.001 | − 0.078 | − 0.286 | 0.057 | < 0.001 |
| 12-week depressive symptoms | - | 0.067 | 0.161 | 0.062 | 0.009 | |||
For the second aim, (Table 3, Aim 2), model fit was adequate (χ2 = 11.37, p = .12; RMSEA = 0.04 (90% CI 0–.09), CFI = 0.98, SRMR = .05). Prenatal depressive symptoms remained a statistically significant predictor of infant health concerns (β = 0.26, SE = 0.07, p < .001; R2 = 0.24, SE = 0.05, p < .001). Concurrent depression was also associated with more infant health concerns (β = 0.16, SE = 0.06, p < .01; R2 = 0.23, SE = 0.05, p < .001).
In the three models computed to address the third aim, prenatal depressive symptoms did not predict gestational age (p = .56), birth weight (p = .46) or breastfeeding (p = .36). In addition, infant health concerns were not predicted by gestational age (p = .39), birth weight (p = .96), or breastfeeding (p = .31). None of the indirect paths from prenatal depression to health concerns were significant (gestational age p = .69; birth weight p = .96; breastfeeding p = .91). Combined findings from all three aims are summarized conceptually in Fig. 1.
Fig. 1.
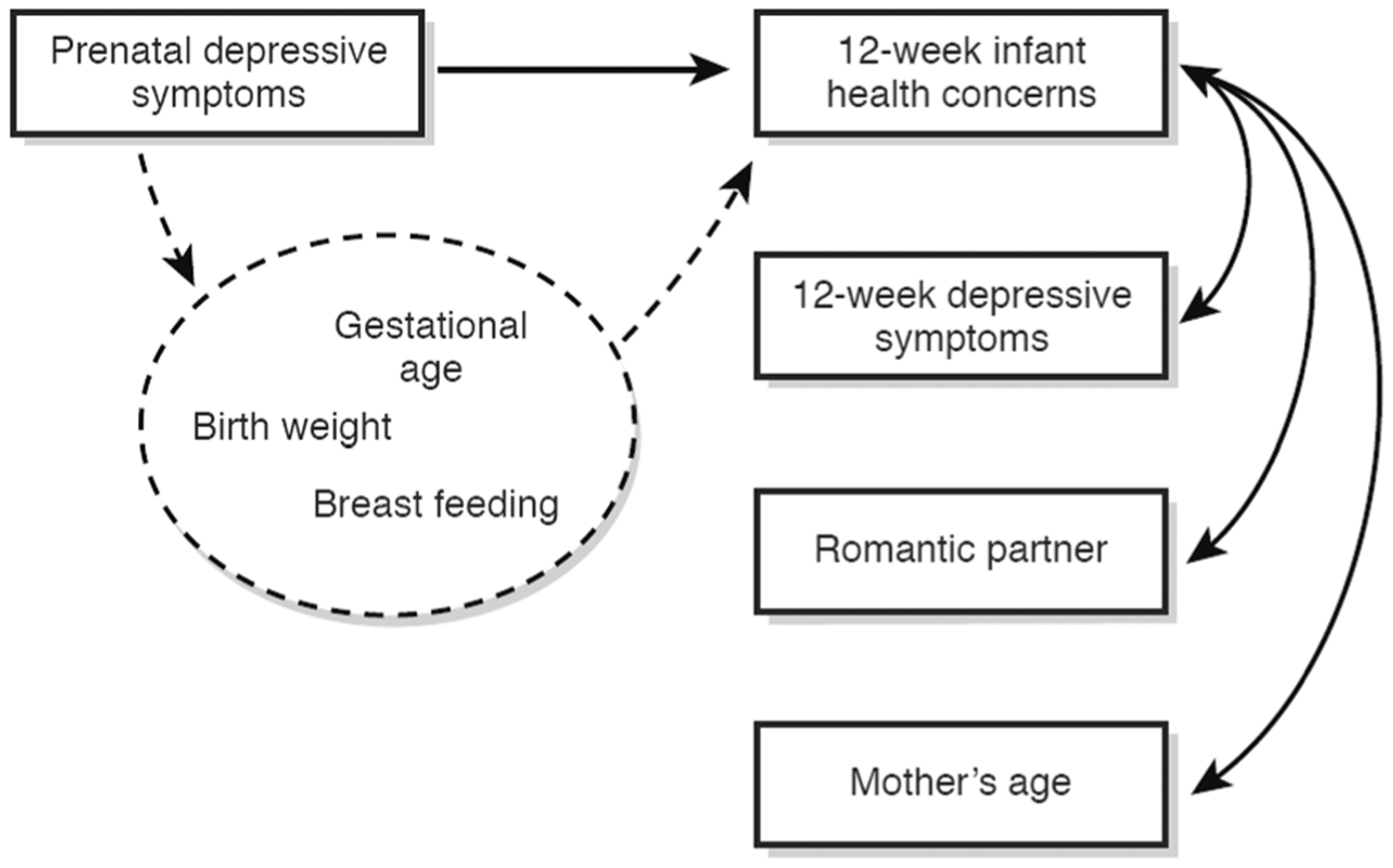
Conceptual model summarizing results from multiple regressions testing 12-week infant health concerns above and beyond 12-week maternal depressive symptoms as well as potential mediators (Aims 1, 2, and 3). Dashed lines indicate nonsignificant associations
Discussion
Low-income and ethnic minority children face health disparities in a number of domains relative to higher SES and ethnic majority children, including a higher lifetime risk of chronic illness (Dominguez et al. 2015). As early social determinants of health have been targeted as a priority for research (NIH Health Disparities Strategic Plan) and fetal origins of adult illness have been identified (Calkins and Devaskar 2011), perinatal depression is a key, possibly modifiable factor in the emergence of health problems among at-risk infants. As hypothesized, we found that higher prenatal maternal depressive symptoms predicted more infant health concerns at 12 weeks of age (rash, colic, cold, fever, cough, ear infections, vomiting, and diarrhea), above and beyond the impact of concurrent maternal depressive symptoms. Contrary to hypotheses, there was no evidence that this association was mediated by birth outcomes or breastfeeding. Findings from this study warrant further investigation to explore other factors that may potentially influence the emergence of health concerns in infants among women who experience prenatal depressive symptoms. It will be important to consider contextual factors affecting women from Mexican American, low-income backgrounds in developing appropriate follow-up studies and interventions designed to treat prenatal depression and mitigate infant health risks.
Prenatal Depressive Symptoms
Although prenatal depression is a known risk factor for disrupted infant development and physiological functioning (Field 2011), surprisingly little research has investigated effects on physical health. Prenatal depression is associated with negative birth outcomes (Field et al. 2004), and antenatal maternal depression has been linked to impaired growth in infants in developing countries (Stewart 2007) as well as more health concerns and more sleep disturbance (Gress-Smith et al. 2012). Our findings support the hypothesis that depressive symptoms may confer risk for infant health concerns, above and beyond concurrent postpartum depressive symptoms.
Physiological development in utero is dependent on inherited susceptibilities and internal and external conditions. In light of our findings, one possible consideration is that excess levels of certain hormones associated with maternal depression may interfere with normal fetal development, referred to as fetal programming (Seckl 2001). Moreover, pregnant women who are depressed may be less likely to engage in positive health practices, such as attending routine prenatal appointments, getting enough sleep, and smoking cessation (Lindgren 2001). Programming likely occurs through several mechanisms, such as altered diet, stress, inflammation, and glucocorticoids (Calkins and Devaskar 2011). Together, the physiological ramifications of elevated psychological depressive symptoms, compounded by less health-promoting behavior, could create teratogenic effects on health and development of the child.
Postpartum Depressive Symptoms
This study offers the advantage of examining depressive symptoms across the perinatal period to clarify influences on infant health. In addition to prenatal effects, higher maternal depressive symptoms during the postpartum period (12 weeks) were associated with more infant health concerns. This is consistent with other findings among low-SES Mexican-American women and their infants (Gress-Smith et al. 2012). Women with prenatal depressive symptoms are more likely to develop postpartum depression, which can also have negative consequences on children, such as emotional and behavior problems, attachment difficulties, cognitive deficits, physical growth and development, and feeding habits and attitudes (Stein et al. 2014). Related, maternal depression affects parenting behaviors (Bornstein et al. 2011), which in turn could affect child outcomes. Thus, women’s mental health throughout the perinatal period should be a priority, not only to support women, but also to promote optimal functioning for their infants.
Birth Outcomes and Breastfeeding
Contrary to other reports (Field et al. 2004; Grote et al. 2010), there was no indication that prenatal depressive symptoms predicted lower birth weight, earlier gestational age, or less breastfeeding. Although there are no available studies that serve as direct comparisons, our findings differ from those that show that poorer birth outcomes predict poorer parent- or physician-rated developmental functioning (Hack et al. 1995) as well as increased objectively measured health care utilization in the first year (Mac Bird et al. 2010). Given the strength in this study that we used objective birth outcomes as well as mother-reported breastfeeding, it is intriguing that we detected no correlation between birth outcomes nor breastfeeding with any of our key variables. It is possible that we did not have sufficient power to detect smaller effects. Nonetheless, another consideration is that Mexican American women may experience risks differently than others.
Although links are well established in Caucasian samples, studies examining Mexican American samples have not found the same associations between prenatal depression and poorer birth outcomes or breastfeeding. Specifically, Hispanic women have lower odds for preterm birth (OR 0.66; 95% CI 0.54–0.80), even in the presence of “risk factors” established for Caucasian samples (Fuentes-Afflick et al. 1999). Additionally, the associations between prenatal depression and breastfeeding appear to be nuanced, and breastfeeding practices among Mexican American women are not well understood (Wambach et al. 2016). For example, Fairlie and colleagues (2009) found that although mothers reporting more symptoms of prenatal depression were less likely to plan to initiate breastfeeding, prenatal depressive symptoms were not actually related to initiation of breastfeeding. Nonetheless, other studies suggest that prenatal depressive symptoms may be related to shorter durations of breastfeeding (Figueiredo et al. 2014; Ystrom 2012). Thus, future studies investigating the impact of breastfeeding on health outcomes would do well to consider more detailed assessments of breastfeeding, such as by including both rates and duration of exclusive breastfeeding.
Clinical Implications
Screening and prevention during the sensitive period of pregnancy are important strategies for reducing disparities and improving maternal and infant health trajectories. Whereas women are commonly screened for postpartum depression after the birth of a child, the present study implicates pregnancy as a critical period for identifying depressed women, as it may be important for improving women’s mental health and reducing health risks for infants. Unfortunately, despite recommendations, psychological screening is not consistently incorporated into routine care (Kingston et al. 2012). Intervening on symptoms during pregnancy could potentially prevent or mitigate damaging effects in utero (Lazinski et al. 2008). Our results suggest a continuum in the link between maternal depressive symptoms and infant health; pregnant women with subclinical depressive symptoms may benefit from intervention. Focusing on mental health and self-care during pregnancy could have lasting effects on their children’s health; communicating this importance may motivate women to seek treatment for their prenatal depressive symptoms (Dunkel Schetter and Tanner 2012).
Limitations and Future Directions
The sample for this study consisted of Mexican American women, most of whom were immigrants; generalizability of findings to other populations may be limited but requires further exploration. In addition, the data consisted of mother-reported depression and infant health as well as objective data on birth outcomes. The primary findings involved variables based on mother-report, raising the possibility of reporting bias or shared method variance. However, this concern is mitigated somewhat by controlling for concurrent maternal depressive symptoms. Related, our measure of infant health concerns does not necessarily reflect underlying physical illness or disorder, and was limited to early infancy. The study would be strengthened by longer follow-up with objective health outcomes, which were not available in the present study.
This study has implications for follow-up research on the effects of prenatal depression on infant health. First, this study used longitudinal methods to examine women’s depressive symptoms before and after birth. Follow-up studies will clarify long-term effects on infant health, which could have repercussions for childhood health and development. Second, this investigation identifies the importance of maternal mental health during the perinatal period. Additional work should replicate these findings and examine the specific aspects of depressive symptoms during pregnancy that may negatively affect infants, as well as potential links between previous depression, prenatal depressive symptoms, and infant health. Third, future research should include additional measures of infant health using medical records, physiological measures, or other objective sources of information. Fourth, future studies should investigate other potential mediators, including negative birth experiences, preterm birth, prenatal substance use, method of delivery, and adverse experiences in infancy, which could inform future interventions. Finally, screening programs should be implemented to identify and mitigate maternal depression during pregnancy and evaluate their efficacy for preventing infant health concerns.
Conclusions
This study offers evidence that prenatal maternal depressive symptoms may be associated with later infant health, above and beyond concurrent maternal depressive symptoms, in low-income Mexican American families. There was no indication that gestational age, birth weight, or breastfeeding mediated this process. Our findings highlight the importance of additional work to examine the environmental and physiological processes that may influence infants during pregnancy and after birth. The prenatal period is critical for child health and may have lasting health ramifications for health across the lifespan; therefore, research efforts must focus on the fetal origins of long-term health to understand its mechanisms and develop and test interventions. Effective screening and therapeutic programs may have the potential to not only improve trajectories of health population-wide, but also reduce health disparities in low income ethnic minority populations.
Significance.
What does this study add?
This prospective investigation found that infants of low-income Mexican-origin women with elevated depressive symptoms during pregnancy had more mother-reported health concerns at 12 weeks of age. The results emphasize the importance of evaluating mental health during pregnancy and its ramifications for children’s health
Acknowledgements
This study is supported by a Grant, R01 NIMH MH083173 (MPIs: Keith Crnic, Nancy Gonzales and Linda Luecken). The first author of this manuscript was supported by an institutional training Grant, T32 MH18387, NIMH. We thank the mothers and infants for their participation; Kirsten Letham, Anne Mauricio, Monica Gutierrez, and Craig Enders, for their assistance with data collection and management; Dr. Dean Coonrod and the Maricopa Integrated Health Systems for their assistance with recruitment; Kim Battista for graphical design assistance; and the interviewers for their commitment and dedication to this project.
Footnotes
Conflict of interest
The authors of this manuscript have no conflicts of interests, including financial interests or gains.
References
- Beijers R, Jansen J, Riksen-Walraven M, & de Weerth C (2010). Maternal prenatal anxiety and stress predict infant illnesses and health complaints. Pediatrics, 126(2), e401–9. 10.1542/peds.2009-3226. [DOI] [PubMed] [Google Scholar]
- Bennett HA, Einarson A, Taddio A, Koren G, & Einarson TR (2004). Prevalence of depression during pregnancy: systematic review. Obstetrics and Gynecology, 103(4), 698–709. 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Hahn C, & Haynes OM (2011). Maternal personality, parenting cognitions, and parenting practices. Developmental Psychology, 47(3), 658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle EM, Poulsen G, Field DJ, Kurinczuk JJ, Wolke D, Alfirevic Z, & Quigley MA (2012). Effects of gestational age at birth on health outcomes at 3 and 5 years of age: population based cohort study. British Medical Journal (Clinical Research Ed.), 344, e896. 10.1136/bmj.e896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins K, & Devaskar SU (2011). Fetal origins of adult disease. Current Problems in Pediatric and Adolescent Health Care, 41(6), 158–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung EK, McCollum KF, Elo IT, Lee HJ, & Culhane JF (2004). Maternal depressive symptoms and infant health practices among low-income women. Pediatrics, 113(6), e523–e529. [DOI] [PubMed] [Google Scholar]
- Chung M, Raman G, Chew P, Magula N, Trikalinos T, & Lau J (2007). Breastfeeding and maternal and infant health outcomes in developed countries. Evidence Report/Technology Assessment, 153, 1–186. [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Holden JM, & Sagovsky R (1987). Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. The British Journal of Psychiatry: The Journal of Mental Science, 150, 782–786. [DOI] [PubMed] [Google Scholar]
- Dominguez K, Penman-Aguilar A, Chang M, Moonesinghe R, Castellanos T, Rodriguez-Lainz A, & Schieber R (2015). Vital signs: Leading causes of death, prevalence of diseases and risk factors, and use of health services among Hispanics in the United States—2009–2013. MMWR Morbidity and Mortality Weekly Report, 64(17), 469–478. [PMC free article] [PubMed] [Google Scholar]
- Dunkel Schetter C, & Tanner L (2012). Anxiety, depression and stress in pregnancy: Implications for mothers, children, research,and practice. Current Opinion in Psychiatry, 25(2), 141–148. 10.1097/YCO.0b013e3283503680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK (2010). Applied missing data analysis. New York: Guil-ford Press. [Google Scholar]
- Fairlie TG, Gillman MW, & Rich-Edwards J (2009). High pregnancy-related anxiety and prenatal depressive symptoms as predictors of intention to breastfeed and breastfeeding initiation. Journal of Women’s Health, 18(7), 945–953. 10.1089/jwh.2008.0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T (2011). Prenatal depression effects on early development: A review. Infant Behavior and Development, 34(1), 1–14. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Dieter J, Hernandez-Reif M, Schanberg S, Kuhn C, et al. (2004). Prenatal depression effects on the fetus and the newborn. Infant Behavior and Development, 27(2), 216–229. [Google Scholar]
- Field T, Hernandez-Reif M, & Diego M (2011). Depressed mothers’newborns are less responsive to animate and inanimate stimuli. Infant and Child Development, 20(1), 94–105. 10.1002/icd.687. [DOI] [Google Scholar]
- Figueiredo B, Canário C, & Field T (2014). Breastfeeding is negatively affected by prenatal depression and reduces postpartum depression. Psychological Medicine, 44(05), 927–936. [DOI] [PubMed] [Google Scholar]
- Fuentes-Afflick E, Hessol NA, & Pérez-Stable EJ (1999). Testing the epidemiologic paradox of low birth weight in Latinos. Archives of Pediatrics & Adolescent Medicine, 153(2), 147–153. [DOI] [PubMed] [Google Scholar]
- Garcia-Esteve L, Ascaso C, Ojuel J, & Navarro P (2003). Validation of the Edinburgh postnatal depression scale (EPDS) in Spanish mothers. Journal of Affective Disorders, 75(1), 71–76. [DOI] [PubMed] [Google Scholar]
- Gress-Smith JL, Luecken LJ, Lemery-Chalfant K, & Howe R (2012). Postpartum depression prevalence and impact on infant health, weight, and sleep in low-income and ethnic minority women and infants. Maternal and Child Health Journal, 16(4), 887–893. [DOI] [PubMed] [Google Scholar]
- Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, & Katon WJ (2010). A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Archives of General Psychiatry, 67(10), 1012–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack M, Klein NK, & Taylor HG (1995). Long-term developmental outcomes of low birth weight infants. The Future of Children, 5, 176–196. [PubMed] [Google Scholar]
- Kajantie E (2006). Fetal origins of stress-related adult disease Stress, obesity, and metabolic syndrome (pp. 11–27) New York: New York Academy of Sciences. [DOI] [PubMed] [Google Scholar]
- Kingston D, Tough S, & Whitfield H (2012). Prenatal and postpartum maternal psychological distress and infant development: a systematic review. Child Psychiatry & Human Development, 43(5), 683–714. [DOI] [PubMed] [Google Scholar]
- Lara MA, Le H, Letechipia G, & Hochhausen L (2009). Prenatal depression in Latinas in the US and Mexico. Maternal and Child Health Journal, 13(4), 567–576. [DOI] [PubMed] [Google Scholar]
- Lazinski MJ, Shea AK, & Steiner M (2008). Effects of maternal prenatal stress on offspring development: A commentary. Archives of Women’s Mental Health, 11(5–6), 363–375. [DOI] [PubMed] [Google Scholar]
- Lindgren K (2001). Relationships among maternal–fetal attachment, prenatal depression, and health practices in pregnancy. Research in Nursing & Health, 24(3), 203–217. [DOI] [PubMed] [Google Scholar]
- Mac Bird T, Bronstein JM, Hall RW, Lowery CL, Nugent R, & Mays GP (2010). Late preterm infants: Birth outcomes and health care utilization in the first year. Pediatrics, 126(2), e311–e319. [DOI] [PubMed] [Google Scholar]
- Midodzi WK, Rowe BH, Majaesic CM, Saunders LD, & Sen-thilselvan A (2010). Early life factors associated with incidence of physician-diagnosed asthma in preschool children: Results from the Canadian Early Childhood Development cohort study. Journal of Asthma, 47(1), 7–13. [DOI] [PubMed] [Google Scholar]
- NIH Health Disparities Strategic Plan. NIH health disparities strategic plan and budget, fiscal years 2009–2013 (extended to FY2016). [Google Scholar]
- Rahman A, Iqbal Z, Bunn J, Lovel H, & Harrington R (2004). Impact of maternal depression on infant nutritional status and illness: A cohort study. Archives of General Psychiatry, 61(9), 946–952. [DOI] [PubMed] [Google Scholar]
- Reiter JP, & Raghunathan TE (2007). The multiple adaptations of multiple imputation. Journal of the American Statistical Association, 102(480), 1462–1471. [Google Scholar]
- Rossiter MD, Colapinto CK, Khan MK, McIsaac JLD, Williams PL, Kirk SF, & Veugelers PJ (2015). Breast, formula and combination feeding in relation to childhood obesity in Nova Scotia, Canada. Maternal and Child Health Journal, 19(9), 2048–2056. [DOI] [PubMed] [Google Scholar]
- Seckl JR (2001). Glucocorticoid programming of the fetus; adult phenotypes and molecular mechanisms. Molecular and Cellular Endocrinology, 185(1), 61–71. [DOI] [PubMed] [Google Scholar]
- Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, et al. (2014). Effects of perinatal mental disorders on the fetus and child. The Lancet, 384(9956), 1800–1819. [DOI] [PubMed] [Google Scholar]
- Stewart RC (2007). Maternal depression and infant growth—A review of recent evidence. Maternal & Child Nutrition, 3(2), 94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambach K, Domian EW, Page-Goertz S, Wurtz H, & Hoffman K (2016). Exclusive breastfeeding experiences among Mexican American women. Journal of Human Lactation: Official Journal of International Lactation Consultant Association, 32(1), 103–111. 10.1177/0890334415599400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystrom E (2012). Breastfeeding cessation and symptoms of anxiety and depression: A longitudinal cohort study. BMC Pregnancy and Childbirth, 12, 36–36. [DOI] [PMC free article] [PubMed] [Google Scholar]


